Bioremediation of Cd-Contaminated Soil around Bauxite with Stimulants and Microorganisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Complex Bacterial Flora
2.2. Preparation of Simulated Soil
2.3. Remediation of Simulated Soil by a Single Stimulant
2.4. Remediation of Simulated Soil by Combinations of Different Stimulants and Complex Bacterial Flora
2.5. Determination of Soil Properties
2.6. Statistical Analysis
3. Results and Discussion
3.1. Repairing Effect of a Single Stimulant on Soil
- (1)
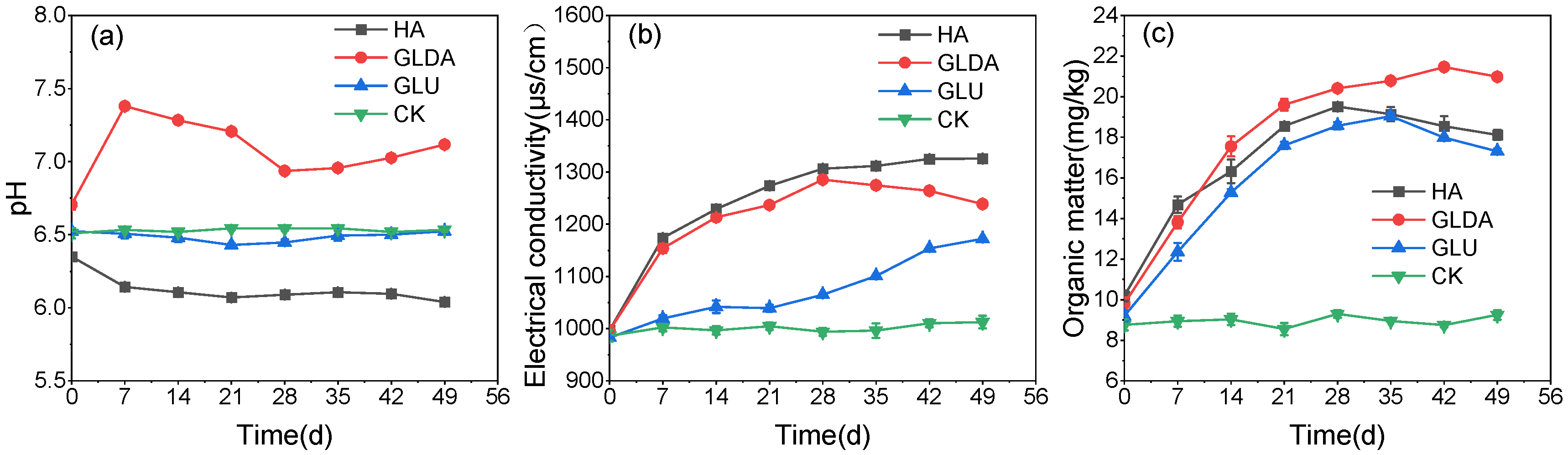
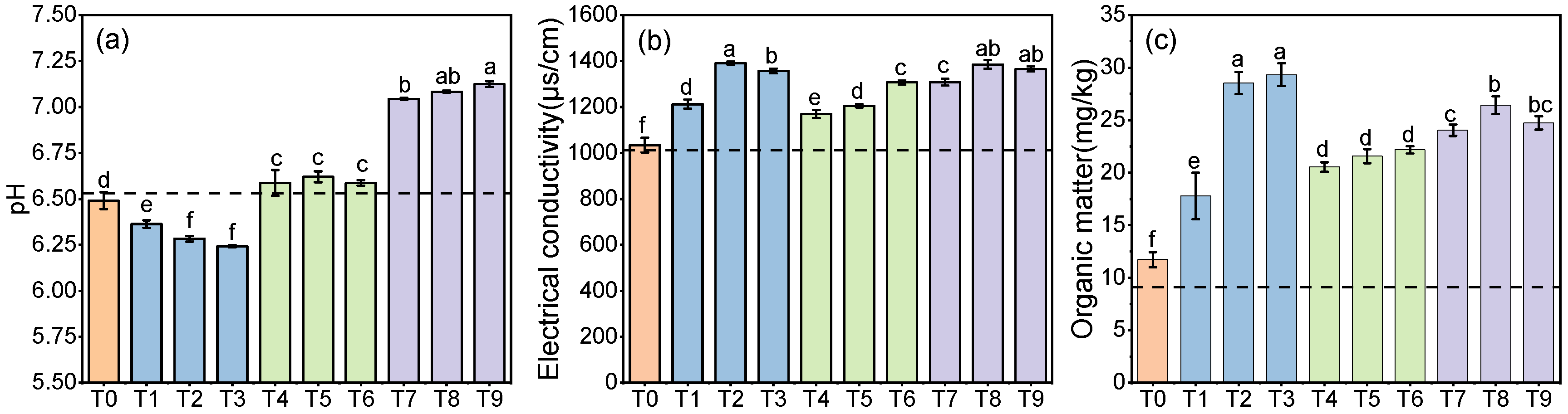
- Changes in physical and chemical properties of soil
- (2)
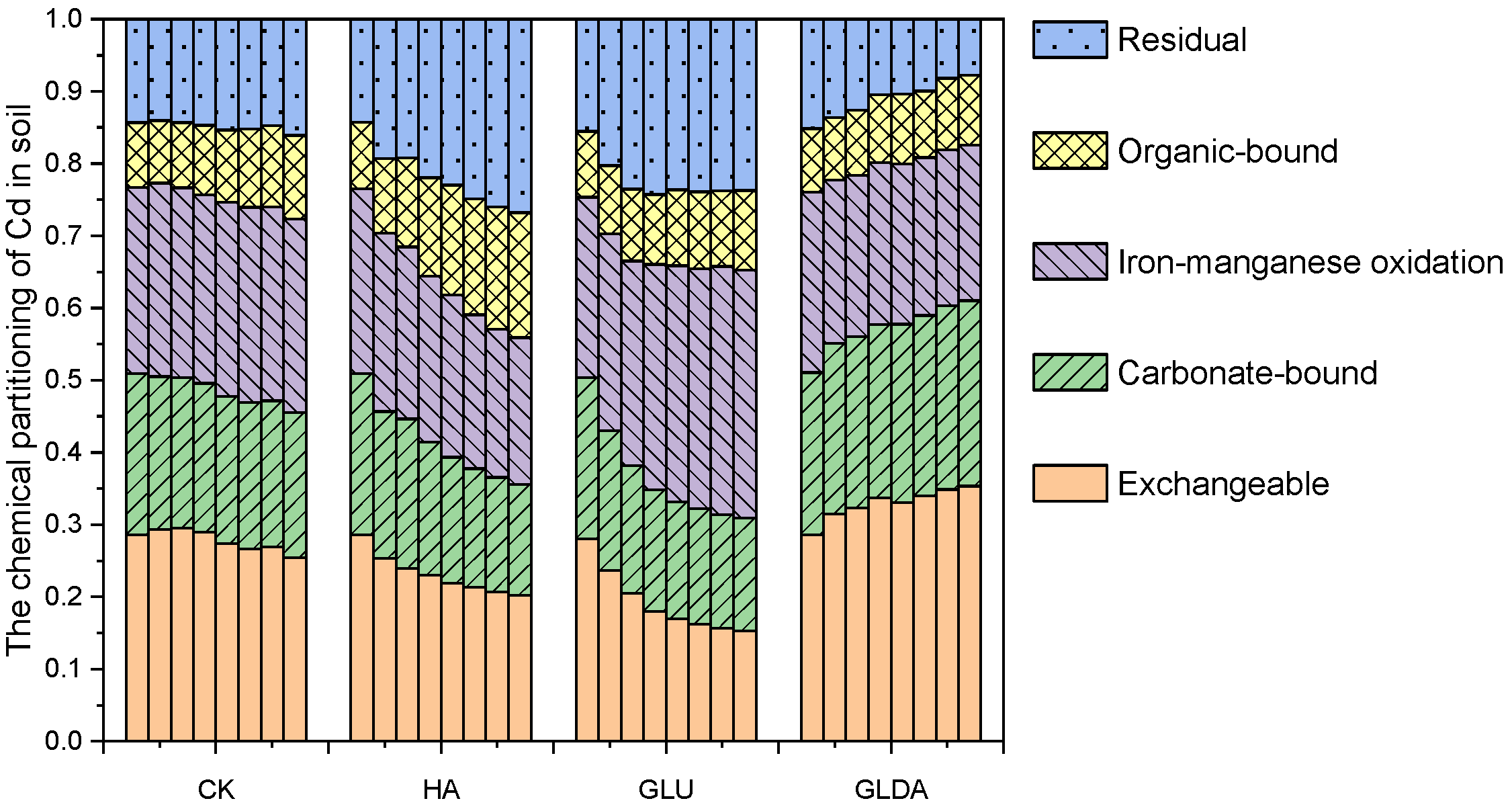
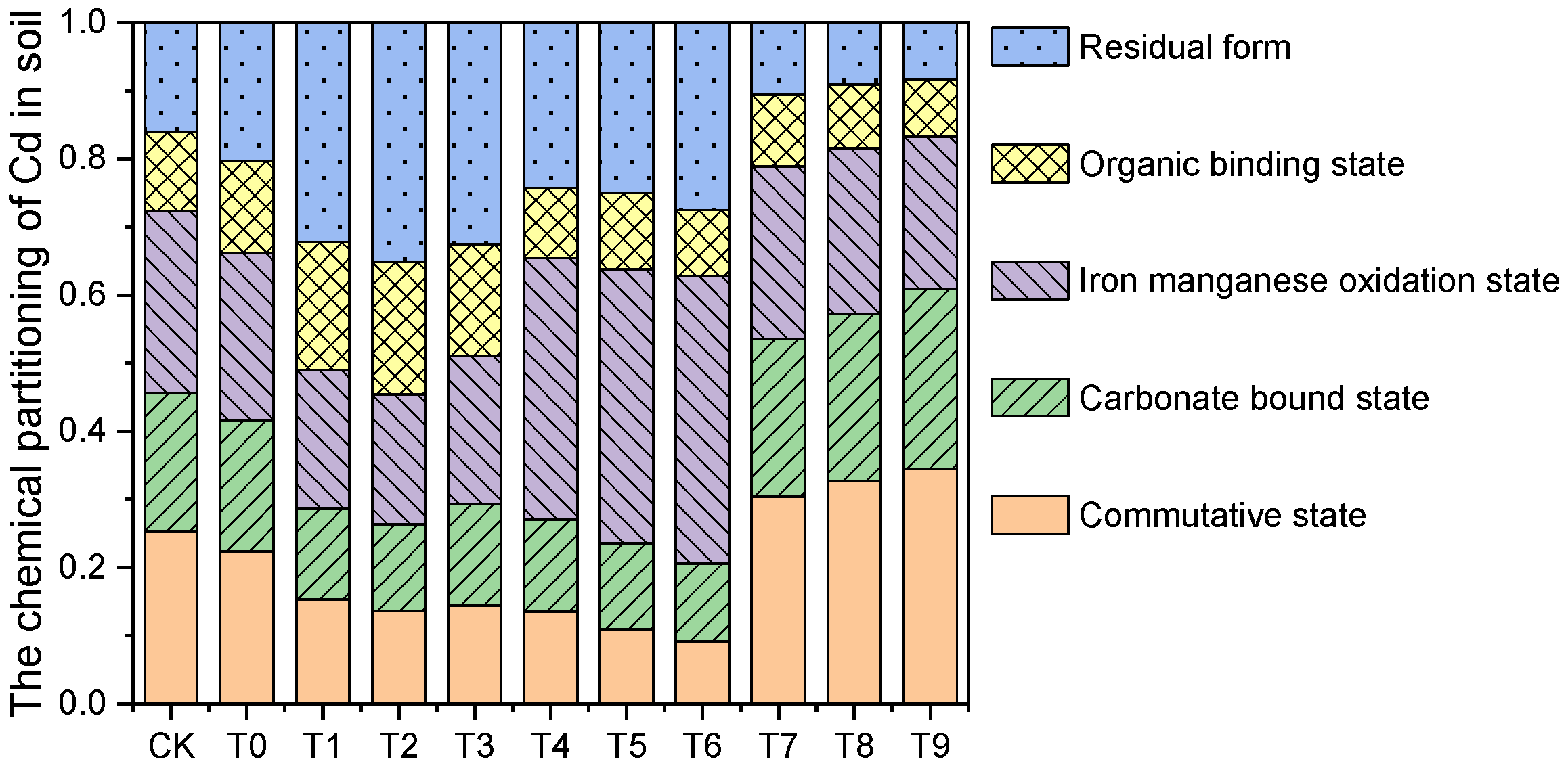
- Transformation of soil Cd morphology
- (3)
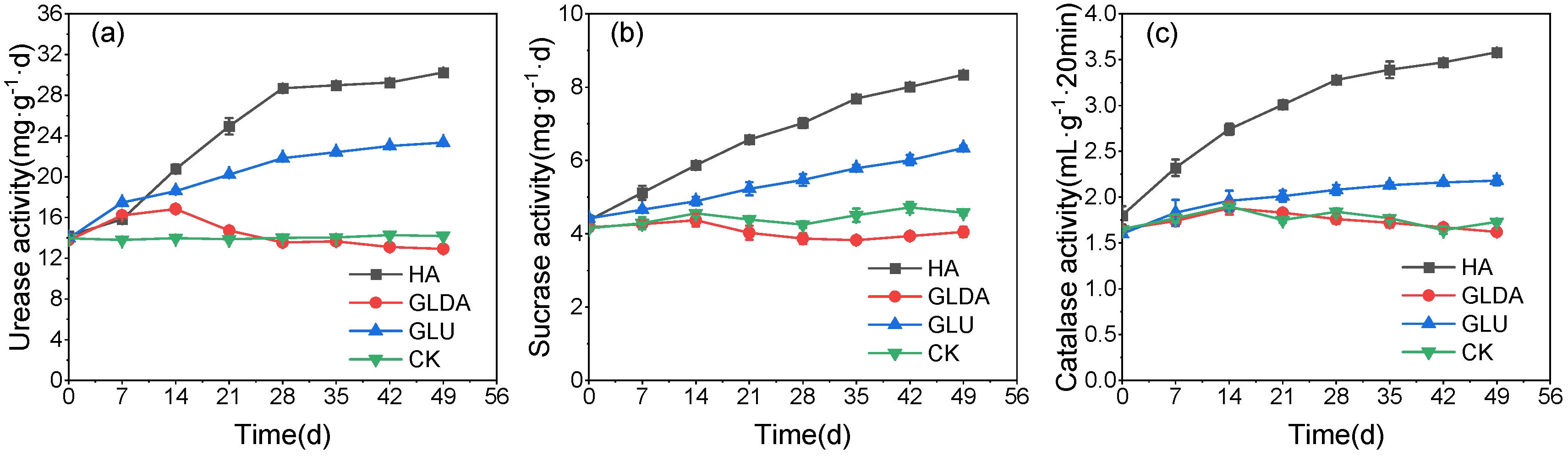
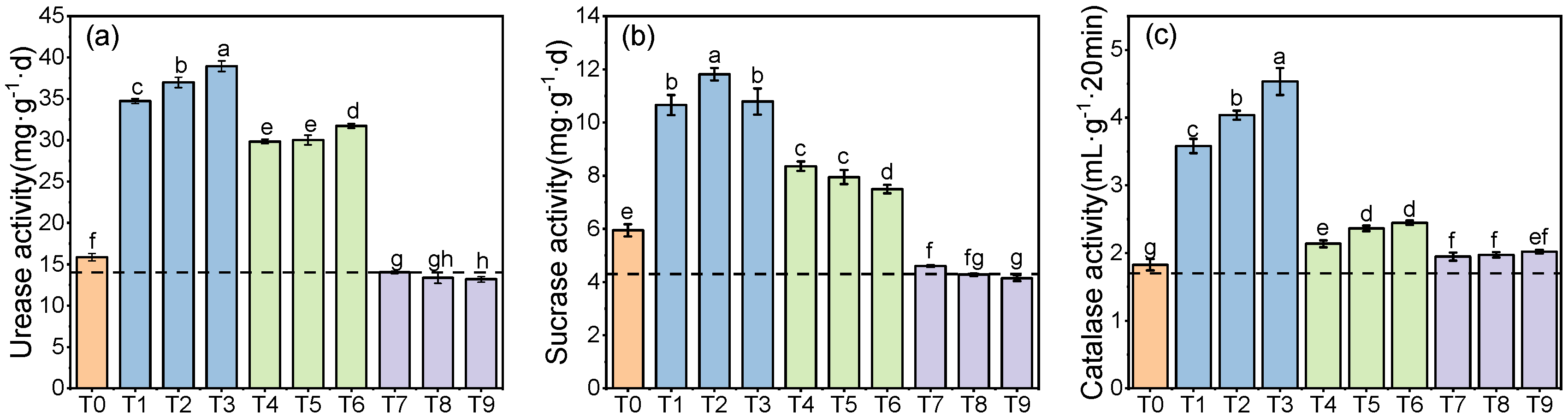
- Changes in soil enzyme activity
- (4)
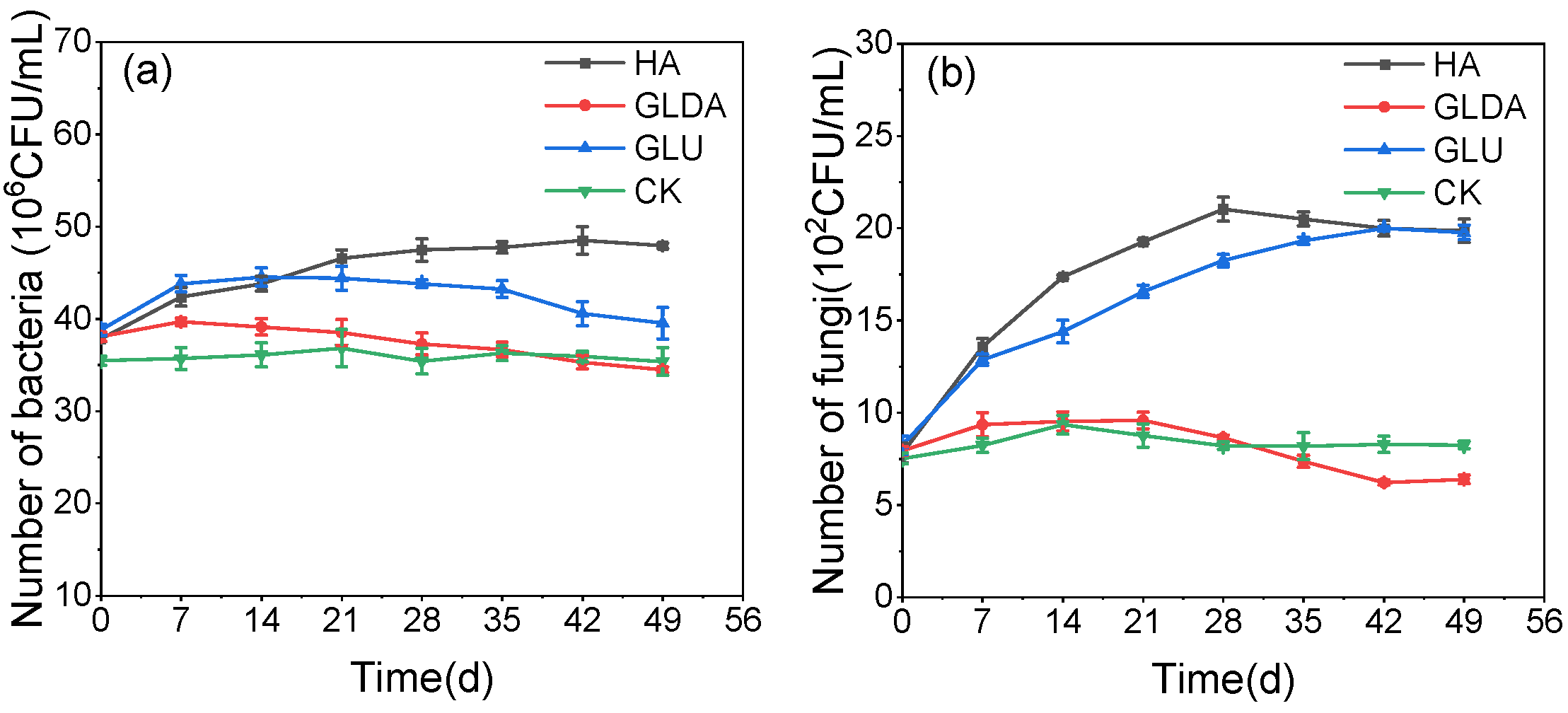
- Changes in soil microbial quantity
3.2. The Effect of a Combination of Different Stimulants and Complex Bacterial Flora on Simulated Soil Remediation
- (1)
- Changes in the physical and chemical properties of soil
- (2)
- Transformation of soil Cd morphology
- (3)
- Changes in soil enzyme activity
- (4)
- Changes in soil microbial quantity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vafeias, M.; Bempelou, A.; Georgala, E.; Davris, P.; Balomenos, E.; Panias, D. Leaching of Ca-Rich slags produced from reductive smelting of bauxite residue with Na2CO3 solutions for alumina extraction: Lab and Pilot Scale Experiments. Minerals 2021, 11, 896. [Google Scholar] [CrossRef]
- Chao, X.; Zhang, T.-A.; Lv, G.; Chen, Y.; Li, X.; Yang, X. Comprehensive Application Technology of Bauxite Residue Treatment in the Ecological Environment: A Review. Bull. Environ. Contam. Toxicol. 2022, 109, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Xiao, B.; Ali, M.U.; Xiao, P.; Zhao, P.; Wang, H.; Bibi, S. Heavy metals pollution from smelting activities: A threat to soil and groundwater. Ecotoxicol. Environ. Saf. 2024, 274, 116189. [Google Scholar] [CrossRef]
- Nie, X. Potential ecological risk assessment of heavy metal pollution in soil reclamation of Xiaoyi Aluminum Mine. Sci. Soil Water Conserv. 2018, 16, 116–122. [Google Scholar]
- Chen, X.; Li, X.; Wu, P.; Zha, X.; Liu, Y.; Wei, T.; Ran, W. The development and utilization of bauxite resources in the Guizhou Province and relevant challenges to the ecology and the environment. Gospod. Surowcami Miner. 2022, 38, 5–30. [Google Scholar]
- Kumar, A.; Subrahmanyam, G.; Mondal, R.; Cabral-Pinto, M.M.S.; Shabnam, A.A.; Jigyasu, D.K.; Malyan, S.K.; Fagodiya, R.K.; Khan, S.A.; Yu, Z.-G. Bio-remediation approaches for alleviation of cadmium contamination in natural resources. Chemosphere 2021, 268, 128855. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Jiang, W.; Wang, X.; Hussain, S.; Ahmad, M.; Maqsood, M.F.; Ali, N.; Ishfaq, M.; Kaleem, M.; Haider, F.U.; et al. Cadmium phytotoxicity, tolerance, and advanced remediation approaches in agricultural soils; a comprehensive review. Front. Plant Sci. 2022, 13, 773815. [Google Scholar] [CrossRef]
- Lee, H.; Sam, K.; Coulon, F.; De Gisi, S.; Notarnicola, M.; Labianca, C. Recent developments and prospects of sustainable remediation treatments for major contaminants in soil: A review. Sci. Total Environ. 2024, 912, 168769. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Smirnov, V.G.; Malyshenko, N.V.; Zherebtsov, S.I. Sorption of metal ions from aqueous solutions by humic substances. Solid Fuel Chem. 2023, 57, 297–306. [Google Scholar] [CrossRef]
- Ratie, G.; Chrastny, V.; Guinoiseau, D.; Marsac, R.; Vankova, Z.; Komarek, M. Cadmium isotope fractionation during complexation with humic acid. Environ. Sci. Technol. 2021, 55, 7430–7444. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, R.; Xu, Z.; Hu, H.; Kalkhajeh, Y.K.; Zhao, Y.; Zhan, L. Application of chelate GLDA for remediating Cd-contaminated farmlands using Tagetes patula L. Environ. Sci. Pollut. Res. 2023, 30, 3774–3782. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.J.; Hussain, M.M.; Albasher, G.; Iqbal, B.; Khan, K.A.; Rahim, R.; Li, G.; Du, D. Glucose input profit soil organic carbon mineralization and nitrogen dynamics in relation to nitrogen amended soils. J. Environ. Manag. 2024, 351, 119715. [Google Scholar] [CrossRef] [PubMed]
- Santini, T.C.; Malcolm, L.I.; Tyson, G.W.; Warren, L.A. pH and organic carbon dose rates control microbially driven bioremediation efficacy in alkaline bauxite residue. Environ. Sci. Technol. 2016, 50, 11164–11173. [Google Scholar] [CrossRef] [PubMed]
- MEP; MOLR; MOA. National Soil Pollution Situation detailed Inspection of Soil Sample Analysis Test Method Technical Regulations [Z]//CHINA. 2017. Available online: https://www.mee.gov.cn/gkml/hbb/bgth/201711/t20171106_425226.htm (accessed on 18 May 2024).
- Han, D.; Zhang, D.; Han, D.; Ren, H.; Wang, Z.; Zhu, Z.; Sun, H.; Wang, L.; Qu, Z.; Lu, W.; et al. Effects of salt stress on soil enzyme activities and rhizosphere microbial structure in salt-tolerant and -sensitive soybean. Sci. Rep. 2023, 13, 17057. [Google Scholar] [CrossRef] [PubMed]
- Sut-Lohmann, M.; Ramezany, S.; Kaestner, F.; Raab, T.; Heinrich, M.; Grimm, M. Using modified Tessier sequential extraction to specify potentially toxic metals at a former sewage farm. J. Environ. Manag. 2022, 304, 114229. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kong, D.; Zhang, B.; Kalkhajeh, Y.K.; Zhao, Y.; Huang, J.; Hu, H. Chemical forms of cadmium in soil and its distribution in French marigold sub-cells in response to chelator GLDA. Sci. Rep. 2022, 12, 17577. [Google Scholar] [CrossRef]
- Vassileva, M.; Malusa, E.; Sas-Paszt, L.; Trzcinski, P.; Galvez, A.; Flor-Peregrin, E.; Shilev, S.; Canfora, L.; Mocali, S.; Vassilev, N. Fermentation strategies to improve soil bio-inoculant production and quality. Microorganisms 2021, 9, 1254. [Google Scholar] [CrossRef]
- Li, J.; Deng, K.; Zhang, W.; Sang, C.; Zhao, Y.; Xv, R. The effect of adding glucose on nitrogen transformation and acidification of fertilizer in red soil farmland. Acta Pedol. Sin. 2021, 58, 162–168. [Google Scholar]
- Masoudi, F.; Shirvani, M.; Shariatmadari, H.; Sabzalian, M.R. Performance of new biodegradable chelants in enhancing phytoextraction of heavy metals from a contaminated calcareous soil. J. Environ. Health Sci. Eng. 2020, 18, 655–664. [Google Scholar] [CrossRef]
- Zhao, K.; Yang, Y.; Peng, H.; Zhang, L.; Zhou, Y.; Zhang, J.; Du, C.; Liu, J.; Lin, X.; Wang, N. Silicon fertilizers, humic acid and their impact on physicochemical properties, availability and distribution of heavy metals in soil and soil aggregates. Sci. Total Environ. 2022, 822, 153483. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Ekinci, M.; Turan, M.; Agar, G.; Dursun, A.; Kul, R.; Alim, Z.; Argin, S. Humic plus Fulvic acid mitigated Cd adverse effects on plant growth, physiology and biochemical properties of garden cress. Sci. Rep. 2021, 11, 8040. [Google Scholar] [CrossRef]
- Bretti, C.; Di Pietro, R.; Cardiano, P.; Gomez-Laserna, O.; Irto, A.; Lando, G.; De Stefano, C. Thermodynamic Solution Properties of a Biodegradable Chelant (L-glutamic-N,N-diacetic Acid, L-GLDA) and Its Sequestering Ability toward Cd2+. Molecules 2021, 26, 7087. [Google Scholar] [CrossRef] [PubMed]
- Meena, A.; Rao, K.S. Assessment of soil microbial and enzyme activity in the rhizosphere zone under different land use/cover of a semiarid region, India. Ecol. Process. 2021, 10, 16. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zhu, Q.; Zhou, J.; Shi, L.; Lu, W.; Bao, L.; Meng, L.; Wu, L.; Zhang, N.; et al. Differences in the activities of six soil enzymes in response to cadmium contamination of paddy soils in high geological background areas. Environ. Pollut. 2024, 346, 123704. [Google Scholar] [CrossRef]
- Zheng, R.; Zhu, J.; Liao, P.; Wang, D.; Wu, P.; Mao, W.; Zhang, Y.; Wang, W. Environmental colloid behaviors of humic acid-Cadmium nanoparticles in aquatic environments. J. Environ. Sci. 2025, 149, 663–675. [Google Scholar] [CrossRef]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the role of humic acids on crop performance and soil health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Haider, F.U.; Maqsood, M.F.; Mohy-Ud-Din, W.; Shabaan, M.; Ahmad, M.; Kaleem, M.; Ishfaq, M.; Aslam, Z.; Shahzad, B. Recent advances in microbial-assisted remediation of cadmium-contaminated soil. Plants 2023, 12, 3147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, Y.; Wang, Z.; Qian, G. Enhancement of humic acid on plant growth in a Cd-contaminated matrix: Performance, kinetics, and mechanism. Environ. Sci. Pollut. Res. 2023, 30, 5677–5687. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Q.; Su, Y. Cadmium Phytoavailability and Enzyme Activity under Humic Acid Treatment in Fluvo-aquic Soil. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 042013. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, G.; Ma, W.; Wu, J.; Gong, Y.; Xu, G. Vegetation degradation impacts soil nutrients and enzyme activities in wet meadow on the Qinghai-Tibet Plateau. Sci. Rep. 2020, 10, 21271. [Google Scholar] [CrossRef] [PubMed]
| Name | pH | Electrical Conductivity | Organic Matter | Cd |
|---|---|---|---|---|
| Simulated soil | 6.53 | 1020 μs/cm | 9.08 mg/kg | 10.35 mg/kg |
| Group | Processing Mode |
|---|---|
| CK | without complex bacterial flora and stimulants |
| T0 | complex bacterial flora |
| T1 | 0.1% HA + complex bacterial flora |
| T2 | 0.5% HA + complex bacterial flora |
| T3 | 1% HA + complex bacterial flora |
| T4 | 0.1% GLU + complex bacterial flora |
| T5 | 0.5% GLU + complex bacterial flora |
| T6 | 1% GLU + complex bacterial flora |
| T7 | 0.1% GLDA + complex bacterial flora |
| T8 | 0.5% GLDA + complex bacterial flora |
| T9 | 1% GLDA + complex bacterial flora |
| Group | Number of Bacteria (106 CFU/mL) | Number of Fungi (102 CFU/mL) |
|---|---|---|
| CK | 35.97 ± 1.43 e | 8.85 ± 0.66 e |
| T0 | 37.01 ± 0.75 d | 10.80 ± 0.53 d |
| T1 | 47.83 ± 1.29 a | 19.69 ± 0.76 b |
| T2 | 48.26 ± 0.66 a | 20.88 ± 0.46 ab |
| T3 | 48.71 ± 0.57 a | 20.76 ± 0.83 ab |
| T4 | 42.56 ± 0.64 c | 20.17 ± 0.45 ab |
| T5 | 42.92 ± 0.47 bc | 20.49 ± 0.35 ab |
| T6 | 44.71 ± 0.80 b | 21.21 ± 0.71 a |
| T7 | 38.00 ± 0.46 d | 15.24 ± 0.29 c |
| T8 | 38.74 ± 0.39 d | 15.32 ± 0.89 c |
| T9 | 39.02 ± 0.46 d | 15.41 ± 0.82 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Chen, X.; Yao, J.; Xiao, L.; Feng, X.; Wu, S. Bioremediation of Cd-Contaminated Soil around Bauxite with Stimulants and Microorganisms. Water 2024, 16, 1910. https://doi.org/10.3390/w16131910
Feng L, Chen X, Yao J, Xiao L, Feng X, Wu S. Bioremediation of Cd-Contaminated Soil around Bauxite with Stimulants and Microorganisms. Water. 2024; 16(13):1910. https://doi.org/10.3390/w16131910
Chicago/Turabian StyleFeng, Luxuan, Xiaofeng Chen, Jinghua Yao, Lei Xiao, Xiujuan Feng, and Shengmin Wu. 2024. "Bioremediation of Cd-Contaminated Soil around Bauxite with Stimulants and Microorganisms" Water 16, no. 13: 1910. https://doi.org/10.3390/w16131910
APA StyleFeng, L., Chen, X., Yao, J., Xiao, L., Feng, X., & Wu, S. (2024). Bioremediation of Cd-Contaminated Soil around Bauxite with Stimulants and Microorganisms. Water, 16(13), 1910. https://doi.org/10.3390/w16131910






