Influencing Factors for the Growth of Cladophora and Its Cell Damage and Destruction Mechanism: Implication for Prevention and Treatment
Abstract
1. Introduction
2. Characterization of the Cell Structure and the Process of Matter-Energy Cycling in Cladophora
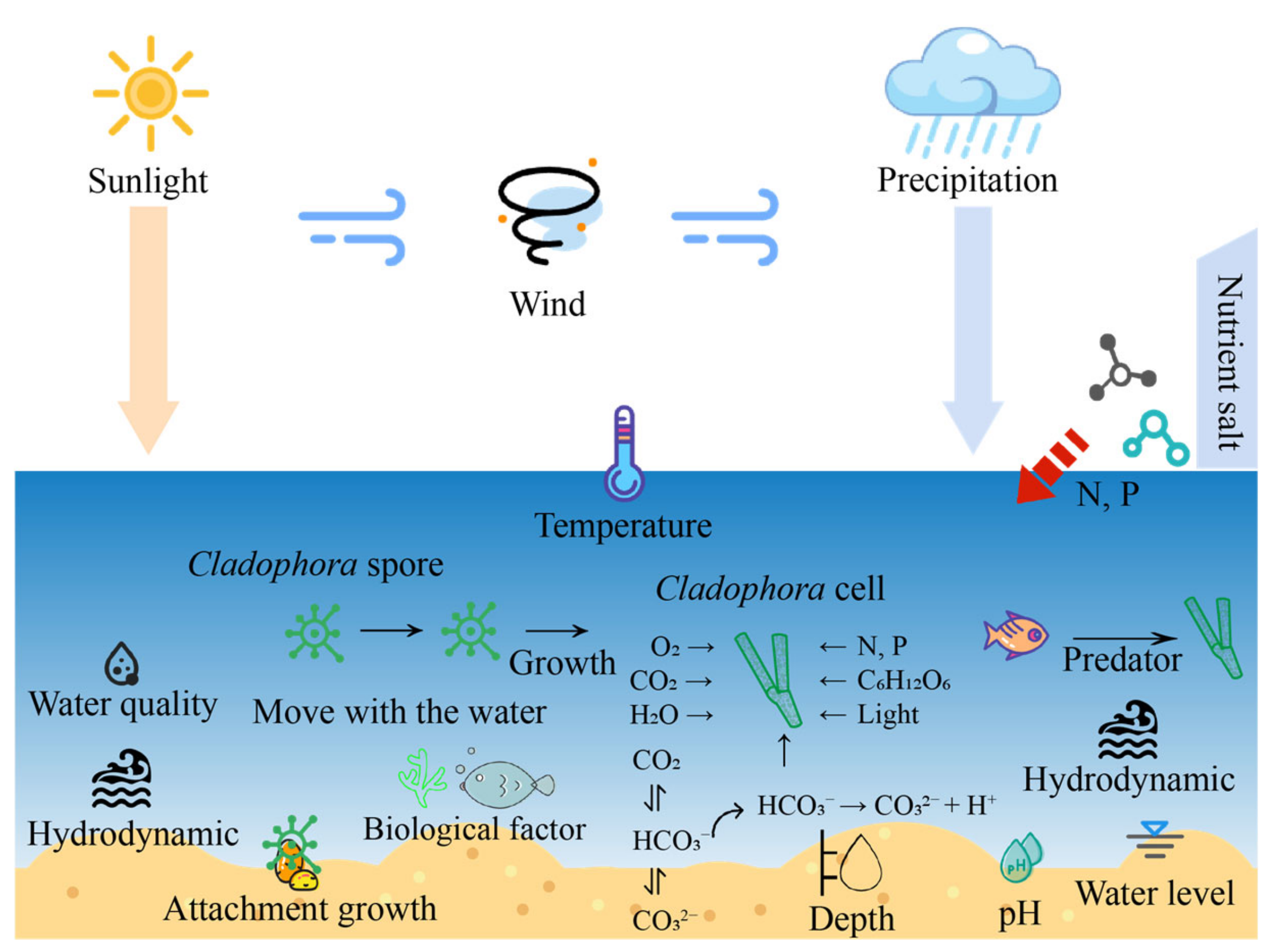
3. Factors Influencing Cell Growth and Dissemination and Critical Processes in Cladophora
4. Mechanisms of Cell Damage Destruction in Cladophora
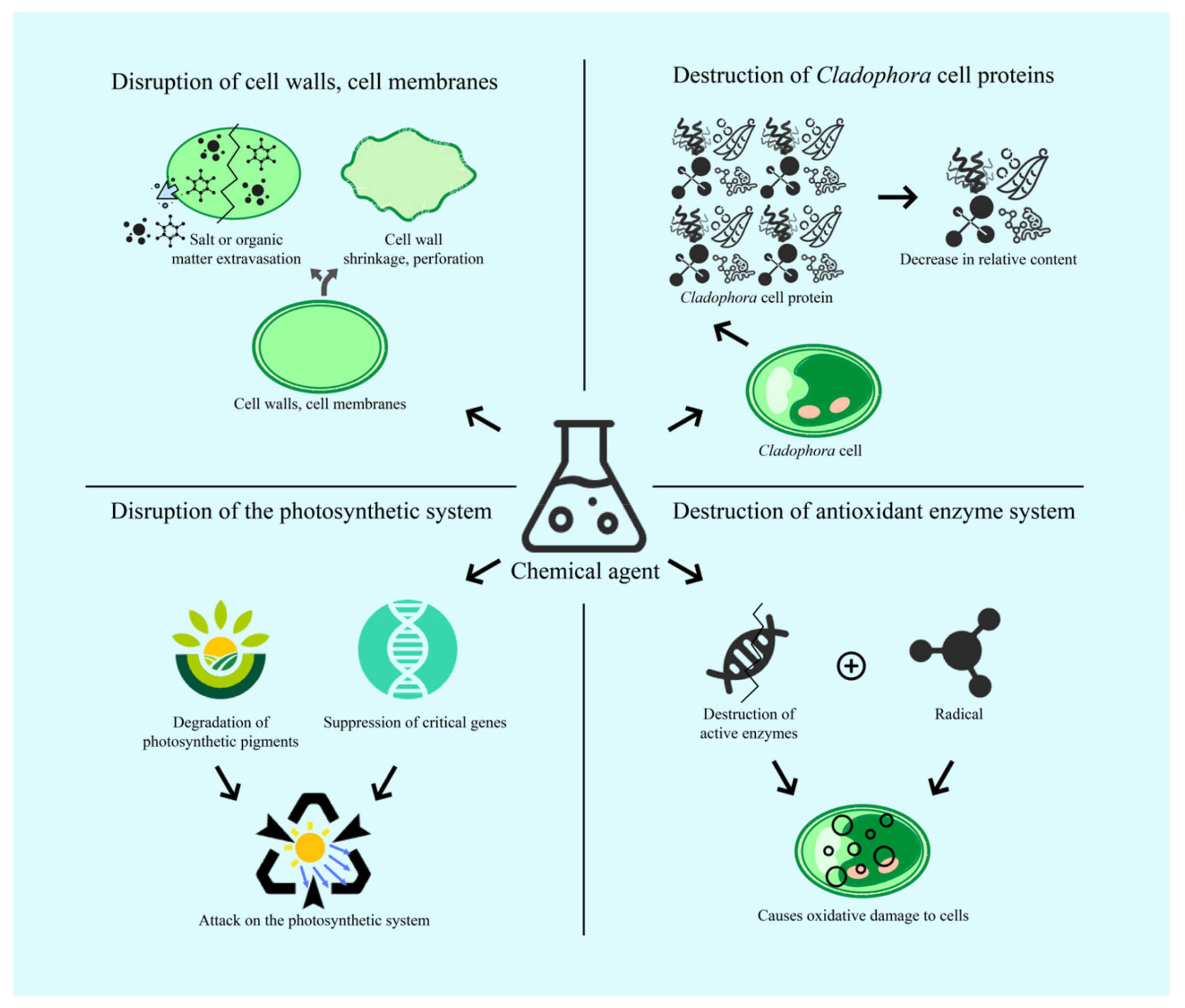
4.1. Mechanism of Cell Damage and Destruction of Cladophora under Physical Action
4.1.1. Disruption of Cell Walls and Cell Membranes
4.1.2. Disruption of the Photosynthetic System
4.1.3. Destruction of Antioxidant Enzyme System
4.2. Mechanisms of Cell Damage and Destruction of Cladophora under Chemical Action
4.2.1. Disruption of Cell Walls and Cell Membranes
4.2.2. Destruction of Algal Cell Proteins
4.2.3. Disruption of the Photosynthetic System
4.2.4. Destruction of Antioxidant Enzyme System
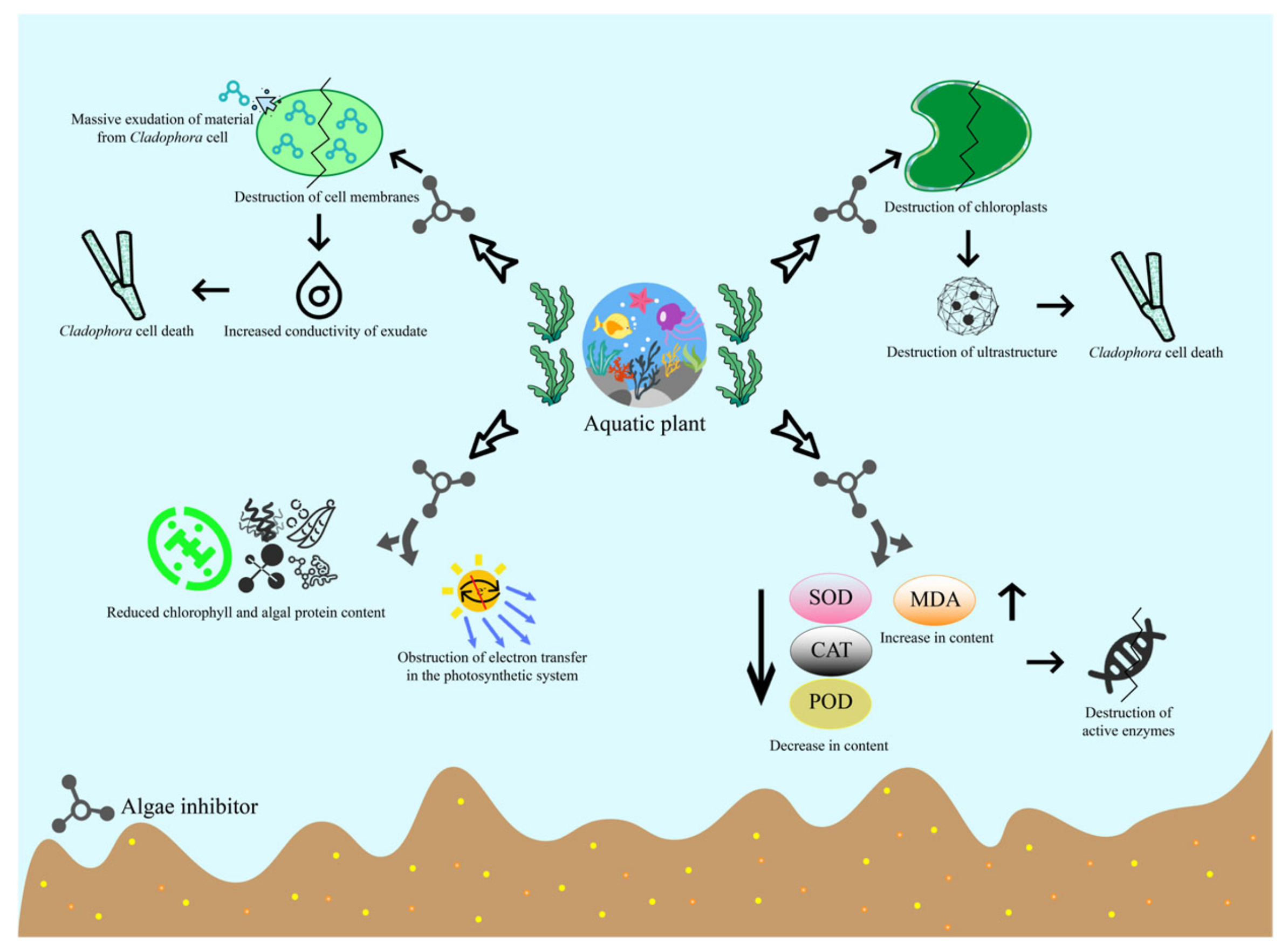
4.3. Mechanisms of Cell Damage and Destruction of Cladophora under Biological Action
4.3.1. Disruption of Cell Walls and Cell Membranes
4.3.2. Destruction of Ultrastructure
4.3.3. Disruption of the Photosynthetic System
4.3.4. Destruction of Antioxidant Enzyme System
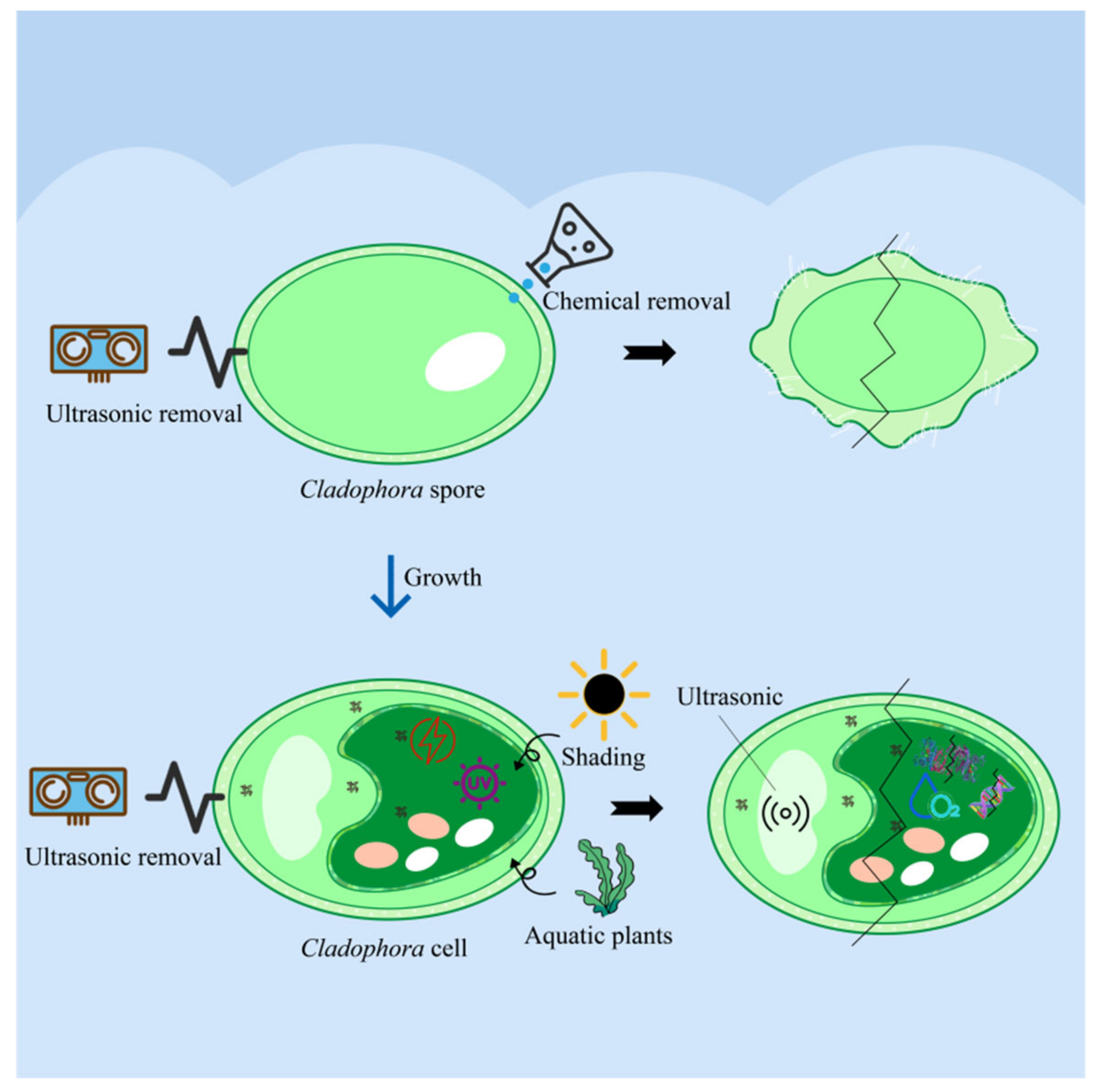
5. Integrated Control and Potential Utilization Pathways for Cladophora Cells
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, L.; Fang, L.; Zhang, Z.; Chang, X.; Penny, D.; Zhong, B. Chloroplast Phylogenomic Inference of Green Algae Relationships. Sci. Rep. 2016, 6, 20528. [Google Scholar] [CrossRef] [PubMed]
- Burlacot, A.; Dao, O.; Auroy, P.; Cuiné, S.; Li-Beisson, Y.; Peltier, G. Alternative photosynthesis pathways drive the algal CO2-concentrating mechanism. Nature 2022, 605, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tran, P.Q.; Kieft, K.; Anantharaman, K. Genome diversification in globally distributed novel marine Proteobacteria is linked to environmental adaptation. ISME J. 2020, 14, 2060–2077. [Google Scholar] [CrossRef] [PubMed]
- Lawton, R.J.; Cole, A.J.; Roberts, D.A.; Paul, N.A.; de Nys, R. The industrial ecology of freshwater macroalgae for biomass applications. Algal Res. 2017, 24, 486–491. [Google Scholar] [CrossRef]
- Cui, J.; Xu, H.; Cui, Y.; Song, C.; Qu, Y.; Zhang, S.; Zhang, H. Improved eutrophication model with flow velocity-influence function and application for algal bloom control in a reservoir in East China. J. Environ. Manag. 2023, 348, 119209. [Google Scholar] [CrossRef] [PubMed]
- Peller, J.; Nevers, M.B.; Byappanahalli, M.; Nelson, C.; Ganesh Babu, B.; Evans, M.A.; Kostelnik, E.; Keller, M.; Johnston, J.; Shidler, S. Sequestration of microfibers and other microplastics by green algae, Cladophora, in the US Great Lakes. Environ. Pollut. 2021, 276, 116695. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yuan, X.; Xiong, X.; Ao, H.; Wu, C.; Liu, G.; Zhu, H. Cladophora as ecological engineer: A new test from the largest lake of Qinghai-Tibet plateau with filamentous algal blooms. Water Biol. Secur. 2024, 3, 100210. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Geng, G.; Wang, J.-J.; Xin, Y.; Zhang, Q.; Cao, D.-J.; Ma, Y.-H. The remediation potential and kinetics of cadmium in the green alga Cladophora rupestris. Environ. Sci. Pollut. Res. 2019, 26, 775–783. [Google Scholar] [CrossRef]
- Cao, D.-J.; Shi, X.-D.; Li, H.; Xie, P.-P.; Zhang, H.-M.; Deng, J.-W.; Liang, Y.-G. Effects of lead on tolerance, bioaccumulation, and antioxidative defense system of green algae, Cladophora. Ecotoxicol. Environ. Saf. 2015, 112, 231–237. [Google Scholar] [CrossRef]
- Li, B.; Yin, Y.; Kang, L.; Feng, L.; Liu, Y.; Du, Z.; Tian, Y.; Zhang, L. A review: Application of allelochemicals in water ecological restoration—Algal inhibition. Chemosphere 2021, 267, 128869. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xiong, X.; Ao, H.; Wu, C.; He, Y.; Hu, Z.; Liu, G. Cladophora reblooming after half a century: Effect of climate change-induced increases in the water level of the largest lake in Tibetan Plateau. Environ. Sci. Pollut. Res. 2020, 27, 42175–42181. [Google Scholar] [CrossRef]
- Guo, L.L.; Li, L.-J.; Li, D.-H.; Li, G.-B. The characteristics of Cladophora community and its influencing factors in a eutrophic lake. China Environ. Sci. 2017, 37, 4667–4674. [Google Scholar]
- Hao, M.-Y.; Zhu, H.; Xiong, X.; He, Y.-B.; Ao, H.-Y.; Yu, G.-L.; Wu, C.-X.; Liu, G.-X.; Luo, Z.; Liu, J.-T.; et al. Analysis on the distribution and origin of Cladophora in the nearshore water of Qinghai lake. Acta Hydrobiol. Sin. 2020, 44, 1152–1158. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, S.; Peng, X.; Liu, B.; Zhang, X.; Ge, F.; Zhou, Q.; Wu, Z. Potential ecological implication of Cladophora oligoclora decomposition: Characteristics of nutrient migration, transformation, and response of bacterial community structure. Water Res. 2021, 190, 116741. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, J.; Lei, X.; Zhang, D.; Peng, Q.; Wang, J.; Zhu, B. Assessment of the spatiotemporal water quality variations in the Middle Route of China’s South-to-North Water Diversion Project by multivariate analysis. Environ. Sci. Pollut. Res. 2023, 30, 44206–44222. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Yang, T.; Gao, W.; Liu, Y.; Xu, C.; Yang, Y. Prevention and control of algae residue deposition in long-distance water conveyance project. Environ. Pollut. 2024, 344, 123294. [Google Scholar] [CrossRef] [PubMed]
- Raeder, U.; Ruzicka, J.; Goos, C. Characterization of the light attenuation by periphyton in lakes of different trophic state. Limnologica 2010, 40, 40–46. [Google Scholar] [CrossRef]
- Patrick, R.; Rhyne, C.F.; Richardson, R.W.; Larson, R.A.; Bott, T.L.; Rogenmuser, K. The Potential for Biological Controls of Cladophora Glomerata; EPA 600/3-83-065: 153; United States Environmental Protection Agency: Washington, DC, USA, 1983. [Google Scholar]
- Liu, H.; Chen, S.; Zhang, H.; Wang, N.; Ma, B.; Liu, X.; Niu, L.; Yang, F.; Xu, Y.; Zhang, X. Effects of copper sulfate algaecide on the cell growth, physiological characteristics, the metabolic activity of Microcystis aeruginosa and raw water application. J. Hazard. Mater. 2023, 445, 130604. [Google Scholar] [CrossRef]
- Munir, M.; Qureshi, R.; Bibi, M.; Khan, A.M. Pharmaceutical aptitude of Cladophora: A comprehensive review. Algal Res. 2019, 39, 101476. [Google Scholar] [CrossRef]
- Michalak, I.; Messyasz, B. Concise review of Cladophora spp.: Macroalgae of commercial interest. J. Appl. Phycol. 2021, 33, 133–166. [Google Scholar] [CrossRef]
- Bar-Shai, N.; Sharabani-Yosef, O.; Zollmann, M.; Lesman, A.; Golberg, A. Seaweed cellulose scaffolds derived from green macroalgae for tissue engineering. Sci. Rep. 2021, 11, 11843. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.; Zachgo, S. The Evolution of Cell Division: From Streptophyte Algae to Land Plants. Trends Plant Sci. 2016, 21, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Whitton, B.A. Biology of Cladophora in freshwaters. Water Res. 1970, 4, 457–476. [Google Scholar] [CrossRef]
- Wellman, C.H. 3—Origin, function and development of the spore wall in early land plants. In The Evolution of Plant Physiology; Hemsley, A.R., Poole, I., Eds.; Academic Press: Oxford, UK, 2004; pp. 43–63. [Google Scholar]
- Jarvis, P.; López-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 2013, 14, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Rousso, B.Z.; Bertone, E.; Stewart, R.; Aguiar, A.; Chuang, A.; Hamilton, D.P.; Burford, M.A. Chlorophyll and phycocyanin in-situ fluorescence in mixed cyanobacterial species assemblages: Effects of morphology, cell size and growth phase. Water Res. 2022, 212, 118127. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.C.; Monaco, M.E.; Herdendorf, C.E. Minimum Light Requirements for Substrate Colonization by Cladophora glomerata. J. Great Lakes Res. 1991, 17, 536–542. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, K. Photosynthetically active and UV radiation act in an antagonistic way in regulating buoyancy of Arthrospira (Spirulina) platensis (cyanobacterium). Environ. Exp. Bot. 2009, 66, 265–269. [Google Scholar] [CrossRef]
- Graham, J.M.; Auer, M.T.; Canale, R.P.; Hoffmann, J.P. Ecological Studies and Mathematical Modeling of Cladophora in Lake Huron: 4. Photosynthesis and Respiration as Functions of Light and Temperature. J. Great Lakes Res. 1982, 8, 100–111. [Google Scholar] [CrossRef]
- Turner, M.A.; Schindler, D.W.; Graham, R.W. Photosynthesis-irradiance relationships of epilithic algae measured in the laboratory and in situ. In Proceedings of the Periphyton of Freshwater Ecosystems, Växjö, Sweden, 14–17 September 1982; Springer: Dordrecht, The Netherlands, 1983; pp. 73–87. [Google Scholar]
- Parr, L.B.; Perkins, R.G.; Mason, C.F. Reduction in photosynthetic efficiency of Cladophora glomerata, induced by overlying canopies of Lemna spp. Water Res. 2002, 36, 1735–1742. [Google Scholar] [CrossRef]
- Wong, S.L.; Clark, B.; Kirby, M.; Kosciuw, R.F. Water Temperature Fluctuations and Seasonal Periodicity of Cladophora and Potamogeton in Shallow Rivers. J. Fish. Res. Board Can. 1978, 35, 866–870. [Google Scholar] [CrossRef]
- Stewart, T.; Lowe, R. Benthic Algae of Lake Erie (1865–2006): A Review of Assemblage Composition, Ecology, and Causes and Consequences of Changing Abundance. Ohio J. Sci. 2008, 108, 82–94. [Google Scholar]
- Song, Y. Hydrodynamic impacts on algal blooms in reservoirs and bloom mitigation using reservoir operation strategies: A review. J. Hydrol. 2023, 620, 129375. [Google Scholar] [CrossRef]
- Patil, R.; Wei, Y.; Pullar, D.; Shulmeister, J. Effects of change in streamflow patterns on water quality. J. Environ. Manag. 2022, 302, 113991. [Google Scholar] [CrossRef] [PubMed]
- Kuhlisch, C.; Shemi, A.; Barak-Gavish, N.; Schatz, D.; Vardi, A. Algal blooms in the ocean: Hot spots for chemically mediated microbial interactions. Nat. Rev. Microbiol. 2024, 22, 138–154. [Google Scholar] [CrossRef]
- Higgins, S.N.; Todd Howell, E.; Hecky, R.E.; Guildford, S.J.; Smith, R.E. The Wall of Green: The Status of Cladophora glomerata on the Northern Shores of Lake Erie’s Eastern Basin, 1995–2002. J. Great Lakes Res. 2005, 31, 547–563. [Google Scholar] [CrossRef]
- Higgins, S.N.; Pennuto, C.M.; Howell, E.T.; Lewis, T.W.; Makarewicz, J.C. Urban influences on Cladophora blooms in Lake Ontario. J. Great Lakes Res. 2012, 38, 116–123. [Google Scholar] [CrossRef]
- Yang, W.; Xu, M.; Li, R.; Zhang, L.; Deng, Q. Estimating the ecological water levels of shallow lakes: A case study in Tangxun Lake, China. Sci. Rep. 2020, 10, 5637. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.E.; Davis, K.; McColl, R.; Stanley, M.S.; Day, J.G.; Semião, A.J.C. Nitrogen uptake by the macro-algae Cladophora coelothrix and Cladophora parriaudii: Influence on growth, nitrogen preference and biochemical composition. Algal Res. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Scott, N.H.; Robert, E.H.; Stephanie, J.G. Environmental Controls of Cladophora Growth Dynamics in Eastern Lake Erie: Application of the Cladophora Growth Model (CGM). J. Great Lakes Res. 2006, 32, 629–644. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, S.; Raza, M.A.; Iqbal, N.; Asghar, M.A.; Raza, A.; Fan, Y.-F.; Mumtaz, M.; Shoaib, M.; Ansar, M.; et al. Crop photosynthetic response to light quality and light intensity. J. Integr. Agric. 2021, 20, 4–23. [Google Scholar] [CrossRef]
- Chevalier, P.; Proulx, D.; Lessard, P.; Vincent, W.F.; de la Noüe, J. Nitrogen and phosphorus removal by high latitude mat-forming cyanobacteria for potential use in tertiary wastewater treatment. J. Appl. Phycol. 2000, 12, 105–112. [Google Scholar] [CrossRef]
- Tsutsui, I.; Miyoshi, T.; Aue-umneoy, D.; Songphatkaew, J.; Meeanan, C.; Klomkling, S.; Sukchai, H.; Pinphoo, P.; Yamaguchi, I.; Ganmanee, M.; et al. High tolerance of Chaetomorpha sp. to salinity and water temperature enables survival and growth in stagnant waters of central Thailand. Int. Aquat. Res. 2015, 7, 47–62. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Lehmuskero, A.; Skogen Chauton, M.; Boström, T. Light and photosynthetic microalgae: A review of cellular- and molecular-scale optical processes. Prog. Oceanogr. 2018, 168, 43–56. [Google Scholar] [CrossRef]
- Ren, Y.; Shi, W.; Chen, J.; Li, J. Water quality drives the reconfiguration of riverine planktonic microbial food webs. Environ. Res. 2024, 249, 118379. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Liu, Z.-Y.; Han, Z.-Y.; Yuan, Y.; Shao, Y.; Feng, X.-Q.; Weitz, D.A. Regulation of cell attachment, spreading, and migration by hydrogel substrates with independently tunable mesh size. Acta Biomater. 2022, 141, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Yamazaki, T.; Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Kibuye, F.A.; Zamyadi, A.; Wert, E.C. A critical review on operation and performance of source water control strategies for cyanobacterial blooms: Part I-chemical control methods. Harmful Algae 2021, 109, 102099. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.; Shan, K.; Zheng, L.; Song, L. Influence of sunlight on the proliferation of cyanobacterial blooms and its potential applications in Lake Taihu, China. J. Environ. Sci. 2014, 26, 626–635. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, X.; Ren, B.; Zhang, Z.; Deng, X.; Yin, W.; Zhou, S.; Yang, S. Algae removal characteristics of the ultrasonic radiation enhanced drinking water treatment process. J. Water Process Eng. 2023, 55, 104154. [Google Scholar] [CrossRef]
- Mascia, M.; Vacca, A.; Palmas, S. Electrochemical treatment as a pre-oxidative step for algae removal using Chlorella vulgaris as a model organism and BDD anodes. Chem. Eng. J. 2013, 219, 512–519. [Google Scholar] [CrossRef]
- Zhan, M.-M.; Liu, P.-R.; Liu, X.-Y.; Hong, Y.; Xie, X. Inactivation and Removal Technologies for Algal-Bloom Control: Advances and Challenges. Curr. Pollut. Rep. 2021, 7, 392–406. [Google Scholar] [CrossRef]
- Phull, S.S.; Newman, A.P.; Lorimer, J.P.; Pollet, B.; Mason, T.J. The development and evaluation of ultrasound in the biocidal treatment of water. Ultrason. Sonochem. 1997, 4, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, Y.; Wang, K.; Tan, Y.; Bing, X.; Jiang, J.; Fang, W.; Chen, L.; Liao, H. Principles and research progress of physical prevention and control technologies for algae in eutrophic water. iScience 2024, 27, 109990. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, Z.; Cai, S.; Xia, S.; Zhang, X. The non-thermal influences of ultrasound on cell membrane: A molecular dynamics study. J. Mol. Struct. 2024, 1299, 137140. [Google Scholar] [CrossRef]
- Khan, W.; Park, J.W.; Maeng, S.K. Fluorescence descriptors for algal organic matter and microalgae disintegration during ultrasonication. J. Water Process Eng. 2022, 45, 102517. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, L.; Guo, Z.; Wang, G.; Xu, J.; Zheng, Z.; Sun, K.; Guo, F. Lipid peroxidation in osteoarthritis: Focusing on 4-hydroxynonenal, malondialdehyde, and ferroptosis. Cell Death Discov. 2023, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; King, P.M.; Wu, X.; Mason, T.J.; Joyce, E.M. Effect of ultrasonic frequency and power on the disruption of algal cells. Ultrason. Sonochem. 2015, 24, 165–171. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, W.; Li, L.; Wei, X.; Li, H.; Gao, N.; Yao, J. Evaluation of ultrasound as a preventative algae-controlling strategy: Degradation behaviors and character variations of algal organic matter components during sonication at different frequency ranges. Chem. Eng. J. 2021, 426, 130891. [Google Scholar] [CrossRef]
- Wu, X.; Joyce, E.M.; Mason, T.J. Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies. Water Res. 2012, 46, 2851–2858. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Watanabe, M.; Ikeuchi, M. Phycobilisome: Architecture of a light-harvesting supercomplex. Photosynth. Res. 2013, 116, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Cui, Y.; Yuan, W. Ultrasound for microalgal cell disruption and product extraction: A review. Ultrason. Sonochem. 2022, 87, 106054. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, Z.; Kong, Y.; Li, Y.; Zhou, Y.; Shi, X.; Shi, X. Effects of ultrasound on Microcystis aeruginosa cell destruction and release of intracellular organic matter. Ultrason. Sonochem. 2020, 63, 104909. [Google Scholar] [CrossRef]
- Tang, J.W.; Wu, Q.Y.; Hao, H.W.; Chen, Y.; Wu, M. Effect of 1.7 MHz ultrasound on a gas-vacuolate cyanobacterium and a gas-vacuole negative cyanobacterium. Colloids Surf. B Biointerfaces 2004, 36, 115–121. [Google Scholar] [CrossRef]
- Lewandowska, S.; Dziergowska, K.; Galek, R.; Michalak, I. Cladophora glomerata extracts produced by Ultrasound-Assisted Extraction support early growth and development of lupin (Lupinus angustifolius L.). Sci. Rep. 2023, 13, 17867. [Google Scholar] [CrossRef]
- Mallick, N.; Mohn, F.H. Reactive oxygen species: Response of algal cells. J. Plant Physiol. 2000, 157, 183–193. [Google Scholar] [CrossRef]
- Krehbiel, J.D.; Schideman, L.C.; King, D.A.; Freund, J.B. Algal cell disruption using microbubbles to localize ultrasonic energy. Bioresour. Technol. 2014, 173, 448–451. [Google Scholar] [CrossRef]
- Tan, X.; Shu, X.; Guo, J.; Parajuli, K.; Zhang, X.; Duan, Z. Effects of Low-Frequency Ultrasound on Microcystis aeruginosa from Cell Inactivation to Disruption. Bull. Environ. Contam. Toxicol. 2018, 101, 117–123. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhandari, G.; Turco, R.F.; Aminikhoei, Z.; Bhatt, K.; Simsek, H. Algae in wastewater treatment, mechanism, and application of biomass for production of value-added product. Environ. Pollut. 2022, 309, 119688. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hu, Y.; Qiu, G.; Li, Q.; Wang, G. Complexation of copper algaecide and algal organic matter in algae-laden water: Insights into complex metal–organic interactions. Environ. Pollut. 2023, 333, 122032. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, S.; Zhou, W.; Yuan, W.; Wang, D. Algal cell lysis by bacteria: A review and comparison to conventional methods. Algal Res. 2020, 46, 101794. [Google Scholar] [CrossRef]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal cellulose, production and potential use in plastics: Challenges and opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, J.; Wang, S.; Liu, X.; Li, M.; Mann, S.; Huang, X. Algal cell bionics as a step towards photosynthesis-independent hydrogen production. Nat. Commun. 2023, 14, 1872. [Google Scholar] [CrossRef] [PubMed]
- Vaahtera, L.; Schulz, J.; Hamann, T. Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 2019, 5, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zamyadi, A.; Zhang, W.; Dumée, L.F.; Gao, L. Algae-based water treatment: A promising and sustainable approach. J. Water Process Eng. 2022, 46, 102630. [Google Scholar] [CrossRef]
- Geada, P.; Moreira, C.; Silva, M.; Nunes, R.; Madureira, L.; Rocha, C.M.R.; Pereira, R.N.; Vicente, A.A.; Teixeira, J.A. Algal proteins: Production strategies and nutritional and functional properties. Bioresour. Technol. 2021, 332, 125125. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Zavadskiy, S.P.; Astakhov, D.V.; Terentiev, A.A. Lipid peroxidation: Reactive carbonyl species, protein/DNA adducts, and signaling switches in oxidative stress and cancer. Biochem. Biophys. Res. Commun. 2023, 687, 149167. [Google Scholar] [CrossRef]
- Li, D.; Kang, X.; Chu, L.; Wang, Y.; Song, X.; Zhao, X.; Cao, X. Algicidal mechanism of Raoultella ornithinolytica against Microcystis aeruginosa: Antioxidant response, photosynthetic system damage and microcystin degradation. Environ. Pollut. 2021, 287, 117644. [Google Scholar] [CrossRef]
- Kato, K.; Shinoda, T.; Nagao, R.; Akimoto, S.; Suzuki, T.; Dohmae, N.; Chen, M.; Allakhverdiev, S.I.; Shen, J.-R.; Akita, F.; et al. Structural basis for the adaptation and function of chlorophyll f in photosystem I. Nat. Commun. 2020, 11, 238. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Ooka, H.; Chiba, Y.; Nakamura, R. Thermodynamic principle to enhance enzymatic activity using the substrate affinity. Nat. Commun. 2023, 14, 4860. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Yesankar, P.J.; Dwivedi, A.; Qureshi, A. Biotic control of harmful algal blooms (HABs): A brief review. J. Environ. Manag. 2020, 268, 110687. [Google Scholar] [CrossRef]
- Yang, K.; Chen, Q.; Zhang, D.; Zhang, H.; Lei, X.; Chen, Z.; Li, Y.; Hong, Y.; Ma, X.; Zheng, W.; et al. The algicidal mechanism of prodigiosin from Hahella sp. KA22 against Microcystis aeruginosa. Sci. Rep. 2017, 7, 7750. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, C.; Cresson, P.; MacKenzie, K.; Fontaine, V.; Loots, C.; Delegrange, A.; Lefebvre, S. Insights into planktonic food-web dynamics through the lens of size and season. Sci. Rep. 2024, 14, 1684. [Google Scholar] [CrossRef]
- Vincent, F.; Gralka, M.; Schleyer, G.; Schatz, D.; Cabrera-Brufau, M.; Kuhlisch, C.; Sichert, A.; Vidal-Melgosa, S.; Mayers, K.; Barak-Gavish, N.; et al. Viral infection switches the balance between bacterial and eukaryotic recyclers of organic matter during coccolithophore blooms. Nat. Commun. 2023, 14, 510. [Google Scholar] [CrossRef]
- Sinang, S.C.; Daud, N.; Kamaruddin, N.; Poh, K.B. Potential growth inhibition of freshwater algae by herbaceous plant extracts. Acta Ecol. Sin. 2019, 39, 229–233. [Google Scholar] [CrossRef]
- Abudaqqa, W.S.K.; Madhuranthakam, C.M.R.; Chaalal, O. Algae-based membrane bioreactors: A mini review on their progress and processes for wastewater treatment. J. Water Process Eng. 2024, 59, 104937. [Google Scholar] [CrossRef]
- Hua, Q.; Liu, Y.-G.; Yan, Z.-L.; Zeng, G.-M.; Liu, S.-B.; Wang, W.-J.; Tan, X.-F.; Deng, J.-Q.; Tang, X.; Wang, Q.-P. Allelopathic effect of the rice straw aqueous extract on the growth of Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2018, 148, 953–959. [Google Scholar] [CrossRef]
- Yu, H.; Lei, P.; Ma, J.; Jin, J.; Ma, Y.; Fang, Y.; Zeng, G.; Zhang, K.; Jin, L.; Sun, D. The potential of white-rot fungi for algal control: Mechanisms, Strategies, and Challenges. Environ. Res. 2023, 236, 116738. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Niu, Y. The allelopathic effect of para-hydroxybenzoic acid on the gene expression of photosynthesis and respiration in Solanum lycopersicum. Curr. Plant Biol. 2022, 32, 100261. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Z.; Luo, Y.; Bai, Y.; Fan, J. Bioremediation of phenolic pollutants by algae—Current status and challenges. Bioresour. Technol. 2022, 350, 126930. [Google Scholar] [CrossRef]
- Woodson, J.D. Control of chloroplast degradation and cell death in response to stress. Trends Biochem. Sci. 2022, 47, 851–864. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, H.; Tang, S.; Peng, H.; Yin, D.; Yang, Y.; Liu, Z.; Dang, Z. Physiological responses of Microcystis aeruginosa against the algicidal bacterium Pseudomonas aeruginosa. Ecotoxicol. Environ. Saf. 2016, 127, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M. Chapter 2—Fundamental physiological processes: Photosynthesis, light-harvesting complex, and carbon-concentrating mechanisms. In Cyanobacterial Physiology; Kageyama, H., Waditee-Sirisattha, R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 17–28. [Google Scholar]
- Rochaix, J.-D. Regulation of photosynthetic electron transport. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, L.; Zhao, L.; Qian, C.; Lun, F.; Wang, C.; Zheng, H.; Tang, B.; Cheng, Y.; Guo, X. Inhibitory effects and oxidative damages in Cladophora sp. (Cladophoraceae) exposed to berberine. Aquac. Rep. 2022, 27, 101357. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Zhang, W.; Luo, D.-L.; Jiang, H.-J.; Wu, X.-H.; Sun, K.; Dai, C.-C. Fungal endophyte promotes plant growth and disease resistance of Arachis hypogaea L. by reshaping the core root microbiome under monocropping conditions. Microbiol. Res. 2023, 277, 127491. [Google Scholar] [CrossRef]
- Prazukin, A.V.; Anufriieva, E.V.; Shadrin, N.V. Biomass of Cladophora (Chlorophyta, Cladophorales) is a promising resource for agriculture with high benefits for economics and the environment. Aquac. Int. 2024, 32, 3637–3673. [Google Scholar] [CrossRef]
- Dorella, M.; Romagnoli, F.; Gruduls, A.; Collotta, M.; Tomasoni, G. Design of a biogas plant fed with Cladophora Sp. algae and wheat straw. Energy Procedia 2018, 147, 458–466. [Google Scholar] [CrossRef]
- Sharmila, D.; Rebecca, J. GC-MS Analysis of esters of fatty acid present in biodiesel produced from Cladophora vagabunda. J. Chem. Pharm. Res. 2012, 4, 4883–4887. [Google Scholar]
- Anh, N.T.N.; Hai, T.N.; Hien, T.T.T. Effects of partial replacement of fishmeal protein with green seaweed (Cladophora spp.) protein in practical diets for the black tiger shrimp (Penaeus monodon) postlarvae. J. Appl. Phycol. 2018, 30, 2649–2658. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, K.; Bing, X.; Tan, Y.; Zhou, Q.; Jiang, J.; Zhu, Y. Influencing Factors for the Growth of Cladophora and Its Cell Damage and Destruction Mechanism: Implication for Prevention and Treatment. Water 2024, 16, 1890. https://doi.org/10.3390/w16131890
Wang Y, Wang K, Bing X, Tan Y, Zhou Q, Jiang J, Zhu Y. Influencing Factors for the Growth of Cladophora and Its Cell Damage and Destruction Mechanism: Implication for Prevention and Treatment. Water. 2024; 16(13):1890. https://doi.org/10.3390/w16131890
Chicago/Turabian StyleWang, Yuyao, Kuo Wang, Xiaojie Bing, Yidan Tan, Qihao Zhou, Juan Jiang, and Yuanrong Zhu. 2024. "Influencing Factors for the Growth of Cladophora and Its Cell Damage and Destruction Mechanism: Implication for Prevention and Treatment" Water 16, no. 13: 1890. https://doi.org/10.3390/w16131890
APA StyleWang, Y., Wang, K., Bing, X., Tan, Y., Zhou, Q., Jiang, J., & Zhu, Y. (2024). Influencing Factors for the Growth of Cladophora and Its Cell Damage and Destruction Mechanism: Implication for Prevention and Treatment. Water, 16(13), 1890. https://doi.org/10.3390/w16131890





