Effects of pH on the Photocatalytic Activity and Degradation Mechanism of Rhodamine B over Fusiform Bi Photocatalysts under Visible Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Fusiform Bi
2.3. Characterizations
2.4. Photocatalytic Activity
3. Results and Discussion
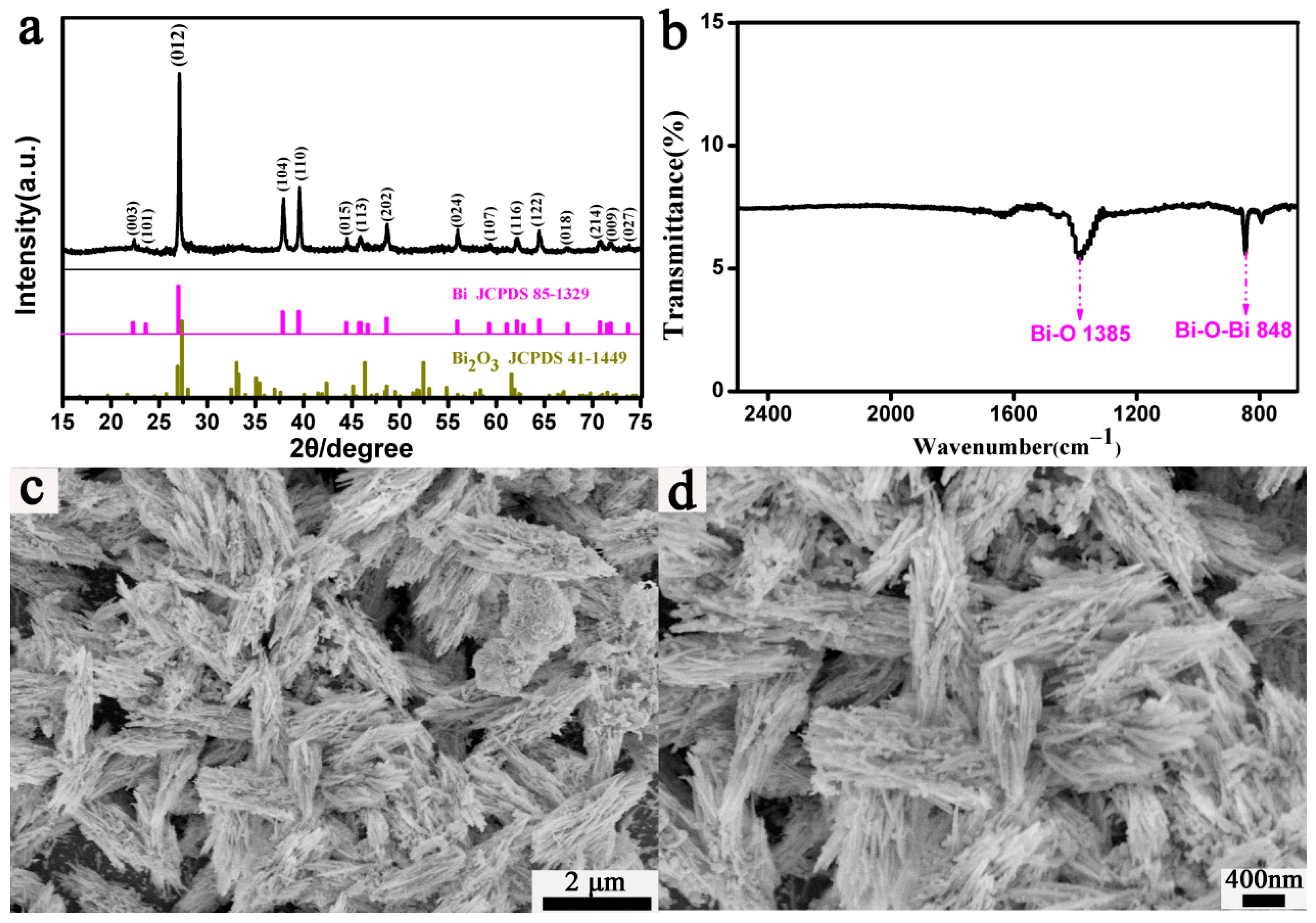
3.1. Fusiform Bi Characterization
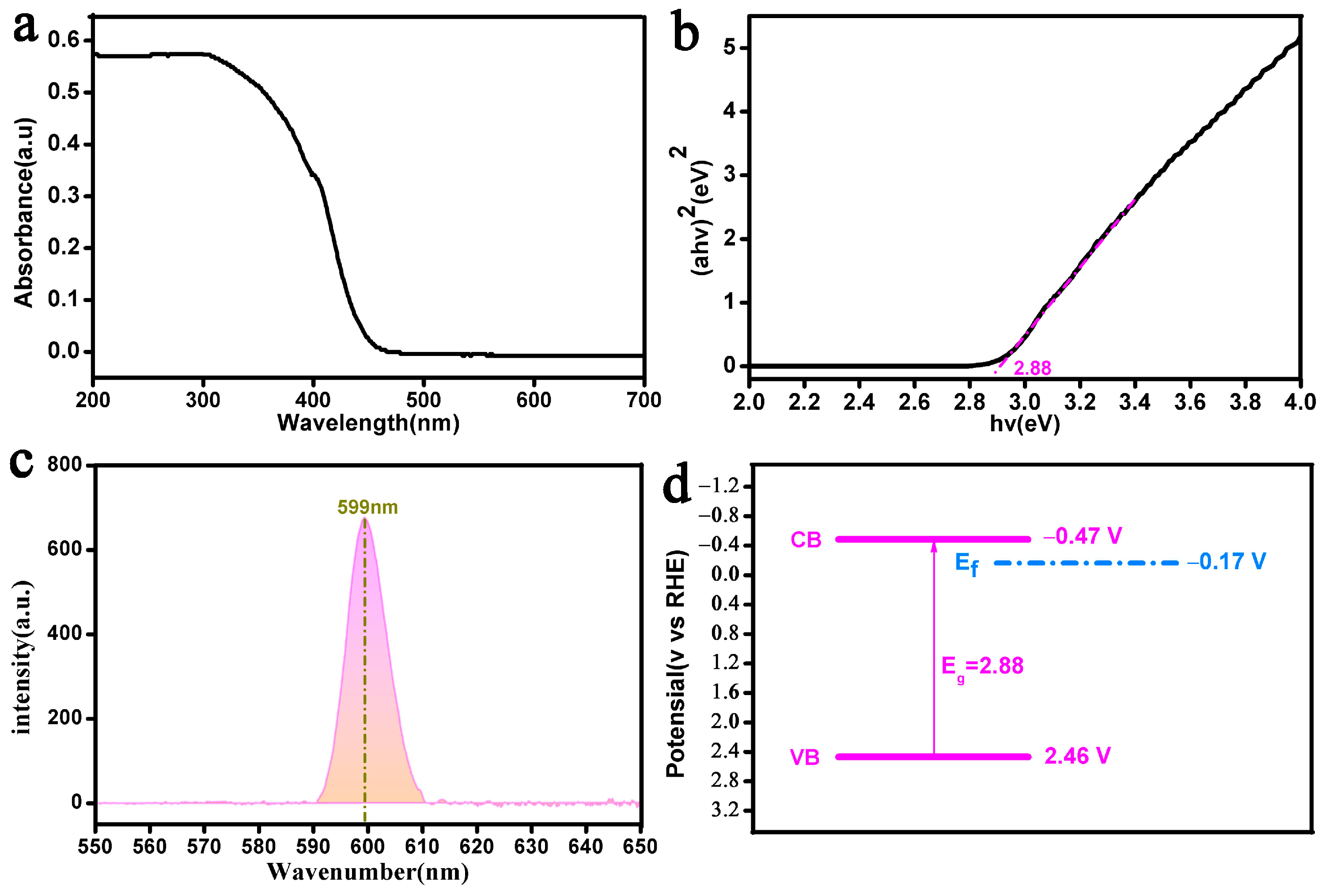
3.2. Analysis of Optical Properties
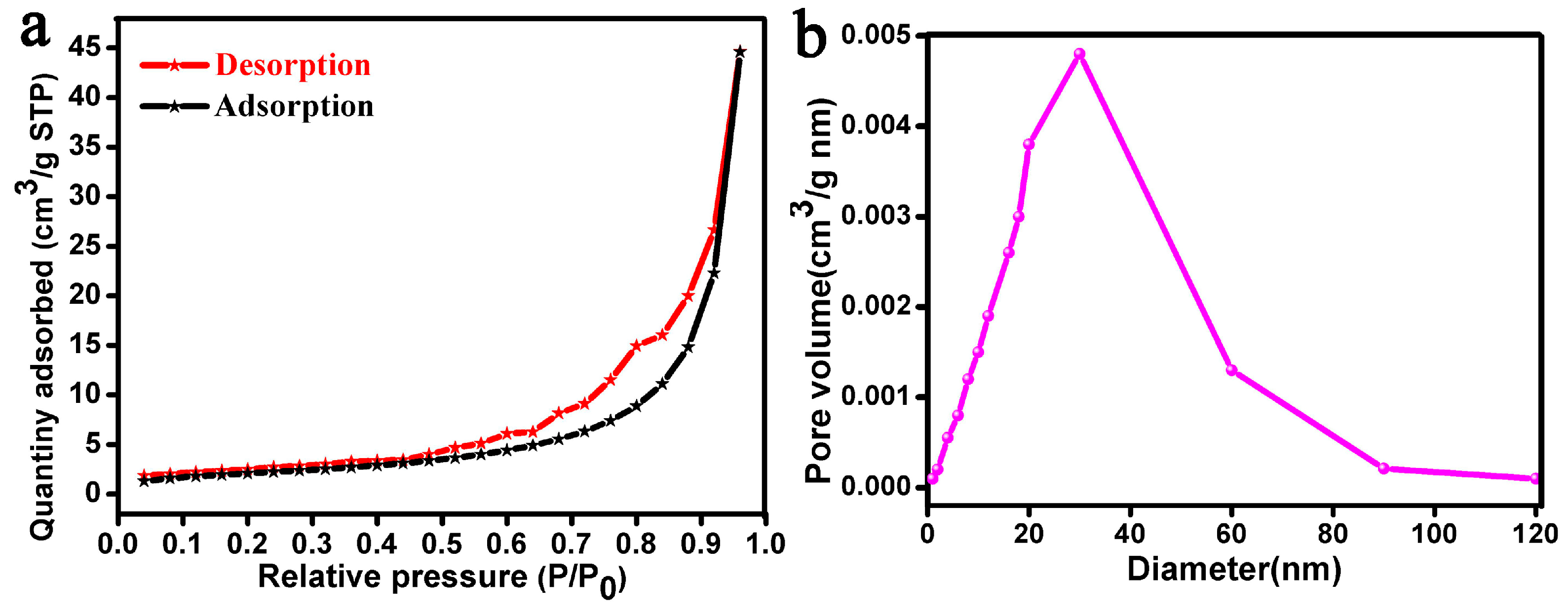
3.3. BET Analysis and Adsorption
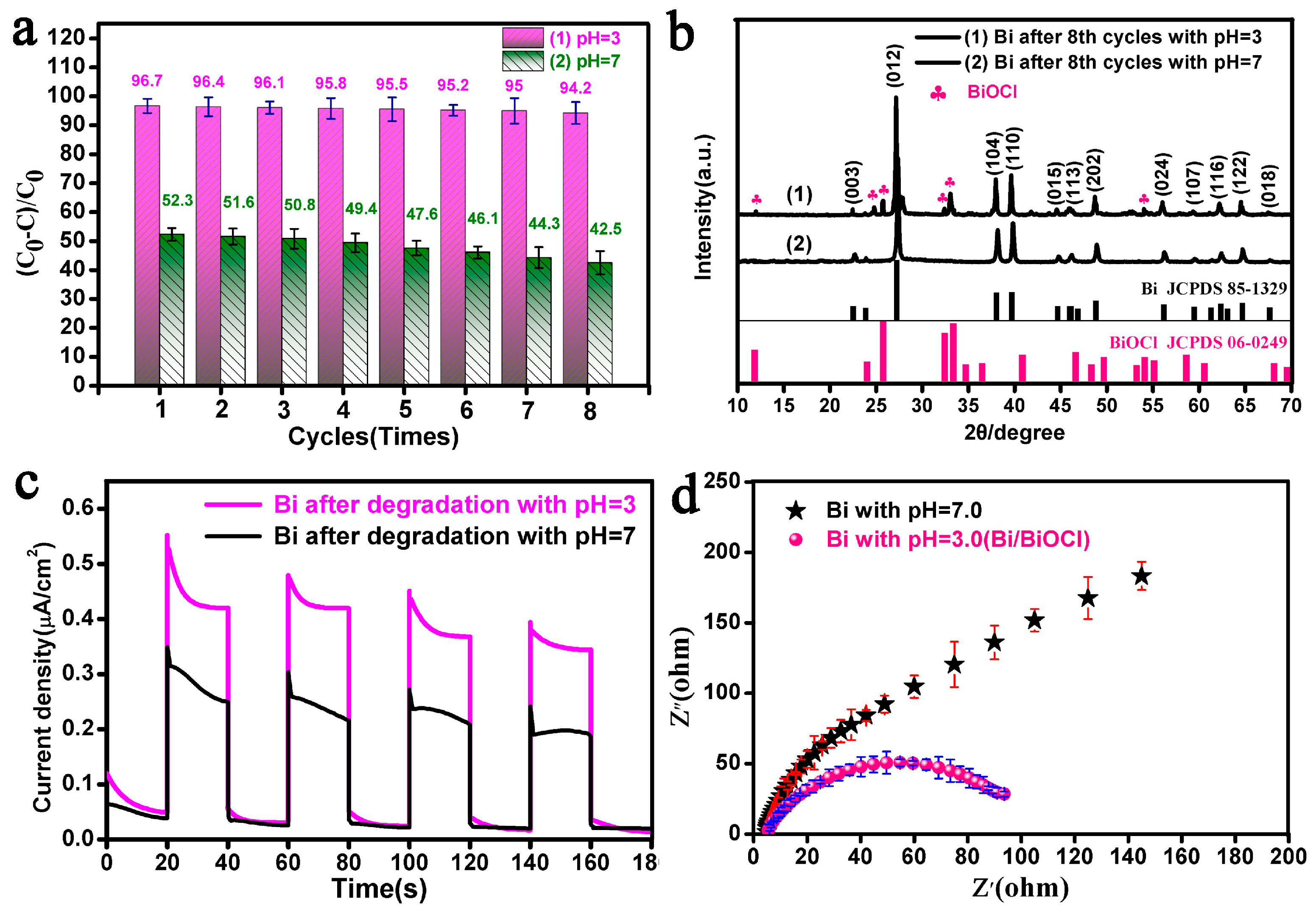
3.4. Photodegradation of RhB over Fusiform Bi
3.5. XPS and FTIR Analyses
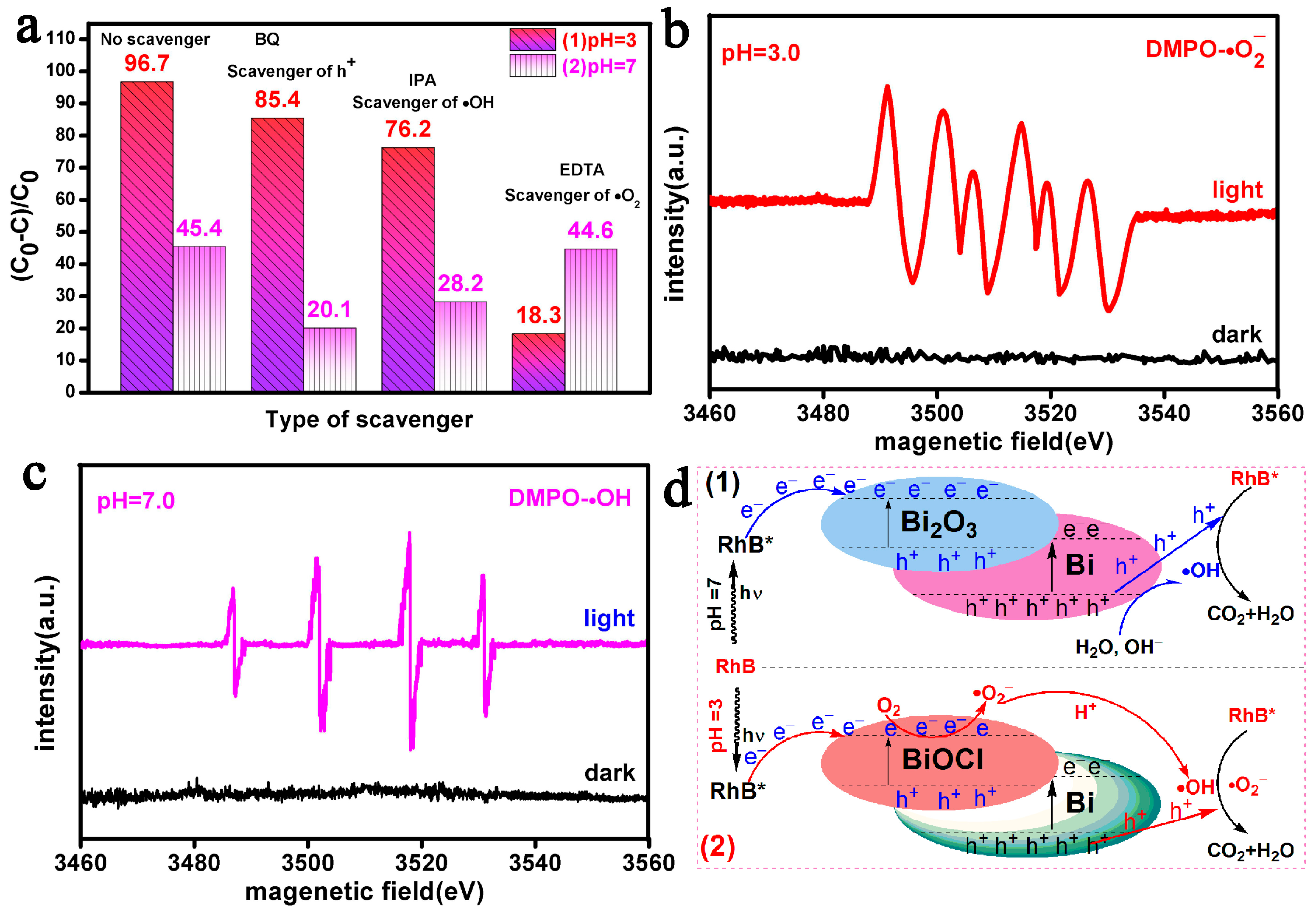
3.6. Potential Photocatalytic Mechanisms
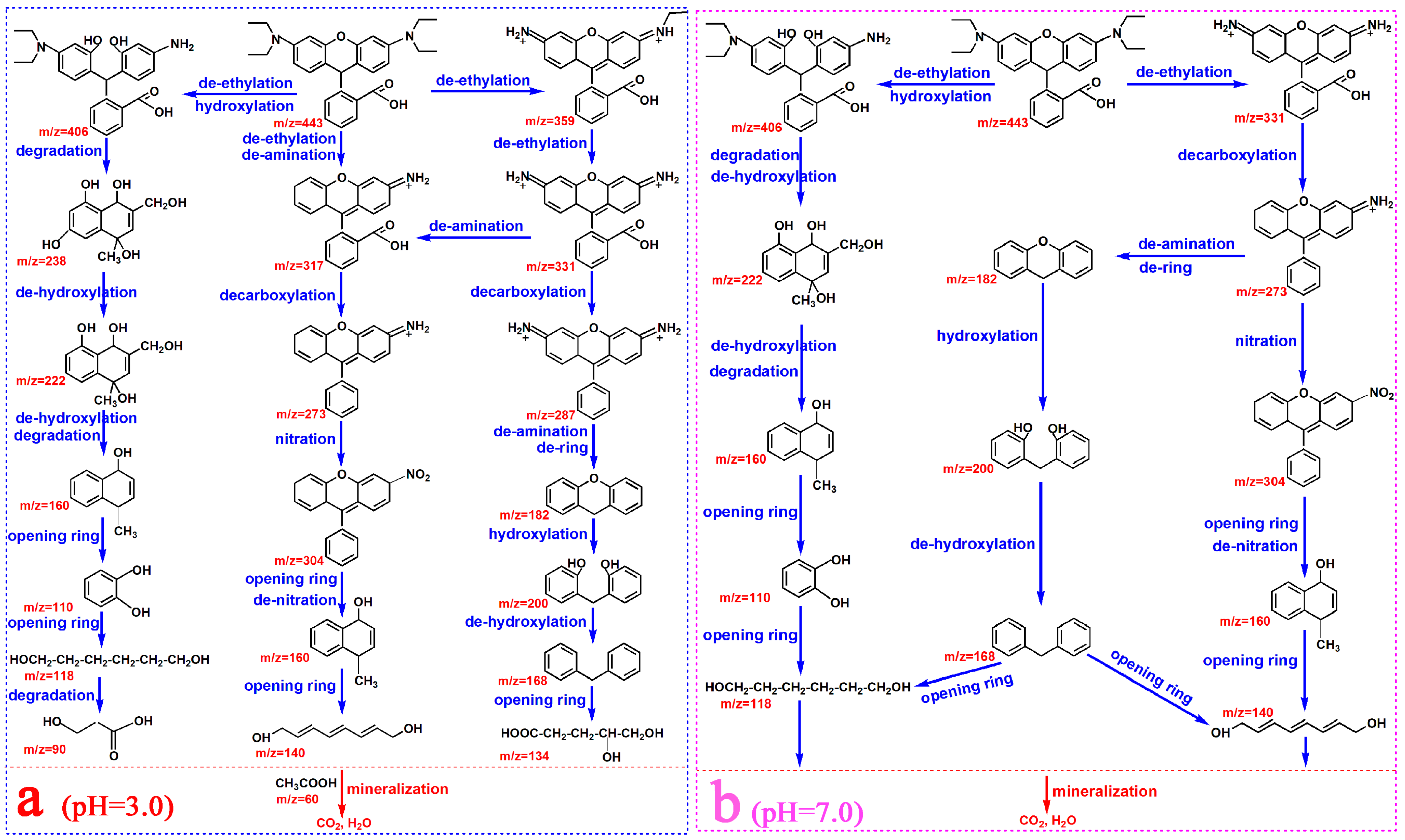
3.7. Degradation Pathways of RhB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| RhB | Rhodamine B |
| COD | Chemical oxygen demand |
| TOC | Total organic carbon |
| pH | Potential of hydrogen |
| Superoxide anion radical | |
| •OH | Hydroxyl radical |
| e− | Electron |
| h+ | Hole |
| VB | Valence band |
| CB | Conduction band |
| RHE | Reversible hydrogen electrode |
| SSA | Specific surface area |
| EIS | Electrochemical impedance spectroscopy |
| EPR | Electron paramagnetic resonance |
| m/z | Mass charge ratio |
| Eg | Band gap energy value |
| SPR | Surface plasmon resonance |
References
- Parsa, S.M.; Norozpour, F.; Elsheikhc, A.H.; Kabeel, A.E. Solar desalination/purification (solar stills, humidification-dehumidification, solar disinfection) in high altitude during COVID19: Insights of gastrointestinal manifestations and systems’mechanism. J. Hazard. Mater. Adv. 2023, 10, 100259. [Google Scholar] [CrossRef]
- Parsa, S.M. Mega-scale desalination efficacy (Reverse Osmosis, Electrodialysis, Membrane Distillation, MED, MSF) during COVID-19: Evidence from salinity, pretreatment methods, temperature of operation. J. Hazard. Mater. Adv. 2023, 9, 100217. [Google Scholar] [CrossRef] [PubMed]
- Parsa, S.M.; Norouzpour, F.; Shoeibi, S.; Shahsavar, A.; Aberoumand, S.; Said, Z.; Guo, W.S.; Ngo, H.H.; Ni, B.-J.; Afrand, M.; et al. A comprehensive study to find the optimal fraction of nanoparticle coated at the interface of solar desalination absorbers: 5E and GHGs analysis in different seasons. Sol. Energ. Mat. Sol. C 2023, 256, 112308. [Google Scholar] [CrossRef]
- Parsa, S.M.; Rahbar, A.; Koleini, M.H.; Aberoumand, S.; Afrand, M.; Amidpour, M. A renewable energy-driven thermoelectric-utilized solar still with external condenser loaded by silver/nanofluid for simultaneously water disinfection and desalination. Desalination 2020, 480, 114354. [Google Scholar] [CrossRef]
- Al-Buriahi, A.K.; Al-Gheethi, A.A.; Kumar, P.S.; Mohamed, R.M.S.R.; Yusof, H.; Alshalif, A.F.; Khalifa, N.A. Elimination of rhodamine B from textile wastewater using nanoparticle photocatalysts: A review for sustainable approaches. Chemosphere 2022, 287, 132162. [Google Scholar] [CrossRef]
- Venkatraman, S.K.; Vijayakumar, N.; Bal, D.K.; Mishra, A.; Gupta, B.; Mishra, V.; Wysokowski, M.; Koppala, S.; Swamiappan, S. Degradation of environmentally harmful textile dye rhodamine B using silicate ceramic photocatalysts. Inorg. Chem. Commun. 2022, 142, 109674. [Google Scholar] [CrossRef]
- Danish, M.; Khanday, W.A.; Hashim, R.; Sulaiman, N.S.B.; Akhtar, M.N.; Nizami, M. Application of optimized large surface area date stone (Phoenix dactylifera) activated carbon for rhodamin B removal from aqueous solution: Box-Behnken design approach. Ecotoxicol. Environ. Saf. 2017, 139, 280–290. [Google Scholar] [CrossRef]
- Lim, S.; Shi, J.L.; Gunten, U.; McCurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef]
- Lal, M.; Sharma, P.; Singh, L.; Ram, C. Photocatalytic degradation of hazardous Rhodamine B dye using sol-gel mediated ultrasonic hydrothermal synthesized of ZnO nanoparticles. Results Eng. 2023, 17, 100890. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Bhatt, U.; Soni, V. Toxic effects of Rhodamine B on antioxidant system and photosynthesis of Hydrilla verticillata. J. Hazard. Mater. Lett. 2022, 3, 100069. [Google Scholar] [CrossRef]
- Çelik, M.S.; Çetinus, Ş.A.; Yenidünya, A.F.; Çetinkaya, S.; Tüzün, B. Biosorption of Rhodamine B dye from aqueous solution by Rhus coriaria L. plant: Equilibrium, kinetic, thermodynamic and DFT calculations. J. Mol. Struct. 2023, 1272, 134158. [Google Scholar] [CrossRef]
- Souza, F.H.M.; Leme, V.F.C.; Costa, G.O.B.; Castro, K.C.; Giraldi, T.R.; Andrade, G.S.S. Biosorption of Rhodamine B using a low-cost biosorbent prepared from inactivated aspergillus oryzae cells: Kinetic, equilibrium and thermodynamic studies. Water Air Soil. Pollut. 2020, 242, 231. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Azhar, Q.M.; Kumar, P.S.; Yusuf, A.A.; Al-Buriahi, A.K.; Mohamed, R.M.S.R.; Al-shaibani, M.M. Sustainable approaches for removing Rhodamine B dye using agriculturalwaste adsorbents: A review. Chemosphere 2022, 287, 132080. [Google Scholar] [CrossRef] [PubMed]
- Saigl, Z.M. Various adsorbents for removal of Rhodamine B dye: A review. Indones. J. Chem. 2021, 21, 1039–1056. [Google Scholar] [CrossRef]
- Deng, D.L.; Li, Y.; Wu, M.Z.; Song, Y.; Huang, Q.J.; Duan, Y.Q.; Chang, Y.; Zhao, Y.Y.; He, C.L. Electrocatalytic degradation of Rhodamine B on the Sb-doped SnO2/Ti electrode in alkaline medium. ACS Omega 2023, 8, 48480–48490. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.F.; Zhu, H.; Lin, M.; Wu, D.E.; Yao, J.G.; Sun, T.Y.; Ma, X.J.; Xia, Y.J. Highly-efficient removal of Rhodamine B using a flow-through electrocatalytic filtration system: Characteristics, efficiency and mechanism. J. Electroanal. Chem. 2023, 946, 117742. [Google Scholar] [CrossRef]
- Ma, X.L.; Duan, D.M.; Chen, X.; Feng, X.M.; Ma, Y.H. A polysaccharide-based bioflocculant BP50-2 from banana peel waste: Purification, structure and flocculation performance. Int. J. Biol. Macromol. 2022, 205, 604–614. [Google Scholar] [CrossRef]
- Vadivel, M.; Jayakumar, S.; Philip, J. Rapid removal of rhodamine dye from aqueous solution using casein-surfactant complexes: Role of casein-surfactant interaction. J. Disper. Sci. Technol. 2022, 43, 928–943. [Google Scholar] [CrossRef]
- Saruchi; Kumar, V.; Kaith, B.S.; Jindal, R. Synthesis of hybrid ion exchanger for Rhodamine B dye removal: Equilibrium, kinetic and thermodynamic studies. Ind. Eng. Chem. Res. 2016, 55, 10492–10499. [Google Scholar] [CrossRef]
- Thakur, V.; Singh, S.; Kumar, P.; Rawat, S.; Srivastava, V.C.; Lo, S.-L.; Štangar, U.L. Photocatalytic behaviors of bismuth-based mixed oxides: Types, fabrication techniques and mineralization mechanism of antibioticsm. Chem. Eng. J. 2023, 475, 146100. [Google Scholar] [CrossRef]
- Jain, S.; Mittal, A.; Kumari, V.; Sharma, A.; Jindal, J.; Makgwane, P.R.; Kumar, V.; Kumar, N.; Kumari, K. A facile synthesized Z-scheme Bi2O3/SnS/Ag ternary nanocomposite: An expedited visible photocatalysis by plasmonic silver for efficient organic decontamination. Opt. Mater. 2023, 145, 114434. [Google Scholar] [CrossRef]
- Astuti, Y.; Elesta, P.P.; Widodo, D.S.; Widiyandari, H.; Balgis, R. Hydrazine and urea fueled-solution combustion method for Bi2O3 synthesis: Characterization of physicochemical properties and photocatalytic activity. Bull. Chem. React. Eng. Catal. 2020, 15, 104–111. [Google Scholar] [CrossRef]
- Kansaard, T.; Ishihara, K.N.; Pecharapa, W. Structural, optical and photo-induced catalytic properties of derived-Leucoxene/BiVO4 composite prepared by sonochemical process. Optik 2022, 267, 169665. [Google Scholar] [CrossRef]
- Monisha, K.; Kavipriya, S.; Silambarasan, A.; Arulmozhi, R.; Abirami, N.; Ramesh, R. Hydrothermal synthesis of hierarchically structured cobalt doped bismuth tungstate with improved photocatalytic activity. Optik 2020, 206, 164366. [Google Scholar] [CrossRef]
- Khalid, N.R.; Ishtiaq, H.; Ali, F.; Tahir, M.B.; Naeem, S.; Ul-Hamid, A.; Ikram, M.; Iqbal, T.; Kamal, M.R.; Alrobei, H.; et al. Synergistic effects of Bi and N doped on ZnO nanorods for efficient photocatalysis. Mater. Chem. Phys. 2022, 289, 126423. [Google Scholar] [CrossRef]
- Chen, L.Q.; Tian, L.J.; Zhao, X.; Hu, Z.; Fan, J.J.; Lv, K.L. SPR effect of Au nanoparticles on the visible photocatalytic RhB degradation and NO oxidation over TiO2 hollow nanoboxes. Arab. J. Chem. 2022, 13, 4404–4416. [Google Scholar] [CrossRef]
- Chen, X.G.; Zhu, L.; Ma, Z.P.; Wang, M.L.; Zhao, R.; Zou, Y.Y.; Fan, Y.J. Ag nanoparticles decorated ZnO nanorods as multifunctional SERS substrates for ultrasensitive detection and catalytic degradation of Rhodamine B. Nanomaterials 2022, 12, 2394. [Google Scholar] [CrossRef]
- Pinchujit, S.; Phuruangrat, A.; Wannapop, S.; Sakhon, T.; Kuntalue, B.; Thongtem, T.; Thongtem, S. Bi2WO6 thin nanoplates decorated with Pt nanoparticles possessing enhanced visible-light-driven photocatalytic performance for Rhodamine B degradation. Russ. J. Inorg. Chem. 2022, 67, S199–S209. [Google Scholar] [CrossRef]
- Nevinskas, I.; Stanionyte, S.; Devenson, J.; Krotkus, A. Direct bandgap dependence of bismuth films on their thickness. J. Appl. Phys. 2022, 132, 055301. [Google Scholar] [CrossRef]
- Huang, H.; Liu, G.S.; Wang, X.H. Bismuth microsphere for photo-assisted nitrate removal: Experimental and theoretical investigations. Chem. Eng. J. 2022, 431, 133239. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yang, Z.W.; Wang, L.S.; Chao, L. Carbon covered Ag and Bi nanoparticles uniformly dispersed in porous carbon matrix: Synergistic effect for removal of RhB. Mater. Lett. 2020, 275, 128099. [Google Scholar] [CrossRef]
- Zhang, F.N.; Zhao, Y.; Li, Y.W.; Wu, G.J.; Zhao, J.Z. CTAB induced hierarchical bismuth microspheres for visible-light photocatalytic study. J. Colloid Interf. Sci. 2017, 505, 519–527. [Google Scholar] [CrossRef]
- Ozer, M.S.; Eroglu, Z.; Yalin, A.S.; Kılıç, M.; Rothlisberger, U.; Metin, O. Bismuthene as a versatile photocatalyst operating under variable conditions for the photoredox C-H bond functionalization. Appl. Catal. B 2022, 304, 120957. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Y.; Wang, F.; Dong, F.; Li, W.; Li, H.; Patzke, G.R. From semiconductors to semimetals: Bismuth as a photocatalyst for NO oxidation in air. J. Mater. Chem. 2014, 2, 11065–11072. [Google Scholar] [CrossRef]
- Zhang, M.L.; Ke, J.; Xu, D.S.; Zhang, X.Y.; Liu, H.Y.; Wang, Y.R.; Yu, J.X. Construction of plasmonic Bi/Bismuth oxycarbonate/Zinc bismuth oxide ternary heterojunction for enhanced charge carrier separation and photocatalytic performances. J. Colloid Interf. Sci. 2022, 615, 663–673. [Google Scholar] [CrossRef]
- Zhang, L.P.; Ghimire, P.; Phuriragpitikhon, J.; Jiang, B.J.; Gonçalves, A.A.S.; Jaroniec, M. Facile formation of metallic bismuth/bismuth oxide heterojunction on porous carbon with enhanced photocatalytic activity. J. Colloid Interf. Sci. 2018, 513, 82–91. [Google Scholar] [CrossRef]
- Khavar, A.H.C.; Mahjoub, A.R.; Najafi, S. Enhanced photocatalytic degradation of tetracycline by improving electron flux at the Schottky junction between Bi nanoparticles and MoS2-anchored RGO. J. Photoch. Photobio. A Chem. 2024, 447, 115270. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.L.; Zhao, C.Y.; Nie, E.; Wang, J.Q.; Zhou, J.; Zhao, Q. Efficient dye contaminant elimination and simultaneously electricity production via a Bi-doped TiO2 photocatalytic fuel cell. Nanomaterials 2022, 12, 210. [Google Scholar] [CrossRef]
- Yue, C.L.; Zhu, L.L.; Qiu, Y.X.; Du, Z.L.; Qiu, J.L.; Liu, F.Q.; Wang, F.H. Recent advances of plasmonic elemental Bi based photocatalysts in environmental remediation and energy conversion. J. Clean. Prod. 2023, 392, 136017. [Google Scholar] [CrossRef]
- Song, Y.K.; Bao, Z.Q.; Gu, Y.Y. Photocatalytic enhancement strategy with the introduction of metallic Bi: A review on Bi/semiconductor photocatalysts. Chem. Rec. 2023, 24, e202300307. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, C.L.; Huang, R.; Peng, H.; Tang, X.D. Investigation of optical and photocatalytic properties of bismuth nanospheres prepared by a facile thermolysis method. J. Phys. Chem. C 2014, 118, 1155–1160. [Google Scholar] [CrossRef]
- Li, Y.L.; Zhu, J.Z. Synthesis of Bi nanoparticles supported on activated carbon for efficient photocatalytic degradation of high concentration Rhodamine B. Micro Nano Lett. 2020, 15, 627–631. [Google Scholar] [CrossRef]
- Naing, H.H.; Li, Y.; Ghasemi, J.B.; Wang, J.T.; Zhang, G.K. Enhanced visible-light-driven photocatalysis of in-situ reduced of bismuth on BiOCl nanosheets and montmorillonite loading: Synergistic effect and mechanism insight. Chemosphere 2022, 304, 135354. [Google Scholar] [CrossRef]
- Astuti, Y.; Andianingrum, R.; Haris, A.; Darmawan, A. The role of H2C2O4 and Na2CO3 as precipitating agents on the physichochemical properties and photocatalytic activity of bismuth oxide. Open Chem. 2020, 18, 129–137. [Google Scholar] [CrossRef]
- Astuti, Y.; Amri, D.; Widodo, D.S.; Widiyandari, H.; Balgis, R.; Ogi, T. Effect of fuels on the physicochemical properties and photocatalytic activity of bismuth oxide, synthesized using solution combustion method. Int. J. Technol. 2020, 11, 26–36. [Google Scholar] [CrossRef]
- Astuti, Y.; Listyani, B.M.; Suyati, L.; Darmawan, A. Bismuth oxide prepared by sol-gel method: Variation of physicochemical characteristics and photocatalytic activity due to difference in calcination temperature. Indones. J. Chem. 2021, 21, 108–117. [Google Scholar] [CrossRef]
- Landi, S.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid. State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Ye, F.; Qian, J.; Xia, J.J.; Li, L.F.; Wang, S.J.; Zeng, Z.X.; Mao, J.; Ahamad, M.; Xiao, Z.R.; Zhang, Q.R. Efficient photoelectrocatalytic degradation of pollutants over hydrophobic carbon felt loaded with Fe-doped porous carbon nitride via direct activation of molecular oxygen. Environ. Res. 2024, 249, 118497. [Google Scholar] [CrossRef]
- Wang, P.L.; Fan, S.Y.; Li, X.Y.; Duan, J.; Zhang, D.K. Modulating the molecular structure of graphitic carbon nitride for identifying the impact of the piezoelectric effect on photocatalytic H2O2 production. ACS Catalysis 2023, 13, 9515–9523. [Google Scholar] [CrossRef]
- Rong, J.; Zhang, T.; Qiu, F.; Chen, M. Structural evolution of hierarchical porous NiO/Al2O3 composites and their application for removal of dyes by adsorption. Korean J. Chem. Eng. 2017, 34, 41–53. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, J.J.; Wang, H.T.; Zhang, A.M.; Li, J.P.; Yan, M.F.; Guo, T.Y. Shaddock peel-derived N-doped carbon quantum dots coupled with ultrathin BiOBr square nanosheets with boosted visible light response for high-efficiency photodegradation of RhB. Environ. Pollut. 2023, 325, 121424. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.; Basu, S.; Barman, S. Efficient eradication of antibiotic and dye by C-dots@zeolite nanocomposites: Performance evaluation, and degraded products analysis. Chemosphere 2022, 298, 134260. [Google Scholar] [CrossRef]
- Tun, P.P.; Wang, J.T.; Khaing, T.T.; Wu, X.Y.; Zhang, G.K. Fabrication of functionalized plasmonic Ag loaded Bi2O3/montmorillonite nanocomposites for efficient photocatalytic removal of antibiotics and organic dyes. J. Alloys Compd. 2020, 818, 152836. [Google Scholar] [CrossRef]
- Sharma, A.; Mitta, A.; Sharma, S.; Tahir, M.; Parmar, D.; Singh, P.; Kumar, N. Integrating Ni, Pt, and Pd on biphasic Cu-doped Bi2O3 for physicochemical characteristics and superior light driven elimination of pollutants. Catal. Surv. Asia 2024, 28, 101–116. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, L.; Zhang, Y.; Chen, Y.; Wang, S.; Li, L.; Long, Y.; Jiang, F. Synthesis and characterization of α/β-Bi2O3 with enhanced photocatalytic activity for 17α- Ethynylestradiol. Ceram. Int. 2017, 43, 7627–7635. [Google Scholar] [CrossRef]
- Bera, K.K.; Chakraborty, M.; Mondal, M.; Banik, S.; Bhattacharya, S.K. Synthesis of α-β Bi2O3 heterojunction photocatalyst and evaluation of reaction mechanism for degradation of RhB dye under natural sunlight. Ceram. Int. 2020, 46, 7667–7680. [Google Scholar]
- Bera, K.K.; Majumdar, R.; Chakraborty, M.; Bhattacharya, S.K. Phase control synthesis of α, β and α/β Bi2O3 hetero-junction with enhanced and synergistic photocatalytic activity on degradation of a toxic dye, Rhodamine-B under natural sunlight. J. Hazard. Mater. 2018, 352, 182–191. [Google Scholar]
- Mittal, A.; Sharma, S.; Kumari, V.; Yadav, S.; Chauhan, N.S.; Kumar, N. Highly efficient, visible active TiO2/CdS/ZnS photocatalyst, study of activity in an ultra low energy consumption LED based photo reactor. J. Mater. Sci. Mater. Electron. 2019, 30, 17933–17946. [Google Scholar]
- Sakkas, V.A.; Arabatzis, I.M.; Konstantinou, I.K.; Dimou, A.D.; Albanis, T.A.; Falaras, P. Metolachlor photocatalytic degradation using TiO2 photocatalysts. Appl. Catal. B 2004, 49, 195–205. [Google Scholar] [CrossRef]
- Park, S.K.; Shin, H. Effect of HCl and H2SO4 treatment of TiO2 powder on the photosensitized degradation of aqueous rhodamine B under visible light. J. Nanosci. Nanotechnol. 2014, 14, 8122–8128. [Google Scholar] [CrossRef] [PubMed]
- Jayaweera, P.M.; Sanjeewa, T.; Tennakone, K. Formation of ion-associated complexes between SPADNS and rhodamine B: Antenna effect and enhancement of photovoltaic properties. Sol. Energ. Mat. Sol. C 2007, 91, 944–950. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Z.; Zhang, H.; Lu, H.; Qiu, Y. Efficient degradation of organic pollutants by peroxymonosulfate activated with MgCuFe-layered double hydroxide. RSC Adv. 2019, 9, 2284–2291. [Google Scholar] [CrossRef]
- Li, W.; Wu, P.-X.; Zhu, Y.; Huang, Z.-J.; Lu, Y.-H.; Li, Y.-W.; Dang, Z.; Zhu, N.-W. Catalytic degradation of bisphenol A by CoMnAl mixed metal oxides catalyzed peroxymonosulfate: Performance and mechanism. Chem. Eng. J. 2015, 279, 93–102. [Google Scholar] [CrossRef]
- Kong, B.; Zeng, T.X.; Wang, W.T. The n-type and p-type conductivity mechanisms of the bulk BiOCl photocatalyst from hybrid density functional theory calculations. Phys. Chem. Chem. Phys. 2021, 23, 19841. [Google Scholar] [CrossRef]
- Raza, A.; Qumar, U.; Haider, A.; Naz, S.; Haider, J.; Ul-Hamid, A.; Ikram, M.; Ali, S.; Goumri-Said, S.; Kanoun, M.B. Liquid-phase exfoliated MoS2 nanosheets doped with p-type transition metals: A comparative analysis of photocatalytic and antimicrobial potential combined with density functional theory. Dalton Trans. 2021, 50, 6598–6619. [Google Scholar] [CrossRef]
- Wu, S.Q.; Wang, J.B.; Li, Q.C.; Huang, Z.A.; Rao, Z.Q.; Zhou, Y. Bi/BiOCl Nanosheets Enriched with Oxygen Vacancies to Enhance Photocatalytic CO2 Reduction. Trans. Tianjin Univ. 2021, 27, 155–164. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Z.X.; Shang, Z.C.; Wang, X.H. One-step solution combustion synthesis of Bi/BiOCl nanosheets: Reaction mechanism and photocatalytic RhB degradation. J. Phys. Chem. Solids 2023, 174, 111172. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Sun, H.; Guo, F.; Shi, W. One-step simple green method to prepare carbon-doped graphitic carbon nitride nanosheets for boosting visible-light photocatalytic degradation of tetracycline. J. Chem. Technol. Biotechnol. 2021, 96, 3122–3133. [Google Scholar] [CrossRef]
- Shi, W.; Wang, J.; Yang, S.; Lin, X.; Guo, F.; Shi, J. Fabrication of a ternary carbon dots/CoO/g-C3N4 nanocomposite photocatalyst with enhanced visible-light-driven photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2020, 95, 2129–2138. [Google Scholar] [CrossRef]
- Zhao, D.X.; Lu, G.P.; Cai, C. Efficient visible-light-driven Suzuki coupling reaction over Co-doped BiOCl/Ce-doped Bi2O2CO3 composites. Green. Chem. 2021, 23, 1823–1833. [Google Scholar] [CrossRef]
- Nie, G.D.; Lu, X.F.; Lei, J.Y.; Yang, L.; Wang, C. Facile and controlled synthesis of bismuth sulfide nanorods-reduced graphene oxide composites with enhanced supercapacitor performance. Electrochim. Acta 2015, 154, 24–30. [Google Scholar] [CrossRef]
- Zatsepin, A.F.; Kuznetsova, Y.A.; Zatsepin, D.A.; Wong, C.-H.; Law, W.-C.; Tang, C.-Y.; Gavrilov, N.V.; Boukhwalov, D.V. Bismuth-doped gadolinium oxide films for UV-Vis multicolor conversion: Combined XPS, DFT and photoluminescence study. J. Alloys Compd. 2023, 949, 169815. [Google Scholar] [CrossRef]
- Wang, S.; Yin, H.S.; Li, P.H.; Ding, J.; Wang, L.S.; Zhou, Y.L.; Wang, J. Controlled preparation of Bi/BiOCl with enhanced catalytic activity for organic pollutant under visible light using one-pot hydrothermal technology. Chemosphere 2022, 307, 136188. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Yin, H.S.; Wang, S.; Wang, J.; Ai, S.Y. Ni2+-assisted catalytic one-step synthesis of Bi/BiOCl/Bi2O2CO3 heterojunction with enhanced photocatalytic activity under visible light. Appl. Catal. B-Environ. 2022, 305, 121039. [Google Scholar] [CrossRef]
- Song, D.X.; Li, M.X.; Yang, F.; Yu, M.H.; Li, Z.Z.; Chen, J.; Zhang, X.S.; Zhou, W. Plasmon Bi in-situ anchored on BiOCl nanosheets assembled microspheres towards optimized photothermal-photocatalytic performance. Chinese Chem. Lett. 2024, 35, 108591. [Google Scholar] [CrossRef]
- Sun, X.; Zhai, H.J.; Sun, Z.; Liu, Z.; Yin, Z.M.; Zhang, S.; Shi, L.; Qu, X.F. From one to three: In-situ transformation of Bi3O4Cl to Bi/BiOCl/Bi3O4Cl core-shell nanocomposites with highly photocatalytic activities. Surf. Interfaces 2023, 40, 103017. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, J.C.; Feng, X.Y.; Liu, J.R.; Zong, S.; Liu, L.L.; Fang, Y.X. Outstanding photo-thermo synergy in aerobic oxidation of cyclohexane by bismuth tungstate-bismuth oxychloride high-low heterojunction. J. Colloid Interf. Sci. 2023, 651, 304–318. [Google Scholar] [CrossRef]
- Saeed, M.; Alwadai, N.; Farhat, L.B.; Baig, A.; Nabgan, W.; Iqbal, M. Co3O4-Bi2O3 heterojunction: An effective photocatalyst for photodegradation of rhodamine B dye. Arab. J. Chem. 2022, 15, 103732. [Google Scholar] [CrossRef]
- Gupta, G.; Kaur, M.; Kansal, S.K.; Umar, A.; Ibrahim, A.A. α-Bi2O3 nanosheets: An efficient material for sunlight-driven photocatalytic degradation of Rhodamine B. Ceram. Int. 2022, 48, 29580–29588. [Google Scholar] [CrossRef]
- Bouziani, A.; Park, J.; Ozturk, A. Effects of fluorination and thermal shock on the photocatalytic activity of Bi2O3 nanopowders. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127049. [Google Scholar] [CrossRef]
- Pizarro-Castillo, L.; Mera, A.C.; Cabello-Guzmán, G.; Bernal, C.; Bizarro, M.; Carrasco, C.; Blesa, M.-J.; Rodríguez, C.A. The effect of sintering temperature on the properties of the BiOCl films for potential application in DSSC. Ceram. Int. 2023, 49, 16305–16313. [Google Scholar] [CrossRef]
- Younis, S.; Taj, A.; Mujahid, A.; Yazdi, A.A.; Xu, J.; Bhatti, H.N.; Khan, W.S.; Bajwa, S.Z. Ultrathin 2D bismuth oxychloride nanosheets as novel catalytic interfaces for detection of biomolecules. Mater. Sci. Eng. B 2022, 278, 115651. [Google Scholar] [CrossRef]
- Kanagaraj, T.; Kumar, P.S.M.; Thomas, R.; Kulandaivelu, R.; Subramani, R.; Mohamed, R.N.; Lee, S.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Novel pure α-, β-, and mixed-phase α/β-Bi2O3 photocatalysts for enhanced organic dye degradation under both visible light and solar irradiation. Environ. Res. 2022, 205, 112439. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Makgwane, P.R.; Kumari, V.; Kumari, K.; Kataria, J.; Kumar, N. Ag@AgCl/Cu2+-Bi2O3 nanocomposite for decontamination of Rhodamine B: Adsorption, kinetics, thermodynamics, and photocatalytic aspects. Eur. Phys. J. Plus 2022, 137, 825. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Shen, Z.R.; Xin, Z.K.; Hu, Z.F.; Ji, H.M. Interfacial charge dominating major active species and degradation pathways: An example of carbon based photocatalyst. J. Colloid Interf. Sci. 2019, 554, 743–751. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Ma, D.; He, G.; Pan, S. Effects of pH on the Photocatalytic Activity and Degradation Mechanism of Rhodamine B over Fusiform Bi Photocatalysts under Visible Light. Water 2024, 16, 2389. https://doi.org/10.3390/w16172389
Chen Y, Ma D, He G, Pan S. Effects of pH on the Photocatalytic Activity and Degradation Mechanism of Rhodamine B over Fusiform Bi Photocatalysts under Visible Light. Water. 2024; 16(17):2389. https://doi.org/10.3390/w16172389
Chicago/Turabian StyleChen, Yuli, Dechong Ma, Guowen He, and Sai Pan. 2024. "Effects of pH on the Photocatalytic Activity and Degradation Mechanism of Rhodamine B over Fusiform Bi Photocatalysts under Visible Light" Water 16, no. 17: 2389. https://doi.org/10.3390/w16172389
APA StyleChen, Y., Ma, D., He, G., & Pan, S. (2024). Effects of pH on the Photocatalytic Activity and Degradation Mechanism of Rhodamine B over Fusiform Bi Photocatalysts under Visible Light. Water, 16(17), 2389. https://doi.org/10.3390/w16172389







