Abstract
This study investigated the tolerance of resting eggs of Eurytemora pacifica to storage under low temperatures, which is of particular interest in light of the recent use of nauplii as living food in aquaculture, other than conditions experienced also in the wild during winter cold events in the Northern Hemisphere. Sediment samples collected in August 2020 were used to store the resting eggs at two different freezing temperatures (−5 and −20 °C) for five different durations (1, 3, 6, 9, and 12 months). The mean hatching success rates of the resting eggs after one month of storage were 85.3 ± 1.5% (−5 °C) and 85.0 ± 3.6% (−20 °C), with no significant difference between freezing temperatures. However, significant differences emerged over time, with the mean hatching success rate remaining at 85.0 ± 3.6% at −5 °C after three months, while it dropped sharply to 1.7 ± 2.1% at −20 °C. For the non-freezing conditions, the hatching success at 10 °C increased gradually over the one-month incubation period, ultimately reaching 71.0%. These findings demonstrate the remarkable cold tolerance of E. pacifica but also indicate a limit to this tolerance at longer durations. These results underscore the importance of considering the adoption of storage freezing for resting eggs to be used for aquaculture and also suggest the possibility of the species better surviving the extreme weather events in comparison with other species.
1. Introduction
Climate change, which is caused by the release of large amounts of greenhouse gases from human industrial activities, is expected to lead to a global average temperature increase of 1.4 to 5.8 °C from 1990 to 2100 [1]. Recent studies, however, have reported the sudden occurrence of cold waves in the Northern Hemisphere, and these extreme cold weather events have been attributed to a decrease in Arctic Sea ice and atmospheric changes brought about by climate change [2,3,4]. According to the IPCC Assessment Report (AR6), climate change is projected to increase the frequency and intensity of extreme weather events, such as heat waves, extreme cold minima, heavy rains, and droughts. In the face of this rapid change, there has been a growing interest in extreme weather events [5].
Zooplankton, particularly calanoid copepods, are integral to marine ecosystems, serving as a crucial link in the transfer of energy from primary producers to higher trophic levels. Their role in the biological pump is pivotal for regulating atmospheric carbon dioxide levels [6,7,8,9]. Copepoda species, with their short life cycles and sensitivity to environmental changes, are excellent indicators of marine and climatic fluctuations [10,11]. Their life histories, involving seasonal diapause to survive unfavorable conditions, are ecologically significant [12,13,14,15]. The “egg bank” formed by resting eggs in bottom sediment plays a crucial role in the rapid recovery of copepod populations under favorable conditions [15,16].
Climate change affects species distribution, as well as their life cycles, population dynamics, and ecosystem functions [17,18,19,20]. This phenomenon is having severe impacts on marine biota, such as reducing biological richness and posing an extinction threat not seen in millions of years [21]. Moreover, although the effects of global warming on living organisms have been widely studied [22,23,24], the impacts of extreme weather events, such as extreme cold, have not been sufficiently explored. Temperature, salinity, light, and food quality are known to influence the egg hatching success in Copepoda Calanoida [25,26,27,28], yet the effects of low temperatures have not been sufficiently studied. In addition, eggs’ resistance to extreme conditions is basic for their use as food in aquaculture, because they can be moved around the planet as market products and hatch nauplii when necessary (as living food for fish juveniles) [29,30].
Eurytemora pacifica Sato, 1913 (Copepoda, Calanoida), is a species prevalent in estuaries and coastal regions, including brackish and neritic areas in Korea. It is known for its adaptability to varying environmental conditions [31,32,33,34]. In Gamak Bay, Korea, E. pacifica dominates the zooplankton in winter, from February to April, disappearing after late April, utilizing resting eggs as a survival strategy in seasonally changing coastal environments [35,36]. This species is also unusually (for marine Calanoida) equipped with egg sacs, a feature that seems to be correlated with the adaptation to internal and fresher waters [37]. This reproductive strategy allows E. pacifica to withstand changes in unstable coastal environments and greatly contributes to its predominance in certain marine confined environments, like Gamak Bay in Korea [34]. The adaptation of such a copepod to winter and/or extremely low temperatures, together with its abundance in coastal waters, sees proposed it as an interesting source of living food for marine aquaculture purposes.
This study aims to examine the response of E. pacifica resting eggs to cold temperatures. We investigate the effects of freezing for different durations (1, 3, 6, 9, and 12 months) at two different temperatures (−5 and −20 °C) for a proposal of market importance, and the effects of freezing conditions on the hatching rate of E. pacifica, to better understand its adaptation to episodic cold waves in natural conditions.
2. Materials and Methods
2.1. Sediment Sampling
A piston core sampler (64 mm internal diameter, 50 cm length) was used to collect five sediment samples from 5 randomly selected points in Gamak Bay, South Korea, on 5 August 2020 (Figure 1). In our study area, during the period of investigation, we recorded a surface water temperature of 28.2 °C and a bottom layer temperature of 22.9 °C. The salinity levels were 27.2‰ at the surface and 31.4‰ at the bottom. The average depth of Gamak Bay is 10 m, and the sediments were identified as muddy [38]. The sediment samples were placed in a dark-treated icebox and immediately transferred to the laboratory. The first 3 cm of each sediment core was isolated and then stored in a 100 mL dilution tube. The tubes were stored at −5 and −20 °C for 1, 3, 6, 9, and 12 months, with three replicates (three tubes) per temperature. The tubes were covered with aluminum foil to block any external light.
Figure 1.
Map of South Korea. The black square indicates the sampling location for the sediment used in this study.
2.2. Isolation of Resting Eggs
The extraction of Eurytemora pacifica resting eggs from sediment samples followed a meticulous process. Initially, each sediment sample, stored under varying freezing conditions, was thawed and incubated at a constant temperature of 3 °C for 24 h to facilitate ice melting and egg separation. After incubation, we utilized a refined sugar flotation method adapted from Onbé [39]. This thawed sediment was passed through a 40 µm mesh sieve, a deviation from Onbé’s original 100 µm mesh, to ensure finer separation. The material retained on the sieve was carefully transferred into a 50 mL conical tube containing a dense sugar solution (specific gravity: 1 g/mL). The mixture was then homogenized and centrifuged at 3000 rpm for 5 min, resulting in a supernatant rich in copepod eggs. The supernatant was promptly filtered through the same 40 µm mesh sieve to wash away sugar residues and other fine particulates. The crucial step in our method involved the manual sorting of E. pacifica eggs under a stereomicroscope (Nikon SMZ 1000; Nikon, Japan). Eggs attributed to E. pacifica were individually identified based on distinct morphological characteristics (Figure 2). Unlike other eggs that are typically spherical, E. pacifica eggs are faceted with each facet surrounded by a crested chorion. These identified eggs were selectively isolated using a precision transfer pipette.
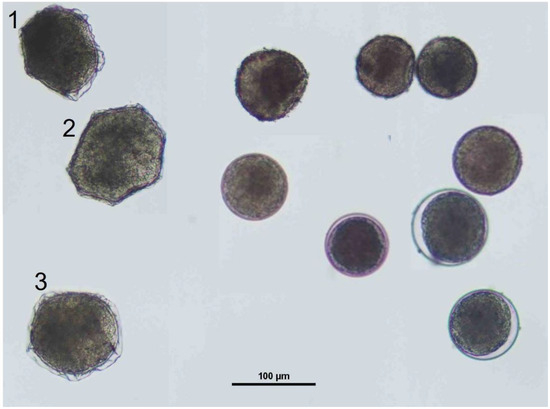
Figure 2.
The various copepod eggs observed under a stereomicroscope. Numbers 1, 2, and 3 indicate Eurytemora pacifica eggs. The remaining eggs are unidentified calanoid copepod eggs.
The resting eggs of E. pacifica were observed under a stereomicroscope (Nikon SMZ 1000; Nikon, Japan) and transferred to another Petri dish containing filtered seawater using a transfer pipette. Observations requiring a high degree of magnification were performed using a light microscope (LM, Nikon eclipse E200, Nikon, Tokyo, Japan). The sizes of the resting eggs of E. pacifica within the LM images were determined using the ImageJ v. 1.32 software. Through this rigorous and methodical approach, we ensured the isolation and accurate identification of E. pacifica resting eggs.
2.3. Egg Hatching Success Experiment
To monitor the egg hatching success, the resting eggs of E. pacifica, isolated by frozen sediments and thawed at 3 °C, were incubated at 10 °C and salinity 32 in 6-well plates, with each well containing 10 mL of filtered seawater and 20 eggs. Such conditions corresponded to the natural winter conditions of Gamak Bay [28]. A total of 100 resting eggs (divided into 5 different wells) were used for each test, and one test was conducted for each freeze incubation time (1, 3, 6, 9, and 12 months). The same test number was set up for the two freeze conditions (−5 and −20 °C), for a total of 1.000 eggs. The resting eggs were monitored every 24 h for 30 days to check for hatching. The seawater in the multi-well plate was changed once a day. Additionally, a control group (100 eggs), was set up with eggs not previously frozen, reared at 10 °C and monitored daily for one month to assess the hatching success over time. A certain number of nauplii (15 individuals) reached adulthood and confirmed in all the cases their appurtenance to the species E. pacifica. Additionally, 24 nauplii and 32 copepodites were observed (they did not reach the adulthood). The remaining 29 eggs did not hatch.
2.4. Statistical Analyses
The hatching success data were transformed using the arcsine transformation to improve normality. Generalized linear models (GLMs) analyzed the effects of freezing storage duration and temperature on hatching success. The deviance value (%D) indicated the percentage of variability in the dependent variable explained by the independent variables. For linear regression analysis, the relationship between freezing storage duration and hatching success was examined by fitting a linear regression line to the arcsine-transformed data. Normality was checked using the Shapiro–Wilk test, and homoscedasticity (constant variance) was confirmed through visual inspection of residual plots. All statistical analyses were performed using R (version 4.5) with relevant packages such as stats and ggplot2. Data were presented as mean ± standard error (SE).
3. Results
There was a significant difference in the hatching success of resting eggs of E. pacifica depending on the length of the freezing storage period (Figure 3). The mean egg hatching success (a total of 100 eggs per condition) after freezing was 85.3 ± 1.5% at −5 °C and 85.0 ± 3.6% at −20 °C after one month of storage, and there was no significant difference between the two treatments. The mean hatching success after three months at −5 °C was 85.0 ± 3.6%, which was not significantly different from that observed after one month of storage. After six months of storage, the hatching success of the eggs stored at −5 °C decreased to 81.7 ± 2.1%, and further decreased to 29.3 ± 5.5% after 12 months. In contrast, the hatching success of the eggs stored at −20 °C decreased abruptly to 1.7 ± 2.1% after three months of storage. A trend of zero hatching success continued for −20 °C storage conditions beyond the three-month period, characterizing also six, nine, and 12 months.
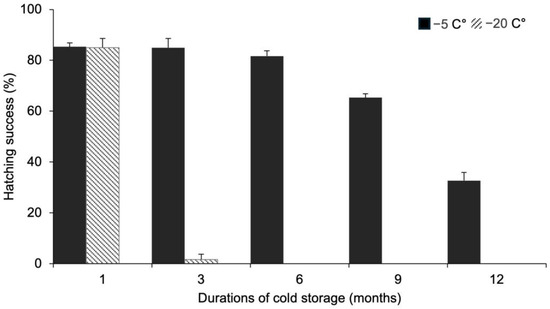
Figure 3.
Mean hatching success of resting eggs of Eurytemora pacifica stored at different temperatures for varying durations.
The cumulative hatching success of resting eggs stored at 10 °C (control group) increased rapidly over 7 days, reaching a final hatching success of 71.0% (Table 1; Figure 4). Resting eggs stored at −5 °C began hatching on day 4 after one month of storage and on day 12 after three months, ultimately achieving a hatching rate of approximately 85%. However, after nine months, hatching was delayed, and after 12 months of storage, the hatching rate declined to 29.3%. In contrast, resting eggs stored at −20 °C began hatching on day 15 after one month of storage, reaching 85%. However, after 3 months, the hatching rate plummeted to 1.7%, and no significant hatching occurred after six months of storage.

Table 1.
Hatching success and development of resting eggs of Eurytemora pacifica at 10 °C (control group). Results after 30 days of control.
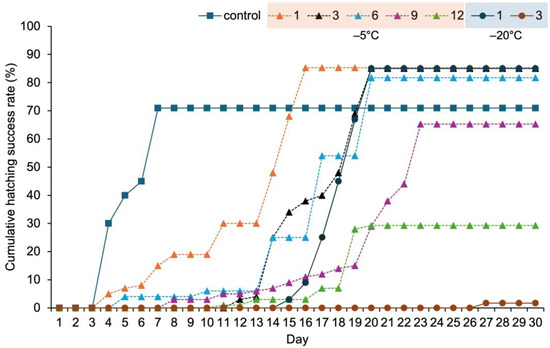
Figure 4.
Cumulative hatching success of Eurytemora pacifica resting eggs at various temperatures.
Generalized linear models (GLMs) showed that the effects of the length of the freezing storage period, and its interaction with the storage temperature, on hatching success were highly significant (p < 0.01). Linear regression analysis on arcsine-transformed data demonstrated a significant negative linear relationship between freezing storage duration and hatching success at both temperatures (Figure 5). The hatching success of the resting eggs stored at −5 °C decreased with increasing storage duration (p < 0.01), starting from the 6th month. Similarly, the hatching success of the resting eggs stored at −20 °C also showed a negative linear relationship with storage duration (p < 0.01), in this case starting from the 2nd month.
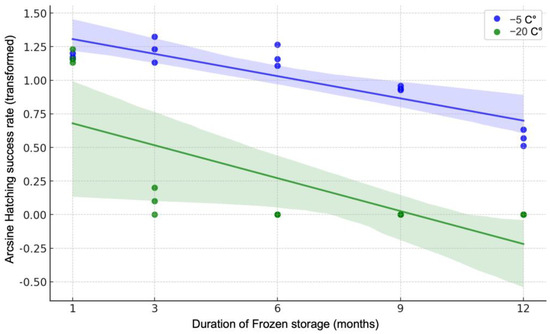
Figure 5.
Arcsine-transformed hatching success rate of Eurytemora pacifica resting eggs at different durations of frozen storage. The hatching success rates were monitored at −5 °C (blue circles) and −20 °C (green circles) over a period of 12 months. The shaded areas represent the 95% confidence intervals for the linear regression lines.
4. Discussion
Several studies have investigated the ability of copepod resting eggs to tolerate freezing temperatures. Some authors [40,41] reported that resting eggs from Eurytemora affinis, Acartia tonsa, Acartia clausi (later reclassified as Acartia teclae), and Centropages hamatus, could not withstand freezing at −15 °C. However, other experiments have demonstrated that the resting eggs of the freshwater copepod Diaptomus stagnalis can withstand freezing temperatures as low as −25 °C and can also survive at low temperatures (3–5 °C) for longer periods [40,42,43]. These findings suggest that the ability of copepod resting eggs to tolerate freezing temperatures may vary depending on the species and the specific environmental conditions.
In this study, we investigated the impact of freezing for variable durations (1, 3, 6, 9, and 12 months) at different temperatures (−5 and −20 °C) on the egg hatching success of the copepod E. pacifica, one of the most abundant copepod species in Gamak Bay (South Korea). Our findings demonstrated that one month of exposure to freezing conditions (−5 and −20 °C) does not compromise the hatching success of E. pacifica resting eggs, which was very close to the hatching obtained from eggs maintained under environmental conditions (the control group). More prolonged exposures to freezing temperatures significantly reduced hatching success, and the longest exposure durations resulted in more severe effects. Notably, the hatching success in the control group (10 °C) began earlier than in the experimental groups stored at −5 °C and −20 °C. Despite this earlier onset, the control group did not maintain the higher hatching rates observed in the frozen groups. Although the initial hatching rates in the control group were comparable to those in the frozen groups, they eventually plateaued and remained lower than the final hatching rates in the experimental groups (Figure 4). These results suggest that freezing temperature may have played a role in reducing microbial competition and thus contributed to the higher hatching success observed in the frozen groups. After 12 months of freezing, the hatching success of the eggs stored at −5 °C was still present, although it was reduced to 29.3%. These findings are consistent with previous studies that demonstrated the negative impacts of low temperatures on copepod eggs [44,45]. However, the underlying physiological or biochemical changes responsible for the reduced hatching success, particularly at −20 °C, were not fully explored in this study. Employing chemical and histological techniques could help identify these changes, providing deeper insights into the mechanisms at play. Nonetheless, we find these results highly encouraging, as they support our objective of proposing E. pacifica resting eggs as a viable material for the production of live food (nauplii) for juvenile fish in aquaculture.Storage at −5 °C is optimal for the conservation and delivery of resting eggs around the geographic area requiring their use, and one–three months of time without sensible losses, ensures also the coverage of long distances. The rearing at −20 °C, on the contrary, is not convenient from the yield point of view, and also in consideration of the higher energy cost of such a treatment.
Drillet et al. [46] showed that Acartia tonsa eggs stored at 2–3 °C for up to 12 months maintained a high hatching success rate, though the success rate diminished with extended storage periods. Similarly, Choi et al. [47] demonstrated that A. sinjiensis eggs stored at 4 ± 1 °C for up to 180 days had a hatching success rate greater than 40% after 90 days under various salinity conditions, with the rate decreasing over longer storage periods. Their research highlights the practical applications of optimizing cold storage conditions for copepod eggs in aquaculture, emphasizing the importance of adjusting storage conditions to maintain egg quality over time and avoid potential issues from using deteriorated eggs as live feed [46,47]. Cold preservation of resting eggs does not damage their potential hatching success and possibly helps their defense against microbes, making it a valuable technique for the aquaculture market [43]. Therefore, our findings suggest that E. pacifica resting eggs may be considered among the best options in the field of the management of viable food for aquaculture, because the already-used rotifer resting eggs (Brachionus sp.) and Anostraca cysts (Artemia sp.) ensure the availability of hatchling prey that are either very small or very large, respectively, for fish fry.
One limitation of our study is that E. pacifica is a species with egg sacs, which introduces challenges in studying resting eggs independently. Typically, the mother carries the eggs in sacs until they are ready to hatch or be released. This trait may influence their response to environmental conditions differently compared to species without egg sacs. Additionally, E. pacifica is known to occur in Gamak Bay during the winter and spring seasons, indicating a certain degree of adaptability to colder temperatures. Brylinski et al. [32] noted that E. pacifica females exhibit cyclomorphosis, producing both winged and wingless forms that also differ in their ability to lay subitaneous and diapause eggs. This reproductive strategy could affect their adaptability to varying environmental conditions. Furthermore, dormant stages of many aquatic invertebrates, including copepods, have been shown to survive extreme environmental conditions such as freezing and desiccation due to protective egg envelopes and biochemical adaptations [48]. For example, the dormant stages of Artemia can withstand temperatures as low as −196 °C [49]. Redden and Daborn [50] found that some copepod species, such as Eurytemora herdmani, require passage through a predator’s gut to enhance hatching success, suggesting that reproductive strategies involve behaviors able to offer egg-carrying females to predators (fish) to significantly impact egg viability. This highlights the necessity of addressing the unique reproductive traits of egg-carrying species in future studies.
In our study area of Gamak Bay, we found a significant abundance of eggs in the sediment, indicating their origin from diverse calanoid copepods residing in the region. Choi et al. [51] also suggested that Calanoida might contribute to the egg abundance in the sediments, although they did not identify the eggs at the species level. Recent studies by Moon et al. [34] and Moon and Oh [52] have identified the predominant Copepoda species in the area, which include Paracalanus parvus, E. pacifica, Oithona similis, Acartia omorii, A. erythraea, A. ohtsukai, A. sinjiensis, Centropages abdominalis, Pseudodiaptomus marinus, and Calanus sinicus in Gamak Bay. Importantly, some of these species are known to produce resting eggs [35,52,53,54]. While it is fairly straight forward to differentiate between E. pacifica and Acartia species, the task of distinguishing between the eggs of the Acartia species present in Gamak Bay, based purely on microscopic observations, proved to be quite challenging. This difficulty is compounded by the fact that the external morphology of Calanoida eggs can be influenced by environmental conditions [55], and each species can display more than one type of egg surface [56,57]. In addition, Paracalanus parvus s. l. is recognized as a consistently prevalent species throughout the year in Gamak Bay [52], and it might not produce resting eggs, also because they are too fragile to withstand the abrasion caused by sediment [58,59]. Furthermore, O. similis (Cyclopoida) is known to rest as juveniles, not as eggs. Among the Gamak Bay species, P. marinus shares egg-carrying behavior with E. pacifica, suggesting that both species might employ similar strategies for the survival of their eggs. Considering these factors, while our study primarily focused on the resting eggs of E. pacifica, it is important to acknowledge a limitation related to species identification based solely on egg morphology. Although E. pacifica was identified through distinctive morphological features, and all hatched individuals were confirmed as E. pacifica, it remains possible that eggs from other species may not have hatched under our conditions. This limitation highlights the need for more reliable methods, such as genetic analysis, to ensure accurate species identification in future studies. Furthermore, our findings suggest that future research should include multiple species to better understand the effects of freezing on embryo development. Although E. pacifica does not thrive in glacial areas, examining a broader range of species, including those with different reproductive strategies and ecological niches, could provide more comprehensive insights into the effects of freezing on copepod resting eggs and their possible use as a source of viable food in aquaculture.
Notably, some spiny patterns in copepod eggs have been reported to exhibit considerable variability within species, and even within individual clutches [60,61]. To confirm the species of the eggs, we cultured them and observed them developing into E. pacifica adults after approximately 30 days from the hatch. This method allowed us to accurately identify the species and ensured the reliability of our observations. Although no zooplankton species with eggs morphologically similar to those of E. pacifica have been identified in the local zooplankton community [34,35], there is a need for further research on other species and their responses to extreme temperatures. The recognizability of resting eggs in the sediments has a certain importance in the frame of viable food management for aquacultural purposes, because the sediment could be a more abundant source of eggs than single females with egg sacs. The next step could be the quantification of the cost–benefits of a procedure of isolating from sediments, as opposed to the rearing of copepods to obtain eggs directly from water tanks where they thrive.
5. Conclusions
This study provides information on the impacts of low temperatures on the hatching success of E. pacifica and highlights the possible use of frozen resting eggs as a source of nauplii in aquaculture. Prolonged exposure (more than six months at −5 °C, and more than three months at −20 °C) to low temperatures negatively impacts hatching success, and longer exposure durations or colder temperatures have more severe effects. The high hatching rate (>80%) after one–three months of freezing, and the persistence of hatching also after a long time at −5 °C, allow us to consider E. pacifica as one of the best candidates to supply fresh and viable prey to juvenile aquaculture fish. This study also allowed us to discover that the period of freezing of eggs even ameliorates the hatching success after one month, probably due to the biocide effect of the freeze on the microbiota, diversely trying to obtain energy from eggs. This study also raises important questions about the adaptive capacity of copepods and the effectiveness of bet-hedging strategies under rapidly changing climate conditions. Therefore, further research is needed to better understand the mechanisms and limits of bet-hedging strategies in copepod populations, as well as to develop effective conservation and management strategies for marine ecosystems in the face of climate change.
Author Contributions
Conceptualization, S.Y.C.; methodology, S.Y.C. and H.Y.S.; software, S.Y.C.; validation, S.Y.C., S.H.Y. and H.Y.S.; formal analysis, S.Y.C. and E.H.L.; investigation, S.Y.C. and E.H.L.; resources, S.Y.C. and E.H.L.; data curation, S.Y.C. and M.H.S.; writing—original draft preparation, S.Y.C. and H.Y.S.; writing—review and editing, S.Y.C., G.B., K.Y.K., H.Y.S., M.-C.J., K.W.P. and S.H.Y.; visualization, S.Y.C.; supervision, S.H.Y.; project administration, S.H.Y.; funding acquisition, S.H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Korea institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries, Korea (RS-2018-KS181192) and the National Institute of Fisheries Sciences (NIFS) grant (Countermeasure study of harmful organism to fisheries damage; R2024040).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Karl, T.R.; Trenbeth, K.E. Modern global climate change. Science 2003, 302, 1719–1723. [Google Scholar] [CrossRef] [PubMed]
- Orsolini, Y.J.; Senan, R.; Vitart, F.; Balsamo, G.; Weisheimer, A.; Doblas-Reyes, F.J. Influence of the Eurasian snow on the negative North Atlantic Oscillation in subseasonal forecasts of the cold winter 2009/2010. Clim. Dyn. 2016, 47, 1325–1334. [Google Scholar] [CrossRef]
- Ashfold, M.J.; Latif, M.T.; Samah, A.A.; Mead, M.I.; Harris, N.R.P. Influence of northeast monsoon cold surges on air quality in Southeast Asia. Atmos. Environ. 2017, 166, 498–509. [Google Scholar] [CrossRef][Green Version]
- Salman, S.A.; Shahid, S.; Ismail, T.; Chung, E.; Al-Abadi, A.M. Long-term trends in daily temperature extremes in Iraq. Atmos. Res. 2017, 198, 97–107. [Google Scholar] [CrossRef]
- Walsh, J.E.; Ballinger, T.J.; Euskirchen, E.S.; Hanna, E.; Mård, J.; Overland, J.E.; Vihma, T. Extreme weather and climate events in northern areas: A review. Earth-Sci. Rev. 2020, 209, 103324. [Google Scholar] [CrossRef]
- Kleppel, G.S. On the diets of calanoid copepods. Mar. Ecol. Prog. Ser. 1993, 99, 183. [Google Scholar] [CrossRef]
- Turner, J.T. The importance of small planktonic copepods and their roles in pelagic marine food webs. Zool. Stud. 2004, 43, 255–266. [Google Scholar]
- Kwon, E.Y.; Primeau, F.; Sarmiento, J.L. The impact of remineralization depth on the air–sea carbon balance. Nat. Geosci. 2009, 2, 630–635. [Google Scholar] [CrossRef]
- Parekh, P.; Dutkiewicz, S.; Follows, M.J.; Ito, T. Atmospheric carbon dioxide in a less dusty world. Geophys. Res. Lett. 2006, 33, 2–5. [Google Scholar] [CrossRef]
- Hays, G.C.; Richardson, A.J.; Robinson, C. Climate change and marine plankton. Trends Ecol. Evol. 2005, 20, 337–344. [Google Scholar] [CrossRef]
- Hooff, R.C.; Peterson, W.T. Copepod biodiversity as an indicator of changes in ocean and climate conditions of the northern California current ecosystem. Limnol. Oceanogr. 2006, 51, 2607–2620. [Google Scholar] [CrossRef]
- Marcus, N.H. Abundance in bottom sediments and hatching requirements of eggs of Centropages hamatus (Copepoda: Calanoida) from the Alligator Harbor region, Florida. Biol. Bull. 1989, 176, 142–146. [Google Scholar] [CrossRef]
- Marcus, N.H. Calanoid copepod, cladoceran, and rotifer eggs in sea-bottom sediments of northern Californian coastal waters: Identification, occurrence and hatching. Mar. Biol. 1990, 105, 413–418. [Google Scholar] [CrossRef]
- Næss, T. Marine calanoid resting eggs in Norway: Abundance and distribution of two copepod species in the sediment of an enclosed marine basin. Mar. Biol. 1991, 110, 261–266. [Google Scholar] [CrossRef]
- Belmonte, G.; Rubino, F. Resting cysts from coastal marine plankton. Oceanogr. Mar. Biol. 2019, 57, 1–88. [Google Scholar]
- Marcus, N.H.; Lutz, R.; Burnett, W.; Cable, P. Age, viability, and vertical distribution of zooplankton resting eggs from an anoxic basin: Evidence of an egg bank. Limnol. Oceanogr. 1994, 39, 154–158. [Google Scholar] [CrossRef]
- Stempniewicz, L.; Błachowiak-Samołyk, K.; Węsławski, J.M. Impact of climate change on zooplankton communities, seabird populations and arctic terrestrial ecosystem—A scenario. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 2934–2945. [Google Scholar] [CrossRef]
- Richardson, A.J. In hot water: Zooplankton and climate change. ICES J. Mar. Sci. 2008, 65, 279–295. [Google Scholar] [CrossRef]
- Ekvall, M.K.; Hansson, L.A. Differences in recruitment and life-history strategy alter zooplankton spring dynamics under climate-change conditions. PLoS ONE 2012, 7, e44614. [Google Scholar] [CrossRef]
- Villarino, E.; Chust, G.; Licandro, P.; Butenschön, M.; Ibaibarriaga, L.; Larrañaga, A.; Irigoien, X. Modelling the future biogeography of North Atlantic zooplankton communities in response to climate change. Mar. Ecol. Prog. Ser. 2015, 531, 121–142. [Google Scholar] [CrossRef]
- Penn, J.L.; Deutsch, C. Avoiding ocean mass extinction from climate warming. Science 2022, 376, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.M.; Boyce, D.G.; Hofmann, M.; Matthiessen, B.; Sommer, U.; Worm, B. Effects of sea surface warming on marine plankton. Ecol. Lett. 2014, 17, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Maugendre, L.; Gattuso, J.P.; Louis, J.; De Kluijver, A.; Marro, S.; Soetaert, K.; Gazeau, F. Effect of ocean warming and acidification on a plankton community in the NW Mediterranean Sea. ICES J. Mar. Sci. 2015, 72, 1744–1755. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; O’Malley, R.T.; Boss, E.S.; Westberry, T.K.; Graff, J.R.; Halsey, K.H.; Brown, M.B. Revaluating ocean warming impacts on global phytoplankton. Nat. Clim. Chang. 2016, 6, 323–330. [Google Scholar] [CrossRef]
- Kleppel, G.S.; Burkart, C.A. Egg production and the nutritional environment of Acartia tonsa: The role of food quality in copepod nutrition. ICES J. Mar. Sci. 1995, 52, 297–304. [Google Scholar] [CrossRef]
- Chinnery, F.E.; Williams, J.A. The influence of temperature and salinity on Acartia (Copepoda: Calanoida) nauplii survival. Mar. Biol. 2004, 145, 733–738. [Google Scholar] [CrossRef]
- Holste, L.; Peck, M.A. The effects of temperature and salinity on egg production and hatching success of Baltic Acartia tonsa (Copepoda: Calanoida): A laboratory investigation. Mar. Biol. 2005, 148, 1061–1070. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, E.H.; Shin, S.S.; Lim, Y.H.; Soh, H.Y. Optimal photoperiod for the reproduction of Eurytemora pacifica: Potential live feed for fish larvae. Aquac. Int. 2022, 30, 2389–2401. [Google Scholar] [CrossRef]
- Lavens, P.; Sorgeloos, P. Manual on the Production and Use of Live Food for Aquaculture; FAO Fisheries Technical Papers 361; FAO: Rome, Italy, 1996. [Google Scholar]
- Marcus, N.H. Calanoid copepods, resting eggs, and aquaculture. In Copepods in Aquaculture; Lee, C.S., O’Bryen, P.J., Marcus, N.H., Eds.; Blackwell Publishing: Oxford, UK, 2005; pp. 3–9. [Google Scholar]
- Dodson, S.I.; Skelly, D.A.; Lee, C.E. Out of Alaska: Morphological diversity within the genus Eurytemora from its ancestral Alaskan range (Crustacea, Copepoda). Hydrobiologia 2010, 653, 131–148. [Google Scholar] [CrossRef]
- Brylinski, J.M.; Courcot, L.; David, V.; Sautour, B. Expansion of the North Pacific copepod Eurytemora pacifica Sato, 1913 (Copepoda: Calanoida: Temoridae) along the Atlantic coast of France. Bioinvasions Rec. 2016, 5, 245–250. [Google Scholar] [CrossRef]
- Wang, Y.G.; Tseng, L.C.; Sun, R.X.; Liu, Z.Y.; Lin, M.; Hwang, J.S. Effects of the China Coastal Current on the community structure of planktonic copepods in early spring, with notes on Eurytemora pacifica Sato, 1913 in the western Taiwan Strait. Crustaceana 2020, 93, 487–506. [Google Scholar] [CrossRef]
- Moon, S.Y.; Yoon, H.S.; Soh, H.Y.; Choi, S.D. Environmental factors and variation characteristics of zooplankton communities in Gamak Bay. Ocean Polar Res. 2006, 28, 79–94. [Google Scholar] [CrossRef]
- Moon, S.Y.; Oh, H.J. Seasonal changes in copepod biomass and production in Gamak Bay, Korea. Fish Aquat. Sci. 2021, 24, 171–179. [Google Scholar] [CrossRef]
- Solokhina, E.V. Two forms of Eurytemora pacifica (Crustacea, Copepoda, Calanoida) from the lagoon Gladskovskaya (the Commandor Islands). Zool. Zhurnal. 1992, 82, 137–139. [Google Scholar]
- Belmonte, G. The suspected contradictory role of parental care in the adaption of planktonic Calanoida to temporary freshwater. Water 2021, 13, 100. [Google Scholar] [CrossRef]
- Hwang, D.W.; Kim, P.J.; Jeon, S.B.; Koh, B.S. Geochemical characteristics of intertidal sediment in the semi-enclosed bays of the southern region of Jeollanam Province. Korean J. Fish Aquat. Sci. 2013, 46, 638–648. [Google Scholar] [CrossRef][Green Version]
- Onbe, T. Sugar flotation method for sorting the resting eggs of marine cladocerans and copepods from sea-bottom sediment. Bull. Jpn. Soc. Fish 1978, 41, 1411. [Google Scholar] [CrossRef]
- Zillioux, E.J.; Gonzalez, J.G. Egg dormancy in a neritic calanoid copepod and its implications to overwintering in boreal waters. In Fifth European Marine Biology Symposium; Battagia, B., Ed.; Piccin Editore: Padova, Italy, 1972; pp. 217–230. [Google Scholar]
- Næss, T. Tolerance of marine calanoid resting eggs: Effects of freezing, desiccation and rotenone exposure: A field and laboratory study. Mar. Biol. 1991, 111, 455–459. [Google Scholar] [CrossRef]
- Brewer, R.H. The phenology of Diaptomus stagnalis (Copepoda: Calanoida) the development and the hatching of the egg stage. Physiol. Zool. 1964, 27, 1–20. [Google Scholar] [CrossRef]
- Kaviyarasan, M.; Santhanam, P. A technique on the culture and preservation of marine copepod eggs. Basic Appl. Zooplankton Biol. 2019, 34, 197–208. [Google Scholar] [CrossRef]
- Drillet, G.; Lindley, L.C.; Michels, A.; Wilcox, J.; Marcus, N.H. Improving cold storage of subitaneous eggs of the copepod Acartia tonsa Dana from the Gulf of Mexico (Florida–USA). Aquac. Res. 2007, 38, 457–466. [Google Scholar] [CrossRef]
- Olivotto, I.; Gaiot, G.; Holste, L.; Tulli, F.; Cardinaletti, G.; Piccinetti, C.C.; Carnevali, O. Are Acartia tonsa cold-stored eggs a suitable food source for the marine ornamental species Amphiprion polymnus? A feeding study. Aquac. Nutr. 2012, 18, 685–696. [Google Scholar] [CrossRef]
- Drillet, G.; Iversen, M.H.; Sørensen, T.F.; Ramløv, H.; Lund, T.; Hansen, B.W. Effect of cold storage upon eggs of a calanoid copepod, Acartia tonsa (Dana) and their offspring. Aquaculture 2006, 254, 714–729. [Google Scholar] [CrossRef]
- Choi, S.Y.; Jeon, S.C.; Soh, H.Y. Effects of cold storage and salinity on Acartia sinjiensis (Copepoda: Calanoida) egg hatching. Aquac. Res. 2022, 53, 3568–3574. [Google Scholar] [CrossRef]
- Radzikowski, J. Resistance of dormant stages of planktonic invertebrates to adverse environmental conditions. J. Plankton Res. 2013, 35, 707–723. [Google Scholar] [CrossRef]
- Clegg, J.S.; Trotman, C.N. Physiological and biochemical aspects of Artemia ecology. In Artemia: Basic and Applied Biology; Springer: Dordrecht, The Netherlands, 2002; pp. 129–170. [Google Scholar]
- Redden, A.M.; Daborn, G.R. Viability of subitaneous copepod eggs following fish predation on egg-carrying calanoids. Mar. Ecol. Prog. Ser. 1991, 77, 307–310. [Google Scholar] [CrossRef]
- Choi, S.Y.; Soh, H.Y.; Shin, K.; Jung, S.W.; Jang, M.C. Effects of hypoxia on benthic eggs of calanoid copepods in the Southern Sea of Korea. Front. Mar. Sci. 2023, 10, 1132851. [Google Scholar] [CrossRef]
- Moon, S.Y.; Kim, H.Y.; Oh, H.J. Seasonal variation of the zooplankton community of Gamak Bay, Korea. Korean J. Environ. Biol. 2020, 38, 231–247. [Google Scholar] [CrossRef]
- Park, C.; Ju, S.J.; Park, W.; Kim, H.W.; Lee, S.R.; Park, J.H. The strategy of population maintenance by coastal copepod inferred from seasonal variations in abundance of adults and resting eggs. Ocean Polar Res. 2018, 40, 213–222. [Google Scholar] [CrossRef]
- Uye, S.I.; Kasahara, S.; Onbe, T. Calanoid copepod eggs in sea-bottom muds. IV. Effects of some environmental factors on the hatching of resting eggs. Mar. Biol. 1979, 51, 151–156. [Google Scholar] [CrossRef]
- Gaudy, R. Etude expérimentale de la ponte chez trois espčces de copépodes pélagiques (Centropages typicus, Acartia clausi et Temora stylifera). Mar. Biol. 1971, 9, 65–70. [Google Scholar] [CrossRef]
- Onoue, Y.; Toda, T.; Ban, S. Morphological features and hatching patterns of eggs in Acartia steueri (Crustacea, Copepoda) from Sagami Bay, Japan. Hydrobiologia 2004, 511, 17–25. [Google Scholar] [CrossRef]
- Castellani, C.; Lucas, I.A.N. Seasonal variation in egg morphology and hatching success in the calanoid copepods Temora longicornis, Acartia clausi and Centropages hamatus. J. Plankton Res. 2003, 25, 527–537. [Google Scholar] [CrossRef]
- Marcus, N.H. Planktonic copepods in a sub-tropical estuary: Seasonal patterns in the abundance of adults, copepodites, nauplii, and eggs in the sea bed. Biol. Bull. 1991, 181, 269–274. [Google Scholar] [CrossRef]
- Næss, T. Benthic resting eggs of calanoid copepods in Norwegian enclosures used in mariculture: Abundance, species composition and hatching. Hydrobiologia 1996, 320, 161–168. [Google Scholar] [CrossRef]
- Belmonte, G.; Miglietta, A.; Rubino, F.; Boero, F. Morphological convergence of resting stages produced by planktonic organisms: A review. Hydrobiologia 1997, 335, 159–165. [Google Scholar] [CrossRef]
- Souissi, A.; Souissi, S. Abnormalities in shape and size of Eurytemora affinis (Copepoda, Calanoida) and its eggs under different environmental conditions. Crustaceana 2020, 93, 355–378. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).