Microalgal–Bacteria Biofilm in Wastewater Treatment: Advantages, Principles, and Establishment
Abstract
:1. Introduction
2. Advantages of MBBFs in Comparison with Other Wastewater Treatment Technologies
2.1. Emerging Pollutant Removal
2.2. High Value of Microalgal–Bacterial Biomass
2.3. Carbon Capture
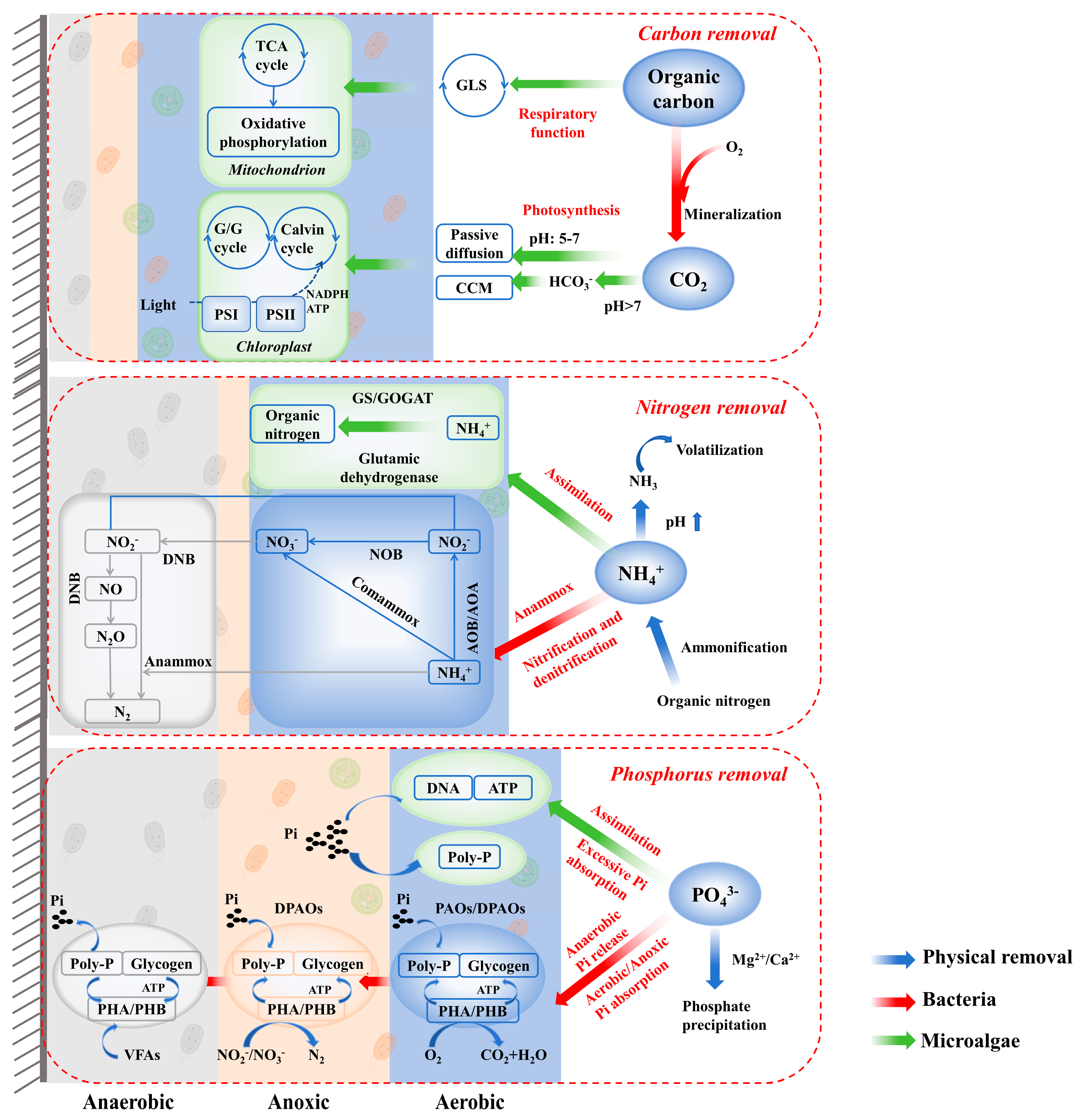
3. Pollutant Removal Pathways and Collaborative Mechanism of MBBFs
3.1. Multiple Pollutant Removal Pathways
3.1.1. Carbon Removal
3.1.2. Nitrogen Removal
3.1.3. Phosphorus Removal
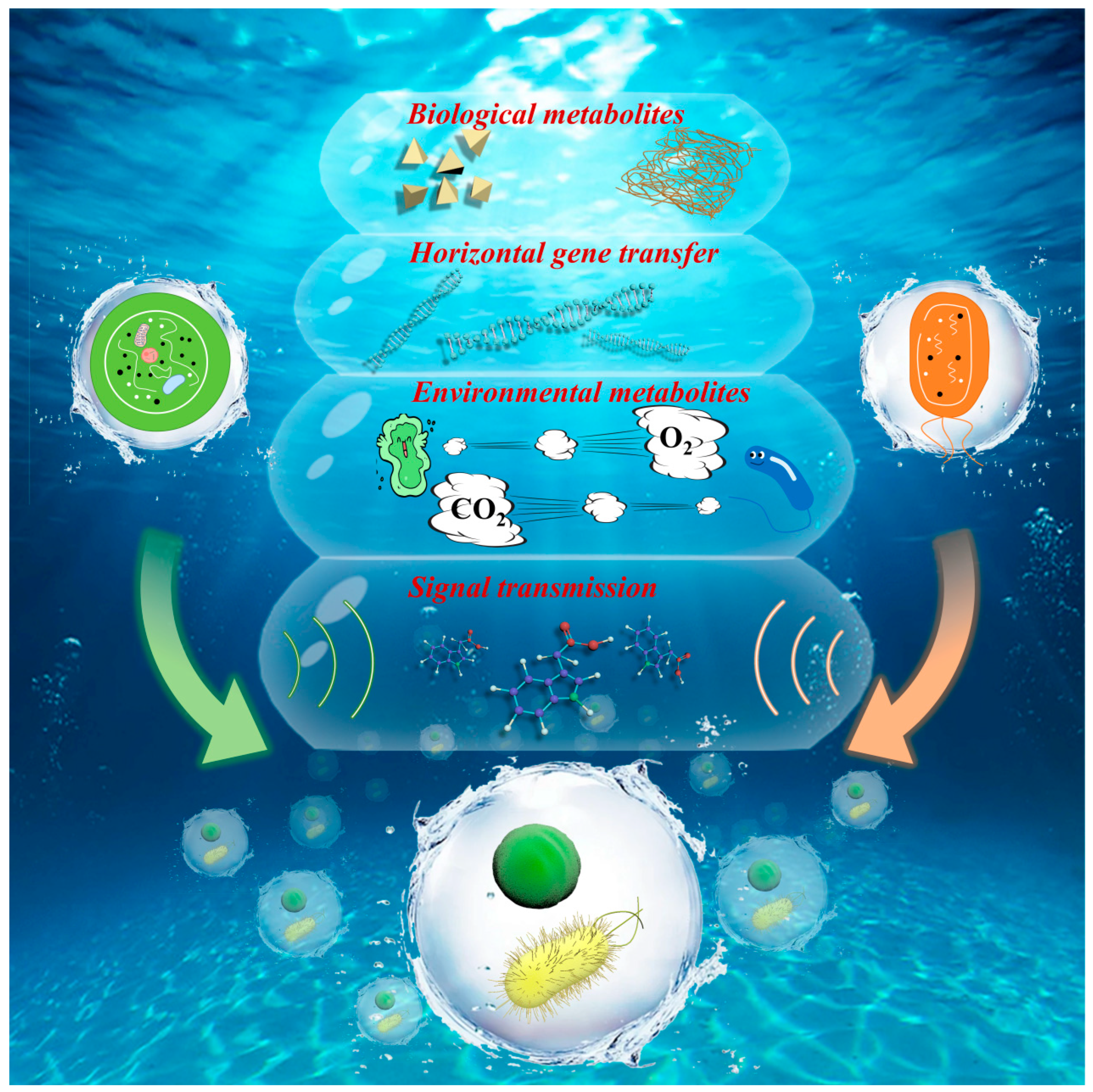
3.2. Interactions between Microalgae and Bacteria in MBBFs
3.2.1. The Synergistic Interactions between Microalgae and Bacteria Enhance Growth Activity and Resistance
3.2.2. Competition and Antagonism between Microalgae and Bacteria
4. The Principle and Process of Establishing an MBBF
4.1. Establishment of an MBC
4.2. MBBF Formation Process
4.2.1. Establishment of Biofilm on Carrier Surface
4.2.2. EPSs Form the Backbone of Biofilms
5. Factors Affecting the Formation of MBBFs
5.1. Environmental Factors
5.2. Biological Factors
5.3. Carrier Factors
6. Photobioreactor Suitable for Implementing MBBF Applications
6.1. Open Photobioreactor
6.1.1. Membrane Photobioreactor
6.1.2. Runway Photobioreactor
6.2. Closed Photobioreactor
6.2.1. Plate Photobioreactor
6.2.2. Column Photobioreactor
6.2.3. Tubular Photobioreactor
7. Challenges and Future Perspectives of MBBF Coupled Wastewater Treatment Systems
7.1. Challenges of MBBF Coupled Wastewater Treatment Systems
7.2. Future Perspectives of MBBF Coupled Wastewater Treatment Systems
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nagarajan, D.; Lee, D.J.; Chen, C.Y.; Chang, J.S. Resource recovery from wastewaters using microalgae-based approaches: A circular bioeconomy perspective. Bioresour. Technol. 2020, 302, 122817. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.P.; Koyande, A.K.; Chew, K.W.; Ho, S.H.; Chen, W.H.; Chang, J.S.; Krishnamoorthy, R.; Banat, F.; Show, P.L. Continuous cultivation of microalgae in photobioreactors as a source of renewable energy: Current status and future challenges. Renew. Sustain. Energy Rev. 2022, 154, 111852. [Google Scholar] [CrossRef]
- Huo, S.; Kong, M.; Zhu, F.; Qian, J.; Huang, D.; Chen, P.; Ruan, R. Co-culture of Chlorella and wastewater-borne bacteria in vinegar production wastewater: Enhancement of nutrients removal and influence of algal biomass generation. Algal Res. 2020, 45, 101744. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Qiu, S.; Li, M.; Yuan, W.; Ge, S. Granular indigenous microalgal-bacterial consortium for wastewater treatment: Establishment strategy, functional microorganism, nutrient removal, and influencing factor. Bioresour. Technol. 2022, 353, 127130. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. A review of the harvesting of micro-algae for biofuel production. Rev. Environ. Sci. Biotechnol. 2012, 12, 165–178. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.Q.; Shi, W.X.; Cui, F.Y.; Lens, P.N.; Tay, J.H. Microalgal-bacterial consortia: From interspecies interactions to biotechnological applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Wu, C.; Muylaert, K.; Vyverman, W.; Yu, H.Q.; Muñoz, R.; Rittmann, B. Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: A review. Bioresour. Technol. 2017, 241, 1127–1137. [Google Scholar] [CrossRef]
- Wang, H.; Hill, R.T.; Zheng, T.; Hu, X.; Wang, B. Effects of bacterial communities on biofuel-producing microalgae: Stimulation, inhibition and harvesting. Crit. Rev. Biotechnol. 2014, 36, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hong, Y. Microalgae biofilm and bacteria symbiosis in nutrient removal and carbon fixation from wastewater: A review. Curr. Pollut. Rep. 2022, 8, 128–146. [Google Scholar] [CrossRef]
- Wolf, G.; Picioreanu, C.; Loosdrecht, M.C. Kinetic modeling of phototrophic biofilms: The PHOBIA model. Biotechnol. Bioeng. 2007, 97, 1064–1079. [Google Scholar] [CrossRef]
- Deng, L.J.; Guo, W.S.; Ngo, H.H.; Zhang, X.B.; Wang, X.C.; Zhang, Q.H.; Chen, R. New functional biocarriers for enhancing the performance of a hybrid moving bed biofilm reactor–membrane bioreactor system. Bioresour. Technol. 2016, 208, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kuang, B.; Zhou, L.; Cai, C.; Long, X.; Zhang, B.; Wang, T. Mariculture wastewater treatment using electroactive bacteria-algae biofilm coupled with siphon aeration. Trans. Chin. Soc. Agric. Eng. 2023, 39, 205–212. [Google Scholar]
- Yao, X.Y.; Sun, J.; Bai, X.Y.; Yuan, Y.; Zhang, Y.P.; Xu, Y.B.; Huang, G.F. A high-efficiency mixotrophic photoelectroactive biofilm reactor (MPBR) for enhanced simultaneous removal of nutrients and antibiotics by integrating light intensity regulation and microbial extracellular electron extraction. J. Environ. Manag. 2022, 325, 116520. [Google Scholar] [CrossRef]
- Zhao, D.; Cheah, W.Y.; Lai, S.H.; Ng, E.P.; Khoo, K.S.; Show, P.L.; Ling, T.C. Symbiosis of microalgae and bacteria consortium for heavy metal remediation in wastewater. J. Environ. 2023, 11, 109943. [Google Scholar] [CrossRef]
- Han, W.; Mao, Y.; Wei, Y.; Shang, P.; Zhou, X. Bioremediation of aquaculture wastewater with algal-bacterial biofilm combined with the production of selenium rich biofertilizer. Water 2020, 12, 2071. [Google Scholar] [CrossRef]
- Maghzian, A.; Aslani, A.; Zahedi, R. A comprehensive review on effective parameters on microalgae productivity and carbon capture rate. J. Environ. Manag. 2024, 355, 120539. [Google Scholar] [CrossRef]
- Rangsayatorn, N.; Upatham, E.S.; Kruatrachue, M.; Pokethitiyook, P.; Lanza, G.R. Phytoremediation potential of Spirulina (Arthrospira) platensis: Biosorption and toxicity studies of cadmium. Environ. Polut. 2002, 199, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Ji, B.; Zhang, M.; Ma, Y.Q.; Gu, J.; Liu, Y. Defensive responses of microalgal-bacterial granules to tetracycline in municipal wastewater treatment. Bioresour. Technol. 2020, 312, 123605. [Google Scholar] [CrossRef]
- Guo, R.X.; Chen, J.Q. Application of alga-activated sludge combined system (AASCS) as a novel treatment to remove cephalosporins. Chem. Eng. J. 2015, 260, 550–556. [Google Scholar] [CrossRef]
- Aydin, S.; Ünlü, İ.D.; Arabacı, D.N.; Duru, Ö.A. Evaluating the effect of microalga Haematococcus pluvialis bioaugmentation on aerobic membrane bioreactor in terms of performance, membrane fouling and microbial community structure. Sci. Total Environ. 2022, 807, 149908. [Google Scholar] [CrossRef]
- Zambrano, J.; García, P.A.; Hernández, F.; Botero, A.M.; Jiménez, J.J.; Irusta, R. Kinetics of the removal mechanisms of veterinary antibiotics in synthetic wastewater using microalgae–bacteria consortia. Environ. Technol. 2023, 29, 103031. [Google Scholar] [CrossRef]
- Rodrigues, D.A.; Cunha, C.C.; Espirito, D.R.; Barros, A.L.; Pereira, A.R.; Queiroz, S.; Fonseca, A.; Cássia, R.J. Removal of cephalexin and erythromycin antibiotics, and their resistance genes, by microalgae-bacteria consortium from wastewater treatment plant secondary effluents. Environ. Sci. Pollut. Res. 2021, 28, 67822–67832. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Essam, T.M.; Ragab, Y.M.; Mourad, F.E. Biodegradation of ketoprofen using a microalgal–bacterial consortium. Biotechnol. Lett. 2016, 38, 1493–1502. [Google Scholar] [CrossRef]
- Orandi, S.; Lewis, D.M.; Moheimani, N.R. Biofilm establishment and heavy metal removal capacity of an indigenous mining algal-microbial consortium in a photo-rotating biological contactor. J. Ind. Microbiol. Biotechnol. 2012, 39, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Zárate, A.M.; Florez, J.Z.; Angulo, E.; Varela, L.; Infante, C.; Barrios, F.; Barraza, B.; Gallardo, D.I.; Valdé, J. Burkholderia tropica as a potential microalgal growth-promoting bacterium in the biosorption of mercury from aqueous solutions. J. Microbiol. Biotechnol. 2017, 27, 1138–1149. [Google Scholar] [CrossRef]
- Goiris, K.; Colen, W.; Wilches, I.; Tamariz, F.; Cooman, L.; Muylaert, K. Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res. 2015, 7, 51–57. [Google Scholar] [CrossRef]
- Hoang, A.T.; Sirohi, R.; Pandey, A.; Nižetić, S.; Lam, S.S.; Chen, W.H.; Luque, R.; Thomas, S.; Arıcı, M.; Pham, V.V. Biofuel production from microalgae: Challenges and chances. Phytochem. Rev. 2022, 22, 1089–1126. [Google Scholar] [CrossRef]
- Ge, S.; Qiu, S.; Tremblay, D.; Viner, K.; Champagne, P.; Jessop, P.G. Centrate wastewater treatment with Chlorella vulgaris: Simultaneous enhancement of nutrient removal, biomass and lipid production. Chem. Eng. J. 2018, 342, 310–320. [Google Scholar] [CrossRef]
- Johnson, D.T.; Taconi, K.A. The glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ. Prog. 2007, 26, 338–348. [Google Scholar] [CrossRef]
- Jiao, H.X.; Tsigkou, K.; Elsamahy, T.; Pispas, K.; Sun, J.Z.; Manthos, G.; Schagerl, M.; Sventzouri, E.; Tohamy, R.; Kornaros, M.; et al. Recent advances in sustainable hydrogen production from microalgae: Mechanisms, challenges, and future perspectives. Ecotoxicol. Environ. Saf. 2024, 270, 115908. [Google Scholar] [CrossRef]
- Hirano, A.; Ueda, R.; Hirayama, S.; Ogushi, Y. CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy 1997, 22, 137–142. [Google Scholar] [CrossRef]
- Safafar, H.; Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef]
- Wilson, D.; Nash, P.; Buttar, H.; Griffiths, K.; Singh, R.; Meester, F.; Horiuchi, R.; Takahashi, T. The role of food antioxidants, benefits of functional foods, and influence of feeding habits on the health of the older person: An overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Wang, S.; Xiao, C.; Ge, S. Assessment of microalgae as a new feeding additive for fruit fly Drosophila melanogaster. Sci. Total Environ. 2019, 667, 455–463. [Google Scholar] [CrossRef]
- Gherabli, A.; Grimi, N.; Lemaire, J.; Vorobiev, E.; Lebovka, N. Extraction of valuable biomolecules from the microalga Haematococcus pluvialis assisted by electrotechnologies. Molecules 2023, 28, 2089. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Kudo, M.; Maeda, H.; Kohno, H.; Tanaka, T.; Miyashita, K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2004, 1675, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Qiu, S.; Abbew, A.W.; Chen, Z.; Liu, Y.; Zuo, J.; Ge, S. Evaluation of nitrogen source, concentration and feeding mode for co-production of fucoxanthin and fatty acids in Phaeodactylum tricornutum. Algal Res. 2022, 63, 102655. [Google Scholar] [CrossRef]
- Morales, M.; Hélias, A.; Bernard, O. Optimal integration of microalgae production with photovoltaic panels: Environmental impacts and energy balance. Biotechnol. Biofuels 2019, 12, 239. [Google Scholar] [CrossRef]
- Tripathi, S.; Choudhary, S.; Meena, A.; Poluri, K.M. Carbon capture, storage, and usage with microalgae: A review. Environ. Chem. Lett. 2023, 21, 2085–2128. [Google Scholar] [CrossRef]
- Valdovinos, E.M.; Barajas, J.; Olán, M.; Petriz, M.A.; Guzmán, A.; Bravo, M.G. Techno-economic study of CO2 capture of a thermoelectric plant using microalgae (Chlorella vulgaris) for production of feedstock for bioenergy. Energies 2020, 13, 413. [Google Scholar] [CrossRef]
- Yao, S.; Lyu, S.; An, Y.; Lu, J.; Gjermansen, C.; Schramm, A. Microalgae-bacteria symbiosis in microalgal growth and biofuel production: A review. J. Appl. Microbiol. 2019, 126, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Y.; Guo, Y.; Koyande, A.K.; Chew, K.W.; Vo, D.V.; Show, P.L. Green technology for the industrial production of biofuels and bioproducts from microalgae: A review. Environ. Chem. Lett. 2020, 18, 1967–1985. [Google Scholar] [CrossRef]
- Liu, H.J.; Were, P.; Li, Q.; Gou, Y.; Hou, Z. Worldwide status of CCUS technologies and their development and challenges in China. Geofluids 2017, 2017, 126505. [Google Scholar] [CrossRef]
- Kong, W.; Shen, B.; Lyu, H.; Kong, J.; Ma, J.; Wang, Z.; Feng, S. Review on carbon dioxide fixation coupled with nutrients removal from wastewater by microalgae. J. Clean. Prod. 2021, 292, 125975. [Google Scholar] [CrossRef]
- Xu, X.; Gu, X.; Wang, Z.; Shatner, W.; Wang, Z. Progress, challenges and solutions of research on photosynthetic carbon sequestration efficiency of microalgae. Renew. Sustain. Energy Rev. 2019, 110, 65–82. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Basílico, G.; Cabo, L.; Magdaleno, A.; Faggi, A. Poultry effluent bio-treatment with Spirodela intermedia and periphyton in mesocosms with water recirculation. Water Air Soil Pollut. 2016, 227, 190. [Google Scholar] [CrossRef]
- González, J.; Barat, R.; Pachés, M.; Murgui, M.; Seco, A.; Ferrer, J. Wastewater nutrient removal in a mixed microalgae-bacteria culture: Effect of light and temperature on the microalgae—Bacteria competition. Environ. Technol. 2017, 39, 503–515. [Google Scholar] [CrossRef]
- Ma, X.; Mi, Y.; Zhao, C.; Wei, Q. A comprehensive review on carbon source effect of microalgae lipid accumulation for biofuel production. Sci. Total Environ. 2022, 806, 151387. [Google Scholar] [CrossRef]
- Simsek, H.; Kasi, M.; Ohm, J.B.; Murthy, S.; Khan, E. Impact of solids retention time on dissolved organic nitrogen and its biodegradability in treated wastewater. Water Res. 2016, 92, 44–51. [Google Scholar] [CrossRef]
- Courtens, E.N.; Spieck, E.; Vilchez, R.; Bodé, S.; Boeckx, P.; Schouten, S.; Jauregui, R.; Pieper, D.H.; Vlaeminck, S.E.; Boon, N. A robust nitrifying community in a bioreactor at 50 °C opens up the path for thermophilic nitrogen removal. ISME J. 2016, 10, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.M.; Camejo, P.; Oshlag, J.Z.; Noguera, D.R. Ammonia-oxidizing microbial communities in reactors with efficient nitrification at low-dissolved oxygen. Water Res. 2015, 70, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, L.; Zhang, J.; Li, J.; Peng, Y. Enhancing nitrogen removal through directly integrating anammox into mainstream wastewater treatment: Advantageous, issues and future study. Bioresour. Technol. 2022, 362, 127827. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Zhang, C.F.; Li, S.N.; Ho, S.H. Converting nitrogen and phosphorus wastewater into bioenergy using microalgae-bacteria consortia: A critical review. Bioresour. Technol. 2021, 342, 126056. [Google Scholar] [CrossRef]
- Sforza, E.; Calvaruso, C.; Rocca, N.; Bertucco, A. Luxury uptake of phosphorus in Nannochloropsis salina: Effect of P concentration and light on P uptake in batch and continuous cultures. Biochem. Eng. J. 2018, 134, 69–79. [Google Scholar] [CrossRef]
- Schmidt, J.J.; Gagnon, G.A.; Jamieson, R.C. Microalgae growth and phosphorus uptake in wastewater under simulated cold region conditions. Ecol. Eng. 2016, 95, 588–593. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Zhang, J.; He, Y.; Zhang, J.; Ge, R. Screening and diversity analysis of aerobic denitrifying phosphate accumulating bacteria cultivated from A2O activated sludge. Processes 2019, 7, 827. [Google Scholar] [CrossRef]
- Zaman, M.; Kim, M.G.; Nakhla, G. Simultaneous partial nitrification and denitrifying phosphorus removal (PNDPR) in a sequencing batch reactor process operated at low DO and high SRT for carbon and energy reduction. Chem. Eng. J. 2021, 425, 131881. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.H.; Cui, S.; Ren, Y.X.; Yu, J.; Chen, N.; Xiao, Q.; Guo, L.K.; Wang, R.H. Simultaneous removal of nitrogen and phosphorous by heterotrophic nitrification-aerobic denitrification of a metal resistant bacterium Pseudomonas putida strain NP5. Bioresour. Technol. 2019, 285, 121360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Pan, L.Q.; Liu, L.P.; Su, C.; Dou, L.; Su, Z.P.; He, Z.Y. Phosphorus and nitrogen removal by a novel phosphate-accumulating organism, Arthrobacter sp. HHEP5 capable of heterotrophic nitrification-aerobic denitrification: Safety assessment, removal characterization, mechanism exploration and wastewater treatment. Bioresour. Technol. 2020, 312, 123633. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chen, Z.P.; Li, M.T.; Qiu, S.; Lv, Z.; Ge, S.J. Principles, challenges, and optimization of indigenous microalgae-bacteria consortium for sustainable swine wastewater treatment. Bioresour. Technol. 2024, 406, 131055. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.R.; Zheng, R.; Feng, Y.M.; Du, W.R.; Xie, C.; Gu, Y.Q.; Liu, S.T. Anammox bacteria adapt to long-term light irradiation in photogranules. Water Res. 2023, 241, 120144. [Google Scholar] [CrossRef]
- Hou, J.; Li, T.F.; Miao, L.Z.; You, G.X.; Xu, Y.; Liu, S.Q. Effects of titanium dioxide nanoparticles on algal and bacterial communities in periphytic biofilms. Environ. Pollut. 2019, 251, 407–414. [Google Scholar] [CrossRef]
- Liu, J.Z.; Tang, J.; Wan, J.J.; Wu, C.; Graham, B.; Kerr, P.G.; Wu, Y.H. Functional sustainability of periphytic biofilms in organic matter and Cu2+ removal during prolonged exposure to TiO2 nanoparticles. J. Hazard. 2019, 370, 4–12. [Google Scholar] [CrossRef]
- Croft, M.T.; Deery, E.; Smith, A.; Warren, M. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Comp. Biochem. Physiol. Part A 2007, 146, S222. [Google Scholar] [CrossRef] [PubMed]
- Durham, B.P.; Sharma, S.; Luo, H.; Smith, C.B.; Amin, S.A.; Bender, S.J.; Dearth, S.P.; Mooy, B.A.S.; Campagna, S.R.; Kujawinski, E.B.; et al. Cryptic carbon and sulfur cycling between surface ocean plankton. Proc. Natl. Acad. Sci. USA 2014, 112, 453–457. [Google Scholar] [CrossRef]
- Shady, A.A.; David, H.G.; Mark, C.H.; Frithjof, C.K.; William, G.S.; Carl, J.C. Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism. Proc. Natl. Acad. Sci. USA 2009, 106, 17071–17076. [Google Scholar]
- Kouzuma, A.; Watanabe, K. Exploring the potential of algae/bacteria interactions. Curr. Opin. Biotechnol. 2015, 33, 125–129. [Google Scholar] [CrossRef]
- Meng, F.S.; Huang, W.W.; Liu, D.F.; Zhao, Y.X.; Huang, W.L.; Lei, Z.F.; Zhang, Z.Y. Application of aerobic granules-continuous flow reactor for saline wastewater treatment: Granular stability, lipid production and symbiotic relationship between bacteria and algae. Bioresour. Technol. 2020, 295, 122291. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.A.; Gomez, G.; Bashan, Y.; Bashan, L.E.; Sessitsch, A. Tryptophan, thiamine and indole-3-acetic acid exchange between Chlorella sorokini and the plant growth-promoting bacterium Azospirillum brasilense. FEMS Microbiol. Ecol. 2016, 92, fiw077. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.A.; Hmelo, L.R.; Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.D.; Zhang, C.F.; Fu, L.; Xu, L.; Cui, X.C.; Li, Q.C.; Crittenden, J.C. Responses of the microalga Chlorophyta sp. to bacterial quorum sensing molecules (N-acylhomoserine lactones): Aromatic protein-induced self-aggregation. Environ. Sci. Technol. 2017, 51, 3490–3498. [Google Scholar] [CrossRef]
- Ou, Z.X.; Chen, X.D.; Wu, X.M.; Zhou, C.R.; Zhang, K.J.; Luo, J.Y.; Fang, F.; Sun, Y.Q.; Li, M.; Feng, Q. N-acyl homoserine lactone mediating initial adhesion of microalgal biofilm formation. Environ. Res. 2023, 233, 116446. [Google Scholar] [CrossRef]
- Hays, S.G.; Patrick, W.G.; Ziesack, M.; Oxman, N.; Silver, P.A. Better together: Engineering and application of microbial symbioses. Curr. Opin. Biotechnol. 2015, 36, 40–49. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Chen, Q.W.; Zhang, J.Y.; Guan, T.S.; Chen, Y.C.; Shi, W.Q. Critical roles of cyanobacteria as reservoir and source for antibiotic resistance genes. Environ. Int. 2020, 144, 106034. [Google Scholar] [CrossRef]
- He, X.Y.; Wang, J.P.; Abdoli, L.L.; Li, H. Mg2+/Ca2+ promotes the adhesion of marine bacteria and algae and enhances following biofilm formation in artificial seawater. Colloids Surf. B Biointerfaces 2016, 146, 289–295. [Google Scholar] [CrossRef]
- González, J.; Montero, P.; Aparicio, S.; Ruano, M.V.; Borrás, L.; Seco, A.; Barat, R. Nitrite inhibition of microalgae induced by the competition between microalgae and nitrifying bacteria. Water Res. 2020, 172, 115499. [Google Scholar] [CrossRef]
- Maddela, N.R.; Sheng, B.; Yuan, S.; Zhou, Z.; Villamar, R.; Meng, F. Roles of quorum sensing in biological wastewater treatment: A critical review. Chemosphere 2019, 221, 616–629. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Beardall, J.; Raven, J.A. Chemically-mediated interactions in microalgae. In The Physiology of Microalgae; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 321–357. [Google Scholar]
- Demuez, M.; González, C.; Ballesteros, M. Algicidal microorganisms and secreted algicides: New tools to induce microalgal cell disruption. Biotechnol. Adv. 2015, 33, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Yu, Z.W.; Hu, Y.B.; Chen, Z.P.; Guo, J.H.; Xia, W.H.; Ge, S.J. An evolved native microalgal consortium-snow system for the bioremediation of biogas and centrate wastewater: Start-up, optimization and stabilization. Water Res. 2021, 196, 117038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Xiong, Z.S.; Yang, L.M.; Ren, Z.; Shao, P.H.; Shi, H.; Xiao, X.; Pavlostathis, S.G.; Fang, L.L.; Luo, X.B. Successful isolation of a tolerant co-flocculating microalgae towards highly efficient nitrogen removal in harsh rare earth element tailings (REEs) wastewater. Water Res. 2019, 166, 115076. [Google Scholar] [CrossRef]
- Lindemann, S.R.; Bernstein, H.C.; Song, H.S.; Fredrickson, J.K.; Fields, M.W.; Shou, W.; Johnson, D.R.; Beliaev, A.S. Engineering microbial consortia for controllable outputs. ISME J. 2016, 10, 2077–2084. [Google Scholar] [CrossRef]
- Sun, X.M.; Ren, L.J.; Bi, Z.Q.; Ji, X.J.; Zhao, Q.Y.; Huang, H. Adaptive evolution of microalgae Schizochytrium sp. under high salinity stress to alleviate oxidative damage and improve lipid biosynthesis. Bioresour. Technol. 2018, 267, 438–444. [Google Scholar] [CrossRef]
- Pang, N.; Bergeron, A.D.; Gu, X.Y.; Fu, X.; Dong, T.; Yao, Y.Q.; Chen, S.L. Recycling of nutrients from dairy wastewater by extremophilic microalgae with high ammonia tolerance. Environ. Sci. Technol. 2020, 54, 15366–15375. [Google Scholar] [CrossRef]
- Liao, Q.; Zhong, N.B.; Zhu, X.; Chen, R.; Wang, Y.Z.; Lee, D.J. Enhancement of hydrogen production by adsorption of Rhodoseudomonas palustris CQK 01 on a new support material. Int. J. Hydrogen Energy 2013, 38, 15730–15737. [Google Scholar] [CrossRef]
- Fu, Q.; Li, Y.S.; Zhong, N.B.; Liao, Q.; Huang, Y.; Xia, A.; Zhu, X.; Hou, Y.P. A novel biofilm photobioreactor using light guide plate enhances the hydrogen production. Int. J. Hydrogen Energy 2017, 42, 27523–27531. [Google Scholar] [CrossRef]
- Nadell, C.D.; Drescher, K.; Wingreen, N.S.; Bassler, B.L. Extracellular matrix structure governs invasion resistance in bacterial biofilms. ISME J. 2015, 9, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Y.T.; Chan, D.J. Physiology of microalgal biofilm: A review on prediction of adhesion on substrates. Bioengineered 2021, 12, 7577–7599. [Google Scholar] [CrossRef]
- Pastore, M.; Santaeufemia, S.; Bertucco, A.; Sforza, E. Light intensity affects the mixotrophic carbon exploitation in Chlorella protothecoides: Consequences on microalgae-bacteria based wastewater treatment. Water Sci. Technol. 2018, 78, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.X.; Tang, D.Y.; Chew, K.W.; Nguyen, T.D.; Ho, H.L.; Tran, T.N. Green biorefinery: Microalgae-bacteria microbiome on tolerance investigations in plants. J. Biotechnol. 2022, 343, 120–127. [Google Scholar]
- Park, J.; Kumar, G.; Bakonyi, P.; Peter, J.; Nemestóthy, N.; Koter, S.; Kujawski, W.; Bélafi-Bakó, K.; Pientka, Z.; Muñoz, R.; et al. Comparative evaluation of CO2 fixation of microalgae strains at various CO2 aeration conditions. Waste Biomass Valoriz. 2021, 12, 2999–3007. [Google Scholar] [CrossRef]
- Wang, M.; Keeley, R.; Zalivina, N.; Halfhide, T.; Scott, K.; Zhang, Q.; van der Steen, P.; Ergas, S.J. Advances in algal-prokaryotic wastewater treatment: A review of nitrogen transformations, reactor configurations and molecular tools. J. Environ. Manag. 2018, 217, 845–857. [Google Scholar] [CrossRef]
- Ji, C.L.; Wang, J.F.; Zhang, W.; Liu, J.L.; Wang, H.; Gao, L.L.; Liu, T.Z. An applicable nitrogen supply strategy for attached cultivation of Aucutodesmus obliquus. J. Appl. Phycol. 2013, 26, 173–180. [Google Scholar] [CrossRef]
- Serôdio, J.; Vieira, S.; Cruz, S. Photosynthetic activity, photoprotection and photoinhibition in intertidal microphytobenthos as studied in situ using variable chlorophyll fluorescence. Cont. Shelf. Res. 2008, 28, 1363–1375. [Google Scholar] [CrossRef]
- Meng, F.S.; Xi, L.M.; Liu, D.F.; Huang, W.W.; Lei, Z.F.; Zhang, Z.Y.; Huang, W.L. Effects of light intensity on oxygen distribution, lipid production and biological community of algal-bacterial granules in photo-sequencing batch reactors. Bioresour. Technol. 2019, 272, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Toninelli, A.E.; Wang, J.F.; Liu, M.S.; Wu, H.; Liu, T.Z. Scenedesmus dimorphus biofilm: Photoefficiency and biomass production under intermittent lighting. Sci. Rep. 2016, 6, 32305. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Zhang, L.L.; Zhang, Z.H.; Liu, T.Z. Feasibility of attached cultivation for polysaccharides production by Porphyridium cruentum. Bioprocess Biosyst. Eng. 2016, 40, 73–83. [Google Scholar] [CrossRef]
- Liu, C.; Shi, Y.W.; Liu, H.P.; Ma, M.R.; Liu, G.Q.; Zhang, R.H.; Wang, W. Insight of co-fermentation of carbon monoxide with carbohydrate-rich wastewater for enhanced hydrogen production: Homoacetogenic inhibition and the role of pH. J. Clean. Prod. 2020, 267, 122027. [Google Scholar] [CrossRef]
- You, X.T.; Zhang, Z.S.; Guo, L.; Liao, Q.R.; Wang, Y.; Zhao, Y.G.; Jin, C.J.; Gao, M.C.; She, Z.L.; Wang, G.C. Integrating acidogenic fermentation and microalgae cultivation of bacterial-algal coupling system for mariculture wastewater treatment. Bioresour. Technol. 2021, 320, 124335. [Google Scholar] [CrossRef] [PubMed]
- Blanken, W.; Schaap, S.; Theobald, S.; Rinzema, A.; Wijffels, R.H.; Janssen, M. Optimizing carbon dioxide utilization for microalgae biofilm cultivation. Biotechnol. Bioeng. 2016, 114, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Q.; Li, Y.Z.; Pei, H.Y. Algal–bacterial consortia for bioproduct generation and wastewater treatment. Renew. Sustain. Energy Rev. 2021, 149, 111395. [Google Scholar] [CrossRef]
- Guo, C.L.; Wang, W.; Duan, D.R.; Zhao, C.Y.; Guo, F.Q. Enhanced CO2 biofixation and protein production by microalgae biofilm attached on modified surface of nickel foam. Bioprocess Biosyst. Eng. 2018, 42, 521–528. [Google Scholar] [CrossRef]
- Roosjen, A.; Boks, N.P.; Mei, H.C.; Busscher, H.J.; Norde, W. Influence of shear on microbial adhesion to PEO-brushes and glass by convective-diffusion and sedimentation in a parallel plate flow chamber. Colloids Surf. B Biointerfaces 2005, 46, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Soini, S.M.; Koskinen, K.T.; Vilenius, M.J.; Puhakka, J.A. Effects of fluid-flow velocity and water quality on planktonic and sessile microbial growth in water hydraulic system. Water Res. 2002, 36, 3812–3820. [Google Scholar] [CrossRef] [PubMed]
- Tijhuis, L.; Huisman, J.L.; Hekkelman, H.D.; Loosdrecht, M.C.; Heijnen, J.J. Formation of nitrifying biofilms on small suspended particles in airlift reactors. Biotechnol. Bioeng. 2004, 47, 585–595. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.T.; Binh, Q.; Bui, X.T.; Ngo, H.H.; Vo, H.N.; Andrew, K.Y.; Vo, T.D.; Guo, W.S.; Lin, C.; et al. Co-culture of microalgae-activated sludge for wastewater treatment and biomass production: Exploring their role under different inoculation ratios. Bioresour. Technol. 2020, 314, 123754. [Google Scholar] [CrossRef]
- Gross, M.; Zhao, X.; Mascarenhas, V.; Wen, Z. Effects of the surface physico-chemical properties and the surface textures on the initial colonization and the attached growth in algal biofilm. Biotechnol. Biofuels 2016, 9, 38. [Google Scholar] [CrossRef]
- Amshawee, S.; Yunus, M.Y.; Vo, D.V.; Tran, N.H. Biocarriers for biofilm immobilization in wastewater treatments: A review. Environ. Chem. Lett. 2020, 18, 1925–1945. [Google Scholar] [CrossRef]
- Luo, S.; Sai, P.; He, Z. Effective algal harvesting by using mesh membrane for enhanced energy recovery in an innovative integrated photobioelectrochemical system. Bioresour. Technol. 2018, 253, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sekar, R.; Venugopalan, V.P.; Satpathy, K.K.; Nair, K.V.; Rao, V.N. Laboratory studies on adhesion of microalgae to hard substrates. Hydrobiologia 2004, 512, 109–116. [Google Scholar] [CrossRef]
- Ozkan, A.; Berberoglu, H. Adhesion of Chlorella vulgaris on Hydrophilic and Hydrophobic Surfaces. In Proceedings of the ASME 2011 International Mechanical Engineering Congress and Exposition, Denver, CO, USA, 11–17 November 2011; ASME: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Liao, H.; Sun, L.; Li, J.; He, D. Bacterial-algal symbiosis biofilm for wastewater treatment: A review. J. Civ. Environ. Eng. 2021, 43, 141–153. [Google Scholar]
- Praveen, P.; Xiao, W.; Lamba, B.; Loh, K.C. Low-retention operation to enhance biomass productivity in an algal membrane photobioreactor. Algal Res. 2019, 40, 101487. [Google Scholar] [CrossRef]
- Hase, R.; Oikawa, H.; Sasao, C.; Morita, M.; Watanabe, Y. Photosynthetic production of microalgal biomass in a raceway system under greenhouse conditions in sendai city. J. Biosci. Bioeng. 2000, 89, 157–163. [Google Scholar] [CrossRef]
- Sun, Z.L.; Sun, L.Q.; Chen, G.Z. Microalgal cultivation and nutrient removal from digested piggery wastewater in a thin-film flat plate photobioreactor. Appl. Biochem. Biotechnol. 2018, 187, 1488–1501. [Google Scholar] [CrossRef]
- Asterio, S.M.; Marie, C.G.; Francisco, G.C.; Grima, E.M.; Chisti, Y. Growth and biochemical characterization of microalgal biomass produced in bubble column and airlift photobioreactors: Studies in fed-batch culture. Enzyme Microb. Technol. 2002, 31, 1015–1023. [Google Scholar]
- Solimeno, A.; Acíen, F.G.; García, J. Mechanistic model for design, analysis, operation and control of microalgae cultures: Calibration and application to tubular photobioreactors. Algal Res. 2017, 21, 236–246. [Google Scholar] [CrossRef]
- Luo, Y.L.; Clech, P.; Henderson, R.K. Assessment of membrane photobioreactor (MPBR) performance parameters and operating conditions. Water Res. 2018, 138, 169–180. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Z.H.; Li, C.; Wang, Y.J.; Jin, W.H.; Deng, Y.B. Concentrated microalgae cultivation in treated sewage by membrane photobioreactor operated in batch flow mode. Bioresour. Technol. 2014, 167, 441–446. [Google Scholar] [CrossRef]
- Ketheesan, B.; Nirmalakhandan, N. Feasibility of microalgal cultivation in a pilot-scale airlift-driven raceway reactor. Bioresour. Technol. 2012, 108, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Radmann, E.M.; Reinehr, C.O.; Costa, J.A. Optimization of the repeated batch cultivation of microalga Spirulina platensis in open raceway ponds. Aquaculture 2007, 265, 118–126. [Google Scholar] [CrossRef]
- Mendoza, J.L.; Granados, M.R.; Godos, I.; Acién, F.G.; Molina, E.; Banks, C.; Heaven, S. Fluid-dynamic characterization of real-scale raceway reactors for microalgae production. Biomass Bioenergy 2013, 54, 267–275. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Casini, D.; Tredici, M.R.; Rodolfi, L.; Bassi, N.; Zittelli, G.C.; Bondioli, P. Review of energy balance in raceway ponds for microalgae cultivation: Re-thinking a traditional system is possible. Appl. Energy 2013, 102, 101–111. [Google Scholar] [CrossRef]
- Sun, Y.H.; Huang, Y.; Liao, Q.; Fu, Q.; Zhu, X. Enhancement of microalgae production by embedding hollow light guides to a flat-plate photobioreactor. Bioresour. Technol. 2016, 207, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.P.; Wolf, L.; Weuster, D. Reaction engineering analysis of Scenedesmus ovalternus in a flat-plate gas-lift photobioreactor. Bioresour. Technol. 2017, 225, 165–174. [Google Scholar] [CrossRef]
- Shi, J.; Podola, B.; Melkonian, M. Application of a prototype-scale Twin-Layer photobioreactor for effective N and P removal from different process stages of municipal wastewater by immobilized microalgae. Bioresour. Technol. 2014, 154, 260–266. [Google Scholar] [CrossRef]
- Slegers, P.M.; Wijffels, R.H.; Straten, G.; Boxtel, A.J. Design scenarios for flat panel photobioreactors. Appl. Energy 2011, 88, 3342–3353. [Google Scholar] [CrossRef]
- Jung, E.E.; Jain, A.; Voulis, N.; Doud, D.F.; Angenent, L.T.; Erickson, D. Stacked optical waveguide photobioreactor for high density algal cultures. Bioresour. Technol. 2014, 171, 495–499. [Google Scholar] [CrossRef]
- Hu, J.Y.; Sato, T. A photobioreactor for microalgae cultivation with internal illumination considering flashing light effect and optimized light-source arrangement. Energy Convers. Manag. 2017, 133, 558–565. [Google Scholar] [CrossRef]
- Nayak, B.K.; Das, D. Improvement of carbon dioxide biofixation in a photobioreactor using Anabaena sp. PCC 7120. Process Biochem. 2013, 48, 1126–1132. [Google Scholar] [CrossRef]
- Pegallapati, A.K.; Nirmalakhandan, N.; Dungan, B.; Holguin, F.O.; Schaub, T. Evaluation of internally illuminated photobioreactor for improving energy ratio. J. Biosci. Bioeng. 2014, 117, 92–98. [Google Scholar] [CrossRef]
- Murray, A.M.; Fotidis, I.A.; Isenschmid, A.; Haxthausen, K.R.; Angelidaki, I. Wirelessly powered submerged-light illuminated photobioreactors for efficient microalgae cultivation. Algal Res. 2017, 25, 244–251. [Google Scholar] [CrossRef]
- Kang, D.; Zhao, Q.C.; Wu, Y.H.; Wu, C.X.; Xiang, W. Removal of nutrients and pharmaceuticals and personal care products from wastewater using periphyton photobioreactors. Bioresour. Technol. 2018, 248, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Binnal, P.; Babu, P.N. Optimization of environmental factors affecting tertiary treatment of municipal wastewater by Chlorella protothecoides in a lab scale photobioreactor. J. Water Process Eng. 2017, 17, 290–298. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Wu, X.; Xue, S.Z.; Liang, K.H.; Cong, W. Study of hydrodynamic characteristics in tubular photobioreactors. Bioprocess Biosyst. Eng. 2012, 36, 143–150. [Google Scholar] [CrossRef]
- Gómez, C.A.; Espinosa, J.J.; Montenegro, L.C.; Boxtel, A.J. Twisted tubular photobioreactor fluid dynamics evaluation for energy consumption minimization. Algal Res. 2017, 27, 65–72. [Google Scholar] [CrossRef]
- Soman, A.; Shastri, Y. Optimization of novel photobioreactor design using computational fluid dynamics. Appl. Energy 2015, 140, 246–255. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Qiu, S.; Li, M.; Ge, S. Enriched functional exoproteins and increased exopolysaccharides with altered molecular conformation mutually promoted indigenous microalgal-bacterial consortium biofilm growth under high light intensity. Chem. Eng. J. 2024, 480, 148056. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Sun, Y.; Zeng, W.; Xia, A.; Zhu, X.; Zhu, X.; Liao, Q. Non-immersed zigzag microalgae biofilm overcoming high turbidity and ammonia of wastewater for muti-pollutants bio-purification. Water Res. 2023, 244, 120499. [Google Scholar] [CrossRef]
- Fu, H.M.; Wang, J.; Ren, H.; Ding, L. Acceleration of start-up of moving bed biofilm reactor at low temperature by adding specialized quorum sensing bacteria. Bioresour. Technol. 2022, 358, 127249. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Hu, Y.; Xu, K.-Q. Impact of cationic substances on biofilm formation from sieved fine particles of anaerobic granular sludge at high salinity. Bioresour. Technol. 2018, 257, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, T.E.; Salazar, M.J.; Weng, L.L.; Palsson, B.O.; Feist, A.M. The emergence of adaptive laboratory evolution as an efficient tool for biological discovery and industrial biotechnology. Metab. Eng. 2019, 56, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Arcila, J.S.; Buitron, G. Influence of solar irradiance levels on the formation of microalgae-bacteria aggregates for municipal wastewater treatment. Algal Res. 2017, 27, 190–197. [Google Scholar] [CrossRef]


| Microalgae–Bacteria Consortium | Emerging Pollutants Types | Pollutant Concentration | Removal Efficiency | Removal Mechanism | Reference |
|---|---|---|---|---|---|
| Mud from Sanyuan Lake and Scenedesmus obliquus FACHB-12 | Chlortetracyc (CTC) | 80 mg/L | CTC: 79.7 ± 2.2% | Biosorption and enzymatic biodegradation | [12] |
| C. vulgaris and B. licheniformis | Oxytetracycline (OTC) and enrofloxacin (EFX) | OTC < 5 mg/L, EFX < 1 mg/L | OTC: 97.84~99.76% EFX: 42.68~42.90% | Photodegradation and biological effects | [13] |
| Sediments mixed with the aquaculture waster for the formation of the MBBF | Se | 115 ± 5 μg/L | Se: 83.74% | Sulfate pathway | [15] |
| H. pluvialis and activated sludge | Sulfamethoxazole (SMX), Tetracycline and Erythromycin (ERY) | ERY (100 mg/L), SMX (100 mg/L) and TET (37.3 mg/L) | SMX: 97.08% ERY: 98.15% TET: 89.73% | Biosorption | [20] |
| Scenedesmus almeriensis biomass was harvested from an HRAP | Tetracycline (TTC), ciprofloxacin (CPF), sulfadiazine (SDZ) and sulfamethoxazole (SMX) | 100 μg/L | TTC: 99.9% CPF: 78.0% SDZ: 52.6% SMX: 5.0% | Biosorption and biodegradation | [21] |
| Chlorella sorokiniana and Brevundimon | Cephalexin (CEP) and Erythromycin (ERY) | 50 μg/L | CEP: 96.54 ± 5.31% ERY: 92.38 ± 3.13% | Biodegradation | [22] |
| Chlorella sp., Spirulina platensis and Artemia sp. | Ketoprofen | 16 mM | degraded up to 16 mM ketoprofen | Biodegradation | [23] |
| Photo-rotating biological contactor: Ulothrix sp. | Cu | 80–100 mg/L | Cu: 50% | Biosorption | [24] |
| Chlorella sp. and B. tropica | Hg | 0.041 mg/L | Hg: 86% | Biosorption | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Li, Z.; Yu, S.; Chen, Z.; Xu, J.; Qiu, S.; Ge, S. Microalgal–Bacteria Biofilm in Wastewater Treatment: Advantages, Principles, and Establishment. Water 2024, 16, 2561. https://doi.org/10.3390/w16182561
Xu S, Li Z, Yu S, Chen Z, Xu J, Qiu S, Ge S. Microalgal–Bacteria Biofilm in Wastewater Treatment: Advantages, Principles, and Establishment. Water. 2024; 16(18):2561. https://doi.org/10.3390/w16182561
Chicago/Turabian StyleXu, Shiling, Zimu Li, Sheng Yu, Zhipeng Chen, Jiajie Xu, Shuang Qiu, and Shijian Ge. 2024. "Microalgal–Bacteria Biofilm in Wastewater Treatment: Advantages, Principles, and Establishment" Water 16, no. 18: 2561. https://doi.org/10.3390/w16182561
APA StyleXu, S., Li, Z., Yu, S., Chen, Z., Xu, J., Qiu, S., & Ge, S. (2024). Microalgal–Bacteria Biofilm in Wastewater Treatment: Advantages, Principles, and Establishment. Water, 16(18), 2561. https://doi.org/10.3390/w16182561






