Research and Application of Water Treatment Technologies for Emerging Contaminants (ECs): A Pathway to Solving Water Environment Challenges
Abstract
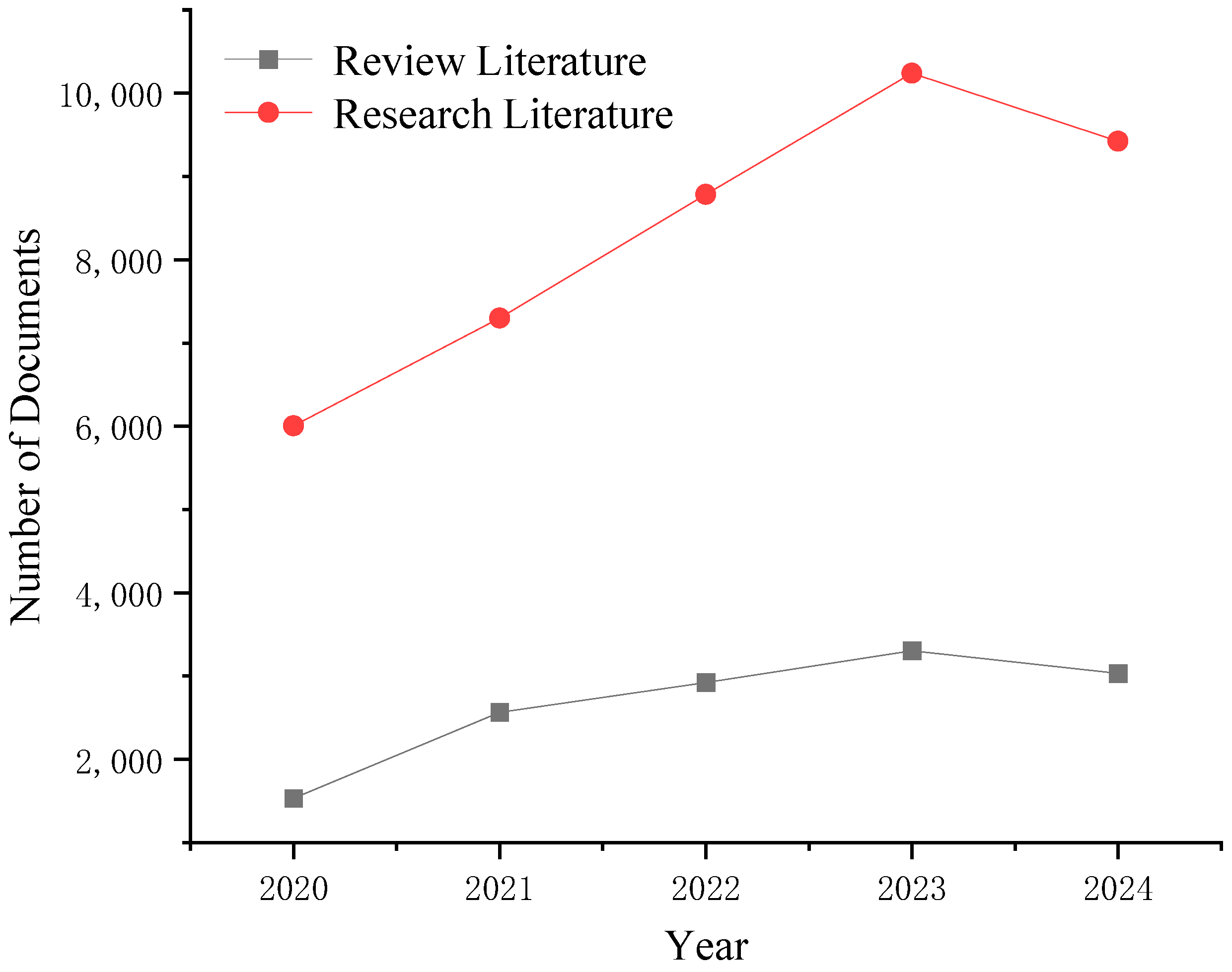
:1. Introduction
2. Water Treatment Technologies for Microplastics
2.1. Physical Methods
2.1.1. Sand Filtration
2.1.2. Adsorption
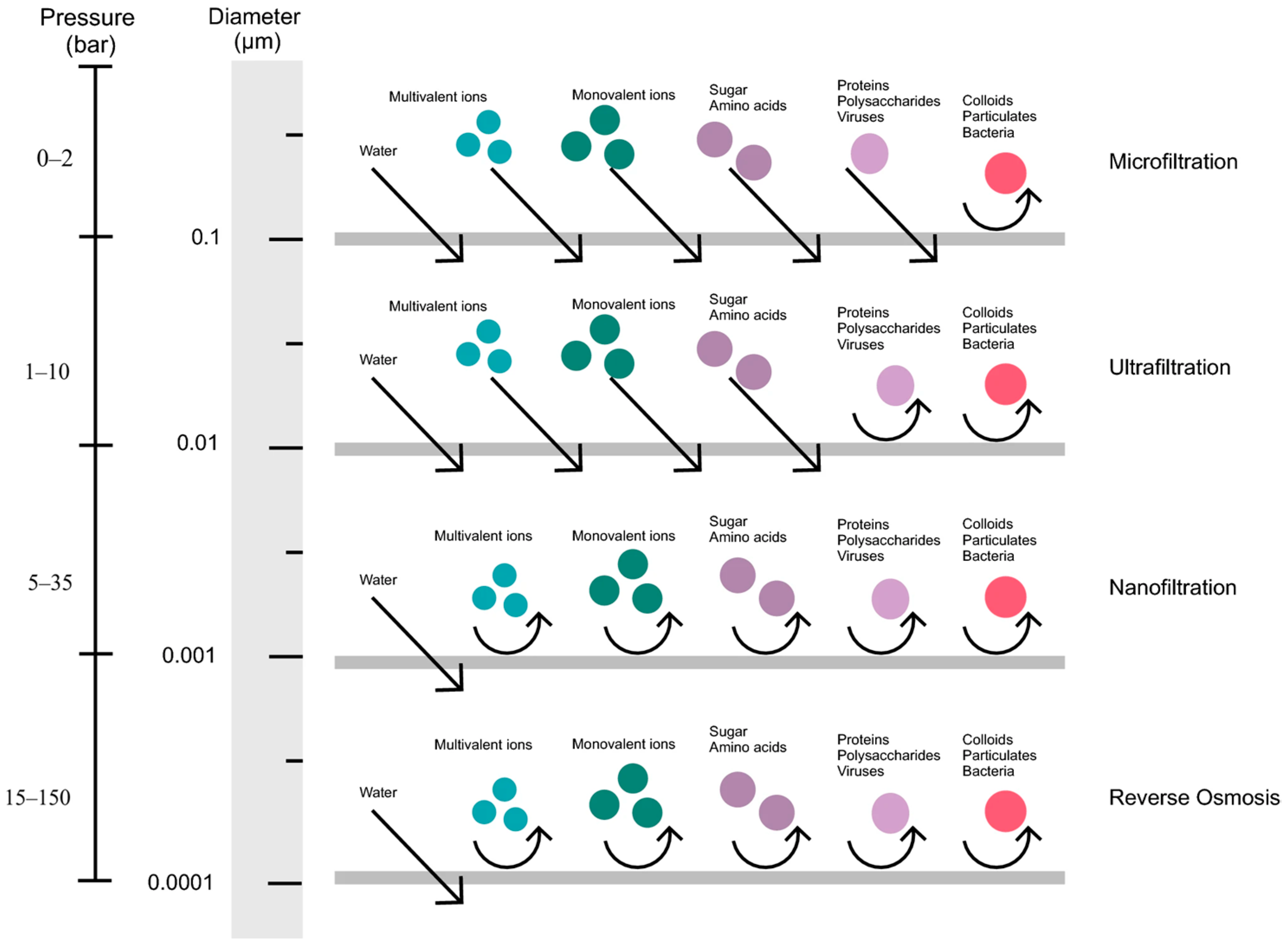
2.1.3. Membrane Separation
2.2. Chemical Methods
2.2.1. Coagulation
| Anode: | M—ne− | → | Mn+ | (1) |
| Mn+ (aq) + nOH− (aq) | → | M(OH)n (s) | (2) | |
| Cathode: | 2H2O (l) + 2e− | → | H2 (g) + 2OH− | (3) |
| 2H+ + 2e− | → | H2 | (5) |
2.2.2. Advanced Oxidation Process
2.2.3. Photocatalysis
2.3. Microbial Degradation
3. Water Treatment Technologies for Drug Residues
3.1. Physical Methods
3.2. Advanced Oxidation Technology
3.2.1. Ozone Oxidation
3.2.2. Photocatalytic Oxidation
3.2.3. Fenton Oxidation
3.3. Biological Treatment Technology
4. Water Treatment Technology for Endocrine Disruptors
4.1. Physical Methods
4.1.1. Adsorption
4.1.2. Membrane Separation Technology
4.2. Chemical Method
4.3. Microbiological Method
5. Future Research Directions and Challenges
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bănăduc, D.; Curtean-Bănăduc, A.; Barinova, S.; Lozano, V.L.; Afanasyev, S.; Leite, T.; Branco, P.; Gomez Isaza, D.F.; Geist, J.; Tegos, A.; et al. Multi-Interacting Natural and Anthropogenic Stressors on Freshwater Ecosystems: Their Current Status and Future Prospects for 21st Century. Water 2024, 16, 1483. [Google Scholar] [CrossRef]
- Singh, S.; Rawat, M.; Malyan, S.K.; Singh, R.; Tyagi, V.K.; Singh, K.; Kashyap, S.; Kumar, S.; Sharma, M.; Panday, B.K.; et al. Global distribution of pesticides in freshwater resources and their remediation approaches. Environ. Res. 2023, 225, 115605. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, E.P.; Caldas, E.D.; Oliveira-Filho, E.C. Pesticides in surface freshwater: A critical review. Environ. Monit. Assess. 2022, 194, 452. [Google Scholar] [CrossRef] [PubMed]
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Kerketta, A.; Sahoo, P.K. A decadal analysis to unravel the global status of emerging contaminants in wastewaters and comparison with the Indian context. Groundw. Sustain. Dev. 2022, 18, 100803. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mentha, S.S.; Misra, Y.; Dwivedi, N. Emerging pollutants of severe environmental concern in water and wastewater: A comprehensive review on current developments and future research. Water-Energy Nexus 2023, 6, 74–95. [Google Scholar] [CrossRef]
- Guimarães, A.T.B.; Charlie-Silva, I.; Malafaia, G. Toxic effects of naturally-aged microplastics on zebrafish juveniles: A more realistic approach to plastic pollution in freshwater ecosystems. J. Hazard. Mater. 2021, 407, 124833. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-Y.; Li, H.; Ren, H.; Zhang, X.; Huang, F.; Zhang, D.; Huang, Q.; Zhang, X. Size-dependent effects of polystyrene nanoplastics on autophagy response in human umbilical vein endothelial cells. J. Hazard. Mater. 2022, 421, 126770. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and environmental pollutants: Key interaction and toxicology in aquatic and soil environments. J. Hazard. Mater. 2022, 422, 126843. [Google Scholar] [CrossRef]
- Alam, F.C.; Sembiring, E.; Muntalif, B.S.; Suendo, V. Microplastic distribution in surface water and sediment river around slum and industrial area (case study: Ciwalengke River, Majalaya district, Indonesia). Chemosphere 2019, 224, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Yin, L.; Li, Z.; Wen, X.; Luo, X.; Hu, S.; Yang, H.; Long, Y.; Deng, B.; Huang, L. Microplastic pollution in the rivers of the Tibet Plateau. Environ. Pollut. 2019, 249, 91–98. [Google Scholar] [CrossRef]
- Lacerda, A.L.D.F.; Rodrigues, L.d.S.; Van Sebille, E.; Rodrigues, F.L.; Ribeiro, L.; Secchi, E.R.; Kessler, F.; Proietti, M.C. Plastics in sea surface waters around the Antarctic Peninsula. Sci. Rep. 2019, 9, 3977. [Google Scholar] [CrossRef] [PubMed]
- Kanhai, L.D.K.; Gardfeldt, K.; Krumpen, T.; Thompson, R.C.; O’Connor, I. Microplastics in sea ice and seawater beneath ice floes from the Arctic Ocean. Sci. Rep. 2020, 10, 5004. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; fan Mao, R.; Guo, X.; Yang, X.; Zhang, Q.; Yang, C. Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci. Total Environ. 2019, 667, 427–434. [Google Scholar] [CrossRef]
- Yan, M.; Nie, H.; Xu, K.; He, Y.; Hu, Y.; Huang, Y.; Wang, J. Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere 2019, 217, 879–886. [Google Scholar] [CrossRef]
- Sulistyowati, L.; Riani, E.; Cordova, M.R. The occurrence and abundance of microplastics in surface water of the midstream and downstream of the Cisadane River, Indonesia. Chemosphere 2022, 291, 133071. [Google Scholar] [CrossRef]
- Nan, B.; Su, L.; Kellar, C.; Craig, N.J.; Keough, M.J.; Pettigrove, V. Identification of microplastics in surface water and Australian freshwater shrimp Paratya australiensis in Victoria, Australia. Environ. Pollut. 2020, 259, 113865. [Google Scholar] [CrossRef]
- Shen, M.; Zeng, Z.; Song, B.; Yi, H.; Hu, T.; Zhang, Y.; Zeng, G.; Xiao, R. Neglected microplastics pollution in global COVID-19: Disposable surgical masks. Sci. Total Environ. 2021, 790, 148130. [Google Scholar] [CrossRef]
- Hu, T.; Shen, M.; Tang, W. Wet wipes and disposable surgical masks are becoming new sources of fiber microplastic pollution during global COVID-19. Environ. Sci. Pollut. Res. 2022, 29, 284–292. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Han, Y.; Tang, Y.; Shi, W.; Du, X.; Sun, S.; Liu, G. Microplastics aggravate the bioaccumulation of two waterborne veterinary antibiotics in an edible bivalve species: Potential mechanisms and implications for human health. Environ. Sci. Technol. 2020, 54, 8115–8122. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Pu, S.; Liu, S.; Bai, Y.; Mandal, S.; Xing, B. Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environ. Pollut. 2020, 261, 114089. [Google Scholar] [CrossRef] [PubMed]
- Turner, A. Heavy metals, metalloids and other hazardous elements in marine plastic litter. Mar. Pollut. Bull. 2016, 111, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J. Different partition of polycyclic aromatic hydrocarbon on environmental particulates in freshwater: Microplastics in comparison to natural sediment. Ecotoxicol. Environ. Saf. 2018, 147, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Arenas, L.R.; Gentile, S.R.; Zimmermann, S.; Stoll, S. Fate and removal efficiency of polystyrene nanoplastics in a pilot drinking water treatment plant. Sci. Total Environ. 2022, 813, 152623. [Google Scholar] [CrossRef] [PubMed]
- Na, S.-H.; Kim, M.-J.; Kim, J.-T.; Jeong, S.; Lee, S.; Chung, J.; Kim, E.-J. Microplastic removal in conventional drinking water treatment processes: Performance, mechanism, and potential risk. Water Res. 2021, 202, 117417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, C.; Li, F.; Chen, L. Fatigue resistance, re-usable and biodegradable sponge materials from plant protein with rapid water adsorption capacity for microplastics removal. Chem. Eng. J. 2021, 415, 129006. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, S.; Zhao, J.; Ma, J.; Wu, H.; Sun, H.; Cheng, S. Experimental study on removal of microplastics from aqueous solution by magnetic force effect on the magnetic sepiolite. Sep. Purif. Technol. 2022, 288, 120564. [Google Scholar] [CrossRef]
- Mohana, A.A.; Farhad, S.; Haque, N.; Pramanik, B.K. Understanding the fate of nano-plastics in wastewater treatment plants and their removal using membrane processes. Chemosphere 2021, 284, 131430. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Ding, Y.; Hu, C.; Liu, H.; Qu, J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci. 2019, 78, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Ao, Z.; Wang, S. Degradation of cosmetic microplastics via functionalized carbon nanosprings. Matter 2019, 1, 745–758. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard. Mater. 2020, 399, 123023. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Uheida, A.; Mejía, H.G.; Abdel-Rehim, M.; Hamd, W.; Dutta, J. Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system. J. Hazard. Mater. 2021, 406, 124299. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Sahu, S.; Abraham, J. Studies on the biodegradation of natural and synthetic polyethylene by Pseudomonas spp. J. Appl. Sci. Environ. Manag. 2010, 14. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Funck, M.; Al-Azzawi, M.S.; Yildirim, A.; Knoop, O.; Schmidt, T.C.; Drewes, J.E.; Tuerk, J. Release of microplastic particles to the aquatic environment via wastewater treatment plants: The impact of sand filters as tertiary treatment. Chem. Eng. J. 2021, 426, 130933. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, X.; Lyu, X.; Gao, B.; Sun, Y.; Wu, J. Combined Effects of Fe/Al Oxyhydroxide Coating and pH on Polystyrene Nanoplastic Transport in Saturated Sand Media. Water Air Soil Pollut. 2022, 233, 2. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Chen, H.; Zhang, M. Retention and transport behavior of microplastic particles in water-saturated porous media. Sci. Total Environ. 2022, 808, 152154. [Google Scholar] [CrossRef] [PubMed]
- Arenas, L.R.; Gentile, S.R.; Zimmermann, S.; Stoll, S. Nanoplastics adsorption and removal efficiency by granular activated carbon used in drinking water treatment process. Sci. Total Environ. 2021, 791, 148175. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Yan, Q.; He, Y.; Wang, X.; Wei, Z.; Liang, D.; Yue, H.; Yun, Y.; Li, G.; Sang, N. Insights into the removal of polystyrene nanoplastics using the contaminated corncob-derived mesoporous biochar from mining area. J. Hazard. Mater. 2022, 433, 128756. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Zheng, H.; Chen, L.; Li, F. Biodegradable and re-usable sponge materials made from chitin for efficient removal of microplastics. J. Hazard. Mater. 2021, 420, 126599. [Google Scholar] [CrossRef]
- Zheng, B.; Li, B.; Wan, H.; Lin, X.; Cai, Y. Coral-inspired environmental durability aerogels for micron-size plastic particles removal in the aquatic environment. J. Hazard. Mater. 2022, 431, 128611. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, S.; Su, Y.; Wu, D.; Zhao, Y.; Xie, B. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chem. Eng. J. 2021, 406, 126804. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Huang, Q.; Chi, Y.; Yan, J.-H. Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J. Hazard. Mater. 2021, 419, 126486. [Google Scholar] [CrossRef]
- Hamzah, S.; Ying, L.Y.; Azmi, A.A.A.R.; Razali, N.A.; Hairom, N.H.H.; Mohamad, N.A.; Harun, M.H.C. Synthesis, characterisation and evaluation on the performance of ferrofluid for microplastic removal from synthetic and actual wastewater. J. Environ. Chem. Eng. 2021, 9, 105894. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Wang, L.; Zhao, X.; Yan, F.; Yang, Y.; Li, G.; Gao, P.; Ji, P. Removal of polystyrene nanoplastics from aqueous solutions using a novel magnetic material: Adsorbability, mechanism, and reusability. Chem. Eng. J. 2022, 430, 133122. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Fehn, S.; Steegmüller, T.; Rauwolf, S.; Löwe, H.; Pflüger-Grau, K.; Berensmeier, S. Immobilization of PETase enzymes on magnetic iron oxide nanoparticles for the decomposition of microplastic PET. Nanoscale Adv. 2021, 3, 4395–4399. [Google Scholar] [CrossRef]
- Poerio, T.; Piacentini, E.; Mazzei, R. Membrane processes for microplastic removal. Molecules 2019, 24, 4148. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Pramanik, S.K.; Monira, S. Understanding the fragmentation of microplastics into nano-plastics and removal of nano/microplastics from wastewater using membrane, air flotation and nano-ferrofluid processes. Chemosphere 2021, 282, 131053. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wu, J.; Lu, J.; Wang, J.; Zhang, C. Fate of microplastics in a coastal wastewater treatment plant: Microfibers could partially break through the integrated membrane system. Front. Environ. Sci. Eng. 2022, 16, 96. [Google Scholar] [CrossRef]
- Tadsuwan, K.; Babel, S. Microplastic abundance and removal via an ultrafiltration system coupled to a conventional municipal wastewater treatment plant in Thailand. J. Environ. Chem. Eng. 2022, 10, 107142. [Google Scholar] [CrossRef]
- Zeri, C.; Adamopoulou, A.; Koi, A.; Koutsikos, N.; Lytras, E.; Dimitriou, E. Rivers and wastewater-treatment plants as microplastic pathways to eastern mediterranean waters: First records for the aegean sea, Greece. Sustainability 2021, 13, 5328. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Bayo, J.; López-Castellanos, J.; Olmos, S. Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar. Pollut. Bull. 2020, 156, 111211. [Google Scholar] [CrossRef] [PubMed]
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/microplastics in water and wastewater treatment processes–origin, impact and potential solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef] [PubMed]
- LaRue, R.J.; Patterson, B.; O’Brien, S.; Latulippe, D.R. Evaluation of membrane fouling by microplastic particles in tertiary wastewater treatment processes. ACS EST Water 2022, 2, 955–966. [Google Scholar] [CrossRef]
- Gan, X.; Lin, T.; Jiang, F.; Zhang, X. Impacts on characteristics and effluent safety of PVDF ultrafiltration membranes aged by different chemical cleaning types. J. Membr. Sci. 2021, 640, 119770. [Google Scholar] [CrossRef]
- Zhang, Y.; Diehl, A.; Lewandowski, A.; Gopalakrishnan, K.; Baker, T. Removal efficiency of micro-and nanoplastics (180 nm–125 μm) during drinking water treatment. Sci. Total Environ. 2020, 720, 137383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, Q.; Li, J.; Li, Q.; Xu, H.; Ye, Q.; Wang, Y.; Shu, S.; Zhang, J. Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: Performance and mechanism. Sci. Total Environ. 2021, 752, 141837. [Google Scholar] [CrossRef] [PubMed]
- Prokopova, M.; Novotna, K.; Pivokonska, L.; Cermakova, L.; Cajthaml, T.; Pivokonsky, M. Coagulation of polyvinyl chloride microplastics by ferric and aluminium sulphate: Optimisation of reaction conditions and removal mechanisms. J. Environ. Chem. Eng. 2021, 9, 106465. [Google Scholar] [CrossRef]
- Ingelsson, M.; Yasri, N.; Roberts, E.P. Electrode passivation, faradaic efficiency, and performance enhancement strategies in electrocoagulation—A review. Water Res. 2020, 187, 116433. [Google Scholar] [CrossRef] [PubMed]
- Perren, W.; Wojtasik, A.; Cai, Q. Removal of microbeads from wastewater using electrocoagulation. ACS Omega 2018, 3, 3357–3364. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zhang, Y.; Almatrafi, E.; Hu, T.; Zhou, C.; Song, B.; Zeng, Z.; Zeng, G. Efficient removal of microplastics from wastewater by an electrocoagulation process. Chem. Eng. J. 2022, 428, 131161. [Google Scholar] [CrossRef]
- Lu, J.; Hou, R.; Wang, Y.; Zhou, L.; Yuan, Y. Surfactant-sodium dodecyl sulfate enhanced degradation of polystyrene microplastics with an energy-saving electrochemical advanced oxidation process (EAOP) strategy. Water Res. 2022, 226, 119277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, H.; Shi, L.; Wu, Z.; Zhang, S.; Wang, S.; Sun, H. Photocatalysis coupling with membrane technology for sustainable and continuous purification of wastewater. Sep. Purif. Technol. 2023, 329, 125225. [Google Scholar] [CrossRef]
- Nabi, I.; Li, K.; Cheng, H.; Wang, T.; Liu, Y.; Ajmal, S.; Yang, Y.; Feng, Y.; Zhang, L. Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film. iScience 2020, 23, 101326. [Google Scholar] [CrossRef]
- Skariyachan, S.; Setlur, A.S.; Naik, S.Y.; Naik, A.A.; Usharani, M.; Vasist, K.S. Enhanced biodegradation of low and high-density polyethylene by novel bacterial consortia formulated from plastic-contaminated cow dung under thermophilic conditions. Environ. Sci. Pollut. Res. 2017, 24, 8443–8457. [Google Scholar] [CrossRef]
- Dang, T.C.H.; Nguyen, D.T.; Thai, H.; Nguyen, T.C.; Hien Tran, T.T.; Le, V.H.; Nguyen, V.H.; Tran, X.B.; Thao Pham, T.P.; Nguyen, T.G. Plastic degradation by thermophilic Bacillus sp. BCBT21 isolated from composting agricultural residual in Vietnam. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015014. [Google Scholar] [CrossRef]
- Yan, F.; Wei, R.; Cui, Q.; Bornscheuer, U.T.; Liu, Y.J. Thermophilic whole-cell degradation of polyethylene terephthalate using engineered Clostridium thermocellum. Microb. Biotechnol. 2021, 14, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Purohit, J.; Chattopadhyay, A.; Teli, B. Metagenomic exploration of plastic degrading microbes for biotechnological application. Curr. Genom. 2020, 21, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc database of metabolic pathways and enzymes—A 2019 update. Nucleic Acids Res. 2019, 48, D445–D453. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Zhu, D.; Sun, J. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; El-Arabi, N.I.; El-Hussein, A.; El-Maaty, S.A.; Abdelhadi, A.A. Reduction of chromium-VI by chromium-resistant Escherichia coli FACU: A prospective bacterium for bioremediation. Folia Microbiol. 2020, 65, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, S.; Bontempi, E.; Ducoli, S.; Vethaak, A.D.; Dey, A.; Federici, S. Biotechnological methods to remove microplastics: A review. Environ. Chem. Lett. 2023, 21, 1787–1810. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999−2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Johnson, A.C.; Keller, V.; Dumont, E.; Sumpter, J.P. Assessing the concentrations and risks of toxicity from the antibiotics ciprofloxacin, sulfamethoxazole, trimethoprim and erythromycin in European rivers. Sci. Total Environ. 2015, 511, 747–755. [Google Scholar] [CrossRef]
- Valcárcel, Y.; Alonso, S.G.; Rodríguez-Gil, J.; Gil, A.; Catalá, M. Detection of pharmaceutically active compounds in the rivers and tap water of the Madrid Region (Spain) and potential ecotoxicological risk. Chemosphere 2011, 84, 1336–1348. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.; Murby, E.; Kolpin, D.W.; Costanzo, S. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.J. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Managaki, S.; Murata, A.; Takada, H.; Tuyen, B.C.; Chiem, N.H. Distribution of macrolides, sulfonamides, and trimethoprim in tropical waters: Ubiquitous occurrence of veterinary antibiotics in the Mekong Delta. Environ. Sci. Technol. 2007, 41, 8004–8010. [Google Scholar] [CrossRef] [PubMed]
- WHO. Pharmaceuticals in Drinking-Water. 2012. Available online: https://iris.who.int/bitstream/handle/10665/44630/?sequence=1 (accessed on 10 June 2024).
- Hampshire, N. Pharmaceuticals and Personal Care Products in Drinking Water and Aquatic Environments—Answers to Frequently Asked Questions. Available online: https://www.des.nh.gov/organization/commissioner/pip/factsheets/dwgb/documents/dwgb-22-28.pdf (accessed on 10 June 2024).
- Marx, C.; Mühlbauer, V.; Krebs, P.; Kuehn, V. Environmental risk assessment of antibiotics including synergistic and antagonistic combination effects. Sci. Total Environ. 2015, 524, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Rooklidge, S.J.; Miner, J.R.; Kassim, T.A.; Nelson, P.O. Antimicrobial contaminant removal by multistage slow sand filtration. J.-Am. Water Work. Assoc. 2005, 97, 92–100. [Google Scholar] [CrossRef]
- Košutić, K.; Dolar, D.; Ašperger, D.; Kunst, B. Removal of antibiotics from a model wastewater by RO/NF membranes. Sep. Purif. Technol. 2007, 53, 244–249. [Google Scholar] [CrossRef]
- Tian, S.-Q.; Wang, L.; Liu, Y.-L.; Yang, T.; Huang, Z.-S.; Wang, X.-S.; He, H.-Y.; Jiang, J.; Ma, J. Enhanced permanganate oxidation of sulfamethoxazole and removal of dissolved organics with biochar: Formation of highly oxidative manganese intermediate species and in situ activation of biochar. Environ. Sci. Technol. 2019, 53, 5282–5291. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.M.; GÖbel, A.; Joss, A.; Hermann, N.; LÖffler, D.; McArdell, C.S.; Ried, A.; Siegrist, H.; Ternes, T.A.; von Gunten, U. Oxidation of pharmaceuticals during ozonation of municipal wastewater effluents: A pilot study. Environ. Sci. Technol. 2005, 39, 4290–4299. [Google Scholar] [CrossRef]
- Mohammadi, S.; Sohrabi, M.; Golikand, A.N.; Fakhri, A. Preparation and characterization of zinc and copper co-doped WO3 nanoparticles: Application in photocatalysis and photobiology. J. Photochem. Photobiol. B Biol. 2016, 161, 217–221. [Google Scholar] [CrossRef]
- Çağlar Yılmaz, H.; Akgeyik, E.; Bougarrani, S.; El Azzouzi, M.; Erdemoğlu, S. Photocatalytic degradation of amoxicillin using Co-doped TiO2 synthesized by reflux method and monitoring of degradation products by LC–MS/MS. J. Dispers. Sci. Technol. 2020, 41, 414–425. [Google Scholar] [CrossRef]
- Cui, K.-P.; Yang, T.-T.; Chen, Y.-H.; Weerasooriya, R.; Li, G.-H.; Zhou, K.; Chen, X. Magnetic recyclable heterogeneous catalyst Fe3O4/g-C3N4 for tetracycline hydrochloride degradation via photo-Fenton process under visible light. Environ. Technol. 2022, 43, 3341–3354. [Google Scholar] [CrossRef] [PubMed]
- Batt, A.L.; Kim, S.; Aga, D.S. Comparison of the occurrence of antibiotics in four full-scale wastewater treatment plants with varying designs and operations. Chemosphere 2007, 68, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, Y.; Mei, J.; Ding, Y.; Wang, X. Preparation of poly(divinylbenzene-co-methyl acrylate) adsorbent with tunable surface hydrophilicity for atrazine removal. Surf. Interfaces 2024, 49, 104371. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, J.; Duan, T.; Wu, Q.; Yang, X.; Wang, C.; Wang, Z. In site preparation of core-shell magnetic triazine functionalized hyper-crosslinking polymers as adsorbent for efficient enrichment of benzoyl urea insecticides. Food Control 2023, 151, 109819. [Google Scholar] [CrossRef]
- Adams, C.; Wang, Y.; Loftin, K.; Meyer, M. Removal of antibiotics from surface and distilled water in conventional water treatment processes. J. Environ. Eng. 2002, 128, 253–260. [Google Scholar] [CrossRef]
- Zhu, X.; Li, C.; Li, J.; Xie, B.; Lü, J.; Li, Y. Thermal treatment of biochar in the air/nitrogen atmosphere for developed mesoporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2018, 263, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, H.; Min, L.; Ren, C. Biochars change the sorption and degradation of thiacloprid in soil: Insights into chemical and biological mechanisms. Environ. Pollut. 2018, 236, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Fan, H.; Yi, G.; Zhang, Z.; Zhang, X.; Li, P.; Zhang, C.; Chen, L.; Zhang, Y.; Sun, Q. Fabrication of Ag particles deposited BiVO4 photoanode for significantly efficient visible-light driven photoelectrocatalytic degradation of β-naphthol. J. Environ. Chem. Eng. 2022, 10, 107221. [Google Scholar] [CrossRef]
- Martimiano do Prado, T.; Lindo Silva, F.; Grosseli, G.; Sergio Fadini, P.; Fatibello-Filho, O.; Cruz de Moraes, F. Using BiVO4/CuO-based photoelectrocatalyzer for 4-nitrophenol degradation. Materials 2020, 13, 1322. [Google Scholar] [CrossRef] [PubMed]
- Ao, M.M.; Liu, L.; Wei, J.; Song, Y.; Chen, T.; Xiong, Z.; Lai, B.; Chen, Z. Ozone oxidation mechanism and degradation pathway of β-lactam antibiotics. J. Civ. Environ. Eng. 2021, 43, 187–196. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.H.; Lawrence, J.; Tosine, H.M. Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Linsebigler, A.L.; Lu, G.; Yates Jr, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Li, H.; Ji, H.; Liu, J.; Liu, W.; Li, F.; Shen, Z. Interfacial modulation of ZnIn2S4 with high active Zr-S4 sites for boosting photocatalytic activation of oxygen and degradation of emerging contaminant. Appl. Catal. B Environ. 2023, 328, 122481. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.; Fourcade, F.; Bellakhal, N.; Dachraoui, M.; Hauchard, D.; Amrane, A. Biodegradability improvement of sulfamethazine solutions by means of an electro-Fenton process. Water Air Soil Pollut. 2012, 223, 2023–2034. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, D.; Chen, Y.; Hu, Y. Enhanced degradation of triclosan in heterogeneous E-Fenton process with MOF-derived hierarchical Mn/Fe@PC modified cathode. Chem. Eng. J. 2020, 384, 123324. [Google Scholar] [CrossRef]
- Zhang, Y.; He, T.; Ding, S.; Li, H.; Song, W.; Ding, J.; Lu, J. Photo-fenton degradation of RhB via transition metal oxides composite catalyst Fe3O4/CuO under visible light optimized using response surface methodology. Mater. Technol. 2022, 37, 2347–2359. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, P.; An, W.; Liu, L.; Liang, Y.; Cui, W. In-situ Fe-doped g-C3N4 heterogeneous catalyst via photocatalysis-Fenton reaction with enriched photocatalytic performance for removal of complex wastewater. Appl. Catal. B Environ. 2019, 245, 130–142. [Google Scholar] [CrossRef]
- Yongjun, W.; Ping, C.; Chonghui, Z.; Yonghui, Y.; Yong-tao, L.; Wei, C. Application of Circulation-type Aerobic Biochemical Tank for Treatment of Antibiotic Wastewater. China Water Wastewater 2012, 28, 132–134. [Google Scholar]
- Lang, W. Study on Interaction Mechanism between Cefotaxime Sodium and Microorganisms in Normal Temperature Anaerobic Antibiotic Wastewater Treatment Process; Guangzhou University: Guangzhou, China, 2022. [Google Scholar]
- Jihui, S. Pilot study on treatment of antibiotic wastewater by CASB anaerobic bioreactor. Water Wastewater Eng. 2016, 42, 53–56. [Google Scholar]
- Han, Y.; Yang, L.; Chen, X.; Cai, Y.; Zhang, X.; Qian, M.; Chen, X.; Zhao, H.; Sheng, M.; Cao, G. Removal of veterinary antibiotics from swine wastewater using anaerobic and aerobic biodegradation. Sci. Total Environ. 2020, 709, 136094. [Google Scholar] [CrossRef]
- Lisco, G.; Giagulli, V.A.; Iovino, M.; Guastamacchia, E.; Pergola, G.D.; Triggiani, V. Endocrine-disrupting chemicals: Introduction to the theme. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2022, 22, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nag, R.; Brunton, N.P.; Siddique, M.A.B.; Harrison, S.M.; Monahan, F.J.; Cummins, E. Human health risk assessment of bisphenol A (BPA) through meat products. Environ. Res. 2022, 213, 113734. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Qin, L.; Xie, X.; Gao, B.; Sun, J.; Li, A. Distribution of endocrine-disrupting chemicals in colloidal and soluble phases in municipal secondary effluents and their removal by different advanced treatment processes. Chemosphere 2019, 219, 730–739. [Google Scholar] [CrossRef]
- Murray, A.; Örmeci, B.; Lai, E.P. Use of sub-micron sized resin particles for removal of endocrine disrupting compounds and pharmaceuticals from water and wastewater. J. Environ. Sci. 2017, 51, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Egea-Corbacho Lopera, A.; Gutiérrez Ruiz, S.; Quiroga Alonso, J.M. Removal of emerging contaminants from wastewater using reverse osmosis for its subsequent reuse: Pilot plant. J. Water Process Eng. 2019, 29, 100800. [Google Scholar] [CrossRef]
- Juhola, R.; Heponiemi, A.; Tuomikoski, S.; Hu, T.; Vielma, T.; Lassi, U. Preparation of novel Fe catalysts from industrial by-products: Catalytic wet peroxide oxidation of bisphenol A. Top. Catal. 2017, 60, 1387–1400. [Google Scholar] [CrossRef]
- Kasonga, T.K.; Coetzee, M.A.; Van Zijl, C.; Momba, M.N.B. Removal of pharmaceutical’estrogenic activity of sequencing batch reactor effluents assessed in the T47D-KBluc reporter gene assay. J. Environ. Manag. 2019, 240, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Vieira, W.T.; de Farias, M.B.; Spaolonzi, M.P.; da Silva, M.G.C.; Vieira, M.G.A. Removal of endocrine disruptors in waters by adsorption, membrane filtration and biodegradation. A review. Environ. Chem. Lett. 2020, 18, 1113–1143. [Google Scholar] [CrossRef]
- Guo, J.-X.; Pan, J.; Wang, J.; Wang, F.; Shi, H.-X. Study of the adsorption of endocrine disruptor compounds on typical filter materials using a quartz crystal microbalance. Environ. Sci. Pollut. Res. 2019, 26, 20499–20509. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ma, R.; Jiao, C.; Hao, L.; Wang, C.; Wu, Q.; Wang, Z. Magnetic mesoporous polymelamine-formaldehyde resin as an adsorbent for endocrine disrupting chemicals. Microchim. Acta 2018, 185, 19. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, C.; Wu, J.; Luo, Y. Adsorptive Removal of Bisphenol A Using N-Doped Biochar Made of Ulva prolifera. Water Air Soil Pollut. 2017, 228, 327. [Google Scholar] [CrossRef]
- Zielińska, M.; Cydzik-Kwiatkowska, A.; Bułkowska, K.; Bernat, K.; Wojnowska-Baryła, I. Treatment of Bisphenol A-Containing Effluents from Aerobic Granular Sludge Reactors with the Use of Microfiltration and Ultrafiltration Ceramic Membranes. Water Air Soil Pollut. 2017, 228, 282. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Hu, Z.; Huang, S. Combined Process of Ozone Oxidation and Ultrafiltration as an Effective Treatment Technology for the Removal of Endocrine-Disrupting Chemicals. Appl. Sci. 2018, 8, 1240. [Google Scholar] [CrossRef]
- Guo, H.; Peng, L.E.; Yao, Z.; Yang, Z.; Ma, X.; Tang, C.Y. Non-Polyamide Based Nanofiltration Membranes Using Green Metal–Organic Coordination Complexes: Implications for the Removal of Trace Organic Contaminants. Environ. Sci. Technol. 2019, 53, 2688–2694. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Zheng, K.; Sun, Y.; Gong, S.; Jiang, G.; Zheng, X.; Yu, Z. Degradation of sulfamethoxazole in aqueous solution by dielectric barrier discharge plasma combined with Bi2WO6-rMoS2 nanocomposite: Mechanism and degradation pathway. Chemosphere 2019, 222, 872–883. [Google Scholar] [CrossRef]
- Moussavi, G.; Pourakbar, M.; Shekoohiyan, S.; Satari, M. The photochemical decomposition and detoxification of bisphenol A in the VUV/H2O2 process: Degradation, mineralization, and cytotoxicity assessment. Chem. Eng. J. 2018, 331, 755–764. [Google Scholar] [CrossRef]
- Goulart de Araujo, L.; Santos, F.d.S.; Teixeira, A.C.S.C. Degradation of bisphenol A by the UV and UV/H2O2 processes: Evaluation of process variables through experimental design. Can. J. Chem. Eng. 2017, 95, 2278–2285. [Google Scholar] [CrossRef]
- Chen, W.; Zou, C.; Liu, Y.; Li, X. The experimental investigation of bisphenol A degradation by Fenton process with different types of cyclodextrins. J. Ind. Eng. Chem. 2017, 56, 428–434. [Google Scholar] [CrossRef]
- Becker, D.; Rodriguez-Mozaz, S.; Insa, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.-P.; Sanchez-Marcano, J.; Misovic, A.; Oehlmann, J.r. Removal of endocrine disrupting chemicals in wastewater by enzymatic treatment with fungal laccases. Org. Process Res. Dev. 2017, 21, 480–491. [Google Scholar] [CrossRef]
- Křesinová, Z.; Linhartová, L.; Filipová, A.; Ezechiáš, M.; Mašín, P.; Cajthaml, T. Biodegradation of endocrine disruptors in urban wastewater using Pleurotus ostreatus bioreactor. New Biotechnol. 2018, 43, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ruan, Z.; Liu, J.; Liu, C.; Zhang, F.; Linhardt, R.J.; Li, L. Complete degradation of bisphenol A and nonylphenol by a composite of biogenic manganese oxides and Escherichia coli cells with surface-displayed multicopper oxidase CotA. Chem. Eng. J. 2019, 362, 897–908. [Google Scholar] [CrossRef]
- Bai, X.; Acharya, K. Removal of seven endocrine disrupting chemicals (EDCs) from municipal wastewater effluents by a freshwater green alga. Environ. Pollut. 2019, 247, 534–540. [Google Scholar] [CrossRef]


| Microplastic Removal Technology | Source of Pollutant | Removed Objects | Removal Efficiency | Advantages | Disadvantages | Reference | |
|---|---|---|---|---|---|---|---|
| Physical Methods | sand filtration | Lake Geneva | nm PS | 88.1% | Low cost, easy to operate, reduced burden of follow-up processes | Limited removal efficiency and frequent material replacement | [26,27] |
| Han River | 10 μm PS | 83.4% | |||||
| adsorption | Laboratory Homemade Pollutant | 1 μm PS | 81.2% | Highly reusable, low cost, easy to operate, no toxic chemicals, can handle microplastics with particle sizes from 1 to 5 µm. | Low selectivity and complex adsorbent preparation | [28,29] | |
| Homemade PE solution in the laboratory | 48 μm PE | 97.3% | |||||
| membrane separation | Laboratory Homemade Pollutants | <0.5 mm PE | 13–91% | High removal efficiency, stable separation effect, easy operation, small footprint | Membrane contamination clogging, high cost | [30] | |
| Chemical methods | coagulation | Laboratory Homemade Pollutants | <5 mm PE | <15% | Fast, low energy consumption, easy to operate, suitable for microplastics above 10 µm in particle size | Low removal efficiency of microplastics with low density and small particle size, high coagulant demand, high mud yield | [31,32] |
| Yangtze River | 5–10 μm PET | 44.9–75% | |||||
| advanced oxidation | Facial cleanser solution | PE | 54 wt% | High degradation capacity, good treatment effect, improved biodegradability | High cost, degradation system under confined conditions, temporarily in the laboratory stage | [33,34] | |
| Homemade PVC solution in the laboratory | PVC | 56 wt% | |||||
| photocatalysis | Exfoliating scrub | PE | 2.86 mL% | Fast reaction speed, high processing efficiency, suitable for processing nanometer-sized microplastics | High cost, long time to achieve high removal efficiency, still being explored at the industrial level | [35,36] | |
| Homemade PP solution in the laboratory | PP | Lessen 65% | |||||
| Microbiological method | microbial degradation | Combination of virgin plastic vegetable starch (6%), organic minerals, and vegetable extracts | Natural PE | 46.2% | High processing efficiency, low operating costs, no secondary pollution | Complicated and time-consuming pretreatment of microplastics with light, heat, and chemical oxidizers | [37] |
| Drug Residues Removal Technology | Source of Pollutants | Removed Objects | Removal Efficiency | Advantages | Disadvantages | Reference | |
|---|---|---|---|---|---|---|---|
| Physical methods | Sand filtration | The North Santiam River | Trimethoprim removal | >99% | Low cost, easy to operate, renewable | Limited removal efficiency, large footprint, possible sludge generation | [88] |
| Lincomycin | <25% | ||||||
| Sulfonamide antimicrobials | <4% | ||||||
| Membrane filtration | Wastewater from veterinary drug manufacturing plants | Levamisole, Sulfaguanidine, Sulfadiazine, etc. | >98.5% | Highly efficient removal, small footprint, flexible operation | High cost, membrane contamination problems, high energy consumption | [89] | |
| Adsorption | Homemade SMX solution in the laboratory | SMX | 97% | High removal efficient, simple operation, selective adsorption | Adsorbent regeneration, cost issues | [90] | |
| Advanced oxidation technology | Ozone oxidation | Urban wastewater | Macrolides, sulfonamide antibiotics, estrogens, etc. | 90~99% | Fast reaction rate, high oxidizing ability, easy to control the reaction process | Low solubility, low mass transfer efficiency, and O3 utilization | [91] |
| Photocatalytic oxidation | Homemade Gentamycin solution in the laboratory | Gentamycin | 95% | Wide range of applications, mild conditions, green, efficient, economical, and environmentally friendly | Most catalysts require UV light for effective activation | [92,93] | |
| Homemade AMX solution in the laboratory | AMX | 100% | |||||
| Fenton oxidation | Homemade TC solution in the laboratory | TC | 99.8% | High degradation efficiency and easy operation | Higher costs, corrosion-prone installations, and large quantities of iron-containing sludge | [94] | |
| Biological treatment technology | Wastewater treatment plants | Removal of CIP by activated sludge | 50%~60% | Cost-effective, sustainable, no secondary pollution | Long processing time, highly affected by temperature | [95] | |
| Drug Residues Removal Technology | Source of Pollutants | Removed Objects | Removal Efficiency | Advantages | Disadvantages | Reference | |
|---|---|---|---|---|---|---|---|
| Physical methods | Adsorption | Laboratory Homemade Pollutants | E2, EE2, BPA and DES | 98%, 80%, 87%, and 97% | Efficient removal, easy operation, wide range of applications | Cost issues, secondary contamination, adsorbent lifetime | [121] |
| Membrane Separation Technology | WWTP in Medina Sidonia | Caffeine, theobromine, theophylline, amoxicillin, and penicillin G | 100% | High separation efficiency, automated operation, modularizable design | Membrane contamination, high energy consumption | [122] | |
| Chemical Method | Homemade BPA solution in the laboratory | BPA | 83% | Fast degradation and high flexibility | Chemical reagent costs, possible by-products, operational safety issues | [123] | |
| Microbiological Method | Laboratory Homemade Pollutants | CBZ, DCF, and IBP | 89.8%, 95.8%, and 91.4% | Environmentally friendly, cost-effective, sustainable | Impact of long processing time, environmental factors | [124] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Gao, X.; Zuo, Y. Research and Application of Water Treatment Technologies for Emerging Contaminants (ECs): A Pathway to Solving Water Environment Challenges. Water 2024, 16, 1837. https://doi.org/10.3390/w16131837
Wang H, Gao X, Zuo Y. Research and Application of Water Treatment Technologies for Emerging Contaminants (ECs): A Pathway to Solving Water Environment Challenges. Water. 2024; 16(13):1837. https://doi.org/10.3390/w16131837
Chicago/Turabian StyleWang, Hongqiang, Xing Gao, and Yanqiu Zuo. 2024. "Research and Application of Water Treatment Technologies for Emerging Contaminants (ECs): A Pathway to Solving Water Environment Challenges" Water 16, no. 13: 1837. https://doi.org/10.3390/w16131837
APA StyleWang, H., Gao, X., & Zuo, Y. (2024). Research and Application of Water Treatment Technologies for Emerging Contaminants (ECs): A Pathway to Solving Water Environment Challenges. Water, 16(13), 1837. https://doi.org/10.3390/w16131837






