Optimization of Culture Conditions for Microalgae Treatment Fly Ash Leachate System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Pre Cultivation and Pretreatment of Microalgae
2.3. Experimental Group Setup
2.3.1. Sterilized/Unsterilized
2.3.2. Dilution Ratio of Leachate
2.3.3. Nutrients
2.4. Measurement of Indicators
2.5. Data Processing and Analysis
3. Results and Discussion
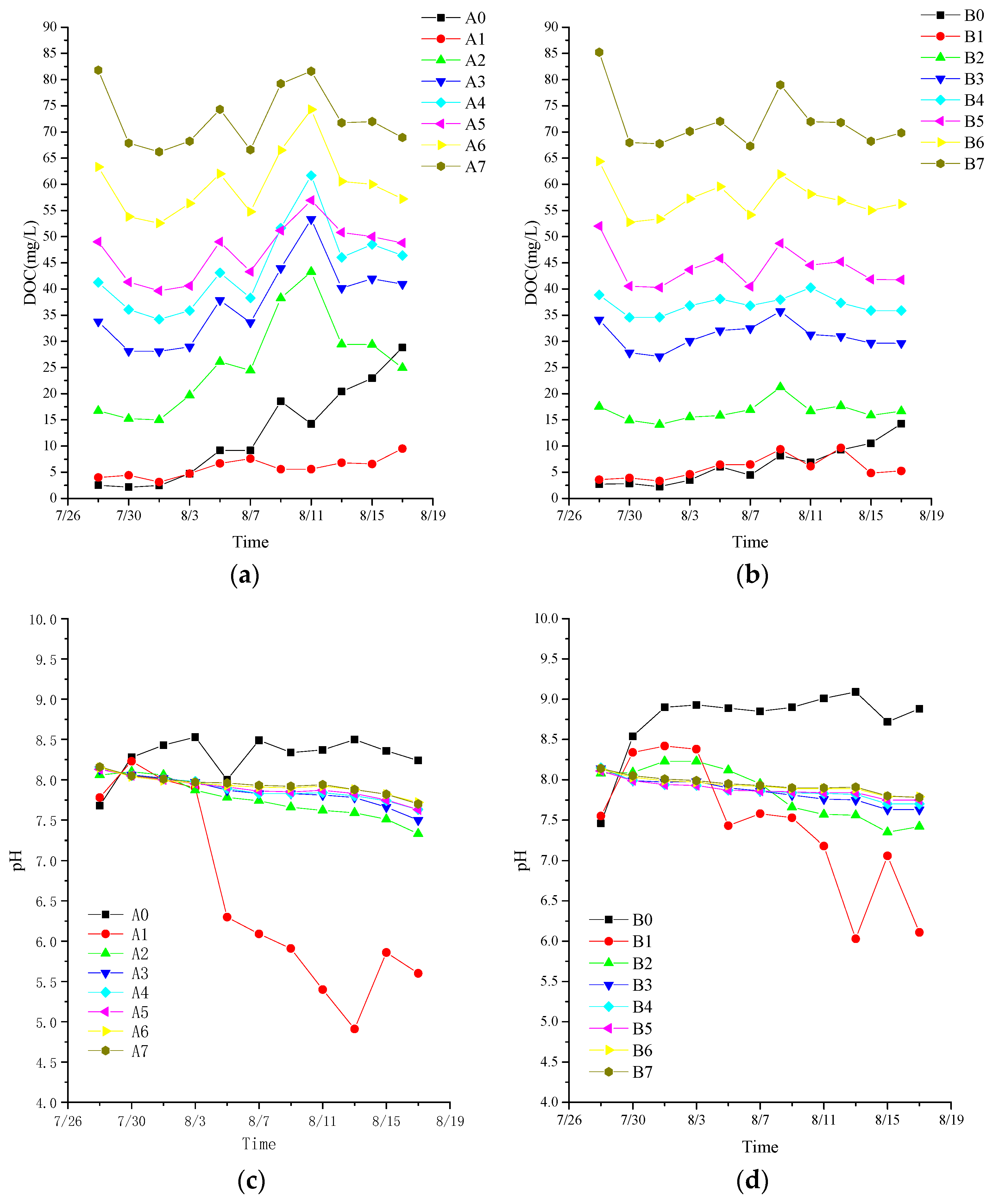
3.1. Effect of Sterilization on Algal Treatment of Leachate
3.1.1. Effect of Sterilization of Leachate on Algal Growth
3.1.2. Effect of Sterilization of Leachate on Pollutant Removal Efficiency
3.2. Effect of Initial Leachate Concentration on Algal Treatment
3.2.1. Algal Growth under Different Concentrations of Leachate
3.2.2. Influence of Initial Concentration of Leachate on Pollutant Removal Efficiency
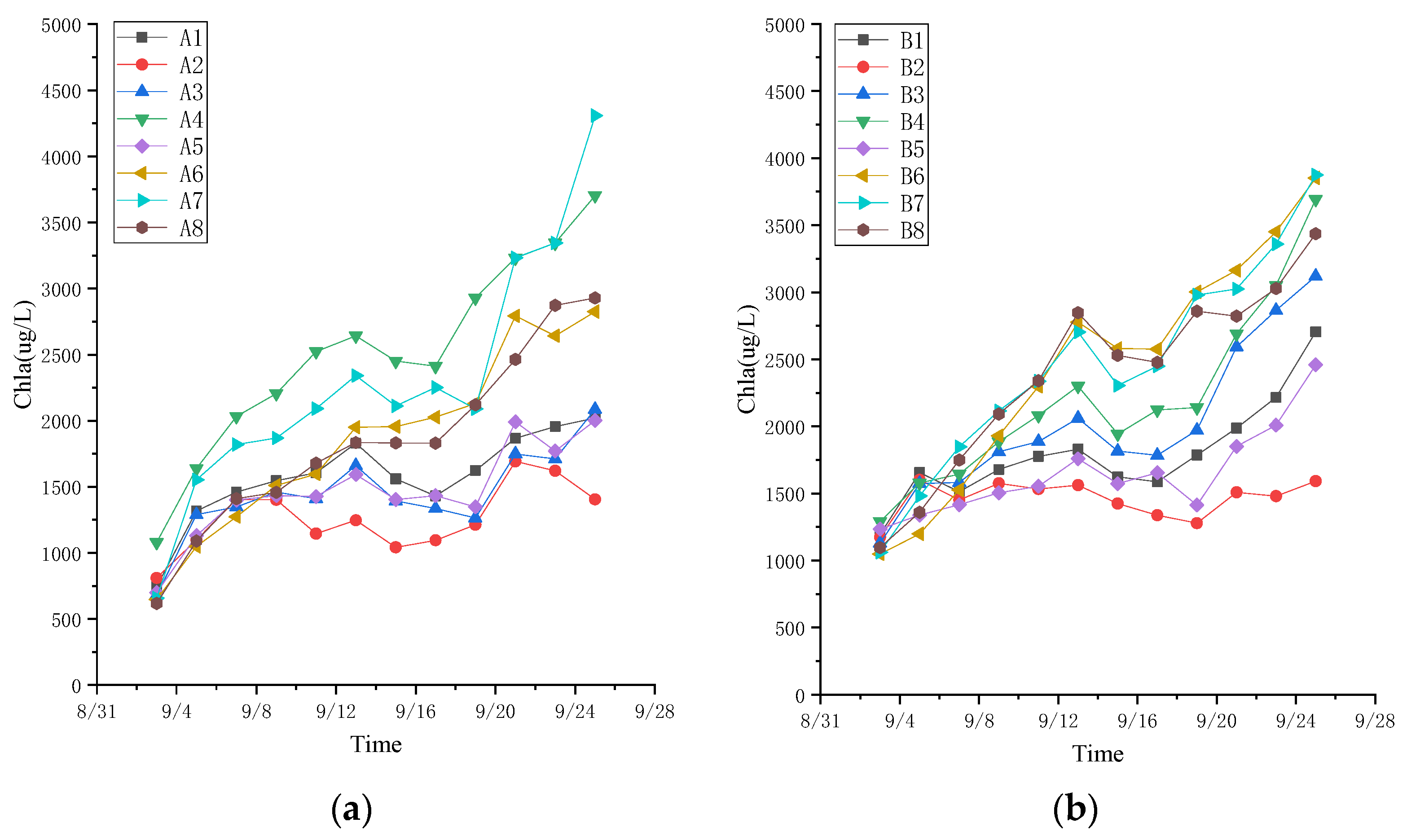
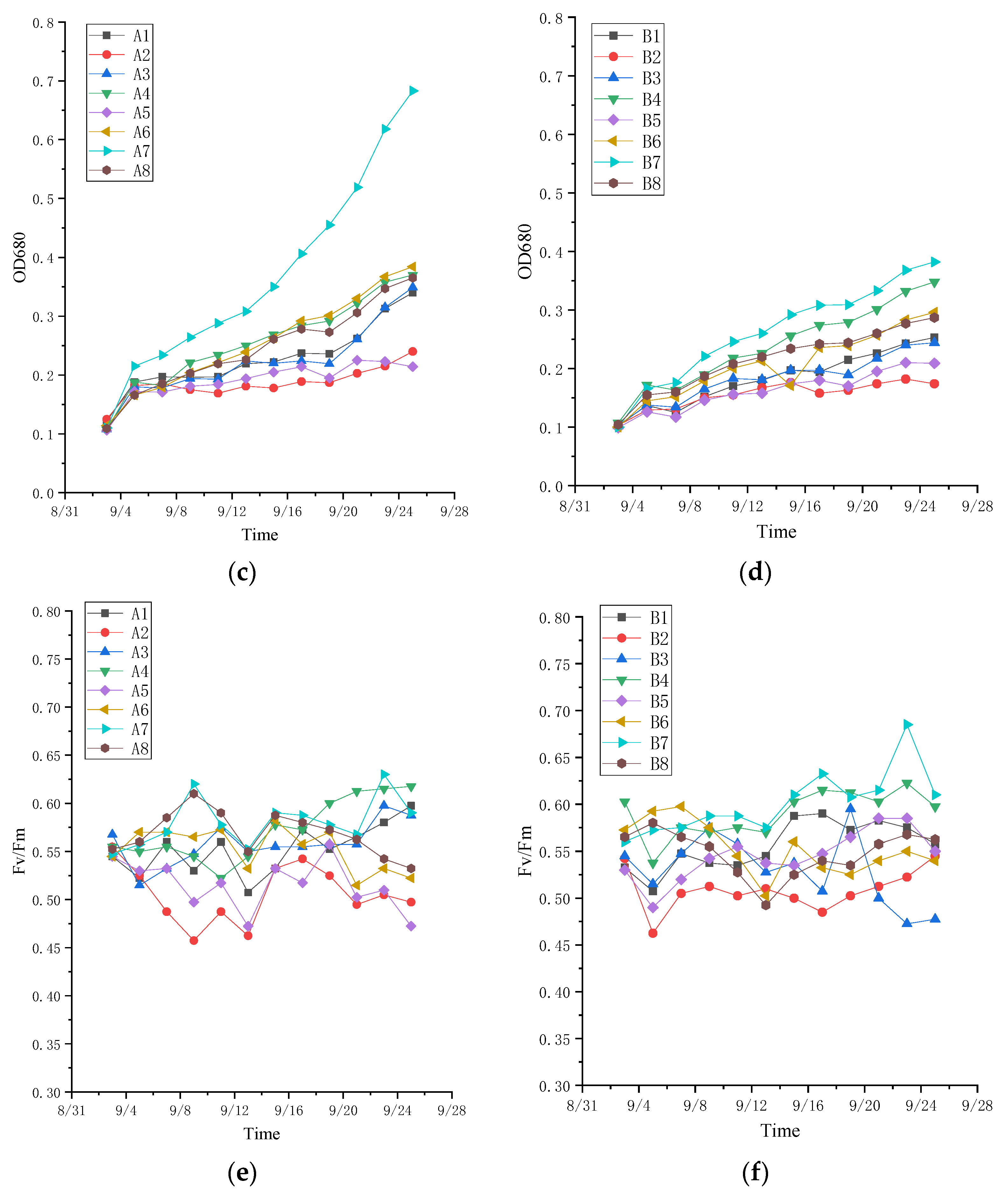
3.3. Effects of Additional Nutrients on Microalgae Treatment of Leachate System
3.3.1. Growth of Algae in the Presence of Added Nutrients
3.3.2. Effect of Added Nutrients on the Efficiency of Pollutant Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Kirkelund, G.M.; Jensen, P.E.; Ottosen, L.M. Comparison of different MSWI fly ash treatment processes on the thermal behavior of As, Cr, Pb and Zn in the ash. Waste Manag. 2017, 68, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Y.; Luo, G.Q.; Liu, H.; Qiao, Y.; Xu, M.H.; Yao, H. Fate of chromium during thermal treatment of municipal solid waste incineration (MSWI) fly ash. Proc. Combust. Inst. 2013, 34, 2795–2801. [Google Scholar] [CrossRef]
- Li, H.Y.; Gao, P.P.; Ni, H.G. Emission characteristics of parent and halogenated PAHs in simulated municipal solid waste incineration. Sci. Total Environ. 2019, 665, 11–17. [Google Scholar] [CrossRef]
- Ma, W.C.; Chen, D.M.; Pan, M.H.; Gu, T.B.; Zhong, L.; Chen, G.Y.; Yan, B.B.; Cheng, Z.J. Performance of chemical chelating agent stabilization and cement solidification on heavy metals in MSWI fly ash: A comparative study. J. Environ. Manag. 2019, 247, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.P.; Cheng, Q.Y.; Liu, M.J.; Li, L.; Ru, Y.; Yan, D.H. Industrial disposal processes for treatment of polychlorinated dibenzo-p-dioxins and dibenzofurans in municipal solid waste incineration fly ash. Chemosphere 2020, 243, 125351. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Du, B.; Baheiduola, A.; Geng, C.; Liu, J.G. HCB dechlorination combined with heavy metals immobilization in MSWI fly ash by using n-Al/CaO dispersion mixture. J. Hazard. Mater. 2020, 392, 122510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.K.; Li, A.M.; Wang, X.X.; Zhang, L. Stabilization/solidification of municipal solid waste incineration fly ash via co-sintering with waste-derived vitrified amorphous slag. Waste Manag. 2016, 56, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Huber, F.; Fellner, J. Integration of life cycle assessment with monetary valuation for resource classification: The case of municipal solid waste incineration fly ash. Resour. Conserv. Recycl. 2018, 139, 17–26. [Google Scholar] [CrossRef]
- Mathews, G.; Sinnan, R.; Young, M. Evaluation of reclaimed municipal solid waste incinerator sands in concrete. J. Clean. Prod. 2019, 229, 838–849. [Google Scholar] [CrossRef]
- Ke, H.; Zhang, C.S.; Hu, J.; Qin, R.; Chen, Y.M.; Lan, J.W. Evaluation of leachate production and level in municipal solid waste landfills considering secondary compression. Environ. Sci. Pollut. Res. 2022, 29, 20542–20555. [Google Scholar] [CrossRef]
- Budi, S.; Suliasih, B.A.; Othman, M.S.; Heng, L.Y.; Surif, S. Toxicity identification evaluation of landfill leachate using fish, prawn and seed plant. Waste Manag. 2016, 55, 231–237. [Google Scholar] [CrossRef]
- Toufexi, E.; Tsarpali, V.; Efthimiou, I.; Vidali, M.S.; Vlastos, D.; Dailianis, S. Environmental and human risk assessment of landfill leachate: An integrated approach with the use of cytotoxic and genotoxic stress indices in mussel and human cells. J. Hazard. Mater. 2013, 260, 593–601. [Google Scholar] [CrossRef]
- Kamal, A.; Makhatova, A.; Yergali, B.; Baidullayeva, A.; Satayeva, A.; Kim, J.; Inglezakis, V.J.; Poulopoulos, S.G.; Arkhangelsky, E. Biological Treatment, Advanced Oxidation and Membrane Separation for Landfill Leachate Treatment: A Review. Sustainability 2022, 14, 14427. [Google Scholar] [CrossRef]
- Han, M.N.; Duan, X.G.; Cao, G.L.; Zhu, S.S.; Ho, S.H. Graphitic nitride -catalyzed advanced oxidation processes (AOPs) for landfill leachate treatment: A mini review. Process Saf. Environ. 2020, 139, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.K.; Chen, Y.T.; Liu, Y.L.; Bowden, J.A.; Townsend, T.G.; Solo-Gabriele, H.M. Comparison of the PFAS and physical-chemical parameter fluctuations between an ash landfill and a MSW landfill. Waste Manag. 2024, 174, 558–567. [Google Scholar] [CrossRef]
- Yoriya, S.; Tepsri, P. Investigation of Metal and Trace Elements of Cenospheres from Lignite High-Calcium Fly Ash (Thailand). Water 2021, 13, 2935. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, X.S.; Dong, L.; Nai, C.X.; Liu, Y.Q.; Huang, Q.F. Long-term dynamics of leachate production, leakage from hazardous waste landfill sites and the impact on groundwater quality and human health. Waste Manag. 2018, 82, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Kholomyeva, M.; Vurm, R.; Tajnaiova, L.; Sir, M.; Maslova, M.; Koci, V. Phycoremediation of Landfill Leachate with Desmodesmus subspicatus: A Pre-Treatment for Reverse Osmosis. Water 2020, 12, 1755. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.S.; Tan, F.X.; Wu, D.J. Treatment of Landfill Leachate Using Activated Sludge Technology: A Review. Archaea 2018, 2018, 1039453. [Google Scholar] [CrossRef] [PubMed]
- El-Fadel, M.; Hashisho, J. A comparative examination of MBR and SBR performance for the treatment of high-strength landfill leachate. J. Air Waste Manag. 2014, 64, 1073–1084. [Google Scholar] [CrossRef]
- Zheng, D.Y.; Sun, Y.J.; Li, H.J.; Lu, S.; Shan, M.J.; Xu, S.W. Multistage A-O Activated Sludge Process for Paraformaldehyde Wastewater Treatment and Microbial Community Structure Analysis. J. Chem. 2016, 2016, 2746715. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, B.; Wang, Q.; Meng, X.; Wu, Q.; Zheng, T.; Huhe, T. Designing Multi-Stage 2 A/O-MBR Processes for a Higher Removal Rate of Pollution in Wastewater. Membranes 2022, 12, 377. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Willoughby, N. Effect of CO2 aeration on cultivation of microalgae in luminescent photobioreactors. Biomass Bioenergy 2016, 85, 168–177. [Google Scholar] [CrossRef]
- Feng, S.R.; Liu, F.; Zhu, S.N.; Xu, Z.B.; Qin, L.; Feng, P.Z.; Wang, Z.M.; Chen, H.; Guo, W.S.; Ngo, H.H. Role of hydraulic retention time in integration of microalgae and activated sludge process for nutrient recycle from diluted dairy liquid digestate. Chem. Eng. J. 2024, 484, 149538. [Google Scholar] [CrossRef]
- Emalya, N.; Mairiza, L.; Bilqis, P.Z.; Suhendrayatna, S.; Munawar, E.; Yunardi, Y. Removal of Organic and Nitrogen Compounds from Domestic Landfill Leachate by Microalgae. Biointerface Res. Appl. Chem. 2023, 13, 131. [Google Scholar] [CrossRef]
- Li, Y.P.; Wu, X.X.; Liu, Y.; Taidi, B. Immobilized microalgae: Principles, processes and its applications in wastewater treatment. World J. Microbiol. Biotechnol. 2024, 40, 150. [Google Scholar] [CrossRef] [PubMed]
- Garza-Valverde, E.; García-Gómez, C.; Nápoles-Armenta, J.; Samaniego-Moreno, L.; Martínez-Orozco, E.; De La Mora-Orozco, C. Nejayote and Food Waste Leachate as a Medium for Scenedesmus acutus and Haematococcus pluvialis Production: A Mixture Experimental Design. Water 2024, 16, 1314. [Google Scholar] [CrossRef]
- Ji, M.K.; Abou-Shanab, R.A.I.; Kim, S.H.; Salama, E.; Lee, S.H.; Kabra, A.N.; Lee, Y.S.; Hong, S.; Jeon, B.H. Cultivation of microalgae species in tertiary municipal wastewater supplemented with CO for nutrient removal and biomass production. Ecol. Eng. 2013, 58, 142–148. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, T.Y.; Dao, G.H.; Xu, X.Q.; Wang, X.X.; Hu, H.Y. Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl. Microbiol. Biotechnol. 2017, 101, 2659–2675. [Google Scholar] [CrossRef]
- Muradov, N.; Taha, M.; Miranda, A.F.; Wrede, D.; Kadali, K.; Gujar, A.; Stevenson, T.; Ball, A.S.; Mouradov, A. Fungal-assisted algal flocculation: Application in wastewater treatment and biofuel production. Biotechnol. Biofuels 2015, 8, 24. [Google Scholar] [CrossRef]
- Barka, A.; Amira, A.B.; Francis, F.; Blecker, C. Physicochemical characterization of colored soluble protein fractions extracted from Spirulina (Spirulina platensis). Food Sci. Technol. Int. 2018, 24, 651–663. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, Z.Z.; Yan, K.; Wang, Z.L.; Torres, O.L.; Guo, R.X.; Chen, J.Q. A new disposal method for systematically processing of ceftazidime: The intimate coupling UV/algae-algae treatment. Chem. Eng. J 2017, 314, 152–159. [Google Scholar] [CrossRef]
- Guo, W.Q.; Zheng, H.S.; Li, S.; Du, J.S.; Feng, X.C.; Yin, R.L.; Wu, Q.L.; Ren, N.Q.; Chang, J.S. Removal of cephalosporin antibiotics 7-ACA from wastewater during the cultivation of lipid-accumulating microalgae. Bioresour. Technol. 2016, 221, 284–290. [Google Scholar] [CrossRef]
- Xue, X.D.; Chen, B.H.; Wang, H.; Fang, C.R.; Long, Y.Y.; Hu, L.F. Antibiotics in the municipal solid waste incineration plant leachate treatment process. Chem. Ecol. 2021, 37, 633–645. [Google Scholar] [CrossRef]
- Hernández-García, A.; Velásquez-Orta, S.B.; Novelo, E.; Yáñez-Noguez, I.; Monje-Ramírez, I.; Ledesma, M.T.O. Wastewater-leachate treatment by microalgae: Biomass, carbohydrate and lipid production. Ecotox. Environ. Saf. 2019, 174, 435–444. [Google Scholar] [CrossRef] [PubMed]
- El Ouaer, M.; Kallel, A.; Kasmi, M.; Hassen, A.; Trabelsi, I. Tunisian landfill leachate treatment using Chlorella sp.: Effective factors and microalgae strain performance. Arab. J. Geosci. 2017, 10, 457. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Arashiro, L.; Santos, L.H.M.L.M.; Insa, S.; Rodríguez-Mozaz, S.; Barceló, D.; Ferrer, I.; Garfí, M. Fate of priority pharmaceuticals and their main metabolites and transformation products in microalgae-based wastewater treatment systems. J. Hazard. Mater. 2020, 390, 121771. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Water quality―Determination of Ammonia Nitrogen―Nessler’s Reagent Spectrophotometry: HJ 535-2009. 1 April 2010. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201001/t20100112_184155.shtml (accessed on 7 July 2024).
- Ministry of Ecology and Environment of the People’s Republic of China. Water Quality-Determination of Total Nitrogen-Alkaline Potassium Persulfate Digestion UV Spectrophotometric Method: HJ636-2012. 1 June 2012. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201203/t20120307_224383.shtml (accessed on 7 July 2024).
- Ministry of Ecology and Environment of the People’s Republic of China. Water Quality-Determination of Total Phospho-Rus-Ammonium Molybdate Spectrophotometric Method: GB 11893-89. 1 July 1990. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/199007/t19900701_67131.shtml (accessed on 7 July 2024).
- Zhejiang Analysis and Testing Association. Water Quality—Determination of the Chemical Oxygen Demand—Precast Reagent- Spectrophotometric Method: T/ZJATA 0001-2020. 1 September 2024. Available online: https://m.antpedia.com/standard/1721532541-1.html (accessed on 7 July 2024).
- Oh, S.; Chan, K.S. Chlorophyll a Fluorescence Response to Mercury Stress in the Freshwater Microalga Chlorella Vulgaris. J. Environ. Sci. Int. 2013, 22, 705–715. [Google Scholar] [CrossRef]
- Oh, S.; Chan, K.S. Assessment of Heavy Metal Effects on the Freshwater Microalga, Chlorella vulgaris, by Chlorophyll Fluorescence Analysis. J. Environ. Sci. Int. 2015, 24, 1591–1600. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Standard for Pollution Control on the Hazardous Waste Landfill: GB 18598—2019. 1 June 2020. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/gthw/gtfwwrkzbz/201910/t20191012_737241.shtml (accessed on 7 July 2024).
- Ministry of Ecology and Environment of the People’s Republic of China. Water Quality-Determination of Copper, Zinc, Lead and Cadmium-Atomlc Absorption Spectrometry: GB 7475-87. 1 August 1987. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/198708/t19870801_66850.shtml (accessed on 7 July 2024).
- Ministry of Ecology and Environment of the People’s Republic of China. Water Quality-Determination of Mercury, Arsenic, Selenium, Bismuth and Antimony-Atomic Fluorescence Spectrometry: HJ 694—2014. 1 July 2014. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201403/t20140319_269361.shtml (accessed on 7 July 2024).
- Long, S.X.; Hamilton, P.B.; Wang, C.A.; Li, C.L.; Xue, X.Y.; Zhao, Z.W.; Wu, P.Z.; Gu, E.R.; Uddin, M.M.; Li, B.A.; et al. Bioadsorption, bioaccumulation and biodegradation of antibiotics by algae and their association with algal physiological state and antibiotic physicochemical properties. J. Hazard. Mater. 2024, 468, 133787. [Google Scholar] [CrossRef]





| Components | Mother Liquor Concentration | Dosage |
|---|---|---|
| NaNO3 | 15.00 g/100 mL dH2O | 10 mL/L |
| K2HPO4 | 2.00 g/500 mL dH2O | 10 mL/L |
| MgSO4·7H2O | 3.75 g/500 mL dH2O | 10 mL/L |
| CaCl2·2H2O | 1.80 g/500 mL dH2O | 10 mL/L |
| Citric acid | 0.30 g/500 mL dH2O | 10 mL/L |
| Ferric ammonium citrate | 0.30 g/500 mL dH2O | 10 mL/L |
| EDTANa2 | 0.05 g/500 mL dH2O | 10 mL/L |
| Na2CO3 | 1.00 g/500 mL dH2O | 10 mL/L |
| A5 (trace metal solution) * | 1 mL/L |
| Components | Concentration |
|---|---|
| H3BO3 | 2.86 g/L dH2O |
| MnCl2·4H2O | 1.86 g/L dH2O |
| ZnSO4·7H2O | 0.22 g/L dH2O |
| Na2MoO4·2H2O | 0.39 g/L dH2O |
| CuSO4·5H2O | 0.08 g/L dH2O |
| CO(NO3)2·6H2O | 0.05 g/L dH2O |
| Group | Algae Species | K2HPO4·3H2O | MgSO4·7H2O | Ammonium Ferric Citrate | Trace Elements | Leachate Gradient | Sterilization Status |
|---|---|---|---|---|---|---|---|
| A0 | C. vulgaris | NA | NA | NA | NA | BG11 | NA |
| A1 | C. vulgaris | + | + | − | − | 1% leachate | Sterilized |
| A2 | C. vulgaris | − | − | − | + | 10% leachate | Non-sterilized |
| A3 | C. vulgaris | + | + | − | + | 20% leachate | NA |
| A4 | C. vulgaris | + | − | + | + | 25% leachate | NA |
| A5 | C. vulgaris | − | − | − | − | 30% leachate | NA |
| A6 | C. vulgaris | − | + | + | + | 40% leachate | NA |
| A7 | C. vulgaris | + | − | + | − | 50% leachate | NA |
| A8 | C. vulgaris | − | + | + | − | NA | NA |
| B0 | S. obliquus | NA | NA | NA | NA | BG11 | NA |
| B1 | S. obliquus | + | + | − | − | 1% leachate | Sterilized |
| B2 | S. obliquus | − | − | − | + | 10% leachate | Non-sterilized |
| B3 | S. obliquus | + | + | − | + | 20% leachate | NA |
| B4 | S. obliquus | + | − | + | + | 25% leachate | NA |
| B5 | S. obliquus | − | − | − | − | 30% leachate | NA |
| B6 | S. obliquus | − | + | + | + | 40% leachate | NA |
| B7 | S. obliquus | + | − | + | − | 50% leachate | NA |
| B8 | S. obliquus | − | + | + | − | NA | NA |
| C0 | Algae-free control | NA | NA | NA | NA | NA | NA |
| C1 | Algae-free control | NA | NA | NA | NA | 1% leachate | Sterilized |
| C2 | Algae-free control | NA | NA | NA | NA | 10% leachate | Non-sterilized |
| C3 | Algae-free control | NA | NA | NA | NA | 20% leachate | NA |
| C4 | Algae-free control | NA | NA | NA | NA | 25% leachate | NA |
| C5 | Algae-free control | NA | NA | NA | NA | 30% leachate | NA |
| C6 | Algae-free control | NA | NA | NA | NA | 40% leachate | NA |
| C7 | Algae-free control | NA | NA | NA | NA | 50% leachate | NA |
| Group | A1 | A2 | B1 | B2 |
|---|---|---|---|---|
| Algae species | C. vulgaris | S. obliquus | ||
| Percolate | Sterilized | Non-sterilized | Sterilized | Non-sterilized |
| Chla growth rate (%) | 315.0 ± 14.0 | 300.3 ± 4.8 | 77.0 ± 9.4 | 18.3 ± 6.0 |
| OD680 growth rate (%) | 356.3 ± 25.2 | 326.0 ± 39.2 | 201.6 ± 36.1 | 173.7 ± 18.6 |
| Fv/Fm change rate (%) | −24.4 ± 1.0 | −25.0 ± 2.3 | 6.4 ± 2.5 | 10.2 ± 7 |
| Group | A1 | A2 | B1 | B2 |
|---|---|---|---|---|
| Algae species | C. vulgaris | S. obliquus | ||
| percolate | Sterilization | Non-sterilized | Sterilization | Non-sterilized |
| DOC growth rate (%) | 125.3 ± 13.7 | 122.7 ± 19.3 | 47.9 ± 3.4 | 43.9 ± 5.3 |
| NH3-N removal rate (%) | 67.1 ± 1.3 | 66.8 ± 0.5 | 68.1 ± 1.7 | 67.7 ± 0.9 |
| TN removal rate (%) | 55.2 ± 1.0 | 53.5 ± 0.9 | 57.0 ± 0.5 | 56.2 ± 0.8 |
| TP removal rate (%) | 88.1 ± 1.0 | 85.9 ± 0.9 | 92.7 ± 0.6 | 92.4 ± 1.7 |
| Group | A0 | A1 | A2 | A3 | A4 | A5 | A6 | A7 |
|---|---|---|---|---|---|---|---|---|
| Leachate concentration | BG11 | 1% | 10% | 20% | 25% | 30% | 40% | 50% |
| Initial concentration (mg/L) | 6.37 | 10.62 | 42.87 | 79.38 | 96.38 | 110.14 | 143.64 | 177.40 |
| The final concentration (mg/L) | 4.37 | 0.67 | 27.37 | 49.12 | 64.38 | 76.13 | 100.39 | 115.64 |
| Removal rate% | 23.82 | 93.96 | 37.31 | 35.50 | 33.85 | 35.11 | 29.47 | 36.58 |
| Group | B0 | B1 | B2 | B3 | B4 | B5 | B6 | B7 |
| Leachate concentration | BG11 | 1% | 10% | 20% | 25% | 30% | 40% | 50% |
| Initial concentration (mg/L) | 7.62 | 11.62 | 45.12 | 80.13 | 92.38 | 114.14 | 151.65 | 185.15 |
| The final concentration (mg/L) | 0.97 | 0.72 | 17.08 | 48.62 | 66.88 | 81.63 | 106.14 | 124.89 |
| Removal rate% | 85.58 | 93.52 | 68.18 | 38.19 | 27.56 | 27.33 | 27.96 | 31.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Pang, W.; Wang, C.; Chen, Q.; Ke, Q.; Wang, Q. Optimization of Culture Conditions for Microalgae Treatment Fly Ash Leachate System. Water 2024, 16, 2223. https://doi.org/10.3390/w16162223
Zhao R, Pang W, Wang C, Chen Q, Ke Q, Wang Q. Optimization of Culture Conditions for Microalgae Treatment Fly Ash Leachate System. Water. 2024; 16(16):2223. https://doi.org/10.3390/w16162223
Chicago/Turabian StyleZhao, Rong, Wenjing Pang, Chuanhua Wang, Qiongzhen Chen, Qiang Ke, and Qi Wang. 2024. "Optimization of Culture Conditions for Microalgae Treatment Fly Ash Leachate System" Water 16, no. 16: 2223. https://doi.org/10.3390/w16162223
APA StyleZhao, R., Pang, W., Wang, C., Chen, Q., Ke, Q., & Wang, Q. (2024). Optimization of Culture Conditions for Microalgae Treatment Fly Ash Leachate System. Water, 16(16), 2223. https://doi.org/10.3390/w16162223





