Water Storage–Discharge Relationship with Water Quality Parameters of Carhuacocha and Vichecocha Lagoons in the Peruvian Puna Highlands
Abstract
1. Introduction
2. Materials and Methods
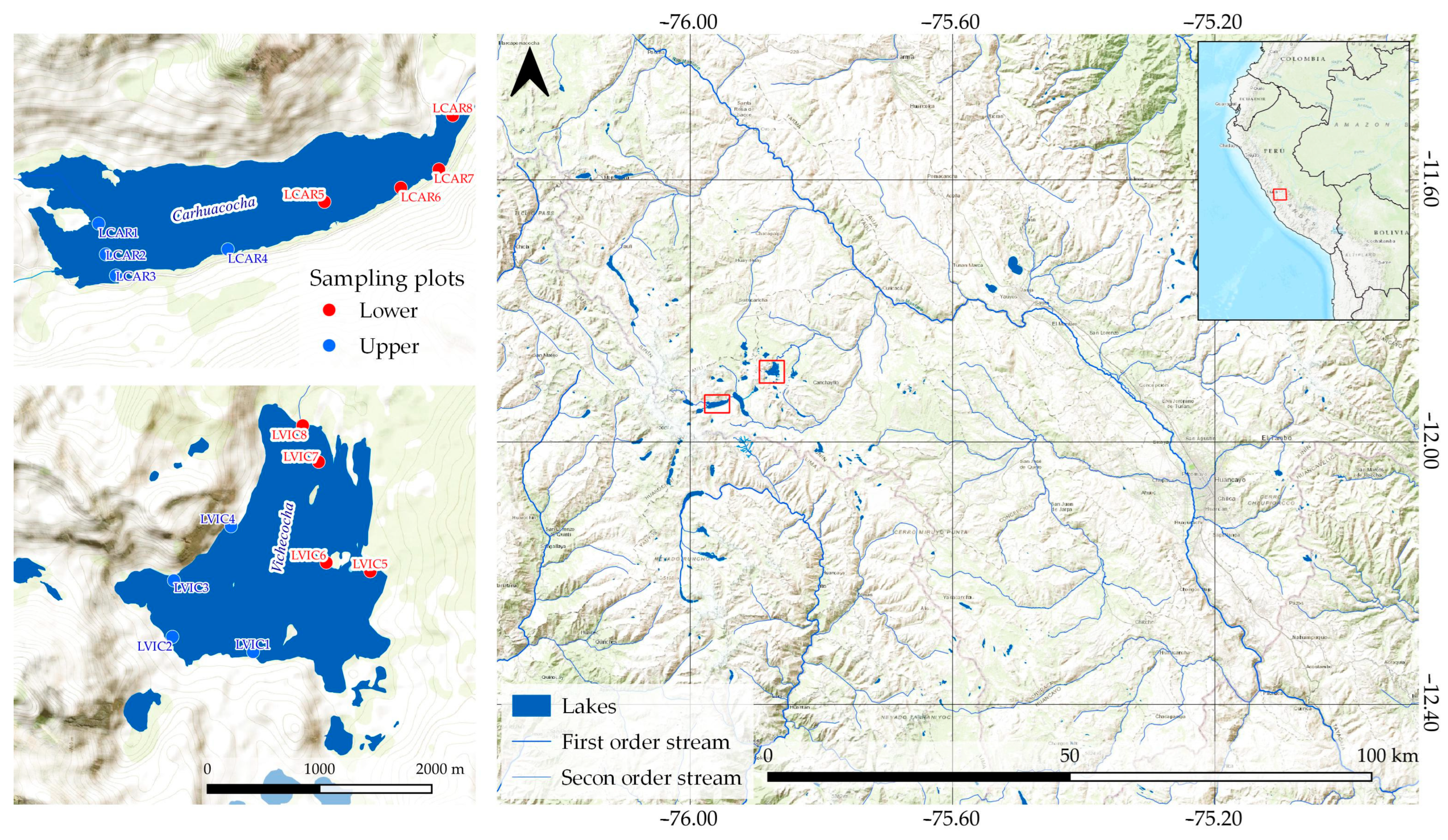
2.1. Study Area
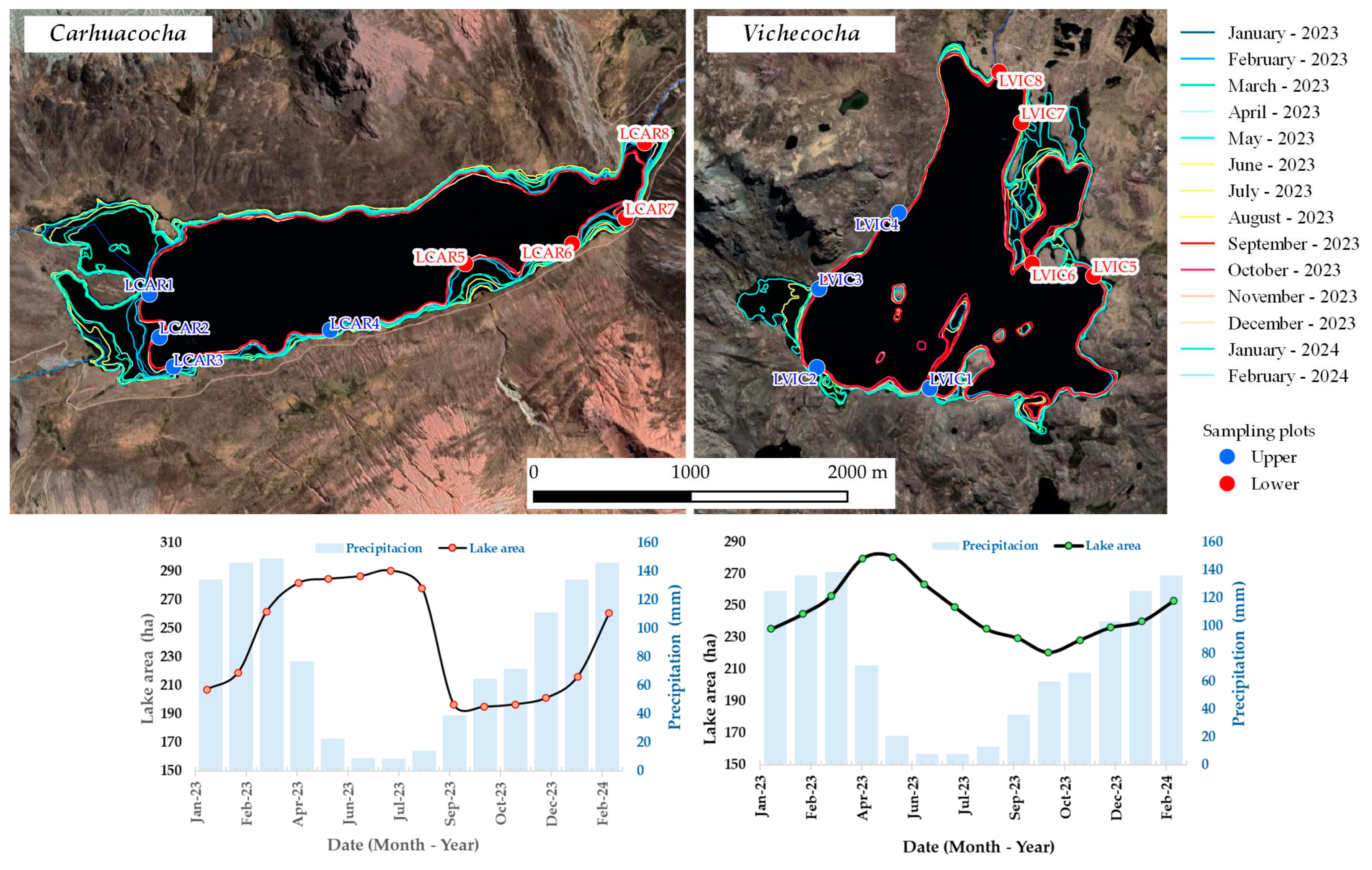
2.2. Water Extension
2.3. Sampling and Analytical Procedure
2.4. Data Analysis
Water Quality Assessment
3. Results
3.1. Spatial Variability of Water Parameters Content
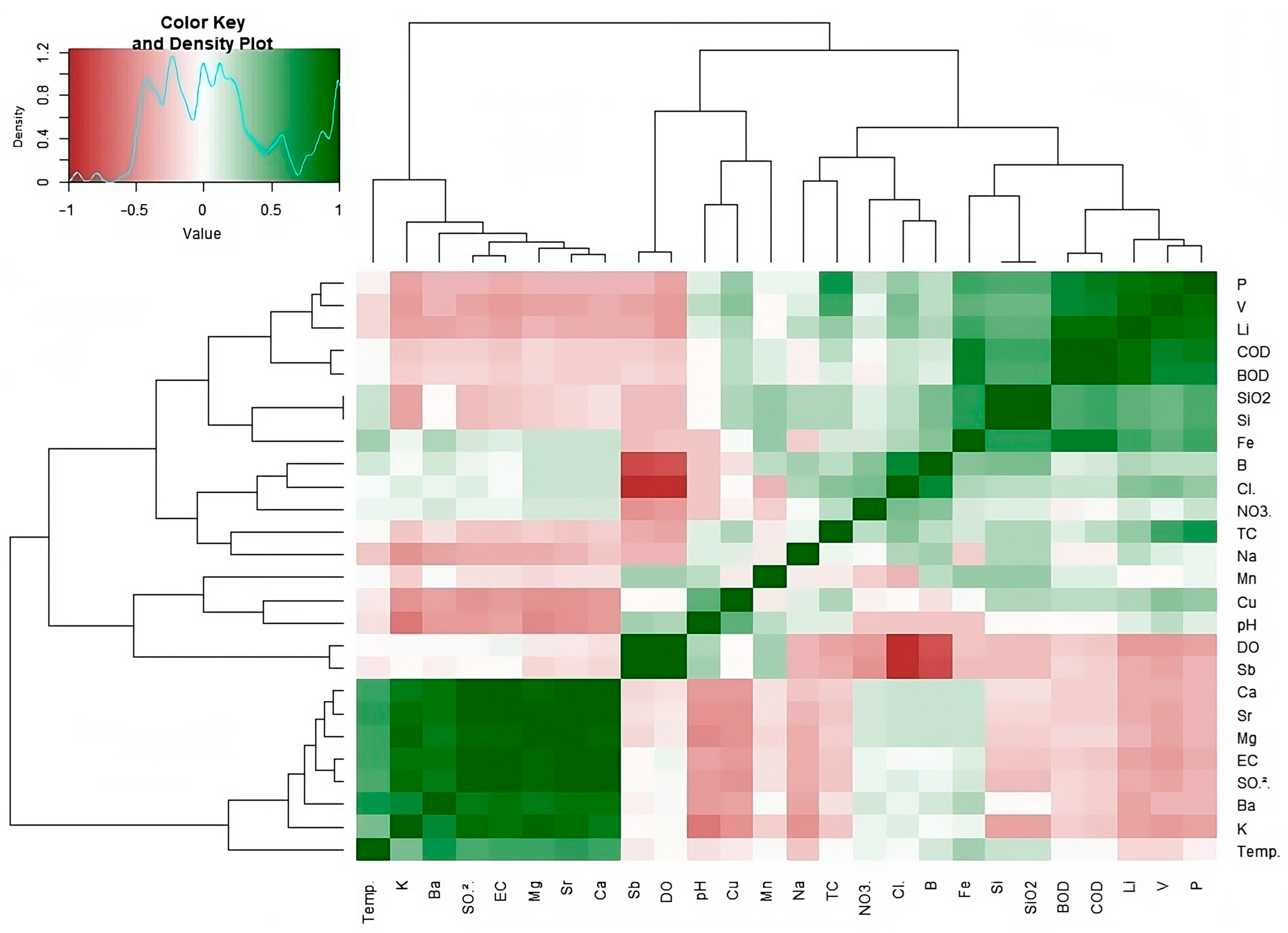
3.2. Correlation Analysis
3.3. Water Quality
3.4. Analysis of Main Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riveros, J.C.; Germaná, C.; Álvarez, C. Un Frágil Ciclo—Agua, Energía y Población En Lima; WWF: Lima, Perú, 2014; p. 68. [Google Scholar]
- Glavovic, B.C.; Smith, G.P. Adapting to Climate Change: Lessons from Natural Hazards Planning; Springer Science: Berlin/Heidelberg, Germany, 2014; ISBN 9789401786300. [Google Scholar]
- Hommes, L.; Boelens, R. Urbanizing Rural Waters: Rural-Urban Water Transfers and the Reconfiguration of Hydrosocial Territories in Lima. Polit. Geogr. 2017, 57, 71–80. [Google Scholar] [CrossRef]
- Silva, Y.; Takahashi, K.; Cruz, N.; Trasmonte Soto, G.L.; Mosquera, K.; Nickl, E.; Chavez, R.; Segura, B.; Lagos, P. Variability and Climate Change in the Mantaro River Basin, Central Peruvian Andes. In Proceedings of the International Conference on Southern Hemisphere Meteorology and Oceanography (ICSHMO), Foz do Iguacu, Brazil, 24–28 April 2006; pp. 407–419. [Google Scholar]
- Callañaupa Gutierrez, S.; Segura Cajachagua, H.; Saavedra Huanca, M.; Flores Rojas, J.; Silva Vidal, Y.; Cuxart, J. Seasonal Variability of Daily Evapotranspiration and Energy Fluxes in the Central Andes of Peru Using Eddy Covariance Techniques and Empirical Methods. Atmos. Res. 2021, 261, 105760. [Google Scholar] [CrossRef]
- Behmel, S.; Damour, M.; Ludwig, R.; Rodriguez, M.J. Water Quality Monitoring Strategies—A Review and Future Perspectives. Sci. Total Environ. 2016, 571, 1312–1329. [Google Scholar] [CrossRef] [PubMed]
- Custodio, M.; Peñaloza, R.; Chanamé, F.; Yaranga, R.; Pantoja, R. Assessment of the Aquatic Environment Quality of High Andean Lagoons Using Multivariate Statistical Methods in Two Contrasting Climatic Periods. J. Ecol. Eng. 2018, 19, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Ministerio del Ambiente [MINAM]. Estandares de Calidad Ambiental (ECA) Para Aguas; El Peruano Official Newspaper, 6–9: Lima, Peru, 2017; pp. 10–19. [Google Scholar]
- Autoridad Nacional del Agua (ANA). Aprueban La Clasificación de Los Cuerpos de Agua Continentales Superficiales; El Peruano Official Newspaper, 9: Lima, Peru, 2018; Volume 21, pp. 10–13. [Google Scholar]
- Panhwar, M.Y.; Panhwar, S.; Keerio, H.A.; Khokhar, N.H.; Shah, S.A.; Pathan, N. Water Quality Analysis of Old and New Phuleli Canal for Irrigation Purpose in the Vicinity of Hyderabad, Pakistan. Water Pract. Technol. 2022, 17, 529–536. [Google Scholar] [CrossRef]
- ELECTROPERU. Memoria Descriptiva Infraestructura Hidráulica Operada Por ELECTROPERU S.A. Cuenca Rio Mantaro; ELECTROPERU: Lima, Peru, 2015. [Google Scholar]
- Custodio, M.; Peñaloza, R.; Alvarado, J.; Chanamé, F.; Maldonado, E. Surface Water Quality in the Mantaro River Watershed Assessed after the Cessation of Anthropogenic Activities Due to the Covid-19 Pandemic. Polish J. Environ. Stud. 2021, 30, 3005–3018. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-Scale Geospatial Analysis for Everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- McFeeters, S.K. The Use of the Normalized Difference Water Index (NDWI) in the Delineation of Open Water Features. Int. J. Remote Sens. 1996, 17, 1425–1432. [Google Scholar] [CrossRef]
- Peñaloza, R.; Custodio, M.; Cacciuttolo, C.; Chanamé, F.; Cano, D.; Solorzano, F. Human Health Risk Assessment for Exposure to Heavy Metals via Dietary Intake of Rainbow Trout in the Influence Area of a Smelting Facility Located in Peru. Toxics 2023, 11, 764. [Google Scholar] [CrossRef]
- Lipps, W.C.; Braun-Howland, E.B.; Baxter, T.E. Standard Methods for the Examination of Water and Wastewater, 24th ed.; Association, A.P.H., Ed.; American Public Health Association: Wahington, DC, USA, 2023. [Google Scholar]
- Kaiser, H.F. Analysis of Factorial Simplicity. Psychometrika 1974, 39, 31–36. [Google Scholar] [CrossRef]
- Canadian Council of Ministers of the Environment. Canadian Water Quality Guidelines for the Protection of Aquatic Life: CCME Water Quality Index 1.0; Technical Report; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2001; pp. 1–13. [Google Scholar]
- Xiao, J.; Lv, G.; Chai, N.; Hu, J.; Jin, Z. Hydrochemistry and Source Apportionment of Boron, Sulfate, and Nitrate in the Fen River, a Typical Loess Covered Area in the Eastern Chinese Loess Plateau. Environ. Res. 2022, 206, 112570. [Google Scholar] [CrossRef] [PubMed]
- Trach, R.; Trach, Y.; Kiersnowska, A.; Markiewicz, A.; Lendo-Siwicka, M.; Rusakov, K. A Study of Assessment and Prediction of Water Quality Index Using Fuzzy Logic and ANN Models. Sustainability 2022, 14, 5656. [Google Scholar] [CrossRef]
- MINAM. Environmental Quality Standards (EQS) for Water: DS N° 004-2017-MINAM; El Peruano. Off. Newsp. 6: Lima, Peru, 2017. [Google Scholar]
- Meng, Z.; Yang, Y.; Qin, Z.; Huang, L. Evaluating Temporal and Spatial Variation in Nitrogen Sources along the Lower Reach of Fenhe River (Shanxi Province, China) Using Stable Isotope and Hydrochemical Tracers. Water 2018, 10, 231. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, S.; Lu, W.; Yang, W.; Li, S. Water Quality Assessment, Possible Origins and Health Risks of Toxic Metal(Loid)s in Five Cascade Reservoirs in the Upper Mekong. J. Clean. Prod. 2024, 441, 141049. [Google Scholar] [CrossRef]
- Custodio, M.; Chanamé, F.; Pizarro, S.; Cruz, D. Quality of the Aquatic Environment and Diversity of Benthic Macroinvertebrates of High Andean Wetlands of the Junín Region, Peru. Egypt. J. Aquat. Res. 2018, 44, 195–202. [Google Scholar] [CrossRef]
- Arenas-Sánchez, A.; Dolédec, S.; Vighi, M.; Rico, A. Effects of Anthropogenic Pollution and Hydrological Variation on Macroinvertebrates in Mediterranean Rivers: A Case-Study in the Upper Tagus River Basin (Spain). Sci. Total Environ. 2021, 766, 144044. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, G.; Zhang, Q.; Zhang, Z. Multi-Scale Analysis of Relationship between Landscape Pattern and Urban River Water Quality in Different Seasons. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- de Mello, K.; Taniwaki, R.H.; de Paula, F.R.; Valente, R.A.; Randhir, T.O.; Macedo, D.R.; Leal, C.G.; Rodrigues, C.B.; Hughes, R.M. Multiscale Land Use Impacts on Water Quality: Assessment, Planning, and Future Perspectives in Brazil. J. Environ. Manag. 2020, 270, 110879. [Google Scholar] [CrossRef]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Xiang, R.; Wang, L.; Li, H.; Tian, Z.; Zheng, B. Water Quality Variation in Tributaries of the Three Gorges Reservoir from 2000 to 2015. Water Res. 2021, 195, 116993. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Qi, W. Assessing the Water Quality in Urban River Considering the Influence of Rainstorm Flood: A Case Study of Handan City, China. Ecol. Indic. 2024, 160, 111941. [Google Scholar] [CrossRef]
- Mohamed, H.M.; Khalil, M.T.; El-Zeiny, A.M.; Khalifa, N.; Kafrawy, S.B.E.; Emam, W.W.M. Trophic State and Potential Productivity Assessment for Qaroun Lake Using Spatial Techniques. Environ. Monit. Assess. 2023, 195, 1–13. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Used Instrumentation and Methods/Solutions | Accuracy (Sensitivity) | Range Test |
| pH | HANNA ® HI98129 | ±0.05 | 0–14 |
| Conductivity (EC, µS/cm) | ±1 | 0–3999 | |
| Temperature (T, °C) | ±0.5 | 0.0–60.0 | |
| Dissolved oxygen (DO, mg/L) | HANNA ® HI9146−10, | ±0.06 | 0.00–45.00 |
| Chemical oxygen demand (COD, mg/L) | Chemical Oxygen Demand, Closed Reflux Colorimetric Method SMEWW−APHA−AWWA–WEF Part 5220 D. 23 rd. 2017. | ±0.4 | 0–2 |
| Biochemical oxygen demand (BOD5, mg/L) | Biochemical Oxygen Demand (BOD). 5−Day BOD Test SMEWW−APHA−AWWA–WEF Part 5210B. 24th Ed. 2022. | ±2 | 0–5 |
| Thermotolerant coliforms (TC, NMP/100 mL) | Multiple-Tube defermentation Technique for Members of the Coliform Group. E. coli Procedure Using Fluorogenic Substrate. Simultaneous Determination of Thermotolerant Coliforms and E. coli. | ±1.8 | −− |
| Chloride (Cl−, mg/L) | Determination of inorganic anions by ion chromatography Environmental Protection Agency. Methods for Chemicals Analysis (EPA) | ||
| Nitrate (NO3−, mg/L) | ±0.02 | 0.02–0.300 | |
| Sulfate (SO42−, mg/L) | ±0.2 | 2–70 | |
| Phosphorus (P, mg/L) | Ascorbic Acid Method SMEWW−APHA−AWWA–WEF Part 4500–P B (Item 5) y E, 24th Ed. 2023. | ±0.002 | |
| Aluminum (Al, mg/L) | Determination of trace Elements in Water and Waste Inductively Coupled Plasma–Mass spectrometry. Method 200.8 Revision 5.4 1994 | ±0.001 | |
| Bario (Ba, mg/L) | ±0.00008 | ||
| Boron (B, mg/L) | ±0.0003 | ||
| Calcium (Ca, mg/L) | ±0.001 | ||
| Copper (Cu, mg/L) | ±0.0001 | ||
| Strontium (Sr, mg/L) | ±0.00002 | ||
| Iron (Fe, mg/L) | ±0.001 | ||
| Lithium (Li, mg/L) | ±0.00003 | ||
| Magnesium (Mg, mg/L) | ±0.0006 | ||
| Manganese (Mn, mg/L) | ±0.00002 | ||
| Potassium (K, mg/L) | ±0.003 | ||
| Silica (SiO2, mg/L) | ±0.001 | ||
| Silicon (Si, mg/L) | ±0.0002 | ||
| Sodium (Na, mg/L) | ±0.0003 | ||
| Vanadium (V, mg/L) | ±0.0001 |
| Parameter | Carhuacocha | Vichecocha | EQS Water—Conservation of the Aquatic Environment | ||||||
| Storage | Discharge | Storage | Discharge | ||||||
| Upper | Low | Upper | Low | Upper | Low | Upper | Low | ||
| pH | 8.3500 ± 0.3836 a | 8.5225 ± 0.2405 a | 8.1250 ± 0.0954 b | 8.1900 ± 0.1227 ab | 8.6575 ± 0.2061 a | 9.0925 ± 0.2965 a | 8.7075 ± 0.3917 a | 8.6825 ± 0.5789 a | 6.5–9.0 |
| Temperature (°C) | 11.8500 ± 2.1825 a | 13.5250 ± 2.4446 a | 13.9250 ± 1.8998 a | 14.0250 ± 0.7500 a | 11.6250 ± 1.5218 a | 12.5000 ± 0.5354 b | 12.3250 ± 0.6850 b | 10.5000 ± 0.4967 ab | ∆ 3 |
| DO (mg/L) | 7.6775 ± 0.1420 a | 7.9125 ± 0.3836 a | 2.2525 ± 0.1335 b | 2.2225 ± 0.4665 b | 7.7075 ± 0.2317 a | 8.1050 ± 0.3743 a | 1.6400 ± 0.3222 b | 1.4700 ± 0.2082 b | ≥5 |
| EC (µS/cm) | 195.2900 ± 78.5408 a | 249.0000 ± 3.1623 a | 235.0000 ± 36.3043 a | 236.5000 ± 8.3865 a | 113.7500 ± 3.5940 a | 118.0000 ± 10.9545 a | 102.0000 ± 9.3452 b | 106.7500 ± 1.8930 b | 1000 |
| BOD (mg/L) | 4.9900 ± 0.0000 a | 4.9900 ± 0.0000 a | 4.9900 ± 0.0000 a | 4.9900 ± 0.0000 a | 4.9900 ± 0.0000 a | 4.9900 ± 0.0000 b | 5.6950 ± 1.1530 a | 4.9900 ± 0.0000 a | 5 |
| COD (mg/L) | 1.9900 ± 0.0000 a | 1.9900 ± 0.0000 a | 1.9900 ± 0.0000 a | 1.9900 ± 0.0000 a | 1.9900 ± 0.0000 a | 1.9900 ± 0.0000 a | 2.2675 ± 0.5550 a | 1.9900 ± 0.0000 a | 40 |
| TC(NMP/100 mL) | 2.4750 ± 1.3500 ab | 1.7900 ± 0.0000 ab | 2.4675 ± 1.3550 a | 2.4675 ± 1.3550 b | 1.7900 ± 0.0000 a | 1.7900 ± 0.0000 b | 8.2750 ± 3.5132 a | 2.4675 ± 1.3550 a | 1000 |
| P (mg/L) | 0.0059 ± 0.0000 a | 0.0059 ± 0.0000 a | 0.0059 ± 0.0000 ab | 0.0059 ± 0.0000 b | 0.0059 ± 0.0000 a | 0.0059 ± 0.0000 b | 0.0220 ± 0.0109 a | 0.0079 ± 0.0040 a | 0.035 |
| Cl− (mg/L) | 0.3232 ± 0.4445 a | 0.1045 ± 0.0013 a | 0.9900 ± 0.0000 b | 0.9900 ± 0.0000 b | 0.1085 ± 0.0013 a | 0.1125 ± 0.0013 a | 0.9900 ± 0.0000 b | 0.9900 ± 0.0000 b | −− |
| NO3− (mg/L) | 0.1870 ± 0.1680 a | 0.1292 ± 0.1605 ab | 0.4175 ± 0.3931 b | 0.5600 ± 0.4968 ab | 0.1648 ± 0.0773 ab | 0.1320 ± 0.1000 a | 0.4250 ± 0.3779 b | 0.4125 ± 0.3958 b | 13 |
| SO42− (mg/L) | 44.9525 ± 19.3041 a | 57.1100 ± 0.6914 ab | 56.4250 ± 13.8454 b | 54.8250 ± 0.6602 a | 19.0875 ± 0.2617 ab | 19.3650 ± 0.2525 a | 16.6500 ± 2.1810 c | 18.7000 ± 0.2000 bc | −− |
| SiO2 (mg/L) | 3.1677 ± 0.6338 a | 3.6868 ± 0.0823 a | 4.8045 ± 0.8920 b | 4.1830 ± 0.1450 b | 4.9533 ± 0.3605 a | 4.4910 ± 0.2667 b | 5.3595 ± 1.1776 ab | 4.1242 ± 0.5154 b | −− |
| Al (mg/L) | 0.0029 ± 0.0000 a | 0.0029 ± 0.0000 a | 0.0932 ± 0.0455 b | 0.0257 ± 0.0180 b | 0.0442 ± 0.0655 a | 0.0680 ± 0.0232 b | 0.1735 ± 0.2116 b | 0.0150 ± 0.0109 ab | −− |
| Ba (mg/L) | 0.0089 ± 0.0040 a | 0.0132 ± 0.0019 ab | 0.0128 ± 0.0039 ab | 0.0138 ± 0.0023 b | 0.0056 ± 0.0021 a | 0.0039 ± 0.0004 ab | 0.0058 ± 0.0057 b | 0.0019 ± 0.0012 b | 0.7 |
| B (mg/L) | 0.0009 ± 0.0000 a | 0.0009 ± 0.0000 a | 0.0174 ± 0.0013 b | 0.0168 ± 0.0004 b | 0.0091 ± 0.0113 a | 0.0009 ± 0.0000 a | 0.0126 ± 0.0023 b | 0.0144 ± 0.0011 ab | −− |
| Ca (mg/L) | 0.0090 ± 0.0000 a | 0.0090 ± 0.0000 ab | 0.0002 ± 0.0000 bc | 0.0002 ± 0.0000 c | 0.0090 ± 0.0000 a | 0.0090 ± 0.0000 ab | 0.0002 ± 0.0000 b | 0.0002 ± 0.0000 b | −− |
| Cu (mg/L) | 0.0002 ± 0.0000 a | 0.0002 ± 0.0000 a | 0.0007 ± 0.0002 b | 0.0008 ± 0.0001 b | 0.0002 ± 0.0000 ab | 0.0085 ± 0.0055 a | 0.0057 ± 0.0008 a | 0.0025 ± 0.0025 b | 0.1 |
| Sr (mg/L) | 0.1846 ± 0.0821 a | 0.2434 ± 0.0044 a | 0.2809 ± 0.0646 ab | 0.2629 ± 0.0094 b | 0.0790 ± 0.0031 a | 0.0765 ± 0.0012 b | 0.0695 ± 0.0111 b | 0.0827 ± 0.0056 ab | −− |
| Fe (mg/L) | 0.0360 ± 0.0094 a | 0.0437 ± 0.0250 ab | 0.0720 ± 0.0488 b | 0.0988 ± 0.0153 b | 0.0510 ± 0.0442 a | 0.0302 ± 0.0075 b | 0.1143 ± 0.1330 b | 0.0055 ± 0.0006 b | 5 |
| Li (mg/L) | 0.0001 ± 0.0000 a | 0.0001 ± 0.0000 ab | 0.0001 ± 0.0000 b | 0.0001 ± 0.0000 b | 0.0001 ± 0.0000 a | 0.0001 ± 0.0000 a | 0.0023 ± 0.0027 b | 0.0014 ± 0.0003 b | −− |
| Mg (mg/L) | 2.3093 ± 0.8920 ab | 2.9712 ± 0.0830 a | 3.5375 ± 0.3057 bc | 3.2715 ± 0.1133 c | 0.8914 ± 0.0328 a | 0.9264 ± 0.0448 a | 0.8728 ± 0.0796 a | 0.9498 ± 0.1216 a | −− |
| Mn (mg/L) | 0.0059 ± 0.0021 a | 0.0090 ± 0.0022 a | 0.0086 ± 0.0048 a | 0.0057 ± 0.0023 a | 0.0251 ± 0.0269 a | 0.0127 ± 0.0087 b | 0.0091 ± 0.0066 b | 0.0014 ± 0.0015 b | −− |
| K (mg/L) | 0.7888 ± 0.0763 a | 0.7480 ± 0.0157 b | 0.8875 ± 0.0561 a | 0.7685 ± 0.0471 a | 0.1752 ± 0.0439 a | 0.1942 ± 0.0688 b | 0.1305 ± 0.0515 ab | 0.2115 ± 0.0583 ab | −− |
| Si (mg/L) | 1.4782 ± 0.2958 a | 1.7203 ± 0.0385 a | 2.2422 ± 0.4163 b | 1.9520 ± 0.0677 b | 2.3116 ± 0.1682 a | 2.0958 ± 0.1244 b | 2.5010 ± 0.5495 ab | 1.9246 ± 0.2407 b | −− |
| Na (mg/L) | 1.1654 ± 0.3604 a | 1.4573 ± 0.0237 a | 1.5747 ± 0.3018 ab | 1.5274 ± 0.0297 b | 1.8290 ± 0.0181 a | 1.8021 ± 0.0833 b | 1.9106 ± 0.2413 b | 2.9350 ± 1.2264 b | −− |
| V (mg/L) | 0.0003 ± 0.0000 a | 0.0003 ± 0.0000 a | 0.0003 ± 0.0000 ab | 0.0003 ± 0.0000 b | 0.0003 ± 0.0000 a | 0.0003 ± 0.0000 b | 0.0017 ± 0.0008 a | 0.0008 ± 0.0006 a | −− |
| Event | Lagoon | Sampling Site | F1 | F2 | F3 | CCME WQI | WQI according to Color |
| Storage | Carhuacocha | 1 | − | − | − | 100.00 | Excellent |
| 2 | − | − | − | 100.00 | Excellent | ||
| 3 | − | − | − | 100.00 | Excellent | ||
| 4 | − | − | − | 100.00 | Excellent | ||
| 5 | − | − | − | 100.00 | Excellent | ||
| 6 | − | − | − | 100.00 | Excellent | ||
| 7 | − | − | − | 100.00 | Excellent | ||
| 8 | − | − | − | 100.00 | Excellent | ||
| Vichecocha | 1 | − | − | − | 100.00 | Excellent | |
| 2 | − | − | − | 100.00 | Excellent | ||
| 3 | − | − | − | 100.00 | Excellent | ||
| 4 | − | − | − | 100.00 | Excellent | ||
| 5 | − | − | − | 100.00 | Excellent | ||
| 6 | − | − | − | 100.00 | Excellent | ||
| 7 | 9.09 | 9.09 | 0.23 | 93.00 | Good | ||
| 8 | 9.09 | 9.09 | 0.43 | 93.00 | Good | ||
| Discharge | Carhuacocha | 1 | 9.09 | 9.09 | 17.19 | 88.00 | Good |
| 2 | 9.09 | 9.09 | 15.92 | 88.00 | Good | ||
| 3 | 9.09 | 9.09 | 16.38 | 88.00 | Good | ||
| 4 | 9.09 | 9.09 | 17.79 | 87.00 | Good | ||
| 5 | 9.09 | 9.09 | 17.12 | 88.00 | Good | ||
| 6 | 9.09 | 9.09 | 13.88 | 89.00 | Good | ||
| 7 | 9.09 | 9.09 | 17.19 | 88.00 | Good | ||
| 8 | 9.09 | 9.09 | 21.29 | 86.00 | Good | ||
| Vichecocha | 1 | 18.18 | 18.18 | 21.24 | 81.00 | Good | |
| 2 | 9.09 | 9.09 | 26.67 | 83.00 | Good | ||
| 3 | 9.09 | 9.09 | 22.34 | 85.00 | Good | ||
| 4 | 9.09 | 9.09 | 18.29 | 87.00 | Good | ||
| 5 | 9.09 | 9.09 | 20.90 | 86.00 | Good | ||
| 6 | 9.09 | 9.09 | 23.02 | 85.00 | Good | ||
| 7 | 9.09 | 9.09 | 27.14 | 83.00 | Good | ||
| 8 | 18.18 | 18.18 | 24.52 | 79.00 | Fair |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizarro, S.; Custodio, M.; Solórzano-Acosta, R.; Contreras, D.; Verástegui-Martínez, P. Water Storage–Discharge Relationship with Water Quality Parameters of Carhuacocha and Vichecocha Lagoons in the Peruvian Puna Highlands. Water 2024, 16, 2505. https://doi.org/10.3390/w16172505
Pizarro S, Custodio M, Solórzano-Acosta R, Contreras D, Verástegui-Martínez P. Water Storage–Discharge Relationship with Water Quality Parameters of Carhuacocha and Vichecocha Lagoons in the Peruvian Puna Highlands. Water. 2024; 16(17):2505. https://doi.org/10.3390/w16172505
Chicago/Turabian StylePizarro, Samuel, Maria Custodio, Richard Solórzano-Acosta, Duglas Contreras, and Patricia Verástegui-Martínez. 2024. "Water Storage–Discharge Relationship with Water Quality Parameters of Carhuacocha and Vichecocha Lagoons in the Peruvian Puna Highlands" Water 16, no. 17: 2505. https://doi.org/10.3390/w16172505
APA StylePizarro, S., Custodio, M., Solórzano-Acosta, R., Contreras, D., & Verástegui-Martínez, P. (2024). Water Storage–Discharge Relationship with Water Quality Parameters of Carhuacocha and Vichecocha Lagoons in the Peruvian Puna Highlands. Water, 16(17), 2505. https://doi.org/10.3390/w16172505









