The Conditions for the Formation of Strontium in the Water of Ancient Silicate Deposits Near the Arctic Coast of Russia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
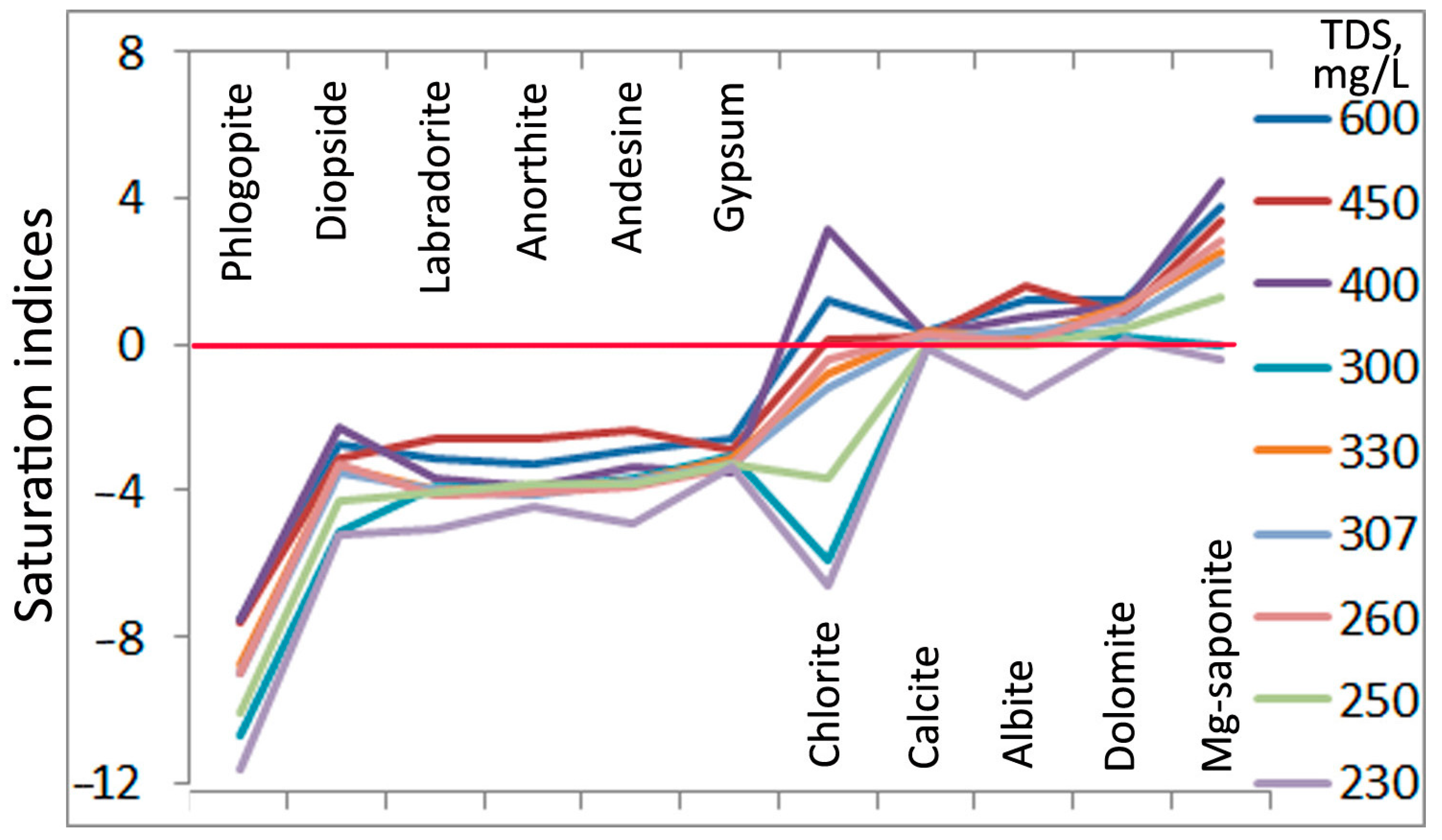
3.1. Chemical Composition and Saturation of Groundwater in Relation to the Main Rock-Forming Minerals
3.2. Isotopic Composition and Residence Time of Groundwater in the Aquifer
4. Discussion
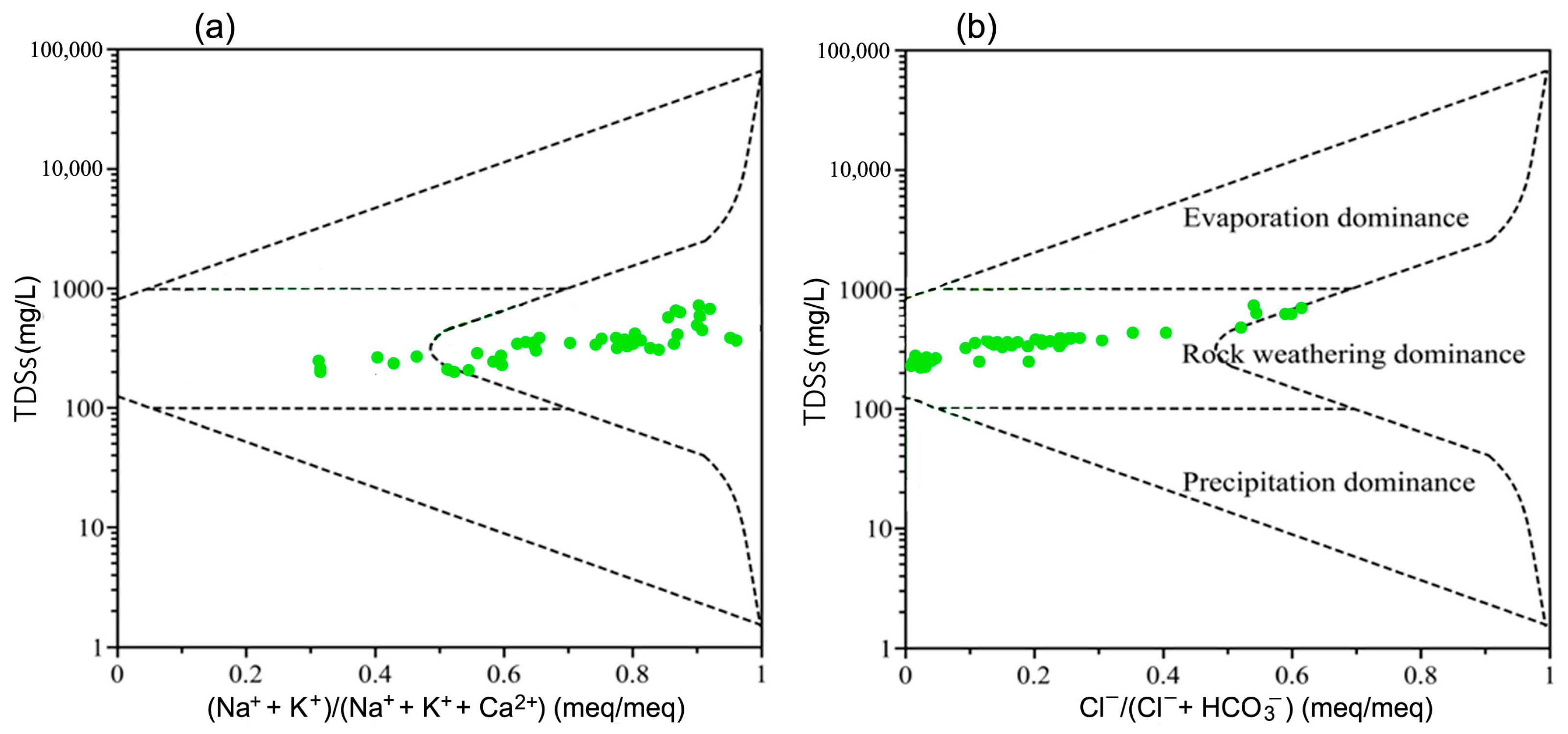
4.1. Formation of Groundwater Composition
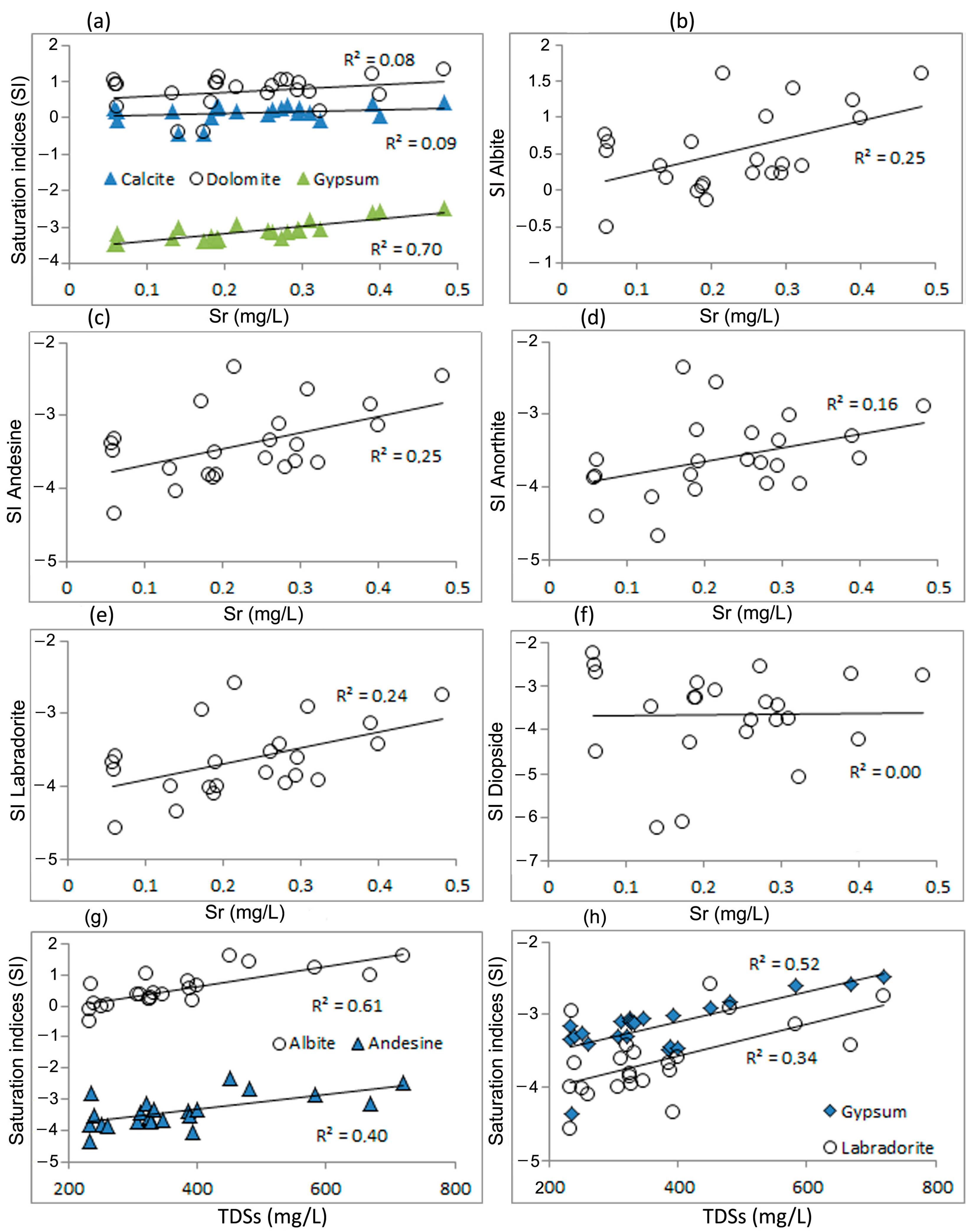
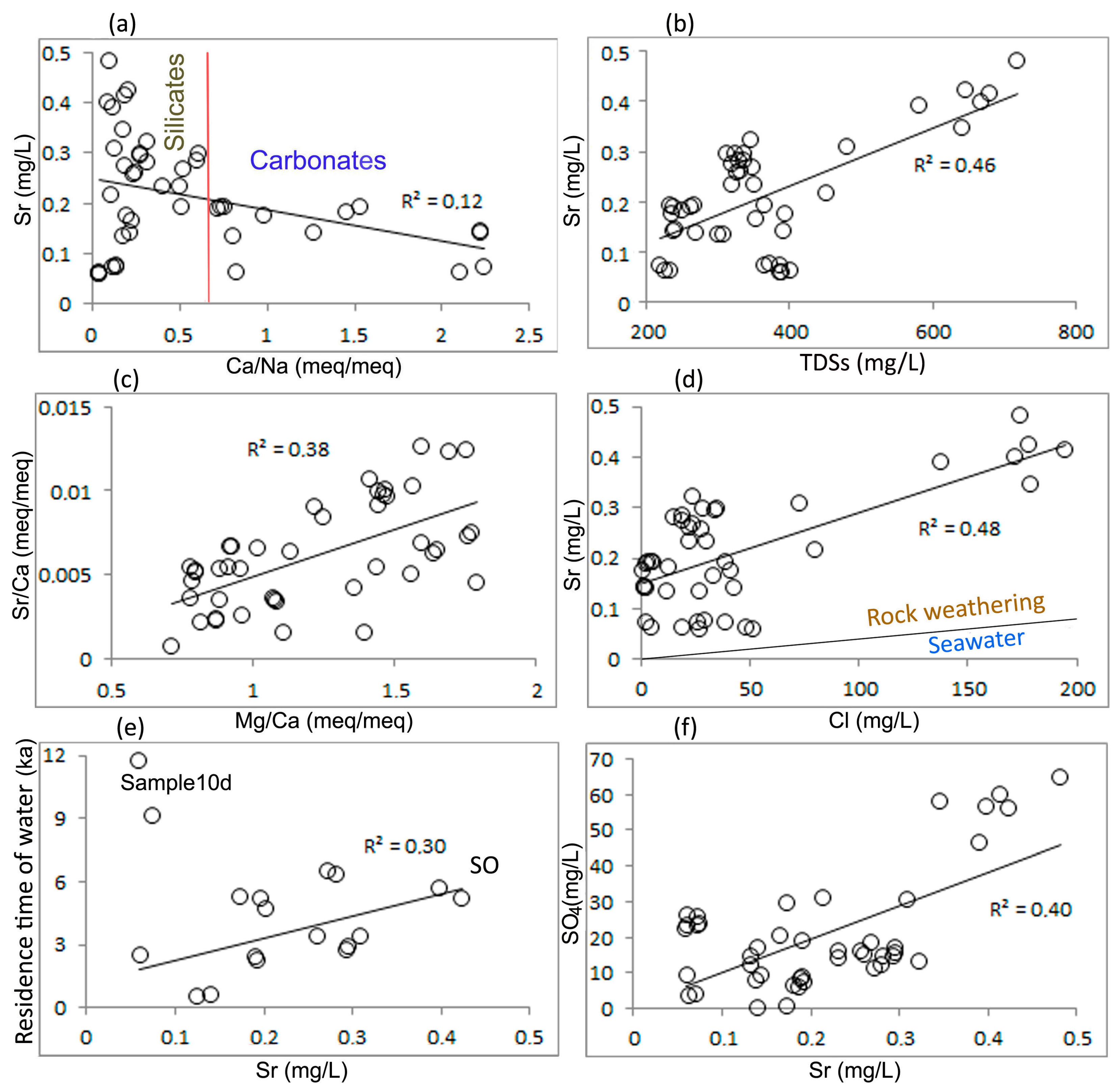
4.2. Sources of Strontium in Groundwater and Processes of Transition of Strontium into Water
4.3. Statistical Estimates
4.4. The Danger of Strontium When Using Groundwater for Drinking
5. Conclusions
- Minimum concentrations of strontium were characteristic of the least mineralized waters and appeared there mainly due to the dissolution of carbonates. After their saturation with respect to calcite, the process of the dissolution of carbonates was replaced by their precipitation.
- Increased strontium concentrations were observed in more mineralized waters where silicate dissolution increased. The incongruent dissolution of aluminosilicates led to the appearance of new clay minerals in the aquifer. Together with iron hydroxides, newly formed calcium and clay carbonates provided the possibilities for sorption and ion exchange processes.
- There was an increase in the strontium–calcium ratio associated with the dedolomitization process.
- The transfer of strontium into water due to the dissolution of gypsum inclusions in cement was limited.
- The contribution of seawater was significant in increasing sodium and chlorine concentrations. The strontium content increased due to seawater by approximately 15–20%.
- The influence of the duration of the water–rock interaction on strontium concentrations in groundwater was expressed in the fact that over a thousand years they increased by 0.1 mg/L, which is 20–30 times less than in the waters of carbonate sediments located 100 km to the east.
- An assessment of the non-carcinogenic risk to human health of contact with the groundwater showed the safety of using the studied groundwater for drinking purposes.
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Musgrove, M. The occurrence and distribution of strontium in U.S. groundwater. Appl. Geochem. 2021, 126, 104867. [Google Scholar] [CrossRef]
- Heine, F.; Zosseder, K.; Einsiedl, F. Hydrochemical Zoning and Chemical Evolution of the Deep Upper Jurassic Thermal Groundwater Reservoir Using Water Chemical and Environmental Isotope Data. Water 2021, 13, 1162. [Google Scholar] [CrossRef]
- Li, D.; Gan, S.; Li, J.; Dong, Z.; Long, Q.; Qiu, S.; Zhou, Y.; Lu, C. Hydrochemical Characteristics and Formation Mechanism of Strontium-Rich Groundwater in Shijiazhuang, North China Plain. J. Chem. 2021, 5547924. [Google Scholar] [CrossRef]
- Liang, C.; Wang, W.; Ke, X.; Ou, A.; Wang, D. Hydrochemical Characteristics and Formation Mechanism of Strontium-Rich Groundwater in Tianjiazhai, Fugu, China. Water 2022, 14, 1874. [Google Scholar] [CrossRef]
- Malov, A.I. Features of the Formation of Strontium Pollution of Drinking Groundwater and Associated Health Risks in the North-West of Russia. Water 2023, 15, 3846. [Google Scholar] [CrossRef]
- Huang, T.; Ma, B. The Origin of Major Ions of Groundwater in a Loess Aquifer. Water 2019, 11, 2464. [Google Scholar] [CrossRef]
- Mughal, A.; Sultan, K.; Ashraf, K.; Hassan, A.; Zaman, Q.U.; Haider, F.U.; Shahzad, B. Risk Analysis of Heavy Metals and Groundwater Quality Indices in Residential Areas: A Case Study in the Rajanpur District, Pakistan. Water 2022, 14, 3551. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, X.; Zhou, Y.; Yan, Y.; Li, Y.; Ouyang, S.; Liu, F.; Zhong, J. Hydrogeochemistry and Precursory Anomalies in Thermal Springs of Fujian (Southeastern China) Associated with Earthquakes in the Taiwan Strait. Water 2021, 13, 3523. [Google Scholar] [CrossRef]
- Yu, M.; Wang, G.; Ma, F.; Zhang, W.; Lin, W.; Zhu, X.; Zhang, H. Geochemical Characteristics of Geothermal Fluids of a Deep Ancient Buried Hill in the Xiong’an New Area of China. Water 2022, 14, 3182. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Mu, W.; Zhu, G. Using Isotopes (H, O, and Sr) and Major Ions to Identify Hydrogeochemical Characteristics of Groundwater in the Hongjiannao Lake Basin, Northwest China. Water 2020, 12, 1467. [Google Scholar] [CrossRef]
- Yang, N.; Su, C.; Liu, W.; Zhao, L. Occurrences and mechanisms of strontium-rich groundwater in Xinglong County, northern China: Insight from hydrogeological and hydrogeochemical evidence. Hydrogeol. J. 2022, 30, 2043–2057. [Google Scholar] [CrossRef]
- Swarzenski, P.W.; Baskaran, M.; Rosenbauer, R.J.; Edwards, B.D.; Land, M. A Combined Radio- and Stable-Isotopic Study of a California Coastal Aquifer System. Water 2013, 5, 480–504. [Google Scholar] [CrossRef]
- Khandare, A.L.; Validandi, V.; Rajendran, A.; Singh, T.G.; Thingnganing, L.; Kurella, S.; Nagaraju, R.; Dheeravath, S.; Vaddi, N.; Kommu, S.; et al. Health risk assessment of heavy metals and strontium in groundwater used for drinking and cooking in 58 villages of Prakasam district, Andhra Pradesh, India. Environ. Geochem. Health 2020, 42, 3675–3701. [Google Scholar] [CrossRef]
- Bui, D.T.; Khosravi, K.; Karimi, M.; Busico, G.; Khozani, Z.S.; Nguyen, H.; Mastrocicco, M.; Tedesco, D.; Cuoco, E.; Kazakis, N. Enhancing nitrate and strontium concentration prediction in groundwater by using new data mining algorithm. Sci. Total Environ. 2020, 715, 136836. [Google Scholar] [CrossRef]
- Ding, D.; Kong, L.; Jiang, D.; Wei, J.; Cao, S.; Li, X.; Zheng, L.; Deng, S. Source apportionment and health risk assessment of chemicals of concern in soil, water and sediment at a large strontium slag pile area. J. Environ. Manag. 2022, 304, 114228. [Google Scholar] [CrossRef] [PubMed]
- Keesari, T.; Sabarathinam, C.; Sinha, U.K.; Pethaperumal; Thilagavathi, R.; Kamaraj, P. Fate and transport of strontium in groundwater from a layered sedimentary aquifer system. Chemosphere 2022, 307, 136015. [Google Scholar] [CrossRef]
- Plechacek, A.; Scott, S.R.; Gotkowitz, M.B.; Ginder-Vogel, M. Strontium and radium occurrence at the boundary of a confined aquifer system. Appl. Geochem. 2022, 142, 105332. [Google Scholar] [CrossRef]
- Malov, A.I.; Zykov, S.B. Study of the Mobilization of Uranium Isotopes in a Sandstone Aquifer in Combination with Groundwater Data. Water 2020, 12, 112. [Google Scholar] [CrossRef]
- Malov, A.I.; Sidkina, E.S.; Ershova, D.D.; Cherkasova, E.V.; Druzhinin, S.V. Time regularities of strontium concentration in drinking groundwater distant from the sea coast. Env. Geochem. Health 2023, 45, 8097–8118. [Google Scholar] [CrossRef]
- Malyshev, V.I.; Bakhur, A.E.; Manuylova, L.I. Methods for Measuring the Volumetric Activity of Uranium Isotopes (234, 238) in Natural Water Sample Alpha Spectrometry with Radiochemical Separation; RIMR: Moscow, Russia, 1999. (In Russian) [Google Scholar]
- Fröhlich, K. Dating of old groundwater using uranium isotopes—Principles and applications. In Isotope Methods for Dating Old Groundwate; IAEA: Vienna, Austria, 2013; pp. 153–178. [Google Scholar]
- Garrels, R.M.; Christ, C.L. Solutions, Minerals, and Equilibria; Jones and Bartlett Publishers: Boston, MA, USA, 1990. [Google Scholar]
- Krainov, S.R.; Ryzhenko, B.N.; Shvets, V.M. Geochemistry of Groundwater. Fundamental, Applied and Environmental Aspects; CenterLitNefteGaz: Moskow, Russia, 2012. (In Russian) [Google Scholar]
- Malov, A.I. Evolution of the groundwater chemistry in the coastal aquifers of the south-eastern White Sea area (NW Russia) using 14C and 234U-238U dating. Sci. Total Environ. 2018, 616–617, 1208–1223. [Google Scholar] [CrossRef]
- Han, L.-F.; Plummer, N. Revision of Fontes and Garnier’s model for the initial 14C content of dissolved inorganic carbon used in groundwater dating. Chem. Geol. 2013, 351, 105–114. [Google Scholar] [CrossRef]
- Han, L.-F.; Plummer, N. A review of single-sample-based models and other approaches for radiocarbon dating of dissolved inorganic carbon in groundwater. Earth Sci. Rev. 2016, 152, 119–142. [Google Scholar] [CrossRef]
- Ingerson, E.; Pearson, F.J. Estimation of age and rate of motion of groundwater by the 14C method. In Recent Researches in the Fields of Hydrosphere; Atmosphere and Nuclear Geochemistry: Tokyo, Japan, 1964; pp. 263–283. [Google Scholar]
- Pearson, F.J., Jr.; Hanshaw, B.B. Sources of dissolved carbonate species in groundwater and their effects on carbon-14 dating. In Isotope Hydrology; IAEA: Vienna, Austria, 1970; pp. 271–286. [Google Scholar]
- Reimer, P.J.; Austin, W.E.N.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Ramsey, C.B.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 ka cal BP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Stuiver, M.; Reimer, P.J.; Reimer, R.W. CALIB 8.2 [WWW Program]. 2021. Available online: http://calib.org (accessed on 16 February 2021).
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Sci. New Ser. 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Shvartsev, S.L. Interaction of water with aluminosilicate rocks. Geol. Geophys. 1991, 12, 16–50. (In Russian) [Google Scholar]
- Shvartsev, S.L. Hydrogeochemistry of the Hypergenesis Zone; Nedra: Moscow, Russia, 1998. (In Russian) [Google Scholar]
- Shao, H.B.; Ray, J.R.; Jun, Y.S. Dissolution and Precipitation of Clay Minerals under Geologic CO2 Sequestration Conditions: CO2-Brine-Phlogopite Interactions. Environ. Sci. Technol. 2010, 44, 5999–6005. [Google Scholar] [CrossRef]
- Cappelli, C.; Lamarca-Irisarri, D.; Camas, J.; Huertas, F.J.; Van Driessche, A.E. In situ observation of biotite (001) surface dissolution at pH 1 and 9.5 by advanced optical microscopy. Beilstein J. Nanotechnol. 2015, 6, 665–673. [Google Scholar] [CrossRef]
- Wang, J.; Yagi, M.; Tamagawa, T.; Hirano, H.; Watanabe, N. Reactivity and Dissolution Characteristics of Naturally Altered Basalt in CO2-Rich Brine: Implications for CO2 Mineralization. ACS Omega 2024, 9, 4429–4438. [Google Scholar] [CrossRef]
- Magaritz, M.; Kafri, U. Stable isotope and Sr2+/Ca2+ evidence of diagenetic dedolomitization in a schizohaline environment: Cenomanian of northern Israel. Sediment. Geol. 1981, 28, 29–41. [Google Scholar] [CrossRef]
- Musgrove, M.; Banner, J.L. Controls on the spatial and temporal variability of vadose dripwater geochemistry: Edwards aquifer, central Texas. Geochem. Cosmochim. Acta 2004, 68, 1007–1020. [Google Scholar] [CrossRef]
- Pärn, J.; Raidla, V.; Vaikmäe, R.; Martma, T.; Ivask, J.; Mokrik, R.; Erg, K. The recharge of glacial meltwater and its influence on the geochemical evolution of groundwater in the Ordovician-Cambrian aquifer system, northern part of the Baltic Artesian Basin. Appl. Geochem. 2016, 72, 125–135. [Google Scholar] [CrossRef]
- Gerber, C.; Vaikmäe, R.; Aeschbach, W.; Babre, A.; Jiang, W.; Leuenberger, M.; Lu, Z.-T.; Mokrik, R.; Müller, P.; Raidla, V.; et al. Using 81Kr and noble gases to characterize and date groundwater and brines in the Baltic Artesian Basin on the one-million-year timescale. Geochim. Cosmochim. Acta 2017, 205, 187–210. [Google Scholar] [CrossRef]
- Malov, A.I.; Sidkina, E.S.; Cherkasova, E.V. The Influence of DOC on the Migration Forms of Elements and Their Sedimentation from River Waters at an Exploited Diamond Deposit (NW Russia). Water 2023, 15, 2160. [Google Scholar] [CrossRef]
- Riley, J.P.; Skirrow, G. (Eds.) Chemical Oceanography; Academic Press: London, UK, 1965. [Google Scholar]
- USEPA. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites; OSWER 9355.4-24; U.S. Environmental Protection Agency: Washington, DC, USA, 2002.
- USEPA. Risk Assessment Guidance for Superfund; Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment). Final. EPA/540/R/99/005; U.S. Environmental Protection Agency: Washington, DC, USA, 2004. [Google Scholar]
- USEPA. Health Effects Support Document for Strontium; EPA 820-P-14-0012014; U.S. Environmental Protection Agency: Washington, DC, USA, 2014.
- Nazir, M.A.; Yasar, A.; Bashir, M.A.; Siyal, S.H.; Najam, T.; Javed, M.S.; Ahmad, K.; Hussain, S.; Anjum, S.; Hussain, E.; et al. Quality assessment of the noncarbonated-bottled drinking water: Comparison of their treatment techniques. Int. J. Environ. Anal. Chem. 2020, 102, 8195–8206. [Google Scholar] [CrossRef]
- Malov, A.I. Assessment of water supply to the East European Arctic agglomeration from groundwater, taking into account their quality and health risks. Environ. Pollut. 2024, 360, 124636. [Google Scholar] [CrossRef]
- SanPiN. Sanitary and Epidemiological Rules and Regulations "Drinking Water. Hygienic Requirements for Water Quality in Centralized Drinking Water Supply Systems. Quality Control"; Ministry of Health of the Russian Federation: Moscow, Russia, 2001; p. 67. (In Russian)
- GB 5749-2022; Standards for Drinking Water Quality. National Standard of the People’s Republic of China: Beijing, China, 2022; p. 10. Available online: https://faolex.fao.org/docs/pdf/chn222304.pdf (accessed on 29 July 2024).
- WHO. Guidelines For Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2022; p. 614.



| SiO2 | TiO2 | Al2O3 | Fe2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | P2O5 | Cr2O3 | V2O5 | LOI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sa 1 | 91.5 | 0.38 | 3.59 | 1.08 | 0.22 | 0.04 | 0.48 | 0.50 | 0.10 | 1.11 | 0.04 | 0.02 | 0.01 | 0.99 |
| Si 2 | 72.0 | 0.81 | 12.8 | 5.30 | 0.30 | 0.14 | 1.24 | 0.48 | 0.14 | 2.94 | 0.07 | 0.02 | 0.01 | 3.66 |
| Parameter | Unit | Mean | Max | Min | Std. Dev. |
|---|---|---|---|---|---|
| Depth | m | 140 | 180 | 80.0 | 21.3 |
| pH | unit | 8.45 | 9.20 | 7.80 | 0.37 |
| TDS | mg·L−1 | 368 | 719 | 219 | 132 |
| Na+ | mg·L−1 | 68.8 | 199 | 2.03 | 49.4 |
| K+ | mg·L−1 | 4.03 | 9.14 | 1.54 | 1.37 |
| Ca2+ | mg·L−1 | 17.5 | 37.2 | 3.66 | 7.61 |
| Mg2+ | mg·L−1 | 11.9 | 18.8 | 3.78 | 3.70 |
| Cl− | mg·L−1 | 44.8 | 195 | 1.10 | 54.7 |
| SO42− | mg·L−1 | 20.6 | 64.7 | 0.04 | 16.5 |
| HCO3− | mg·L−1 | 201 | 239 | 146 | 19.6 |
| Sr | µg.L−1 | 212 | 481 | 58.4 | 112 |
| (Ca) | (Ca-Na) | (Ca-Mg) | (Mg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Well ID | Gypsum | Calcite | Anorthite | Labradorite | Andesine | Albite | Dolomite | Diopside | Chlorite | Phlogopite | Mg-Saponite |
| 21d | −2.60 | 0.39 | −3.29 | −3.13 | −2.85 | 1.23 | 1.21 | −2.74 | 1.24 | −7.54 | 3.78 |
| 22d | −2.91 | 0.20 | −2.56 | −2.58 | −2.34 | 1.6 | 0.84 | −3.09 | 0.13 | −7.63 | 3.39 |
| 16h | −3.02 | −0.45 | −4.66 | −4.34 | −4.03 | 0.18 | −0.39 | −6.24 | −8.96 | −10.73 | −1.56 |
| 10d | −3.48 | 0.27 | −3.88 | −3.66 | −3.37 | 0.77 | 1.03 | −2.25 | 3.15 | −7.57 | 4.46 |
| 26d | −3.05 | −0.08 | −3.96 | −3.91 | −3.65 | 0.34 | 0.18 | −5.1 | −5.92 | −10.69 | −0.01 |
| 36d | −3.12 | 0.34 | −3.96 | −3.96 | −3.71 | 0.24 | 1.04 | −3.39 | −0.77 | −8.76 | 2.54 |
| 24d | −3.29 | 0.18 | −4.13 | −4.00 | −3.72 | 0.34 | 0.69 | −3.48 | −1.20 | −9.01 | 2.31 |
| 28d | −3.39 | 0.26 | −4.03 | −4.10 | −3.86 | 0.04 | 0.95 | −3.28 | −0.40 | −9.03 | 2.83 |
| Ch | −3.26 | 0.00 | −3.83 | −4.02 | −3.81 | −0.01 | 0.41 | −4.31 | −3.69 | −10.07 | 1.29 |
| 21d | −2.58 | 0.06 | −3.61 | −3.42 | −3.13 | 0.98 | 0.62 | −4.24 | −2.89 | −8.85 | 1.57 |
| 21d | −2.47 | 0.41 | −2.88 | −2.74 | −2.46 | 1.61 | 1.33 | −2.77 | 1.29 | −7.22 | 3.96 |
| Ae | −3.30 | 0.25 | −3.67 | −3.42 | −3.12 | 1.02 | 1.03 | −2.55 | 2.47 | −7.44 | 4.10 |
| 22d | −2.82 | 0.13 | −3.02 | −2.91 | −2.64 | 1.40 | 0.72 | −3.74 | −1.80 | −8.35 | 2.41 |
| 10d | −3.44 | 0.19 | −3.85 | −3.76 | −3.49 | 0.54 | 0.91 | −2.53 | 2.70 | −7.43 | 4.08 |
| 10d | −3.46 | 0.19 | −3.62 | −3.58 | −3.32 | 0.66 | 0.91 | −2.71 | 2.11 | −8.12 | 3.83 |
| 26d | −3.09 | 0.27 | −3.36 | −3.61 | −3.40 | 0.36 | 0.96 | −3.45 | −0.49 | −8.34 | 2.63 |
| 26d | −3.06 | 0.16 | −3.71 | −3.84 | −3.62 | 0.23 | 0.76 | −3.80 | −1.59 | −9.19 | 2.06 |
| 36d | −3.12 | 0.21 | −3.26 | −3.52 | −3.33 | 0.41 | 0.86 | −3.79 | −1.47 | −8.68 | 2.14 |
| 36d | −3.09 | 0.12 | −3.63 | −3.80 | −3.58 | 0.23 | 0.67 | −4.07 | −2.31 | −9.27 | 1.66 |
| 28d | −3.34 | 0.32 | −3.64 | −3.99 | −3.81 | −0.14 | 1.13 | −2.92 | 1.20 | −8.01 | 3.48 |
| 28d | −3.29 | 0.25 | −3.21 | −3.67 | −3.51 | 0.08 | 0.98 | −3.26 | 0.17 | −8.27 | 3.05 |
| Ch | −4.36 | −0.43 | −2.36 | −2.95 | −2.81 | 0.67 | −0.41 | −6.09 | −8.67 | −11.7 | −1.06 |
| Br | −3.16 | −0.05 | −4.41 | −4.56 | −4.34 | −0.51 | 0.32 | −4.51 | −3.81 | −10.8 | 0.85 |
| Mean | −3.16 | 0.14 | −3.59 | −3.63 | −3.39 | 0.53 | 0.73 | −3.67 | −1.28 | −8.81 | 2.34 |
| Sample ID | U (µg/L) | 234U/238U (unit) | 14C (pmc) | δ13C (‰) | 14C Age (cal BP, ka) a | 234U-238U Age (ka) b |
|---|---|---|---|---|---|---|
| Ae-2013 | 2.53 ± 0.05 | 5.26 ± 0.68 | NA | NA | NC | 6.5 ± 1.0 |
| Br-2013 | 4.36 ± 0.09 | 1.97 ± 0.29 | NA | NA | NC | 2.5 ± 0.4 |
| 28d-2013 | 3.27 ± 0.06 | 2.11 ± 0.32 | NA | NA | NC | 2.2 ± 0.3 |
| 28d-2014 | 2.99 ± 0.06 | 2.39 ± 0.36 | 58.40 ± 0.89 | −11.0 | modern | 2.4 ± 0.4 |
| 28d-2021 | 0.65 ± 0.01 | 4.08 ± 0.59 | 30.07 ± 0.44 | −11.7 | 4.7 ± 0.36 | NC |
| 26d-2013 | 2.44 ± 0.05 | 3.04 ± 0.45 | NA | NA | NC | 2.9 ± 0.4 |
| 26d-2013 | 2.41 ± 0.05 | 2.86 ± 0.43 | NA | NA | NC | 2.7 ± 0.4 |
| 36d-2013 | 1.99 ± 0.04 | 4.34 ± 0.65 | NA | NA | NC | 3.4 ± 0.2 |
| 36d-2014 | 2.00 ± 0.04 | 4.81 ± 0.62 | 25.01 ± 0.47 | −11.7 | 6.34 ± 0.36 | NC |
| 10d-2013 | 14.14 ± 0.28 | 2.39 ± 0.36 | NA | NA | NC | 11.7 ± 1.6 |
| 22d-2014 | 6.37 ± 0.13 | 1.63 ± 0.24 | 24.86 ± 0.43 | −10.1 | 5.22 ± 0.39 | NC |
| 22d-2021 | 3.30 ± 0.06 | 3.23 ± 0.45 | 26.62 ± 0.32 | −11.0 | 5.20 ± 0.44 | NC |
| 10d-2014 | 13.35 ± 0.27 | 2.14 ± 0.31 | NA | NA | NC | 9.1 ± 1.2 |
| 22d-2013 | 4.47 ± 0.09 | 2.28 ± 0.34 | NA | NA | NC | 3.4 ± 0.5 |
| 21d-2013 | 9.60 ± 0.19 | 1.99 ± 0.30 | NA | NA | NC | 5.7 ± 0.8 |
| 21d-2014 | 10.38 ± 0.21 | 1.84 ± 0.27 | NA | NA | NC | 5.2 ± 0.8 |
| 21d-2021 | 1.80 ± 0.04 | 3.64 ± 0.47 | 45.17 ± 0.66 | −11.61 | 0.5 ± 0.07 | NC |
| 16h-2012 | 1.12 ± 0.02 | 1.93 ± 0.28 | NA | NA | NC | 0.56 ± 0.1 |
| h | pH | TDS | Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | Sr | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| h | 1 | 0.04 | 0.85 a | 0.82 | 0.58 | 0.04 | 0.17 | 0.83 | 0.84 | 0.52 | 0.56 |
| pH | 1 | 0.22 | 0.35 b | 0.20 | −0.71 | −0.71 | 0.11 | 0.26 | 0.37 | −0.16 | |
| TDS | 1 | 0.98 | 0.60 | −0.03 | 0.13 | 0.97 | 0.98 | 0.62 | 0.68 | ||
| Na+ | 1 | 0.62 | −0.21 | 0.02 | 0.92 | 0.97 | 0.66 | 0.60 | |||
| K+ | 1 | −0.31 | 0.03 | 0.54 | 0.57 | 0.47 | 0.28 | ||||
| Ca2+ | 1 | 0.70 | 0.10 | −0.02 | −0.32 | 0.23 | |||||
| Mg2+ | 1 | 0.28 | 0.11 | −0.33 | 0.49 | ||||||
| Cl− | 1 | 0.97 | 0.43 | 0.69 | |||||||
| SO42− | 1 | 0.55 | 0.63 | ||||||||
| HCO3− | 1 | 0.37 | |||||||||
| Sr | 1 |
| Parameter | Component | |
|---|---|---|
| 1 | 2 | |
| h, m | 0.89 | 0.07 |
| pH | 0.18 | −0.86 |
| TDS | 0.99 | −0.02 |
| Na+ | 0.97 | −0.19 |
| K+ | 0.66 | −0.22 |
| Ca2+ | −0.03 | 0.89 |
| Mg2+ | 0.17 | 0.91 |
| Cl− | 0.96 | 0.13 |
| SO42− | 0.97 | −0.03 |
| HCO3− | 0.64 | −0.43 |
| Sr | 0.72 | 0.39 |
| % of variance | 54.4 | 25.4 |
| Sum 79.8% | ||
| Non-Carcinogenic Risks for Adults | Non-Carcinogenic Risks for Children | |
|---|---|---|
| Well ID | Hazard Index (HI) for Strontium | |
| 21d | 0.0187 | 0.0549 |
| 22d | 0.0099 | 0.0290 |
| 16h | 0.0064 | 0.0188 |
| 10d | 0.0031 | 0.0090 |
| 36d | 0.0119 | 0.0348 |
| 26d | 0.0117 | 0.0342 |
| Ae | 0.0125 | 0.0366 |
| 24d | 0.0061 | 0.0178 |
| 28d | 0.0082 | 0.0242 |
| Ch | 0.0073 | 0.0215 |
| Br | 0.0028 | 0.0083 |
| Bl | 0.0033 | 0.0095 |
| HI | 0.0087 | 0.0250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malov, A.I. The Conditions for the Formation of Strontium in the Water of Ancient Silicate Deposits Near the Arctic Coast of Russia. Water 2024, 16, 2369. https://doi.org/10.3390/w16172369
Malov AI. The Conditions for the Formation of Strontium in the Water of Ancient Silicate Deposits Near the Arctic Coast of Russia. Water. 2024; 16(17):2369. https://doi.org/10.3390/w16172369
Chicago/Turabian StyleMalov, Alexander I. 2024. "The Conditions for the Formation of Strontium in the Water of Ancient Silicate Deposits Near the Arctic Coast of Russia" Water 16, no. 17: 2369. https://doi.org/10.3390/w16172369
APA StyleMalov, A. I. (2024). The Conditions for the Formation of Strontium in the Water of Ancient Silicate Deposits Near the Arctic Coast of Russia. Water, 16(17), 2369. https://doi.org/10.3390/w16172369








