Effects of Dry Periods on Nitrogen and Phosphorus Removal in Runoff Infiltration Devices and Their Biological Succession Patterns
Abstract
:1. Introduction
2. Materials and Methods
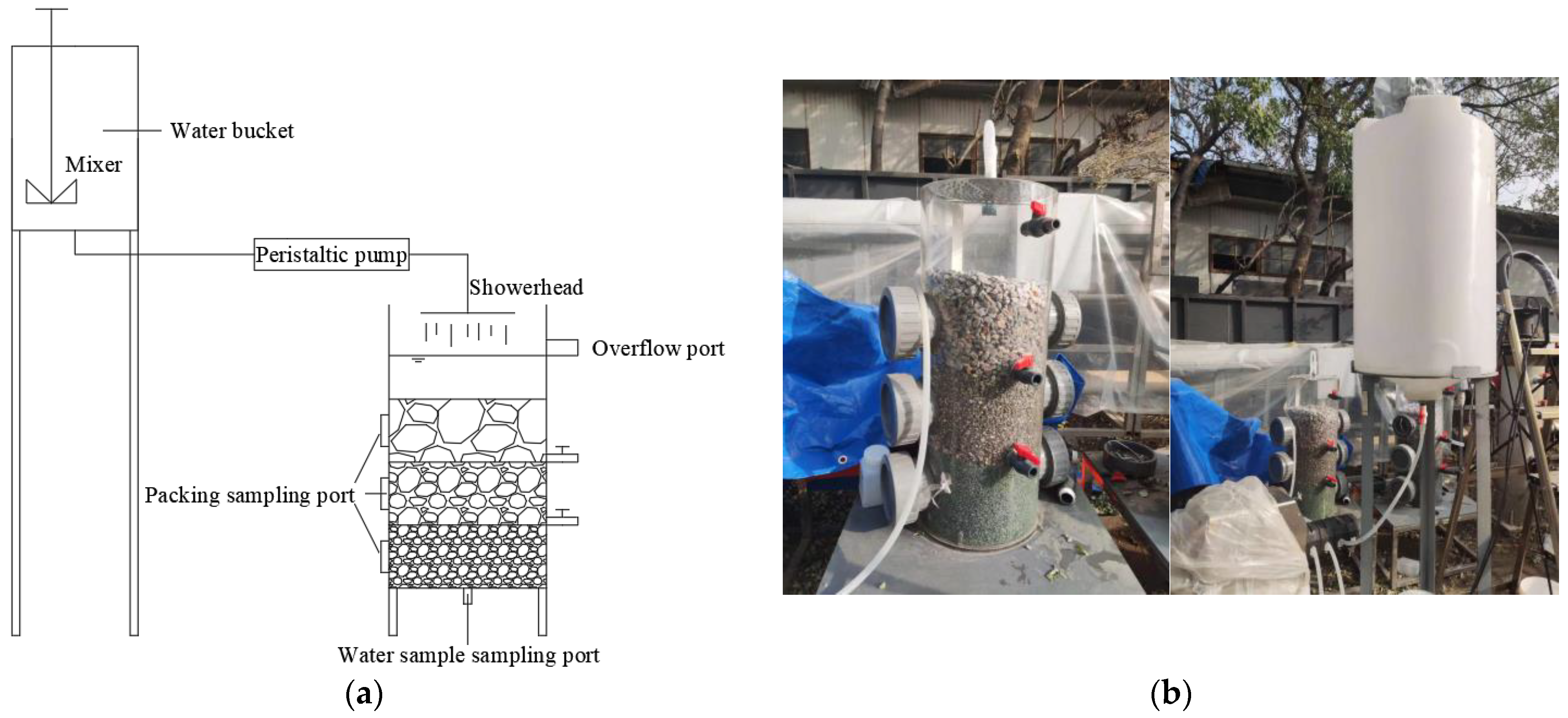
2.1. Experimental Materials and Equipment
2.2. Experimental Design
2.3. Sample Collection Method
2.4. Sample Index Determination Method
2.5. Data Analysis Methods
2.5.1. Pollutant Total Reduction Rate
2.5.2. Material Balance Equation
3. Results and Discussion
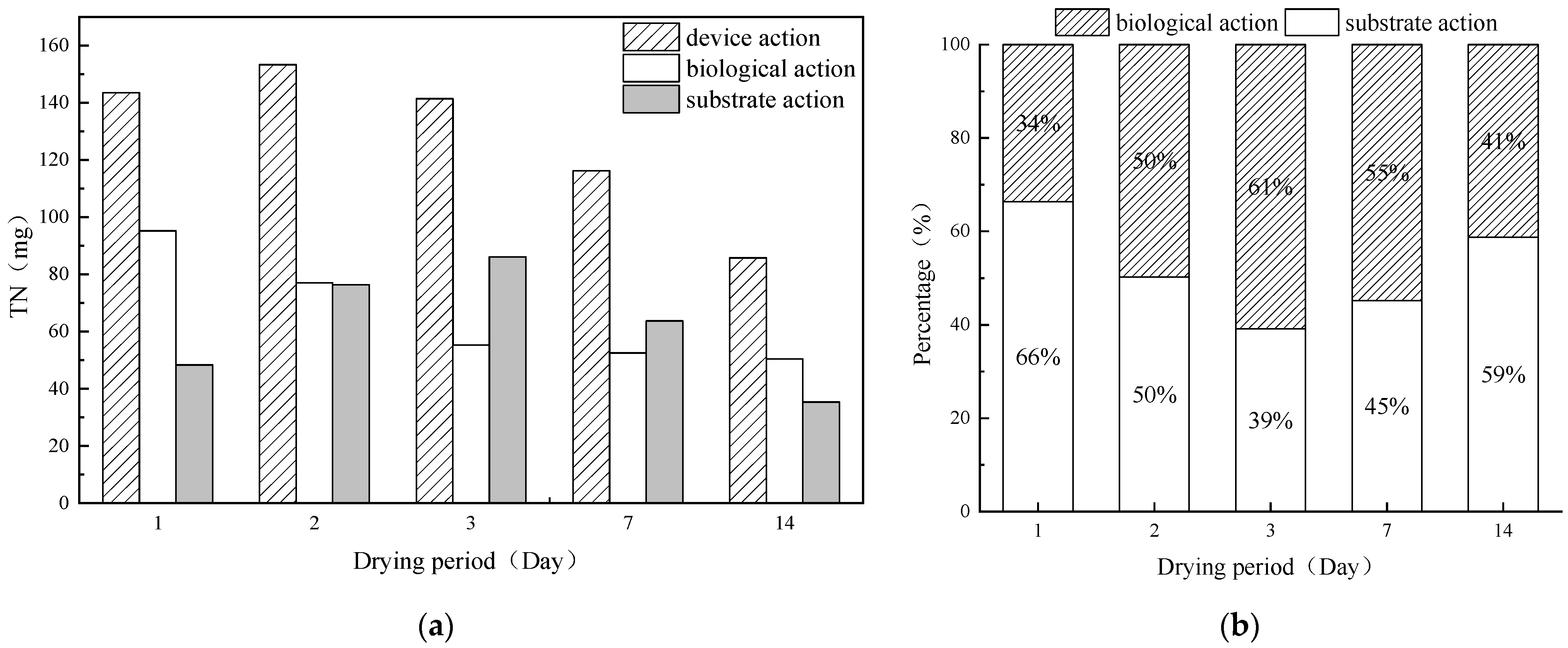
3.1. Trends in Nitrogen and Phosphorus Removal from Runoff Infiltration Devices during Various Dry Periods
3.1.1. The Removal Trend of Total Nitrogen by Runoff Infiltration Devices
3.1.2. The Removal Trend of Total Phosphorus by Runoff Infiltration Devices
3.2. Microbial Succession Patterns on the Surface of Runoff Infiltration Devices Media during Various Dry Periods
3.2.1. Microbial Sequences and Alpha Diversity on Substrate Surfaces
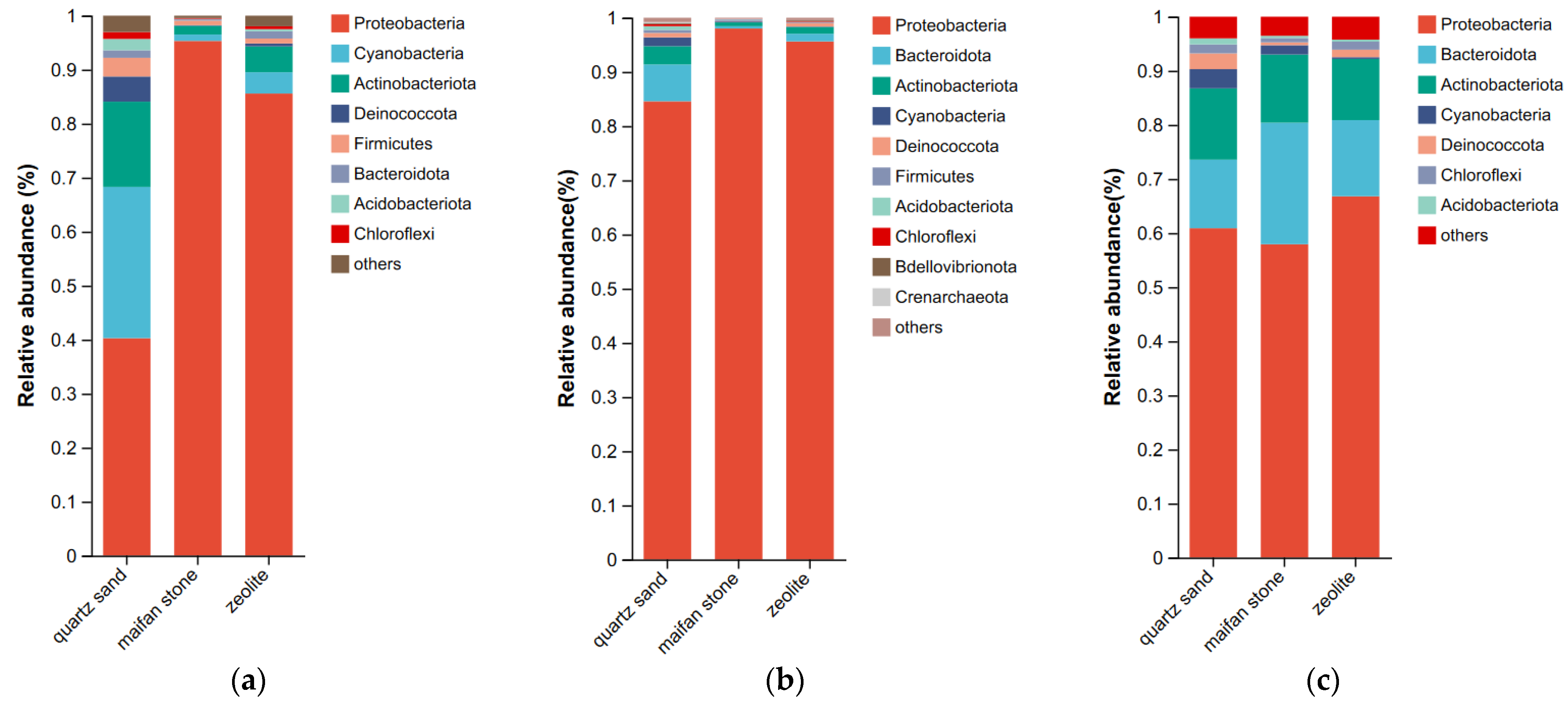
3.2.2. Composition of Microbial Community Structure at the Phylum Levels
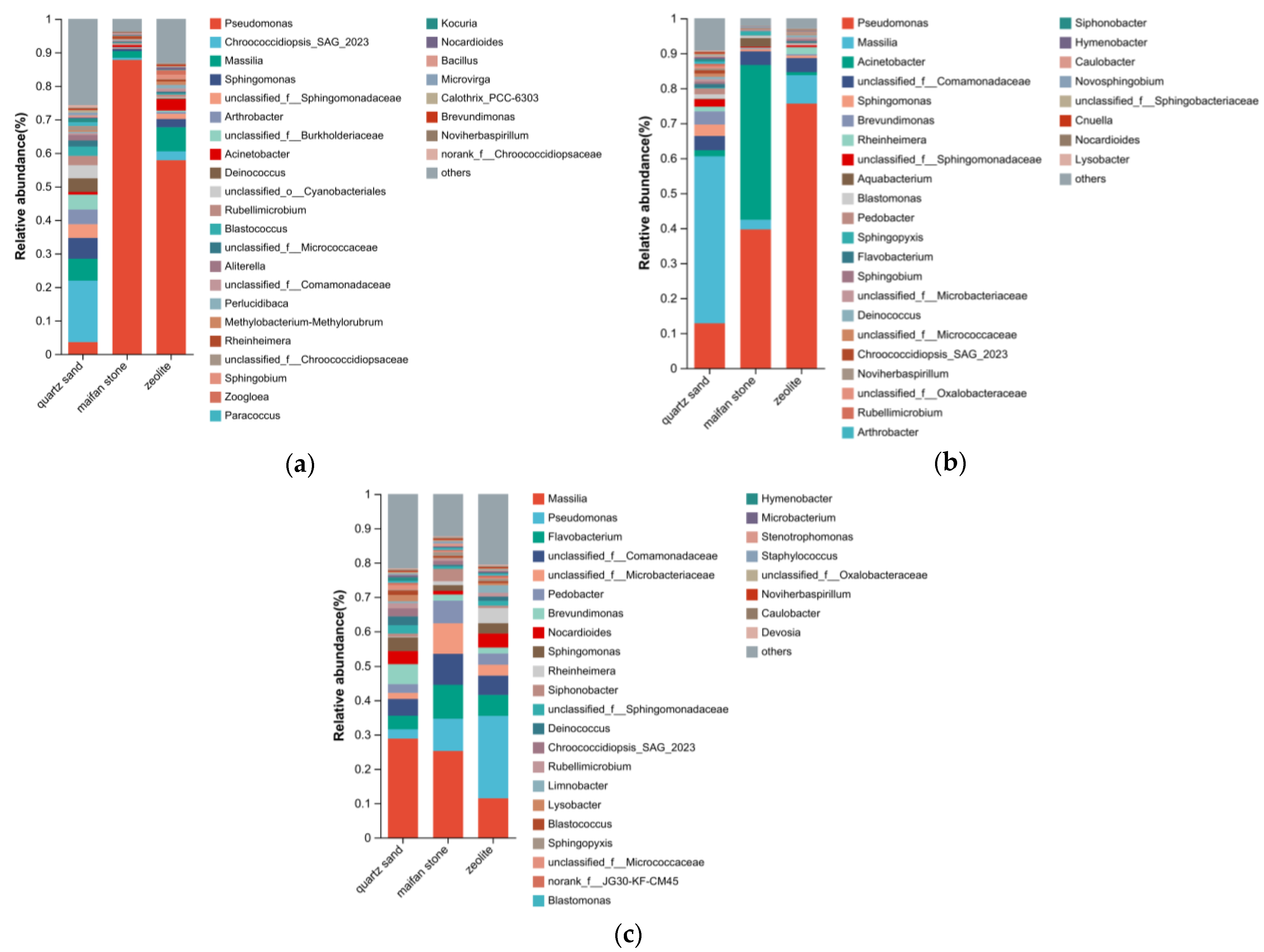
3.2.3. Composition of Microbial Community Structure at the Genus Levels
3.3. Response of Substrate Microbial Communities to Nitrogen and Phosphorus Pollutants Under Different Drying Periods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Xue, C.; Li, J.; Wang, W. Nitrogen and Phosphorus Release Characteristics of Pipeline Sediments on Entering Different Water Bodies. Water 2023, 15, 1903. [Google Scholar] [CrossRef]
- Moazzem, S.; Bhuiyan, M.; Muthukumaran, S.; Fagan, J.; Jegatheesan, V. Microbiome Wetlands in Nutrient and Contaminant Removal. Curr. Pollut. Rep. 2023, 9, 694–709. [Google Scholar] [CrossRef]
- Li, B.; Yang, Y.; Chen, J.; Wu, Z.; Liu, Y.; Xie, S. Nitrifying activity and ammonia-oxidizing microorganisms in a constructed wetland treating polluted surface water. Sci. Total Environ. 2018, 628, 310–318. [Google Scholar] [CrossRef]
- Liu, L.; Wang, F.; Xu, S.; Sun, W.; Wang, Y.; Ji, M. Woodchips bioretention column for stormwater treatment: Nitrogen removal performance, carbon source and microbial community analysis. Chemosphere 2021, 285, 131519. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Removal of nutrients in constructed wetlands for wastewater treatment through plant harvesting—Biomass and load matter the most. Ecol. Eng. 2020, 155, 105962. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, W.; Cai, N.; Li, P. Effects of Bioretention with Oyster Shell as Filler on Pollutants Removal from Urban Surface Runoff. J. Soil Water Conserv. 2019, 33, 128–133+139. (In Chinese) [Google Scholar]
- Li, Y.; Li, H.; Xu, X.; Gong, X.; Zhou, Y. Application of subsurface wastewater infiltration system to on-site treatment of domestic sewage under high hydraulic loading rate. Water Sci. Eng. 2015, 8, 49–54. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuang, Z.; Li, J.; Sun, H.; Che, W. Using a Laboratory Column Experiment to Explore the Influence of an Antecedent Dry Period on the Nutrient Removal of a Bioretention Filter. Water Air Soil Pollut. 2024, 235, 131. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.; Davis, A.P.; Liu, Y. Dissolved inorganic nitrogen behavior and fate in bioretention systems: Role of vegetation and saturated zones. J. Environ. Eng. 2019, 145, 04019074. [Google Scholar] [CrossRef]
- Xiong, J.; He, Y.; Bai, X.; Wang, X. Removal effect of phosphorus in rain-runoff by the media-improved bioretention tank. J. Environ. Eng. 2019, 13, 2164–2172. (In Chinese) [Google Scholar]
- Wang, X.; Chen, Y.; Huang, R.; Chen, Z. Effect of immobilized bacteria and algae on enhanced nitrogen and phosphorus removal in bioretention tank. J. Environ. Eng. Technol. 2023, 13, 2117–2125. (In Chinese) [Google Scholar]
- Zhang, F.; Yang, X.; Li, F.; Luo, J.; Ren, X. Research Progress in Chemical Fungicides for Oilfield Water Treatment. Contemp. Chem. Res. 2024, 9–12. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, X.; Gao, J.; Zhao, S.; Lei, Y.; Yuan, Y.; He, C.; Gao, C.; Deng, L. Hexavalent chromium removal from aqueous solution by adsorption on modified zeolites coated with Mg-layered double hydroxides. Environ. Sci. Pollut. Res. 2019, 26, 32928–32941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, C.; Fan, Y.; Lv, X.; Ji, J.; Ge, L.; Wu, J. Effect of influent C/N rations on nitrite accumulation and microbial community in the partial denitrification process. China Environ. Sci. 2022, 42, 620–628. (In Chinese) [Google Scholar] [CrossRef]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef]
- Su, J.f.; Bai, Y.h.; Huang, T.l.; Wei, L.; Gao, C.y.; Wen, Q. Multifunctional modified polyvinyl alcohol: A powerful biomaterial for enhancing bioreactor performance in nitrate, Mn(II) and Cd(II) removal. Water Res. 2020, 168, 115152. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, J.; Xie, J.; Tan, Z.; Wei, C.; Zhu, S. Investigation of the nitrogen removal performance and microbial community structure in a full-scale A/O1/H/O2 coking wastewater treatment system. Lett. Appl. Microbiol. 2023, 76, ovac015. [Google Scholar] [CrossRef]
- Yang, W.; Deng, Z.; Xu, Y.; Wang, Z.; Li, W. Study on the effect of photosynthetic bacteria on the structure and function of microbial communities in activated sludge systems. China Environ. Sci. 2024, 44, 1314–1323. (In Chinese) [Google Scholar] [CrossRef]
- Meng, J.; Liu, S.; Qiu, X.; Zhou, R. Bacterial Community Structure of Typical Lakee Sediments in Yinchuan City and Its Response to Heavy Metals. Chin. J. Environ. Sci. 2023, 45, 2727–2740. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, Z.; Pei, Y. Nutrient removal and microbial community structure in an artificial-natural coupled wetland system. Process Saf. Environ. Prot. 2021, 147, 1160–1170. [Google Scholar] [CrossRef]
- Du, L.; Chen, Q.; Liu, P.; Zhang, X.; Wang, H.; Zhou, Q.; Xu, D.; Wu, Z. Phosphorus removal performance and biological dephosphorization process in treating reclaimed water by Integrated Vertical-flow Constructed Wetlands (IVCWs). Bioresour. Technol. 2017, 243, 204–211. [Google Scholar] [CrossRef]
- Vera, A.; Wilson, F.P.; Cupples, A.M. Predicted functional genes for the biodegradation of xenobiotics in groundwater and sediment at two contaminated naval sites. Appl. Microbiol. Biotechnol. 2022, 106, 835–853. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, Y.; Zhang, W.; Zhou, H.; Chen, X.; Li, Y.; Wei, D.; Wei, Z. Roles of bacterial community in the transformation of organic nitrogen toward enhanced bioavailability during composting with different wastes. Bioresour. Technol. 2019, 285, 121326. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Wang, H.; Mu, T.; Chen, J.; Luo, H.; He, B.-J. Characterization and microbial mechanism of pollutant removal from stormwater runoff in the composite filler bioretention system. J. Water Reuse Desalination 2024, 14, 95–114. [Google Scholar] [CrossRef]
- He, Q.; Xie, Z.; Tang, M.; Fu, Z.; Ma, J.; Wang, H.; Zhang, W.; Zhang, H.; Wang, M.; Hu, J.; et al. Insights into the simultaneous nitrification, denitrification and phosphorus removal process for in situ sludge reduction and potential phosphorus recovery. Sci. Total Environ. 2021, 801, 149569. [Google Scholar]
- Bixiao, J.; Huining, Z.; Lun, Z.; Jing, Y.; Kefeng, Z.; Xin, Y.; Jianqing, M.; Yongxing, Q. Effect of the rapid increase of salinity on anoxic-oxic biofilm reactor for treatment of high-salt and high-ammonia–nitrogen wastewater. Bioresour. Technol. 2021, 337, 125363. [Google Scholar]
- Naylor, D.; Derr, D.C. Drought Stress and Root-Associated Bacterial Communities. Front. Plant Sci. 2018, 8, 2223. [Google Scholar] [CrossRef]
- Liu, T.; Wang, M.; Wu, X.; Jiao, Y.; Wen, J. Analysis of microbial community structure and functionality during biodrying of storage sludge. J. Saf. Environ. 2023, 23, 2477–2486. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, M.; Xue, S.; Zhou, S.; Jiao, Y.; Tian, X. Nitrogen and phosphorusas major factors shaping microbial community structures in surface sediments in Xiangxi River, Three Gorges Reservoir. Lake Sci. 2023, 35, 473–486. (In Chinese) [Google Scholar]


| Name | Fungicide | Rainfall Duration (min) | Rainfall Intensity (mm/h) | Design Inflow Rate (L) |
|---|---|---|---|---|
| JZ | Add | 60 | 23.5 | 31 |
| CY | Do not add | 60 | 23.5 | 31 |
| Drying Period | Media | Diversity Indicators | Richness Indicators | Coverage | |||
|---|---|---|---|---|---|---|---|
| Shannon | Simpson | Sobs | Ace | Chao | |||
| 3 day | quartz sand | 5.44 | 0.0189 | 1436 | 1436.00 | 1436.00 | 1 |
| maifan stone | 2.04 | 0.3476 | 481 | 481.18 | 481.00 | 0.999 | |
| zeolite | 3.63 | 0.1254 | 644 | 644.31 | 644.10 | 0.999 | |
| 7 day | quartz sand | 3.69 | 0.1725 | 957 | 958.04 | 957.06 | 0.999 |
| maifan stone | 2.59 | 0.1572 | 363 | 363.00 | 363.00 | 0.999 | |
| zeolite | 2.94 | 0.1235 | 553 | 553.54 | 553.01 | 1 | |
| 14 day | quartz sand | 5.02 | 0.0635 | 1365 | 1367.48 | 1365.35 | 0.999 |
| maifan stone | 4.60 | 0.0287 | 922 | 922.00 | 922.00 | 1 | |
| zeolite | 5.10 | 0.0243 | 1328 | 1334.94 | 1330.64 | 0.999 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.; Xue, C.; Li, J.; Wang, W.; Du, X.; Gong, Y.; Zhao, Y.; Liang, M.; Ren, Y. Effects of Dry Periods on Nitrogen and Phosphorus Removal in Runoff Infiltration Devices and Their Biological Succession Patterns. Water 2024, 16, 2372. https://doi.org/10.3390/w16172372
He T, Xue C, Li J, Wang W, Du X, Gong Y, Zhao Y, Liang M, Ren Y. Effects of Dry Periods on Nitrogen and Phosphorus Removal in Runoff Infiltration Devices and Their Biological Succession Patterns. Water. 2024; 16(17):2372. https://doi.org/10.3390/w16172372
Chicago/Turabian StyleHe, Tian, Chonghua Xue, Junqi Li, Wenhai Wang, Xiaoli Du, Yongwei Gong, Yimeng Zhao, Manman Liang, and Yaxin Ren. 2024. "Effects of Dry Periods on Nitrogen and Phosphorus Removal in Runoff Infiltration Devices and Their Biological Succession Patterns" Water 16, no. 17: 2372. https://doi.org/10.3390/w16172372





