Effects of Formulated Pellet Feed or Live Fish Food on the Intestinal and Aquaculture Water Microbial Communities in Goldfish, Carassius auratus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Goldfish and Housing
2.2. Experimental Diets
2.3. Feeding Trials
2.4. Growth Performance and Sample Collection
2.5. DNA Extraction and High-Throughput Sequencing
2.6. Data Processing and Analysis
3. Results
3.1. Growth Performance of Goldfish
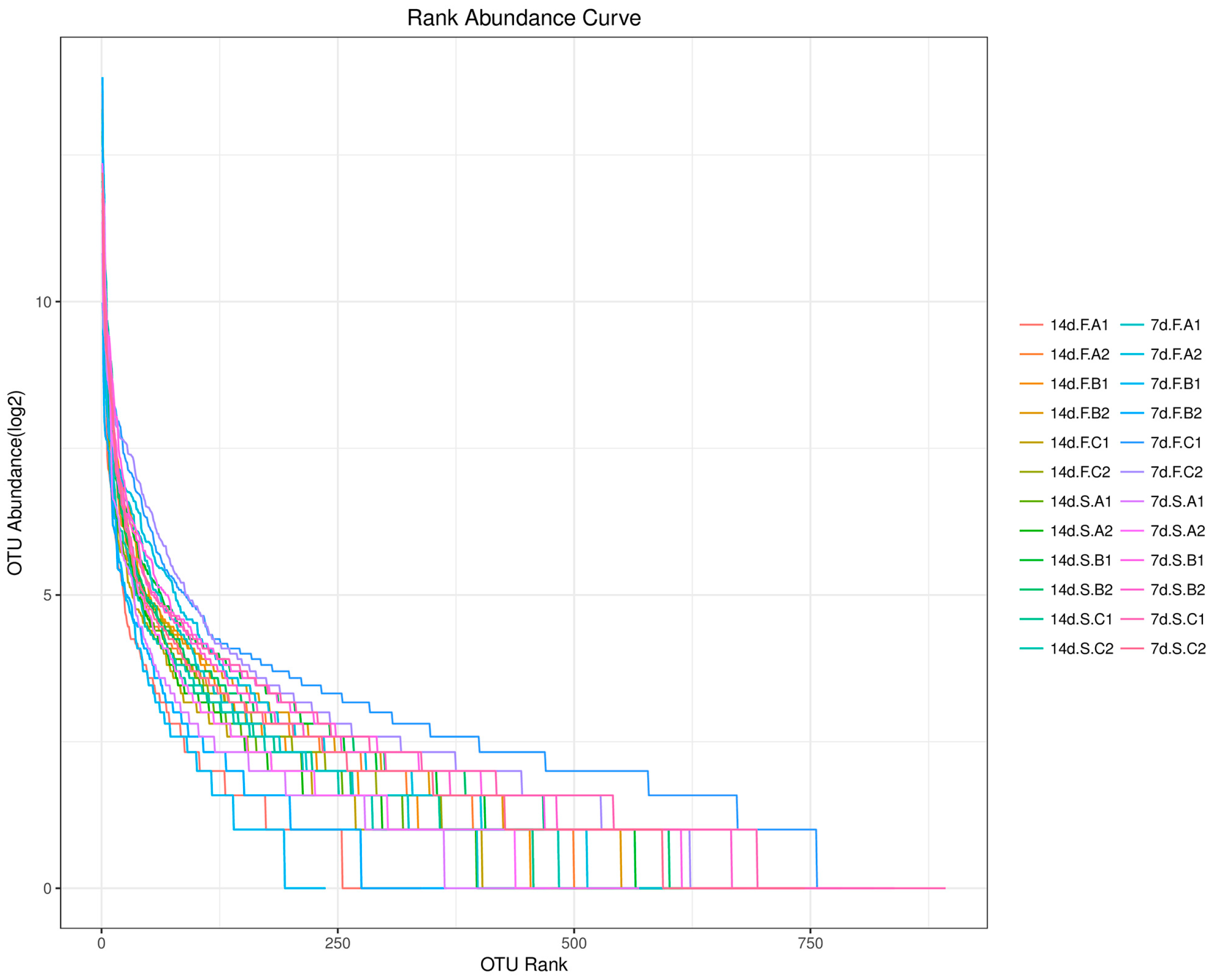
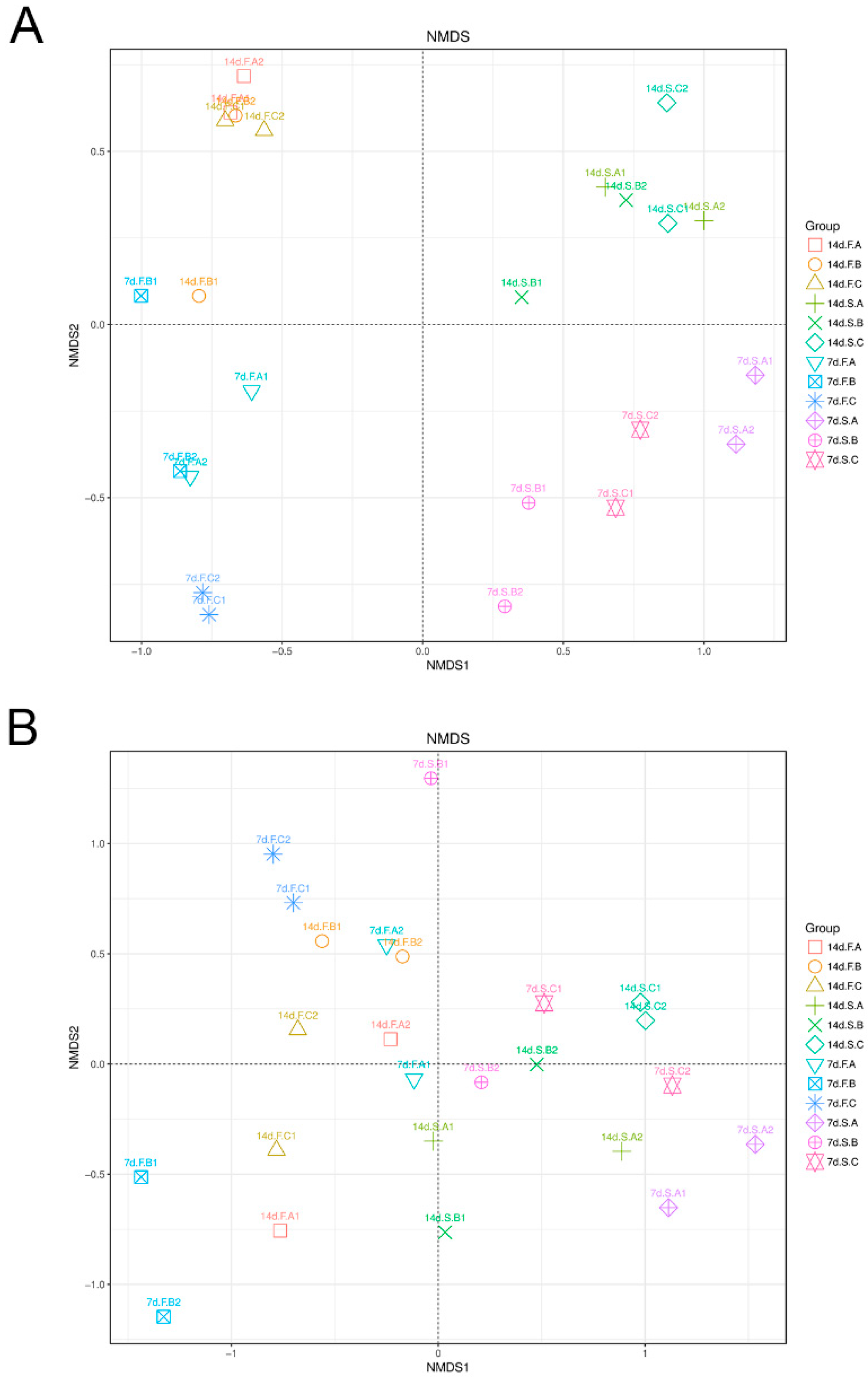
3.2. Impact of Different Feeds on the Bacterial Communities in the Goldfish Intestines and Aquaculture Water
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Banerjee, G.; Ray, A.K. Bacterial symbiosis in the fish gut and its role in health and metabolism. Symbiosis 2017, 72, 1–11. [Google Scholar] [CrossRef]
- Vlková, E.; Kalous, L.; Bunešová, V.; Rylková, K.; Světlíková, R.; Rada, V. Occurrence of bifidobacteria and lactobacilli in digestive tract of some freshwater fishes. Biologia 2012, 67, 411–416. [Google Scholar] [CrossRef]
- Ringø, E.; Harikrishnan, R.; Soltani, M.; Ghosh, K. The Effect of Gut Microbiota and Probiotics on Metabolism in Fish and Shrimp. Animals 2022, 12, 3016. [Google Scholar] [CrossRef] [PubMed]
- Hayatgheib, N.; Moreau, E.; Calvez, S.; Lepelletier, D.; Pouliquen, H. A review of functional feeds and the control of Aeromonas infections in freshwater fish. Aquac. Int. 2020, 28, 1083–1123. [Google Scholar] [CrossRef]
- Nguyen, J.; Lara-Gutiérrez, J.; Stocker, R. Environmental fluctuations and their effects on microbial communities, populations and individuals. FEMS Microbiol. Rev. 2021, 45, fuaa068. [Google Scholar] [CrossRef]
- Yadav, M.; Verma, M.K.; Chauhan, N.S. A review of metabolic potential of human gut microbiome in human nutrition. Arch. Microbiol. 2018, 200, 203–217. [Google Scholar] [CrossRef]
- Ahern, P.; Faith, J.; Gordon, J. Mining the Human Gut Microbiota for Effector Strains that Shape the Immune System. Immunity 2014, 40, 815–823. [Google Scholar] [CrossRef]
- Philipp, E.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar]
- Finotello, F.; Mastrorilli, E.; Di Camillo, B. Measuring the diversity of the human microbiota with targeted next-generation sequencing. Brief. Bioinform. 2018, 19, 679–692. [Google Scholar] [CrossRef]
- Ghanbari, M.; Kneifel, W.; Domig, K.J. A new view of the fish gut microbiome: Advances from next-generation sequencing. Aquaculture 2015, 448, 464–475. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Gasco, L.; Gai, F.; Maricchiolo, G.; Genovese, L.; Ragonese, S.; Bottari, T.; Caruso, G. Fishmeal Alternative Protein Sources for Aquaculture Feeds. In Feeds for the Aquaculture Sector: Current Situation and Alternative Sources; Gasco, L., Gai, F., Maricchiolo, G., Genovese, L., Ragonese, S., Bottari, T., Caruso, G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–28. [Google Scholar]
- Luthada-Raswiswi, R.; Mukaratirwa, S.; O’Brien, G. Animal Protein Sources as a Substitute for Fishmeal in Aquaculture Diets: A Systematic Review and Meta-Analysis. Appl. Sci. 2021, 11, 3854. [Google Scholar] [CrossRef]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.; et al. Nutritional Composition of Black Soldier Fly Larvae (Hermetia illucens L.) and Its Potential Uses as Alternative Protein Sources in Animal Diets: A Review. Insects 2022, 13, 831. [Google Scholar] [CrossRef]
- Melenchón, F.; Larrán, A.M.; de Mercado, E.; Hidalgo, M.C.; Cardenete, G.; Barroso, F.G.; Fabrikov, D.; Lourenço, H.M.; Pessoa, M.F.; Tomás-Almenar, C. Potential use of black soldier fly (Hermetia illucens) and mealworm (Tenebrio molitor) insectmeals in diets for rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2021, 27, 491–505. [Google Scholar] [CrossRef]
- Sealey, W.; Gaylord, G.; Barrows, F.; Tomberlin, J.; McGuire, M.; Ross, C.; St-Hilaire, S. Sensory Analysis of Rainbow Trout, Oncorhynchus mykiss, Fed Enriched Black Soldier Fly Prepupae, Hermetia illucens. J. World Aquac. Soc. 2011, 42, 1–134. [Google Scholar] [CrossRef]
- Mandall, R.N.; Kar, S.; Chakrabarti, P.P.; Chattopadhyay, D.N.; Paul, B.N.; Adhikari, S.; Maity, J.; Pillai, B.R. Production of tubifex—A new dimension of aquaculture in feeding juvenile fish. Aquac. Asia Mag. 2018, 22, 19–22. [Google Scholar]
- Lim, L.C.; Dhert, P.; Sorgeloos, P. Recent developments in the application of live feeds in the freshwater ornamental fish culture. Aquaculture 2003, 227, 319–331. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Maradonna, F.; Faheem, M.; Harikrishnan, R.; Devi, G.; Ringø, E.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Carnevali, O. Sustainable Ornamental Fish Aquaculture: The Implication of Microbial Feed Additives. Animals 2023, 13, 1583. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, P.; Wang, W.; Wen, H.; Zhang, Y. Effects of artificial compound feed and fresh food on growth and immunity of red carp, Carassius auratus. Sci. Fish Farming 2013, 4, 71–72. [Google Scholar]
- Chen, D.; Zhang, Q.; Tang, W.; Huang, Z.; Wang, G.; Wang, Y.; Shi, J.; Xu, H.; Lin, L.; Li, Z.; et al. The evolutionary origin and domestication history of goldfish (Carassius auratus). Proc. Natl. Acad. Sci. USA 2020, 117, 29775–29785. [Google Scholar] [CrossRef]
- Brown, C.; Wolfenden, D.; Sneddon, L. Goldfish (Carassius auratus). In Companion Animal Care and Welfare; Wiley: Hoboken, NJ, USA, 2018; pp. 467–478. [Google Scholar]
- The Ministry of Agriculture of the People’s Republic of China. Determination of moisture in feedstuffs. In PRC National Standard; China Standards Press: Beijing, China, 2014. [Google Scholar]
- The Ministry of Agriculture of the People’s Republic of China. Determination of crude protein in feeds-Kjeldahl method. In PRC National Standard; China Standards Press: Beijing, China, 2018. [Google Scholar]
- Kirk, P.L. Kjeldahl method for total nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- The Ministry of Agriculture of the People’s Republic of China. Determination of crude fat in feeds. In PRC National Standard; China Standards Press: Beijing, China, 2006. [Google Scholar]
- Luque de Castro, M.D.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Qin, J.G. A single phase of food deprivation provoked compensatory growth in barramundi Lates calcarifer. Aquaculture 2003, 224, 169–179. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, B.; Meng, L.-J.; Gao, J.-Z.; Chen, Z.-Z. Dynamic changes of gut microbiota of discus fish (Symphysodon haraldi) at different feeding stages. Aquaculture 2021, 531, 735912. [Google Scholar] [CrossRef]
- McCormick, M.I.; Molony, B.W. Effects of feeding history on the growth characteristics of a reef fish at settlement. Mar. Biol. 1992, 114, 165–173. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Liang, H.; Ye, L.; Lan, L.; Lu, F.; Wang, Q.; Lei, T.; Yang, X.; Cui, P.; et al. Differences in Alpha Diversity of Gut Microbiota in Neurological Diseases. Front. Neurosci. 2022, 16, 879318. [Google Scholar] [CrossRef]
- White, J.R.; Nagarajan, N.; Pop, M. Statistical Methods for Detecting Differentially Abundant Features in Clinical Metagenomic Samples. PLoS Comput. Biol. 2009, 5, e1000352. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, Q.; Xie, S.; Hu, W.; Yu, Y.; Hu, Z. Gut Microbiota Contributes to the Growth of Fast-Growing Transgenic Common Carp (Cyprinus carpio L.). PLoS ONE 2013, 8, e64577. [Google Scholar] [CrossRef]
- Marchesi, J.; Adams, D.; Fava, F.; Hermes, G.; Hirschfield, G.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2015, 65, 330. [Google Scholar] [CrossRef]
- Hamlin, H.; Hunt von Herbing, I.; Kling, L.J. Histological and morphological evaluations of digestive tract and associated organs of haddock throughout post-hatching ontogeny. J. Fish Biol. 2005, 57, 716–732. [Google Scholar]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota Regulate Intestinal Absorption and Metabolism of Fatty Acids in the Zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, J.; C Peterson, B.; Swanson, K.; Small, B. Ontogenetic Characterization of the Intestinal Microbiota of Channel Catfish through 16S rRNA Gene Sequencing Reveals Insights on Temporal Shifts and the Influence of Environmental Microbes. PLoS ONE 2016, 11, e0166379. [Google Scholar] [CrossRef] [PubMed]
- Gang, Y.; Qing, J.S.; Hongzhong, C.; Chungen, W.; Baoqing, H.; Mo, P.; Liusheng, P.; Jianguo, Y.; Lifeng, L. Changes in microbiota along the intestine of grass carp (Ctenopharyngodon idella): Community, interspecific interactions, and functions. Aquaculture 2019, 498, 151–161. [Google Scholar]
- Ghanbari, M.; Shahraki, H.; Kneifel, W.; Domig, K. A first insight into the intestinal microbiota of snow trout (Schizothorax zarudnyi). Symbiosis 2017, 72, 183–193. [Google Scholar] [CrossRef]
- Carda Diéguez, M.; Mira, A.; Fouz, B. Pyrosequencing survey of intestinal microbiota diversity in cultured sea bass (Dicentrarchus labrax) fed functional diets. FEMS Microbiol. Ecol. 2014, 87, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chen, C.; Jia, L.; He, X.; Zhang, B. Comparison of the intestinal microbiota composition and function in healthy and diseased Yunlong Grouper. AMB Express 2019, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Lahav, O.; Massada, I.B.; Yackoubov, D.; Zelikson, R.; Mozes, N.; Tal, Y.; Tarre, S. Quantification of anammox activity in a denitrification reactor for a recirculating aquaculture system. Aquaculture 2009, 288, 76–82. [Google Scholar] [CrossRef]
- Butbunchu, N.; Pathom-Aree, W. Actinobacteria as Promising Candidate for Polylactic Acid Type Bioplastic Degradation. Front. Microbiol. 2019, 10, 2834. [Google Scholar] [CrossRef]
- Kim, P.S.; Shin, N.-R.; Lee, J.-B.; Kim, M.-S.; Whon, T.W.; Hyun, D.-W.; Yun, J.-H.; Jung, M.-J.; Kim, J.Y.; Bae, J.-W. Host habitat is the major determinant of the gut microbiome of fish. Microbiome 2021, 9, 166. [Google Scholar] [CrossRef]
- Cottrell, M.T.; Kirchman, D.L. Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl. Environ. Microbiol. 2000, 66, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Sandi, W.; Thomas, W.; Steven, S.; John, D.; Frederic, B.; P Brett, K.; Timothy, W.; Wiens, G.D.; Kevin, S.; Rawls, J.F. Aquacultured rainbow trout (Oncorhynchus mykiss) possess a large core intestinal microbiota that is resistant to variation in diet and rearing density. Appl. Environ. Microbiol. 2013, 79, 4974–4984. [Google Scholar]
- Pérez-Pascual, D.; Estellé, J.; Dutto, G.; Rodde, C.; Bernardet, J.-F.; Marchand, Y.; Duchaud, E.; Przybyla, C.; Ghigo, J.-M. Growth Performance and Adaptability of European Sea Bass (Dicentrarchus labrax) Gut Microbiota to Alternative Diets Free of Fish Products. Microorganisms 2020, 8, 1346. [Google Scholar] [CrossRef] [PubMed]


| Group | Time | Numbers of Taxon with Significant Difference | |
|---|---|---|---|
| Phylum | Genus | ||
| FA vs. FB | 7 d | 3 | 22 |
| FA vs. FC | 4 | 21 | |
| SA vs. SB | 5 | 16 | |
| SA vs. SC | 2 | 9 | |
| FA vs. FB | 14 d | 0 | 10 |
| FA vs. FC | 1 | 6 | |
| SA vs. SB | 0 | 9 | |
| SA vs. SC | 0 | 6 | |
| Group | Time | Simpson | Chao1 | ACE | Shannon |
|---|---|---|---|---|---|
| FA | 7 d | 0.83 ± 0.09 a | 568.55 ± 61.05 ab | 576.86 ± 64.82 ab | 5.13 ± 0.69 abc |
| FB | 0.60 ± 0.19 a | 336.54 ± 82.34 a | 349.43 ± 84.35 a | 2.75 ± 0.47 a | |
| FC | 0.98 ± 0.00 a | 774.56 ± 63.98 b | 776.05 ± 61.74 b | 7.28 ± 0.20 c | |
| FA | 14 d | 0.74 ± 0.16 a | 525.55 ± 145.04 ab | 542.32 ± 138.20 ab | 3.90 ± 1.22 ab |
| FB | 0.92 ± 0.01 a | 678.61 ± 75.11 ab | 696.85 ± 73.33 b | 5.49 ± 0.15 bc | |
| FC | 0.78 ± 0.09 a | 583.06 ± 112.24 ab | 593.15 ± 104.88 ab | 4.23 ± 0.66 ab |
| Group | Time | Simpson | Chao1 | ACE | Shannon |
|---|---|---|---|---|---|
| SA | 7 d | 0.88 ± 0.00 a | 592.18 ± 40.40 a | 605.80 ± 55.92 a | 4.53 ± 0.24 a |
| SB | 0.95 ± 0.01 c | 899.11 ± 19.88 b | 923.45 ± 25.70 b | 6.25 ± 0.23 b | |
| SC | 0.92 ± 0.02 abc | 917.24 ± 105.83 b | 935.57 ± 93.58 b | 5.80 ± 0.45 b | |
| SA | 14 d | 0.81 ± 0.01 d | 540.69 ± 103.69 a | 550.98 ± 113.98 a | 4.28 ± 0.09 a |
| SB | 0.93 ± 0.01 bc | 757.05 ± 55.57 ab | 777.58 ± 44.07 ab | 5.80 ± 0.02 b | |
| SC | 0.90 ± 0.01 ab | 623.58 ± 119.62 ab | 625.90 ± 114.36 a | 4.90 ± 0.19 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Huang, Q.; Huang, Z.; Hong, Y. Effects of Formulated Pellet Feed or Live Fish Food on the Intestinal and Aquaculture Water Microbial Communities in Goldfish, Carassius auratus. Water 2024, 16, 2486. https://doi.org/10.3390/w16172486
Huang Y, Huang Q, Huang Z, Hong Y. Effects of Formulated Pellet Feed or Live Fish Food on the Intestinal and Aquaculture Water Microbial Communities in Goldfish, Carassius auratus. Water. 2024; 16(17):2486. https://doi.org/10.3390/w16172486
Chicago/Turabian StyleHuang, Yi, Qiang Huang, Zhiqiu Huang, and Yuhang Hong. 2024. "Effects of Formulated Pellet Feed or Live Fish Food on the Intestinal and Aquaculture Water Microbial Communities in Goldfish, Carassius auratus" Water 16, no. 17: 2486. https://doi.org/10.3390/w16172486




