Abstract
In the face of current challenges related to climate change, maintaining the appropriate quality of freshwater becomes crucial. This study examined the effectiveness of removing heavy metals (Cu(II) and Co(II)) using Chlorella vulgaris biosorbents (dietary supplements in the form of powder). This study determined the parameters of the biosorbent (point of zero charge (PZC) analysis using scanning electron microscopy with back-scattered electron (SEM-BSE) and Fourier transform infrared spectroscopy (FT-IR) analysis). Batch tests were also performed to determine the kinetic constants and adsorption equilibrium of Cu(II) and Co(II) ions. Based on the conducted research, it was found that a pseudo-second-order equation describes the kinetics of the biosorption process. Among the studied adsorption isotherms, the Langmuir and Freundlich models fit best. The results indicate that single-layer adsorption took place and Chlorella vulgaris is a microporous adsorbent. The maximum sorption capacity in the single-component system for Cu(II) and Co(II) was 30.3 mg·g−1 and 9.0 mg·g−1, respectively. In contrast, in the binary system, it was 20.8 mg·g−1 and 19.6 mg·g−1 (extended Langmuir model) and 23.5 mg·g−1 and 19.6 mg·g−1 (Jain-Snoeyinka model). Chlorella vulgaris is an effective biosorbent for removing heavy metals from freshwater. This technology offers an ecological and economical solution for improving water quality, making it a promising alternative to traditional purification methods.
1. Introduction
Adsorption and ion exchange processes are methods of removing heavy metal ions that are used in both traditional methods and biosorption techniques. Biosorbents can remove heavy metals with comparable efficiency to physical methods, which makes biosorption an attractive alternative to traditional methods [1,2,3,4,5]. Biosorption can occur in two ways, passively, using dead biomass, and actively, through bioaccumulation involving living microorganisms. Biosorption is a physicochemical process in which metal ions are adsorbed onto the surface of a sorbent. Biosorption is the first step in the bioaccumulation process, which in turn requires the participation of living microorganisms and involves the transport of contaminants into the cell [2,6,7]. Any biomass can bind metal ions, but the manner and capacity of this binding can vary depending on the type of biomass. Biosorbents can be different forms of biomass, such as moss, leaves, trees, algae, bacteria, fungi, or yeast [8]. Each of these groups can vary in their ability to bind heavy metals. The structure and chemical composition of the cell wall play a key role in the amount of heavy metals bound. The ability of the biomass to effectively bind metals, its ability to renew itself, and its availability are key characteristics to consider when selecting a biosorbent. In addition to sorption capacity, economic considerations are also important. Biosorbents, which are costly, should have high pollutant removal efficiency and the ability to be easily regenerated [6].
In the context of searching for effective and economical biosorbents, microalgae deserve special attention. Chlorella vulgaris (C. vulgaris) is one of the most well-known and widely studied microalgae species due to its large specific surface area, the rich chemical composition of the cell wall, and its ability to accumulate pollutants [9]. Additionally, microalgae can be easily cultivated on a large scale in various environmental conditions, which makes them an accessible and renewable source of biomass. Their flexibility in adapting to different conditions makes them competitive compared to other biosorbents. C. vulgaris can be cultivated in autotrophic, mixotrophic, or heterotrophic conditions, providing flexibility in biomass production growth media [8,10]. In addition, microalgae can photosynthesize, which not only supports their growth but also contributes to a reduction in carbon dioxide, which gives additional ecological value to their use in biosorption processes [11]. Chlorella is widely produced and used worldwide, with the largest producers being Japan, Taiwan, and South Korea. Chlorella is used for biofuel, animal feed, and dietary supplements, among other things [6,7,8]. Chlorella vulgaris has a rich chemical composition including proteins (approx. 60%), polyunsaturated omega-3 fatty acids, polysaccharides (including β-1,3-glucan), vitamins, and minerals. Clinical studies suggest that supplementation with C. vulgaris brings health benefits such as supporting the treatment of hyperlipidemia, hyperglycemia, asthma, ulcers, and hemorrhoids and protects against oxidative stress and cancer [12,13]. A diverse morphological structure characterizes Chlorella, so it is often used in the food, pharmaceutical, cosmetics, and aquaculture industries [14]. In addition, it can also be used as a biosorbent for removing organic compounds [15,16,17] and heavy metals [15,18,19,20,21,22]. Pollution of fresh waters with metals is one of the most serious environmental problems due to their toxicity and ability to bioaccumulate in the food chain of aquatic organisms [23].
Considering the above advantages of C. vulgaris, in this work we focused on studying its ability for biosorption of two heavy metals, namely cobalt and copper. The choice of these metals was deliberate, as they are common environmental contaminants, and their excess can lead to serious health problems and ecosystem degradation. Although cobalt and copper are essential trace elements, they become toxic at higher concentrations [18,24,25]. Therefore, understanding the mechanisms by which C. vulgaris can effectively remove these metals from polluted waters is crucial for developing new, sustainable methods of freshwater purification. This study aimed to investigate the biosorption in the single-component and binary system of copper and cobalt using C. vulgaris. Detailed studies were conducted to determine the kinetics and equilibrium of the biosorption process. The results of our research can contribute to the further development of bioremediation technologies, offering ecological and effective solutions to the problem of environmental pollution with heavy metals.
2. Materials and Methods
2.1. Subject of Research: Algal Biomass
Chlorella vulgaris biomass was used to prepare biosorbents, which is a commercial product sold as a dietary supplement (Bio Planet, Superfoods).
2.2. Analysis of Algal Biomass Properties
2.2.1. Determination of Point of Zero Charge (PZC) of Chlorella vulgaris
A total of 0.5 g of C. vulgaris powder was weighed into 10 Erlenmeyer flasks. Each flask had 50 mL of NaNO3 added at a concentration of 0.1 M. The pH in each flask was then determined so that the values were 2, 3, 4, 5, 6, 7, 8, 9, 10, and 11. In total, 0.1 M HNO3 and 0.1 M NaOH were used to determine the pH values. The samples were left on an Elpin Plus laboratory shaker for 24 h. After this time, the pH values in each sample were measured again [26]. Based on the obtained initial and final pH values, the value of ∆pH = pH1 − pH0 was calculated.
2.2.2. Scanning Electron Microscopy with Back-Scattered Electron (SEM-BSE)
The morphological and textural observation of the surface was made by scanning electron microscope (SEM) (TESCAN VEGA 3, Brno, Czech Republic). The SEM was used also with a back-scattered electron detector (BSE) (INCA x-act, Oxford Instruments, High Wycombe, UK) to broaden the scope of the element content analysis.
2.2.3. Fourier Transform Infrared Spectroscopy FT-IR
The FTIR spectra of C. vulgaris were obtained with an Alpha spectrometer (Bruker, Billerica, MA, USA). The experiments were conducted using the transmission method, where samples were pressed with potassium bromide. The compressed adsorbent samples were mixed with KBr, maintaining a consistent ratio of 0.25% adsorbent weight to KBr weight, and then pressed into pellets. The FTIR spectra were employed in a spectral range of 4000 to 400 cm−1 [27].
2.3. Batch Studies of the Adsorption Process
2.3.1. Equilibrium Studies
A total of 6 conical flasks of 250 mL capacity were prepared. They were labeled accordingly and 0.5 g of C. vulgaris was weighed into each. Copper and cobalt solutions of given concentrations were added to each sample: 50, 100, 200, 600, 2000, and 5000 mg·L−1. Then, 2 drops of 1 M NaOH were added to maintain an alkaline pH. The prepared flasks were shaken for 2 h, and then the contents of the flasks were filtered through a medium quantitative filter 055 FILTRAK (Chem-Land). The obtained filtrate was further analyzed and the precipitate was discarded.
2.3.2. Determination of Cu(II) and Co(II) Concentration by Flame Atomic Absorption Spectrometry (F-AAS)
A PERKIN ELMER model 3100 F-AAS spectrometer was used to determine the concentration of Cu(II) and Co(II) in the aqueous phase after adsorption. A hollow cathode lamp for copper and cobalt was used as the radiation source. The measurement was carried out with acetylene–air flame activation. Initially, calibration of the apparatus was performed on standard solutions. The number of ions adsorbed by the algal biomass and the percentage of adsorption were calculated using the following Formulas (1) and (2), respectively:
where q is the adsorption capacity (in mg·g−1), C0 and Ce are, respectively, the initial concentration of the metal ion in solution and after adsorption over a specified period (in mg·L−1), V (in L) is the volume of solution, and m (in grams), is the amount of C. vulgaris used.
2.3.3. Adsorption Isotherm Models in a Single-Component and Binary System
The adsorption isotherm in the studied system was obtained by applying the Freundlich [28], Langmuir [29], Brunauer Emmett, and Teller (BET) [30] equal (Table 1). The coefficient of determination (R2) and the Chi-square statistic reduced by the number of degrees of freedom (χ2/DoF) were used to define the fit of the models to the experiment’s results. The adsorption isotherm in the studied binary system was obtained using the Jain–Snoeyink and extended Langmuir [31] equations (Table 2).

Table 1.
Lists of adsorption isotherm models in a single-component system [14,28,29,30].

Table 2.
Lists of adsorption isotherm models in a binary system [31].
2.3.4. Kinetic Studies
An array of 6 flasks corresponding to times of 15, 30, 60, 90, 120, and 180 min was prepared. A total of 0.5 g of C. vulgaris and 50 mL solution of Cu(II) and Co(II) ions at 600 mg·L−1 were weighed into each. 2 drops of 1M NaOH were added to maintain an alkaline pH (pH = 8). The prepared C. vulgaris solutions were shaken for the appropriate time, and then the contents of the flasks were filtered through a medium quantitative filter 055 FILTRAK (Chem-Land). The adsorption process was carried out at 20 °C. To study the mechanism of Cu(II) and Co(II) ion adsorption, pseudo-first-order, and pseudo-second-order kinetic models were used, and the rate-controlling stage, i.e., intra-particle diffusion, was also determined [32,33,34,35,36,37] (Table 3).

Table 3.
Lists of kinetic models [32,33,34,35,36,37].
3. Results and Discussion
3.1. Characterization of the Biosorbent
The value of the zero point of the Chlorella vulgaris load determined by the suspension method was 8.0 (Figure 1). The zero point of electric charge (PZC) is the point of intersection of the relationship ∆pH = f(pH0) with the OX axis and is, therefore, equivalent to the zero point of this function. The point of zero electrical charge is the pH for which the electrical charge of a surface or suspended solid in water is zero. Knowing the PZC value makes it possible to determine the type of groups that predominate on the surface of the adsorbent and their presumed interactions with other ions. As the pH value of C. vulgaris is above the determined PZC, this means that its surface is negatively charged and will, therefore, have a higher cation exchange capacity [26].
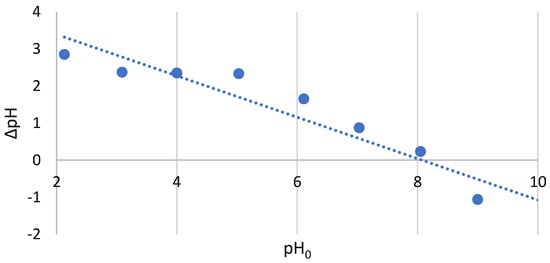
Figure 1.
PZC of Chlorella vulgaris.
The structure of C. vulgaris is shown in Figure 2. The photos were taken using an SEM VEGA3 TESCAN microscope at various resolutions. These photos are characterized by a large depth of field, which allows for an accurate assessment of porosity on the surface. The structure of the tested biological material can be described as an aggregate of particles of various sizes and microspheres in the form of irregular shapes [38,39,40]. Based on microscopic C. vulgaris images, BSE spectra were made, thanks to which the elemental composition of the biosorbent was determined. The results indicate that C. vulgaris mainly contained carbon and oxygen, and trace amounts of phosphorus, sulfur, silicon, calcium, and magnesium in its composition (Figure 3).

Figure 2.
Chlorella vulgaris image obtained with the VEGA3 TESCAN microscope (magnification 500× and 3000×).
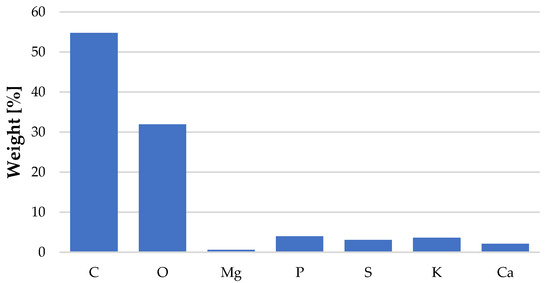
Figure 3.
Elemental composition of Chlorella vulgaris.
FTIR analysis showed the presence of different absorption bands on the surface of the biosorbent (Figure 4). A broad band at about 3485 cm−1 belongs to the –NH and –OH groups, with a stretching frequency of about 2945 cm−1 attributed to –CH. The band at 1674 cm−1 shows the frequency of the carbonyl group (C=O), then the band at 1267 cm−1 was assigned to the –CH3 group [41]. The other lower bands were assigned to the C–N and C=S groups. After the absorption of Co(II) and Cu(II) ions, the various vibrational frequencies decreased, confirming the bond formation by Co(II) and Cu(II) ions to carboxyl, carbonyl, hydroxyl, amine, and amide groups [42].
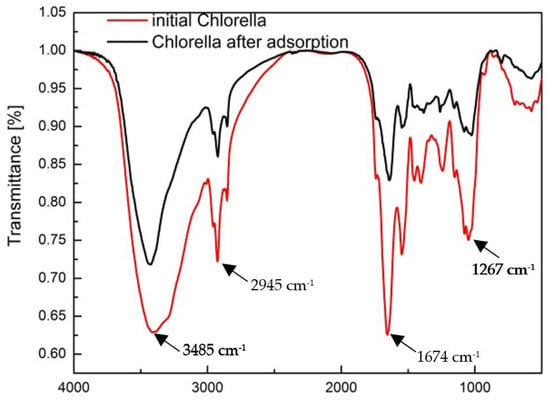
Figure 4.
FTIR spectra of initial Chlorella vulgaris and after Co(II) and Cu(II) ion adsorption.
3.2. Sorption Batch Model
Isotherms determine the equilibrium between the concentration of adsorbate in the solid phase and its concentration in the liquid phase. We can obtain information on the maximum adsorption capacity based on the course of isotherms. In addition, they provide information on the power of the sorbent or the amount required to remove a unit mass of pollutants under the conditions studied [10,38]. Experimental data were processed using various adsorption isotherms models in the solid–liquid system (Table 4). The comparison of isothermal models for adsorbed Cu(II) and Co(II) ions is presented in Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10.

Table 4.
The values of isotherm adsorption parameters for Cu(II) and Co(II) ions by Chlorella vulgaris in a single-component system.
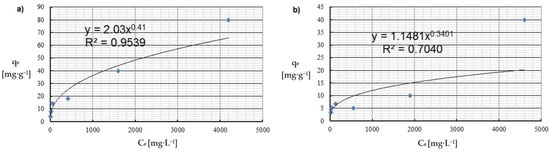
Figure 5.
Freundlich isotherm of: (a) Cu(II) and (b) Co(II) ions (pH = 8, Ce = 50–5000 mg·L−1, t = 2 h).
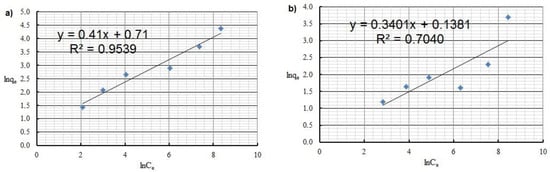
Figure 6.
The logarithmic form of Freundlich isotherm of: (a) Cu(II) and (b) Co(II) ions (pH = 8, Ce = 50–5000 mg·L−1, t = 2 h).
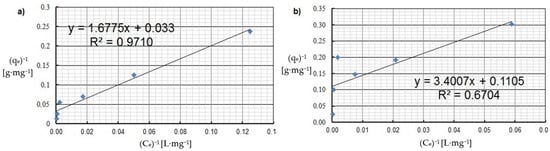
Figure 7.
Langmuir isotherm of: (a) Cu(II) and (b) Co(II) ions (pH = 8, Ce = 50–5000 mg·L−1, t = 2 h).
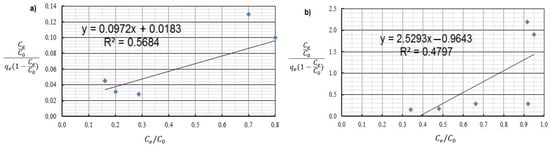
Figure 8.
BET isotherm of: (a) Cu(II) and (b) Co(II) ions (pH = 8, Ce = 50–5000 mg·L−1, t = 2 h).
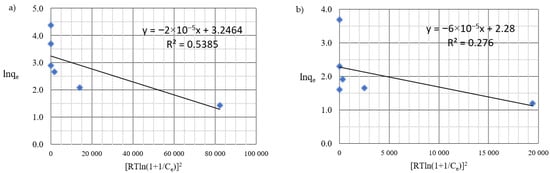
Figure 9.
D-R isotherm of: (a) Cu(II) and (b) Co(II) ions (pH = 8, Ce = 50–5000 mg·L−1, t = 2 h).
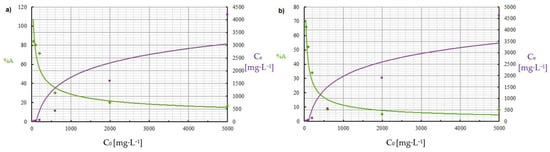
Figure 10.
Effect of initial concentration of: (a) Cu(II) and (b) Co(II) ions on the adsorption process (pH = 8, Ce = 50–5000 mg·L−1, t = 2 h).
By analyzing adsorption isotherms, it was possible to adjust the model accordingly and reflect the adsorption studies carried out for Cu(II) and Co(II) ions using Chlorella vulgaris. Each graph shows the equilibrium between the concentration of the adsorbate in the solid phase and its concentration in the liquid phase. Comparing these results, it can be concluded that a more efficient adsorption process occurred for Cu(II) ions. The maximum adsorption capacity for Cu(II) and Co(II) ions was obtained according to the Langmuir model. The maximum adsorption capacity was about 30.3 mg·g−1 and 9 mg·g−1 for Cu(II) and Co(II) ions, respectively. Equilibrium experiments showed that C. vulgaris’s selectivity towards Cu(II) ions is greater than that of Co(II) ions, which is related to their hydrated ionic radius and first hydrolysis equilibrium constant. For the BET isotherm, qmax values were also compared. For adsorption of Cu(II) ions, a value of qmax = 0.018 mg·g−1 was obtained, and for adsorption of Co(II) ions, qmax = 0.280 mg·g−1 (Table 4). On this basis, it was concluded that the efficiency of the adsorption process for this isotherm is poor, especially in the first case. Another isotherm discussed is the Freundlich isotherm, for which the dimensionless parameter n allows the determination of the intensity of adsorption; if this parameter is in the range 1 < n < 10, it means that the adsorption process is effective and efficient. In the case of the adsorption process of Cu(II) ions, a value of n = 2.4 was obtained, while for Co(II) ions a value of n = 2.9 was obtained. Based on this, it was concluded that the process was efficient, as it fell within the above-described range (Figure 5 and Figure 6). Then, calculating the inverse of the parameter n, information about the degree of diversity of sorption sites on the sorbent surface was obtained. The results were obtained for Cu(II) ions 1/n = 0.42, for Co(II) ions 1/n = 0.34. These values are closer to zero than unity, which allows us to predict that the adsorption surface with C. vulgaris is significantly homogeneous. The coefficients of determination for each isotherm were also compared. The results obtained were: R2 = 0.9539 and 0.7040 for the Freundlich isotherm (Figure 5 and Figure 6), R2 = 0.9710 and 0.6700 for the Langmuir isotherm (Figure 7), R2 = 0.5684 and 0.4802 for the BET isotherm (Figure 8), and R2 = 0.539 and 0.276 for the R-D isotherm (Figure 9). The Freundlich and Langmuir isotherms showed the best coefficient of determination. However, the experimental data did not correlate so well with the D-R model (Figure 9). The D-R model often fits the data at high concentrations well but has poor performance at low metal ion concentrations. The energy (E) was determined to be 0.14 kJ·mol−1 and 0.09 kJ·mol−1 for Cd(II) and Cu(II) indicating that physisorption may play a significant role in the metal adsorption process.
For binary mixtures, isotherm Jain–Snoeyinka and extended Langmuir models allow direct calculation of the concentrations of adsorbed components based on knowledge of isotherms in a single-component system. The total amount adsorbed can be determined based on the sum of the concentrations of the adsorbed components (Table 5).

Table 5.
The values of isotherm adsorption parameters for Cu(II) and Co(II) ions by Chlorella vulgaris in a binary system.
Adsorption proceeded rapidly in the initial phase and gradually slowed down once equilibrium was reached. This phenomenon is very common due to the saturation of the available active surface centers. The experiments showed that an equilibrium state was reached within 2 h and 30 min for Cu(II) and Co(II) ions, respectively after which saturation of the adsorbent surface can be expected to occur (Figure 11).
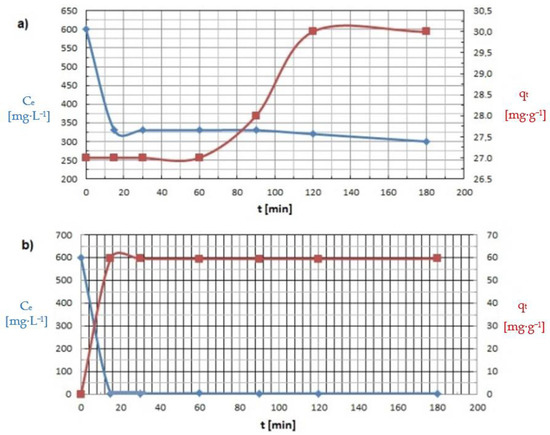
Figure 11.
Influence of mixing time on the capacity adsorption (qt) of: (a) Cu(II) and (b) Co(II) ions (pH = 8, Ce = 600 mg·L−1, t = 15–180 min).
3.3. Kinetic Adsorption
To investigate the mechanism and determine the rate of the adsorption process, pseudo-first-order and pseudo-second-order kinetic models, and diffusion within the particles were developed. Both the adsorbent and the adsorbate molecules could participate in the adsorption of Cu(II) and Co(II) ions on the C. vulgaris (Table 6).

Table 6.
Kinetic model constants and linear correlation coefficients for the adsorption system studied.
Based on the graphs produced and the kinetic model results tabulated, it appears that the adsorption process of both copper and cobalt ions is best described by a pseudo-second-order equation. Analysis of the PSO model yielded R2 determination coefficients approaching unity—0.9979 for Cu(II) ion adsorption, and 0.9746 for Co(II) ion adsorption (Figure 12). In the case of a PFO model, the coefficient of R2 is much smaller than 1. The kinetic curves do not follow a linear path, so the experimental results do not satisfy an equation of this order (Figure 13).
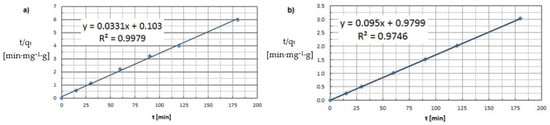
Figure 12.
Pseudo-second-order kinetic model (a) Cu(II) and (b) Co(II) (pH = 8, Ce = 600 mg·L−1, t = 15–180 min.
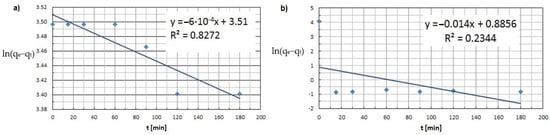
Figure 13.
Pseudo-first-order kinetic model (a) Cu(II) and (b) Co(II) (pH = 8, Ce = 600 mg·L−1, t = 15–180 min.
The intra-particle diffusion model shows that there are two or three separate steps in the sorption process, namely external diffusion and intra-particle diffusion. The non-linear course of the entire adsorption process indicates multi-step adsorption of Cu(II) ions by C. vulgaris. The fit of the multilinear dependence of qt concerning t1/2 is shown in Figure 14. It can be observed that there are two or even three distinct stages in the adsorption of copper ions. The sharp linear progression of the first part of the process is related to the diffusive boundary layer (film), so-called external diffusion, external surface adsorption, or external mass transfer effect. The second stage describes the gradual adsorption, surface diffusion, and adsorption on the pore surface, while the third linear relationship is responsible for the diffusion into the pore, which represents the final stage of the equilibrium state, where adsorption becomes very slow, stable, and assumes a maximum value.
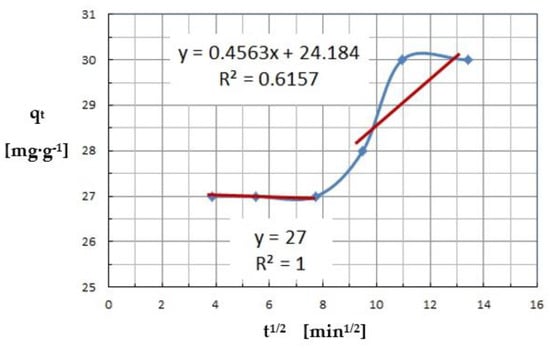
Figure 14.
Intra-particle diffusion kinetic model for Cu(II) (pH = 8, Ce = 600 mg·L−1, t = 15–180 min.
Chlorella vulgaris is one of the biosorbents that can be used in wastewater treatment processes thanks to its favorable physicochemical properties and ability to bind metals. Studies indicate that C. vulgaris effectively bioremediates water from pollutants, constituting a promising alternative to traditional treatment methods. Adsorption, as a process characterized by simplicity, low operating costs, and the availability of various adsorbents, including biosorbents of natural origin, is particularly promising in the removal of heavy metals from aqueous solutions. Different values of adsorption capacity have been reported in the scientific literature depending on the adsorbent used, experimental conditions, and specific chemical and physical properties of the metal ions tested (Table 7).

Table 7.
Adsorption capacity of different adsorbents for the removal of Cu(II) and Co(II).
4. Conclusions
In this study, Chlorella vulgaris was used as an easily available sorbent for the removal of Cu(II) and Co(II) ions in one- and two-component systems. Among the analyzed isotherm models, the Freundlich and Langmuir isotherms best fit the indicated experimental points. The coefficient of determination for the Freundlich isotherm was R2 = 0.95 and 0.70, and for the Langmuir isotherm R2 = 0.97 and 0.6 for Cu(II) and Co(II) ions, respectively. The obtained results indicate that single-layer adsorption occurred and Chlorella vulgaris is a microporous adsorbent. The value of the dimensionless parameter n in the Freundlich equation indicates that the adsorption process is effective, and the adsorption surface is homogeneous. Cu(II) ions) were retained more effectively on the surface of Chlorella vulgaris (qmax = 30.3 mg·g−1 than Co(II) ions (qmax = 9.0 mg·g−1). However, in the binary system, these values were 20.8 and 19.6 [mg/g] for the extended Langmuir model, and 23.5 and 19.6 mg·g−1 for the Jain-Snoeyinka model for Cu(II) and Co(II). The adsorbent surface was saturated with adsorbed anions after 2 h for Cu(II) and 0.5 h for Co(II). The obtained results indicate that copper ions had easier access to the activity centers of Chlorella vulgaris. The analysis of the intraparticle diffusion model showed that the sorption process of heavy metal ions is controlled by diffusion in the pores. The obtained sorption kinetic data were well expressed by a pseudo-second-order model, while they showed a very poor fit to a pseudo-first-order model, as evidenced by the analysis of the linear regression coefficient values. Based on the results obtained, it can be concluded that Chlorella vulgaris becomes an attractive material for purifying water containing Cu(II) and Co(II) ions.
Author Contributions
Conceptualization, E.S.; methodology, E.S. and D.P. (Dariusz Pająk); validation, E.S.; formal analysis, E.S., A.D. and D.P. (Dorota Papciak); investigation, E.S. and D.P. (Dariusz Pająk); data curation, E.S.; writing—original draft preparation, E.S. and A.D.; writing—review and editing, A.D. and D.P. (Dorota Papciak); visualization, E.S. and A.D.; supervision, E.S., D.P. (Dorota Papciak) and A.D.; project administration, E.S. All authors have read and agreed to the published version of the manuscript.
Funding
Financed by the Minister of Science and Higher Education Republic of Poland within the program “Regional Excellence Initiative”.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and Removal of Heavy Metal Ions: A Review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Hadi, B.; El-Naas, M.H. Biosorption of Heavy Metals: Potential and Applications of Yeast Cells for Cadmium Removal. In Environmental Contaminants: Ecological Implications and Management; Bharagava, R.N., Ed.; Microorganisms for Sustainability; Springer: Singapore, 2019; Volume 14, pp. 237–271. [Google Scholar]
- Chen, X.; Tian, Z.; Cheng, H.; Xu, G.; Zhou, H. Adsorption Process and Mechanism of Heavy Metal Ions by Different Components of Cells, Using Yeast (Pichia Pastoris) and Cu2+ as Biosorption Models. RSC Adv. 2021, 11, 17080–17091. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of Heavy Metal Ions from Aqueous System by Ion-Exchange and Biosorption Methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Lavado-Meza, C.; Fernandez-Pezua, M.C.; Gamarra-Gómez, F.; Sacari-Sacari, E.; Angeles-Suazo, J.; Dávalos-Prado, J.Z. Single and Binary Removals of Pb(II) and Cd(II) with Chemically Modified Opuntia ficus indica Cladodes. Molecules 2023, 28, 4451. [Google Scholar] [CrossRef] [PubMed]
- Hansda, A.; Kumar, V.; Anshumali. A Comparative Review towards Potential of Microbial Cells for Heavy Metal Removal with Emphasis on Biosorption and Bioaccumulation. World J. Microbiol. Biotechnol. 2016, 32, 170. [Google Scholar] [CrossRef]
- León-Vaz, A.; León, R.; Giráldez, I.; Vega, J.M.; Vigara, J. Impact of heavy metals in the microalga Chlorella sorokiniana and assessment of its potential use in cadmium bioremediation. Aquat. Toxicol. 2021, 239, 105941. [Google Scholar] [CrossRef]
- Machado, L.; Carvalho, G.; Pereira, R.N. Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass. Biomass 2022, 2, 80–102. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Dutta, S.; Banerjee, I.; Pohrmen, C.B.; Kumar, V. Photosynthetic Microalgae–Based Carbon Sequestration and Generation of Biomass in Biorefinery Approach for Renewable Biofuels for a Cleaner Environment. Biomass Conv. Bioref. 2023, 13, 7403–7421. [Google Scholar] [CrossRef]
- Panahi, Y.; Darvishi, B.; Jowzi, N.; Beiraghdar, F.; Sahebkar, A. Chlorella vulgaris: A Multifunctional Dietary Supplement with Diverse Medicinal Properties. Curr. Pharm. Des. 2016, 22, 164–173. [Google Scholar] [CrossRef]
- Adamakis, I.-D.; Lazaridis, P.A.; Terzopoulou, E.; Torofias, S.; Valari, M.; Kalaitzi, P.; Rousonikolos, V.; Gkoutzikostas, D.; Zouboulis, A.; Zalidis, G.; et al. Cultivation, characterization, and properties of Chlorella vulgaris microalgae with different lipid contents and effect on fast pyrolysis oil composition. Environ. Sci. Pollut. Res. 2018, 25, 23018–23032. [Google Scholar] [CrossRef]
- Kua, T.L.; Kooh, M.R.R.; Dahri, M.K.; Zaidi, N.A.H.M.; Lu, Y.; Lim, L.B.L. Aquatic Plant, Ipomoea Aquatica, as a Potential Low-Cost Adsorbent for the Effective Removal of Toxic Methyl Violet 2B Dye. Appl. Water Sci. 2020, 10, 243. [Google Scholar] [CrossRef]
- Liu, D.; Yang, W.; Lv, Y.; Li, S.; Qv, M.; Dai, D.; Zhu, L. Pollutant removal and toxic response mechanisms of freshwater microalgae Chlorella sorokiniana under exposure of tetrabromobisphenol A and cadmium. Chem. Eng. J. 2023, 461, 142065. [Google Scholar] [CrossRef]
- Tarlan, E.; Dilek, F.B.; Yetis, U. Effectiveness of Algae in the Treatment of a Wood-Based Pulp and Paper Industry Wastewater. Bioresour. Technol. 2002, 84, 1–5. [Google Scholar] [CrossRef]
- Xie, L.; Zhou, L.; Liu, T.; Xu, X. Degradation of disperse blue 2BLN by oleaginous C. sorokiniana XJK. RSC Adv. 2016, 6, 106935–106944. [Google Scholar] [CrossRef]
- Ramesh, B.; Saravanan, A.; Kumar, P.S.; Yaashikaa, P.R.; Thamarai, P.; Shaji, A.; Rangasamy, G. A review on algae biosorption for the removal of hazardous pollutants from wastewater: Limiting factors, prospects and recommendations. Environ. Pollut. 2023, 327, 121572. [Google Scholar] [CrossRef]
- Goher, M.E.; Abd El-Monem, A.M.; Abdel-Satar, A.M.; Ali, M.H.; Hussian, A.-E.M.; Napiórkowska-Krzebietke, A. Biosorption of Some Toxic Metals from Aqueous Solution Using Non-Living Algal Cells of Chlorella Vulgaris. J. Elem. 2016, 21, 703–714. [Google Scholar]
- Lapeñas, L.A.; Peña-Bahamonde, J.; Faria, L.U.S.; De Luna, M.D.G.; Rodrigues, D.F. Removing Heavy Metal Ions from Wastewater by Chlorella Sorokiniana Coupled to Manganese-Doped Magnetic Ferrite Nanoparticles. J. Hazard. Mater. Lett. 2023, 4, 100082. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Use of Algae for Removing Heavy Metal Ions from Wastewater: Progress and Prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Alharbi, R.M.; Sholkamy, E.N.; Alsamhary, K.I.; Abdel-Raouf, N.; Ibraheem, I.B.M. Optimization Study of the Capacity of Chlorella Vulgaris as a Potential Bio-Remediator for the Bio-Adsorption of Arsenic (III) from Aquatic Environments. Toxics 2023, 11, 439. [Google Scholar] [CrossRef]
- Mohammad Ali, M.; Hossain, D.; Al-Imran, A.; Khan, M.S.; Begum, M.; Hasan Osman, M. Environmental Pollution with Heavy Metals: A Public Health Concern. In Heavy Metals—Their Environmental Impacts and Mitigation; Khaled Nazal, M., Zhao, H., Eds.; IntechOpen: London, UK, 2021. [Google Scholar]
- Mahey, S.; ·Kumar, R.; Sharma, M.; Kumar, V.; Bhardwaj, R. A critical review on toxicity of cobalt and its bioremediation strategies. SN Appl. Sci. 2020, 2, 1279. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Prevalence of Cobalt in the Environment and Its Role in Biological Processes. Biology 2023, 12, 1335. [Google Scholar] [CrossRef]
- Al-Maliky, E.A.; Gzar, H.A.; Al-Azawy, M.G. Determination of Point of Zero Charge (PZC) of Concrete Particles Adsorbents. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1184, p. 012004. [Google Scholar]
- Sočo, E.; Papciak, D.; Michel, M.M.; Pająk, D.; Domoń, A.; Kupiec, B. Characterization of the Physical, Chemical, and Adsorption Properties of Coal-Fly-Ash–Hydroxyapatite Composites. Minerals 2021, 11, 774. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Bruanuer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–316. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Removal of Cadmium(II) and Zinc(II) Metal Ions from Binary Aqueous Solution by Rice Husk Ash. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 172–184. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Savastru, E.; Bulgariu, D.; Zamfir, C.-I.; Bulgariu, L. Application of Saccharomyces Cerevisiae in the Biosorption of Co(II), Zn(II) and Cu(II) Ions from Aqueous Media. Water 2022, 14, 976. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of Methylene Blue from Aqueous Solution by Sewage Sludge-Derived Biochar: Adsorption Kinetics, Equilibrium, Thermodynamics and Mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Nasanjargal, S.; Munkhpurev, B.-A.; Kano, N.; Kim, H.-J.; Ganchimeg, Y. The Removal of Chromium(VI) from Aqueous Solution by Amine-Functionalized Zeolite: Kinetics, Thermodynamics, and Equilibrium Study. J. Environ. Protect. 2021, 12, 654–675. [Google Scholar] [CrossRef]
- Gunorubon, A.J.; Chukwunonso, N. Adsorption of Heavy Metals: Mechanisms, Kinetics, Equilibrium and Thermodynamics Studies of Fe3+ Ion Removal from Aqueous Solutions Using Periwinkle Shell Activated Carbon. Adv. Chem. Eng. Sci. 2018, 8, 775–805. [Google Scholar] [CrossRef]
- Rojas, J.; Suarez, D.; Moreno, A.; Silva-Agredo, J.; Torres-Palma, R.A. Kinetics, Isotherms and Thermodynamic Modeling of Liquid Phase Adsorption of Crystal Violet Dye onto Shrimp-Waste in Its Raw, Pyrolyzed Material and Activated Charcoals. Appl. Sci. 2019, 9, 5337. [Google Scholar] [CrossRef]
- Ali, A.; Zhang, N.; Santos, R.M. Mineral Characterization Using Scanning Electron Microscopy (SEM): A Review of the Fundamentals, Advancements, and Research Directions. Appl. Sci. 2023, 13, 12600. [Google Scholar] [CrossRef]
- Dery, B.; Zaixiang, L. Scanning Electron Microscopy (SEM) as an Effective Tool for Determining the Morphology and Mechanism of Action of Functional Ingredients. Food Rev. Int. 2023, 39, 2007–2026. [Google Scholar] [CrossRef]
- Sun, C.; Lux, S.; Müller, E.; Meffert, M.; Gerthsen, D. Versatile application of a modern scanning electron microscope for materials characterization. J. Mater. Sci. 2020, 55, 13824–13835. [Google Scholar] [CrossRef]
- Duygu, D.Y.; Udoh, A.U.; Ozer, T.B.; Akbulut, A.; Erkaya, I.A.; Yildiz, K.; Guler, D. Fourier Transform Infrared (FTIR) Spectroscopy for Identification of Chlorella vulgaris Beijerinck 1890 and Scenedesmus obliquus (Turpin) Kützing 1833. Afr. J. Biotechnol. 2012, 11, 3817–3824. [Google Scholar]
- Tattibayeva, Z.; Tazhibayeva, S.; Kujawski, W.; Zayadan, B.; Musabekov, K. Peculiarities of adsorption of Cr(VI) ions on the surface of Chlorella vulgaris ZBS1 algae cells. Helion 2022, 8, e10468. [Google Scholar] [CrossRef]
- Ramana, D.K.V.; Jamuna, K.; Satyanarayana, B.; Venkateswarlu, B.; Rao, M.M.; Seshaiah, K. Removal of Heavy Metals from Aqueous Solutions Using Activated Carbon Prepared from Cicer Arietinum. Toxicol. Environ. Chem. 2010, 92, 1447–1460. [Google Scholar] [CrossRef]
- Areco, M.M.; Hanela, S.; Duran, J.; Dos Santos Afonso, M. Biosorption of Cu(II), Zn(II), Cd(II) and Pb(II) by Dead Biomasses of Green Alga Ulva Lactuca and the Development of a Sustainable Matrix for Adsorption Implementation. J. Hazard. Mater. 2012, 213–214, 123–132. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; Freire, S.J.; Santos, L.V.S.; Palladino, F.; Jacob, R.S. Biosorption of Copper Ions from Aqueous Solution Using Chlorella Pyrenoidosa: Optimization, Equilibrium and Kinetics Studies. Microchem. J. 2019, 145, 119–129. [Google Scholar] [CrossRef]
- Do Nascimento, J.M.; De Oliveira, J.D.; Rizzo, A.C.L.; Leite, S.G.F. Biosorption Cu (II) by the Yeast Saccharomyces Cerevisiae. Biotechnol. Rep. 2019, 21, e00315. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Application of the Biochar Derived from Orange Peel for Effective Biosorption of Copper and Cadmium in Batch Studies: Isotherm Models and Kinetic Studies. Arab. J. Geosci. 2019, 12, 46. [Google Scholar] [CrossRef]
- Bouzidi, A.; Djedid, M.; Ad, C.; Benalia, M.; Hafez, B.; Elmsellem, H. Biosorption of Co (II) Ions from Aqueous Solutions Using Selected Local Luffa Cylindrica: Adsorption and Characterization Studies. Mor. J. Chem. 2021, 9, 156–167. [Google Scholar]
- Vilvanathan, S.; Shanthakumar, S. Modeling of Fixed-Bed Column Studies for Removal of Cobalt Ions from Aqueous Solution Using Chrysanthemum Indicum. Res. Chem. Intermed. 2017, 43, 229–243. [Google Scholar] [CrossRef]
- Tofan, L.; Teodosiu, C.; Paduraru, C.; Wenkert, R. Cobalt (II) Removal from Aqueous Solutions by Natural Hemp Fibers: Batch and Fixed-Bed Column Studies. Appl. Surf. Sci. 2013, 285, 33–39. [Google Scholar] [CrossRef]
- Galedar Biosorption of ternary cadium, niciel and cobalt ions from aqueous solution onto saccharomyces cerevisiae cells: Batch and column studies. Am. J. Biochem. Biotechnol. 2013, 9, 47–60. [CrossRef]
- Hymavathi, D.; Prabhakar, G. Studies on the Removal of Cobalt(II) from Aqueous Solutions by Adsorption with Ficus Benghalensis Leaf Powder through Response Surface Methodology. Chem. Eng. Commun. 2017, 204, 1401–1411. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).