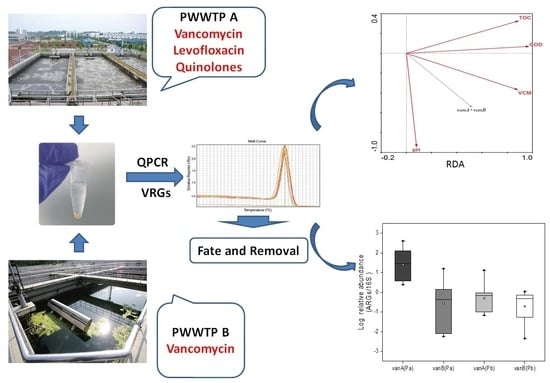
Fate and Proliferation of Vancomycin Resistance Genes in Two Typical Pharmaceutical Wastewater Treatment Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of PWWTPs and Sample Collection
2.2. Sample Preparation and DNA Extraction
2.3. Construction of qPCR Calibration Curve
2.4. Real-Time PCR
2.5. Statistical Analysis
3. Results and Discussion
3.1. Prevalence of ARGs in PWWTPs
3.2. Fate of ARGs in Different Wastewater Treatment Processes
3.2.1. Effects of Different Treatments
3.2.2. Mass Balance Analysis
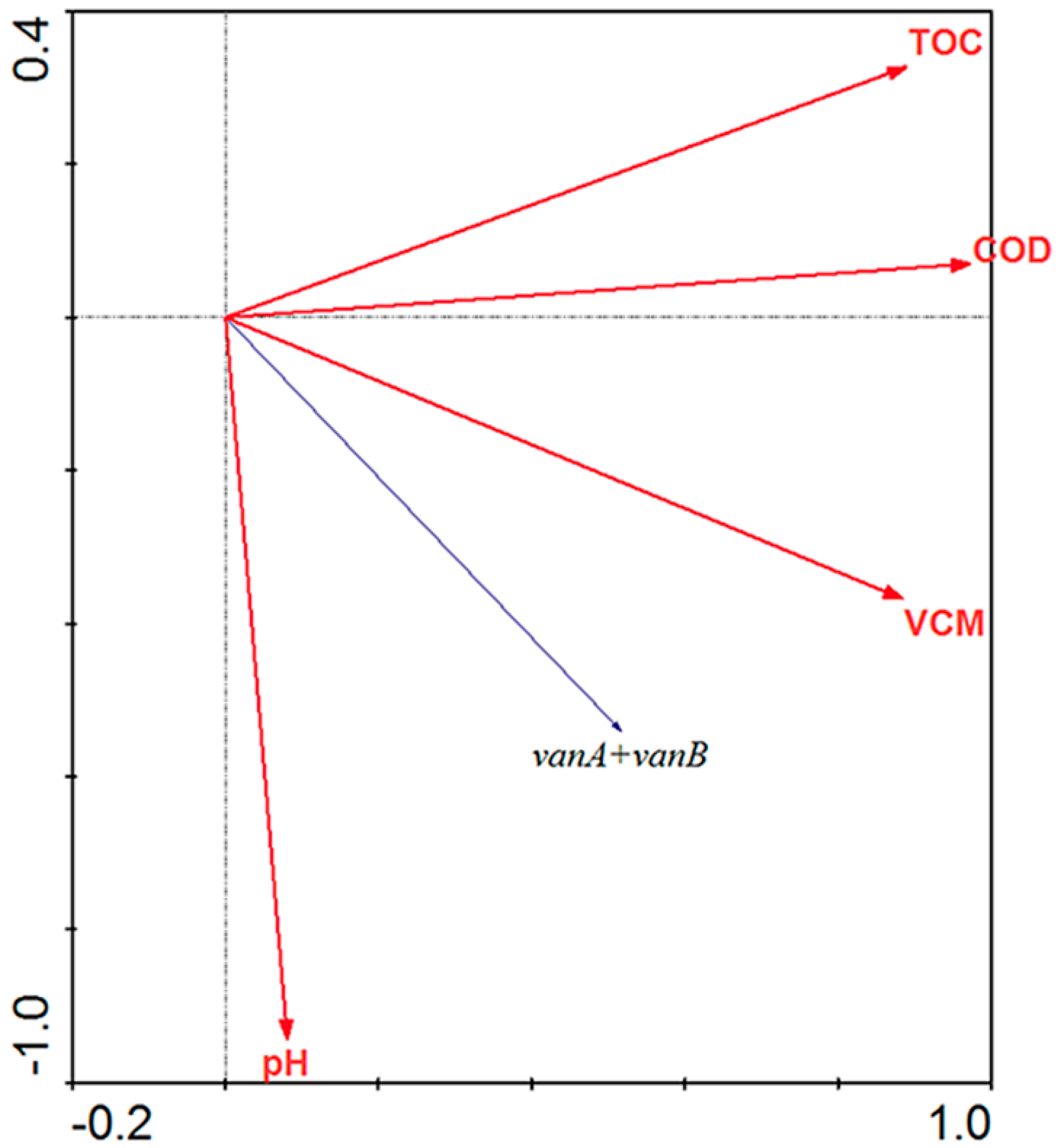
3.2.3. Redundancy Analysis
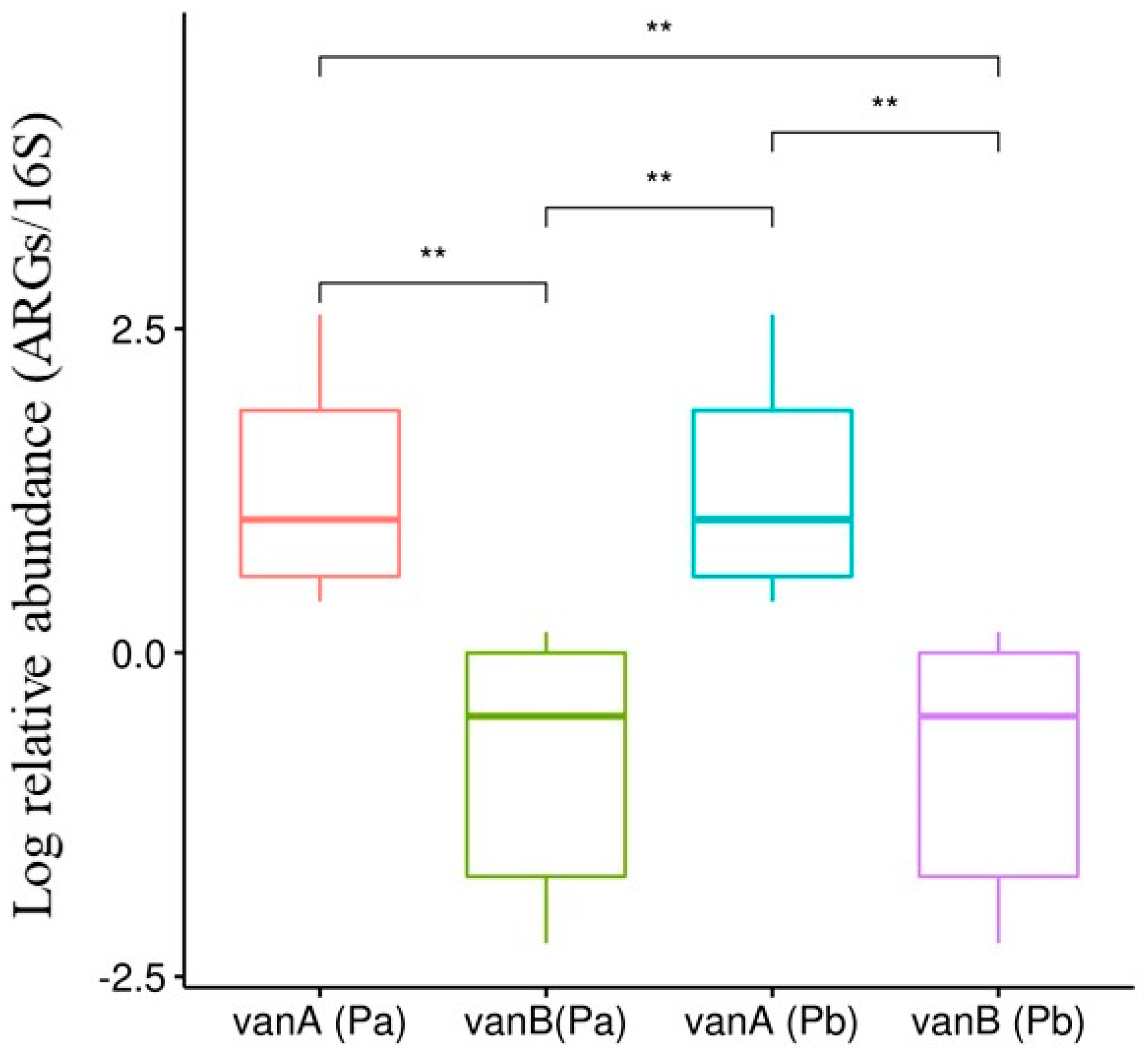
3.3. Selection and Proliferation of Different ARG Subtypes in PWWTPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chavers, L.S.; Moser, S.A.; Funkhouser, E.; Benjamin, W.; Chavers, P.; Stamm, A.; Waites, K.; Nys, S.; Bruinsma, N.; Filius, P.; et al. Association between antecedent intravenous antimicrobial exposure and isolation of vancomycin-resistant enterococci. Microb. Drug Resist. 2003, 9 (Suppl. S1), 69–77. [Google Scholar] [CrossRef] [PubMed]
- Shankar, N.; Baghdayan, A.S.; Gilmore, M.S. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature 2002, 417, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Sahlström, L.; Rehbinder, V.; Albihn, A.; Aspan, A.; Bengtsson, B. Vancomycin resistant enterococci (VRE) in Swedish sewage sludge. Acta Vet. Scand. 2009, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.; Coque, T.M.; Hammerum, A.M.; Hope, R.; Hryniewicz, W.; Johnson, A.; Klare, I.; Kristinsson, K.G.; Leclercq, R.; Lester, C.H.; et al. Emergence and spread of vancomycin resistance among enterococci in Europe. Eurosurveillance 2008, 13, 19046. [Google Scholar] [CrossRef] [PubMed]
- Werner, G. Current trends of emergence and spread of vancomycin-resistant enterococci. In Antibiotic Resistant Bacteria-A Continuous Challenge in the New Millennium; IntechOpen: Rijeka, Croatia, 2012; pp. 303–354. [Google Scholar]
- Oravcova, V.; Hadelova, D.; Literak, I. Vancomycin-resistant Enterococcus faecium with vanA gene isolated for the first time from wildlife in Slovakia. Vet. Microbiol. 2016, 194, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, K.; Kayayama, Y.; Matsuo, M.; Aiba, Y.; Saito, M.; Hishinuma, T.; Iwamoto, A. Vancomycin-intermediate resistance in Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2014, 2, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.A.; Khan, M.A.; Sharmin, T.; Mazumder, M.H.H.; Chowdhury, A.S. Identification of putative drug targets in Vancomycin-resistant Staphylococcus aureus (VRSA) using computer aided protein data analysis. Gene 2016, 575, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Arthur, M.; Courvalin, P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob. Agents Chemother. 1993, 37, 1563–1571. [Google Scholar] [CrossRef]
- Wilcks, A.; van Hoek, A.H.A.M.; Joosten, R.G.; Jacobsen, B.B.; Aarts, H.J. Persistence of DNA studied in different ex vivo and in vivo rat models simulating the human gut situation. Food Chem. Toxicol. 2004, 42, 493–502. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China, 2020. Announcement No. 560. Available online: http://www.moa.gov.cn/gk/tzgg_1/gg/202001/t20200106_6334375.htm (accessed on 15 January 2023). (In Chinese)
- Kemeç, Z.; Kahya, Y.; Atay, A.; Demir, M.; Gürel, A. Vancomycin dependent pancytopenia-a rare side effect: A case report. Int. J. Med. Rev. Case Rep. 2019, 3, 339. [Google Scholar] [CrossRef]
- Selvaraj, P.K.; Khasawneh, F.A. Linear IgA bullous dermatosis: A rare side effect of vancomycin. Ann. Saudi Med. 2013, 33, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, E.; Egilmez, A.I. Eosinophilia; side effect of vancomycin which is not often detected: Case report. Medicine 2018, 7, 453–455. [Google Scholar] [CrossRef]
- Qiu, P.; Guo, X.; Zhang, Y.; Chen, X.; Wang, N. Occurrence, fate, and risk assessment of vancomycin in two typical pharmaceutical wastewater treatment plants in Eastern China. Environ. Sci. Pollut. Res. 2016, 23, 16513–16523. [Google Scholar] [CrossRef] [PubMed]
- Pal, C.; Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genom. 2015, 16, 964. [Google Scholar] [CrossRef]
- Takashi, F.; Reina, H.; Tohru, M. Quantification of vancomycin-resistant enterococci and corresponding resistance genes in a sewage treatment plant. J. Environ. Sci. Health. Part A Toxic/Hazard. Subst. Environ. Eng. 2015, 50, 989–995. [Google Scholar]
- Novo, A.; André, S.; Viana, P.; Nunes, O.C.; Manaia, C.M. Antibiotic resistance, antimicrobial residues and bacterial community composition in urban wastewater. Water Res. 2013, 47, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.J.; Zhao, R.X.; Wan, J.J.; Li, B.; Shen, Y.; Zhang, S.; Luo, G. Metagenomic analysis reveals the fate of antibiotic resistance genes in two-stage and one-stage anaerobic digestion of waste activated sludge. J. Hazard. Mater. 2021, 406, 124595. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Z.Y.; Gao, J.; Xie, Y.; Li, L.; Qin, S.; Wang, Q.; Mao, D.; Luo, Y. Simultaneous removal of antibiotics and antibiotic resistance genes from pharmaceutical wastewater using the combinations of up-flow anaerobic sludge bed, anoxic-oxic tank, and advanced oxidation technologies. Water Res. 2019, 159, 511–520. [Google Scholar] [CrossRef]
- Li, Z.Y.; Dai, R.B.; Yang, B.C.; Chen, M.; Wang, X.; Wang, Z. An electrochemical membrane biofilm reactor for removing sulfonamides from wastewater and suppressing antibiotic resistance development: Performance and mechanisms. J. Hazard. Mater. 2021, 404, 124198. [Google Scholar] [CrossRef]
- Pei, R.; Kim, S.C.; Carlson, K.H.; Pruden, A. Effect of river landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Water Res. 2006, 40, 2427–2435. [Google Scholar] [CrossRef]
- Mao, D.; Yu, S.; Rysz, M.; Luo, Y.; Yang, F.; Li, F.; Hou, J.; Mu, Q.; Alvarez, P.J.J. Prevalence and proliferation of antibiotic resistance genes in two municipal wastewater treatment plants. Water Res. 2015, 85, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, B.; Zou, S.; Fang, H.H.; Zhang, T. Fate of antibiotic resistance genes in sewage treatment plant revealed by metagenomic approach. Water Res. 2014, 62, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.R.; Novais, C.; Tedim, A.P.; Francia, M.V.; Baquero, F.; Peixe, L.; Coque, T.M. Microevolutionary events involving narrow host plasmids influences local fixation of vancomycin-resistance in Enterococcus populations. PLoS ONE 2013, 8, e60589. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, D.; Mu, Q.; Luo, Y. Fate and proliferation of typical antibiotic resistance genes in five full-scale pharmaceutical wastewater treatment plants. Sci. Total Environ. 2015, 526, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Yan, Z.; Zhang, Y.; Xu, W.; Kong, D.; Shan, Z.; Wang, N. Behavior of antibiotic resistance genes under extremely high-level antibiotic selection pressures in pharmaceutical wastewater treatment plants. Sci. Total Environ. 2018, 612, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Wong, K.; Xagoraraki, I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res. 2011, 45, 681–693. [Google Scholar] [CrossRef]
- Breazeal, M.V.R.; Novak, J.T.; Vikesland, P.J.; Pruden, A. Effect of wastewater colloids on membrane removal of antibiotic resistance genes. Water Res. 2013, 47, 130–140. [Google Scholar] [CrossRef]
- Tao, C.W.; Hsu, B.M.; Ji, W.T.; Hsu, T.-K.; Kao, P.-M.; Hsu, C.-P.; Shen, S.-M.; Shen, T.-Y.; Wan, T.-J.; Huang, Y.-L. Evaluation of five antibiotic resistance genes in wastewater treatment systems of swine farms by real-time PCR. Sci. Total Environ. 2014, 496, 116–121. [Google Scholar] [CrossRef]
- Lin, H.; Li, H.; Chen, L.; Li, L.; Yin, L.; Lee, H.; Yang, Z. Mass loading and emission of thirty-seven pharmaceuticals in a typical municipal wastewater treatment plant in Hunan Province, Southern China. Ecotoxicol. Environ. Saf. 2018, 147, 530–536. [Google Scholar] [CrossRef]
- Aydin, S.; Ince, B.; Ince, O. Development of antibiotic resistance genes in microbial communities during long-term operation of anaerobic reactors in the treatment of pharmaceutical wastewater. Water Res. 2015, 83, 337–344. [Google Scholar] [CrossRef]
- Börjesson, S.; Mattsson, A.; Lindgren, P.E. Genes encoding tetracycline resistance in a full-scale municipal wastewater treatment plant investigated during one year. J. Water Health 2010, 8, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.A.; Mouton, J.W.; Lewis, D.A.; Nichols, T.; Ison, C.A.; Livermore, D.M. Cephalosporin MIC creep among gonococci: Time for a pharmacodynamic rethink? J. Antimicrob. Chemother. 2010, 65, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.C.; Guillaume, L.; David, L.; Kim, H.; Robin, K.; Tung, C.K.; Pourmand, N.; Austin, R.H. Acceleration of emergence of bacterial antibiotic resistance in connected microenvironment. Science 2011, 333, 1764–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.X.; Yu, K.; Zhang, J.Y.; Zhang, G.; Huang, J.; Ma, L.; Deng, C.; Li, X.; Li, B. Deciphering the mobility and bacterial hosts of antibiotic resistance genes under antibiotic selection pressure by metagenomic assembly and binning approaches. Water Res. 2020, 186, 116318. [Google Scholar] [CrossRef] [PubMed]
- Koteva, K.; Hong, H.J.; Wang, X.D.; Nazi, I.; Hughes, D.; Naldrett, M.J.; Buttner, M.J.; Wright, G.D. A vancomycin photoprobe identifies the histidine kinase VanSsc as a vancomycin receptor. Nat. Chem. Biol. 2010, 6, 327–329. [Google Scholar] [CrossRef]
- Charlebois, A.; Jalbert, L.A.; Harel, J.; Masson, L.; Archambault, M. Characterization of genes encoding for acquired bacitracin resistance in Clostridium perfringens. PLoS ONE 2012, 7, e44449. [Google Scholar] [CrossRef]
- Yuan, Q.B.; Guo, M.T.; Yang, J. Monitoring and assessing the impact of wastewater treatment on release of both antibiotic-resistant bacteria and their typical genes in a Chinese municipal wastewater treatment plant. Environ. Sci. Process. Impacts 2014, 16, 1930–1937. [Google Scholar] [CrossRef]
- Klare, I.; Konstabel, C.; Mueller-Bertling, S.; Werner, G.; Strommenger, B.; Kettlitz, C.; Borgmann, S.; Schulte, B.; Jonas, D.; Serr, A.; et al. Spread of ampicillin/vancomycin-resistant Enterococcus faecium of the epidemic-virulent clonal complex-17 carrying the genes esp and hyl in German hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 815–825. [Google Scholar] [CrossRef]
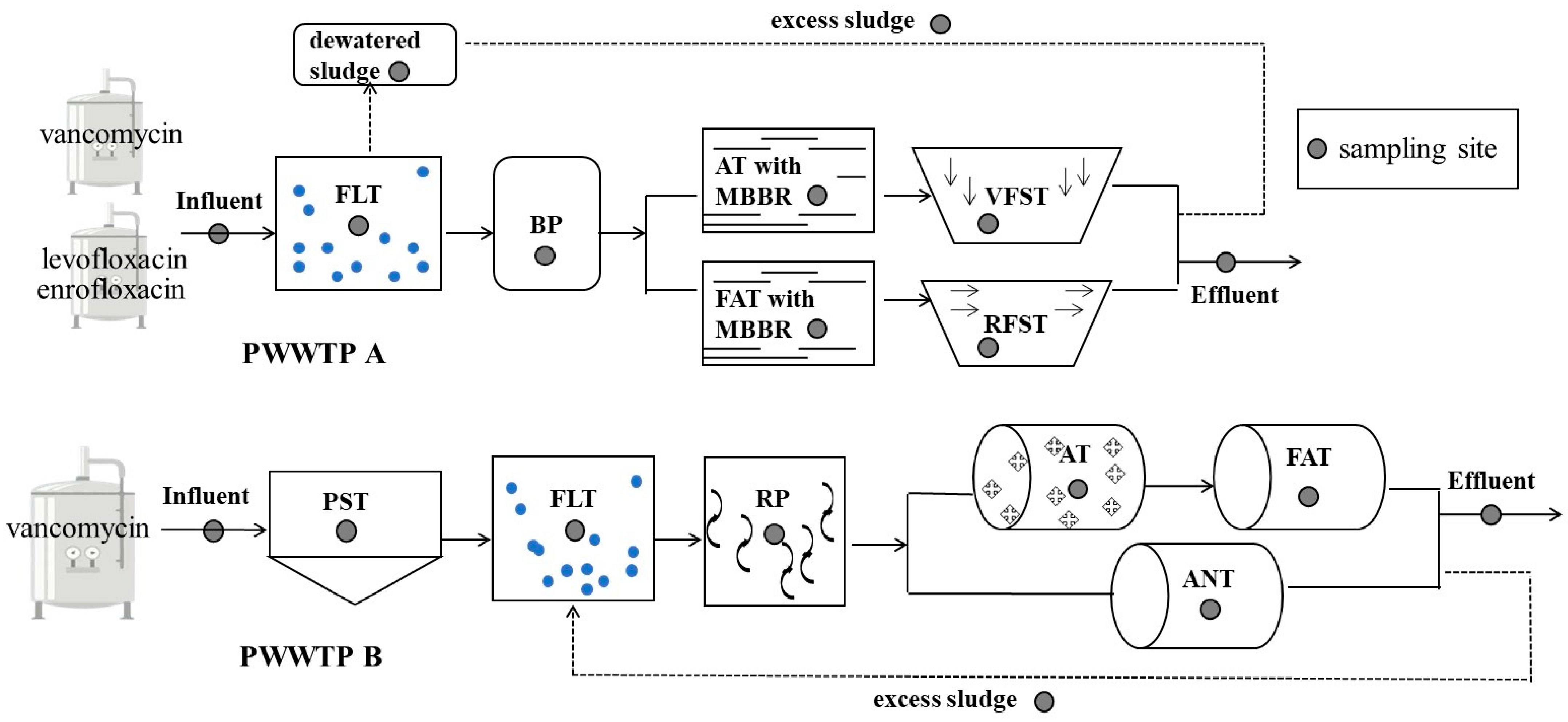

| PWWTPs | Latitude and Longitude | Influent (m3/d) | Effluent (m3/d) | Sludge (ton/d) | Influent/Effluent COD (mg/L) | Influent/Effluent NH3-N (mg/L) | Influent/Effluent pH | Antibiotics Contained |
|---|---|---|---|---|---|---|---|---|
| A | 29°30′12″ N, 120°54′42″ E | 1200 | 1200 | 42 | 11,000/289 | 300/3 | 9.49/7.50 | vancomycin, levofloxacin, enrofloxacin |
| B | 28°40′33″ N, 121°27′56″ E | 2880 | 2880 | 90 | 15,000/500 | 200/10 | 6.35/8.16 | vancomycin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Zhang, X.; Ni, N.; Shi, M.; Wang, N. Fate and Proliferation of Vancomycin Resistance Genes in Two Typical Pharmaceutical Wastewater Treatment Plants. Water 2024, 16, 114. https://doi.org/10.3390/w16010114
Guo X, Zhang X, Ni N, Shi M, Wang N. Fate and Proliferation of Vancomycin Resistance Genes in Two Typical Pharmaceutical Wastewater Treatment Plants. Water. 2024; 16(1):114. https://doi.org/10.3390/w16010114
Chicago/Turabian StyleGuo, Xinyan, Xiaohui Zhang, Ni Ni, Mali Shi, and Na Wang. 2024. "Fate and Proliferation of Vancomycin Resistance Genes in Two Typical Pharmaceutical Wastewater Treatment Plants" Water 16, no. 1: 114. https://doi.org/10.3390/w16010114
APA StyleGuo, X., Zhang, X., Ni, N., Shi, M., & Wang, N. (2024). Fate and Proliferation of Vancomycin Resistance Genes in Two Typical Pharmaceutical Wastewater Treatment Plants. Water, 16(1), 114. https://doi.org/10.3390/w16010114