Carbon Capture and Resource Utilization by Algal–Bacterial Consortium in Wastewater Treatment: A Mini-Review
Abstract
1. Introduction
2. Carbon Emissions in Wastewater Treatment Processes
2.1. CO2 Emissions
2.2. N2O Emissions
2.3. CH4 Emissions
3. CO2 Capture and Utilization in the Wastewater Treatment Processes
3.1. The Methods of CO2 Capture and Utilization
3.2. CO2 Capture and Utilization along with Wastewater Treatment
3.2.1. CO2 Capture and Utilization by Higher Plants
3.2.2. CO2 Absorption and Transformation by Microalgae
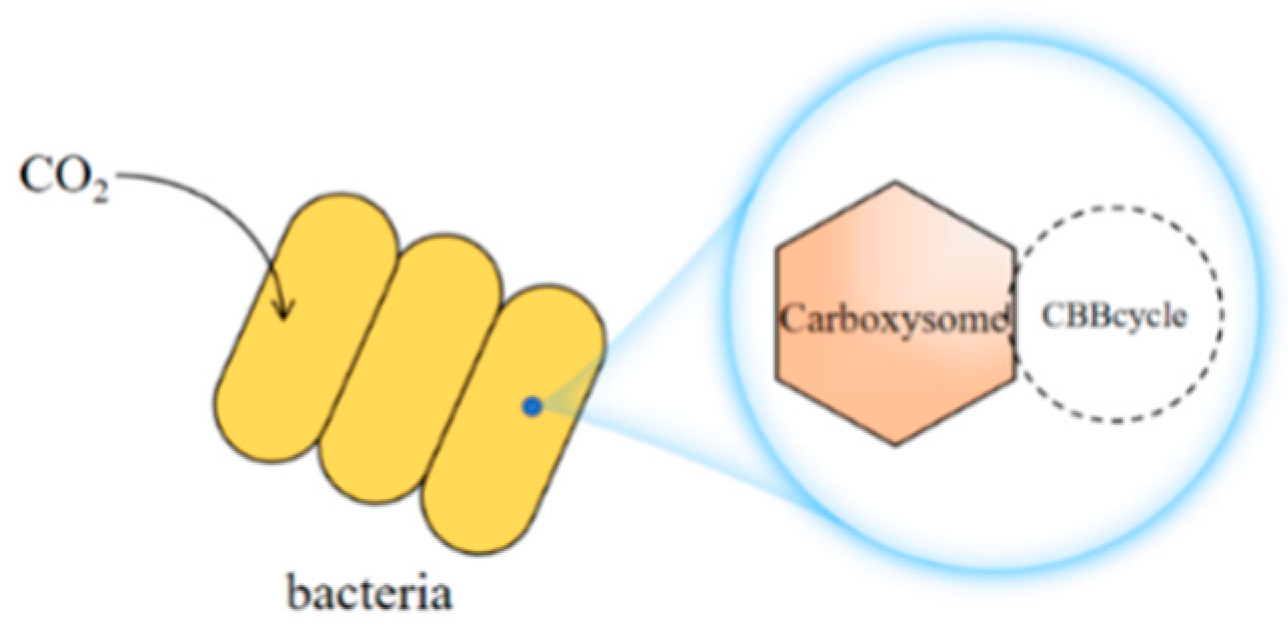
3.2.3. CO2 Capture and Transformation by Bacteria
3.2.4. Carbon Capture in Constructed Wetlands
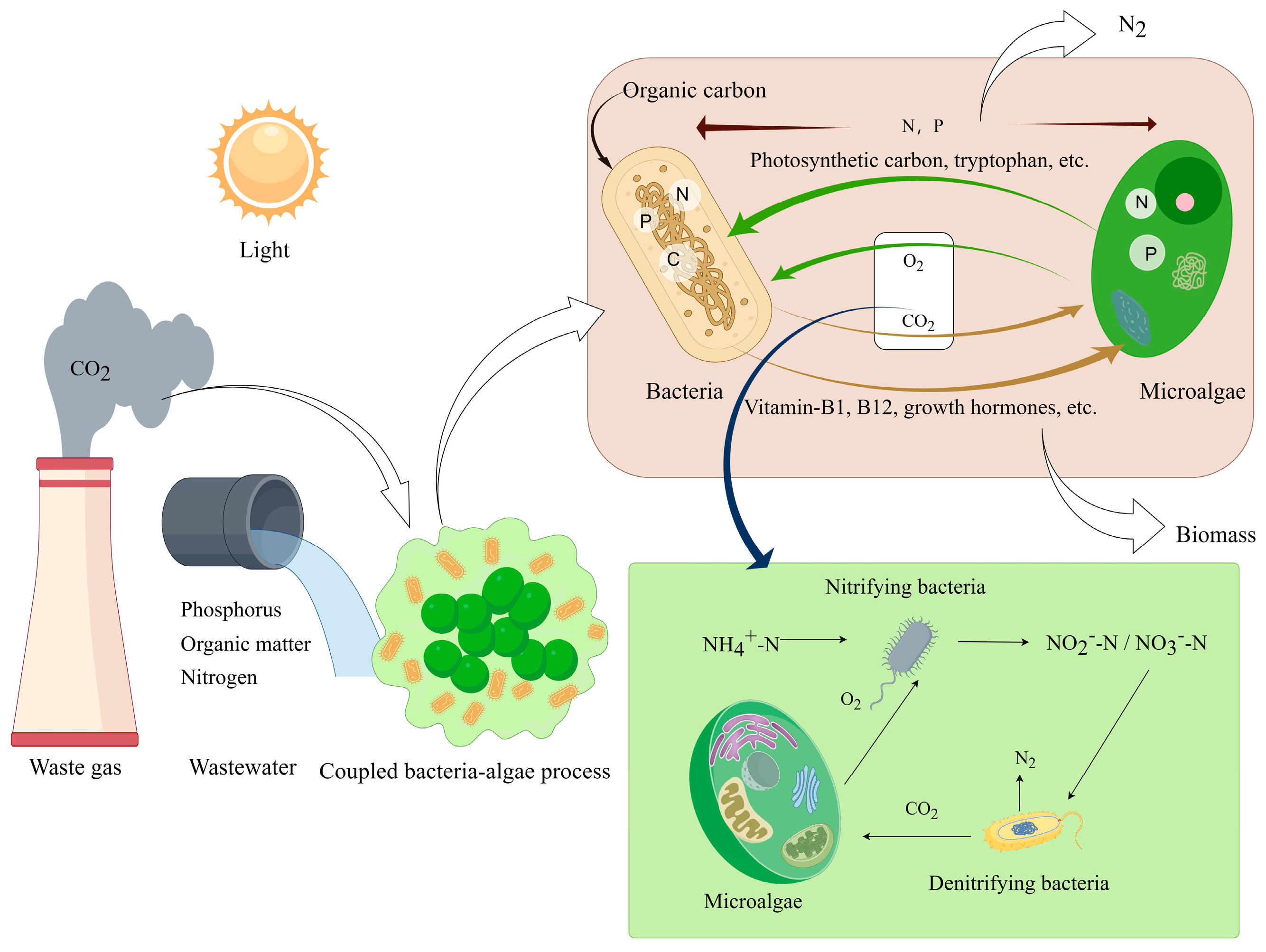
3.2.5. Carbon Storage by Algal-Bacterial Consortium
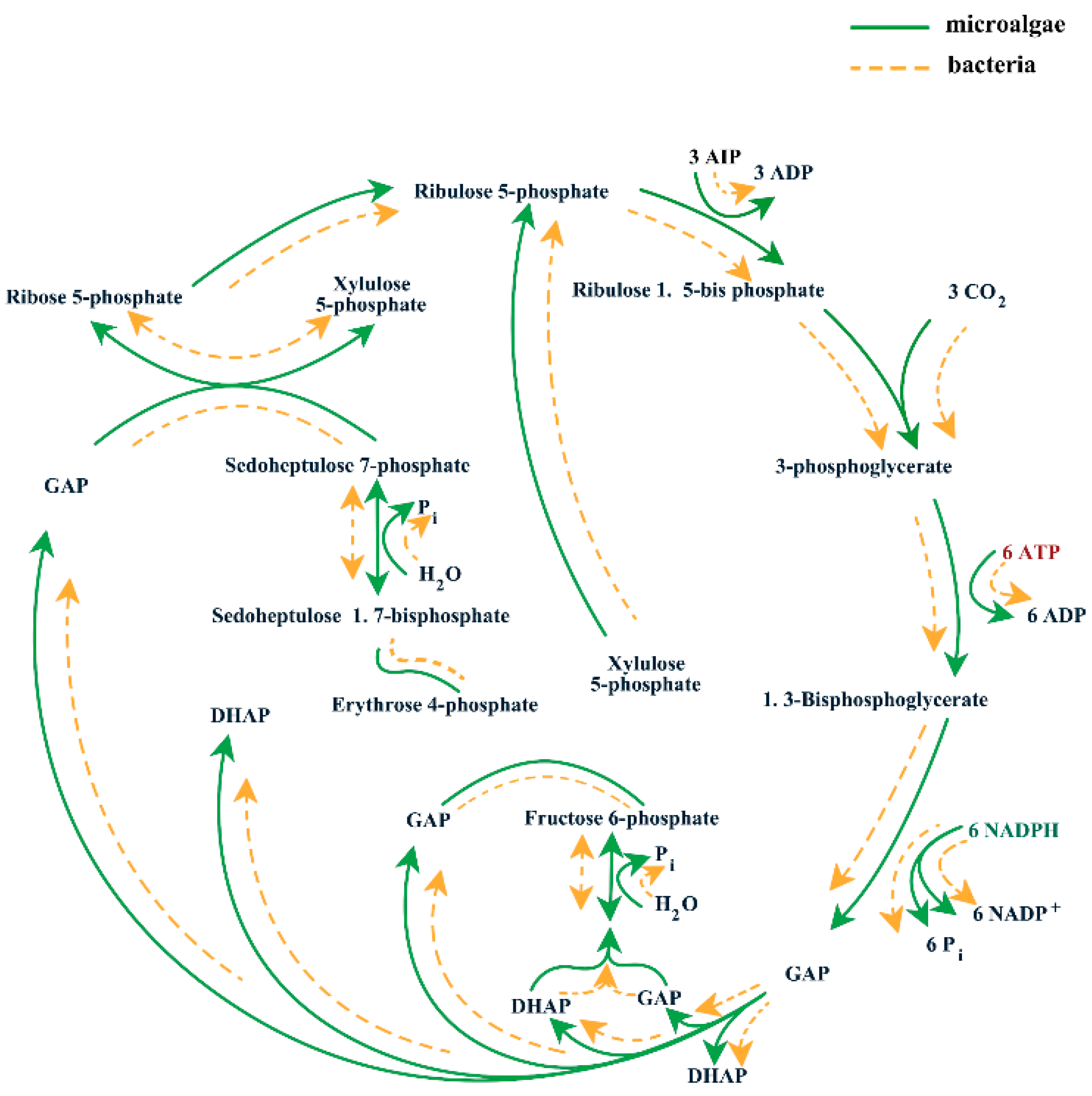
4. CO2 Capture in ABC System
5. Environmental and Energy Benefits of ABC Systems in Wastewater Treatment
5.1. Removal of Nitrogen, Phosphorus, and Other Pollutants
5.2. Reducing the Energy Consumption of Aeration
5.3. Enhancing Carbon Capture to Mitigate GHG Emissions
6. Valuable Biomass and Energy Generation from ABC Sludge
6.1. Separation of Lipids, Carbohydrates, and Proteins
| Species | Yield Rate mg/L·d | Lipid % | Protein % | Carbohydrate % | Ref. |
|---|---|---|---|---|---|
| Chlorella | 126.9 | 10.6 | 17.3 | 25.1 | [95] |
| Leptolyngbya | 52.8 | 10.0 | 15.2 | 22.0 | |
| Leptolyngbya & Chlorella | 196.7 | 18.1 | 20.4 | 30.7 | |
| Chlorella & Ettlia | 500 | 11.0 | 40.0 | 19.5 | [96] |
| Ettlia | 260 | 11.8 | 51.1 | 13.3 | |
| Chlorella | 440 | 11.8 | 34.0 | 20.3 | |
| S. sp. NIT18 | 24.9 | 6.4 | 19.7 | 33.7 | [93] |
| S. obliquus FACHB-416, C. vulgaris FACHB-32 & O. tenuis FACHB-1052 | 6.8–14.2 g/m2·d | 12.5–19.8 | 35.3–42.6 | 28.7–33.1 | [136] |
| C. sp. 46-4 | 26 | 21.1 | - | - | [150] |
| BGS or ABGS | 145.4–173.3 | 5.5–8.1 | 34.4–39.3 | - | [151] |
6.2. Anaerobic Digestion for Energy Generation
6.3. Anaerobic Digestion for Nutrients Recycling
7. Overcoming Environmental and Technological Challenges of the ABC Process
7.1. Addressing Low-Temperature Suppression
7.2. Addressing the Discrepancy between Light Source and WWTP Footprint
7.3. The Puzzle of Harmful Substance Accumulation
7.4. The Importance of Accurate and Reliable Process Models
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.K.; Meyer, K.C.; Funk, W.E. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- Szulejko, J.E.; Kumar, P.; Deep, A.; Kim, K.-H. Global warming projections to 2100 using simple CO2 greenhouse gas modeling and comments on CO2 climate sensitivity factor. Atmos. Pollut. Res. 2017, 8, 136–140. [Google Scholar] [CrossRef]
- Crippa, M.; Guizzardi, D.; Pagani, F.; Banja, M.; Muntean, M.; Schaaf, E.; Becker, W.; Monforti-Ferrario, F.; Quadrelli, R.; Risquez Martin, A.; et al. GHG Emissions of All World Countries; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Wang, D.; Ye, W.; Wu, G.; Li, R.; Guan, Y.; Zhang, W.; Wang, J.; Shan, Y.; Hubacek, K. Greenhouse gas emissions from municipal wastewater treatment facilities in China from 2006 to 2019. Sci. Data 2022, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Sala-Garrido, R.; Maziotis, A.; Mocholi-Arce, M.; Molinos-Senante, M. Assessing eco-efficiency of wastewater treatment plants: A cross-evaluation strategy. Sci. Total Environ. 2023, 900, 165839. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Das, S.; Ghosh, S. CO2 Capture and Utilization (CCU) in Coal-Fired Power Plants: Prospect of In Situ Algal Cultivation. In Sustainable Energy Technology and Policies; Springer: Berlin/Heidelberg, Germany, 2018; pp. 231–254. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z. Advances in the biological fixation of carbon dioxide by microalgae. J. Chem. Technol. Biotechnol. 2021, 96, 1475–1495. [Google Scholar] [CrossRef]
- Yong, J.J.; Chew, K.W.; Khoo, K.S.; Show, P.L.; Chang, J.S. Prospects and development of algal-bacterial biotechnology in environmental management and protection. Biotechnol. Adv. 2021, 47, 107684. [Google Scholar] [CrossRef] [PubMed]
- Loke Show, P. Global market and economic analysis of microalgae technology: Status and perspectives. Bioresour. Technol. 2022, 357, 127329. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, X.; Cai, W.; Lei, Z.; Shimizu, K.; Zhang, Z.; Utsumi, M.; Lee, D.-J. Response of algal-bacterial granular system to low carbon wastewater: Focus on granular stability, nutrients removal and accumulation. Bioresour. Technol. 2018, 268, 221–229. [Google Scholar] [CrossRef]
- Zhu, S.; Qin, L.; Feng, P.; Shang, C.; Wang, Z.; Yuan, Z. Treatment of low C/N ratio wastewater and biomass production using co-culture of Chlorella vulgaris and activated sludge in a batch photobioreactor. Bioresour. Technol. 2019, 274, 313–320. [Google Scholar] [CrossRef]
- Rosli, S.S.; Amalina Kadir, W.N.; Wong, C.Y.; Han, F.Y.; Lim, J.W.; Lam, M.K.; Yusup, S.; Kiatkittipong, W.; Kiatkittipong, K.; Usman, A. Insight review of attached microalgae growth focusing on support material packed in photobioreactor for sustainable biodiesel production and wastewater bioremediation. Renew. Sustain. Energy Rev. 2020, 134, 110306. [Google Scholar] [CrossRef]
- Qv, M.; Dai, D.; Liu, D.; Wu, Q.; Tang, C.; Li, S.; Zhu, L. Towards advanced nutrient removal by microalgae-bacteria symbiosis system for wastewater treatment. Bioresour. Technol. 2023, 370, 128574. [Google Scholar] [CrossRef]
- Pooja, K.; Priyanka, V.; Rao, B.C.S.; Raghavender, V. Cost-effective treatment of sewage wastewater using microalgae Chlorella vulgaris and its application as bio-fertilizer. Energy Nexus 2022, 7, 100122. [Google Scholar] [CrossRef]
- Lu, L.; Guest, J.S.; Peters, C.A.; Zhu, X.; Rau, G.H.; Ren, Z.J. Wastewater treatment for carbon capture and utilization. Nat. Sustain. 2018, 1, 750–758. [Google Scholar] [CrossRef]
- Bai, R.L.; Jin, L.; Sun, S.R.; Cheng, Y.; Wei, Y. Quantification of greenhouse gas emission from wastewater treatment plants. Greenh. Gases Sci. Technol. 2022, 12, 587–601. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, T.; Liu, J.; Sun, Y.; Liu, Y.; Zhao, Q.; Tian, T. The comprehensive measurement method of energy conservation and emission reduction in the whole process of urban sewage treatment based on carbon emission. Environ. Sci. Pollut. Res. 2021, 28, 56727–56740. [Google Scholar] [CrossRef] [PubMed]
- Du, W.-J.; Lu, J.-Y.; Hu, Y.-R.; Xiao, J.; Yang, C.; Wu, J.; Huang, B.; Cui, S.; Wang, Y.; Li, W.-W. Spatiotemporal pattern of greenhouse gas emissions in China’s wastewater sector and pathways towards carbon neutrality. Nat. Water 2023, 1, 166–175. [Google Scholar] [CrossRef]
- EPA. Draft Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2022; EPA 430-D-24-001; U.S. Environmental Protection Agency: Washington, DC, USA, 2024.
- GIO; MOE. National Greenhouse Gas Inventory Document of JAPAN 2024; Center for Global Environmental Research, Earth System Division, National Institute for Environmental Studies: Tsukuba, Japan, 2024. [Google Scholar]
- Climate Change Committee. The Sixth Carbon Budget Methodology Report; Climate Change Committee: London, UK, 2020. [Google Scholar]
- Australian Government, Department of Climate Change, Energy, the Environment and Water. National Inventory Report 2022. Volume I. 2024. Available online: https://www.dcceew.gov.au/climate-change/publications/national-inventory-reports (accessed on 10 July 2024).
- European Parliament. Climate Action in the Netherlands: Latest State of Play; European Parliament: Strasbourg, France, 2021.
- Shrestha, A.; Bhattarai, T.N.; Ghimire, S.; Mainali, B.; Treichel, H.; Paudel, S.R. Estimation of greenhouse gases emission from domestic wastewater in Nepal: A scenario-based analysis applicable for developing countries. Chemosphere 2022, 300, 134501. [Google Scholar] [CrossRef] [PubMed]
- Parravicini, V.; Nielsen, P.H.; Thornberg, D.; Pistocchi, A. Evaluation of greenhouse gas emissions from the European urban wastewater sector, and options for their reduction. Sci. Total Environ. 2022, 838, 156322. [Google Scholar] [CrossRef]
- Noyola, A.; Paredes, M.G.; Morgan-Sagastume, J.M.; Güereca, L.P. Reduction of Greenhouse Gas Emissions From Municipal Wastewater Treatment in Mexico Based on Technology Selection. CLEAN–Soil Air Water 2016, 44, 1091–1098. [Google Scholar] [CrossRef]
- Nayeb, H.; Mirabi, M.; Motiee, H.; Alighardashi, A.; Khoshgard, A. Estimating greenhouse gas emissions from Iran’s domestic wastewater sector and modeling the emission scenarios by 2030. J. Clean. Prod. 2019, 236, 117673. [Google Scholar] [CrossRef]
- Pahunang, R.R.; Buonerba, A.; Senatore, V.; Oliva, G.; Ouda, M.; Zarra, T.; Muñoz, R.; Puig, S.; Ballesteros, F.C.; Li, C.-W.; et al. Advances in technological control of greenhouse gas emissions from wastewater in the context of circular economy. Sci. Total Environ. 2021, 792, 148479. [Google Scholar] [CrossRef] [PubMed]
- Khabiri, B.; Ferdowsi, M.; Buelna, G.; Jones, J.P.; Heitz, M. Bioelimination of low methane concentrations emitted from wastewater treatment plants: A review. Crit. Rev. Biotechnol. 2021, 42, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kansal, A. Energy and GHG accounting for wastewater infrastructure. Resour. Conserv. Recycl. 2018, 128, 499–507. [Google Scholar] [CrossRef]
- Lekkas, T.D.; Stasinakis, A.; Dimopoulou, A.; Noutsopoulos, C.; Mamais, D. Wastewater treatment process impact on energy savings and greenhouse gas emissions. Water Sci. Technol. 2015, 71, 303–308. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, L.; Wang, D.; Zhou, Y.; Yang, X. Recent advances in nitrous oxide production and mitigation in wastewater treatment. Water Res. 2020, 184, 116168. [Google Scholar] [CrossRef] [PubMed]
- Vasilaki, V.; Massara, T.M.; Stanchev, P.; Fatone, F.; Katsou, E. A decade of nitrous oxide (N2O) monitoring in full-scale wastewater treatment processes: A critical review. Water Res. 2019, 161, 392–412. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Zhao, Y.; Koch, K.; Wells, G.F.; Zheng, M.; Yuan, Z.; Ye, L. Insights into Nitrous Oxide Mitigation Strategies in Wastewater Treatment and Challenges for Wider Implementation. Environ. Sci. Technol. 2021, 55, 7208–7224. [Google Scholar] [CrossRef]
- Ocko, I.B.; Sun, T.; Shindell, D.; Oppenheimer, M.; Hristov, A.N.; Pacala, S.W.; Mauzerall, D.L.; Xu, Y.; Hamburg, S.P. Acting rapidly to deploy readily available methane mitigation measures by sector can immediately slow global warming. Environ. Res. Lett. 2021, 16, 054042. [Google Scholar] [CrossRef]
- Delre, A.; Mønster, J.; Scheutz, C. Greenhouse gas emission quantification from wastewater treatment plants, using a tracer gas dispersion method. Sci. Total Environ. 2017, 605–606, 258–268. [Google Scholar] [CrossRef]
- Song, C.; Zhu, J.-J.; Willis, J.L.; Moore, D.P.; Zondlo, M.A.; Ren, Z.J. Methane Emissions from Municipal Wastewater Collection and Treatment Systems. Environ. Sci. Technol. 2023, 57, 2248–2261. [Google Scholar] [CrossRef]
- Zamparas, M. The role of resource recovery technologies in reducing the demand of fossil fuels and conventional fossil-based mineral fertilizers. In Low Carbon Energy Technologies in Sustainable Energy Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–24. [Google Scholar] [CrossRef]
- Zhao, G.; Garrido-Baserba, M.; Reifsnyder, S.; Xu, J.-C.; Rosso, D. Comparative energy and carbon footprint analysis of biosolids management strategies in water resource recovery facilities. Sci. Total Environ. 2019, 665, 762–773. [Google Scholar] [CrossRef]
- Fan, Y.; Wei, F. Contributions of Natural Carbon Sink Capacity and Carbon Neutrality in the Context of Net-Zero Carbon Cities: A Case Study of Hangzhou. Sustainability 2022, 14, 2680. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.G.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef] [PubMed]
- Ariluoma, M.; Ottelin, J.; Hautamäki, R.; Tuhkanen, E.-M.; Mänttäri, M. Carbon sequestration and storage potential of urban green in residential yards: A case study from Helsinki. Urban For. Urban Green. 2021, 57, 126939. [Google Scholar] [CrossRef]
- Raza, A.; Gholami, R.; Rezaee, R.; Rasouli, V.; Rabiei, M. Significant aspects of carbon capture and storage—A review. Petroleum 2019, 5, 335–340. [Google Scholar] [CrossRef]
- Wang, B.; Shi, S.; Wang, S.; Qiu, L.; Zhang, X. Optimal design for cryogenic structured packing column using particle swarm optimization algorithm. Cryogenics 2019, 103, 102976. [Google Scholar] [CrossRef]
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Ullah, N.; Inamuddin; Ramakrishna, S. Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog. Energy Combust. Sci. 2020, 80, 100849. [Google Scholar] [CrossRef]
- Pires, J.C.M.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Nocito, F.; Dibenedetto, A. Atmospheric CO2 mitigation technologies: Carbon capture utilization and storage. Curr. Opin. Green Sustain. Chem. 2020, 21, 34–43. [Google Scholar] [CrossRef]
- Mikulčić, H.; Ridjan Skov, I.; Dominković, D.F.; Wan Alwi, S.R.; Manan, Z.A.; Tan, R.; Duić, N.; Hidayah Mohamad, S.N.; Wang, X. Flexible Carbon Capture and Utilization technologies in future energy systems and the utilization pathways of captured CO2. Renew. Sustain. Energy Rev. 2019, 114, 109338. [Google Scholar] [CrossRef]
- Yelishala, S.C.; Kannaiyan, K.; Sadr, R.; Wang, Z.; Levendis, Y.A.; Metghalchi, H. Performance maximization by temperature glide matching in energy exchangers of cooling systems operating with natural hydrocarbon/CO2 refrigerants. Int. J. Refrig. 2020, 119, 294–304. [Google Scholar] [CrossRef]
- Cormos, A.-M.; Dinca, C.; Petrescu, L.; Andreea Chisalita, D.; Szima, S.; Cormos, C.-C. Carbon capture and utilisation technologies applied to energy conversion systems and other energy-intensive industrial applications. Fuel 2018, 211, 883–890. [Google Scholar] [CrossRef]
- Roefs, P.; Moretti, M.; Welkenhuysen, K.; Piessens, K.; Compernolle, T. CO2-enhanced oil recovery and CO2 capture and storage: An environmental economic trade-off analysis. J. Environ. Manag. 2019, 239, 167–177. [Google Scholar] [CrossRef]
- Ghiat, I.; Al-Ansari, T. A review of carbon capture and utilisation as a CO2 abatement opportunity within the EWF nexus. J. CO2 Util. 2021, 45, 101432. [Google Scholar] [CrossRef]
- Were, D.; Kansiime, F.; Fetahi, T.; Cooper, A.; Jjuuko, C. Carbon Sequestration by Wetlands: A Critical Review of Enhancement Measures for Climate Change Mitigation. Earth Syst. Environ. 2019, 3, 327–340. [Google Scholar] [CrossRef]
- Hillmann, E.R.; Rivera-Monroy, V.H.; Nyman, J.A.; La Peyre, M.K. Estuarine submerged aquatic vegetation habitat provides organic carbon storage across a shifting landscape. Sci. Total Environ. 2020, 717, 137217. [Google Scholar] [CrossRef]
- Bao, Q.; Liu, Z.; Zhao, M.; Hu, Y.; Li, D.; Han, C.; Zeng, C.; Chen, B.; Wei, Y.; Ma, S.; et al. Role of carbon and nutrient exports from different land uses in the aquatic carbon sequestration and eutrophication process. Sci. Total Environ. 2022, 813, 151917. [Google Scholar] [CrossRef]
- Ge, Z.; Wei, D.; Zhang, J.; Hu, J.; Liu, Z.; Li, R. Natural pyrite to enhance simultaneous long-term nitrogen and phosphorus removal in constructed wetland: Three years of pilot study. Water Res. 2019, 148, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Baptestini, G.C.F.; Matos, A.T.; Martinez, M.A.; Borges, A.C.; Matos, M.P. Hydraulic Conductivity Variability in Horizontal Subsurface Flow Constructed Wetlands. Eng. Agríc. 2017, 37, 333–342. [Google Scholar] [CrossRef]
- Zheng, F.; Fang, J.; Guo, F.; Yang, X.; Liu, T.; Chen, M.; Nie, M.; Chen, Y. Biochar based constructed wetland for secondary effluent treatment: Waste resource utilization. Chem. Eng. J. 2022, 432, 134377. [Google Scholar] [CrossRef]
- Jia, L.; Gou, E.; Liu, H.; Lu, S.; Wu, S.; Wu, H. Exploring Utilization of Recycled Agricultural Biomass in Constructed Wetlands: Characterization of the Driving Force for High-Rate Nitrogen Removal. Environ. Sci. Technol. 2019, 53, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Viswanaathan, S.; Perumal, P.K.; Sundaram, S. Integrated Approach for Carbon Sequestration and Wastewater Treatment Using Algal–Bacterial Consortia: Opportunities and Challenges. Sustainability 2022, 14, 1075. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Jin, W.; Tu, R.; Han, S.-F.; Eid, M.; Eladel, H. Microalgal biomass production as a sustainable feedstock for biodiesel: Current status and perspectives. Renew. Sustain. Energy Rev. 2016, 64, 596–606. [Google Scholar] [CrossRef]
- Mao, G.; Shi, K.; Zhang, C.; Li, J.; Chen, S.; Wang, P. Biodiesel Fuel from Chlorella vulgaris and Effects of Its Low-Level Blends on the Performance, Emissions, and Combustion Characteristics of a Nonroad Diesel Engine. J. Energy Eng. 2020, 146, 04020016. [Google Scholar] [CrossRef]
- Mennella, L.; Tosco, D.; Alberti, F.; Cembalo, L.; Crescimanno, M.; Del Giudice, T.; Galati, A.; Moglie, M.; Scardera, A.; Schifani, G.; et al. Perspectives and challenges of small scale plant microalgae cultivation. Evidences from Southern Italy. Algal Res. 2020, 45, 101693. [Google Scholar] [CrossRef]
- Gao, F.; Peng, Y.-Y.; Li, C.; Yang, G.-J.; Deng, Y.-B.; Xue, B.; Guo, Y.-M. Simultaneous nutrient removal and biomass/lipid production by Chlorella sp. in seafood processing wastewater. Sci. Total Environ. 2018, 640–641, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kiran, B.; Venkata Mohan, S. Photosynthetic transients in Chlorella sorokiniana during phycoremediation of dairy wastewater under distinct light intensities. Bioresour. Technol. 2021, 340, 125593. [Google Scholar] [CrossRef]
- Tran, D.T.; Van Do, T.C.; Nguyen, Q.T.; Le, T.G. Simultaneous removal of pollutants and high value biomaterials production by Chlorella variabilis TH03 from domestic wastewater. Clean Technol. Environ. Policy 2020, 23, 3–17. [Google Scholar] [CrossRef]
- Wang, J.-H.; Zhang, T.-Y.; Dao, G.-H.; Xu, X.-Q.; Wang, X.-X.; Hu, H.Y. Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl. Microbiol. Biotechnol. 2017, 101, 2659–2675. [Google Scholar] [CrossRef]
- Guilhen, J.; Al Bitar, A.; Sauvage, S.; Parrens, M.; Martinez, J.-M.; Abril, G.; Moreira-Turcq, P.; Sánchez-Pérez, J.-M. Denitrification and associated nitrous oxide and carbon dioxide emissions from the Amazonian wetlands. Biogeosciences 2020, 17, 4297–4311. [Google Scholar] [CrossRef]
- Farrelly, D.J.; Everard, C.D.; Fagan, C.C.; McDonnell, K.P. Carbon sequestration and the role of biological carbon mitigation: A review. Renew. Sustain. Energy Rev. 2013, 21, 712–727. [Google Scholar] [CrossRef]
- Kumar, M.; Sundaram, S.; Gnansounou, E.; Larroche, C.; Thakur, I.S. Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: A review. Bioresour. Technol. 2018, 247, 1059–1068. [Google Scholar] [CrossRef]
- Sillman, J.; Nygren, L.; Kahiluoto, H.; Ruuskanen, V.; Tamminen, A.; Bajamundi, C.; Nappa, M.; Wuokko, M.; Lindh, T.; Vainikka, P.; et al. Bacterial protein for food and feed generated via renewable energy and direct air capture of CO2: Can it reduce land and water use? Glob. Food Secur. 2019, 22, 25–32. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Zhang, Y.; Lv, M.; Joël Roland Kinhoun, J.; Qian, T.; Fan, B. Constructed wetland treatment of source separated washing wastewater in rural areas of southern China. Sep. Purif. Technol. 2021, 272, 118725. [Google Scholar] [CrossRef]
- Fu, G.; Yu, T.; Huangshen, L.; Han, J. The influence of complex fermentation broth on denitrification of saline sewage in constructed wetlands by heterotrophic nitrifying/aerobic denitrifying bacterial communities. Bioresour. Technol. 2018, 250, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, H.; Yan, B.; Shutes, B.; Xing, D.; Banuelos, G.; Cheng, R.; Wang, X. Greenhouse gas emissions and wastewater treatment performance by three plant species in subsurface flow constructed wetland mesocosms. Chemosphere 2020, 239, 124795. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, R.; Jia, L.; Wu, H. Can biochar application improve nitrogen removal in constructed wetlands for treating anaerobically-digested swine wastewater? Chem. Eng. J. 2020, 379, 122273. [Google Scholar] [CrossRef]
- Yang, R.; Liu, M.; Yang, Q. Microbial fuel cell affected the filler pollution accumulation of constructed wetland in the lab-scale and pilot-scale coupling reactors. Chem. Eng. J. 2022, 429, 132208. [Google Scholar] [CrossRef]
- Ji, X.; Jiang, M.; Zhang, J.; Jiang, X.; Zheng, Z. The interactions of algae-bacteria symbiotic system and its effects on nutrients removal from synthetic wastewater. Bioresour. Technol. 2018, 247, 44–50. [Google Scholar] [CrossRef]
- Huang, W.; Li, B.; Zhang, C.; Zhang, Z.; Lei, Z.; Lu, B.; Zhou, B. Effect of algae growth on aerobic granulation and nutrients removal from synthetic wastewater by using sequencing batch reactors. Bioresour. Technol. 2015, 179, 187–192. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of Microalgae in Global CO2 Sequestration: Physiological Mechanism, Recent Development, Challenges, and Future Prospective. Sustainability 2021, 13, 13061. [Google Scholar] [CrossRef]
- Kumar, M.; Ghosh, P.; Khosla, K.; Thakur, I.S. Biodiesel production from municipal secondary sludge. Bioresour. Technol. 2016, 216, 165–171. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Dulta, K.; Kurniawan, S.B.; Omoarukhe, F.O.; Ewuzie, U.; Eshiemogie, S.O.; Ojo, A.U.; Abdullah, S.R.S. Progress in Microalgae Application for CO2 Sequestration. Clean. Chem. Eng. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Liu, L.-N. Advances in the bacterial organelles for CO2 fixation. Trends Microbiol. 2022, 30, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Gudmundsson, S.; Wichuk, K.; Palsson, S.; Palsson, B.O.; Salehi-Ashtiani, K.; Brynjólfsson, S. Sugar-stimulated CO2 sequestration by the green microalga Chlorella vulgaris. Sci. Total Environ. 2019, 654, 275–283. [Google Scholar] [CrossRef]
- Yadav, G.; Karemore, A.; Dash, S.K.; Sen, R. Performance evaluation of a green process for microalgal CO2 sequestration in closed photobioreactor using flue gas generated in-situ. Bioresour. Technol. 2015, 191, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.A.; Camargo, E.C.; Crnkovic, P.C.G.M.; Lombardi, A.T. Physiological and Biochemical Responses of Chlorella vulgaris to Real Cement Flue Gas Under Controlled Conditions. Water Air Soil Pollut. 2018, 229, 259. [Google Scholar] [CrossRef]
- García-Cubero, R.; Moreno-Fernández, J.; García-González, M. Potential of Chlorella vulgaris to Abate Flue Gas. Waste Biomass Valorization 2017, 9, 2015–2019. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, Z.; Zhou, J.; Cen, K. Improving the CO2 fixation rate by increasing flow rate of the flue gas from microalgae in a raceway pond. Korean J. Chem. Eng. 2017, 35, 498–502. [Google Scholar] [CrossRef]
- Van, T.; Do, C.; Dinh, C.T.; Dang, M.T.; Dang Tran, T.; Giang Le, T. A novel flat-panel photobioreactor for simultaneous production of lutein and carbon sequestration by Chlorella sorokiniana TH01. Bioresour. Technol. 2022, 345, 126552. [Google Scholar] [CrossRef]
- Azizi, M.; Moteshafi, H.; Hashemi, M. A novel CO2 steady feeding based on the pH steady strategy data in the Haematococcus pluvialis cultivation to maximize the cell growth and carbon bio-sequestration. Bioresour. Technol. 2020, 314, 123752. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Shukla, S.P.; Rathi Bhuvaneswari, G.; Kumar, S.; Xavier, M.; Kumar, M. High value pigment production and carbon sequestration through wastewater grown Spirulina (Arthrospira) platensis: A green technology for wastewater utilization. Waste Manag. Bull. 2023, 1, 1–10. [Google Scholar] [CrossRef]
- Ma, S.; Yu, Y.; Cui, H.; Yadav, R.S.; Li, J.; Feng, Y. Unsterilized sewage treatment and carbohydrate accumulation in Tetradesmus obliquus PF3 with CO2 supplementation. Algal Res. 2020, 45, 101741. [Google Scholar] [CrossRef]
- Schipper, K.; Al Muraikhi, M.; Alghasal, G.S.H.S.; Saadaoui, I.; Bounnit, T.; Rasheed, R.; Dalgamouni, T.; Al Jabri, H.M.S.J.; Wijffels, R.H.; Barbosa, M.J. Potential of novel desert microalgae and cyanobacteria for commercial applications and CO2 sequestration. J. Appl. Phycol. 2019, 31, 2231–2243. [Google Scholar] [CrossRef]
- Upendar, G.; Singh, S.; Chakrabarty, J.; Chandra Ghanta, K.; Dutta, S.; Dutta, A. Sequestration of carbon dioxide and production of biomolecules using cyanobacteria. J. Environ. Manag. 2018, 218, 234–244. [Google Scholar] [CrossRef]
- Bilanovic, D.; Holland, M.; Starosvetsky, J.; Armon, R. Co-cultivation of microalgae and nitrifiers for higher biomass production and better carbon capture. Bioresour. Technol. 2016, 220, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Satpati, G.G.; Pal, R. Co-Cultivation of Leptolyngbya tenuis (Cyanobacteria) and Chlorella ellipsoidea (Green alga) for Biodiesel Production, Carbon Sequestration, and Cadmium Accumulation. Curr. Microbiol. 2021, 78, 1466–1481. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.; Ryu, A.J.; Jeong, K.J.; Lee, B.; Chang, Y.-K. Co-cultivation of two freshwater microalgae species to improve biomass productivity and biodiesel production. Energy Convers. Manag. 2019, 196, 640–648. [Google Scholar] [CrossRef]
- Ferro, L.; Colombo, M.; Posadas, E.; Funk, C.; Muñoz, R. Elucidating the symbiotic interactions between a locally isolated microalga Chlorella vulgaris and its co-occurring bacterium Rhizobium sp. in synthetic municipal wastewater. J. Appl. Phycol. 2019, 31, 2299–2310. [Google Scholar] [CrossRef]
- Dao, G.-H.; Wu, G.-X.; Wang, X.-X.; Zhang, T.-Y.; Zhan, X.-M.; Hu, H.-Y. Enhanced microalgae growth through stimulated secretion of indole acetic acid by symbiotic bacteria. Algal Res. 2018, 33, 345–351. [Google Scholar] [CrossRef]
- Fuentes, J.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-del-Valle, M.; Vílchez, C. Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xue, Z.; Sun, S.; Zhao, C.; Liu, J.; Liu, J.; Zhao, Y. Co-culturing microalgae with endophytic bacteria increases nutrient removal efficiency for biogas purification. Bioresour. Technol. 2020, 314, 123766. [Google Scholar] [CrossRef] [PubMed]
- Maghzian, A.; Aslani, A.; Zahedi, R. A comprehensive review on effective parameters on microalgae productivity and carbon capture rate. J. Environ. Manag. 2024, 355, 120539. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Xi, L.; Liu, D.; Huang, W.; Lei, Z.; Zhang, Z.; Huang, W. Effects of light intensity on oxygen distribution, lipid production and biological community of algal-bacterial granules in photo-sequencing batch reactors. Bioresour. Technol. 2019, 272, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Akizuki, S.; Kishi, M.; Cuevas-Rodríguez, G.; Toda, T.J.W.R. Effects of different light conditions on ammonium removal in a consortium of microalgae and partial nitrifying granules. Water Res. 2019, 171, 115445. [Google Scholar] [CrossRef]
- Cheng, C.L.; Lo, Y.C.; Huang, K.L.; Nagarajan, D.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Effect of pH on biomass production and carbohydrate accumulation of Chlorella vulgaris JSC-6 under autotrophic, mixotrophic, and photoheterotrophic cultivation. Bioresour. Technol. 2022, 351, 127021. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yuan, Q.; Rein, A. Removal of nitrogen from wastewater using microalgae and microalgae–bacteria consortia. Cogent Environ. Sci. 2017, 2, 1275089. [Google Scholar] [CrossRef]
- Rezvani, F.; Sarrafzadeh, M.-H.; Ebrahimi, S.; Oh, H.-M. Nitrate removal from drinking water with a focus on biological methods: A review. Environ. Sci. Pollut. Res. 2017, 26, 1124–1141. [Google Scholar] [CrossRef] [PubMed]
- Sepehri, A.; Sarrafzadeh, M.-H. Activity enhancement of ammonia-oxidizing bacteria and nitrite-oxidizing bacteria in activated sludge process: Metabolite reduction and CO2 mitigation intensification process. Appl. Water Sci. 2019, 9, 131. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Microalgae–bacteria biofilms: A sustainable synergistic approach in remediation of acid mine drainage. Appl. Microbiol. Biotechnol. 2017, 102, 1131–1144. [Google Scholar] [CrossRef]
- Katam, K.; Bhattacharyya, D. Simultaneous treatment of domestic wastewater and bio-lipid synthesis using immobilized and suspended cultures of microalgae and activated sludge. J. Ind. Eng. Chem. 2019, 69, 295–303. [Google Scholar] [CrossRef]
- Luo, L.; Lin, X.; Zeng, F.; Luo, S.; Chen, Z.; Tian, G. Performance of a novel photobioreactor for nutrient removal from piggery biogas slurry: Operation parameters, microbial diversity and nutrient recovery potential. Bioresour. Technol. 2019, 272, 421–432. [Google Scholar] [CrossRef]
- Huang, L.; Lu, Z.; Xie, T.; Wang, L.; Mo, C. Nitrogen and phosphorus removal by coupling Anaerobic ammonia oxidation reaction with algal-bacterial symbiotic system. J. Environ. Chem. Eng. 2022, 10, 108905. [Google Scholar] [CrossRef]
- Jiang, W.; Ma, Y.; Nie, Z.; Wang, N.; Yu, G.; Shi, X.; Bian, D. Improving nitrogen and phosphorus removal and sludge reduction in new integrated sewage treatment facility by adjusting biomass concentration. J. Water Process Eng. 2022, 50, 103203. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Xu, G.; Gao, F. Simultaneous nitritation, denitritation and phosphorus removal in an algal-bacterial consortium system treating low-strength mariculture wastewater. J. Water Process Eng. 2022, 49, 103056. [Google Scholar] [CrossRef]
- Ji, X.; Li, H.; Zhang, J.; Saiyin, H.; Zheng, Z. The collaborative effect of Chlorella vulgaris-Bacillus licheniformis consortia on the treatment of municipal water. J. Hazard. Mater. 2019, 365, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhu, L.; Ji, B. Deciphering the effect of light intensity on microalgal-bacterial granular sludge process for non-aerated municipal wastewater treatment. Algal Res. 2021, 58, 102437. [Google Scholar] [CrossRef]
- Qiao, S.; Hou, C.; Wang, X.; Zhou, J. Minimizing greenhouse gas emission from wastewater treatment process by integrating activated sludge and microalgae processes. Sci. Total Environ. 2020, 732, 139032. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.L.; Park, J.; Ralph, P.J.; Craggs, R.J. Improved microalgal productivity and nutrient removal through operating wastewater high rate algal ponds in series. Algal Res. 2020, 47, 101850. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Economou, C.N.; Stefanidou, N.; Moustaka-Gouni, M.; Genitsaris, S.; Aggelis, G.; Tekerlekopoulou, A.G.; Vayenas, D.V. A semi-continuous algal-bacterial wastewater treatment process coupled with bioethanol production. J. Environ. Manag. 2023, 326, 116717. [Google Scholar] [CrossRef]
- Qi, F.; Jia, Y.; Mu, R.; Ma, G.; Guo, Q.; Meng, Q.; Yu, G.; Xie, J. Convergent community structure of algal–bacterial consortia and its effects on advanced wastewater treatment and biomass production. Sci. Rep. 2021, 11, 21118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huo, H.; Meng, F. Partial Nitrification Algal-Bacterial Granule System Cultivation: Performance, Lipid Production and Biological Community. Water Air Soil Pollut. 2020, 231, 236. [Google Scholar] [CrossRef]
- Marazzi, F.; Bellucci, M.; Fantasia, T.; Ficara, E.; Mezzanotte, V. Interactions between Microalgae and Bacteria in the Treatment of Wastewater from Milk Whey Processing. Water 2020, 12, 297. [Google Scholar] [CrossRef]
- Huo, S.; Kong, M.; Zhu, F.; Qian, J.; Huang, D.; Chen, P.; Ruan, R. Co-culture of Chlorella and wastewater-borne bacteria in vinegar production wastewater: Enhancement of nutrients removal and influence of algal biomass generation. Algal Res. 2020, 45, 101744. [Google Scholar] [CrossRef]
- Siwek, M.; Edgecock, T. Application of electron beam water radiolysis for sewage sludge treatment—A review. Environ. Sci. Pollut. Res. 2020, 27, 42424–42448. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jiao, S.; Ma, M.; Peng, S. Microbial fuel cell system: A promising technology for pollutant removal and environmental remediation. Environ. Sci. Pollut. Res. 2020, 27, 6749–6764. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, Y.; Yuan, F.; Yang, Q. Optimization and control strategies of aeration in WWTPs: A review. J. Clean. Prod. 2023, 418, 138008. [Google Scholar] [CrossRef]
- Khiewwijit, R.; Temmink, H.; Rijnaarts, H.; Keesman, K.J. Energy and nutrient recovery for municipal wastewater treatment: How to design a feasible plant layout? Environ. Model. Softw. 2015, 68, 156–165. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Y.; Li, X.; Luo, P.; Wang, H.; Wang, X.; Wu, J.; Li, F. Energy Self-sufficient Wastewater Treatment Plants: Feasibilities and Challenges. Energy Procedia 2017, 105, 3741–3751. [Google Scholar] [CrossRef]
- Peng, L.; Lan, C.Q.; Zhang, Z. Evolution, detrimental effects, and removal of oxygen in microalga cultures: A review. Environ. Prog. Sustain. Energy 2013, 32, 982–988. [Google Scholar] [CrossRef]
- Liang, W.; Yu, C.; Ren, H.; Geng, J.; Ding, L.; Xu, K. Minimization of nitrous oxide emission from CASS process treating low carbon source domestic wastewater: Effect of feeding strategy and aeration rate. Bioresour. Technol. 2015, 198, 172–180. [Google Scholar] [CrossRef]
- Kyung, D.; Kim, M.; Chang, J.; Lee, W. Estimation of greenhouse gas emissions from a hybrid wastewater treatment plant. J. Clean. Prod. 2015, 95, 117–123. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Ngo, H.H.; Guo, W.; Nghiem, L.D.; Qian, G.; Liu, Q.; Liu, J.; Chen, Z.; Bui, X.T.; Mainali, B. Assessing the environmental impacts and greenhouse gas emissions from the common municipal wastewater treatment systems. Sci. Total Environ. 2021, 801, 149676. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Z.; Qi, Y.; Song, C.; Chen, G. The interactions of algae-activated sludge symbiotic system and its effects on wastewater treatment and lipid accumulation. Bioresour. Technol. 2019, 292, 122017. [Google Scholar] [CrossRef]
- Choix, F.J.; López-Cisneros, C.G.; Méndez-Acosta, H.O. Azospirillum brasilense Increases CO2 Fixation on Microalgae Scenedesmus obliquus, Chlorella vulgaris, and Chlamydomonas reinhardtii Cultured on High CO2 Concentrations. Microb. Ecol. 2018, 76, 430–442. [Google Scholar] [CrossRef]
- Tanikawa, D.; Syutsubo, K.; Watari, T.; Miyaoka, Y.; Hatamoto, M.; Iijima, S.; Fukuda, M.; Nguyen, N.B.; Yamaguchi, T. Greenhouse gas emissions from open-type anaerobic wastewater treatment system in natural rubber processing factory. J. Clean. Prod. 2016, 119, 32–37. [Google Scholar] [CrossRef]
- Liu, J.; Tian, H.; Luan, X.; Zhou, X.; Chen, X.; Xu, S.; Kang, X. Submerged anaerobic membrane bioreactor for low-concentration domestic sewage treatment: Performance and membrane fouling. Environ. Sci. Pollut. Res. 2019, 27, 6785–6795. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Z.; Zhu, L.; Ye, T.; Zuo, J.; Li, X.; Xiao, B.; Jin, S. Vertical-algal-biofilm enhanced raceway pond for cost-effective wastewater treatment and value-added products production. Water Res. 2018, 139, 144–157. [Google Scholar] [CrossRef]
- Xu, K.; Zou, X.; Xue, Y.; Qu, Y.; Li, Y. The impact of seasonal variations about temperature and photoperiod on the treatment of municipal wastewater by algae-bacteria system in lab-scale. Algal Res. 2021, 54, 102175. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Riddicka, B.; Li, R.; Able, J.; Boakye-Boaten, N.; Shahbazi, A. Sustainable Production of Algal Biomass and Biofuels Using Swine Wastewater in North Carolina, US. Sustainability 2016, 8, 477. [Google Scholar] [CrossRef]
- Choi, K.-J.; Han, T.H.; Yoo, G.; Cho, M.H.; Hwang, S.-J. Co-culture Consortium of Scenedesmus dimorphus and Nitrifiers Enhances the Removal of Nitrogen and Phosphorus from Artificial Wastewater. KSCE J. Civ. Eng. 2017, 22, 3215–3221. [Google Scholar] [CrossRef]
- Sial, A.; Zhang, B.; Zhang, A.; Liu, K.; Imtiaz, S.A.; Yashir, N. Microalgal–Bacterial Synergistic Interactions and Their Potential Influence in Wastewater Treatment: A Review. BioEnergy Res. 2020, 14, 723–738. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.-O.; et al. Wastewater based microalgal biorefinery for bioenergy production: Progress and challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef] [PubMed]
- Sutapa, B.M.; Dhruti, A.; Gopa, R.B. Pharmacological, pharmaceutical, cosmetic and diagnostic applications of sulfated polysaccharides from marine algae and bacteria. Afr. J. Pharm. Pharmacol. 2017, 11, 68–77. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Gururani, P.; Singh, N.; Gautam, P.; Vlaskin, M.S.; Kumar, V. Review on microalgae protein and its current and future utilisation in the food industry. Int. J. Food Sci. Technol. 2024, 59, 473–480. [Google Scholar] [CrossRef]
- Geada, P.; Moreira, C.; Silva, M.; Nunes, R.; Teixeira, J.A. Algal proteins: Production strategies and nutritional and functional properties. Bioresour. Technol. 2021, 332, 125125. [Google Scholar] [CrossRef]
- Acquah, C.; Tibbetts, S.M.; Pan, S.; Udenigwe, C. Nutritional quality and bioactive properties of proteins and peptides from microalgae. In Handbook of Microalgae-Based Processes and Products; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Shahid, A.; Khan, F.; Ahmad, N.; Farooq, M.; Mehmood, M.A. Microalgal Carbohydrates and Proteins: Synthesis, Extraction, Applications, and Challenges. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 433–468. [Google Scholar] [CrossRef]
- Durmaz, Y.; Kilicli, M.; Toker, O.S.; Konar, N.; Palabiyik, I.; Tamtürk, F. Using spray-dried microalgae in ice cream formulation as a natural colorant: Effect on physicochemical and functional properties. Algal Res. 2020, 47, 101811. [Google Scholar] [CrossRef]
- Olguín, E.J.; Sánchez-Galván, G.; Arias-Olguín, I.I.; Melo, F.J.; González-Portela, R.E.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-Based Biorefineries: Challenges and Future Trends to Produce Carbohydrate Enriched Biomass, High-Added Value Products and Bioactive Compounds. Biology 2022, 11, 1146. [Google Scholar] [CrossRef]
- Mu, R.; Jia, Y.; Ma, G.; Liu, L.; Hao, K.; Qi, F.; Shao, Y. Advances in the use of microalgal–bacterial consortia for wastewater treatment: Community structures, interactions, economic resource reclamation, and study techniques. Water Environ. Res. 2021, 93, 1217–1230. [Google Scholar] [CrossRef]
- Berthold, D.E.; Shetty, K.G.; Jayachandran, K.; Laughinghouse, H.D.; Gantar, M. Enhancing algal biomass and lipid production through bacterial co-culture. Biomass Bioenergy 2019, 122, 280–289. [Google Scholar] [CrossRef]
- Liu, W.; Ji, Y.; Long, Y.; Huang, W.; Zhang, C.; Wang, H.; Xu, Y.; Lei, Z.; Huang, W.; Liu, D. The role of light wavelengths in regulating algal-bacterial granules formation, protein and lipid accumulation, and microbial functions. J. Environ. Manag. 2023, 337, 117750. [Google Scholar] [CrossRef] [PubMed]
- Sittijunda, S.; Sitthikitpanya, N.; Plangklang, P.; Reungsang, A. Two-Stage Anaerobic Codigestion of Crude Glycerol and Micro-Algal Biomass for Biohydrogen and Methane Production by Anaerobic Sludge Consortium. Fermentation 2021, 7, 175. [Google Scholar] [CrossRef]
- Rathinavelu, V.; Kulandaivel, A.; Pandey, A.K.; Bhatt, R.; De Poures, M.V.; Hossain, I.; Seikh, A.H.; Iqbal, A.; Murugan, P. Production of green hydrogen from sewage sludge / algae in agriculture diesel engine: Performance Evaluation. Heliyon 2024, 10, e23988. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, H.; Kang, S.; Kim, K.; Park, J. Shockwave pre-treatment enhances the physicochemical availability and anaerobic mono- and co-digestion of highly concentrated algae. J. Environ. Chem. Eng. 2022, 10, 108993. [Google Scholar] [CrossRef]
- Khanthong, K.; Kadam, R.; Kim, T.; Park, J. Synergetic effects of anaerobic co-digestion of food waste and algae on biogas production. Bioresour. Technol. 2023, 382, 129208. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Li, X.; Zhang, D.; Chen, Y.; Dai, L. Simultaneous enhancement of methane production and methane content in biogas from waste activated sludge and perennial ryegrass anaerobic co-digestion: The effects of pH and C/N ratio. Bioresour. Technol. 2016, 216, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Ding, W.; Shi, Z.; Zhao, S. Methane enhancement through co-digestion of chicken manure and thermo-oxidative cleaved wheat straw with waste activated sludge: A C/N optimization case. Bioresour. Technol. 2016, 211, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Selvaratnam, T.; Pegallapati, A.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Lammers, P.J.; van Voorhies, W. Feasibility of algal systems for sustainable wastewater treatment. Renew. Energy 2015, 82, 71–76. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Wei, W.; Wang, Y.; Ni, B.-J. Enhancing methane production from algae anaerobic digestion using diatomite. J. Clean. Prod. 2021, 315, 128138. [Google Scholar] [CrossRef]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Bio/Technol. 2015, 14, 727–759. [Google Scholar] [CrossRef]
- Song, Y.; Ahmad, S.F.; Abou Houran, M.; Agrawal, M.K.; Nutakki, T.U.K.; Siddiqui, M.R.; Albani, A.; Su, Q. Multi-variable study of a novel multigeneration system using biogas separation unit and LNG cold energy utilization, producing electricity, cooling, heat, fresh water, liquid CO2, biomethane, and methanol. Process Saf. Environ. Prot. 2023, 180, 616–638. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wallace, H.M.; Xu, C.Y.; Zwieten, L.V.; Weng, Z.H.; Xu, Z.; Che, R.; Tahmasbian, I.; Hu, H.W.; Bai, S.H. The effects of short term, long term and reapplication of biochar on soil bacteria. Sci. Total Environ. 2018, 636, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kam, Y.L.; Agutaya, J.K.C.N.; Quitain, A.T.; Ogasawara, Y.; Sasaki, M.; Lam, M.K.; Yusup, S.; Assabumrungrat, S.; Kida, T. In-situ transesterification of microalgae using carbon-based catalyst under pulsed microwave irradiation. Biomass Bioenergy 2023, 168, 106662. [Google Scholar] [CrossRef]
- Sengupta, S.; Nawaz, T.; Beaudry, J. Nitrogen and Phosphorus Recovery from Wastewater. Curr. Pollut. Rep. 2015, 1, 155–166. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.R.; Thamarai, P.; Abirami, B.; George, C.S. A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef]


| Country | CH4 Emission (MMT CO2eq/yr) | N2O Emission (MMT CO2eq/yr) | Total Emissions (MMT CO2eq/yr) | Ref. |
|---|---|---|---|---|
| China | - | - | 53.0 | [18] |
| U.S. | 20.8 | 21.9 | - | [19] |
| Japan | - | - | 3.499 | [20] |
| UK | - | - | 5.0 | [21] |
| Australia | - | - | 2.97 | [22] |
| Netherlands | - | - | 1.95 | [23] |
| Nepal | 3.48 | 3.48 | 3.83 | [24] |
| Europe | - | - | 35.0 | [25] |
| Mexico | 11.12 | - | 12.4 | [26] |
| Iran | 3.36 | 0.49 | 4.83 | [27] |
| Method | Cost | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|
| Biological method | Low | Energy neutralization | Low capacity, many influenced factors | [40] |
| Artificial pre-combustion | High, 24–52 Euros/t CO2 | Compact equipment, high CO2 concentration and energy saving | Secondary pollution | [43,44,45,46] |
| Artificial post-combustion | Simple operation, wide application and least investment | High energy consumption, low CO2 concentration | ||
| Artificial oxygen-rich combustion | Highest CO2 concentration | High energy consumption |
| Species | Reactor | CO2 % | Yield g SS/L·d | C-Fixation mg CO2/L·d | Ref. | |
|---|---|---|---|---|---|---|
| C. Vulgaris | A | BCPBR | 2.5 | 1860 | 3510 | [83] |
| Chlorella sp. | A | BCPBR | Air | 212–216 | 191–201 | [84] |
| C. vulgaris | A | PBR | 8–9 | 1190–1350 | - | [85] |
| C. vulgaris | A | BCPBR | 12 | 502 | 919 | [86] |
| N. oculata | A | Raceway | 10–14 | 17,100 | 31,900 | [87] |
| C. sorokiniana TH01 | A | FPPBR | 5 | 284–469 | - | [88] |
| H. pluvialis | A | PBR | 5 | 250 | 613 | [89] |
| S. platensis | A | PBR | - | 68.4–78.4 | 107.3–122.9 | [90] |
| T. obliquus PF3 | A | CPBR | 10 | 310 | 550–552 | [91] |
| L. sp. QUCCCM 56 | B | PBR | - | 81.8–101 | 130.7–165.3 | [92] |
| S. sp. NIT18 | B | PBR | 10 | 22.5 | 69.4% | [93] |
| B | PBR | 15 | 370 | 71.0% | ||
| C. vulgaris & nitrifier | AB | PBR | Air | 531 | - | [94] |
| L. tenuis & C. ellipsoidea | AB | PBR | Air | 192.3–201.1 | 2540–2720 | [95] |
| C. ellipsoidea (A)/ L. tenuis (B) | 1:1 | BCPBR | 2 | 470–610 | - | [96] |
| 1:4 | BCPBR | 2 | 570–590 | - | ||
| 1:8 | BCPBR | 2 | 680–720 | - | ||
| 1:16 | BCPBR | 2 | 540–560 | - | ||
| Reactor | Wastewater | TN | TP | Ref. | ||
|---|---|---|---|---|---|---|
| Inf. (mg/L) | Removal Rate (%) | Inf. (mg/L) | Removal Rate (%) | |||
| UABR-PSBR | Swine | 580–951 | 95.0 | 10–17 | 91.0 | [111] |
| MPSR | Rural | 56.9 | 89.9 | 2.1–4.6 | 98.2 | [112] |
| PSBR | Aquaculture | 6–14 | 78.4 | 0.4–0.7 | 68.2 | [113] |
| PBR | Municipal | 70–80 | 88.8 | 6–6.5 | 84.9 | [114] |
| PSBR | Synthetic | 30 | 80.7 | 5 | 73.9 | [115] |
| MA/AS | Synthetic | 20 | 87.0 | 2 | 99.6 | [116] |
| PBR | Synthetic | 31.23 | 88.9 | 5.0 | 80.3 | [77] |
| HRAP | Digested | 38.1 | 73.8 | 5.0 | 89.8 | [117] |
| PBR | Brewery | 96.2 | 94.2 | 8.6 | 75.2 | [118] |
| PSBR | Secondary | 20–40 | 73.7 | 3–5 | 94.4 | [119] |
| PSBR | Synthetic | 50–200 | 71.3 | 10 | - | [120] |
| PBR | Whey processing | 52 | 88.0 | 17 | 69 | [121] |
| PBR | Vinegar processing | 20.5 | 78.7 | 7.4 | 74.8 | [122] |
| Technology | Species | Aeration | GHG Emission | Ref. |
|---|---|---|---|---|
| OAC | Bacteria | yes | 57.7–60.8% CH4 329–423 mgN2O/L 14.5–31.5% CO2 | [134] |
| AAO | Bacteria | yes | CH4, N2O, CO2 | [17] |
| MBR | Bacteria | yes | CH4, N2O, CO2 | [135] |
| SBR | Bacteria | yes | CH4, N2O, CO2 | [26] |
| CWs | Plants and bacteria | yes | 582 mg CO2/m2·h 22 mg CH4/m2·h 37 mg N2O/m2·h | [74] |
| Raceway | Algae | no | CO2 | [136] |
| PBR | Algae and bacteria | yes | CO2 | [137] |
| MA/AS | Algae and bacteria | no | 2% CO2 | [116] |
| HARP | Algae and bacteria | no | 0.7 kg CO2/m3 | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.; Wang, S.; Yang, H.; Sun, Y.; Chen, Z.; Xu, G.; Zhang, C. Carbon Capture and Resource Utilization by Algal–Bacterial Consortium in Wastewater Treatment: A Mini-Review. Water 2024, 16, 2197. https://doi.org/10.3390/w16152197
Yu T, Wang S, Yang H, Sun Y, Chen Z, Xu G, Zhang C. Carbon Capture and Resource Utilization by Algal–Bacterial Consortium in Wastewater Treatment: A Mini-Review. Water. 2024; 16(15):2197. https://doi.org/10.3390/w16152197
Chicago/Turabian StyleYu, Ting, Siya Wang, Hui Yang, Yuxin Sun, Zhongtai Chen, Guangjing Xu, and Cuiya Zhang. 2024. "Carbon Capture and Resource Utilization by Algal–Bacterial Consortium in Wastewater Treatment: A Mini-Review" Water 16, no. 15: 2197. https://doi.org/10.3390/w16152197
APA StyleYu, T., Wang, S., Yang, H., Sun, Y., Chen, Z., Xu, G., & Zhang, C. (2024). Carbon Capture and Resource Utilization by Algal–Bacterial Consortium in Wastewater Treatment: A Mini-Review. Water, 16(15), 2197. https://doi.org/10.3390/w16152197






