Efficient Degradation of Untreated Complex Cellulosic Substrates by Newly Isolated Aerobic Paenibacillus Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Enrichment of Aerobic Consortium
2.2. Screening of Cellulose-Degrading Bacteria
2.3. Molecular Identification of Cellulose-Degrading Bacteria
2.4. Growth Estimation and Analysis of Cellulose Degradation
2.5. Biodegradation of Agricultural Residue
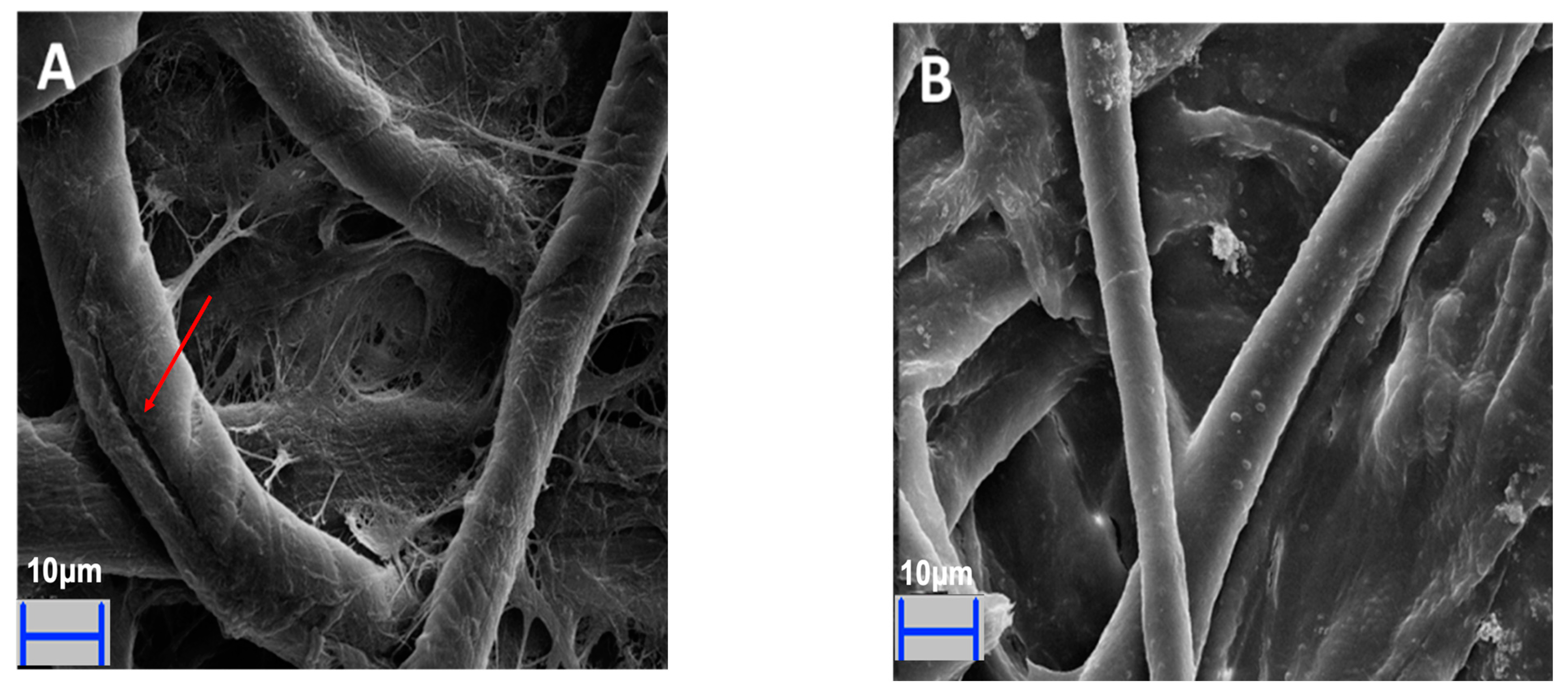
2.6. Scanning Electron Microscope (SEM) Analysis
3. Results
3.1. Development of Cellulose-Degrading Consortium
3.2. Screening and Identification of Cellulose-Degrading Bacteria
3.3. Biodegradation of Cellulosic Substrates by Paenibacillus sp. C7
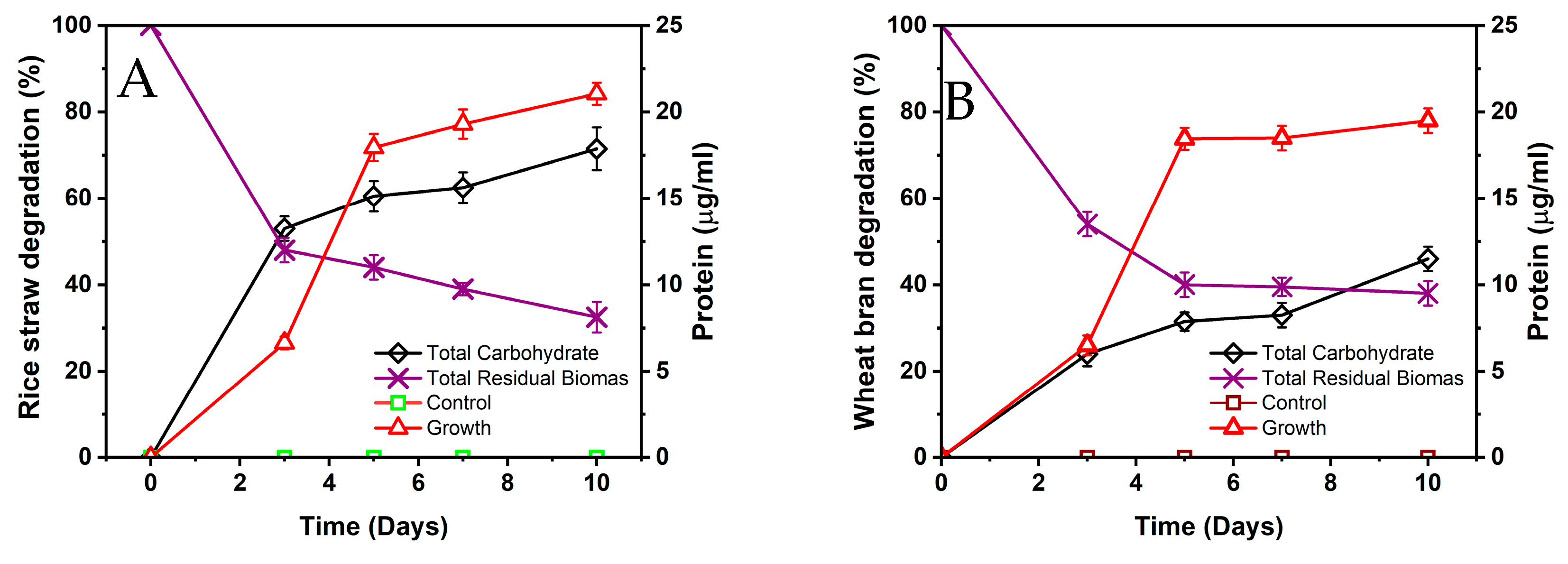
3.4. Biodegradation of Agricultural Residues by Paenibacillus sp. C7
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taherzadeh, M.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Bayer, E.A.; Lamed, R.; Himmel, M.E. The Potential of Cellulases and Cellulosomes for Cellulosic Waste Management. Curr. Opin. Biotechnol. 2007, 18, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Raj, C.S.; Arul, S.; Sendilvelan, S.; Saravanan, C.G. Bio Gas from Textile Cotton Waste—An Alternate Fuel for Diesel Engines. Open Waste Manag. J. 2009, 2, 1–5. [Google Scholar] [CrossRef]
- Thompson, G.; Swain, J.; Kay, M.; Forster, C.F. The Treatment of Pulp and Paper Mill Effluent: A Review. Bioresour. Technol. 2001, 77, 275–286. [Google Scholar] [CrossRef]
- Yeber, M.C.; Silva, T. The Ability of a Bacterial Strain to Remove a Phenolic Structure as an Approach to Pulp and Paper Mill Wastewater Treatment: Optimization by Experimental Design. Water 2022, 14, 3296. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A Critical Review on Analysis in Pretreatment of Lignocelluloses: Degree of Polymerization, Adsorption/Desorption, and Accessibility. Bioresour. Technol. 2016, 203, 348–356. [Google Scholar] [CrossRef]
- Mohammed, A.H. Conversion of Lignocellulosic Material into Fermentable Sugars. Al-Khwarizmi Eng. J. 2012, 12, 141–153. [Google Scholar] [CrossRef]
- Wilson, D.B. Microbial Diversity of Cellulose Hydrolysis. Curr. Opin. Microbiol. 2011, 14, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Hofsten, B.V.; Edberg, N. Estimating the Rate of Degradation of Cellulose Fibers in Water. Oikos 1972, 23, 29. [Google Scholar] [CrossRef]
- Warnock, W.; Davis, K.; Wolf, D.; Gbur, E. Biodegradation of Three Cellulosic Fabrics in Soil. Summ. Ark. Cotton Res. 2009, 2009, 208–211. [Google Scholar]
- Weimer, P.J. Cellulose Degradation by Ruminal Microorganisms. Crit. Rev. Biotechnol. 1992, 12, 189–223. [Google Scholar] [CrossRef]
- Insam, H.; Franke-Whittle, I.; Goberna, M. Microbes in Aerobic and Anaerobic Waste Treatment. In Microbes at Work: From Wastes to Resources; Insam, H., Franke-Whittle, I., Goberna, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–34. ISBN 978-3-642-04043-6. [Google Scholar]
- Weimer, P.J. Degradation of Cellulose and Hemicellulose by Ruminal Microorganisms. Microorganisms 2022, 10, 2345. [Google Scholar] [CrossRef]
- Saye, L.M.; Navaratna, T.A.; Chong, J.P.; O’Malley, M.A.; Theodorou, M.K.; Reilly, M. The Anaerobic Fungi: Challenges and Opportunities for Industrial Lignocellulosic Biofuel Production. Microorganisms 2021, 9, 694. [Google Scholar] [CrossRef]
- Sanchez, W.; Piccini, B.; Ditche, J.-M.; Porcher, J.-M. Assessment of Seasonal Variability of Biomarkers in Three-Spined Stickleback (Gasterosteus aculeatus L.) from a Low Contaminated Stream: Implication for Environmental Biomonitoring. Environ. Int. 2008, 34, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.; Brune, D. Anaerobic Co-Digestion of Algal Sludge and Waste Paper to Produce Methane. Bioresour. Technol. 2007, 98, 130–134. [Google Scholar] [CrossRef]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-State Anaerobic Digestion for Methane Production from Organic Waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic Digestion of Lignocellulosic Biomass: Challenges and Opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef]
- Sodhi, K.K.; Singh, C.K. Recent Development in the Sustainable Remediation of Antibiotics: A Review. Total Environ. Res. Themes 2022, 3, 100008. [Google Scholar] [CrossRef]
- Chauhan, A.; Islam, F.; Imran, A.; Ikram, A.; Zahoor, T.; Khurshid, S.; Shah, M.A. A Review on Waste Valorization, Biotechnological Utilization, and Management of Potato. In Food Science & Nutrition; Wiley Online Library: Hoboken, NJ, USA, 2023; Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/fsn3.3546 (accessed on 16 June 2024).
- Sharma, D.; Joshi, B.; Bhatt, M.R.; Joshi, J.; Malla, R.; Bhattarai, T.; Sreerama, L. Isolation of Cellulolytic Organisms from the Gut Contents of Termites Native to Nepal and Their Utility in Saccharification and Fermentation of Lignocellulosic Biomass. J. Biomass-Biofuel 2015, 2, 11–20. [Google Scholar] [CrossRef]
- Itzhaki, R.F.; Gill, D.M. A Micro-Biuret Method for Estimating Proteins. Anal. Biochem. 1964, 9, 401–410. [Google Scholar] [CrossRef]
- Kumar, M.; Revathi, K.; Khanna, S. Biodegradation of Cellulosic and Lignocellulosic Waste by Pseudoxanthomonas Sp R-28. Carbohydr. Polym. 2015, 134, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.A.; Sizova, M.V.; Lynd, L.R. Diversity of Bacteria and Glycosyl Hydrolase Family 48 Genes in Cellulolytic Consortia Enriched from Thermophilic Biocompost. Appl. Environ. Microbiol. 2010, 76, 3545–3553. [Google Scholar] [CrossRef] [PubMed]
- Wongwilaiwalin, S.; Rattanachomsri, U.; Laothanachareon, T.; Eurwilaichitr, L.; Igarashi, Y.; Champreda, V. Analysis of a Thermophilic Lignocellulose Degrading Microbial Consortium and Multi-Species Lignocellulolytic Enzyme System. Enzym. Microb. Technol. 2010, 47, 283–290. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, Y.; Wang, X.; Qu, Y.; Li, D.; He, W.; Kim, B.H. Degradation of Raw Corn Stover Powder (RCSP) by an Enriched Microbial Consortium and Its Community Structure. Bioresour. Technol. 2011, 102, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yan, L.; Cui, Z.; Gao, Y.; Wang, Y.; Jing, R. Characterization of a Microbial Consortium Capable of Degrading Lignocellulose. Bioresour. Technol. 2011, 102, 9321–9324. [Google Scholar] [CrossRef] [PubMed]
- Lazuka, A.; Auer, L.; Bozonnet, S.; Morgavi, D.P.; O’Donohue, M.; Hernandez-Raquet, G. Efficient Anaerobic Transformation of Raw Wheat Straw by a Robust Cow Rumen-Derived Microbial Consortium. Bioresour. Technol. 2015, 196, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-M.; Xu, X.; Ruan, L.-W. Enrichment and Characterization of an Anaerobic Cellulolytic Microbial Consortium SQD-1.1 from Mangrove Soil. Appl. Microbiol. Biotechnol. 2014, 98, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Kinet, R.; Destain, J.; Hiligsmann, S.; Thonart, P.; Delhalle, L.; Taminiau, B.; Daube, G.; Delvigne, F. Thermophilic and Cellulolytic Consortium Isolated from Composting Plants Improves Anaerobic Digestion of Cellulosic Biomass: Toward a Microbial Resource Management Approach. Bioresour. Technol. 2015, 189, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Haruta, S.; Cui, Z.; Huang, Z.; Li, M.; Ishii, M.; Igarashi, Y. Construction of a Stable Microbial Community with High Cellulose-Degradation Ability. Appl. Microbiol. Biotechnol. 2002, 59, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Lu, Y.; Guo, P.; Wang, X.; Mochidzuki, K.; Cui, Z. Bioconversion of Un-Pretreated Lignocellulosic Materials by a Microbial Consortium XDC-2. Bioresour. Technol. 2013, 136, 481–487. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, K.; Huang, M.; Hector, S.B.; Liu, B.; Tong, C.; Liu, Q.; Zeng, J.; Gao, Y.; Xu, T.; et al. Involvement of Fenton Chemistry in Rice Straw Degradation by the Lignocellulolytic Bacterium Pantoea Ananatis Sd-1. Biotechnol. Biofuels 2016, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Egwuatu, T.F.; Appeh, O.G. Isolation and Characterization of Filter Paper Degrading Bacteria from the Guts of Coptotermes Formosanus. J. Bioremediation Biodegrad. 2018, 9, 440. [Google Scholar]



| Consortium | Degradation Percentage of Filter Paper | Number of Days | Temp (°C) | Reference |
|---|---|---|---|---|
| MC1 (aerobic and anaerobic) | 79 | 4 | 50 | [31] |
| MC3F (aerobic and anaerobic) | 54.7 | 7 | 50 | [25] |
| H-C (aerobic and anaerobic) | 81 | 8 | 40 | [26] |
| Con R (aerobic) | 83 | 15 | 37 | [23] |
| Bac-3 (aerobic) | 95 | 30 | 30 | [34] |
| CSW (aerobic) | 91 | 7 | 37 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, R.S.; Pragati; He, W.; Li, C.; Mishra, J.; Feng, Y. Efficient Degradation of Untreated Complex Cellulosic Substrates by Newly Isolated Aerobic Paenibacillus Species. Water 2024, 16, 1800. https://doi.org/10.3390/w16131800
Yadav RS, Pragati, He W, Li C, Mishra J, Feng Y. Efficient Degradation of Untreated Complex Cellulosic Substrates by Newly Isolated Aerobic Paenibacillus Species. Water. 2024; 16(13):1800. https://doi.org/10.3390/w16131800
Chicago/Turabian StyleYadav, Ravi Shankar, Pragati, Weihua He, Chao Li, Juhi Mishra, and Yujie Feng. 2024. "Efficient Degradation of Untreated Complex Cellulosic Substrates by Newly Isolated Aerobic Paenibacillus Species" Water 16, no. 13: 1800. https://doi.org/10.3390/w16131800








