The Hydrogeochemical Processes of Groundwater in the Bieletan Area, the Western Potash Production Region in China
Abstract
1. Introduction
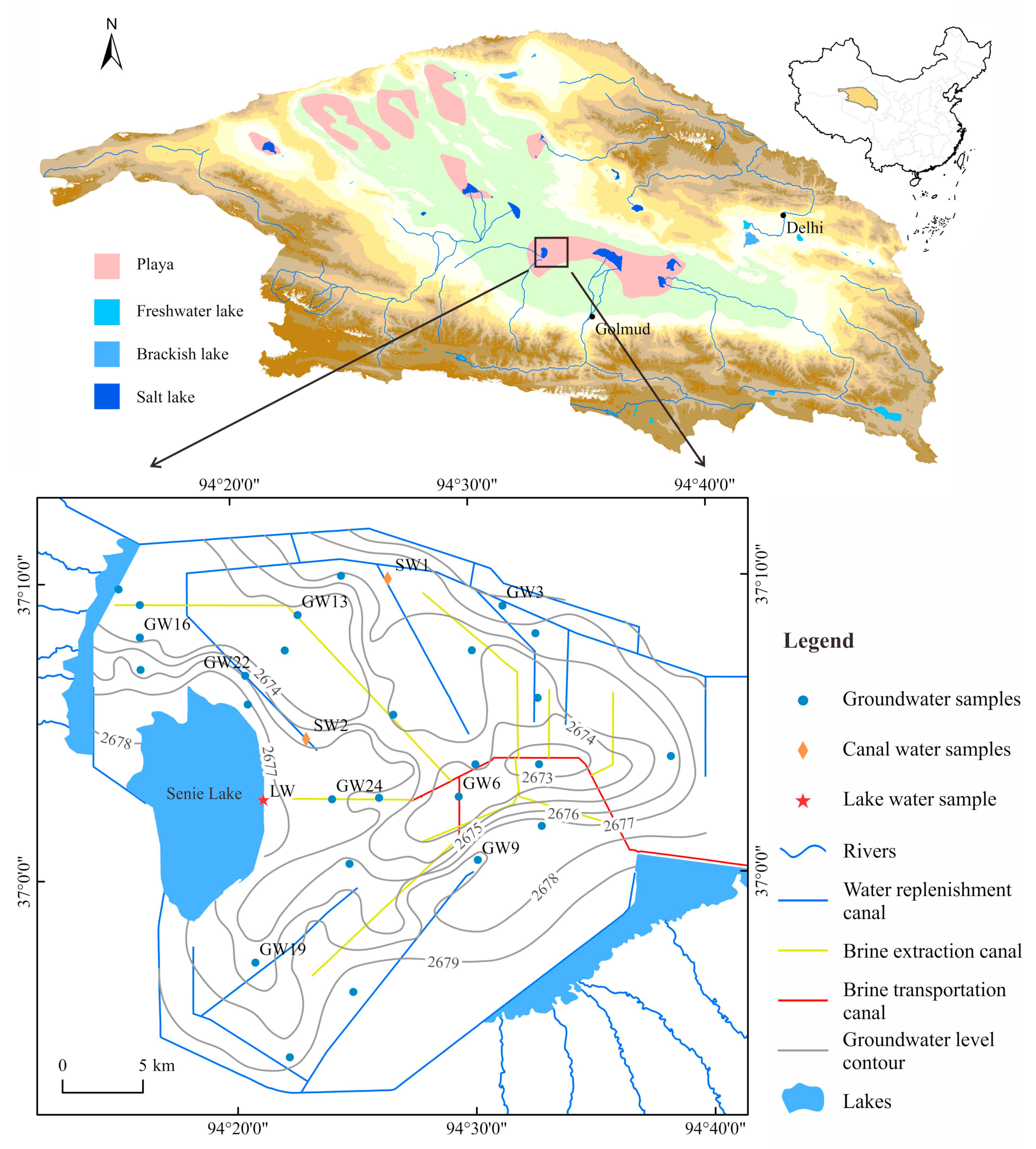
2. Study Area
2.1. Location and Climate
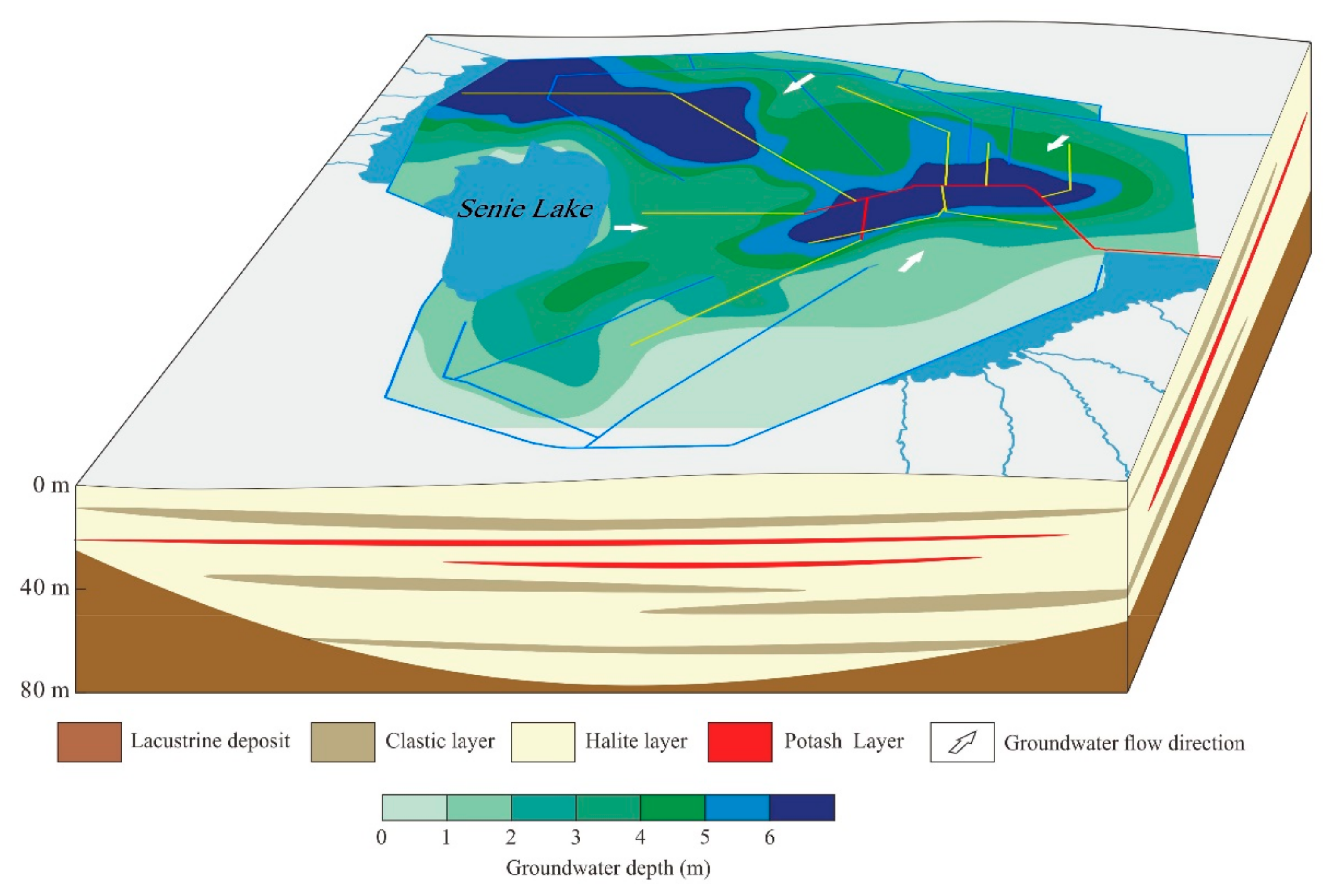
2.2. Geological and Hydrogeological Settings
3. Material and Methods
3.1. Sampling Collection
3.2. Saturation Index
3.3. Hydrogeochemical Modeling
4. Results
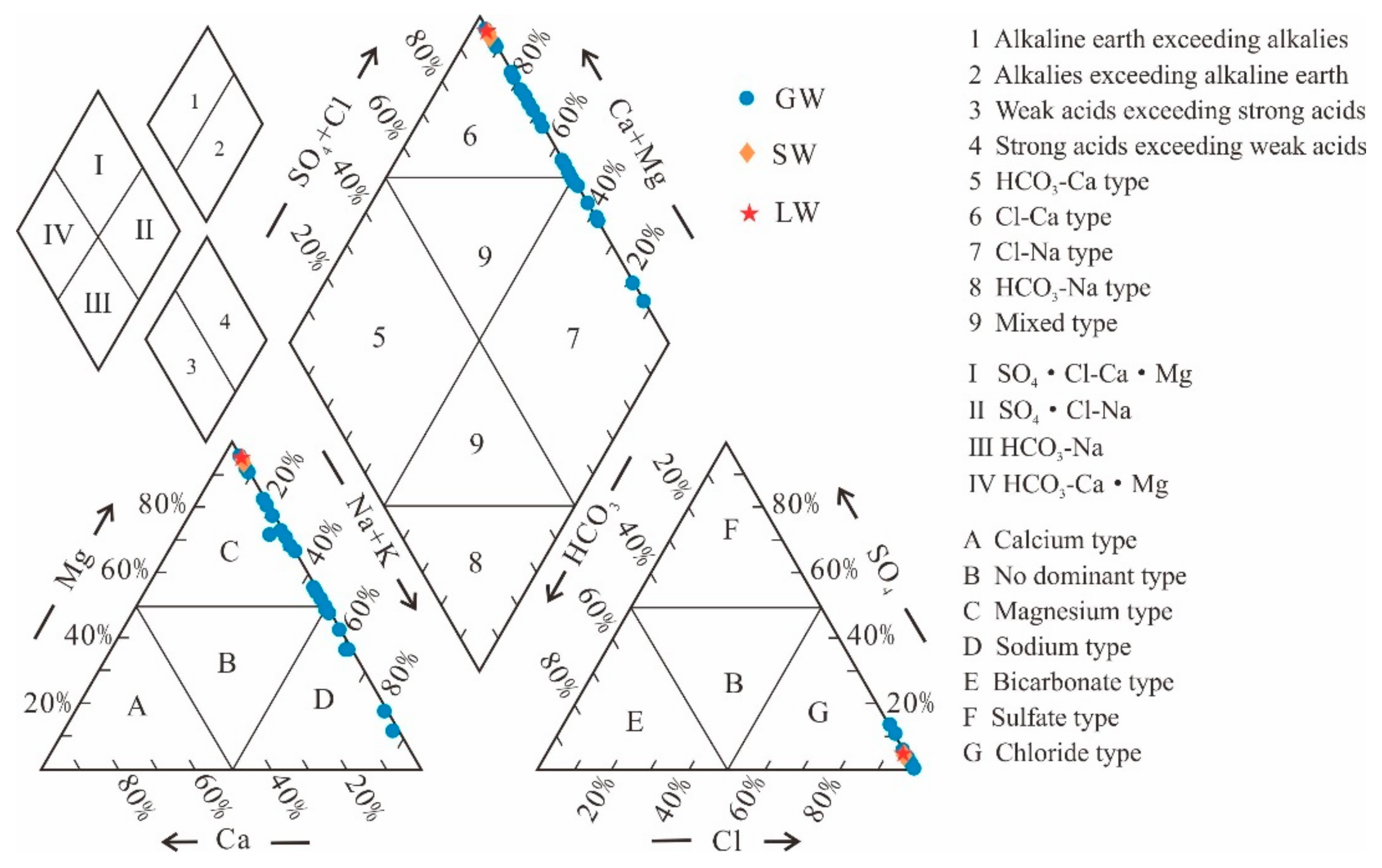
4.1. Characteristics of Major Indices
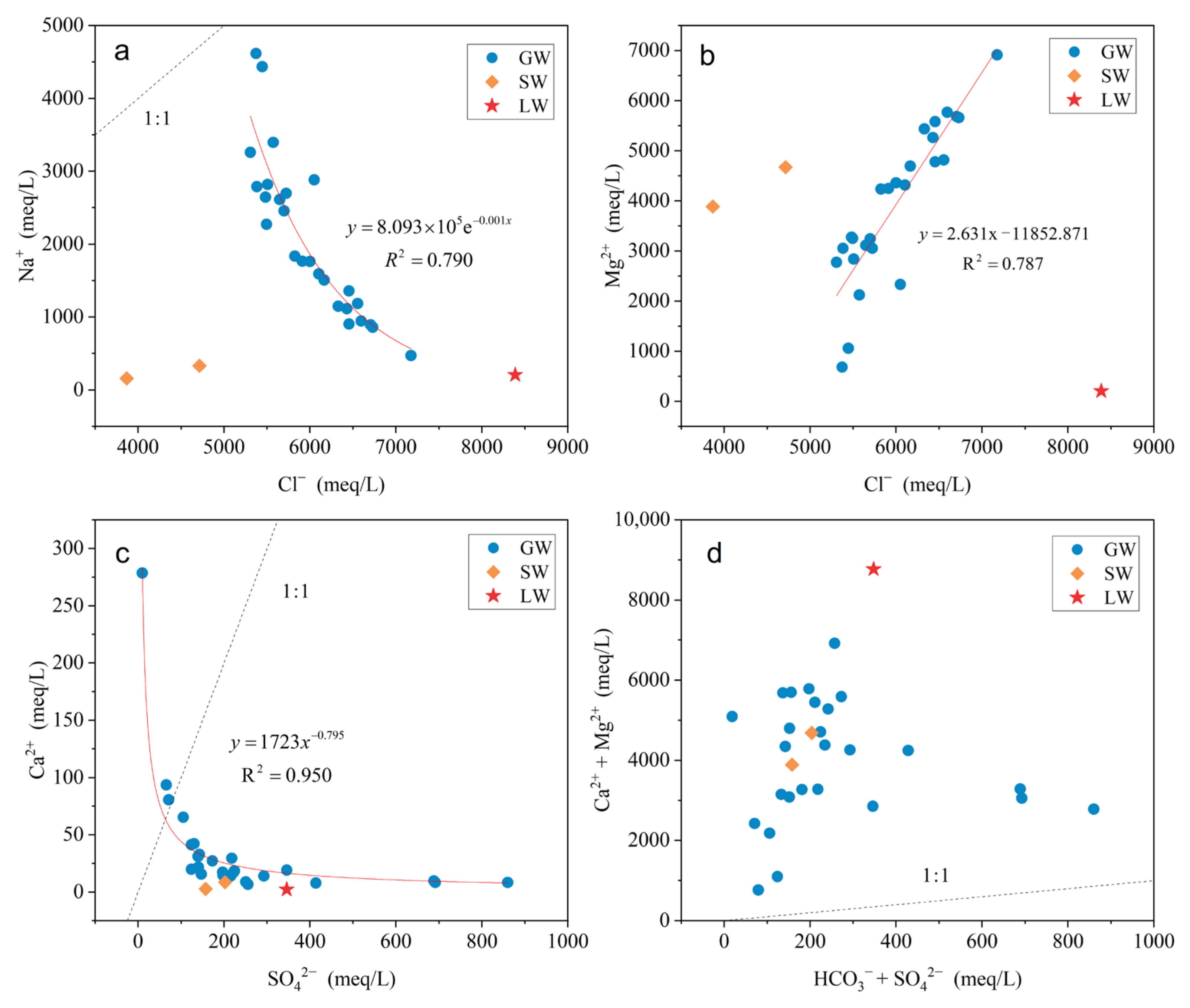
4.2. Correlation Analysis
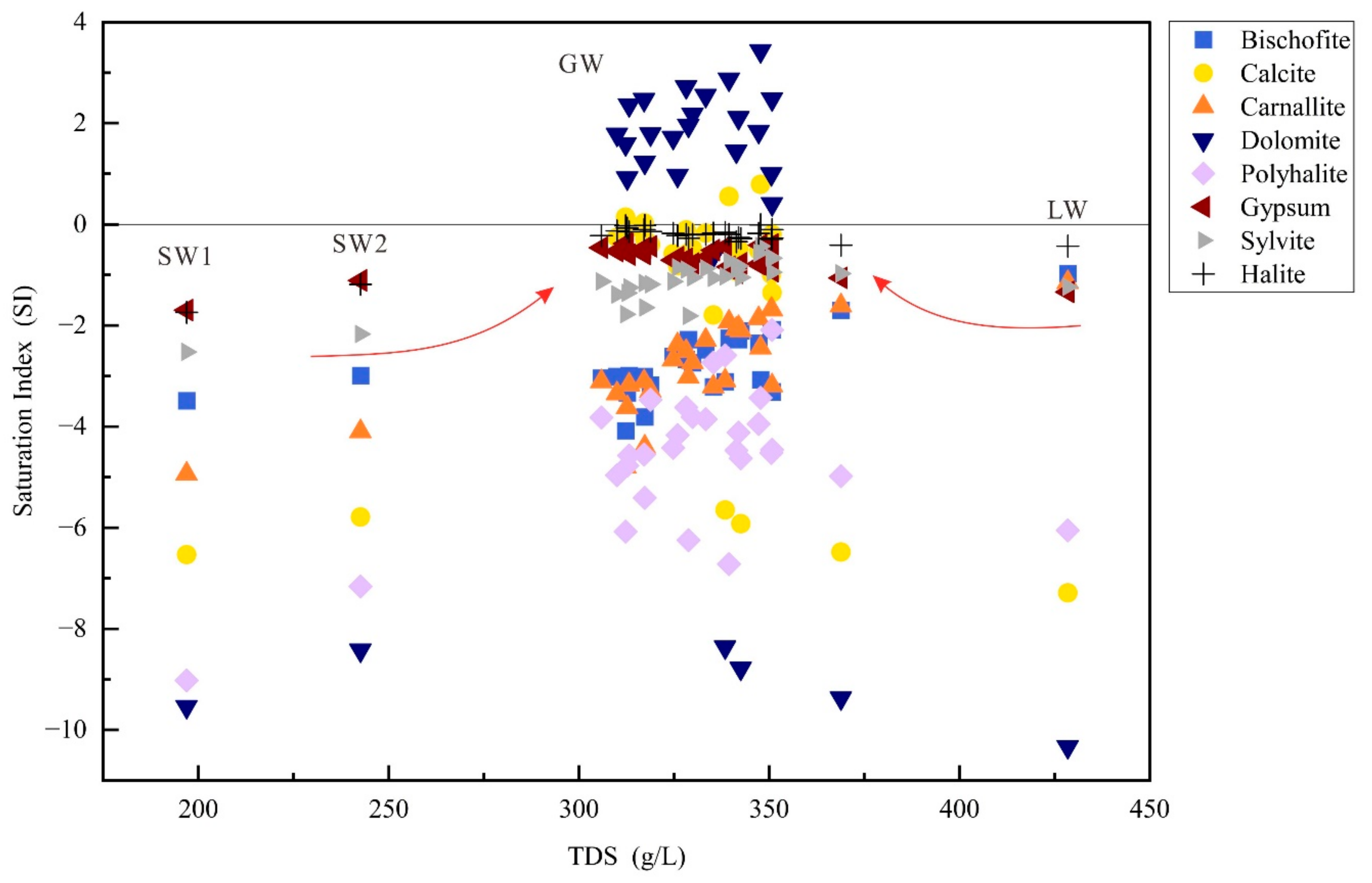
4.3. Saturation Index (SI)
4.4. Hydrochemical Processes: Inverse Modeling
5. Discussion
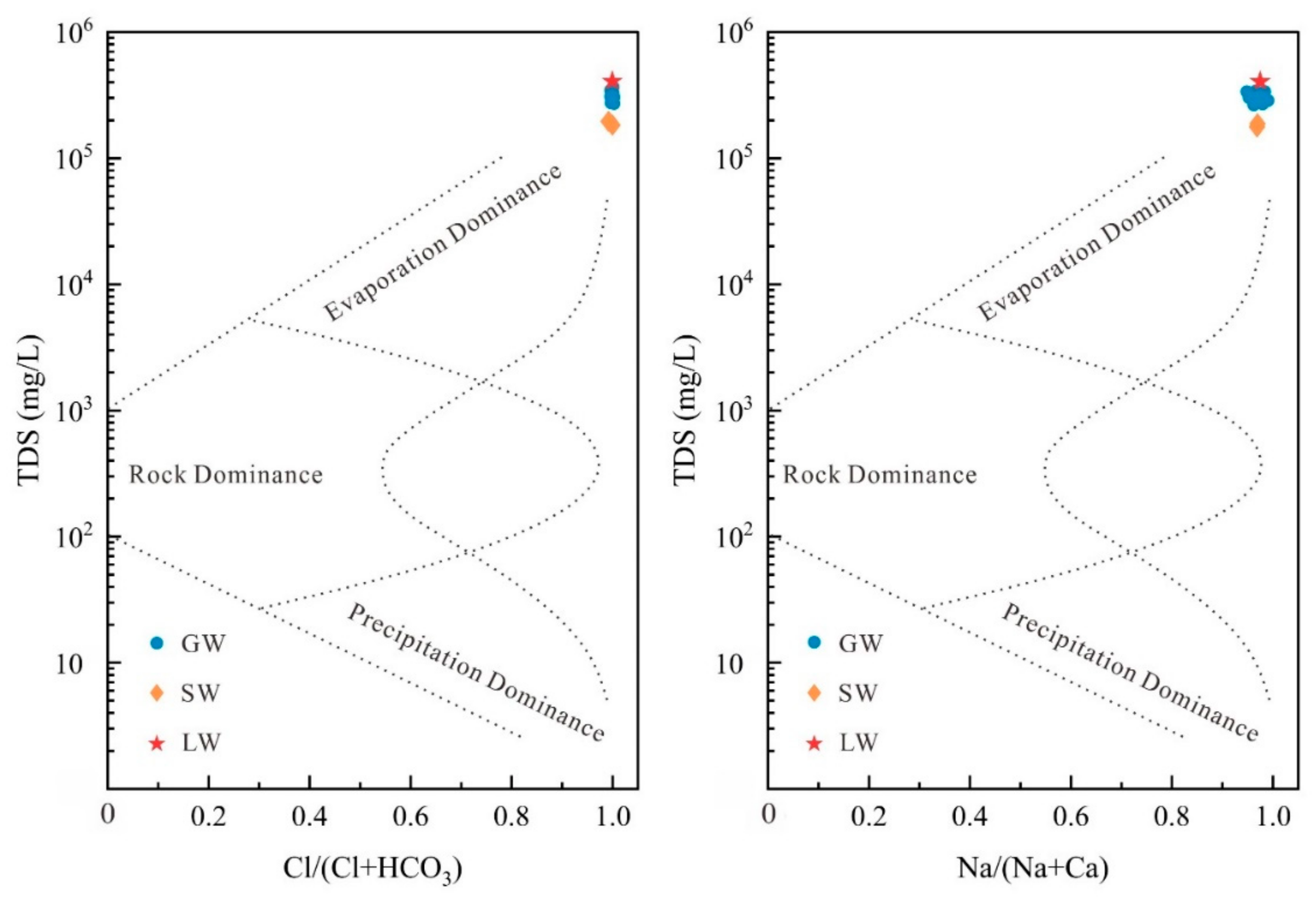
5.1. Hydrogeochemistry
5.2. Water–Rock Interaction
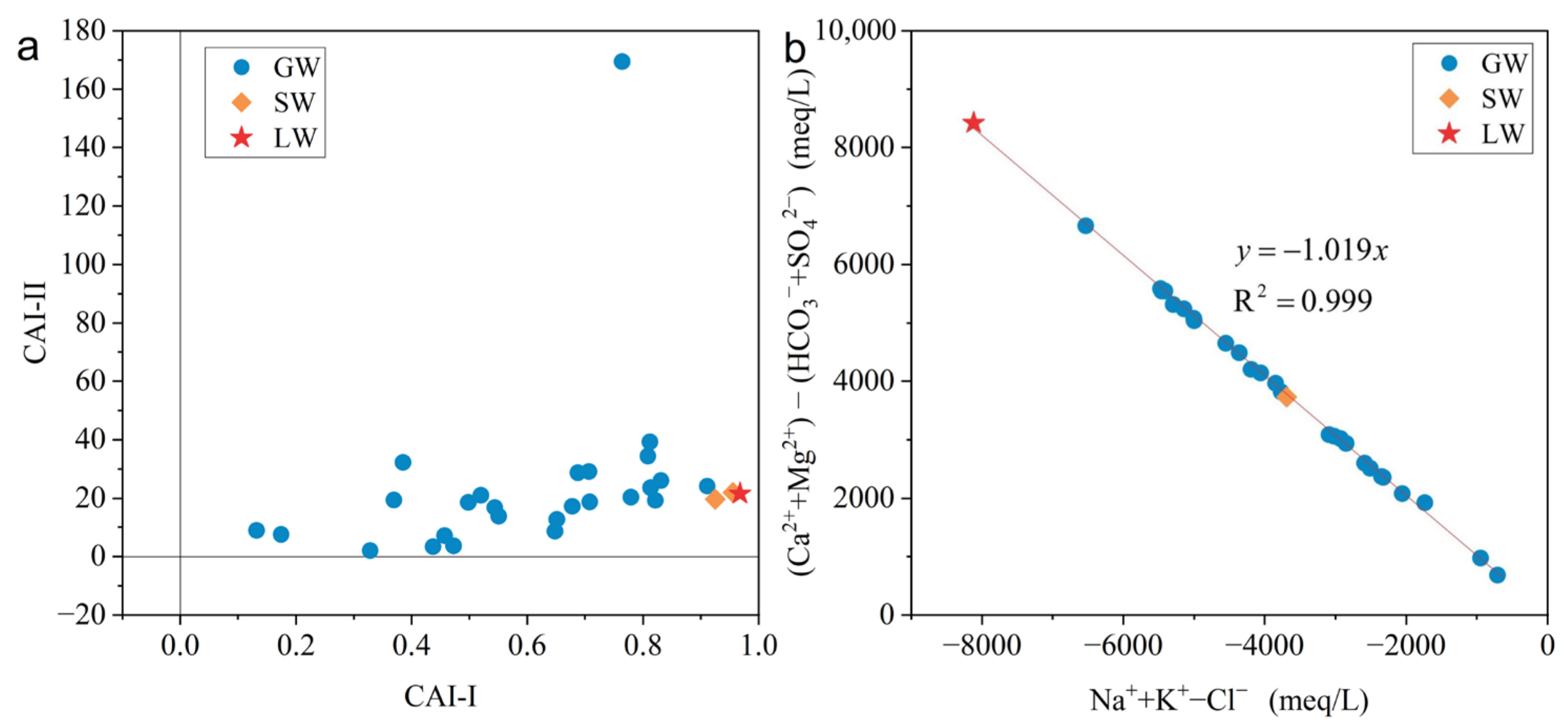
5.3. Cation Exchange Interaction
5.4. Hydrogeochemical Processes
6. Conclusions
- Groundwater (GW) cation concentrations are ranked as Na+ > Mg2+ > K+ > Ga2+, while in the case of lake water (LW) and stream water (SW), cation concentrations are Mg2+ > Na+ > K+ > Ga2+. The anion concentrations in GW are ranked as Cl− > SO42− > HCO3− > CO32−, whereas in LW and SW, the order is Cl− > SO42− > CO32− > HCO3−. The mean total dissolved solids (TDS) values for GW and SW are 332.03 g/L and 219.73 g/L, respectively, while for LW, it is 428.46 g/L. The hydrochemical types of GW are classified as Cl-Mg type and Cl-Na type, whereas LW and SW are categorized as Cl-Mg type.
- Evaporation is the primary mechanism governing the formation of GW, LW, and SW. Cation exchange and mineral precipitation/dissolution are identified as the primary factors influencing water chemistry. Minerals in LW and SW are generally undersaturated, while in GW, apart from dolomite, calcite, and halite, which are saturated or near-saturated, other minerals remain undersaturated.
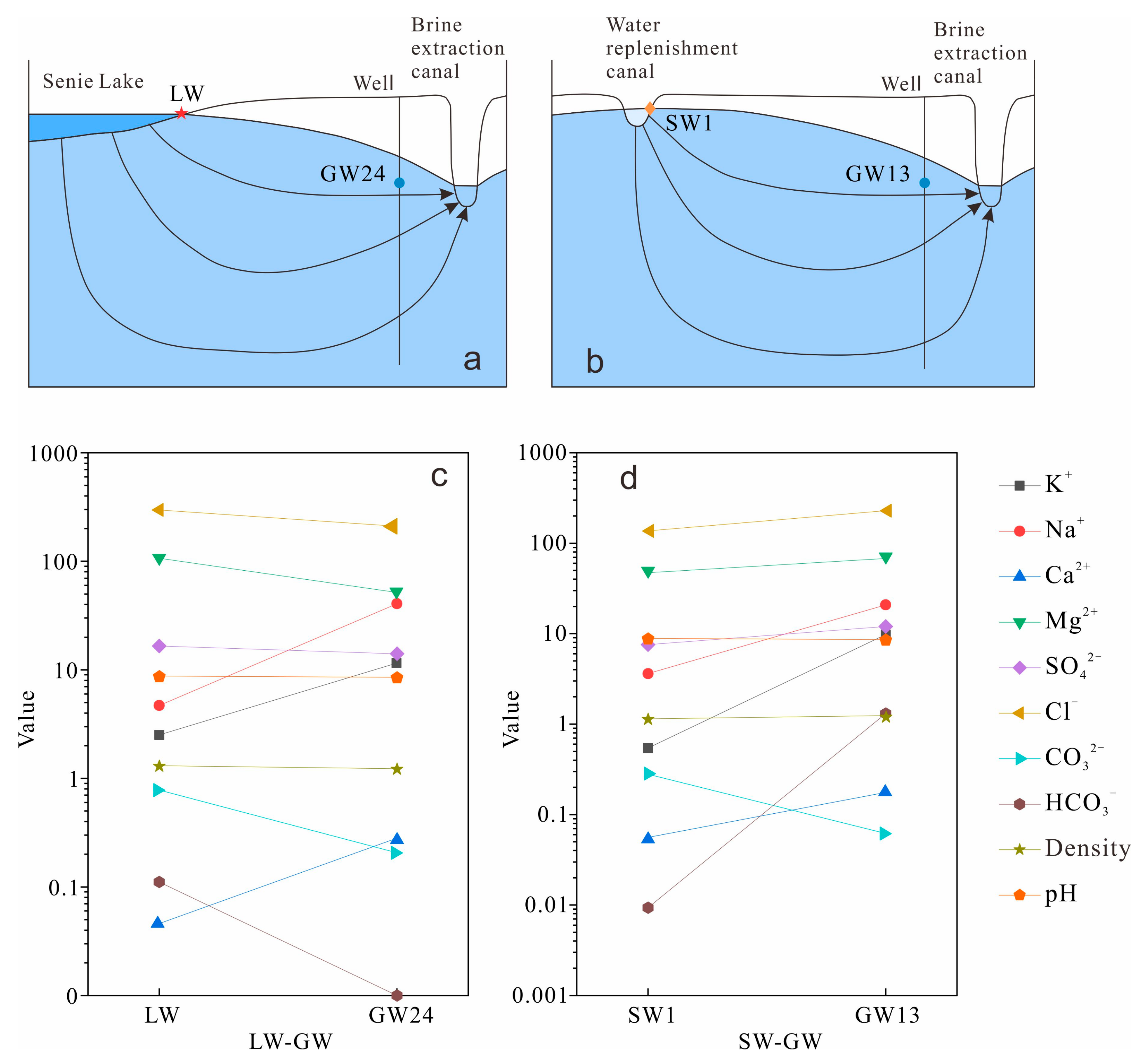
- GW receives recharge from two distinct sources, LW and SW. These two different water bodies undergo distinct hydrogeochemical processes during groundwater recharge. The rates of cation exchange and mineral dissolution are faster in LW, where mineral precipitation predominantly occurs, whereas mineral dissolution is more prevalent in SW.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, Y.; Hu, H.; Ye, C.; Zhao, Y.; Ma, L.; Zhang, J.; Yan, Q.; Lin, Y. Evaluation on liquefaction effect of potassium dissolution extraction from low-grade solid potash ore in Qarhan Salt Lake, northern of Tibetan Plateau. Carbonates Evaporites 2024, 39, 56. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Bhateria, R.; Jain, D. Water quality assessment of lake water: A review. Sustain. Water Resour. Manag. 2016, 2, 161–173. [Google Scholar] [CrossRef]
- Rawashdeh, R.A.; Xavier-Oliveira, E.; Maxwell, P. The potash market and its future prospects. Resour. Policy 2016, 47, 154–163. [Google Scholar] [CrossRef]
- Dmitrieva, D.; Ilinova, A.; Kraslawski, A. Strategic management of the potash industry in Russia. Resour. Policy 2017, 52, 81–89. [Google Scholar] [CrossRef]
- al Rawashdeh, R.; Maxwell, P. Analysing the world potash industry. Resour. Policy 2014, 41, 143–151. [Google Scholar] [CrossRef]
- Song, X.; Geng, Y.; Zhang, Y.; Zhang, X.; Gao, Z.; Li, M. Dynamic potassium flows analysis in China for 2010–2019. Resour. Policy 2022, 78, 102803. [Google Scholar] [CrossRef]
- Chen, W.; Geng, Y.; Hong, J.; Yang, D.; Ma, X. Life cycle assessment of potash fertilizer production in China. Resour. Conserv. Recycl. 2018, 138, 238–245. [Google Scholar] [CrossRef]
- Broughton, P.L. Economic geology of southern Saskatchewan potash mines. Ore Geol. Rev. 2019, 113, 103117. [Google Scholar] [CrossRef]
- Kumar Katta, A.; Davis, M.; Kumar, A. Assessment of greenhouse gas mitigation options for the iron, gold, and potash mining sectors. J. Clean. Prod. 2020, 245, 118718. [Google Scholar] [CrossRef]
- Cabello, J. Lithium brine production, reserves, resources and exploration in Chile: An updated review. Ore Geol. Rev. 2021, 128, 103883. [Google Scholar] [CrossRef]
- Bustos-Gallardo, B.; Bridge, G.; Prieto, M. Harvesting Lithium: Water, brine and the industrial dynamics of production in the Salar de Atacama. Geoforum 2021, 119, 177–189. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Miller, C.; Null, S.E.; DeRose, R.J.; Wilcock, P.; Hahnenberger, M.; Howe, F.; Moore, J. Decline of the world’s saline lakes. Nat. Geosci. 2017, 10, 816–821. [Google Scholar] [CrossRef]
- Song, H.; Fan, Q.; Li, Q.; Chen, T.; Yang, H.; Han, C. Recharge processes limit the resource elements of Qarhan Salt Lake in western China and analogues in the evaporite basins. J. Oceanol. Limnol. 2023, 41, 1226–1242. [Google Scholar] [CrossRef]
- Li, R.; Liu, C.; Jiao, P.; Hu, Y.; Liu, W.; Wang, S. Simulation study on the mining conditions of dissolution of low grade solid potash ore in Qarhan Salt Lake. Sci. Rep. 2021, 11, 10539. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Hao, Q.; Luo, Y.; Wang, S.; Dang, X.; Shao, J.; Huang, L. Origin of Brines and Modern Water Circulation Contribution to Qarhan Salt Lake in Qaidam Basin, Tibetan Plateau; EDP Sciences: Les Ulis, France, 2019; Volume 98, p. 12025. [Google Scholar]
- Li, R.; Liu, C.; Jiao, P.; Liu, W.; Tang, D.; Wang, S. The effect of solvent chemistry on potassium dissolution extraction from low-grade solid potash ore in Qarhan Salt Lake, China. Appl. Geochem. 2020, 115, 104550. [Google Scholar] [CrossRef]
- Tziritis, E.; Skordas, K.; Kelepertsis, A. The use of hydrogeochemical analyses and multivariate statistics for the characterization of groundwater resources in a complex aquifer system. A case study in Amyros River basin, Thessaly, central Greece. Environ. Earth Sci. 2016, 75, 339. [Google Scholar] [CrossRef]
- Nematollahi, M.J.; Clark, M.J.R.; Ebrahimi, P.; Ebrahimi, M. Preliminary assessment of groundwater hydrogeochemistry within Gilan, a northern province of Iran. Environ. Monit. Assess. 2018, 190, 242. [Google Scholar] [CrossRef]
- Chidambaram, S.; Anandhan, P.; Prasanna, M.V.; Ramanathan, A.L.; Srinivasamoorthy, K.; Senthil Kumar, G. Hydrogeochemical Modelling for Groundwater in Neyveli Aquifer, Tamil Nadu, India, Using PHREEQC: A Case Study. Nat. Resour. Res. 2012, 21, 311–324. [Google Scholar] [CrossRef]
- Guo, W.; Li, P.; Du, Q.; Zhou, Y.; Xu, D.; Zhang, Z. Hydrogeochemical Processes Regulating the Groundwater Geochemistry and Human Health Risk of Groundwater in the Rural Areas of the Wei River Basin, China. Expo. Health 2024, 16, 291–306. [Google Scholar] [CrossRef]
- Duan, R.; Li, P.; Wang, L.; He, X.; Zhang, L. Hydrochemical characteristics, hydrochemical processes and recharge sources of the geothermal systems in Lanzhou City, northwestern China. Urban Clim. 2022, 43, 101152. [Google Scholar] [CrossRef]
- de Caritat, P.; Bastrakov, E.N.; Jaireth, S.; English, P.M.; Clarke, J.D.A.; Mernagh, T.P.; Wygralak, A.S.; Dulfer, H.E.; Trafford, J. Groundwater geochemistry, hydrogeology and potash mineral potential of the Lake Woods region, Northern Territory, Australia. Aust. J. Earth Sci. 2019, 66, 411–430. [Google Scholar] [CrossRef]
- El Alfy, M.; Lashin, A.; Abdalla, F.; Al-Bassam, A. Assessing the hydrogeochemical processes affecting groundwater pollution in arid areas using an integration of geochemical equilibrium and multivariate statistical techniques. Environ. Pollut. 2017, 229, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Parisi, A.; Alfio, M.R.; Balacco, G.; Güler, C.; Fidelibus, M.D. Analyzing spatial and temporal evolution of groundwater salinization through Multivariate Statistical Analysis and Hydrogeochemical Facies Evolution-Diagram. Sci. Total Environ. 2023, 862, 160697. [Google Scholar] [CrossRef]
- Qin, Z.; Li, Q.; Li, W.; Fan, Q.; Chen, T.; Wu, C.; Wang, J.; Shan, F. Elemental Variations and Mechanisms of Brines in the Context of Large-Scale Exploitation: A Case Study of Xitaijnar Salt Lake, Qaidam Basin. Aquat. Geochem. 2023. [Google Scholar] [CrossRef]
- Fan, Q.; Lowenstein, T.K.; Wei, H.; Yuan, Q.; Qin, Z.; Shan, F.; Ma, H. Sr isotope and major ion compositional evidence for formation of Qarhan Salt Lake, western China. Chem. Geol. 2018, 497, 128–145. [Google Scholar] [CrossRef]
- Guo, J.; Wei, X.; Long, G.; Wang, B.; Fan, H.; Xu, S. Three-dimensional structural model of the Qaidam basin: Implications for crustal shortening and growth of the northeast Tibet. Open Geosci. 2017, 9, 174–185. [Google Scholar] [CrossRef]
- Stober, I.; Zhong, J.; Bucher, K. From freshwater inflows to salt lakes and salt deposits in the Qaidam Basin, W China. Swiss J. Geosci. 2023, 116, 5. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Aravinthasamy, P.; Subramani, T.; Kumar, D.; Setia, R. Investigation of health risks related with multipath entry of groundwater nitrate using Sobol sensitivity indicators in an urban-industrial sector of South India. Environ. Res. 2021, 200, 111726. [Google Scholar] [CrossRef]
- Zhu, G.; Wu, X.; Ge, J.; Liu, F.; Zhao, W.; Wu, C. Influence of mining activities on groundwater hydrochemistry and heavy metal migration using a self-organizing map (SOM). J. Clean. Prod. 2020, 257, 120664. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Qian, H. Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ. Earth Sci. 2015, 73, 8575–8588. [Google Scholar] [CrossRef]
- Morán-Ramírez, J.; Ledesma-Ruiz, R.; Mahlknecht, J.; Ramos-Leal, J.A. Rock–water interactions and pollution processes in the volcanic aquifer system of Guadalajara, Mexico, using inverse geochemical modeling. Appl. Geochem. 2016, 68, 79–94. [Google Scholar] [CrossRef]
- Pérez-Ceballos, R.; Canul-Macario, C.; Pacheco-Castro, R.; Pacheco-Ávila, J.; Euán-Ávila, J.; Merino-Ibarra, M. Regional Hydrogeochemical Evolution of Groundwater in the Ring of Cenotes, Yucatán (Mexico): An Inverse Modelling Approach. Water 2021, 13, 614. [Google Scholar] [CrossRef]
- Guo, Y.; Lyu, Z.; Wang, G.; Ma, L.; Xu, Q.; Huang, X.; Gao, S. Hydrogeochemical simulation of groundwater in Eastern Fengfeng mining area. Coal Geol. Explor. 2016, 44, 101–105. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H. Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ. Earth Sci. 2013, 69, 2211–2225. [Google Scholar] [CrossRef]
- Adimalla, N.; Venkatayogi, S. Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangana State, South India. Appl. Water Sci. 2018, 8, 44. [Google Scholar] [CrossRef]
- Zaidi, F.K.; Nazzal, Y.; Jafri, M.K.; Naeem, M.; Ahmed, I. Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: A case study from northwestern Saudi Arabia. Environ. Monit. Assess. 2015, 187, 607. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, P.; Du, Q.; Yang, Y.; Yue, B. Seasonal Hydrochemical Characteristics, Geochemical Evolution, and Pollution Sources of Lake Sha in an Arid and Semiarid Region of Northwest China. Expo. Health 2023, 15, 231–244. [Google Scholar] [CrossRef]
- Chaudhary, V.; Satheeshkumar, S. Assessment of groundwater quality for drinking and irrigation purposes in arid areas of Rajasthan, India. Appl. Water Sci. 2018, 8, 218. [Google Scholar] [CrossRef]
- Li, P.; He, X.; Guo, W. Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: A case study in Yan’an City on the Loess Plateau of northwest China. Hum. Ecol. Risk Assess. 2019, 25, 11–31. [Google Scholar] [CrossRef]
- Jia, H.; Qian, H.; Zheng, L.; Feng, W.; Wang, H.; Gao, Y. Alterations to groundwater chemistry due to modern water transfer for irrigation over decades. Sci. Total Environ. 2020, 717, 137170. [Google Scholar] [CrossRef] [PubMed]
| Type | ID | pH | K+ | Na+ | Ca2+ | Mg2+ | SO42− | Cl− | TDS | CO32− | HCO3− | Li+ | Density |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GW | GW1 | 8.49 | 4.05 | 60.02 | 0 | 37.81 | 6.24 | 200.54 | 309.96 | 0.15 | 0.18 | 0.09 | 1.21 |
| GW2 | 8.23 | 32.81 | 66.24 | 1.87 | 28.39 | 3.13 | 214.80 | 347.67 | 0 | 0.32 | 0.11 | 1.23 | |
| GW3 | 6.76 | 1.85 | 106.12 | 1.62 | 8.32 | 3.43 | 190.74 | 312.23 | 0 | 0.48 | 0.02 | 1.20 | |
| GW4 | 8.56 | 20.97 | 31.23 | 0.44 | 58.08 | 6.74 | 229.06 | 347.22 | 0.09 | 0.70 | 0.17 | 1.24 | |
| GW5 | 8.61 | 6.79 | 62.04 | 0.66 | 37.12 | 6.84 | 203.21 | 317.09 | 0.04 | 0.54 | 0.09 | 1.22 | |
| GW6 | 8.65 | 5.61 | 56.52 | 0.54 | 39.42 | 8.28 | 202.32 | 313.16 | 0.05 | 0.52 | 0.10 | 1.22 | |
| GW7 | 8.49 | 15.30 | 20.50 | 0.31 | 69.06 | 7.06 | 237.97 | 350.73 | 0.05 | 0.52 | 0.11 | 1.24 | |
| GW8 | 8.57 | 6.70 | 40.53 | 0.34 | 53.01 | 10.65 | 213.02 | 324.77 | 0.04 | 0.77 | 0.09 | 1.23 | |
| GW9 | 8.67 | 1.27 | 26.38 | 0.29 | 66.03 | 9.47 | 224.60 | 328.76 | 0.13 | 0.82 | 0.12 | 1.23 | |
| GW10 | 8.52 | 15.80 | 19.78 | 0.40 | 68.82 | 5.94 | 238.87 | 350.54 | 0.05 | 0.78 | 0.27 | 1.25 | |
| GW11 | 8.55 | 12.01 | 25.62 | 0.28 | 64.00 | 10.33 | 228.17 | 341.97 | 0 | 1.63 | 0.51 | 1.24 | |
| GW12 | 8.63 | 8.39 | 42.20 | 0.16 | 51.48 | 19.85 | 206.78 | 329.87 | 0.08 | 0.90 | 0.22 | 1.23 | |
| GW13 | 8.61 | 9.78 | 20.78 | 0.18 | 67.81 | 12.04 | 229.06 | 341.37 | 0.06 | 1.31 | 0.48 | 1.24 | |
| GW14 | 8.30 | 9.47 | 64.11 | 0.17 | 37.09 | 33.19 | 191.05 | 335.32 | 0 | 0.10 | 0.25 | 1.23 | |
| GW15 | 8.42 | 9.62 | 60.86 | 0.19 | 39.81 | 33.05 | 194.64 | 338.44 | 0.08 | 0.02 | 0.26 | 1.23 | |
| GW16 | 8.44 | 7.78 | 52.24 | 0.59 | 39.50 | 10.48 | 195.09 | 305.91 | 0 | 0 | 0.21 | 1.21 | |
| GW17 | 8.40 | 6.77 | 64.82 | 0.39 | 34.48 | 16.61 | 195.54 | 318.83 | 0.07 | 0 | 0.21 | 1.22 | |
| GW18 | 8.47 | 11.48 | 34.67 | 0.37 | 57.01 | 10.77 | 218.86 | 333.31 | 0.19 | 0 | 0.14 | 1.23 | |
| GW19 | 8.52 | 6.69 | 21.71 | 0.35 | 70.09 | 9.43 | 234.11 | 342.53 | 0.21 | 0.06 | 0.14 | 1.24 | |
| GW20 | 8.61 | 6.62 | 10.82 | 0.13 | 84.04 | 12.27 | 254.74 | 368.80 | 0.32 | 0.09 | 0.18 | 1.26 | |
| GW21 | 8.42 | 2.40 | 101.94 | 0.83 | 12.86 | 5.95 | 193.30 | 317.32 | 0.01 | 0 | 0.02 | 1.21 | |
| GW22 | 7.85 | 4.55 | 78.08 | 1.31 | 25.79 | 5.04 | 197.78 | 312.65 | 0 | 0.06 | 0.07 | 1.21 | |
| GW23 | 8.54 | 12.02 | 74.89 | 0.17 | 33.69 | 41.30 | 188.36 | 350.75 | 0.16 | 0 | 0.32 | 1.24 | |
| GW24 | 8.56 | 11.59 | 40.64 | 0.28 | 51.59 | 14.04 | 209.89 | 328.22 | 0.21 | 0 | 0.18 | 1.23 | |
| GW25 | 8.71 | 12.34 | 36.60 | 0.63 | 52.47 | 6.69 | 216.64 | 325.96 | 0.11 | 0.18 | 0.05 | 1.23 | |
| GW26 | 8.17 | 14.01 | 27.29 | 5.58 | 58.56 | 0.47 | 232.63 | 339.45 | 0 | 0.52 | 0.31 | 1.23 | |
| Min | 6.76 | 1.27 | 10.82 | 0 | 8.32 | 0.47 | 188.36 | 305.91 | 0 | 0 | 0.02 | 1.20 | |
| Max | 8.71 | 32.81 | 106.12 | 5.58 | 84.04 | 41.30 | 254.74 | 368.80 | 0.32 | 1.63 | 0.51 | 1.26 | |
| Mean | 8.41 | 9.87 | 47.95 | 0.70 | 47.94 | 11.89 | 213.14 | 332.03 | 0.08 | 0.40 | 0.18 | 1.23 | |
| SD | 0.38 | 6.58 | 25.21 | 1.09 | 18.58 | 9.84 | 18.31 | 15.88 | 0.08 | 0.44 | 0.12 | 0.01 | |
| CV(%) | 5 | 67 | 53 | 149 | 39 | 83 | 9 | 5 | 97 | 109 | 68 | 1 | |
| SW | SW1 | 8.88 | 0.54 | 3.63 | 0.06 | 47.25 | 7.55 | 137.26 | 196.89 | 0.28 | 0.01 | 0.11 | 1.15 |
| SW2 | 8.81 | 0.90 | 7.62 | 0.17 | 56.78 | 9.70 | 167.28 | 242.58 | 0.47 | 0.09 | 0.12 | 1.18 | |
| mean | 8.85 | 0.72 | 5.62 | 0.11 | 52.02 | 8.62 | 152.27 | 219.73 | 0.37 | 0.05 | 0.11 | 1.16 | |
| LW | LW | 8.75 | 2.52 | 4.70 | 0.05 | 106.5 | 16.61 | 297.79 | 428.46 | 0.78 | 0.11 | 0.22 | 1.31 |
| Indices | Soluble Ions | ||||
|---|---|---|---|---|---|
| Min | Max | Mean | SD | CV (%) | |
| pH | 8.03 | 8.53 | 8.28 | 0.16 | 1.93 |
| K+ | 0.36 | 16.62 | 3.31 | 3.72 | 112.19 |
| Na+ | 2.53 | 313.40 | 150.01 | 117.47 | 78.31 |
| Ca2+ | 0.37 | 11.81 | 5.40 | 3.20 | 59.25 |
| Mg2+ | 0.40 | 20.59 | 8.10 | 6.27 | 77.46 |
| SO42− | 1.96 | 26.80 | 10.32 | 5.74 | 55.57 |
| Cl− | 31.40 | 570.00 | 302.70 | 198.48 | 65.57 |
| CO32− | 0.00 | 0.28 | 0.08 | 0.06 | 77.95 |
| HCO3− | 0.05 | 0.32 | 0.15 | 0.09 | 59.78 |
| Li+ | 0.00 | 0.42 | 0.13 | 0.14 | 110.67 |
| K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | CO32− | HCO3− | Li+ | pH | |
| K+ | 1 | −0.42 | 0.04 | 0.82 ** | −0.4 | 0.45 * | 0.4 | 0.62 ** | 0.59 ** | 0.12 |
| Na+ | 1 | 0.58 ** | −0.76 ** | 0.99 ** | 0.19 | −0.82 ** | −0.87 ** | −0.89 ** | −0.71 ** | |
| Ca2+ | 1 | −0.22 | 0.61 ** | 0.78 ** | −0.47 * | −0.35 | −0.57 ** | −0.47 * | ||
| Mg2+ | 1 | −0.73 ** | 0.19 | 0.79 ** | 0.86 ** | 0.87 ** | 0.57 ** | |||
| Cl− | 1 | 0.21 | −0.79 ** | −0.85 ** | −0.88 ** | −0.71 ** | ||||
| SO42− | 1 | −0.15 | 0.01 | −0.11 | −0.2 | |||||
| CO32− | 1 | 0.80 ** | 0.85 ** | 0.73 ** | ||||||
| HCO3− | 1 | 0.81 ** | 0.63 ** | |||||||
| Li+ | 1 | 0.71 ** | ||||||||
| pH | 1 |
| K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | CO32− | HCO3− | Li+ | TDS | pH | |
| K+ | 1 | −0.32 | −0.07 | 0.28 | 0.38 | −0.01 | 0.01 | 0.16 | 0.48 * | 0.63 ** | −0.01 |
| Na+ | 1 | 0.38 | −0.98 ** | −0.91 ** | −0.06 | −0.44 * | −0.47 * | −0.37 | −0.55 ** | −0.49 * | |
| Ca2+ | 1 | −0.42 * | −0.12 | −0.87 ** | −0.52 ** | −0.08 | −0.55 ** | −0.48 * | −0.44 * | ||
| Mg2+ | 1 | 0.89 ** | 0.11 | 0.48 * | 0.43 * | 0.37 | 0.57 ** | 0.48 * | |||
| Cl− | 1 | −0.21 | 0.33 | 0.49 ** | 0.19 | 0.59 ** | 0.37 | ||||
| SO42− | 1 | 0.44 * | −0.22 | 0.51 ** | 0.19 | 0.28 | |||||
| CO32− | 1 | −0.13 | 0.18 | 0.35 | 0.53 ** | ||||||
| HCO3− | 1 | 0.13 | 0.21 | 0.44 * | |||||||
| Li+ | 1 | 0.54 ** | 0.02 | ||||||||
| TDS | 1 | 0.11 | |||||||||
| pH | 1 |
| Phase | ρ/(mmol/L) | ||
|---|---|---|---|
| Chemical Formula | LW-GW | SW-GW | |
| CaX2 | CaX2 | 18.720 | 2.671 |
| NaX | NaX | −37.450 | −5.342 |
| Bischofite | MgCl2·6H2O | −15.980 | / |
| Calcite | CaCO3 | / | −5.626 |
| Carnallite | KMgCl3·6H2O | −17.860 | 0.278 |
| Dolomite | CaMg(CO3)2 | / | 2.820 |
| Polyhalite | K2MgCa2(SO4)4·2H2O | 8.860 | / |
| Gypsum | CaSO4·2H2O | −36.490 | −0.017 |
| Halite | NaCl | 38.100 | 6.346 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, R.; Chang, L.; Gu, X.; Li, X.; You, X.; Zhang, Q.; Wang, Q. The Hydrogeochemical Processes of Groundwater in the Bieletan Area, the Western Potash Production Region in China. Water 2024, 16, 1833. https://doi.org/10.3390/w16131833
Duan R, Chang L, Gu X, Li X, You X, Zhang Q, Wang Q. The Hydrogeochemical Processes of Groundwater in the Bieletan Area, the Western Potash Production Region in China. Water. 2024; 16(13):1833. https://doi.org/10.3390/w16131833
Chicago/Turabian StyleDuan, Rui, Liang Chang, Xiaofan Gu, Xiaodeng Li, Xiangzhi You, Qunhui Zhang, and Qian Wang. 2024. "The Hydrogeochemical Processes of Groundwater in the Bieletan Area, the Western Potash Production Region in China" Water 16, no. 13: 1833. https://doi.org/10.3390/w16131833
APA StyleDuan, R., Chang, L., Gu, X., Li, X., You, X., Zhang, Q., & Wang, Q. (2024). The Hydrogeochemical Processes of Groundwater in the Bieletan Area, the Western Potash Production Region in China. Water, 16(13), 1833. https://doi.org/10.3390/w16131833







