Characteristics and Significance of Natural Nanoparticles in the Groundwater of the Baotu Spring Area in Jinan, Shandong Province, Eastern China
Abstract
:1. Introduction
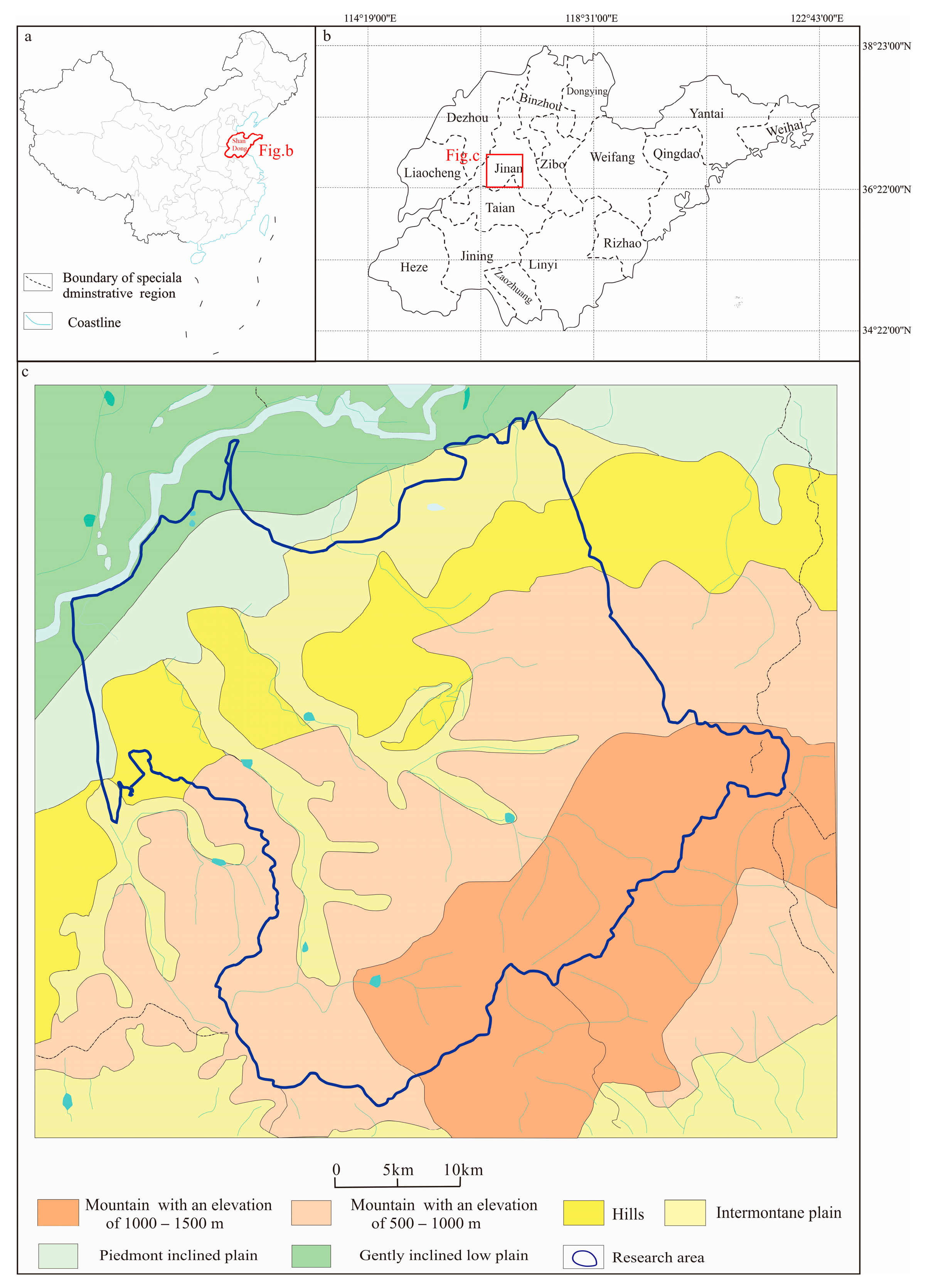
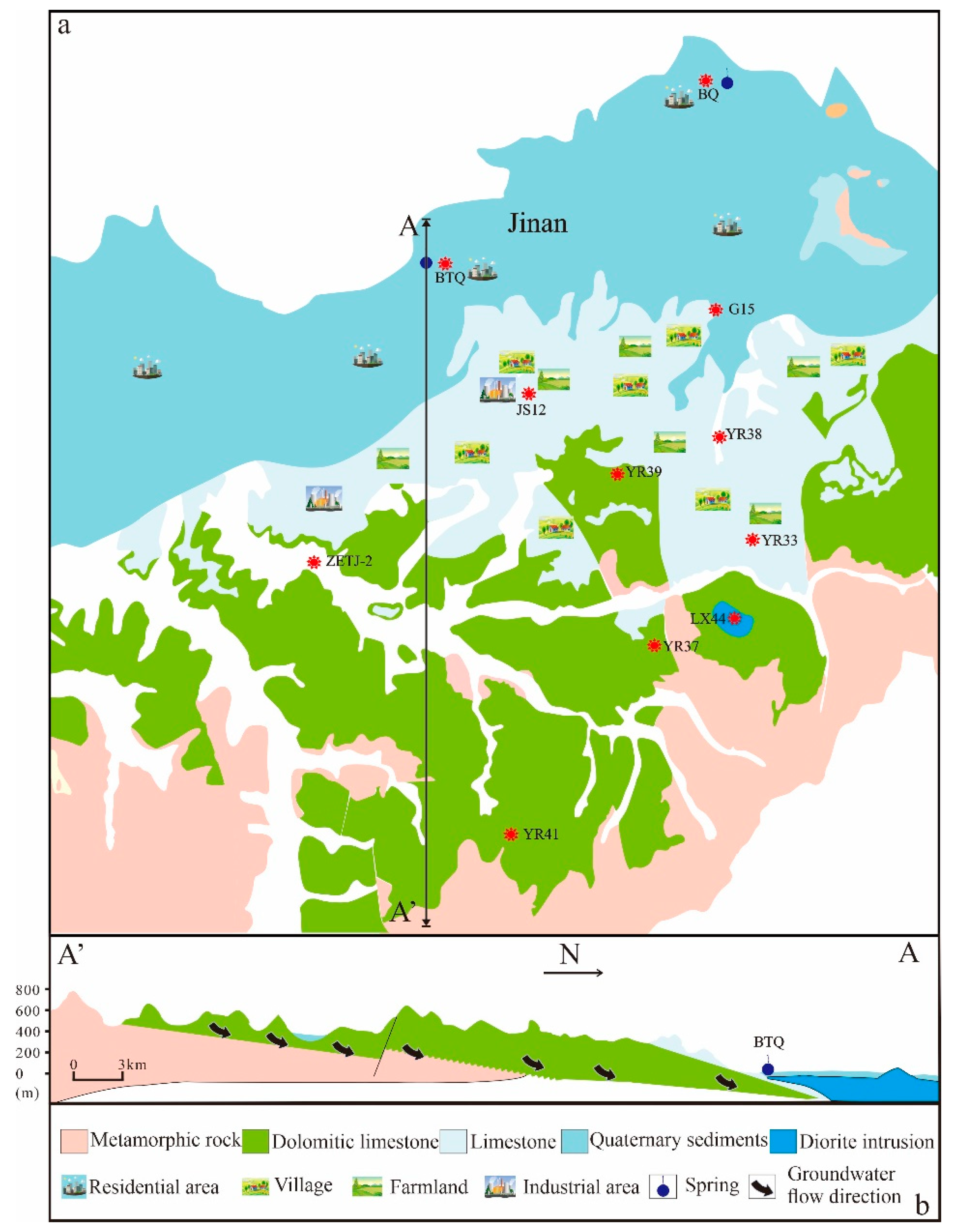
2. Geological Setting
3. Sampling and Analytical Methods
4. Results and Discussion
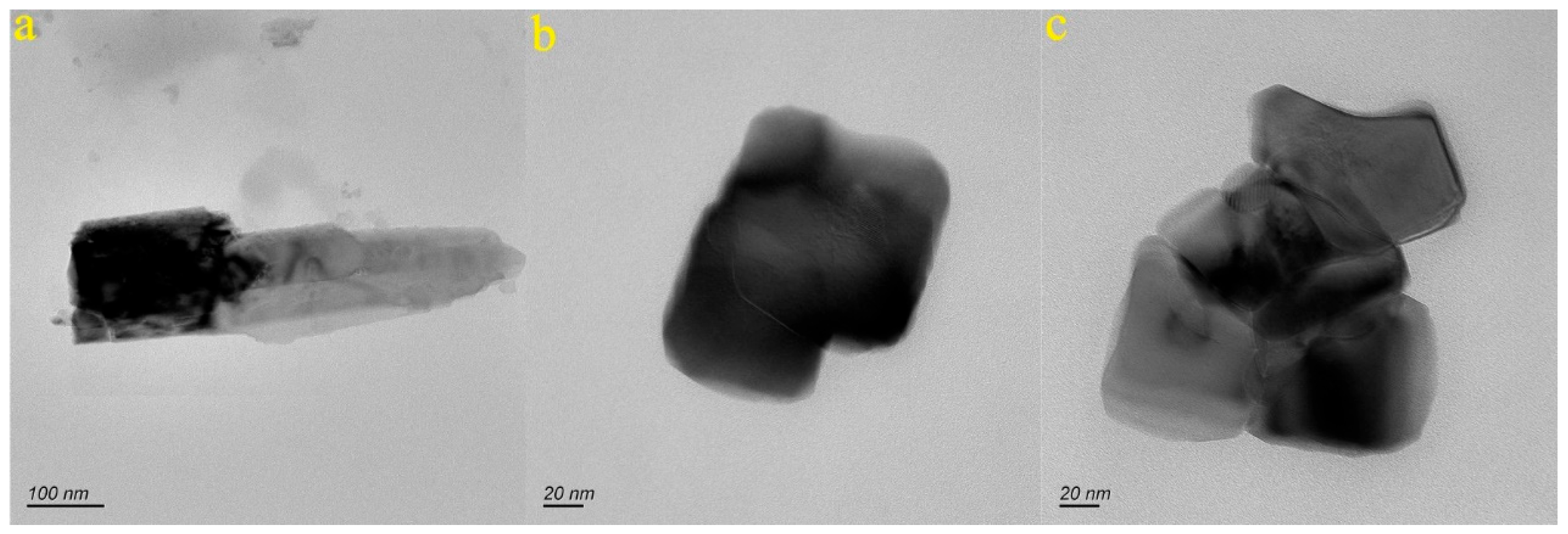
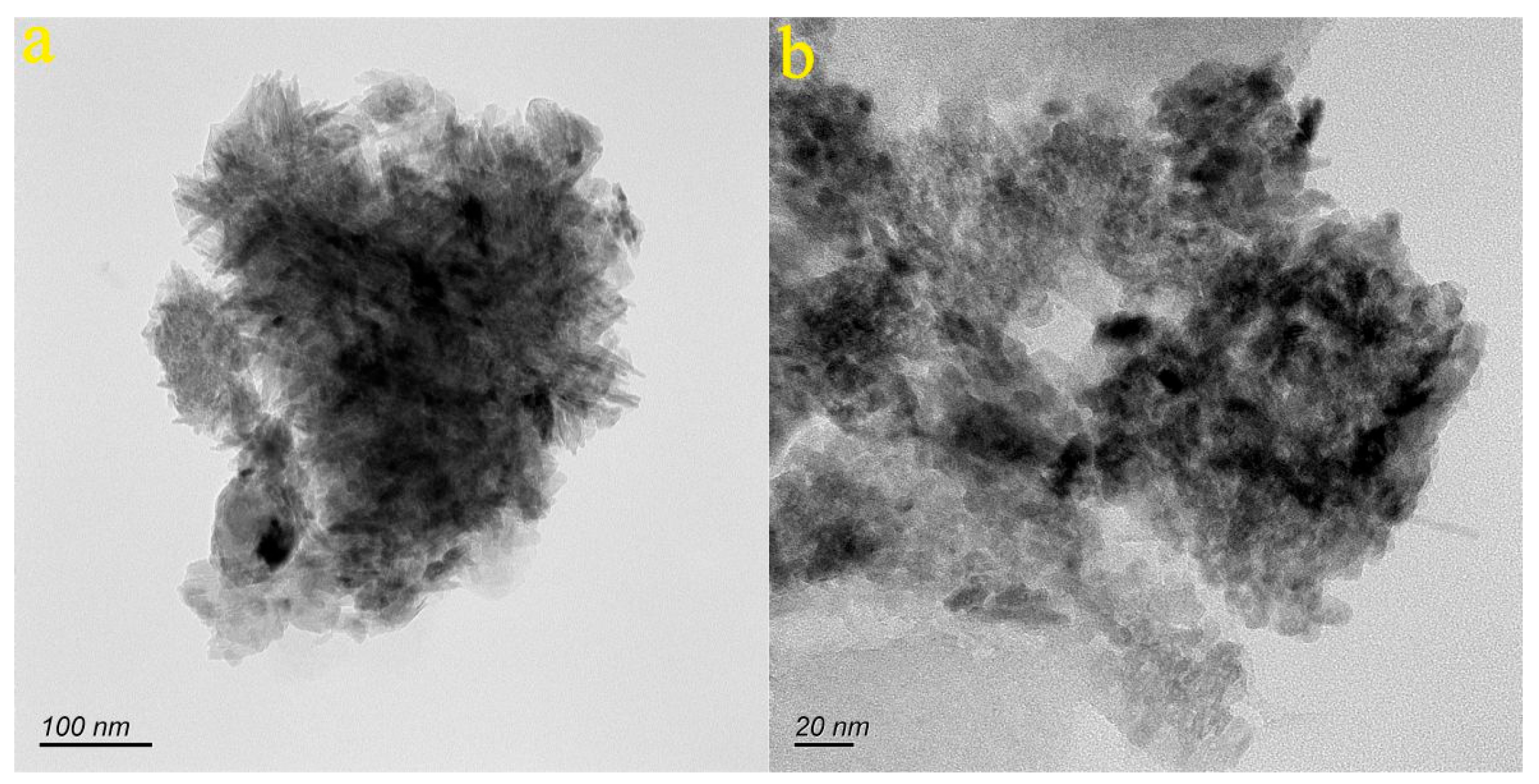
4.1. Characteristics of Nanoparticle in the Groundwater of the Baotu Spring Area
4.2. Comparison of Nanoparticle in the Groundwater from Recharge Area and Discharge Area
4.3. Genesis Nanoparticles in the Groundwater of the Baotu Spring Area
4.4. The Characteristics and Significance of the Composition within Nanoparticles in the Groundwater
4.5. The Application Prospects of Nanoparticles in Researching the Water Cycle
5. Conclusions
- (1)
- A significant number of nanoparticles have been detected in the groundwater samples of the study area. These nanoparticles mainly consist of Ca, Na, Fe, Al, and Si. They typically exhibit granular and irregular shapes, with individual sizes ranging from 76.3 to 621.8 nm. They are predominantly in both amorphous and crystalline forms.
- (2)
- Compared to the characteristics of nanoparticles in indirect recharge area, direct recharge area, and discharge area, the same nanoparticles exhibit certain similarities in morphology, size, structure, aggregation state, and elemental composition, indicating that there are hydraulic connections between the recharge area and discharge area.
- (3)
- Based on the geological background and the composition of nanoparticles, we propose that the nanoparticles observed in this study are formed in a relatively oxidizing environment, likely as a result of fracturing and oxidation processes.
- (4)
- The composition of some nanoparticles is relatively simple, while others are more complex. This suggests that the composition of nanoparticles in the groundwater is closely related to the aquifer and environmental conditions. Nanoparticles can reflect the composition of the aquifer and may serve as indicators of water quality.
- (5)
- The presence of certain metal elements in nanoparticles suggests that nanoparticles can effectively transport metal elements in groundwater, offering new insights into the migration of metal elements in groundwater systems. Nanoparticles can act as carriers of inorganic substances, enhancing their mobility within water systems. Additionally, stable nanoparticles can be detected in both supply and discharge areas. Therefore, nanoparticles can also serve as a novel groundwater tracer agent for assessing water quality.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simonovic, S.P.; Breach, P.A. The Role of Water Supply Development in the Earth System. Water 2020, 12, 3349. [Google Scholar] [CrossRef]
- Wang, J.; He, G.; He, F.; Zhao, Y.; Liu, C. Perceptions and Reflections on the Management of Complex Water Resource Systems. China Water Resour. 2024, 2, 10–16. [Google Scholar]
- Jawadi, H.A.; Sagin, J.; Snow, D.D. A Detailed Assessment of Groundwater Quality in the Kabul Basin, Afghanistan, and Suitability for Future Development. Water 2020, 12, 2890. [Google Scholar] [CrossRef]
- Musgrove, M.; Solder, J.E.; Opsahl, S.P.; Wilson, J.T. Timescales of Water-Quality Change in a Karst Aquifer, South-Central Texas. J. Hydrol. X 2019, 4, 100041. [Google Scholar] [CrossRef]
- Di, C. Research on Comprehensive Technology of Spring Protection in Jinan City. Master’s Thesis, Shandong University, Jinan, China, 2007. [Google Scholar]
- Qi, X.; Li, W.; Li, H.; Yang, L. Teleconnections Between Groundwater Levels, Precipitation, Air Temperature of the Jinan Karst Springs Watershed, and Large-Scale Climatic Patterns. Hydrogeol. Eng. Geol. 2015, 42, 18–28. [Google Scholar] [CrossRef]
- Wang, X. Study on Numerical Simulation of Groundwater in Jinan Spring Area Based on MODFLOW and Evolutionary Algorithm. Master’s Thesis, Hefei University of Technology, Hefei, China, 2017. [Google Scholar]
- Bogatikov, O.A. Inorganic Nanoparticles in Nature. Vestnik-Rossiiskaia Akad. Nauk 2003, 73, 426–428. [Google Scholar]
- Yang, Y.; Zhou, L.; Tou, F.; Sun, X.; Liu, M.; Hochella, M.F., Jr. Nanoparticle: A Unique Geochemical Composition in Environment. Earth Sci. 2018, 43, 1489–1502. [Google Scholar] [CrossRef]
- Palenik, C.S.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Wang, L.; Ewing, R.C. “Invisible” Gold Revealed: Direct Imaging of Gold Nanoparticles in a Carlin-Type Deposit. Am. Mineral. 2004, 89, 1359–1366. [Google Scholar] [CrossRef]
- Hough, R.M.; Noble, R.R.P.; Hitchen, G.J.; Hart, R.; Reddy, S.M.; Saunders, M.; Clode, P.; Vaughan, D.; Lowe, J.; Gray, D.J.; et al. Naturally Occurring Gold Nanoparticles and Nanoplates. Geology 2008, 36, 571–574. [Google Scholar] [CrossRef]
- Griffin, S.; Masood, M.I.; Nasim, M.J.; Sarfraz, M.; Ebokaiwe, A.P.; Schäfer, K.-H.; Keck, C.M.; Jacob, C. Natural Nanoparticles: A Particular Matter Inspired by Nature. Antioxidants 2018, 7, 3. [Google Scholar] [CrossRef]
- Wilson, B.; Dewers, T.; Reches, Z.; Brune, J. Particle Size and Energetics of Gouge from Earthquake Rupture Zones. Nature 2005, 434, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Cao, J. Occurrence and Significance of Natural Ore-Related Ag Nanoparticles in Groundwater Systems. Chem. Geol. 2019, 515, 9–21. [Google Scholar] [CrossRef]
- Lengke, M.; Southam, G. Bioaccumulation of Gold by Sulfate-Reducing Bacteria Cultured in the Presence of Gold(I)-Thiosulfate Complex. Geochim. Cosmochim. Acta 2006, 70, 3646–3661. [Google Scholar] [CrossRef]
- Lähde, A.; Sæunn Gudmundsdottir, S.; Joutsensaari, J.; Tapper, U.; Ruusunen, J.; Ihalainen, M.; Karhunen, T.; Torvela, T.; Jokiniemi, J.; Järvinen, K.; et al. In Vitro Evaluation of Pulmonary Deposition of Airborne Volcanic Ash. Atmos. Environ. 2013, 70, 18–27. [Google Scholar] [CrossRef]
- Wigginton, N.S.; Haus, K.L.; Hochella, M.F., Jr. Aquatic Environmental Nanoparticles. J. Environ. Monit. 2007, 9, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Banfield, J.F.; Zhang, H. Nanoparticles in the Environment. Rev. Mineral. Geochem. 2001, 44, 1–58. [Google Scholar] [CrossRef]
- Bakshi, S.; He, Z.L.; Harris, W.G. Natural Nanoparticles: Implications for Environment and Human Health. Crit. Rev. Environ. Sci. Technol. 2015, 45, 861–904. [Google Scholar] [CrossRef]
- Consani, S.; Carbone, C.; Dinelli, E.; Balić-Žunić, T.; Cutroneo, L.; Capello, M.; Salviulo, G.; Lucchetti, G. Metal Transport and Remobilisation in a Basin Affected by Acid Mine Drainage: The Role of Ochreous Amorphous Precipitates. Environ. Sci. Pollut. Res. 2017, 24, 15735–15747. [Google Scholar] [CrossRef] [PubMed]
- Konrad-Schmolke, M.; Halama, R.; Wirth, R.; Thomen, A.; Klitscher, N.; Morales, L.; Schreiber, A.; Wilke, F.D.H. Mineral Dissolution and Reprecipitation Mediated by an Amorphous Phase. Nat. Commun. 2018, 9, 1637. [Google Scholar] [CrossRef]
- Stefanarou, A.S.; Katzourakis, V.E.; Fu, F.; Malandrakis, A.A.; Chrysikopoulos, C.V. Transport of Thiophanate Methyl in Porous Media in the Presence of Titanium Dioxide Nanoparticles. Water 2023, 15, 1415. [Google Scholar] [CrossRef]
- Georgopoulou, A.P.; Syngouna, V.I.; Chrysikopoulos, C.V. Influence of graphene oxide nanoparticles on the transport and cotransport of biocolloids in saturated porous media. Colloids Surf. B Biointerfaces 2020, 189, 110841. [Google Scholar] [CrossRef] [PubMed]
- Chrysikopoulos, C.V.; Fountouli, T.V. Cotransport of titanium dioxide nanoparticles and formaldehyde in saturated and unsaturated columns packed with quartz sand. Vadose Zone J. 2023, 22, e20175. [Google Scholar] [CrossRef]
- Chrysikopoulos, C.V.; Sotirelis, N.P.; Kallithrakas-Kontos, N.G. Cotransport of graphene oxide nanoparticles and kaolinite colloids in porous media. Transp. Porous Media 2017, 119, 181–202. [Google Scholar] [CrossRef]
- Katzourakis, V.E.; Chrysikopoulos, C.V. Modeling dense-colloid and virus cotransport in three-dimensional porous media. J. Contam. Hydrol. 2015, 181, 102–113. [Google Scholar] [CrossRef]
- Zuo, L.; Zhang, P.; Wang, Y.; Liu, R.; Ma, G. Characteristics and Research Significance of Micro-Nanoparticles in Geothermal Fluids in the Central Area of Shandong Province. Water 2023, 15, 3737. [Google Scholar] [CrossRef]
- Xu, H.; Duan, X.; Gao, Z.; Wang, Q.; Li, W.; Yin, X. Hydrochemical Study of Karst Groundwater in the Jinan Spring Catchment. Hydrogeol. Eng. Geol. 2007, 3, 15–19. [Google Scholar] [CrossRef]
- Cheng, S.C.; Lu, Z.Q.; Zhang, Q.; Hou, H.D.; Xu, J. Research on hydrochemical characteristics and evolution of groundwater sources in west of Jinan city. Shandong Land Resour. 2017, 33, 55–58. [Google Scholar]
- Ronen, D.; Magaritz, M.; Weber, U.; Amiel, A.J. Characterization of suspended particlescollectedin groundwater under natural gradient flow conditions. Water Resour. Res. 1992, 28, 1279–1291. [Google Scholar] [CrossRef]
- Zuo, L.; Li, C.; Zhang, P.; Wang, Y.; Gao, S.; Sun, B.; Liu, R. Characteristics of Natural Ti-Bearing Nanoparticles in Groundwater within Karst Areas of Northern China. Water 2024, 16, 650. [Google Scholar] [CrossRef]
- Liu, R.; Cao, J.J.; Luo, S.Y.; Liao, Y.P.; Chen, J. Particulates in the ascending gas caused by the 2013 Ya’an earthquake: A new matter released by earthquake. Geol. J. 2020, 55, 1216–1226. [Google Scholar] [CrossRef]
- Dou, S.; Xing, L.; Chen, H.; Zhao, Z.; Li, C.; Lei, B. Karst Groundwater Quality Assessment and Health Risk Assessment in Jinan Spring Area. Saf. Environ. Eng. 2022, 29, 112–121. [Google Scholar] [CrossRef]
- Li, C. Identification of Karst Water Flow Paths and Hydrochemical Formation Mechanisms in the Baotu Spring Watershed. Ph.D. Dissertation, China University of Geosciences, Wuhan, China, 2023. [Google Scholar] [CrossRef]
- Benischke, R. Review: Advances in the Methodology and Application of Tracing in Karst Aquifers. Hydrogeol. J. 2021, 29, 67–88. [Google Scholar] [CrossRef]
- Edmunds, W.M. Groundwater Nitrate as a Palaeoenvironmental Indicator. In Geochemistry of the Earth’s Surface. Proceedings of 5th International Symposium on the Geochemistry of the Earth’s Surface, Reykjavik, Iceland, 16–20 August 1999; A. A. Balkema: Rotterdam, The Netherlands, 1999; pp. 35–38. [Google Scholar]
- Edmunds, W.M.; Smedley, P.L. Residence Time Indicators in Groundwater: The East Midlands Triassic Sandstone Aquifer. Appl. Geochem. 2000, 15, 737–752. [Google Scholar] [CrossRef]
- Glynn, P.D.; Plummer, L.N. Geochemistry and the Understanding of Ground-Water Systems. Hydrogeol. J. 2005, 13, 263–287. [Google Scholar] [CrossRef]
- Han, D.; Kohfahl, C.; Song, X.; Xiao, G.; Yang, J. Geochemical and Isotopic Evidence for Palaeo-Seawater Intrusion into the South Coast Aquifer of Laizhou Bay, China. Appl. Geochem. 2011, 26, 863–883. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997; pp. 1–342. ISBN 1-56670-249-6. [Google Scholar]
- Sappa, G.; Barbieri, M.; Ergul, S.; Ferranti, F. Hydrogeological Conceptual Model of Groundwater from Carbonate Aquifers Using Environmental Isotopes (18O, 2H) and Chemical Tracers: A Case Study in Southern Latium Region, Central Italy. J. Water Resour. Prot. 2012, 4, 695–716. [Google Scholar] [CrossRef]
- Penna, D.; Hopp, L.; Scandellari, F.; Allen, S.T.; Benettin, P.; Beyer, M.; Geris, J.; Klaus, J.; Marshall, J.D.; Schwendenmann, L.; et al. Ideas and Perspectives: Tracing Terrestrial Ecosystem Water Fluxes Using Hydrogen and Oxygen Stable Isotopes—Challenges and Opportunities from an Interdisciplinary Perspective. Biogeosciences 2018, 15, 6399–6415. [Google Scholar] [CrossRef]
- Jasechko, S. Global Isotope Hydrogeology―Review. Rev. Geophys. 2019, 57, 835–965. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Pan, G.; Li, X.; Zhang, J.; Liu, Y.; Liang, H. Groundwater-Flow-System Characterization with Hydrogeochemistry: A Case in the Lakes Discharge Area of the Ordos Plateau, China. Hydrogeol. J. 2019, 27, 669–683. [Google Scholar] [CrossRef]
- Stimson, J.; Frape, S.; Drimmie, R.; Rudolph, D. Isotopic and Geochemical Evidence of Regional-Scale Anisotropy and Interconnectivity of an Alluvial Fan System, Cochabamba Valley, Bolivia. Appl. Geochem. 2001, 16, 1097–1114. [Google Scholar] [CrossRef]
- Liang, P.; Shi, T.; Li, J. Nanometer-Size Titanium Dioxide Separation/Preconcentration and FAAS Determination of Trace Zn and Cd in Water Sample. Int. J. Environ. Anal. Chem. 2004, 84, 315–321. [Google Scholar] [CrossRef]
- Engates, K.E.; Shipley, H.J. Adsorption of Pb, Cd, Cu, Zn, and Ni to Titanium Dioxide Nanoparticles: Effect of Particle Size, Solid Concentration, and Exhaustion. Environ. Sci. Pollut. Res. 2011, 18, 386–395. [Google Scholar] [CrossRef] [PubMed]




| Samples | Size (nm) | Concentration (Particles/mL) | Groundwater Type | Area |
|---|---|---|---|---|
| BQ * | 155.9–537.8 | 1.50–4.10 × 105 | Spring | Discharge area |
| BTQ * | 151.5–621.8 | 0.45–1.90 × 105 | Spring | Discharge area |
| LX45 | 86.2–391.2 | 0.32–2.00 × 105 | Karst water | Indirect recharge area |
| ZETJ | 122.1–365.3 | 1.00–5.20 × 105 | Karst water | Indirect recharge area |
| G15 | 188.6–509.2 | 0.98–5.10 × 105 | Karst water | Direct recharge area |
| JS12 * | 76.3–299.4 | 0.31–1.50 × 106 | Karst water | Direct recharge area |
| Samples | C | N | P | O | Ca | S | Cl | Si | Mg | Fe | Al | Na | K | Cr | Mn | Ni | Te |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BQ-1 | 28.63 | 0.32 | 63.45 | 7.44 | 0.17 | ||||||||||||
| BTQ-1 | 35.23 | 45.30 | 19.48 | ||||||||||||||
| BTQ-2 | 36.15 | 6.34 | 39.51 | 16.72 | 1.28 | ||||||||||||
| BTQ-4 | 7.02 | 49.66 | 19.98 | 16.53 | 1.89 | 1.54 | 1.92 | 1.47 | |||||||||
| BTQ-6 | 7.97 | 47.72 | 21.94 | 16.09 | 1.45 | 0.73 | 2.08 | 1.23 | 0.78 | ||||||||
| BTQ-9 | 23.12 | 47.19 | 0.57 | 29.13 | |||||||||||||
| BTQ-11 | 0.95 | ||||||||||||||||
| LX44-1 | 4.84 | 48.18 | 46.99 | ||||||||||||||
| LX44-3 | 12.87 | 16.27 | 0.47 | 1.15 | 43.83 | 10.97 | 11.81 | 1.95 | |||||||||
| YR33-2 | 5.43 | 22.32 | 51.19 | 0.45 | 19.96 | ||||||||||||
| YR38-1 | 29.59 | 52.23 | 18.19 | ||||||||||||||
| YR38-4 | 45.36 | 30.12 | 17.49 | 2.78 | 3.25 | 0.99 | |||||||||||
| YR38-6 | 5.55 | 37.46 | 57.00 | ||||||||||||||
| YR39-2 | 37.74 | 43.82 | 18.06 | 0.39 | |||||||||||||
| YR41-1 | 63.27 | 23.72 | 11.99 | 0.61 | 0.40 | ||||||||||||
| YR41-2 | 34.76 | 22.50 | 19.26 | 1.33 | 0.25 | 0.39 | 2.50 | 1.14 | 0.47 | 17.40 | |||||||
| G15-5 | 13.15 | 49.42 | 19.00 | 15.45 | 0.53 | 0.40 | 1.05 | 1.00 | |||||||||
| ZETJ-2 | 22.50 | 43.00 | 17.26 | 14.75 | 0.21 | 0.62 | 1.28 | 0.39 | |||||||||
| JS12-2 | 10.30 | 48.96 | 1.41 | 0.14 | 0.28 | 5.37 | 0.45 | 32.14 | 0.94 | ||||||||
| JS12-3 | 17.02 | 2.95 | 0.19 | 29.48 | 1.46 | 3.52 | 0.42 | 41.02 | 2.75 | 0.13 | |||||||
| JS12-6 | 25.43 | 15.01 | 0.85 | 36.08 | 10.48 | 8.80 | 2.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Liu, R.; Zhang, P.; Wang, Y.; Zuo, L.; Zhang, X.; Li, C. Characteristics and Significance of Natural Nanoparticles in the Groundwater of the Baotu Spring Area in Jinan, Shandong Province, Eastern China. Water 2024, 16, 1820. https://doi.org/10.3390/w16131820
Hu C, Liu R, Zhang P, Wang Y, Zuo L, Zhang X, Li C. Characteristics and Significance of Natural Nanoparticles in the Groundwater of the Baotu Spring Area in Jinan, Shandong Province, Eastern China. Water. 2024; 16(13):1820. https://doi.org/10.3390/w16131820
Chicago/Turabian StyleHu, Caiping, Rui Liu, Peng Zhang, Yaqin Wang, Lei Zuo, Xiaoheng Zhang, and Changsuo Li. 2024. "Characteristics and Significance of Natural Nanoparticles in the Groundwater of the Baotu Spring Area in Jinan, Shandong Province, Eastern China" Water 16, no. 13: 1820. https://doi.org/10.3390/w16131820






