Systematic Review of Poultry Slaughterhouse Wastewater Treatment: Unveiling the Potential of Nanobubble Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Information Sources and Search
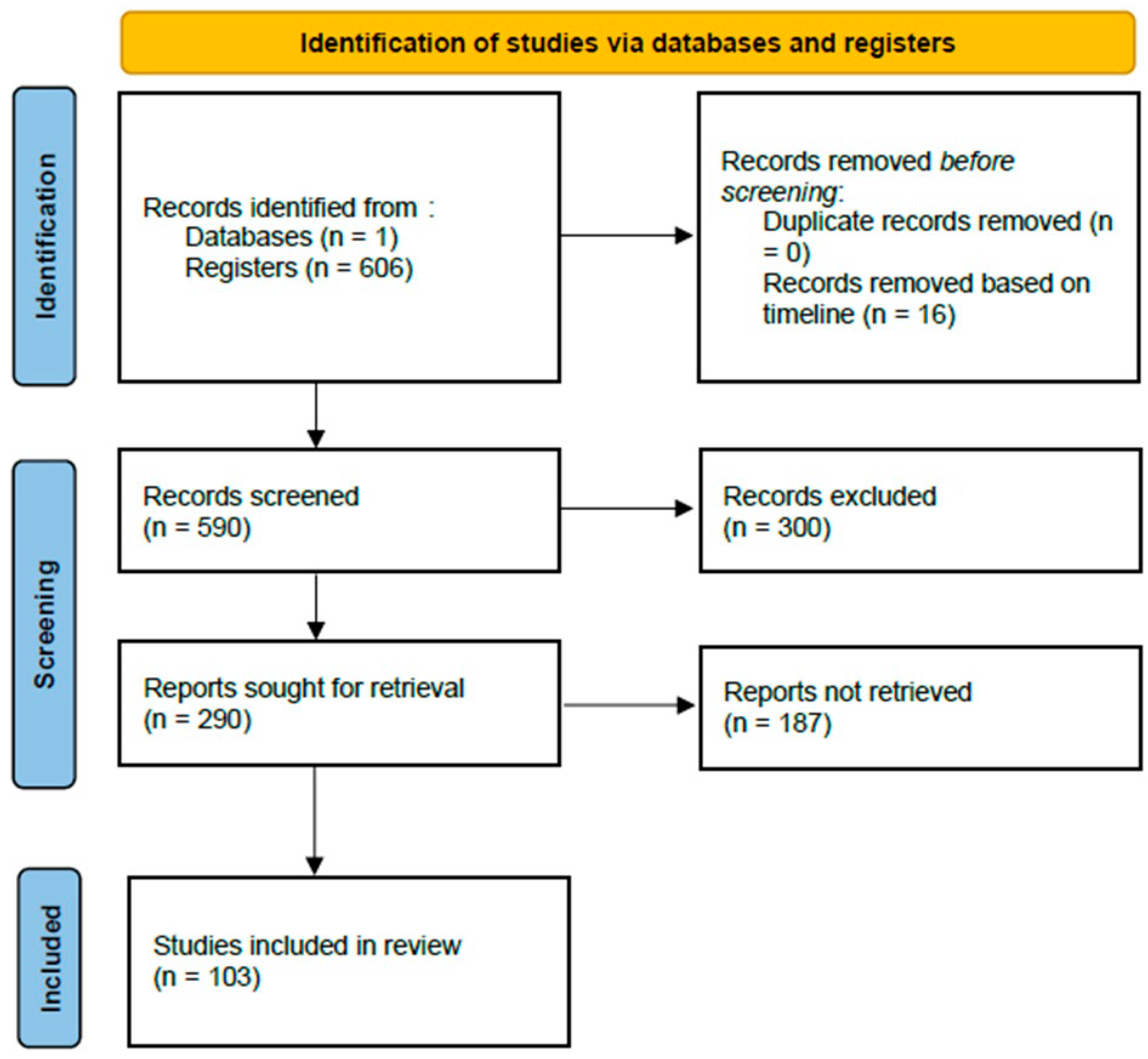
2.2. Selection of Studies
2.3. Data Extraction, Bibliometric Mapping, and Statistical Analysis
3. Nanobubble Technology
3.1. Bubble Size
3.2. Fundamental Properties of NBs
3.2.1. Negative Zeta Potential
3.2.2. Ability to Generate Free Radicals
3.2.3. Gas Mass Transfer
3.2.4. Stability of Nanobubbles
3.3. Generation of Nanobubbles
3.3.1. Mechanical Stirring Method
3.3.2. Venturi-Based Generation
3.3.3. Porous Membrane Method
3.3.4. Acoustic Cavitation Method
3.3.5. Microfluidic Method
3.3.6. Hydrodynamic Cavitation Method
3.4. Application of Nanobubbles in Wastewater Treatment
| Application | Research Focus | Results and Achievements | References |
|---|---|---|---|
| Aeration | Investigation of NB effects on aeration | Improved oxygen transfer efficiency, enhanced DO content, and accelerated pollutant removal. | [46] |
| Floatation | Evaluation of NB impacts on froth flotation | Reduction in bubble rising velocity; improved froth flotation conditions for coarse particles. | [47] |
| Membrane technology | Application of NBs in membrane processes | Improved permeability, reduced fouling, and enhanced efficiency in various membrane technologies. | [48] |
| Ozone oxidation | Use of NBs in ozone treatment | Increased stability, generation of hydroxyl radicals (OH), and improved oxidative efficiency. | [49] |
3.4.1. Nanobubbles in Flotation Technology
3.4.2. Nanobubbles in Aeration
3.4.3. Physiochemical Treatment with Nanobubbles
3.4.4. Advance Oxidation with Ozone Nanobubbles
3.5. Degradation Mechanism of Pollutants by Nanobubbles
3.6. Factors Affecting Pollutant Removal by Nanobubbles
- (1)
- Effect of pH: The degradation of organic pollutants by NBs is influenced by pH levels. Research indicates that acidic conditions enhance the degradation of certain pollutants by NBs, while other studies suggest that an alkaline environment is more effective for different pollutants [73]. For example, NBs best degrade methyl orange, phenol, and rhodamine B under acidic conditions [75]. Conversely, pollutants such as alachlor, benzothiophene, and diethyl phthalate are more effectively degraded by NBs in alkaline conditions [75,76]. This variation is due to the impact of pH on the free radicals produced by NBs and the physical and chemical properties of the pollutants themselves [72]. Thus, the degradation of organic pollutants by NBs involves the dual influence of these factors, which should be comprehensively considered.The pH of PSW can fluctuate, potentially impacting the effectiveness of AOPs. The quality and pH of PSW are affected by the quality of water used during slaughtering, the type of operation during wastewater collection, the sampling methods used by the individuals involved, and the specific cleaning and sanitizing procedures of the abattoir [5,77]. The pH of PSW was reported to vary between 4.9 and 8.1 with a mean of 6.5 [73,78]. To evaluate how pH influences the degradation process, a study needs to be conducted with NBs across various pH levels.
- (2)
- Effect of temperature: Temperature also plays a significant role in the generation of ROS species by NBs and conversely affects the degradation of pollutants. Yu et al. [76] found that in an alkaline NB solution, the concentration of ROS species initially increased and then decreased as the temperature rose, displaying a parabolic trend with a peak concentration at 65 °C. This phenomenon was attributed to the combined effects of temperature on oxygen reactivity, diffusion coefficient, and DO concentration, where ROS levels followed the same trend. In another study, Wang et al. [73] investigated the impact of temperature on the degradation of rhodamine B (RhB) using cavitation-induced and rotating jets. Their findings showed that the degradation efficiency of rhodamine B improved as the temperature increased from 20 °C to 40 °C, but decreased when the temperature rose further from 40 °C to 60 °C.These findings demonstrate that temperature has a dual effect on pollutant removal efficiency by NBs. As the equilibrium vapor pressure increases with temperature, the formation of NBs is promoted, which aids in the generation of OH− and the degradation of organic matter. However, excessively high temperatures cause water vapor to fill the cavitation bubbles, reducing bubble collapse, which hinders the generation of ·OH and the degradation of organic matter [73].
- (3)
- Effect of initial concentrations of pollutants: Ahmadi et al. [79] assessed the impact of different initial COD concentrations (400.0, 600.0, and 800.0 mg L−1) on removal efficiency in the NB aeration system. They found that the removal efficiency decreased as the pollutants’ concentrations (i.e., COD) increased. This decline was attributed to a shortage of DO in the wastewater, which is essential for the oxidation process. Enhancing the oxygen content in the wastewater is crucial. Factors such as the bacterial growth curve, the existing phase, and the sludge volume index (SVI) are highly influential. Similarly, Wang et al. [73] investigated the effect of initial concentrations of RhB (0.1, 1, and 10 mg/L) on their removal efficiency by NBs. The results showed that at a high initial concentration of RhB, the degradation of intermediates (by-products) may compete for the consumption of ROS with the parent RhB compound, leading to a slower reaction rate.
- (4)
- Effect of salinity and other ions: Various constituents in PSW, such as ions, salinity, hardness, and alkalinity, can pose significant challenges for ROS-based AOPs in degrading organic pollutants from wastewater [79]. Some studies have highlighted the impact of foreign ions on the stability of nanobubbles [80]. However, Wang examined the removal efficiency of RhB in the presence of 300 mg/L of background ions, including Ca2⁺, Mg2⁺, HCO₃−, and Cl−. Their findings showed that oxygen nanobubbles can achieve a removal efficiency of RhB exceeding 92%, even in the presence of the background ions. They concluded that the background ions have a negligible impact on degradation by oxygen nanobubbles.
4. Conventional Treatment of Poultry Slaughterhouse Wastewater
4.1. Preliminary Treatment
4.2. Primary Treatment
4.3. Secondary Treatment
4.3.1. Anaerobic Treatment
4.3.2. Aerobic Treatment
5. Nanobubble Application Prospect for PSW Treatment
5.1. Nanobubble Aeration with Enzymes
5.2. Nanobubble Aeration with Ozone
5.3. Aerobic Treatment of PSW with Nanobubbles
6. Conclusions
7. Recommendations and Perspectives for Future Studies
- (1)
- Exploration of novel applications: This review highlighted the effectiveness of NB technology in various wastewater treatment processes, including flotation, aeration, physicochemical treatment, and ozone oxidation. Future studies can explore novel applications of NBs in treating specific types of wastewater, such as PSW.
- (2)
- Optimization of operating conditions: Research is needed to optimize the operating conditions such as pH, temperature, DO, aeration time, and pollutant levels in PSW on the NB performance in treating PSW. Understanding the influence of these parameters on treatment efficiency and energy consumption can lead to more sustainable and cost-effective treatment solutions for PSW.
- (3)
- Integration with advanced treatment methods: NB technology can be integrated with other advanced treatment methods, such as membrane filtration, floatation, and advanced oxidation processes. Future studies can investigate the synergistic effects of combining NBs with these techniques to enhance pollutant removal efficiency.
- (4)
- Economic assessments: Future studies should include comprehensive assessments of NB technology compared to conventional treatment methods. Evaluating factors such as energy consumption and chemical usage can help identify the economic benefits of NB-based treatment for PSW.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bustillo-Lecompte, C.F.; Mehrvar, M. Slaughterhouse wastewater characteristics, treatment, and management in the meat processing industry: A review on trends and advances. J. Environ. Manag. 2015, 161, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Hilares, R.T.; Atoche-Garay, D.F.; Pagaza, D.A.P.; Ahmed, M.A.; Andrade, G.J.C.; Santos, J.C. Promising physicochemical technologies for poultry slaughterhouse wastewater treatment: A critical review. J. Environ. Chem. Eng. 2021, 9, 105174. [Google Scholar] [CrossRef]
- Basitere, M.; Njoya, M.; Rinquest, Z.; Ntwampe, S.K.O.; Sheldon, M.S. Performance evaluation and kinetic parameter analysis for static granular bed reactor (SGBR) for treating poultry slaughterhouse wastewater at mesophilic condition. Water Pract. Technol. 2019, 14, 259–268. [Google Scholar] [CrossRef]
- South African Government. National Water Act 36 of 1998. Available online: https://www.gov.za/documents/national-water-act (accessed on 17 February 2024).
- Njoya, M.; Basitere, M.; Ntwampe, S.K.O. Analysis of the Characteristics of Poultry Slaughterhouse Wastewater (PSW) and Its Treatability. Water Pract. Technol. 2019, 14, 959–970. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and Applications of Microbubble and Nanobubble Technology for Water Treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Rameshkumara, C.; Senthilkumarb, G.; Subalakshmic, R.; Gogoid, R. Generation and characterization of nanobubbles by ionization method for wastewater treatment. Desalination Water Treat. 2019, 164, 98–101. [Google Scholar] [CrossRef]
- Kim, M.S.; Kwak, D.H. Effect of zeta potential on collision attachment coefficient and removal efficiency for dissolved carbon dioxide flotation. Environ. Eng. Sci. 2017, 34, 272–280. [Google Scholar] [CrossRef]
- ISO 20480-1:2017; Fine Bubble Technology—General Principles for Usage and Measurement of Fine Bubbles. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/obp/ui/#iso:std:iso:20480:-1:ed-1:v1:en (accessed on 17 February 2024).
- Gurung, A.; Dahl, O.; Jansson, K. The fundamental phenomena of nanobubbles and their behavior in wastewater treatment technologies. Geosystem Eng. 2016, 19, 133–142. [Google Scholar] [CrossRef]
- Phan, K.K.T.; Truong, T.; Wang, Y.; Bhandari, B. Nanobubbles: Fundamental characteristics and applications in food processing. Trends Food Sci. Technol. 2020, 95, 118–130. [Google Scholar] [CrossRef]
- Sakr, M.; Mohamed, M.M.; Maraqa, M.A.; Hamouda, M.A.; Hassan, A.A.; Ali, J.; Jung, J. A critical review of the recent developments in micro–nano bubbles applications for domestic and industrial wastewater treatment. Alex. Eng. J. 2022, 61, 6591–6612. [Google Scholar] [CrossRef]
- Shangguan, Y.; Yu, S.; Gong, C.; Wang, Y.; Yang, W.; Hou, L. A review of Microbubble and its applications in ozonation. IOP Conf. Ser. Earth Environ. Sci. 2018, 128, 012149. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, D.C.; Han, M. Average size and zeta potential of nanobubbles in different reagent solutions. J. Nanoparticle Res. 2019, 21, 173. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Aluthgun Hewage, S.; Batagoda, J.H. Stability of nanobubbles. Environ. Eng. Sci. 2018, 35, 1216–1227. [Google Scholar] [CrossRef]
- Takahashi, M.; Chiba, K.; Li, P. Free-radical generation from collapsing microbubbles in the absence of a dynamic stimulus. J. Phys. Chem. B 2007, 111, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, A. Fundamentals and applications of micro/nano bubbles. In Proceedings of the 1st International Symposium on Application of High voltage, Plasmas & Micro/Nano Bubbles to Agriculture and Aquaculture, Chiang Mai, Thailand, 5–6 January 2017. [Google Scholar]
- Xiong, X.; Wang, B.; Zhu, W.; Tian, K.; Zhang, H. A review on ultrasonic catalytic microbubbles ozonation processes: Properties, hydroxyl radicals generation pathway and potential in application. Catalysts 2018, 9, 10. [Google Scholar] [CrossRef]
- Zhang, M.; Qiu, L.; Liu, G. Basic characteristics and application of micro-nano Bubbles in water treatment. IOP Conf. Ser. Earth Environ. Sci. 2020, 510, 042050. [Google Scholar] [CrossRef]
- Masuda, N.; Maruyama, A.; Eguchi, T.; Hirakawa, T.; Murakami, Y. Influence of microbubbles on free radical generation by ultrasound in aqueous solution: Dependence of ultrasound frequency. J. Phys. Chem. B 2015, 119, 12887–12893. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, L.; Song, D.; Al-Tabbaa, A. Subsurface transport behavior of Micro-Nano bubbles and potential applications for groundwater remediation. Int. J. Environ. Res. Public Health 2013, 11, 473–486. [Google Scholar] [CrossRef]
- Li, H.; Hu, L.; Song, D.; Lin, F. Characteristics of micro-nano bubbles and potential application in groundwater bioremediation. Water Environ. Res. 2014, 86, 844–851. [Google Scholar] [CrossRef]
- Atkinson, A.J.; Apul, O.G.; Schneider, O.; Garcia-Segura, S.; Westerhoff, P. Nanobubble technologies offer opportunities to improve water treatment. Acc. Chem. Res. 2019, 52, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tang, Y. Application research of micro and nano bubbles in Water Pollution Control. E3S Web Conf. 2019, 136, 06028. [Google Scholar] [CrossRef]
- Azevedo, A.; Oliveira, H.; Rubio, J. Bulk nanobubbles in the mineral and environmental areas: Updating research and applications. Adv. Colloid Interface Sci. 2019, 271, 101992. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, G.; Purusothaman, M.; Rameshkumar, C.; Joy, N.; Sachin, S.; Siva Thanigai, K. Generation and characterization of nanobubbles for heat transfer applications. Mater. Today Proc. 2021, 43, 3391–3393. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Yin, J.; Wang, D. Investigation on the effect of geometrical parameters on the performance of a venturi type bubble generator. Nucl. Eng. Des. 2017, 325, 90–96. [Google Scholar] [CrossRef]
- Zhao, L.; Mo, Z.; Sun, L.; Xie, G.; Liu, H.; Du, M.; Tang, J. A visualized study of the motion of individual bubbles in a venturi-type bubble generator. Prog. Nucl. Energy 2017, 97, 74–89. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, Y.; Chen, L.; Ge, G. Controllable preparation of monodisperse nanobubbles by membrane sieving. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128656. [Google Scholar] [CrossRef]
- Ferrari, A. Fluid dynamics of acoustic and hydrodynamic cavitation in hydraulic power systems. Proc. R. Soc. A Math. Phys. Eng. Sci. 2017, 473, 20160345. [Google Scholar] [CrossRef] [PubMed]
- Nirmalkar, N.; Pacek, A.W.; Barigou, M. Bulk nanobubbles from acoustically cavitated aqueous organic solvent mixtures. Langmuir 2019, 35, 2188–2195. [Google Scholar] [CrossRef]
- Yi-Qiang, F.; Hong-Liang, W.; Ke-Xin, G.; Jing-Ji, L.I.U.; Dong-Ping, C.H.A.I.; Zhang, Y.J. Applications of modular microfluidics technology. Chin. J. Anal. Chem. 2018, 46, 1863–1871. [Google Scholar]
- Zheng, H.; Zheng, Y.; Zhu, J. Recent developments in hydrodynamic cavitation reactors: Cavitation mechanism, reactor design, and applications. Engineering 2022, 19, 180–198. [Google Scholar] [CrossRef]
- Etchepare, R.; Oliveira, H.; Nicknig, M.; Azevedo, A.; Rubio, J. Nanobubbles: Generation using a multiphase pump, properties and features in flotation. Min. Eng. 2017, 112, 19–26. [Google Scholar] [CrossRef]
- Parmar, R.; Majumder, S.K. Microbubble generation and Microbubble-aided transport process intensification—A state-of-the-art report. Chem. Eng. Process. Process Intensif. 2013, 64, 79–97. [Google Scholar] [CrossRef]
- Huang, J.; Sun, L.; Du, M.; Liang, Z.; Mo, Z.; Tang, J.; Xie, G. An investigation on the performance of a micro-scale Venturi Bubble Generator. Chem. Eng. J. 2020, 386, 120980. [Google Scholar] [CrossRef]
- Ulatowski, K.; Sobieszuk, P. Gas nanobubble dispersions as the important agent in Environmental Processes–Generation Methods Review. Water Environ. J. 2020, 34, 772–790. [Google Scholar] [CrossRef]
- Kukizaki, M.; Goto, M. Size control of nanobubbles generated from Shirasu-porous-glass (SPG) membranes. J. Membr. Sci. 2006, 281, 386–396. [Google Scholar] [CrossRef]
- Xu, J.; Salari, A.; Wang, Y.; He, X.; Kerr, L.; Darbandi, A.; de Leon, A.C.; Exner, A.A.; Kolios, M.C.; Yuen, D.; et al. Microfluidic generation of monodisperse nanobubbles by selective gas dissolution. Small 2021, 17, 2100345. [Google Scholar] [CrossRef]
- Alam, H.S.; Sutikno, P.; Soelaiman, T.A.; Sugiarto, A.T. Bulk nanobubbles: Generation using a two-chamber swirling flow nozzle and long-term stability in water. J. Flow Chem. 2021, 12, 161–173. [Google Scholar] [CrossRef]
- Wu, M.; Song, H.; Liang, X.; Huang, N.; Li, X. Generation of micro-nano bubbles by self-developed Swirl-Type micro-nano Bubble generator. Chem. Eng. Process.-Process Intensif. 2022, 181, 109136. [Google Scholar] [CrossRef]
- Temesgen, T.; Bui, T.T.; Han, M.; Kim, T.; Park, H. Micro and nanobubble technologies as a new horizon for water-treatment techniques: A Review. Adv. Colloid Interface Sci. 2017, 246, 40–51. [Google Scholar] [CrossRef]
- Khuntia, S.; Majumder, S.K.; Ghosh, P. Microbubble-aided water and wastewater purification: A Review. Rev. Chem. Eng. 2012, 28, 191–221. [Google Scholar] [CrossRef]
- Kaushik, G.; Chel, A. Microbubble technology: Emerging field for water treatment. Bubble Sci. Eng. Technol. 2014, 5, 33–38. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, G. Mass transfer of nanobubble aeration and its effect on biofilm growth: Microbial Activity and Structural Properties. Sci. Total Environ. 2020, 703, 134976. [Google Scholar] [CrossRef]
- Etchepare, R.; Oliveira, H.; Azevedo, A.; Rubio, J. Separation of emulsified crude oil in saline water by dissolved air flotation with Micro and nanobubbles. Sep. Purif. Technol. 2017, 186, 326–332. [Google Scholar] [CrossRef]
- Dayarathne, H.N.P.; Choi, J.; Jang, A. Enhancement of cleaning-in-place (CIP) of a reverse osmosis desalination process with air micro-nano bubbles. Desalination 2017, 422, 1–4. [Google Scholar] [CrossRef]
- Achar, J.C.; Nam, G.; Jung, J.; Klammler, H.; Mohamed, M.M. Microbubble ozonation of the antioxidant butylated hydroxytoluene: Degradation kinetics and toxicity reduction. Environ. Res. 2020, 186, 109496. [Google Scholar] [CrossRef]
- Kim, M.S.; Han, M.; Kim, T.I.; Lee, J.W.; Kwak, D.H. Effect of nanobubbles for improvement of water quality in freshwater: Flotation model simulation. Sep. Purif. Technol. 2020, 241, 116731. [Google Scholar] [CrossRef]
- Nazari, S.; Shafaei, S.Z.; Shahbazi, B.; Chehreh Chelgani, S. Study relationships between flotation variables and recovery of coarse particles in the absence and presence of nanobubble. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 284–288. [Google Scholar] [CrossRef]
- Fonseca, R.R.; Thompson, J.P.; Franco, I.C.; da Silva, F.V. Automation and control of a dissolved air flotation pilot plant. IFACPapersOnLine 2017, 50, 3911–3916. [Google Scholar] [CrossRef]
- Saththasivam, J.; Loganathan, K.; Sarp, S. An overview of oil–water separation using gas flotation systems. Chemosphere 2016, 144, 671–680. [Google Scholar] [CrossRef]
- Prakash, R.; Majumder, S.K.; Singh, A. Flotation technique: Its mechanisms and design parameters. Chem. Eng. Process 2018, 127, 249–270. [Google Scholar] [CrossRef]
- Lee, J.; Cho, W.C.; Poo, K.M.; Choi, S.; Kim, T.N.; Son, E.B.; Choi, Y.J.; Kim, Y.M.; Chae, K.J. Refractory oil wastewater treatment by dissolved air flotation, electrochemical advanced oxidation process, and magnetic biochar integrated system. J. Water Process Eng. 2020, 36, 101358. [Google Scholar] [CrossRef]
- Xiao, W.; Ke, S.; Quan, N.; Zhou, L.; Wang, J.; Zhang, L.; Dong, Y.; Qin, W.; Qiu, G.; Hu, J. The role of nanobubbles in the precipitation and recovery of organic-phosphine-containing beneficiation wastewater. Langmuir 2018, 34, 6217–6224. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, J.; Remiszewska-Skwarek, A.; Duda, S.; Łagód, G. Aeration process in bioreactors as the main energy consumer in a wastewater treatment plant. Review of solutions and methods of process optimization. Processes 2019, 7, 311. [Google Scholar] [CrossRef]
- Huggins, T.; Fallgren, P.H.; Jin, S.; Ren, Z.J. Energy and performance comparison of microbial fuel cell and conventional aeration treating of wastewater. J. Microb. Biochem. Technol. 2013, S6, 2. [Google Scholar] [CrossRef]
- Leyva, M.; Valverde Flores, J. Reduction of COD and TSS of waste effluents from a sugar industry through the use of air micro-nanobubbles. J. Nanotechnol. 2018, 2, 7. [Google Scholar] [CrossRef]
- Reyes, R.; Valverde Flores, J. Efficiency Of Micro-Nanobubbles for Wastewater Treatment in Puerto Bermúdez, Oxapampa, Pasco. J. Nanotechnol. 2017, 1, 18–24. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L. Research on the nitrogen removal efficiency and mechanism of deep subsurface wastewater infiltration systems by fine bubble aeration. Ecol. Eng. 2017, 107, 33–40. [Google Scholar] [CrossRef]
- Yao, K.; Chi, Y.; Wang, F.; Yan, J.; Ni, M.; Cen, K. The effect of microbubbles on gas-liquid mass transfer coefficient and degradation rate of cod in wastewater treatment. Water Sci. Technol. 2016, 73, 1969–1977. [Google Scholar] [CrossRef]
- Dayarathne, H.N.P.; Jeong, S.; Jang, A. Chemical-free scale inhibition method for seawater reverse osmosis membrane process: Air micro-nano bubbles. Desalination 2019, 461, 1–9. [Google Scholar] [CrossRef]
- Ghadimkhani, A.; Zhang, W.; Marhaba, T. Ceramic membrane defouling (cleaning) by Air Nano bubbles. Chemosphere 2016, 146, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Hung, I. Ultrafine Bubble-Enhanced Ozonation for Water Treatment. Master’s Thesis, The University of Arizona, Tucson, AZ, USA, 2016. [Google Scholar]
- Andinet, T.; Kim, I.; Lee, J.Y. Effect of microbubble generator operating parameters on oxygen transfer efficiency in water. Desalination Water Treat. 2016, 57, 26327–26335. [Google Scholar] [CrossRef]
- Xia, Z.; Hu, L. Treatment of organics contaminated wastewater by ozone micro-nano-bubbles. Water 2018, 11, 55. [Google Scholar] [CrossRef]
- Menendez, D.; Valverde Flores, J. Reduction of hospital wastewater through ozone-air micro-nanobubbles. J. Nanotechnol. 2018, 1, 59. [Google Scholar] [CrossRef]
- Jabesa, A.; Ghosh, P. Removal of diethyl phthalate from water by ozone microbubbles in a pilot plant. J. Environ. Manag. 2016, 180, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Wang, Q.; Zhang, T.; Shi, Z.; Tian, Y.; Shi, S.; Smale, N.; Wang, J. Microbubble enhanced ozonation process for advanced treatment of wastewater produced in acrylic fiber manufacturing industry. J. Hazard. Mater. 2015, 287, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.B.; Xing, X.H.; Yu, A.F.; Sun, X.L.; Jurcik, B. Enhanced treatment of practical textile wastewater by microbubble ozonation. Process Saf. Environ. Prot. 2008, 86, 389–393. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.; Cen, W.; Lyu, T.; Jarvis, P.; Zhang, Y.; Zhang, Y.; Han, Y.; Wang, L.; Pan, G.; et al. Exploring a chemical input free advanced oxidation process based on nanobubble technology to treat organic micropollutants. Environ. Pollut. 2024, 340, 122877. [Google Scholar] [CrossRef]
- Wang, T.; Yang, C.; Sun, P.; Wang, M.; Lin, F.; Fiallos, M.; Khu, S.T. Generation Mechanism of Hydroxyl Free Radicals in Micro–Nanobubbles Water and Its Prospect in Drinking Water. Processes 2024, 12, 683. [Google Scholar] [CrossRef]
- Oktafani, B.; Siami, L.; Hadisoebroto, R.; Tazkiaturrizki, T.; Ratnaningsih, R. The effect of aeration time on chicken slaughterhouse water treatment using gas-SBR. J. Phys. Conf. Ser. 2019, 1402, 033011. [Google Scholar] [CrossRef]
- Khuntia, S.; Majumder, S.K.; Ghosh, P. Quantitative prediction of generation of hydroxyl radicals from ozone microbubbles. Chem. Eng. Res. Des. 2015, 98, 231–239. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Lv, Y.; Wang, S.; Zheng, S.; Du, H.; Zhang, Y. Effect of microbubble diameter, alkaline concentration and temperature on reactive oxygen species concentration. J. Chem. Technol. Biotechnol. 2017, 92, 1738–1745. [Google Scholar] [CrossRef]
- Gutu, L.; Basitere, M.; Harding, T.; Ikumi, D.; Njoya, M.; Gaszynski, C. Multi-Integrated Systems for Treatment of Abattoir Wastewater: A Review. Water 2021, 13, 2462. [Google Scholar] [CrossRef]
- Bustillo-Lecompte, C.; Mehrvar, M.; Quiñones-Bolaños, E. Slaughterhouse wastewater characterisation and treatment: An economic and public health necessity of the meat processing industry in Ontario, Canada. J. Geosci. Environ. Prot. 2016, 4, 175–186. [Google Scholar]
- Ahmadi, M.; Doroodmand, M.M.; Nabi Bidhendi, G.; Torabian, A.; Mehrdadi, N. Efficient Wastewater Treatment via Aeration Through a Novel Nanobubble System in Sequence Batch Reactors. Front. Energy Res. 2022, 10, 884353. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Xu, M.; Cong, S.; Liu, M.; Zou, D. Hydroxylamine facilitated heterogeneous fenton-like reaction by nano micro-electrolysis material for rhodamine B degradation. J. Clean. Prod. 2021, 316, 128136. [Google Scholar] [CrossRef]
- Cao, W.; Mehrvar, M. Slaughterhouse wastewater treatment by combined anaerobic baffled reactor and UV/H2O2 processes. Chem. Eng. Res. Des. 2011, 89, 1136–1143. [Google Scholar] [CrossRef]
- Musa, M.A.; Idrus, S. Physical and biological treatment technologies of slaughterhouse wastewater: A review. Sustainability 2021, 13, 4656. [Google Scholar] [CrossRef]
- Rusten, B.; Rathnaweera, S.S.; Rismyhr, E.; Sahu, A.K.; Ntiako, J. Rotating belt sieves for primary treatment, chemically enhanced primary treatment and secondary solids separation. Water Sci. Technol. 2017, 75, 2598–2606. [Google Scholar] [CrossRef]
- Bhatia, R.K.; Sakhuja, D.; Mundhe, S.; Walia, A. Renewable Energy Products through Bioremediation of Wastewater. Sustainability 2020, 12, 7501. [Google Scholar] [CrossRef]
- De Nardi, I.R.; Del Nery, V.; Amorim, A.K.B.; Dos Santos, N.G.; Chimenes, F. Performances of SBR, chemical-DAF and UV disinfection for poultry slaughterhouse wastewater reclamation. Desalination 2011, 269, 184–189. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation-Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Fotso, S.E. Development of a Dynamic Simulation Model for Equalization Tanks. Master’s Thesis, Faculty of Engineering and the Built Environment, University of Cape Town, Western Cape, South Africa, 2021. [Google Scholar]
- Gidstedt, S.; Betsholtz, A.; Cimbritz, M.; Davidsson, Å.; Hagman, M.; Karlsson, S.; Takman, M.; Svahn, O.; Micolucci, F. Chemically enhanced primary treatment, microsieving, direct membrane filtration and GAC filtration of municipal wastewater: A pilot-scale study. Environ. Technol. 2022, 45, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Philipp, M.; Masmoudi Jabri, K.; Wellmann, J.; Akrout, H.; Bousselmi, L.; Geißen, S.U. Slaughterhouse Wastewater Treatment: A Review on Recycling and Reuse Possibilities. Water 2021, 13, 3175. [Google Scholar] [CrossRef]
- Aziz, A.; Basheer, F.; Sengar, A.; Irfanullah, A.; Khan, S.U.; Farooqi, I.H. Biological wastewater treatment (anaerobic-aerobic) technologies for safe discharge of treated slaughterhouse and meat processing wastewater. Sci. Total Environ. 2019, 686, 681–708. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.; Idrus, S.; Hasfalina, C.; Daud, N. Effect of Organic Loading Rate on Anaerobic Digestion Performance of Mesophilic (UASB) Reactor Using Cattle SlaughterhouseWastewater as Substrate. Int. J. Environ. Res. Public Health 2018, 15, 2220. [Google Scholar] [CrossRef] [PubMed]
- Loganath, R.; Mazumder, D. Performance study on organic carbon, total nitrogen, suspended solids removal and biogas production in hybrid UASB reactor treating real slaughterhouse wastewater. J. Environ. Chem. Eng. 2018, 6, 3474–3484. [Google Scholar] [CrossRef]
- Afridi, Z.U.R.; Jing, W.; Younas, H. Biogas production and fundamental mass transfer mechanism in anaerobic granular sludge. Sustainability 2019, 11, 4443. [Google Scholar] [CrossRef]
- Njoya, M.; Basitere, M.; Ntwampe, S.K.O.; Lim, J.W. Performance evaluation and kinetic modeling of down-flow high-rateanaerobic bioreactors for poultry slaughterhouse wastewater treatment. Environ. Sci. Pollut. Res. 2021, 28, 9529–9541. [Google Scholar] [CrossRef]
- Rinquest, Z.; Basitere, M.; Ntwampe, S.K.O.; Njoya, M. Poultry slaughterhouse wastewater treatment using a static granular bed reactor coupled with single stage nitrification-denitrification and ultrafiltration systems. J. Water Process Eng. 2019, 29, 100778. [Google Scholar] [CrossRef]
- Meyo, H.B.; Njoya, M.; Basitere, M.; Ntwampe, S.K.O.; Kaskote, E. Treatment of poultry slaughterhouse wastewater (PSW) using a pretreatment stage, an expanded granular sludge bed reactor (EGSB), and a membrane bioreactor (MBR). Membranes 2021, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Zhao, J.; Lei, Z.; Shimizu, K.; Zhang, Z. Enhanced energy recovery via separate hydrogen and methane production from two-stage anaerobic digestion of food waste with nanobubble water supplementation. Sci. Total Environ. 2021, 761, 143234. [Google Scholar] [CrossRef]
- Fan, Y.; Lei, Z.; Guo, Z.; Huang, W.; Wang, D.; Wang, X.; Zhang, Z.; Shimizu, K. Enhanced solubilization of solid organics and methane production by anaerobic digestion of swine manure under nano-bubble water addition. Bioresour. Technol. 2020, 299, 122512. [Google Scholar] [CrossRef]
- Fan, Y.; Lei, Z.; Yang, X.; Kobayashi, M.; Adachi, Y.; Zhang, Z.; Shimizu, K. Effect of nano-bubble water on high solid anaerobic digestion of pig manure: Focus on digestion stability, methanogenesis performance and related mechanisms. Bioresour. Technol. 2020, 315, 123793. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Nie, J.; Wang, D.; Zhao, Z.; Kobayashi, M.; Adachi, Y.; Shimizu, K.; Lei, Z.; Zhang, Z. Enhanced hydrolysis of waste activated sludge for methane production via anaerobic digestion under N2-nanobubble water addition. Sci. Total Environ. 2019, 693, 133524. [Google Scholar] [CrossRef]
- Yang, X.; Nie, J.; Wei, Y.; Zhao, Z.; Shimizu, K.; Lei, Z.; Zhang, Z. Simultaneous enhancement on lignin degradation and methane production from anaerobic co-digestion of waste activated sludge and alkaline lignin supplemented with N2- nanobubble water. Bioresour. Technol. Rep. 2020, 11, 100470. [Google Scholar] [CrossRef]
- Wang, X.; Lei, Z.; Shimizu, K.; Zhang, Z.; Lee, D.J. Improved methane production from corn straw using anaerobically digested sludge pre-augmented by nanobubble water. Bioresour. Technol. 2020, 311, 123479. [Google Scholar] [CrossRef]
- Nguyen, D.; Khanal, S.K. A little breath of fresh air into an anaerobic system: How microaeration facilitates anaerobic digestion process. Biotechnol. Adv. 2018, 36, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Wu, Z.; Shrestha, S.; Lee, P.H.; Raskin, L.; Khanal, S.K. Intermittent micro-aeration: New strategy to control volatile fatty acid accumulation in high organic loading anaerobic digestion. Water Res. 2019, 166, 115080. [Google Scholar] [CrossRef]
- Hamawand, I.; Ghadouani, A.; Bundschuh, J.; Hamawand, S.; Al Juboori, R.A.; Chakrabarty, S.; Yusaf, T. A critical review on processes and energy profile of the Australian Meat Processing Industry. Energies 2017, 10, 731. [Google Scholar] [CrossRef]
- Svierzoski, N.D.S.; Matheus, M.C.; Bassin, J.P.; Brito, Y.D.; Mahler, C.F.; Webler, A.D. Treatment of a slaughterhouse wastewater by anoxic-aerobic biological reactors followed by UV-C disinfection and microalgae bioremediation. Water Environ. Res. 2020, 93, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Palomares-Rodríguez, C.; Martínez-Guido, S.I.; Apolinar-Cortés, J.; del Carmen Chávez-Parga, M.; García-Castillo, C.C.; Ponce-Ortega, J.M. Environmental, technical, and economic evaluation of a new treatment for wastewater from slaughterhouses. Int. J. Environ. Res. 2017, 11, 535–545. [Google Scholar] [CrossRef]
- Affes, M.; Aloui, F.; Hadrich, F.; Loukil, S.; Sayadi, S. Effect of bacterial lipase on anaerobic co-digestion of slaughterhouse wastewater and grease in batch condition and continuous fixed-bed reactor. Lipids Health Dis. 2017, 16, 195. [Google Scholar] [CrossRef] [PubMed]
- Jamie, A.; Alshami, A.S.; Maliabari, Z.O.; Ali Ateih, M.; Al Hamouz, O.C.S. Immobilization and enhanced catalytic activity of lipase on modified MWCNT for oily wastewater treatment. Environ. Prog. Sustain. Energy 2016, 35, 1441–1449. [Google Scholar] [CrossRef]
- Ergofito. Ecoflush Eliminated Ammonia. Ecoflush Eliminated Odours. Available online: https://www.ergofito.co.za/home (accessed on 16 February 2024).
- Mdladla, C.T.; Dyosile, P.A.; Njoya, M.; Basitere, M.; Ntwampe, S.K.O.; Kaskote, E. Poultry Slaughterhouse Wastewater Remediation Using a Bio-Delipidation Pre-Treatment Unit Coupled with an Expanded Granular Sludge Bed Reactor. Processes 2021, 9, 1938. [Google Scholar] [CrossRef]
- Dyosile, P.A.; Mdladla, C.; Njoya, M.; Basitere, M.; Ntwampe, S.K.O.; Kaskote, E. Assessment of an Integrated and Sustainable Multistage System for the Treatment of Poultry Slaughterhouse Wastewater. Membranes 2021, 11, 582. [Google Scholar] [CrossRef]
- Koide, R.T.; Nguyen, B.T.; Howard Skinner, R.; Dell, C.J.; Adler, P.R.; Drohan, P.J.; Licht, M.; Matthews, M.B.; Nettles, R.; Ricks, K.; et al. Comparing Biochar Application Methods for Switchgrass Yield and C Sequestration on Contrasting Marginal Lands in Pennsylvania, USA. Bioenergy Res. 2018, 11, 784–802. [Google Scholar] [CrossRef]

| Parameter | Significance | PSW | General Discharge Limits as Set in the National Water Act 36 of 1998 |
|---|---|---|---|
| pH at 25 °C | Measure of acidity and basicity | 6.3–7.3 | 5.5–7.5 |
| COD (mg/L) | Organic substrate for microbial growth | 5126 ± 2534 | 75 |
| TSSs (mg/L) | Measure of particles in wastewater | 1654 ± 1695 | 25 |
| FOG (mg/L) | 715 ± 506 | 2.5 | |
| Ammonium as N (mg/L) | Nutrient source for irrigation | 216 ± 56 | 6 |
| Nitrates as N (mg/L) | Nutrient source for irrigation | 3.33–4.45 | 15 |
| Nitrites as N (mg/L) | Nutrient source for irrigation | - | 15 |
| Total phosphates as P (mg/L) | Nutrient source for irrigation | - | 10 |
| Bubble Property | Macrobubbles | Microbubbles | Nanobubbles |
|---|---|---|---|
| Zeta potential | Low | High | Higher |
| Free radicals | Low | High | Higher |
| Mass transfer efficiency | Low | High | Higher |
| Bubble stability | Unstable | Stable | Stable |
| Rising velocity | Fast | Slow | Slower |
| Rising time | Short | Long | Very long |
| Oxygen transfer process | Inefficient | Efficient | Efficient |
| Internal pressure | Low | High | Higher |
| Method | Principle | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Mechanical stirring | Iterative rotational stirring facilitates bubble formation due to shear forces and turbulence. | Rapid generation; stability for an extended period. | Limited control over size distribution. | [27] |
| Venturi-based | Utilizes converging and diverging flow to induce pressure changes, leading to bubble formation. | Simple design; controllable bubble size with divergent angle and liquid flow rate. | Limited uniformity in bubble size. | [28,29] |
| Porous membrane | Compressed gas is introduced through membrane pores into a liquid phase, generating bubbles on the membrane surface. | Controlled bubble size by adjusting membrane pore size and liquid flow velocity. | The influence of membrane properties on bubble size needs consideration. | [30] |
| Acoustic cavitation | Induces local negative pressure in liquid through high-speed propeller rotation or high-intensity sound waves, forming micro- and nano-scale bubbles. | High energy efficiency; scalability. | Potential for bubble coalescence and fusion; sensitivity to organic solvents. | [31,32] |
| Microfluidics-based | Regulates the flow of mixed gas and liquid in microfluidic chips, resulting in the formation of MBs that evolve into NBs. | Precise control over size and uniformity; adjustable by gas ratio. | Requires specialized equipment; complexity in setup. | [33] |
| Hydrodynamic cavitation | Alters flow velocity to induce cavitation, causing pressure fluctuations and generating NBs. | High energy efficiency, low cost, and scalability. | Requires optimization for specific applications. | [34] |
| Treatment Method | Description | Advantages | Disadvantages | References |
|---|---|---|---|---|
| DAF | Introduction of air to facilitate the separation of FOG and solid materials from wastewater. | 75% removal for FOG, BOD, and TSSs. | High operational and maintenance costs. | [82,85] |
| Chemical Coagulation/flocculation | Addition of chemicals to induce particle aggregation for easier removal. | Effective in treating colloidal and fine particles. | Chemical cost and sludge generation. | [86] |
| Equalization tanks | Balancing and smoothing flow variations and pollutant concentrations before entering treatment processes. | Reduces shock loads to downstream processes. | Requires additional space and monitoring. | [87] |
| Primary filtration | Physical filtration of suspended solids using barriers like sand or cloth. | Effective for fine particle removal. | Regular maintenance and clogging issues. | [88] |
| Anaerobic Digester | Achievement | Advantages | Disadvantages | References |
|---|---|---|---|---|
| DEGBR and SGBR | Attained a 95% reduction in BOD5, COD, and FOG on days of optimal performance for both reactors. | The DEGBR consistently exhibited more substantial biogas production compared to the SGBR. | The SGBR required over 50 days to achieve a 95% removal of FOG, while the DEGBR accomplished this in 14 days. | [94] |
| UASB | Approximately 90% COD removal was achieved at an organic loading rate (OLR) of 0.4 g/L/d, resulting in a biogas production of 5 L/d. | VFA concentration remained low, and HRT of 1 day proved effective in removing more than 70% of COD. | COD removal decreased to less than 50% with an increase in the loading rate to 15 g/L/d. | [91] |
| SGBR is integrated with a single-stage nitrification–denitrification (SND) bioreactor and an ultrafiltration membrane | Average removal efficiencies of 91% for COD, 51% for orthophosphate, 97% for TSSs, and 52% for TDS were attained over a 52-day period. | ufMMs operated in the dead-end filtration mode demonstrated an additional reduction of 65% for COD and 54% for TSSs on average. | The final effluent did not meet the standards for industrial wastewater for PO43− and NH4+-N. | [95] |
| EGSB coupled with a membrane bioreactor (MBR) | Overall system efficiency exceeded 97% for TSSs and COD removal and 97.5% removal efficiency for FOG. | The EGSB’s performance was not affected by varied organic loading rates (OLRs), emphasizing its robustness under different conditions. | FOG removal fluctuated and did not show a consistent improvement | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaskote, E.; Basitere, M.; Mshayisa, V.V.; Sheldon, M.S. Systematic Review of Poultry Slaughterhouse Wastewater Treatment: Unveiling the Potential of Nanobubble Technology. Water 2024, 16, 1933. https://doi.org/10.3390/w16131933
Kaskote E, Basitere M, Mshayisa VV, Sheldon MS. Systematic Review of Poultry Slaughterhouse Wastewater Treatment: Unveiling the Potential of Nanobubble Technology. Water. 2024; 16(13):1933. https://doi.org/10.3390/w16131933
Chicago/Turabian StyleKaskote, Ephraim, Moses Basitere, Vusi Vincent Mshayisa, and Marshall Sheerene Sheldon. 2024. "Systematic Review of Poultry Slaughterhouse Wastewater Treatment: Unveiling the Potential of Nanobubble Technology" Water 16, no. 13: 1933. https://doi.org/10.3390/w16131933
APA StyleKaskote, E., Basitere, M., Mshayisa, V. V., & Sheldon, M. S. (2024). Systematic Review of Poultry Slaughterhouse Wastewater Treatment: Unveiling the Potential of Nanobubble Technology. Water, 16(13), 1933. https://doi.org/10.3390/w16131933









