Elimination of Residual Chemical Oxygen Demand (COD) in a Low-Temperature Post-Denitrifying Moving Bed Biofilm Reactor (MBBR)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactors and Carriers
2.2. Experimental Set-Up
2.3. Synthetic Wastewater and Loads
2.4. Analytical Methods and Measurements
3. Results and Discussion
3.1. Elimination of Residual COD
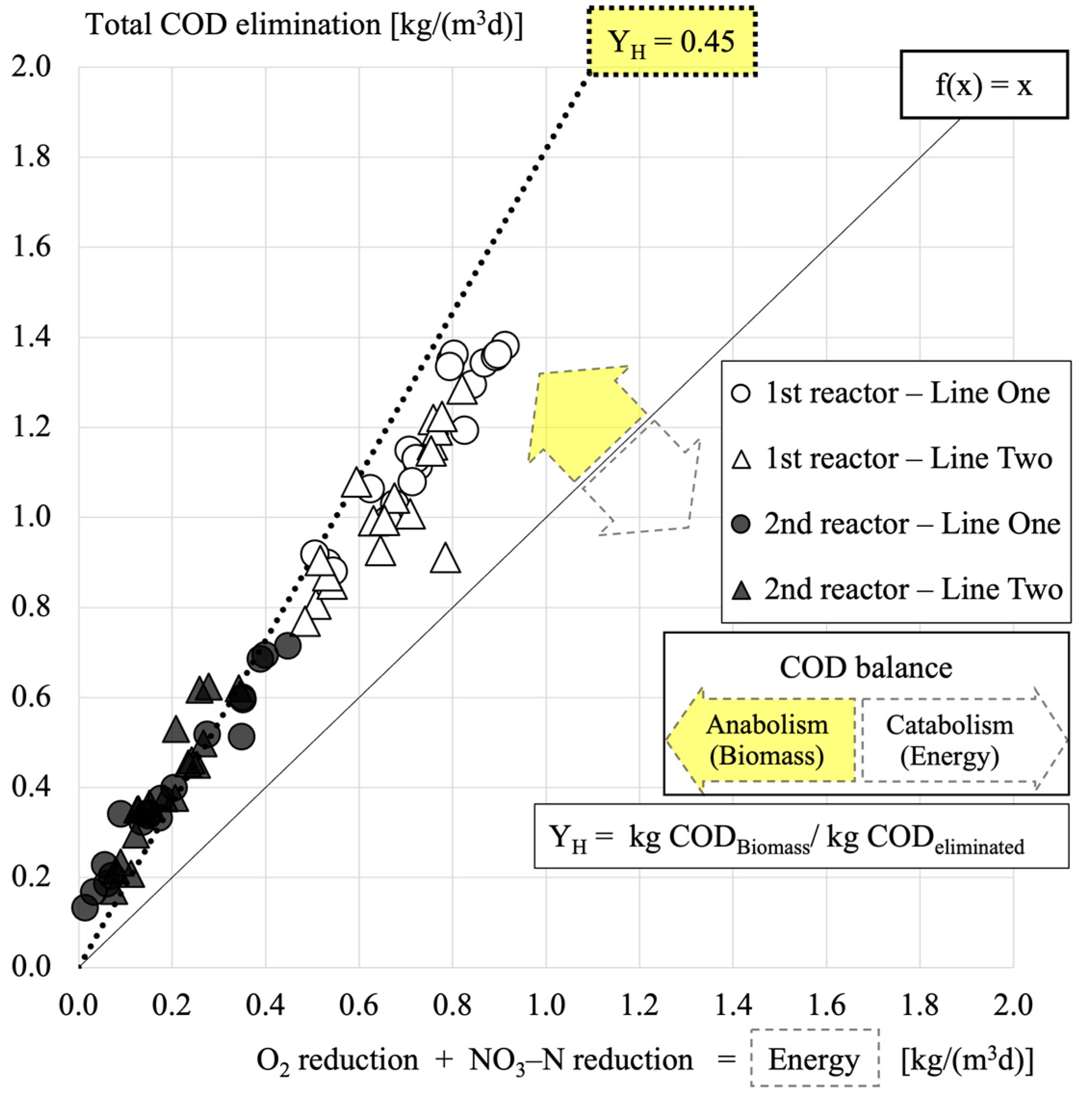
3.2. COD Balance and Excess Sludge Production
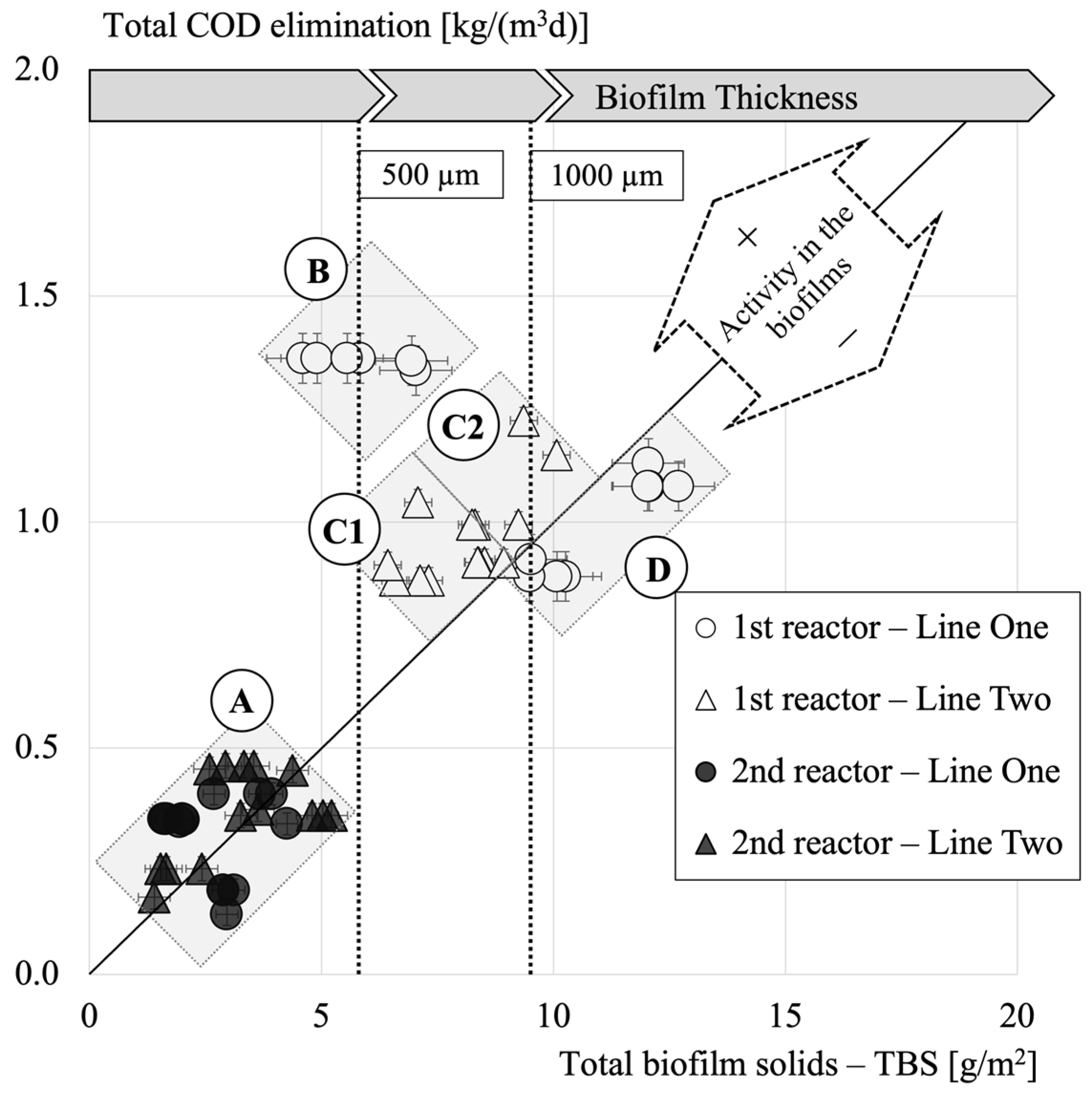
3.3. Biofilm Limitations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ødegaard, H. MBBR and IFAS systems. In Advances in Wastewater Treatment; Mannina, G., Ekama, G., Ødegaard, H., Olsson, G., Eds.; IWA Publishing: London, UK, 2019; ISBN 9781780409719. [Google Scholar]
- Barwal, A.; Chaudhary, R. To study the performance of biocarriers in moving bed biofilm reactor (MBBR) technology and kinetics of biofilm for retrofitting the existing aerobic treatment systems: A review. Rev Env. Sci Biotechnol 2014, 13, 285–299. [Google Scholar] [CrossRef]
- Leiknes, T.; Ødegaard, H. The development of a biofilm membrane bioreactor. Desalination 2007, 202, 135–143. [Google Scholar] [CrossRef]
- Rusten, B.; Hem, L.J.; Ødegaard, H. Nitrification of municipal wastewater in moving-bed biofilm reactors. Water Environ. Res. 1995, 67, 75–86. [Google Scholar] [CrossRef]
- Bassin, J.; Dezotti, M. Moving Bed Biofilm Reactor (MBBR). In Advanced Biological Processes for Wastewater Treatment; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Biswas, K.; Taylor, M.W.; Turner, S.J. Successional development of biofilms in moving bed biofilm reactor (MBBR) systems treating municipal wastewater. Appl. Microbiol. Biotechnol. 2014, 98, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Andreottola, G.; Foladori, P.; Ragazzi, M. Upgrading of a small wastewater treatment plant in a cold climate region using a moving bed biofilm reactor (MBBR) system. Water Sci. Technol. 2000, 41, 177–185. [Google Scholar] [CrossRef]
- Stinson, B.; Peric, M.; Neupane, D.; Laquidara, M.; Locke, E.; Murthy, S.; Bailey, W.; Kharkar, S.; Passarelli, N.; Derminassian, R.; et al. Design and Operating Considerations for a Post Denitrification MBBR to Achieve Limit of Technology Effluent NOx < 1 mg/l and effluent TP < 0.18 mg/L. Proc. Water Environ. Fed. 2009, 2009, 1225–1254. [Google Scholar] [CrossRef]
- Ødegaard, H. New Applications for MBBR and IFAS Systems. In Frontiers in Wastewater Treatment and Modelling: FICWTM 2017; Mannina, G., Ed.; Springer: Cham, Switzerland, 2017; pp. 499–507. ISBN 978-3-319-58421-8. [Google Scholar]
- Hanner, N.; Aspegren, H.; Nyberg, U.; Andersson, B. Upgrading the Sjölunda WWTP according to a novel process concept. Water Sci. Technol. 2003, 47, 1–7. [Google Scholar] [CrossRef]
- Mases, M.; Dimitrova, I.; Nyberg, U.; Gruvberger, C.; Andersson, B. Experiences from MBBR Post-Denitrification Process in Long-term Operation at two WWTPs. Proc. Water Environ. Fed. 2010, 2010, 458–471. [Google Scholar] [CrossRef]
- Neupane, D.; Silverio, R.; Longerbeam, C.; Motsch, S.; McGrath, M.; McGettigan, J.; EuDaly, P.; Ortenzio, L.; Foster, D.; Zhao, H. Challenges in Starting up and Operating Attached Growth Denitrifying Process. Proc. Water Environ. Fed. 2014, 2014, 4649–4669. [Google Scholar] [CrossRef]
- Kopec, L.; Drewnowski, J.; Kopec, A. The application of moving bed biofilm reactor to denitrification process after trickling filters. Water Sci. Technol. 2016, 74, 2909–2916. [Google Scholar] [CrossRef]
- Czarnota, J.; Masłoń, A. Evaluation of the Effectiveness of a Wastewater Treatment Plant with MBBR Technology. Rocz. Ochr. Sr. 2019, 21, 906–925. [Google Scholar]
- Mannina, G.; Viviani, G. Hybrid moving bed biofilm reactors: An effective solution for upgrading a large wastewater treatment plant. Water Sci. Technol. 2009, 60, 1103–1116. [Google Scholar] [CrossRef]
- Leonhard, S.; Schreff, D.; Thoma, K.; Gander, W.; Wichern, M.; Hilliges, R. Single-stage MBBR as post-treatment step for upgrading large WWTPs—Experiences of one-year pilot plant operation. J. Water Process Eng. 2022, 46, 102570. [Google Scholar] [CrossRef]
- Christensen, M.H.; Harremoës, P. Biological denitrification of sewage: A literature review. In Proceedings of the Conference on Nitrogen as a Water Pollutant; Elsevier: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Zhou, X.; Jiang, Z.; Gu, J.; Bi, X.; Liu, J.; Wang, X.; Yang, T.; Shi, X.; Cheng, L.; Huang, S.; et al. Performance characteristics and bacterial community analysis of a novel two-step-feed three-stage A/O-MBBR system for nitrogen removal in municipal wastewater. J. Water Process Eng. 2023, 52, 103513. [Google Scholar] [CrossRef]
- Chai, H.; Xiang, Y.; Chen, R.; Shao, Z.; Gu, L.; Li, L.; He, Q. Enhanced simultaneous nitrification and denitrification in treating low carbon-to-nitrogen ratio wastewater: Treatment performance and nitrogen removal pathway. Bioresour. Technol. 2019, 280, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zhang, Y.; Quan, X.; Li, H. Effects of an electric field and iron electrode on anaerobic denitrification at low C/N ratios. Chem. Eng. J. 2015, 266, 241–248. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, X.; Wen, L.; Jin, X.; Shi, X.; Jin, P. Advanced nutrient removal in a continuous A2/O process based on partial nitrification-anammox and denitrifying phosphorus removal. J. Water Process Eng. 2020, 36, 101245. [Google Scholar] [CrossRef]
- Pang, W.; Li, H.; He, Y.; Zhou, B.; Ji, C.; Li, T.; Wang, X.; Xie, L. Nitrate reduction pathways and microbial community to different COD/NO3−-N ratios in mesophilic and thermophilic denitrification systems. J. Water Process Eng. 2023, 53, 103797. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Li, Z.; Sun, P.; Hui, X.; Fan, X.; Bi, X.; Yang, T.; Huang, S.; Cheng, L.; et al. A novel two-stage anoxic/oxic-moving bed biofilm reactor process for biological nitrogen removal in a full-scale municipal WWTP: Performance and bacterial community analysis. J. Water Process Eng. 2022, 50, 103224. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, F.; Zhou, F.; Xue, Y. Control of COD/N ratio for nutrient removal in a modified membrane bioreactor (MBR) treating high strength wastewater. Bioresour. Technol. 2009, 100, 136–141. [Google Scholar] [CrossRef]
- Piculell, M.; Welander, P.; Jönsson, K.; Welander, T. Evaluating the effect of biofilm thickness on nitrification in moving bed biofilm reactors. Environ. Technol. 2016, 37, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Torresi, E.; Fowler, S.J.; Polesel, F.; Bester, K.; Andersen, H.R.; Smets, B.F.; Plósz, B.G.; Christensson, M. Biofilm Thickness Influences Biodiversity in Nitrifying MBBRs-Implications on Micropollutant Removal. Environ. Sci. Technol. 2016, 50, 9279–9288. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.; Piculell, M.; Modin, O.; Langenheder, S.; Persson, F.; Hermansson, M. Thickness determines microbial community structure and function in nitrifying biofilms via deterministic assembly. Sci. Rep. 2019, 9, 5110. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A. Specific Moving Bed biofilm Reactor in Nutrient Removal from Municipal Wastewater. Ph.D. Thesis, University of Technology, Sydney, Australia, 2013. [Google Scholar]
- van Loosdrecht, M. A more unifying hypothesis for biofilm structures. FEMS Microbiol. Ecol. 1997, 24, 181–183. [Google Scholar] [CrossRef]
- Garcia, K.A.; McLee, P.; Schuler, A.J. Effects of media length on biofilms and nitrification in moving bed biofilm reactors. Biofilm 2022, 4, 100091. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol. Bioeng. 1998, 59, 261–272. [Google Scholar] [CrossRef]
- Stewart, P.S. Diffusion in biofilms. J. Bacteriol. 2003, 185, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Wäsche, S.; Horn, H.; Hempel, D.C. Mass transfer phenomena in biofilm systems. Water Sci. Technol. 2000, 41, 357–360. [Google Scholar] [CrossRef]
- Piculell, M. New Dimensions of Moving Bed Biofilm Carriers: Influence of Biofilm Thickness and Control Possibilities. Ph.D. Thesis, Lund University, Lund, Sweden, 2016. [Google Scholar]
- Rusten, B.; Eikebrokk, B.; Ulgenes, Y.; Lygren, E. Design and operations of the Kaldnes moving bed biofilm reactors. Aquac. Eng. 2006, 34, 322–331. [Google Scholar] [CrossRef]
- Barwal, A.; Chaudhary, R. Impact of carrier filling ratio on oxygen uptake & transfer rate, volumetric oxygen transfer coefficient and energy saving potential in a lab-scale MBBR. J. Water Process Eng. 2015, 8, 202–208. [Google Scholar] [CrossRef]
- Matheus, M.C.; Lourenço, G.R.; Solano, B.A.; Dezotti, M.W.; Bassin, J.P. Assessing the impact of hydraulic conditions and absence of pretreatment on the treatability of pesticide formulation plant wastewater in a moving bed biofilm reactor. J. Water Process Eng. 2020, 36, 101243. [Google Scholar] [CrossRef]
- Kargol, A.K.; Burrell, S.R.; Chakraborty, I.; Gough, H.L. Synthetic wastewater prepared from readily available materials: Characteristics and economics. PLoS Water 2023, 2, e0000178. [Google Scholar] [CrossRef]
- Cokgor, E. Anaerobic treatment of synthetic domestic wastewater. Fresenius Environ. Bull. 1998, 7, 531–538. [Google Scholar]
- Kermani, M.; Bina, B.; Movahedian, H.; Amin, M.M.; Nikaeen, M. Biological Phosphorus and Nitrogen Removal from Wastewater Using Moving Bed Biofilm Process. Iran. J. Biotechnol. 2009, 7, 19–27. [Google Scholar]
- Arbeitsblatt DWA-A 131 Bemessung von Einstufigen Belebungsanlagen; Deutsche Vereinigung für Wasserwirtschaft, Abwasser und Abfall: Hennef, Germany, 2016; ISBN 9783887213312.
- Steinbrenner, C. Biochemische und Molekularbiologische Charakterisierung von Biofilmen des WSB®—Verfahrens im Vergleich zu Belebtschlammverfahren unter Besonderer Berücksichtigung der Nitrifikation. Ph.D. Thesis, TU Dresden, Dresden, Germany, 2011. [Google Scholar]
- Leonhard, S.; Thoma, K.; Schreff, D.; Wichern, M.; Hilliges, R. Rest-Stickstoff-Elimination aus gereinigtem Abwasser im Bypass: Ein modifiziertes Verfahren mit Wirbelschwebebett-Technologie. gwf Wasser|Abwasser 2024, 165, 73–79. [Google Scholar] [CrossRef]
- Monod, J. The Growth of Bacterial Cultures. Annu. Rev. Microbiol. 1949, 3, 371–394. [Google Scholar] [CrossRef]
- Ødegaard, H.; Rusten, B.; Westrum, T. A new moving bed biofilm reactor—Applications and results. Water Sci. Technol. 1994, 29, 157–165. [Google Scholar] [CrossRef]
- Welander, U.; Mattiasson, B. Denitrification at low temperatures using a suspended carrier biofilm process. Water Res. 2003, 37, 2394–2398. [Google Scholar] [CrossRef]
- Comeau, Y.; Henze, M.; van Loosdrecht, M.C.M.; Ekama, G.A.; Brdjanovic, D. Biological Wastewater Treatment: Microbial Metabolism; IWA Publishing: London, UK, 2008; pp. 19–32. ISBN 9781843391883. [Google Scholar]
- Barjenbruch, K.; Hiltbrand, R.; Kosarik, J.M.; Laplante, R.E. Examination of a Lake-Effect Snow Event with the Focus on New Technology; 1997. Available online: https://repository.library.noaa.gov/view/noaa/6688 (accessed on 20 June 2024).
- Mahro, B. Zur Bedeutung des Nitratsauerstoffs bei der biologischen Abwasserreinigung. KA Korresp. Abwasser Abfall 2006, 2006, 916–919. [Google Scholar]
- Vocks, M.; Adam, C.; Lesjean, B.; Gnirss, R.; Kraume, M. Enhanced post-denitrification without addition of an external carbon source in membrane bioreactors. Water Res. 2005, 39, 3360–3368. [Google Scholar] [CrossRef]
- McCarty, P.L. Biological denitrification of wastewaters by addition of organic materials. In Proceedings of the 24th Annual Purdue Industrial Waste Conference, Purdue University, Lafayette, IN, USA, 6–8 May 1969; pp. 1271–1285. [Google Scholar]
- Weissbrodt, D.G.; Laureni, M.; van Loosdrecht, M.C.M.; Comeau, Y. (Eds.) Biological Wastewater Treatment: Principles, Modeling and Design, 2nd ed.; IWA Publishing: London, UK, 2020. [Google Scholar]
- Gujer, W.; Henze, M.; Mino, T.; van Loosdrecht, M. Activated Sludge Model No. 3. Water Sci. Technol. 1999, 39, 183–193. [Google Scholar] [CrossRef]
- Heijnen, J.J.; Robbert Kleerebezem, R. (Eds.) Bioenergetics of Microbial Growth; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]





| Line One | Line Two | |||
|---|---|---|---|---|
| 1st Reactor | 2nd Reactor | 1st Reactor | 2nd Reactor | |
| Reactor volume [L] | 46 | 46 | 46 | 46 |
| Filling ratio [%] | 30 and 40 | 30 | 23 and 30 | 23 |
| Carrier media (Hel-x—Christian Stöhr Ltd.) | HXF17KLL | HXF17KLL | HXF14KLL | HXF14KLL |
| Diameter and length of the carrier [mm] | 17 | 17 | 14 | 14 |
| Total protected surface [m2/reactor] | 6.8 and 9.1 | 6.8 | 6.8 and 8.9 | 6.8 |
| Protected surface [m2/m3Carrier] | 496 | 496 | 644 | 644 |
| Protected surface [m2/m3Volume Reactor] | 148 and 198 | 148 | 148 and 193 | 148 |
| HRT per reactor [min] | 28–55 | 28–55 | 28–55 | 28–55 |
| HRT per line [min] | 56–110 | 56–110 | ||
| Line One and Line Two | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Program | Duration | NO3-N | COD/NO3-N | CODdosed (1) | PO4-P | Dissolved O2 | Temp. | Flow Rate One | Flow Rate Two |
| [d] | [mg/L] | [mg/mg] | [mg/L] | [mg/L] | [mg/L] | [°C] | [L/h] | [L/h] | |
| 1 | 36 | 10 | 4.65 | 46.5 | 0.3 | 7.9 | 12 | 94 | – |
| 2 | 23 | 10 | 4.65 | 46.5 | 0.3 | 7.8 | 14 | 94 | 47 |
| 3 | 18 | 10 | 4.65 | 46.5 | 0.3 | 7.4 | 16 | 94 | 93 |
| 4 | 15 | 10 | 5.55 | 55.5 | 0.3 | 7.3 | 16 | 47 | 47 |
| 5 | 13 | 10 | 4.65 | 46.5 | 0.3 | 6.9 | 16 | 48 | 47 |
| 6 | 15 | 10 | 5.55 | 55.5 | 0.3 | 6.9 | 16 | 48 | 47 |
| 7 | 13 | 10 | 5.55 | 55.5 | 0.3 | 6.6 | 16 | 74 | 74 |
| 8 | 14 | 10 | 5.55 | 55.5 | 0.1 | 6.6 | 16 | 74 | 76 |
| 9 | 7 | 10 | 5.55 | 55.5 | 0.2 | 6.3 | 16 | 75 | 76 |
| 10 | 14 | 14 | 4.65 | 65.1 | 0.3 | 6.4 | 16 | 75 | 76 |
| 11 | 14 | 14 | 4.65 | 65.1 | 0.3 | 6.5 | 16 | 47 | 49 |
| 12 | 14 | 14 | 5.25 | 73.5 | 0.3 | 6.7 | 16 | 47 | 49 |
| 13 | 14 | 14 | 4.65 | 65.1 | 0.3 | 6.7 | 14 | 47 | 49 |
| 14 | 14 | 14 | 4.65 | 65.1 | 0.3 | 6.7 | 14 | 48 | 49 |
| 15 | 29 | 10 | 4.65 | 46.5 | 0.3 | 6.9 | 14 | 47 | 48 |
| 16 (2) | 24 | 10 | 4.65 | 46.5 | 0.3 | 7.4 | 12 | 47 | 48 |
| 17 | 16 | 14 | 5.25 | 73.5 | 0.3 | 7.9 | 12 | 47 | 48 |
| 18 | 13 | 14 | 5.25 | 73.5 | 0.3 | 7.8 | 14 | 47 | 48 |
| 19 | 29 | 14 | 5.25 | 73.5 | 0.3 | 8.0 | 16 | 47 | 48 |
| Ingredient | Wastewater Constituents | Chemical Formula | Producer |
|---|---|---|---|
| Drinking water, temperature-controlled | Water | H2O | Augsburg municipal utilities |
| Sodium nitrate, untreated | NO3-N | NaNO3 | BASF SE, Ludwigshafen, Germany |
| Di-potassium hydrogen phosphate, pure | PO4-P | K2HPO4 | AppliChem GmbH, Darmstadt, Germany |
| Methanol pure 99.5% | Carbon source | CH3OH | häberle Labortechnik GmbH+Co.KG, Lonsee-Ettlenschieß, Germany |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonhard, S.; Wichern, M.; Hilliges, R. Elimination of Residual Chemical Oxygen Demand (COD) in a Low-Temperature Post-Denitrifying Moving Bed Biofilm Reactor (MBBR). Water 2024, 16, 1829. https://doi.org/10.3390/w16131829
Leonhard S, Wichern M, Hilliges R. Elimination of Residual Chemical Oxygen Demand (COD) in a Low-Temperature Post-Denitrifying Moving Bed Biofilm Reactor (MBBR). Water. 2024; 16(13):1829. https://doi.org/10.3390/w16131829
Chicago/Turabian StyleLeonhard, Stephan, Marc Wichern, and Rita Hilliges. 2024. "Elimination of Residual Chemical Oxygen Demand (COD) in a Low-Temperature Post-Denitrifying Moving Bed Biofilm Reactor (MBBR)" Water 16, no. 13: 1829. https://doi.org/10.3390/w16131829








