The Distribution and Succession of Filamentous Algae in the Southern Taihang Catchment under Different Nutrient Regimes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Filamentous Algae Collection, Identification, and Measurement
2.3. Periphytic Algae Collection, Identification, and Measurement
2.4. Macrobenthos Collection, Identification, and Measurement
2.5. Fish Collection, Identification, and Measurement
2.6. Abiotic Conditions
2.7. Data Analysis
2.7.1. Patterns in the Filamentous Algae Community and Environmental Contributions
2.7.2. Generalized Linear Models
2.7.3. Association Network between the Filamentous Algae and Macrobenthos
2.7.4. Other Statistical Analysis
3. Results
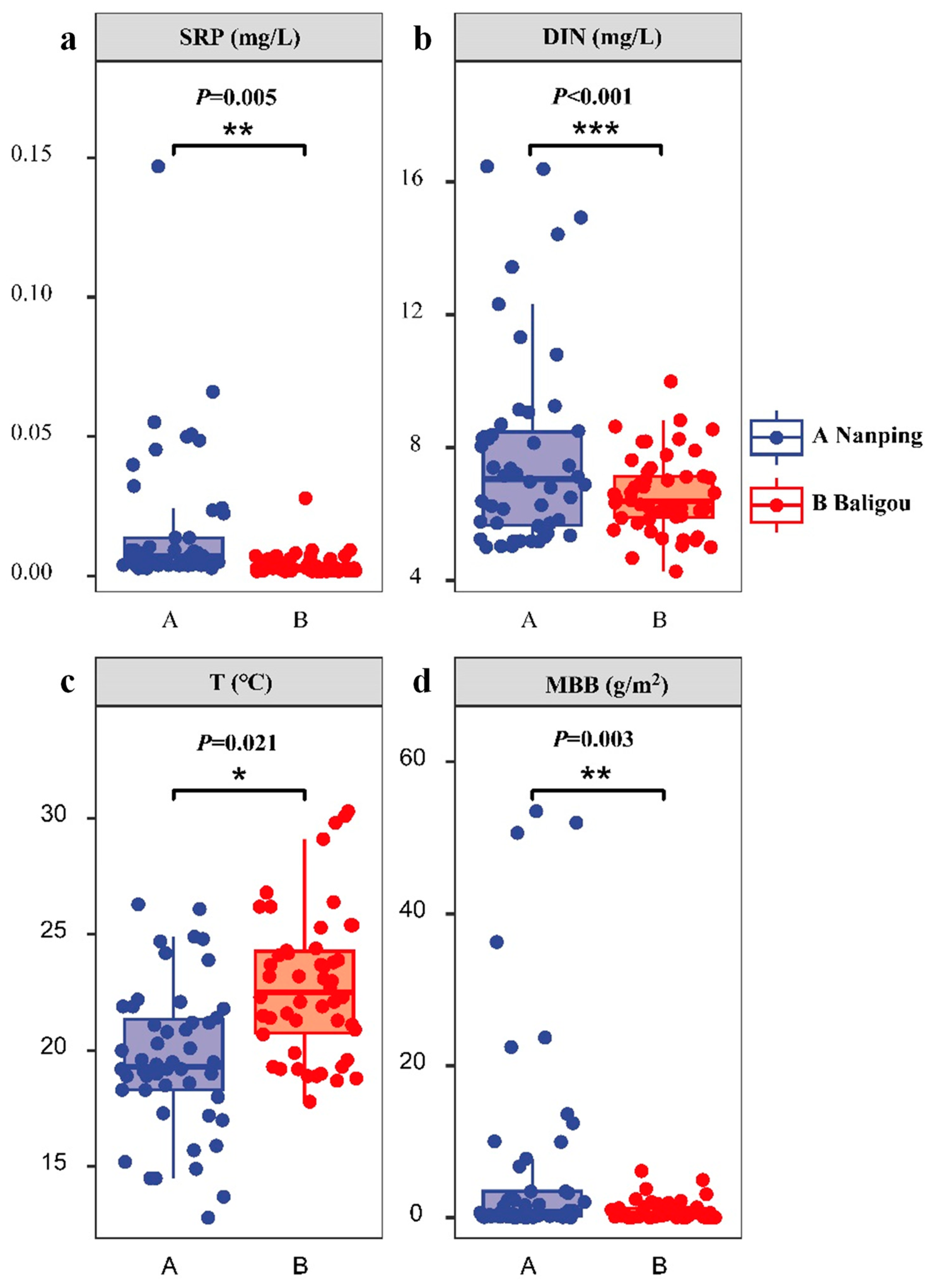
3.1. Characteristics of Nutrients, Other Biotic, and Abiotic Conditions in the Two Streams
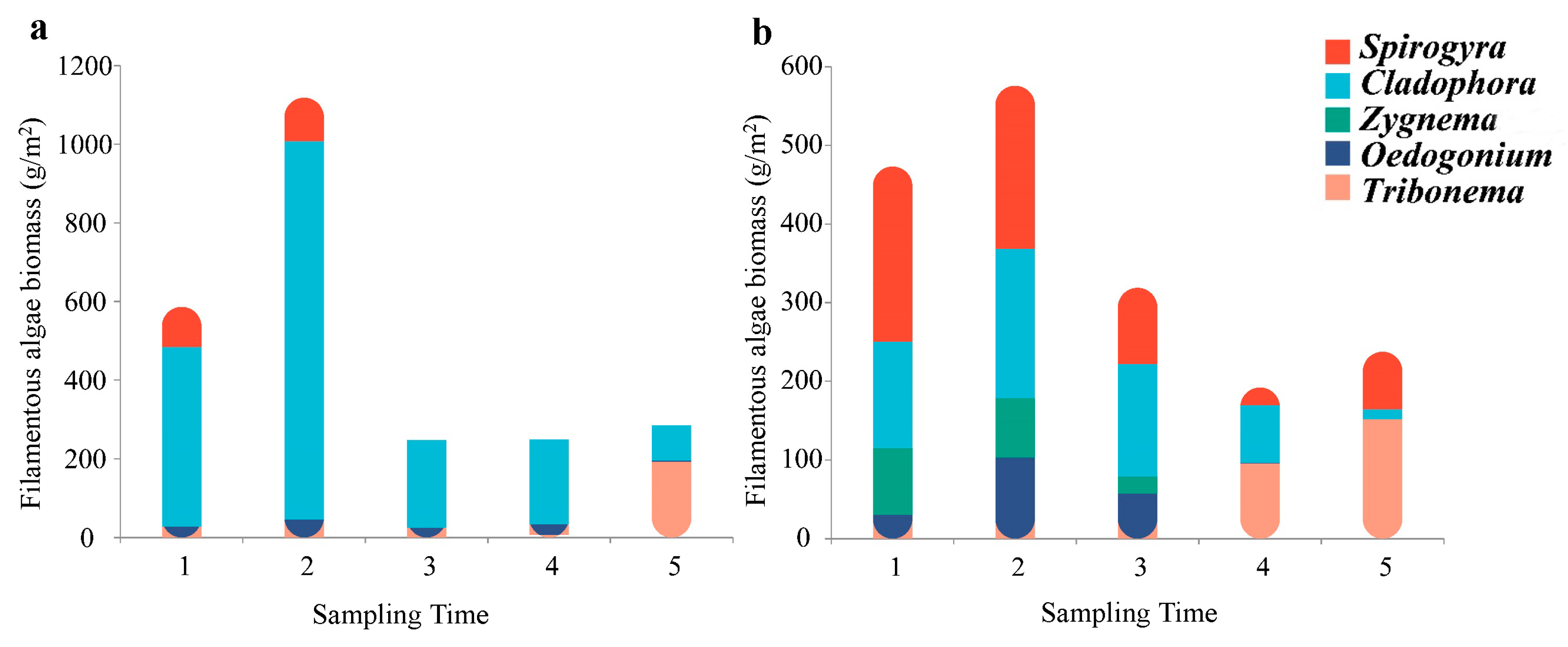
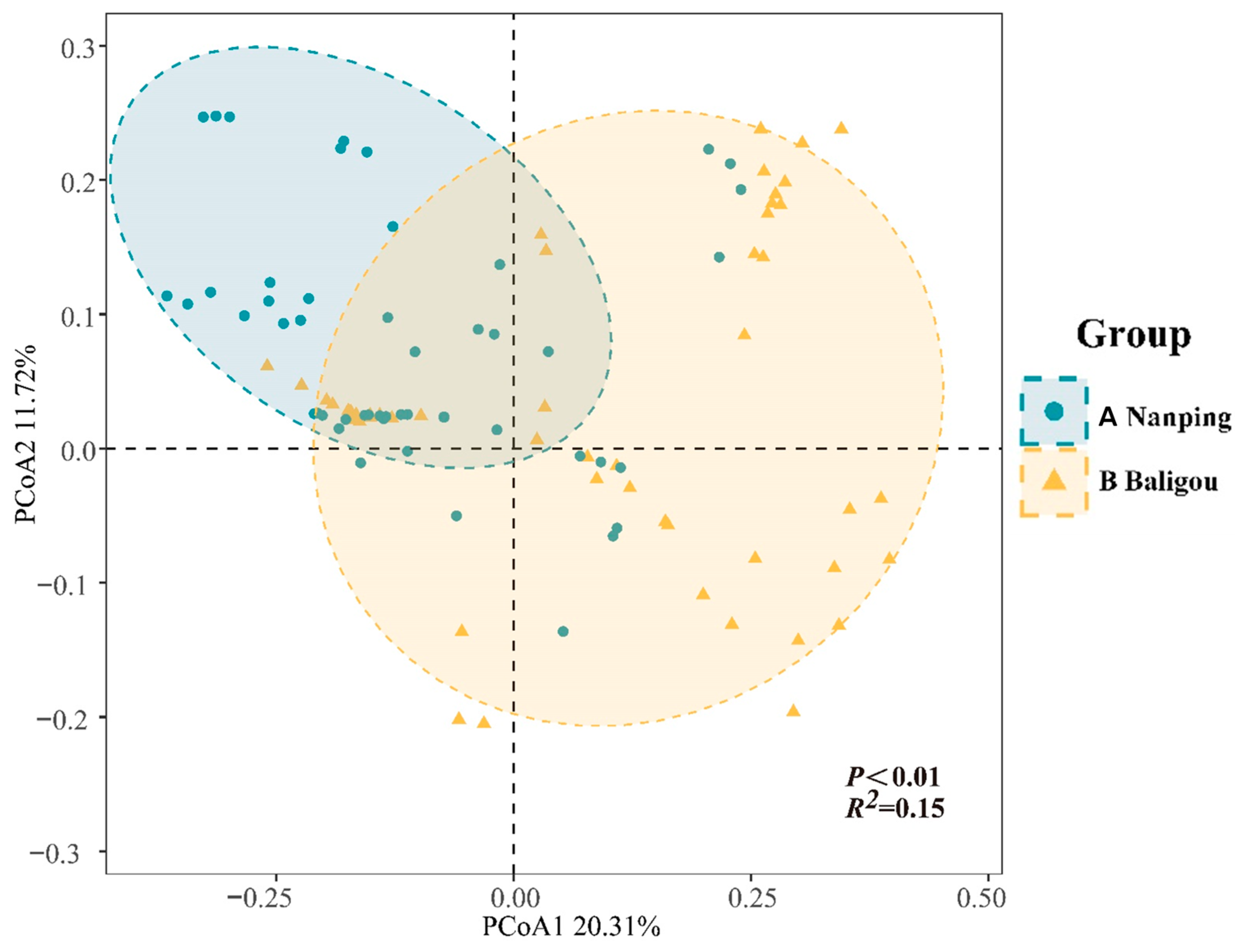
3.2. Distribution Patterns of Filamentous Algae Communities
3.3. Determinants of the Distribution of Filamentous Algae Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Genkai-Kato, M.; Vadeboncoeur, Y.; Liboriussen, L.; Jeppesen, E. Benthic-planktonic coupling, regime shifts, and whole-lake primary production in shallow lakes. Ecology 2012, 93, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Lawniczak-Malińska, A.; Ptak, M.; Celewicz, S.; Choiński, A. Impact of lake morphology and shallowing on the rate of overgrowth in hard-water eutrophic lakes. Water 2018, 10, 1827. [Google Scholar] [CrossRef]
- Kravtsova, L.S.; Izhboldina, L.A.; Khanaev, I.V.; Pomazkina, G.V.; Rodionova, E.V.; Domysheva, V.M.; Sakirko, M.V.; Tomberg, I.V.; Kostornova, T.Y.; Kravchenko, O.S.; et al. Nearshore benthic blooms of filamentous green algae in Lake Baikal. J. Great Lakes Res. 2014, 40, 441–448. [Google Scholar] [CrossRef]
- Dodds, W.; Smith, V. Nitrogen, phosphorus, and eutrophication in streams. Inland Waters 2016, 6, 155–164. [Google Scholar] [CrossRef]
- Oberholster, P.; Somerset, V.; Truter, J.; Botha, A.M. The interplay between environmental conditions and filamentous algae mat formation in two agricultural influenced South African rivers. River Res. Appl. 2017, 33, 388–402. [Google Scholar] [CrossRef]
- Ruley, J.E.; Rusch, K.A. Development of a simplified phosphorus management model for a shallow, subtropical, urban hypereutrophic lake. Ecol. Eng. 2004, 22, 77–98. [Google Scholar] [CrossRef]
- Timoshkin, O.A.; Moore, M.V.; Kulikova, N.N.; Tomberg, I.V.; Malnik, V.V.; Shimaraev, M.N.; Troitskaya, E.S.; Shirokaya, A.A.; Sinyukovich, V.N.; Zaitseva, E.P.; et al. Groundwater contamination by sewage causes benthic algal outbreaks in the littoral zone of Lake Baikal (East Siberia). J. Great Lakes Res. 2018, 44, 230–244. [Google Scholar] [CrossRef]
- McCusker, M.; Dove, A.; Depew, D.; Howell, E.T. Factors affecting Cladophora growth in the eastern basin of Lake Erie: Analysis of a monitoring dataset (2012–2019). J. Great Lakes Res. 2023, 49, 790–808. [Google Scholar] [CrossRef]
- Wehr, J.D.; Sheath, R.G.; Kociolek, J.P. Freshwater algae of North America: Ecology and classification. J. Phycol. 2003, 39, 624–625. [Google Scholar] [CrossRef]
- Khanum, A. An ecological study of freshwater algal mats. Bot. Bull. Acad. Sin. 1982, 23, 89–104. [Google Scholar]
- Pinn, E.; Jones, M. 20. Macroalgal mat development and associated changes in infaunal biodiversity. Proc. Mar. Sci. 2005, 7, 231–237. [Google Scholar] [CrossRef]
- Pikosz, M.; Messyasz, B.; Gąbka, M. Functional structure of algal mat (Cladophora glomerata) in a freshwater in western Poland. Ecol. Indic. 2017, 74, 1–9. [Google Scholar] [CrossRef]
- Pikosz, M.; Messyasz, B. Composition and seasonal changes in filamentous algae in floating mats. Oceanol. Hydrobiol. Stud. 2015, 44, 273–281. [Google Scholar] [CrossRef]
- Çelekli, A.; Kapı, E.; Soysal, Ç.; Arslanargun, H.; Bozkurt, H. Evaluating biochemical response of filamentous algae integrated with different water bodies. Ecotoxicol. Environ. Saf. 2017, 142, 171–180. [Google Scholar] [CrossRef]
- Francoeur, S.N.; Winslow, K.A.P.; Miller, D.; Peacor, S.D. Mussel-derived stimulation of benthic filamentous algae: The importance of nutrients and spatial scale. J. Great Lakes Res. 2017, 43, 69–79. [Google Scholar] [CrossRef]
- Çelekli, A.; Bozkurt, H. Assessing biochemical responses of filamentous algae integrated with surface waters in Yavuzeli-Araban catchment. Ecol. Eng. 2021, 159, 106126. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, X.; Ma, Y.; Zhao, X.; Zhao, T.; Yang, S.; Gao, L. Late Cenozoic geomorphology, geochronology and physiography of Yuntaishan in southern Taihang Mountain, North China. Acta Geol. Sin. 2010, 84, 230–239. [Google Scholar] [CrossRef]
- Han, S.; Yang, Y.; Fan, T.; Xiao, D.; Moiwo, J.P. Precipitation-runoff processes in Shimen hillslope micro-catchment of Taihang Mountain, north China. Hydrol. Process. 2012, 26, 1332–1341. [Google Scholar] [CrossRef]
- Chen, Y.-C. A Simple Method for Isolating Filaments as “Algal Seed Stock” from Monostroma Latissimum (Chlorophyta) Germlings, and Application for Mass Cultivation. J. Phycol. 2011, 48, 246–247. [Google Scholar] [CrossRef]
- Guoxiang Liu, Z.H. Flora Algarum Sinicarum Aquae Dulcis; Science Press: Beijing, China, 2012; Volume 15, pp. 106–121. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Morphology and taxonomy of surirella ovalis and related taxa. Diatom Res. 1987, 2, 77–95. [Google Scholar] [CrossRef]
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Kelly, M.; Cantonati, M. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; Koeltz Botanical Books Schmitten-Oberreifenberg: Glashütten, Germany, 2017; Volume 942. [Google Scholar]
- Tsukazaki, C.; Ishii, K.-I.; Saito, R.; Matsuno, K.; Yamaguchi, A.; Imai, I. Distribution of viable diatom resting stage cells in bottom sediments of the eastern Bering Sea shelf. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2013, 94, 22–30. [Google Scholar] [CrossRef]
- Kahlert, M.; Hasselrot, A.T.; Hillebrand, H.; Pettersson, K. Spatial and temporal variation in the biomass and nutrient status of epilithic algae in Lake Erken, Sweden. Freshw. Biol. 2002, 47, 1191–1215. [Google Scholar] [CrossRef]
- Campos, C.A.; Kennard, M.J.; Júnior, J.F.G. Diatom and macroinvertebrate assemblages to inform management of Brazilian savanna’s watersheds. Ecol. Indic. 2021, 128, 107834. [Google Scholar] [CrossRef]
- Zilli, F.L. Distribution of benthic invertebrate biomass and secondary production in relation to floodplain connectivity in a large river system (Paraná River, Argentina). Int. Rev. Hydrobiol. 2013, 98, 284–293. [Google Scholar] [CrossRef]
- Nelson, J. Fishes of the World, 3rd ed.; John Wiley&SonSINC: Hoboken, NJ, USA, 1994; Volume 3. [Google Scholar] [CrossRef]
- Harley, S.J.; Myers, R.A.; Dunn, A. Is catch-per-unit-effort proportional to abundance? Can. J. Fish. Aquat. Sci. 2001, 58, 1760–1772. [Google Scholar] [CrossRef]
- Gupta, C.; Langer, S.; Dhar, M. Variations in water quality parameters and their correlation with fish catch per unit effort of Bhini Stream, a tributary of River Ravi, Jammu and Kashmir, India. Ecol. Environ. Conserv. 2023, 28, 1967–1975. [Google Scholar] [CrossRef]
- Norton, J.F. Standard Methods for the Examination of Water and Sewage. American Public Health Association: Washington, DC, USA, 2018; Volume 10. [Google Scholar]
- Koutecký, P. MorphoTools: A set of R functions for morphometric analysis. Plant Syst. Evol. 2015, 301, 1115–1121. [Google Scholar] [CrossRef]
- El-Horbaty, Y.S. A Monte Carlo permutation procedure for testing variance components in generalized linear regression models. Comput. Stat. 2023, 39, 2605–2621. [Google Scholar] [CrossRef]
- Dutilleul, P.; Pelletier, B. A valid parametric test of significance for the average R2 in redundancy analysis with spatial data. Spat. Stat. 2016, 19, 21–41. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P.R. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca. hp R package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Hamilton, B.H.; Nickerson, J.A. Correcting for endogeneity in strategic management research. Strateg. Organ. 2003, 1, 51–78. [Google Scholar] [CrossRef]
- Hu, A.; Ju, F.; Hou, L.; Li, J.; Yang, X.; Wang, H.; Mulla, S.I.; Sun, Q.; Bürgmann, H.; Yu, C.P. Strong impact of anthropogenic contamination on the occurrence patterns of a riverine microbial community. Environ. Microbiol. 2017, 19, 4993–5009. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, H.; Dsouza, M.; Lou, J.; He, Y.; Dai, Z.; Brookes, P.C.; Xu, J.; Gilbert, J.A. Geographic patterns of co-occurrence network topological features for soil microbiota at continental scale in eastern China. ISME J. 2016, 10, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Weerman, E.J.; Herman, P.M.J.; van de Koppel, J. Macrobenthos abundance and distribution on a spatially patterned intertidal flat. Mar. Ecol. Prog. Ser. 2011, 440, 95–103. [Google Scholar] [CrossRef]
- Nieoczym, M.; Kloskowski, J. The role of body size in the impact of common carpCyprinus carpioon water quality, zooplankton, and macrobenthos in ponds. Int. Rev. Hydrobiol. 2014, 99, 212–221. [Google Scholar] [CrossRef]
- Lange, K.; Liess, A.; Piggott, J.J.; Townsend, C.R.; Matthaei, C.D. Light, nutrients and grazing interact to determine stream diatom community composition and functional group structure. Freshw. Biol. 2010, 56, 264–278. [Google Scholar] [CrossRef]
- Shimabukuro, E.M.; Henry, R. Macrophytes in tropical shallow lakes: An important food item to benthic entomofauna or an underused resource? Entomol. Sci. 2019, 22, 205–215. [Google Scholar] [CrossRef]
- Filkin, N.R.; Sherwood, A.R.; Vis, M.L. Macroalgae from 23 Stream Segments in the Hawaiian Islands. Pac. Sci. 2003, 57, 421–431. [Google Scholar] [CrossRef][Green Version]
- Bosch, I.; Makarewicz, J.C.; Lewis, T.W.; Bonk, E.A.; Finiguerra, M.; Groveman, B. Management of agricultural practices results in declines of filamentous algae in the lake littoral. J. Great Lakes Res. 2009, 35, 90–98. [Google Scholar] [CrossRef]
- Higgins, S.N.; Malkin, S.Y.; Todd Howell, E.; Guildford, S.J.; Campbell, L.; Hiriart-Baer, V.; Hecky, R.E. An ecological review of cladophora glomerata (Chlorophyta) in the laurentian great lakes. J. Phycol. 2008, 3, 1–7. [Google Scholar] [CrossRef]
- Olsen, S.; Chan, F.; Li, W.; Zhao, S.; Søndergaard, M.; Jeppesen, E. Strong impact of nitrogen loading on submerged macrophytes and algae: A long-term mesocosm experiment in a shallow Chinese lake. Freshwater Biology 2015, 60, 1525–1536. [Google Scholar] [CrossRef]
- Page, M.; Goldhammer, T.; Hilt, S.; Tolentino, S.; Brothers, S. Filamentous algae blooms in a large, clear-water lake: Potential drivers and reduced benthic primary production. Water 2022, 14, 2136. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Ku, C.-R.; Lu, H.-L. Effects of aquatic ecological indicators of sustainable green energy landscape facilities. Ecol. Eng. 2014, 71, 144–153. [Google Scholar] [CrossRef]
- Gladyshev, M.; Gubelit, Y.I. Green tides: New consequences of the eutrophication of natural waters (invited review). Contemp. Probl. Ecol. 2019, 12, 109–125. [Google Scholar] [CrossRef]
- Hawes, I. The seasonal dynamics of Spirogyra in a shallow, maritime Antarctic lake. Polar Biology 1988, 8, 429–437. [Google Scholar] [CrossRef]
- Han, H.; Chen, Y.; Jørgensen, S.E.; Nielsen, S.N.; Hu, W. A system-dynamic model on the competitive growth between Potamogeton malaianus Miq. and Spirogyra sp. Ecol. Model. 2009, 220, 2206–2217. [Google Scholar] [CrossRef]
- Dodds, W.K.; Gudder, D.A. The ecology of Cladophora. J. Phycol. 1992, 28, 415–427. [Google Scholar] [CrossRef]
- Adams, M.S.; Stone, W. Field studies on photosynthesis of Cladophora glomerata (Chlorophyta) in Green bay, Lake Michigan. Ecology 1973, 54, 853–862. [Google Scholar] [CrossRef]
- Cambra, J.; Aboal, M. Filamentous green algae of Spain: Distribution and ecology. Limnetica 1992, 8, 213–220. [Google Scholar] [CrossRef]
- Margalef, R.; Kinne, O. Our Biosphere; Ecology Institute Oldendorf: Oldendorf/Luhe, Germany, 1997; Volume 10. [Google Scholar]
- Gebler, D.; Szoszkiewicz, K. Response of aquatic plants to extreme alterations in river morphology. Water 2022, 14, 3746. [Google Scholar] [CrossRef]
- Frossard, V.; Versanne-Janodet, S.; Aleya, L. Factors supporting harmful macroalgal blooms in flowing waters: A 2-year study in the Lower Ain River, France. Harmful Algae 2014, 33, 19–28. [Google Scholar] [CrossRef]
- Asaeda, T.; Sultana, M.; Manatunge, J.; Fujino, T. The effect of epiphytic algae on the growth and production of Potamogeton perfoliatus L. in two light conditions. Environ. Exp. Bot. 2004, 52, 225–238. [Google Scholar] [CrossRef]
- Ding, Y.-F.; Sun, S.-J.; Feng, J.; Cui, P.; Zhang, D.; Long, Z.-Y. An assessment of lake ecology on the basis of the macrobenthos multi-metric index (MMI) in 11 lakes in the western region of Jilin, China. Water 2021, 13, 235. [Google Scholar] [CrossRef]
- Ye, J.; Tang, Y.; Zhang, X.; Zhong, P.; Liu, Z. Omnivorous shrimp Neocaridina denticulata sinensis enhances the growth of submerged macrophyte Vallisneria denseserrulata. Knowl. Manag. Aquat. Ecosyst. 2019, 5, 32. [Google Scholar] [CrossRef]
- Yang, L.; He, H.; Guan, B.; Yu, J.; Yao, Z.; Zhen, W.; Yin, C.; Wang, Q.; Jeppesen, E.; Liu, Z. Mesocosm experiment reveals a strong positive effect of snail presence on macrophyte growth, resulting from control of epiphyton and nuisance filamentous algae: Implications for shallow lake management. Sci. Total Environ. 2020, 705, 135958. [Google Scholar] [CrossRef]
- Fang, L.; Wong, P.K.; Lin, L.; Lan, C.; Qiu, J.W. Impact of invasive apple snails in Hong Kong on wetland macrophytes, nutrients, phytoplankton and filamentous algae. Freshw. Biol. 2010, 55, 1191–1204. [Google Scholar] [CrossRef]
- Pinowska, A. Effects of snail grazing and nutrient release on growth of the macrophytes Ceratophyllum demersum and Elodea canadensis and the filamentous green alga Cladophora sp. Hydrobiologia 2002, 479, 83–94. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Stoermer, E. Luxury consumption of phosphorus by benthic algae. BioScience 1982, 32, 682–683. [Google Scholar] [CrossRef]
- Huang, R.; Boney, A. Seasonal ecology of littoral epiphytic diatoms on Great Cumbrae Island. Trans. Bot. Soc. Edinb. 1985, 44, 309–322. [Google Scholar] [CrossRef]
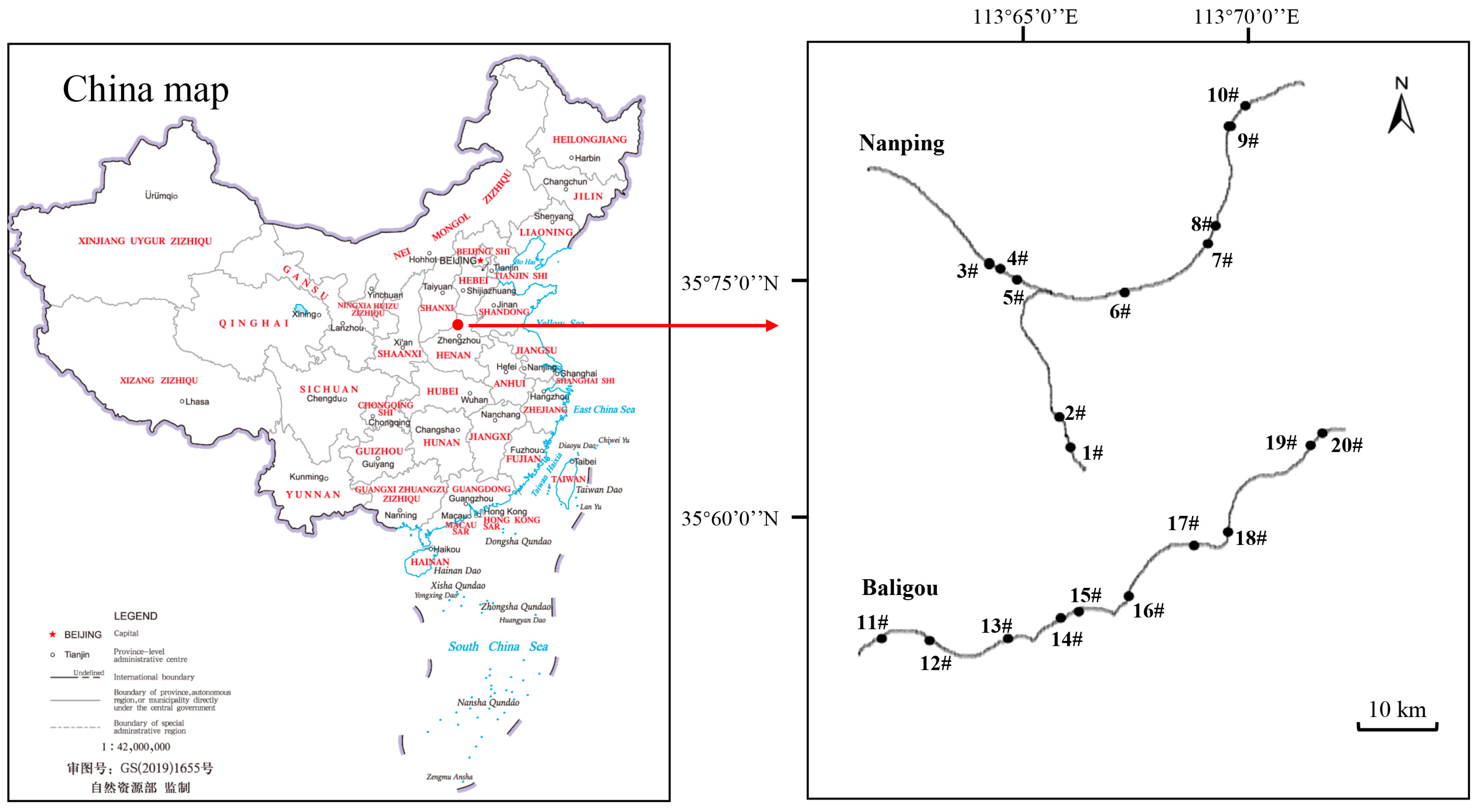




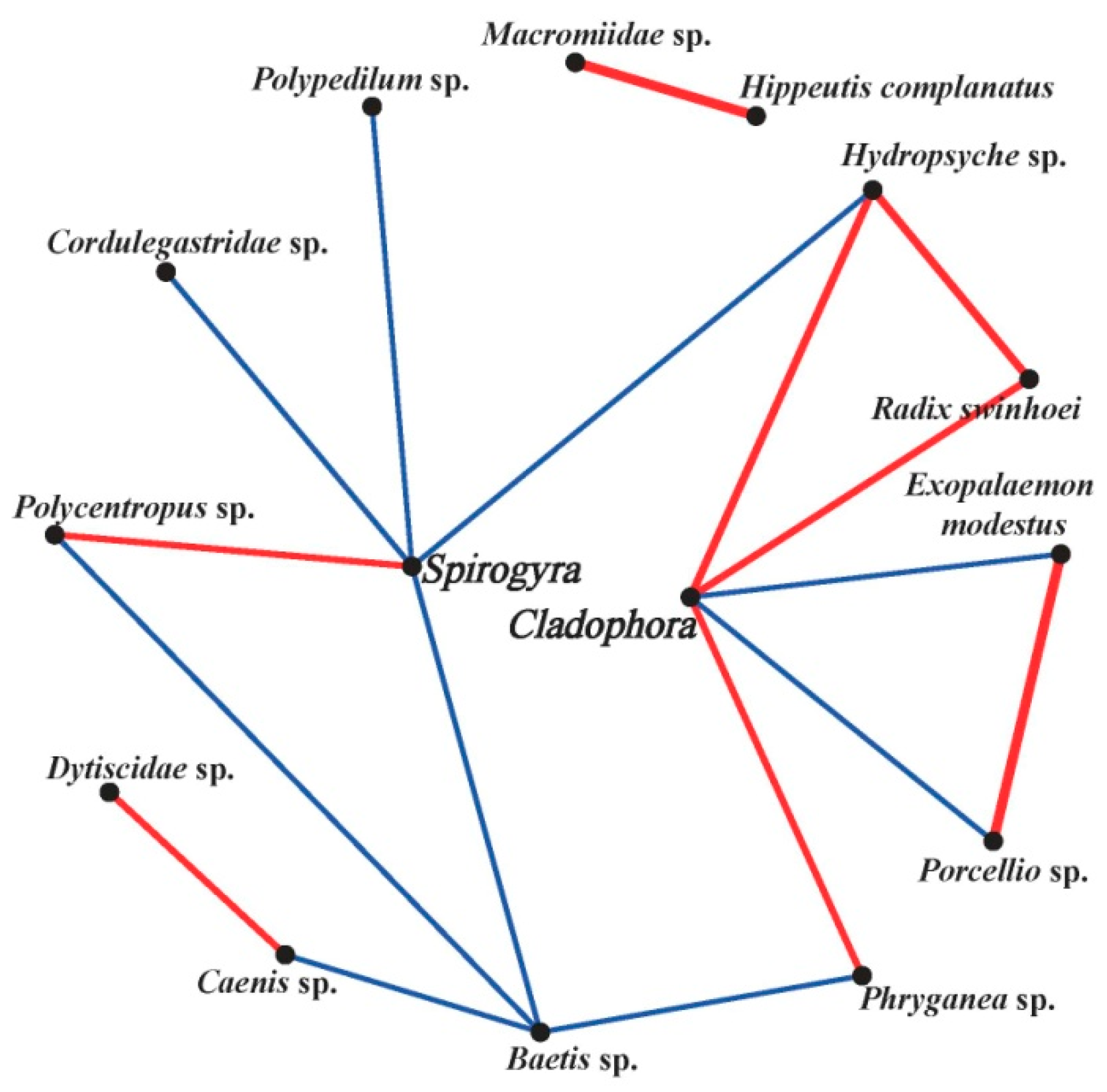
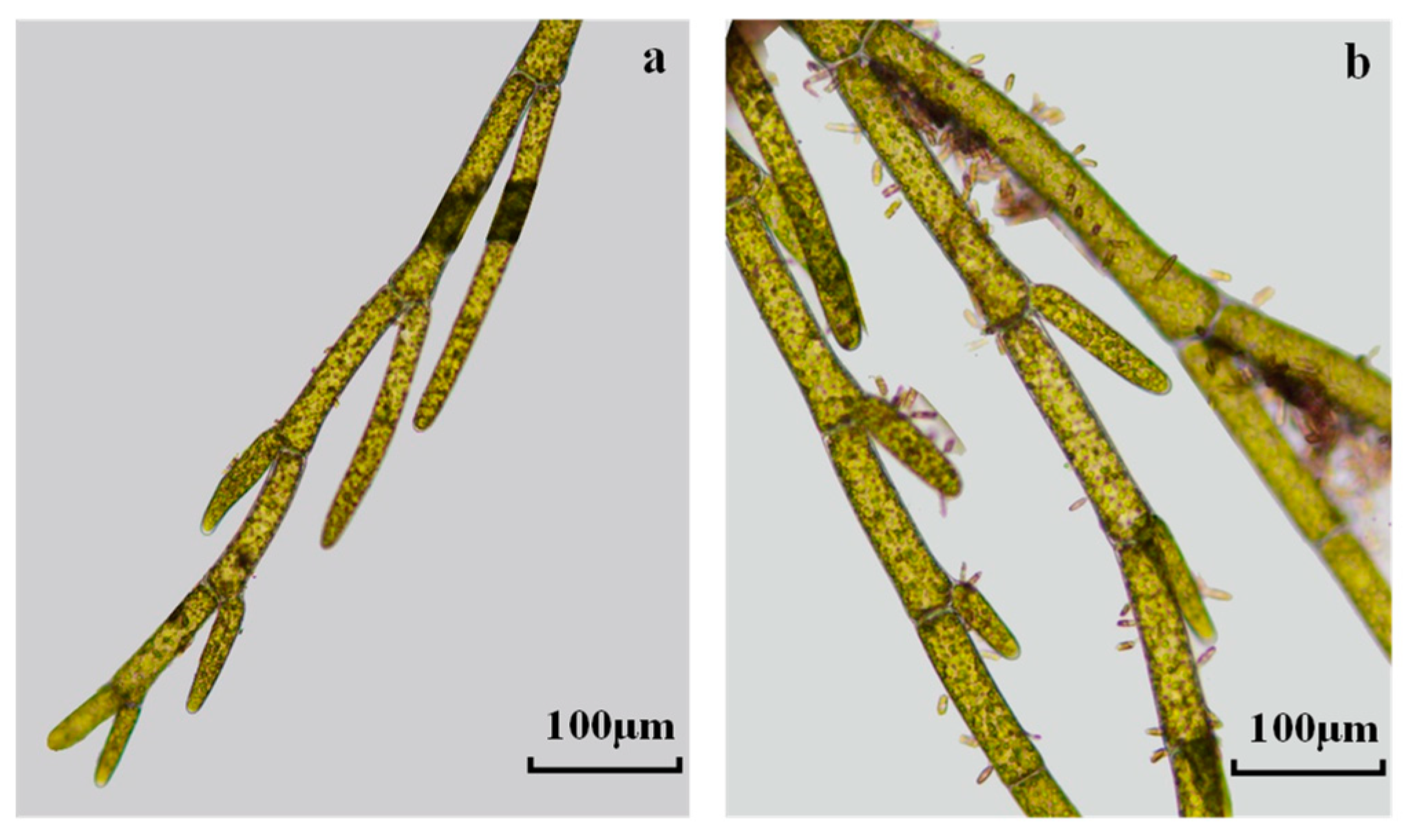
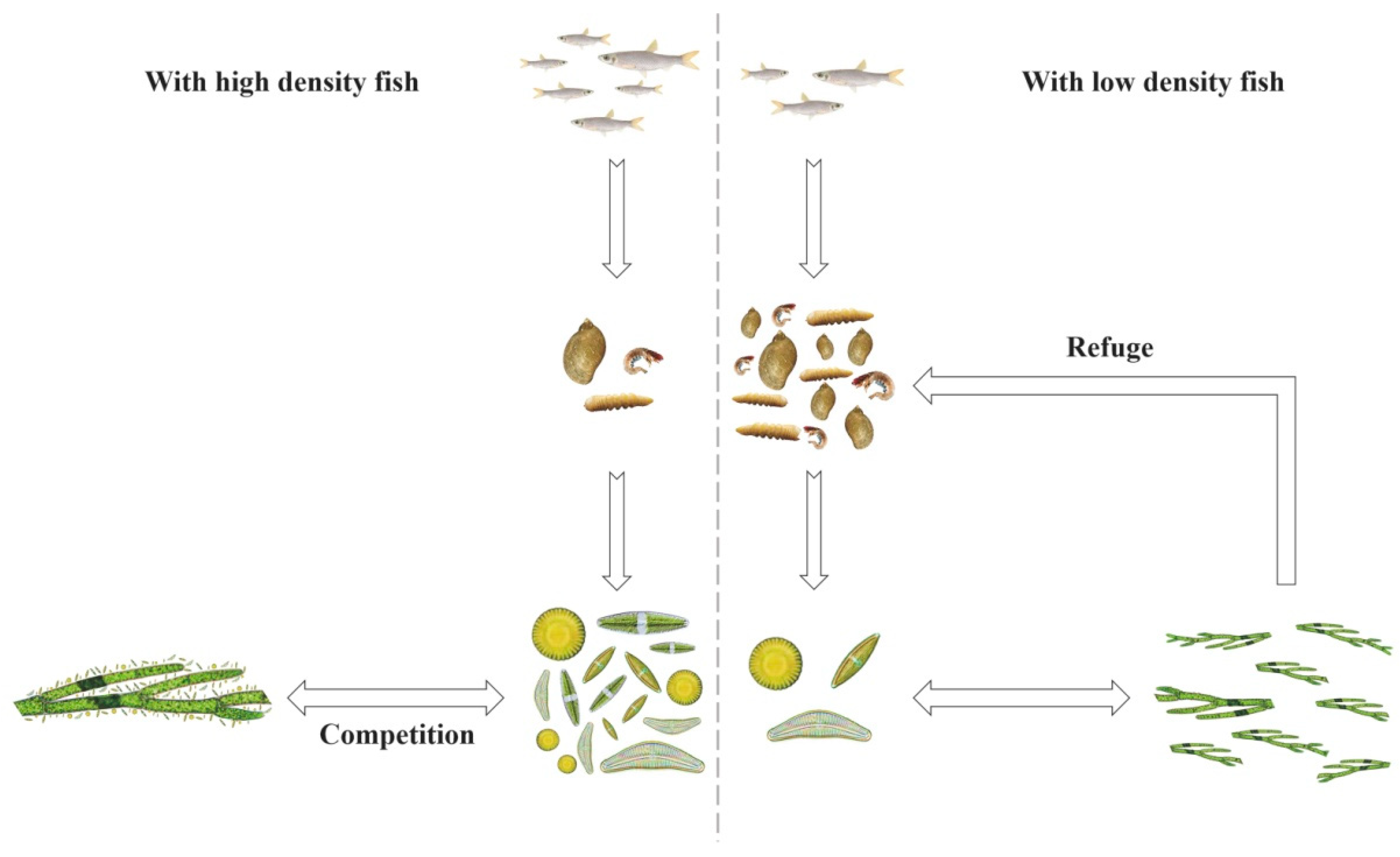
| Classification | Environmental Factors and Biotic Parameters | Nanping | Baligou |
|---|---|---|---|
| Environmental factors | T (°C) | 19.64 ± 3.34 a | 22.82 ± 3.32 b |
| pH | 8.53 ± 0.26 | 8.59 ± 0.25 | |
| DO (mg/L) | 8.95 ± 1.01 | 9.03 ± 1.50 | |
| EC (μs/cm) | 409.86 ± 24.79 | 388.76 ± 27.32 | |
| Hardness (mol/L) | 0.81 ± 0.04 | 0.79 ± 0.12 | |
| V (m/s) | 0.13 ± 0.19 | 0.10 ± 0.16 | |
| Dep (m) | 0.40 ± 0.44 | 0.26 ± 0.24 | |
| Light (μmol/m2·s) | 403.16 ± 493.37 | 641.66 ± 605.37 | |
| Elevation (m) | 718.90 ± 101.61 | 446.20 ± 94.49 | |
| SRP (mg/L) | 0.024 ± 0.06 a | 0.004 ± 0.00 b | |
| DIN (mg/L) | 7.82 ± 3.00 a | 6.60 ± 1.17 b | |
| Fe2+ (mg/L) | 0.11 ± 0.03 | 0.12 ± 0.09 | |
| SiO32− (mg/L) | 216.15 ± 22.69 | 212.84 ± 27.02 | |
| TC (mg/L) | 43.71 ± 5.83 | 40.39 ± 4.66 | |
| IC (mg/L) | 36.64 ± 4.98 | 34.21 ± 3.67 | |
| Filamentous algae biomass (g/m2) | 49.73 ± 70.47 a | 35.95 ± 37.28 b | |
| S. communis biomass (g/m2) | 4.27 ± 16.85 a | 12.48 ± 22.66 b | |
| C. glomerata biomass (g/m2) | 38.88 ± 68.86 a | 11.06 ± 13.46 b | |
| PDA (cells/cm2) | 51897.45 ± 58567.75 | 60180.55 ± 68943.36 | |
| PDB (mg/m2) | 603.01 ± 618.43 | 759.94 ± 918.36 | |
| MBA (cells/m2) | 445.02 ± 514.47 a | 198.65 ± 189.86 b | |
| MBB (g/m2) | 9.35 ± 28.55 a | 0.89 ± 1.29 b | |
| Phoxinus oxycephalus CPUE (g/trap·d) | 2171 ± 871.32 a | 7794 ± 3913.26 b | |
| Total fish CPUE (g/ trap·d) | 3159 ± 340.74 a | 8177 ± 586.73 b |
| Species | Adj-R2 | AIC | BIC | Best Model |
|---|---|---|---|---|
| S. communis biomass | 0.38 | 65.88 | 69.92 | Y = 0.343T + 0.308SRP + 0.249DIN + 0.22Dep − 0.187V + 0.643 |
| C. glomerata biomass | 0.32 | 70.12 | 75.38 | Y = 0.365DIN + 0.322Dep + 0.238MBB + 0.198T + 0.152SRP + 1.782 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Zhang, Y.; Zhang, M.; Lv, X.; Li, Y.; Zhang, J.; Wang, X.; Gao, X.; Zhao, X.; Wang, X. The Distribution and Succession of Filamentous Algae in the Southern Taihang Catchment under Different Nutrient Regimes. Water 2024, 16, 2453. https://doi.org/10.3390/w16172453
Yang B, Zhang Y, Zhang M, Lv X, Li Y, Zhang J, Wang X, Gao X, Zhao X, Wang X. The Distribution and Succession of Filamentous Algae in the Southern Taihang Catchment under Different Nutrient Regimes. Water. 2024; 16(17):2453. https://doi.org/10.3390/w16172453
Chicago/Turabian StyleYang, Bo, Yiguang Zhang, Man Zhang, Xucong Lv, Yuhua Li, Jingxiao Zhang, Xianfeng Wang, Xiaofei Gao, Xueqin Zhao, and Xiufen Wang. 2024. "The Distribution and Succession of Filamentous Algae in the Southern Taihang Catchment under Different Nutrient Regimes" Water 16, no. 17: 2453. https://doi.org/10.3390/w16172453
APA StyleYang, B., Zhang, Y., Zhang, M., Lv, X., Li, Y., Zhang, J., Wang, X., Gao, X., Zhao, X., & Wang, X. (2024). The Distribution and Succession of Filamentous Algae in the Southern Taihang Catchment under Different Nutrient Regimes. Water, 16(17), 2453. https://doi.org/10.3390/w16172453






