A Systematic Literature Review for Addressing Microplastic Fibre Pollution: Urgency and Opportunities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Methodology
3. Results
3.1. Publication Profile
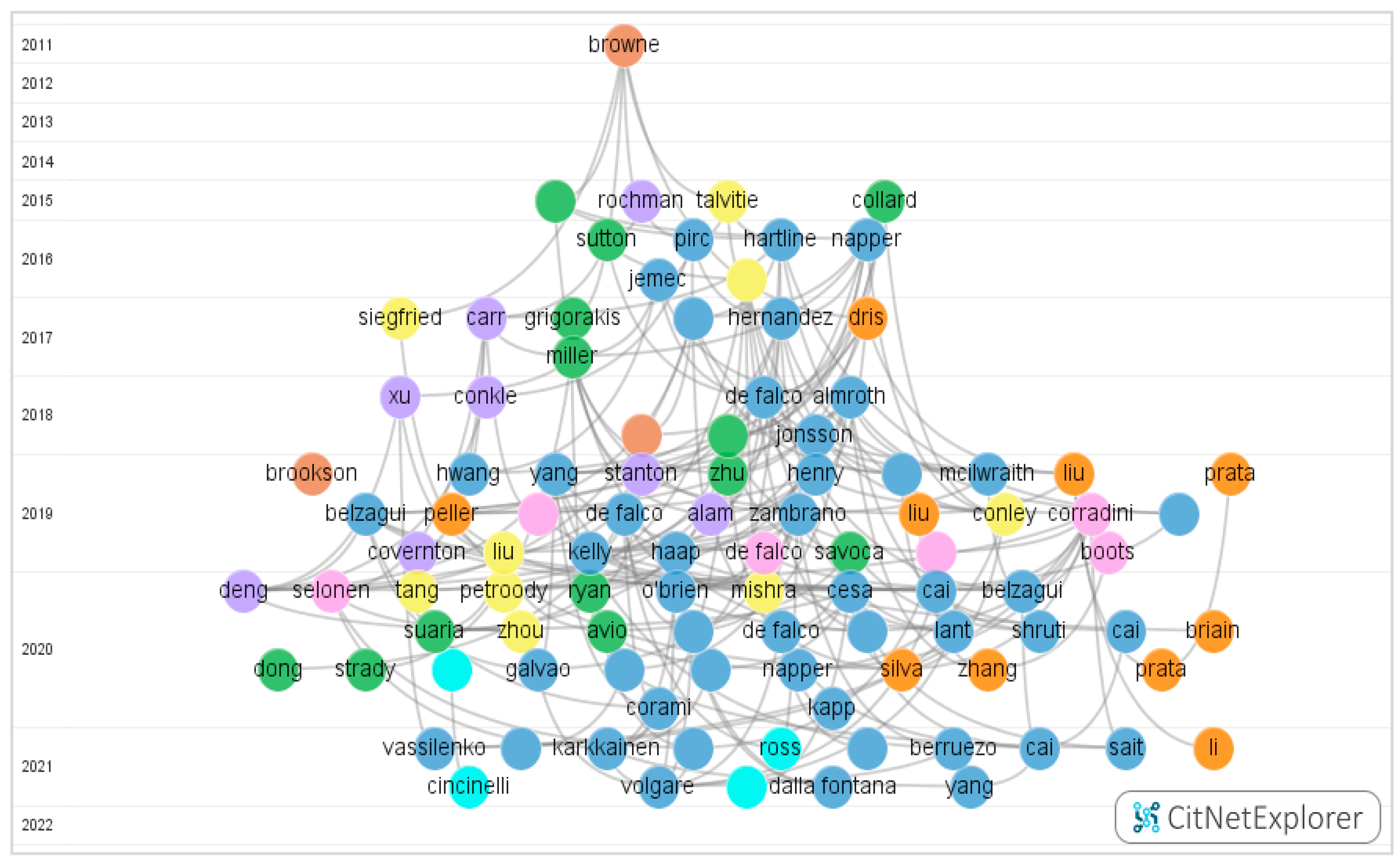
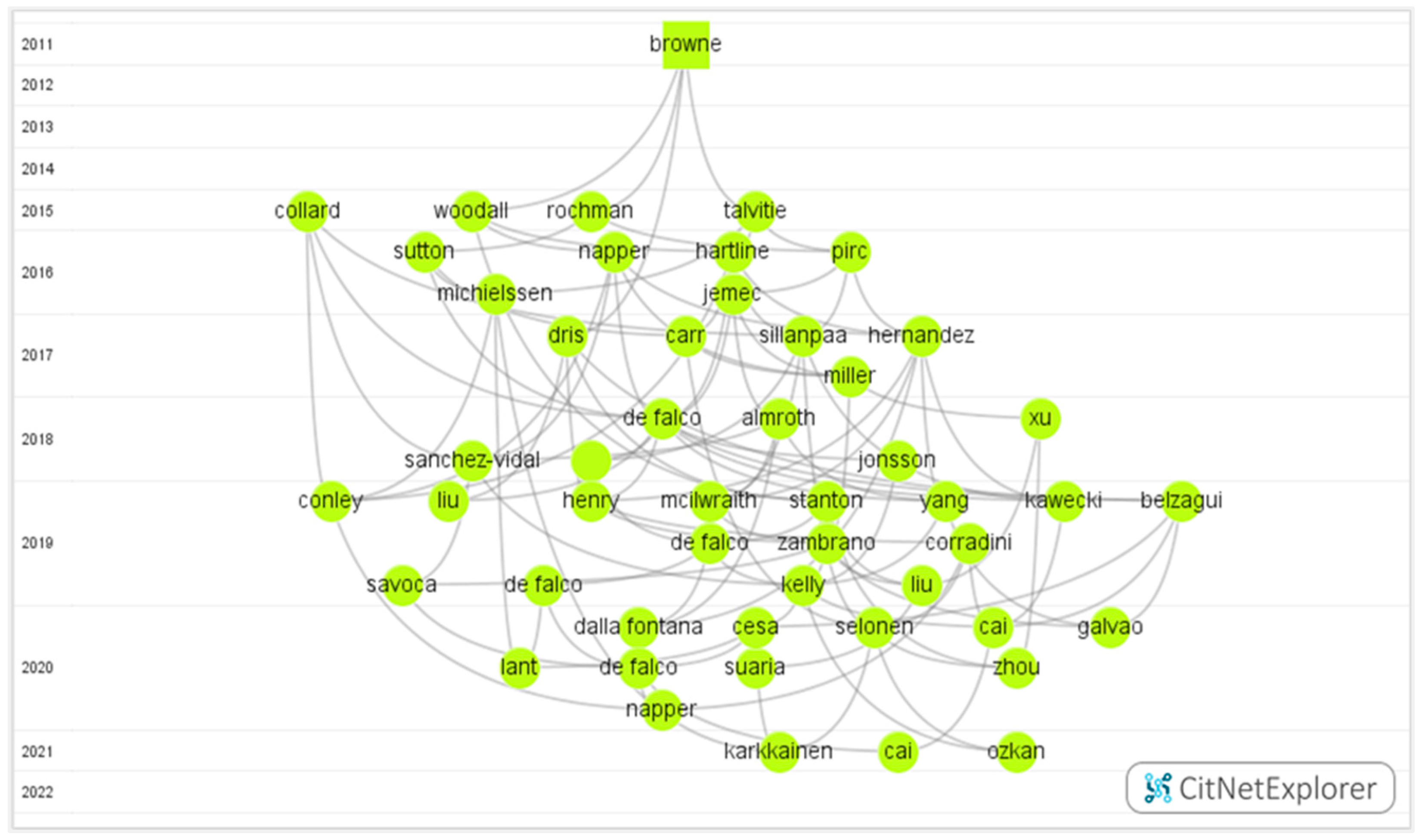
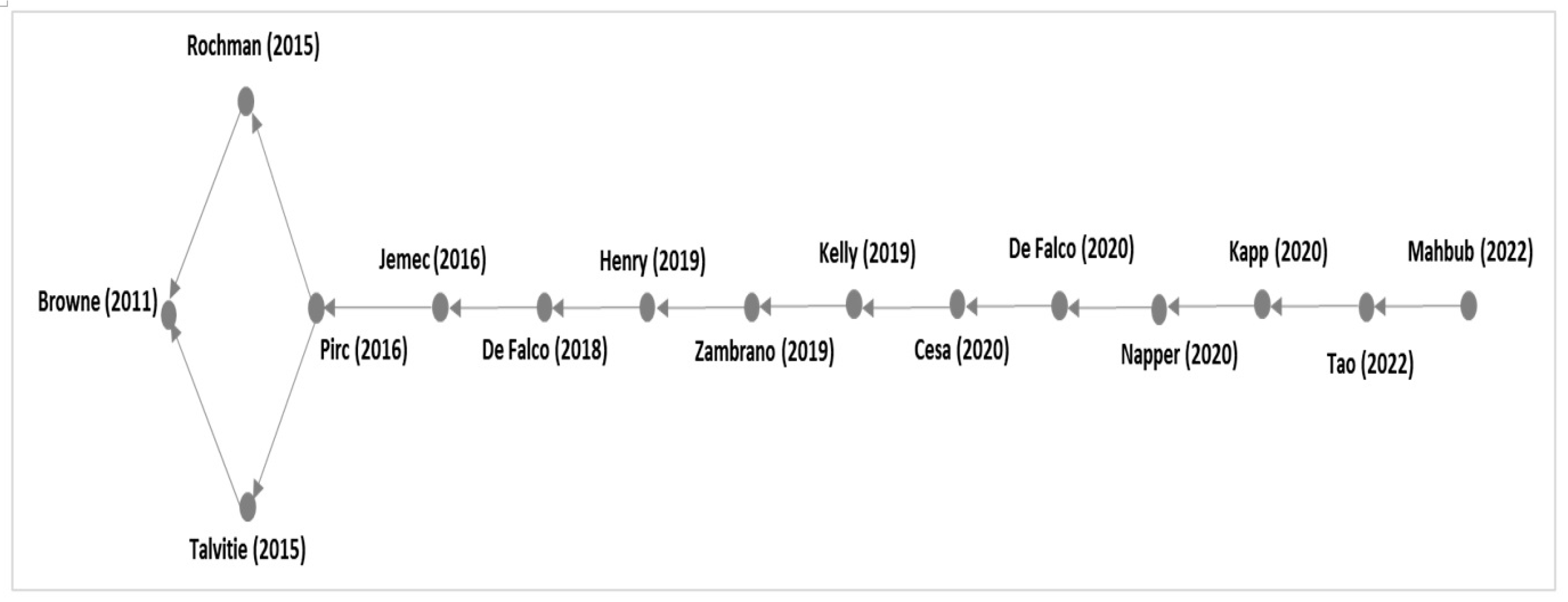
3.2. Citation Network
3.3. Connection between Keywords
3.4. Summary of Each Research Domain
3.4.1. Domestic Laundry and Drying
| Parameters | Articles | Effect on MPF/MF Release |
|---|---|---|
| Textile Parameters | ||
| Structure | [62] | Increase with loose construction |
| [55] | Reduce with compact to loose structure | |
| [63] | Reduce as interlacing coefficient and weft density increase | |
| Composition | [64] | Recycled polyester > virgin polyester |
| [65] | Acrylic > polyester > nylon | |
| Spinning method | [66] | Ring > rotor or air-jet |
| Yarn twist | [62] | Reduce with higher twist |
| [67] | Spun > non-twist filament > hard-twist filament | |
| [55] | Reduce with a higher twist | |
| Fibre length | [55] | Reduce with continuous filament over short staples |
| [53] | Increased release with shorter irregular fibres | |
| [68] | Reduce from staple to textured filament. | |
| Finishing | [47] | Reduce with a pectin-based finish |
| [66] | The processed surface can produce five times more | |
| Cutting | [66] | Scissor-cut 3–31 times higher than laser-cut |
| Washing and Drying Parameters | ||
| Machine type | [59] | The top load releases seven times more than the front load |
| Subsequent washes | [52] | Successive washes decrease emissions |
| [69] | Reduce and typically stabilise from the 4th and 5th cycle | |
| [61] | Reduce after 4 cycles | |
| [53] | Reduce after the peak at 3rd cycle | |
| [17] | Reduce and stabilise from 5th cycle | |
| [67] | Reduce | |
| [68] | Reduce significantly from 5th cycle | |
| [70] | Reduce and stabilise at the 7th cycle | |
| Water volume-to-fabric ratio/washing load decrease | [61] | Increase as the most influential factor |
| [45] | Increase by five times | |
| [60] | Increase as the most influential factor | |
| Washing temperature | [46] | Increase with temperature |
| [61] | No significant effect between 15 and 30 °C and increase at 60 °C | |
| [71] | Increase with temperature | |
| [70] | 1.8 times more if the temperature is increased from 20 to 40 °C | |
| Washing and drying time | [61] | No impact if the increase is from 15 to 60 min |
| [71] | Increase with duration and spin speed | |
| [70] | Increase if duration increases from 30 to 60 min | |
| Using detergent and softener | [52] | Reduce (both detergent and softener) |
| [72] | Reduce (softener only) | |
| [46] | Increase (detergent only) | |
| [61] | No effect (detergent only) | |
| [53] | Reduce (detergent only) | |
| [73] | No significant impact (both detergent and softener) | |
| [71] | Increase (detergent and conditioner) | |
| [74] | Reduce (softener only) | |
| [70] | Increase (detergent only) | |
3.4.2. Test Methodology
3.4.3. Aquatic Ecosystem
3.4.4. Atmospheric Environment
3.4.5. Wastewater Source
3.4.6. Abundance and Distribution
3.4.7. Terrestrial Ecosystem
3.4.8. Hazardous Risk
4. Opportunities
4.1. Interdisciplinary Collaboration
4.2. Textile Parameters
4.3. Laundry Parameters
4.4. Sustainable Chemicals
4.5. Renewable Materials and Circularity
4.6. Wastewater Treatment
4.7. Mitigation Devices
4.8. Standardised Test Method
4.9. Government Interventions
5. Conclusions
5.1. Limitations
5.2. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Cesa, F.S.; Turra, A.; Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017, 598, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Marín, A.V.; Tausif, M. Fragmented fibre (including microplastic) pollution from textiles. Text. Prog. 2021, 53, 123–182. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Tehrani-Bagha, A. A review on microplastic emission from textile materials and its reduction techniques. Polym. Degrad. Stab. 2022, 199, 109901. [Google Scholar] [CrossRef]
- Rathinamoorthy, R.; Balasaraswathi, S.R. Microfiber Pollution Prevention—Mitigation Strategies and Challenges. In Sustainable Textiles; Springer Nature: Singapore, 2022; pp. 205–243. [Google Scholar] [CrossRef]
- Textile Exchange. Preferred Fiber & Materials: Market Report 2022. Volume 60. Available online: https://textileexchange.org/knowledge-center/reports/materials-market-report-2022/ (accessed on 22 August 2019).
- Barrows, A.P.W.; Neumann, C.A.; Berger, M.L.; Shaw, S.D. Grab vs. neuston tow net: A microplastic sampling performance comparison and possible advances in the field. Anal. Methods 2017, 9, 1446–1453. [Google Scholar] [CrossRef]
- Decitex Smart Textiles. Microfiber. Available online: https://www.decitex.com/en/microfiber# (accessed on 16 October 2023).
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; Volume 43. [Google Scholar] [CrossRef]
- Iowe, D. A New Textiles Economy: Redesigning Fashion’s Future; Ellen MacArthur Foundation: Isle of Wight, UK, 2017; pp. 1–150. [Google Scholar]
- Lv, M.; Jiang, B.; Xing, Y.; Ya, H.; Zhang, T.; Wang, X. Recent advances in the breakdown of microplastics: Strategies and future prospectives. Environ. Sci. Pollut. Res. Int. 2022, 29, 65887–65903. [Google Scholar] [CrossRef] [PubMed]
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.J.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.E.; Thompson, R.C. The Deep Sea Is a Major Sink for Microplastic Debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.; Laitala, K.; Klepp, I.G. Microplastic Pollution from Textiles: A Literature Review. 2018. Available online: https://oda.oslomet.no/oda-xmlui/bitstream/handle/20.500.12199/5360/OR1%20-%20Microplastic%20pollution%20from%20textiles%20-%20A%20literature%20review.pdf (accessed on 10 February 2023).
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and Fate of Microplastic Particles in Wastewater Treatment Plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Tao, D.; Zhang, K.; Xu, S.; Lin, H.; Liu, Y.; Kang, J.; Yim, T.; Giesy, J.P.; Leung, K.M.Y.Y. Microfibers Released into the Air from a Household Tumble Dryer. Environ. Sci. Technol. Lett. 2022, 9, 120–126. [Google Scholar] [CrossRef]
- O’Brien, S.; Okoffo, E.D.; O’Brien, J.W.; Ribeiro, F.; Wang, X.; Wright, S.L.; Samanipour, S.; Rauert, C.; Toapanta, T.Y.A.; Albarracin, R.; et al. Airborne Emissions of Microplastic Fibres from Domestic Laundry Dryers. Sci. Total Environ. 2020, 747, 141175. [Google Scholar] [CrossRef]
- Kärkkäinen, N.; Sillanpää, M.; Karkkainen, N.; Sillanpaa, M. Quantification of Different Microplastic Fibres Discharged from Textiles in Machine Wash and Tumble Drying. Environ. Sci. Pollut. Res. 2021, 28, 16253–16263. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric Microplastic Deposition in an Urban Environment and an Evaluation of Transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Castro, J.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.; Cerqueira, M. The Importance of Contamination Control in Airborne Fibers and Microplastic Sampling: Experiences from Indoor and Outdoor Air Sampling in Aveiro, Portugal. Mar. Pollut. Bull. 2020, 159, 111522. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, T.; Yan, M.; Yang, L.; Gong, H.; Wang, W.; Qing, X.; Wang, J. Atmospheric transport and deposition of microplastics in a subtropical urban environment. J. Hazard. Mater. 2021, 416, 126168. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.-P.W.; Galbraith, M.; Ross, P.S. Ingestion of microplastics by zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contam. Toxicol. 2015, 69, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Mizraji, R.; Ahrendt, C.; Perez-Venegas, D.; Vargas, J.; Pulgar, J.; Aldana, M.; Ojeda, F.P.; Duarte, C.; Galbán-Malagón, C.; Patricio Ojeda, F.; et al. Is the Feeding Type Related with the Content of Microplastics in Intertidal Fish Gut? Mar. Pollut. Bull. 2017, 116, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of Airborne Microplastics in Human Lung Tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef] [PubMed]
- Cole, M. A novel method for preparing microplastic fibers. Sci. Rep. 2016, 6, 34519. [Google Scholar] [CrossRef]
- Costa, M.F.; Ivar Do Sul, J.A.; Silva-Cavalcanti, J.S.; Christina, M.; Araújo, B.; Spengler, Â.; Tourinho, P.S. On the Importance of Size of Plastic Fragments and Pellets on the Strandline: A Snapshot of a Brazilian Beach. Environ. Monit. Assess. 2010, 168, 299–304. [Google Scholar] [CrossRef]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment (Part 1). Available online: http://www.gesamp.org/publications/reports-and-studies-no-90 (accessed on 20 February 2023).
- Xu, X.; Hou, Q.; Xue, Y.; Jian, Y.; Wang, L.P. Pollution Characteristics and Fate of Microfibers in the Wastewater from Textile Dyeing Wastewater Treatment Plant. Water Sci. Technol. 2018, 78, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Burke, A.; O’Connor, I.; Officer, R. Microplastic Pollution in the Northeast Atlantic Ocean: Validated and Opportunistic Sampling. Mar. Pollut. Bull. 2014, 88, 325–333. [Google Scholar] [CrossRef] [PubMed]
- AATCC TM212-2021; Test Method for Fiber Fragment Release during Home Laundering. AATCC: Research Triangle Park, NC, USA, 2021.
- ISO/DIS 4484-1; Textiles and Textile Products—Microplastics from Textile Sources—Part 1: Determination of Material Loss from Fabrics during Washing. ISO: Geneva, Switzerland, 2023. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:4484:-1:ed-1:v1:en (accessed on 21 April 2024).
- ISO/DIS 4484-2; Textiles and Textile Products—Microplastics from Textile Sources—Part 2: Qualitative and Quantitative Evaluation of Microplastics. ISO: Geneva, Switzerland, 2023. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:4484:-2:ed-1:v1:en (accessed on 21 April 2024).
- Sanchez-Vidal, A.; Thompson, R.C.; Canals, M.; De Haan, W.P. The Imprint of Microfibres in Southern European Deep Seas. PLoS ONE 2018, 13, e0207033. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Tassin, B. Sources and Fate of Microplastics in Urban Areas: A Focus on Paris Megacity. In Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2018; Volume 58, pp. 69–83. [Google Scholar] [CrossRef]
- Mishra, S.; Rath, C.C.; Das, A.P. Marine Microfiber Pollution: A Review on Present Status and Future Challenges. Mar. Pollut. Bull. 2019, 140, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Brodin, M.; Norin, H.; Hanning, A.-C.; Persson, C.; Okcabol, S. Microplastics from Industrial Laundries—A Laboratory Study of Laundry Effluents. 2018. Available online: http://www.diva-portal.org/smash/get/diva2:1633776/FULLTEXT01.pdf (accessed on 8 July 2019).
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Ballent, A.; Corcoran, P.L.; Madden, O.; Helm, P.A.; Longstaffe, F.J. Sources and Sinks of Microplastics in Canadian Lake Ontario Nearshore, Tributary and Beach Sediments. Mar. Pollut. Bull. 2016, 110, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Vejvar, M.; Lai, K.H.; Lo, C.K.Y. A Citation Network Analysis of Sustainability Development in Liner Shipping Management: A Review of the Literature and Policy Implications. Marit. Policy Manag. 2020, 47, 1–26. [Google Scholar] [CrossRef]
- Colicchia, C.; Strozzi, F. Supply Chain Risk Management: A New Methodology for a Systematic Literature Review. Supply Chain Manag. 2012, 17, 403–418. [Google Scholar] [CrossRef]
- Hummon, N.P.; Dereian, P. Connectivity in a Citation Network: The Development of DNA Theory. Soc. Netw. 1989, 11, 39–63. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Visualizing Bibliometric Networks. In Measuring Scholarly Impact; Springer: Cham, Switzerland, 2014; pp. 285–320. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. CitNetExplorer: A New Software Tool for Analyzing and Visualizing Citation Networks. J. Informetr. 2014, 8, 802–823. [Google Scholar] [CrossRef]
- Mrvar, A.; Batagelj, V. Analysis and Visualization of Large Networks with Program Package Pajek. Complex Adapt. Syst. Model. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Volgare, M.; De Falco, F.; Avolio, R.; Castaldo, R.; Errico, M.E.; Gentile, G.; Ambrogi, V.; Cocca, M. Washing Load Influences the Microplastic Release from Polyester Fabrics by Affecting Wettability and Mechanical Stress. Sci. Rep. 2021, 11, 19479. [Google Scholar] [CrossRef]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Cheng, J.J.; Venditti, R.A. Microfibers Generated from the Laundering of Cotton, Rayon and Polyester Based Fabrics and Their Aquatic Biodegradation. Mar. Pollut. Bull. 2019, 142, 394–407. [Google Scholar] [CrossRef]
- De Falco, F.; Gentile, G.; Avolio, R.; Errico, M.E.; Di Pace, E.; Ambrogi, V.; Avella, M.; Cocca, M. Pectin Based Finishing to Mitigate the Impact of Microplastics Released by Polyamide Fabrics. Carbohydr. Polym. 2018, 198, 175–180. [Google Scholar] [CrossRef]
- Herweyers, L.; Carteny, C.C.; Scheelen, L.; Watts, R.; Du Bois, E. Consumers’ Perceptions and Attitudes toward Products Preventing Microfiber Pollution in Aquatic Environments as a Result of the Domestic Washing of Synthetic Clothes. Sustainability 2020, 12, 2244. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.-C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef]
- Browne, M.A. Sources and Pathways of Microplastics to Habitats. In Marine Anthropogenic Litter; Springer International Publishing: Cham, Switzerland, 2015; Volume 334, pp. 229–244. [Google Scholar] [CrossRef]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.-P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R. Do Wastewater Treatment Plants Act as a Potential Point Source of Microplastics? Preliminary Study in the Coastal Gulf of Finland, Baltic Sea. Water Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [CrossRef]
- Pirc, U.; Vidmar, M.; Mozer, A.; Kržan, A.; Krzan, A.; Kržan, A.; Kr, A. Emissions of Microplastic Fibers from Microfiber Fleece during Domestic Washing. Environ. Sci. Pollut. Res. 2016, 23, 22206–22211. [Google Scholar] [CrossRef] [PubMed]
- Cesa, F.S.; Turra, A.; Checon, H.H.; Leonardi, B.; Baruque-Ramos, J. Laundering and Textile Parameters Influence Fibers Release in Household Washings. Environ. Pollut. 2020, 257, 113553. [Google Scholar] [CrossRef]
- Lykaki, M.; Zhang, Y.Q.; Markiewicz, M.; Brandt, S.; Kolbe, S.; Schrick, J.; Rabe, M.; Stolte, S. The Influence of Textile Finishing Agents on the Biodegradability of Shed Fibres. Green Chem. 2021, 23, 5212–5221. [Google Scholar] [CrossRef]
- De Falco, F.; Cocca, M.; Avella, M.; Thompson, R.C.; De Falco, F.; Cocca, M.; Avella, M.; Thompson, R.C. Microfiber Release to Water, Via Laundering, and to Air, via Everyday Use: A Comparison between Polyester Clothing with Differing Textile Parameters. Environ. Sci. Technol. 2020, 54, 3288–3296. [Google Scholar] [CrossRef] [PubMed]
- Kapp, K.J.; Miller, R.Z. Electric Clothes Dryers: An Underestimated Source of Microfiber Pollution. PLoS ONE 2020, 15, e0239165. [Google Scholar] [CrossRef] [PubMed]
- YarnsandFibers.com. Where Is Polyester Produced in the World? Available online: https://www.yarnsandfibers.com/textile-resources/synthetic-fibers/polyester/polyester-production-raw-materials/where-is-polyester-produced-in-the-world/ (accessed on 12 December 2022).
- Chan, C.K.M.; Park, C.; Chan, K.M.; Mak, D.C.W.; Fang, J.K.H.; Mitrano, D.M. Microplastic Fibre Releases from Industrial Wastewater Effluent: A Textile Wet-Processing Mill in China. Environ. Chem. 2021, 18, 93–100. [Google Scholar] [CrossRef]
- Hartline, N.L.; Bruce, N.J.; Karba, S.N.; Ruff, E.O.; Sonar, S.U.; Holden, P.A. Microfiber Masses Recovered from Conventional Machine Washing of New or Aged Garments. Environ. Sci. Technol. 2016, 50, 11532–11538. [Google Scholar] [CrossRef] [PubMed]
- Rathinamoorthy, R.; Balasaraswathi, S.R. Investigations on the Interactive Effect of Laundry Parameters on Microfiber Release from Polyester Knitted Fabric. Fibers Polym. 2022, 23, 2052–2061. [Google Scholar] [CrossRef]
- Kelly, M.R.; Lant, N.J.; Kurr, M.; Burgess, J.G. Importance of Water-Volume on the Release of Microplastic Fibers from Laundry. Environ. Sci. Technol. 2019, 53, 11735–11744. [Google Scholar] [CrossRef] [PubMed]
- Carney Almroth, B.M.; Åström, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.-K. Quantifying Shedding of Synthetic Fibers from Textiles; A Source of Microplastics Released into the Environment. Environ. Sci. Pollut. Res. 2017, 25, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Berruezo, M.; Bonet-Aracil, M.; Montava, I.; Bou-Belda, E.; Díaz-García, P.; Gisbert-Payá, J.; Gisbert-Paya, J.; Bou-Belda, E.; Díaz-García, P. Preliminary Study of Weave Pattern Influence on Microplastics from Fabric Laundering. Text. Res. J. 2021, 91, 1037–1045. [Google Scholar] [CrossRef]
- Özkan, İ.; Gündoğdu, S. Investigation on the Microfiber Release under Controlled Washings from the Knitted Fabrics Produced by Recycled and Virgin Polyester Yarns. J. Text. Inst. 2021, 112, 264–272. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J.; Kwon, M. The Effect of the Physical and Chemical Properties of Synthetic Fabrics on the Release of Microplastics during Washing and Drying. Polymers 2022, 14(16), 3384. [Google Scholar] [CrossRef]
- Cai, Y.; Mitrano, D.M.; Heuberger, M.; Hufenus, R.; Nowack, B. The Origin of Microplastic Fiber in Polyester Textiles: The Textile Production Process Matters. J. Clean. Prod. 2020, 267, 121970. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, M.; Park, M.-J.; Kim, J. Analysis of Microplastics Released from Plain Woven Classified by Yarn Types during Washing and Drying. Polymers 2021, 13, 2988. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Marín, A.V.; Jabbar, A.; Tausif, M. Fragmented Fiber Pollution from Common Textile Materials and Structures during Laundry. Text. Res. J. 2022, 92, 2265–2275. [Google Scholar] [CrossRef]
- Sillanpää, M.; Sainio, P. Release of Polyester and Cotton Fibers from Textiles in Machine Washings. Environ. Sci. Pollut. Res. 2017, 24, 19313–19321. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, M.S.; Shams, M. Acrylic Fabrics as a Source of Microplastics from Portable Washer and Dryer: Impact of Washing and Drying Parameters. Sci. Total Environ. 2022, 834, 155429. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, A.P. Evaluation of Microfiber Release from Jeans: The Impact of Different Washing Conditions. Environ. Sci. Pollut. Res. 2021, 28, 58570–58582. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Gullo, M.P.; Gentile, G.; Di Pace, E.; Cocca, M.; Gelabert, L.; Brouta-Agnésa, M.; Rovira, A.; Escudero, R.; Villalba, R.; et al. Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ. Pollut. 2018, 236, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Lant, N.J.; Hayward, A.S.; Peththawadu, M.M.D.D.; Sheridan, K.J.; Dean, J.R. Microfiber Release from Real Soiled Consumer Laundry and the Impact of Fabric Care Products and Washing Conditions. PLoS ONE 2020, 15, e0233332. [Google Scholar] [CrossRef] [PubMed]
- Rathinamoorthy, R.; Raja Balasaraswathi, S. Investigations on the Impact of Handwash and Laundry Softener on Microfiber Shedding from Polyester Textiles. J. Text. Inst. 2022, 113, 1428–1437. [Google Scholar] [CrossRef]
- McIlwraith, H.K.; Lin, J.; Erdle, L.M.; Mallos, N.; Diamond, M.L.; Rochman, C.M. Capturing Microfibers—Marketed Technologies Reduce Microfiber Emissions from Washing Machines. Mar. Pollut. Bull. 2019, 139, 40–45. [Google Scholar] [CrossRef]
- Napper, I.E.; Barrett, A.C.; Thompson, R.C. The Efficiency of Devices Intended to Reduce Microfibre Release during Clothes Washing. Sci. Total Environ. 2020, 738, 140412. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.G.; Suaria, G.; Perold, V.; Pierucci, A.; Bornman, T.G.; Aliani, S. Sampling Microfibres at the Sea Surface: The Effects of Mesh Size, Sample Volume and Water Depth. Environ. Pollut. 2020, 258, 113413. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, A.; Mitchell, J.; Arsem, N. Extraction and Identification of Microplastic Particles from Secondary Wastewater Treatment Plant (WWTP) Effluent. Anal. Methods 2017, 9, 1412–1418. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for Sampling and Detection of Microplastics in Water and Sediment: A Critical Review. TrAC-Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Intercomparison Study on Commonly Used Methods to Determine Microplastics in Wastewater and Sludge Samples. Environ. Sci. Pollut. Res. 2019, 26, 12109–12122. [Google Scholar] [CrossRef] [PubMed]
- The Microfibre Consortium. FAQs on the TMC Test Method. 2021. Available online: https://www.microfibreconsortium.com/s/TMC-Test-Method_FAQs-bzch.pdf (accessed on 20 February 2023).
- Conkle, J.L.; Báez Del Valle, C.D.; Turner, J.W. Are We Underestimating Microplastic Contamination in Aquatic Environments? Environ. Manag. 2018, 61, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Covernton, G.A.; Pearce, C.M.; Gurney-Smith, H.J.; Chastain, S.G.; Ross, P.S.; Dower, J.F.; Dudas, S.E. Size and Shape Matter: A Preliminary Analysis of Microplastic Sampling Technique in Seawater Studies with Implications for Ecological Risk Assessment. Sci. Total Environ. 2019, 667, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic Microfibers in the Marine Environment: A Review on Their Occurrence in Seawater and Sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef]
- Carr, S.A. Sources and Dispersive Modes of Micro-Fibers in the Environment. Integr. Environ. Assess. Manag. 2017, 13, 466–469. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Corsi, S.R.; Mason, S.A. Plastic Debris in 29 Great Lakes Tributaries: Relations to Watershed Attributes and Hydrology. Environ. Sci. Technol. 2016, 50, 10377–10385. [Google Scholar] [CrossRef]
- Barrows, A.P.W.; Cathey, S.E.; Petersen, C.W. Marine Environment Microfiber Contamination: Global Patterns and the Diversity of Microparticle Origins. Environ. Pollut. 2018, 237, 275–284. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A First Overview of Textile Fibers, including Microplastics, in Indoor and Outdoor Environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, X.H.; Fang, T.; Xu, P.; Zhu, L.X.; Li, D.J. Source and Potential Risk Assessment of Suspended Atmospheric Microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, Y.; Du, F.; Cai, H.; Wang, G.; Shi, H. Microplastic Fallout in Different Indoor Environments. Environ. Sci. Technol. 2020, 54, 6530–6539. [Google Scholar] [CrossRef] [PubMed]
- Torres-Agullo, A.; Karanasiou, A.; Moreno, T.; Lacorte, S. Airborne Microplastic Particle Concentrations and Characterization in Indoor Urban Microenvironments. Environ. Pollut. 2022, 308, 119707. [Google Scholar] [CrossRef] [PubMed]
- Gaston, E.; Woo, M.; Steele, C.; Sukumaran, S.; Anderson, S. Microplastics Differ between Indoor and Outdoor Air Masses: Insights from Multiple Microscopy Methodologies. Appl. Spectrosc. 2020, 74, 1079–1098. [Google Scholar] [CrossRef] [PubMed]
- Tunahan Kaya, A.; Yurtsever, M.; Çiftçi Bayraktar, S. Ubiquitous Exposure to Microfiber Pollution in the Air. Eur. Phys. J. Plus 2018, 133, 488. [Google Scholar] [CrossRef]
- Abbasi, S.; Turner, A. Dry and Wet Deposition of Microplastics in a Semi-Arid Region (Shiraz, Iran). Sci. Total Environ. 2021, 786, 147358. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in Wastewater Treatment Plants: Detection, Occurrence and Removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Identification of Microplastic in Effluents of Wastewater Treatment Plants Using Focal Plane Array-Based Micro-Fourier-Transform Infrared Imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L.L. Wastewater Treatment Plants as a Pathway for Microplastics: Development of a New Approach to Sample Wastewater-Based Microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Conley, K.; Clum, A.; Deepe, J.; Lane, H.; Beckingham, B. Wastewater Treatment Plants as a Source of Microplastics to an Urban Estuary: Removal Efficiencies and Loading per Capita over One Year. Water Res. X 2019, 3, 100030. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.; Norén, F. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant; Report C55; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2014; Volume 22. [Google Scholar]
- Talvitie, J.; Mikola, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How Well Is Microlitter Purified from Wastewater?—A Detailed Study on the Stepwise Removal of Microlitter in a Tertiary Level Wastewater Treatment Plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to Microplastic Pollution—Removal of Microplastics from Wastewater Effluent with Advanced Wastewater Treatment Technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, K.; Cui, S.; Kang, Y.; An, L.; Lei, K. Removal of Microplastics in Municipal Sewage from China’s Largest Water Reclamation Plant. Water Res. 2019, 155, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Dong, Q.; Zuo, Z.; Liu, Y.; Huang, X.; Wu, W.M. Microplastics in a Municipal Wastewater Treatment Plant: Fate, Dynamic Distribution, Removal Efficiencies, and Control Strategies. J. Clean. Prod. 2019, 225, 579–586. [Google Scholar] [CrossRef]
- Rather, L.J.; Jameel, S.; Dar, O.A.; Ganie, S.A.; Bhat, K.A.; Mohammad, F. Advances in the Sustainable Technologies for Water Conservation in Textile Industries. In Water in Textiles and Fashion; Woodhead Publishing: Sawston, UK, 2019; pp. 175–194. [Google Scholar] [CrossRef]
- Boucher, J.; Billard, G. The Challenges of Measuring Plastic Pollution; Institut Veolia: Aubervilliers, France, 2019. [Google Scholar] [CrossRef]
- Sherrington, C. Plastics in the Marine Environment. Eunomia. Available online: https://www.eunomia.co.uk/reports-tools/plastics-in-the-marine-environment/ (accessed on 10 February 2023).
- Ryberg, M.W.; Laurent, A.; Hauschild, M. Mapping of Global Plastics Value Chain; United Nations Environment Programme: Nairobi, Kenya, 2018; p. 96. [Google Scholar]
- Belzagui, F.; Gutiérrez-Bouzán, C.; Álvarez-Sánchez, A.; Vilaseca, M.; Gutierrez-Bouzan, C.; Alvarez-Sanchez, A.; Vilaseca, M. Textile Microfibers Reaching Aquatic Environments: A New Estimation Approach. Environ. Pollut. 2020, 265, 114889. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.Z.; Watts, A.J.R.; Winslow, B.O.; Galloway, T.S.; Barrows, A.P.W. Mountains to the Sea: River Study of Plastic and Non-Plastic Microfiber Pollution in the Northeast USA. Mar. Pollut. Bull. 2017, 124, 245–251. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Tassin, B. Synthetic and Non-Synthetic Anthropogenic Fibers in a River under the Impact of Paris Megacity: Sampling Methodological Aspects and Flux Estimations. Sci. Total Environ. 2018, 618, 157–164. [Google Scholar] [CrossRef]
- Lusher, A.; Hollman, P.; Mendozal, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO: Rome, Italy, 2017. [Google Scholar]
- Stanton, T.; Johnson, M.; Nathanail, P.; MacNaughtan, W.; Gomes, R.L. Freshwater and airborne textile fibre populations are dominated by ‘natural’, not microplastic, fibres. Sci. Total Environ. 2019, 666, 377–389. [Google Scholar] [CrossRef]
- Sundt, P.; Schulze, P.-E.; Syversen, F. Sources of Microplastic-pollution to the Marine Environment; Project Report M-321|2015; Norwegian Environment Agency. 2014. Available online: https://www.miljodirektoratet.no/globalassets/publikasjoner/M321/M321.pdf (accessed on 10 February 2023).
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended Microplastics in the Surface Water of the Yangtze Estuary System, China: First Observations on Occurrence, Distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef]
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthoropogenic Litter; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of Synthetic Microplastic Plastic Fibres from Domestic Washing Machines: Effects of Fabric Type and Washing Conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.; Kerpen, J.; Prediger, J.; Barkmann, L.; Müller, L. Determination of the Microplastics Emission in the Effluent of a Municipal Wastewater Treatment Plant Using Raman Microspectroscopy. Water Res. X 2019, 2, 100014. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, T.; Abdulazeez, A.; Oladele, E.; Funmilayo, W.E.; Dikko, O.C.; Ja’afar, U.; Salisu, N. Microplastics Abundance, Characteristics, and Risk in Badagry Lagoon in Lagos State, Nigeria. Pollution 2022, 8, 1325–1337. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, L.; Ma, K. Microfiber from Textile Dyeing and Printing Wastewater of a Typical Industrial Park in China: Occurrence, Removal and Release. Sci. Total Environ. 2020, 739, 140329. [Google Scholar] [CrossRef]
- Habib, D.; Locke, D.C.; Cannone, L.J. Synthetic Fibers as Indicators of Municipal Sewage Sludge, Sludge Products, and Sewage Treatment Plant Effluents. Water Air Soil Pollut. 1998, 103, 1–8. [Google Scholar] [CrossRef]
- Zubris, K.A.V.; Richards, B.K. Synthetic Fibers as an Indicator of Land Application of Sludge. Environ. Pollut. 2005, 138, 201–211. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in Sewage Sludge from the Wastewater Treatment Plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of Microplastic Accumulation in Agricultural Soils from Sewage Sludge Disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef] [PubMed]
- Prendergast-miller, M.T.; Katsiamides, A.; Abbass, M.; Sturzenbaum, S.R.; Thorpe, K.L.; Hodson, M.E. Polyester-Derived Micro Fi Bre Impacts on the Soil-Dwelling Earthworm. Environ. Pollut. 2019, 251, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Loureiro, S.; Talluri, V.S.S.L.P.; Dolar, A.; Verweij, R.; Chvojka, J.; Michalcová, A.; Kočí, V.; van Gestel, C.A.M. Microplastic Fibers Influence Ag Toxicity and Bioaccumulation in Eisenia Andrei but Not in Enchytraeus Crypticus. Ecotoxicology 2021, 30, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Selonen, S.; Dolar, A.; Jemec Kokalj, A.; Skalar, T.; Parramon Dolcet, L.; Hurley, R.; van Gestel, C.A.M. Exploring the Impacts of Plastics in Soil—The Effects of Polyester Textile Fibers on Soil Invertebrates. Sci. Total Environ. 2020, 700, 134451. [Google Scholar] [CrossRef] [PubMed]
- Gavigan, J.; Kefela, T.; Macadam-Somer, I.; Suh, S.; Geyer, R. Synthetic Microfiber Emissions to Land Rival Those to Waterbodies and Are Growing. PLoS ONE 2020, 15, e0237839. [Google Scholar] [CrossRef] [PubMed]
- Royer, S.J.; Wiggin, K.; Kogler, M.; Deheyn, D.D. Degradation of Synthetic and Wood-Based Cellulose Fabrics in the Marine Environment: Comparative Assessment of Field, Aquarium, and Bioreactor Experiments. Sci. Total Environ. 2021, 791, 148060. [Google Scholar] [CrossRef] [PubMed]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and Effects of Plastic Additives on Marine Environments and Organisms: A Review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Mathalon, A.; Hill, P. Microplastic Fibers in the Intertidal Ecosystem Surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 2014, 81, 69–79. [Google Scholar] [CrossRef]
- Collard, F.; Gilbert, B.; Eppe, G.; Parmentier, E.; Das, K. Detection of Anthropogenic Particles in Fish Stomachs: An Isolation Method Adapted to Identification by Raman Spectroscopy. Arch. Environ. Contam. Toxicol. 2015, 69, 331–339. [Google Scholar] [CrossRef]
- Keshavarzifard, M.; Zakaria, M.P.; Sharifi, R. Ecotoxicological and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Short-Neck Clam (Paphia Undulata) and Contaminated Sediments in Malacca Strait, Malaysia. Arch. Environ. Contam. Toxicol. 2017, 73, 474–487. [Google Scholar] [CrossRef]
- Michels, J.; Stippkugel, A.; Lenz, M.; Wirtz, K.; Engel, A. Rapid Aggregation of Biofilm-Covered Microplastics with Marine Biogenic Particles. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181203. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of Weathering on Environmental Behavior of Microplastics: Properties, Sorption and Potential Risks. Chemosphere 2020, 242, 125193. [Google Scholar] [CrossRef] [PubMed]
- Lassen, C.; Hansen, S.F.; Magnusson, K.; Norén, F.; Hartmann, N.I.B.; Jensen, P.R.; Nielsen, T.G.; Brinch, A. Microplastics—Occurrence, Effects and Sources of Releases to the Environment in Denmark; The Danish Environmental Protection Agency: Copenhagen, Denmark, 2015. Available online: https://mst.dk/publikationer/2015/november/microplastics-occurrence-effects-and-sources-of-releases-to-the-environment-in-denmark (accessed on 10 February 2023).
- Girard, E.B.; Kaliwoda, M.; Schmahl, W.W.; Wörheide, G.; Orsi, W.D. Biodegradation of Textile Waste by Marine Bacterial Communities Enhanced by Light. Environ. Microbiol. Rep. 2020, 12, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as Contaminants in the Marine Environment: A Review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.J.R.; Urbina, M.A.; Corr, S.; Lewis, C.; Galloway, T.S. Ingestion of Plastic Microfibers by the Crab Carcinus Maenas and Its Effect on Food Consumption and Energy Balance. Environ. Sci. Technol. 2015, 49, 14597–14604. [Google Scholar] [CrossRef]
- Taylor, M.L.; Gwinnett, C.; Robinson, L.F.; Woodall, L.C. Plastic Microfibre Ingestion by Deep-Sea Organisms. Sci. Rep. 2016, 6, 33997. [Google Scholar] [CrossRef] [PubMed]
- Duflos, G.; Dehaut, A.; Cassone, A.-L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; et al. Microplastics in Seafood: Identifying a Protocol for Their Extraction and Characterization. Fate Impact Microplastics Mar. Ecosyst. 2017, 215, 74. [Google Scholar] [CrossRef]
- Weinstein, J.E.; Ertel, B.M.; Gray, A.D. Accumulation and Depuration of Microplastic Fibers, Fragments, and Tire Particles in the Eastern Oyster, Crassostrea Virginica: A Toxicokinetic Approach. Environ. Pollut. 2022, 308, 119681. [Google Scholar] [CrossRef]
- Murano, C.; Vaccari, L.; Casotti, R.; Corsi, I.; Palumbo, A. Occurrence of Microfibres in Wild Specimens of Adult Sea Urchin Paracentrotus Lividus (Lamarck, 1816) from a Coastal Area of the Central Mediterranean Sea. Mar. Pollut. Bull. 2022, 176, 113448. [Google Scholar] [CrossRef]
- Mohsen, M.; Sun, L.; Lin, C.; Huo, D.; Yang, H. Mechanism Underlying the Toxicity of the Microplastic Fibre Transfer in the Sea Cucumber Apostichopus Japonicus. J. Hazard. Mater. 2021, 416, 125858. [Google Scholar] [CrossRef]
- Pradit, S.; Noppradit, P.; Goh, B.P.; Sornplang, K.; Ong, M.C.; Towatana, P. Occurrence of Microplastics and Trace Metals in Fish and Shrimp from Songkhla Lake, Thailand during the COVID-19 Pandemic. Appl. Ecol. Environ. Res. 2021, 19, 1085–1106. [Google Scholar] [CrossRef]
- Esterhuizen, M.; Buchenhorst, L.; Kim, Y.J.; Pflugmacher, S. In Vivo Oxidative Stress Responses of the Freshwater Basket Clam Corbicula Javanicus to Microplastic Fibres and Particles. Chemosphere 2022, 296, 134037. [Google Scholar] [CrossRef] [PubMed]
- Pittura, L.; Nardi, A.; Cocca, M.; De Falco, F.; D’Errico, G.; Mazzoli, C.; Mongera, F.; Benedetti, M.; Gorbi, S.; Avella, M.; et al. Cellular Disturbance and Thermal Stress Response in Mussels Exposed to Synthetic and Natural Microfibers. Front. Mar. Sci. 2022, 9, 1–15. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The Physical Impacts of Microplastics on Marine Organisms: A Review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 Mm to 20 Μm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced Desorption of Persistent Organic Pollutants from Microplastics under Simulated Physiological Conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic Fibers in Atmospheric Fallout: A Source of Microplastics in the Environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Ladewig, S.M.; Bao, S.; Chow, A.T. Natural Fibers: A Missing Link to Chemical Pollution Dispersion in Aquatic Environments. Environ. Sci. Technol. 2015, 49, 12609–12610. [Google Scholar] [CrossRef]
- Acharya, S.; Rumi, S.S.; Hu, Y.; Abidi, N. Microfibers from Synthetic Textiles as a Major Source of Microplastics in the Environment: A Review. Text. Res. J. 2021, 91, 2136–2156. [Google Scholar] [CrossRef]
- Athey, S.N.; Erdle, L.M. Are We Underestimating Anthropogenic Microfiber Pollution? A Critical Review of Occurrence, Methods, and Reporting. Environ. Toxicol. Chem. 2022, 41, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Conservation X labs. PANGAIA x MTIX Microfiber Mitigation. Available online: https://www.microfiberinnovation.org/innovation/pangaia-x-mtix (accessed on 2 April 2022).
- Mermaids. Ocean Clean Wash. Handbook for Zero Microplastics from Textiles and Laundry. 2018. Available online: http://life-mermaids.eu/en/deliverables-mermaids-life-2/ (accessed on 21 January 2022).
- Cotton, L.; Hayward, A.S.; Lant, N.J.; Blackburn, R.S. Improved garment longevity and reduced microfibre release are important sustainability benefits of laundering in colder and quicker washing machine cycles. Dye. Pigment. 2020, 177, 108120. [Google Scholar] [CrossRef]
- Li, J.; Lemstra, P.J.; Ma, P. Chapter 7: Can High-Performance Fibers Be(Come) Bio-Based and Also Biocompostable? Adv. Ind. Eng. Polym. Res. 2022, 5, 117–132. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Zakarauskas, K.; Striūgas, N.; Mohamed, A. A New Strategy for Using Lint-Microfibers Generated from Clothes Dryer as a Sustainable Source of Renewable Energy. Sci. Total Environ. 2021, 762, 143107. [Google Scholar] [CrossRef] [PubMed]
- Plastic Pollution Coalition. 15 Ways to Stop Microfiber Pollution Now. Plastic Pollution Coalition. Available online: https://www.plasticpollutioncoalition.org/blog/2017/3/2/15-ways-to-stop-microfiber-pollution-now (accessed on 13 December 2022).
- Plastic Soup Foundation. Microfiber Pollution: What Solutions for the Oceans? Available online: https://www.oceancleanwash.org/solutions/solutions-for-consumers/ (accessed on 13 December 2022).
- Cowger, W.; Booth, A.M.; Hamilton, B.M.; Thaysen, C.; Primpke, S.; Munno, K.; Lusher, A.L.; Dehaut, A.; Vaz, V.P.; Liboiron, M.; et al. Reporting Guidelines to Increase the Reproducibility and Comparability of Research on Microplastics. Appl. Spectrosc. 2020, 74, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, A.; Meredith, J. The Evolution of the Intellectual Structure of Operations Management—1980–2006: A Citation/Co-Citation Analysis. J. Oper. Manag. 2009, 27, 185–202. [Google Scholar] [CrossRef]
- Daukantienė, V. Analysis of the Sustainability Aspects of Fashion: A Literature Review. Text. Res. J. 2022, 93, 991–1002. [Google Scholar] [CrossRef]
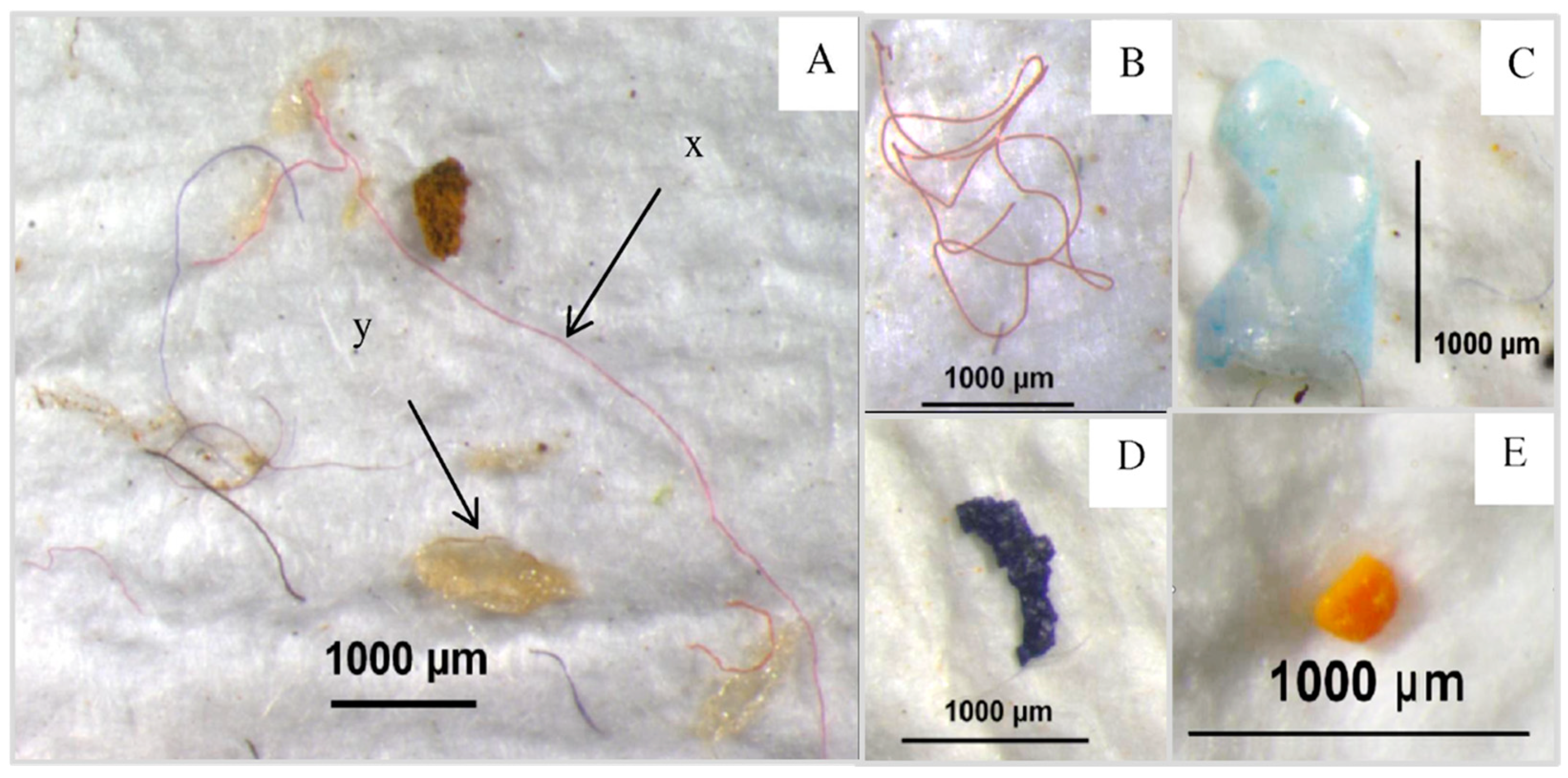
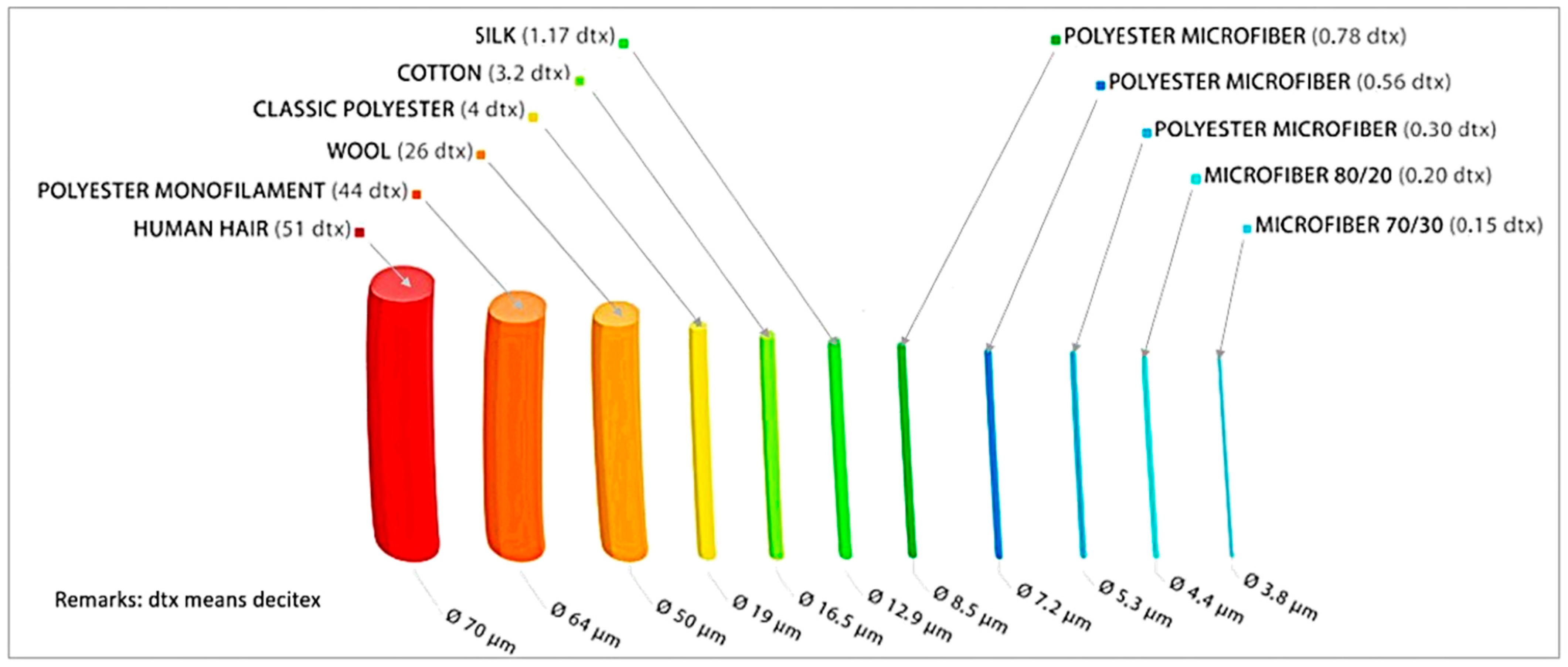



| Keywords | No of Articles |
|---|---|
| TS = (microplastic*) | 8217 |
| TS = (microplastic*) AND TS = (microfibre* or microfiber* or fibre* or fiber*) | 1939 |
| TS = (microfibre* or microfiber*) AND TS = (textile* or clothing* or apparel* or fashion*) | 395 |
| TS = (microplastic*) AND TS= (microfibre* or microfiber* or fibre* or fiber*) AND TS = (textile* or clothing* or apparel* or fashion*) | 219 |
| Name of Journal | No of Publications | % |
|---|---|---|
| Science of the Total Environment | 34 | 16% |
| Environmental Pollution | 27 | 12% |
| Marine Pollution Bulletin | 22 | 10% |
| Environmental Science Technology | 15 | 7% |
| Environmental Science and Pollution Research | 14 | 6% |
| Chemosphere | 9 | 4% |
| Journal of Hazardous Materials | 8 | 4% |
| PLoS ONE | 6 | 3% |
| Frontiers in Marine Science | 4 | 2% |
| Polymers | 4 | 2% |
| Publication Countries | No of Publications | % |
|---|---|---|
| People’s Republic of China | 36 | 16% |
| USA | 34 | 16% |
| England | 27 | 12% |
| Italy | 26 | 12% |
| Canada | 15 | 7% |
| Germany | 12 | 5% |
| Spain | 12 | 5% |
| Switzerland | 11 | 5% |
| Australia | 9 | 4% |
| Finland | 9 | 4% |
| Group No | Colour | No of Publications | Research Domains |
|---|---|---|---|
| 0 | NA | 6 | Scattered Samples |
| 1 | Blue |  85 85 | Domestic laundry and drying |
| 2 | Green |  28 28 | Test methodology |
| 3 | Purple |  22 22 | Aquatic ecosystem |
| 4 | Orange |  21 21 | Atmosphere environment |
| 5 | Yellow |  19 19 | Wastewater source |
| 6 | Brown |  17 17 | Abundance and distribution |
| 7 | Pink |  11 11 | Terrestrial ecosystem |
| 8 | Light Blue |  10 10 | Hazardous nature |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.K.-M.; Lo, C.K.-Y.; Kan, C.-W. A Systematic Literature Review for Addressing Microplastic Fibre Pollution: Urgency and Opportunities. Water 2024, 16, 1988. https://doi.org/10.3390/w16141988
Chan CK-M, Lo CK-Y, Kan C-W. A Systematic Literature Review for Addressing Microplastic Fibre Pollution: Urgency and Opportunities. Water. 2024; 16(14):1988. https://doi.org/10.3390/w16141988
Chicago/Turabian StyleChan, Carmen Ka-Man, Chris Kwan-Yu Lo, and Chi-Wai Kan. 2024. "A Systematic Literature Review for Addressing Microplastic Fibre Pollution: Urgency and Opportunities" Water 16, no. 14: 1988. https://doi.org/10.3390/w16141988
APA StyleChan, C. K.-M., Lo, C. K.-Y., & Kan, C.-W. (2024). A Systematic Literature Review for Addressing Microplastic Fibre Pollution: Urgency and Opportunities. Water, 16(14), 1988. https://doi.org/10.3390/w16141988







