Technological Advancements and Prospects for Near-Zero-Discharge Treatment of Semi-Coking Wastewater
Abstract
:1. Introduction
2. Pretreatment Process
2.1. Deoiling
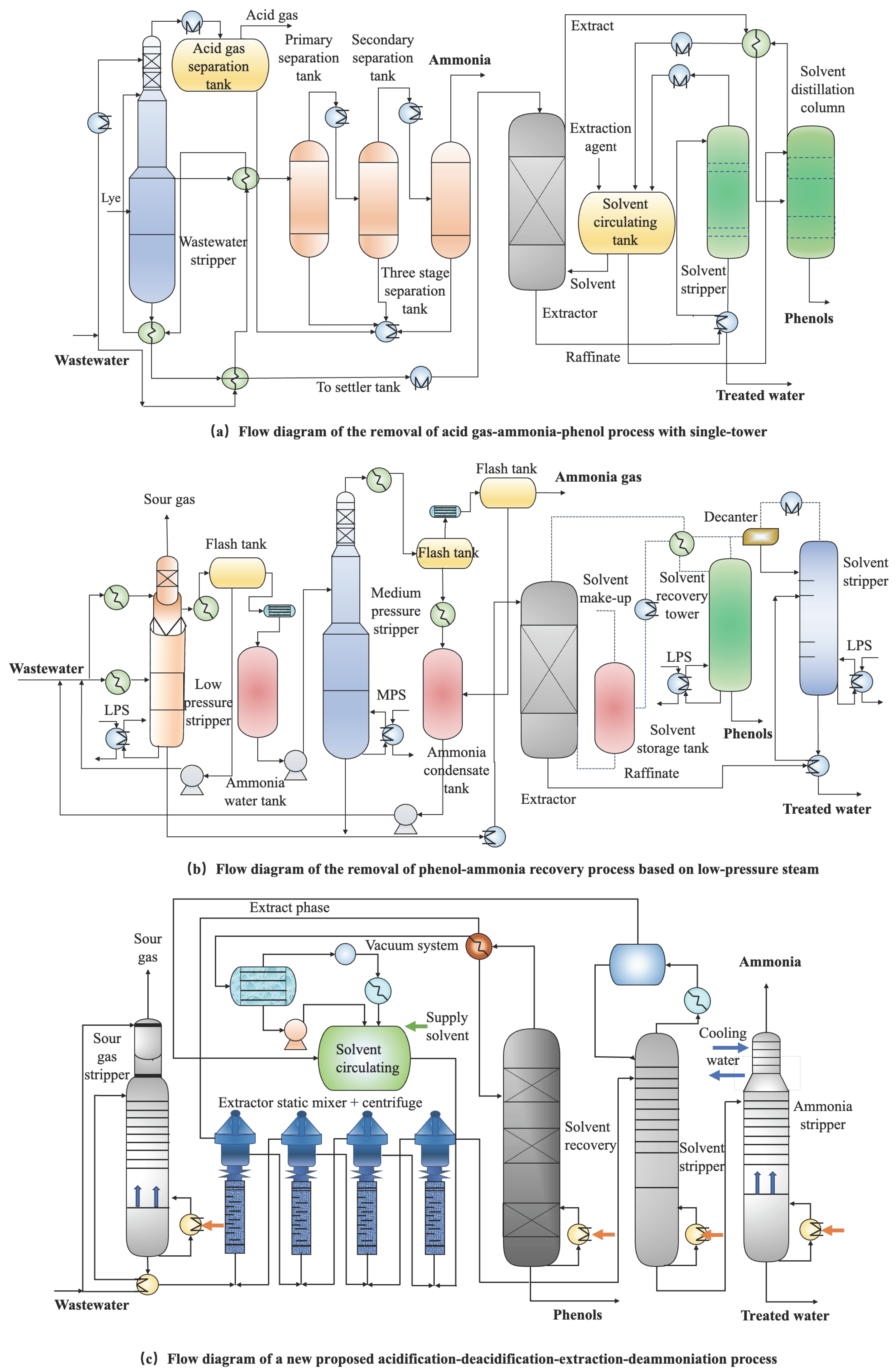
2.2. Phenol and Ammonia Recovery
3. Biochemical Treatment Process
3.1. Physicochemical Pretreatment to Enhance Biodegradability
3.2. Conventional Methods
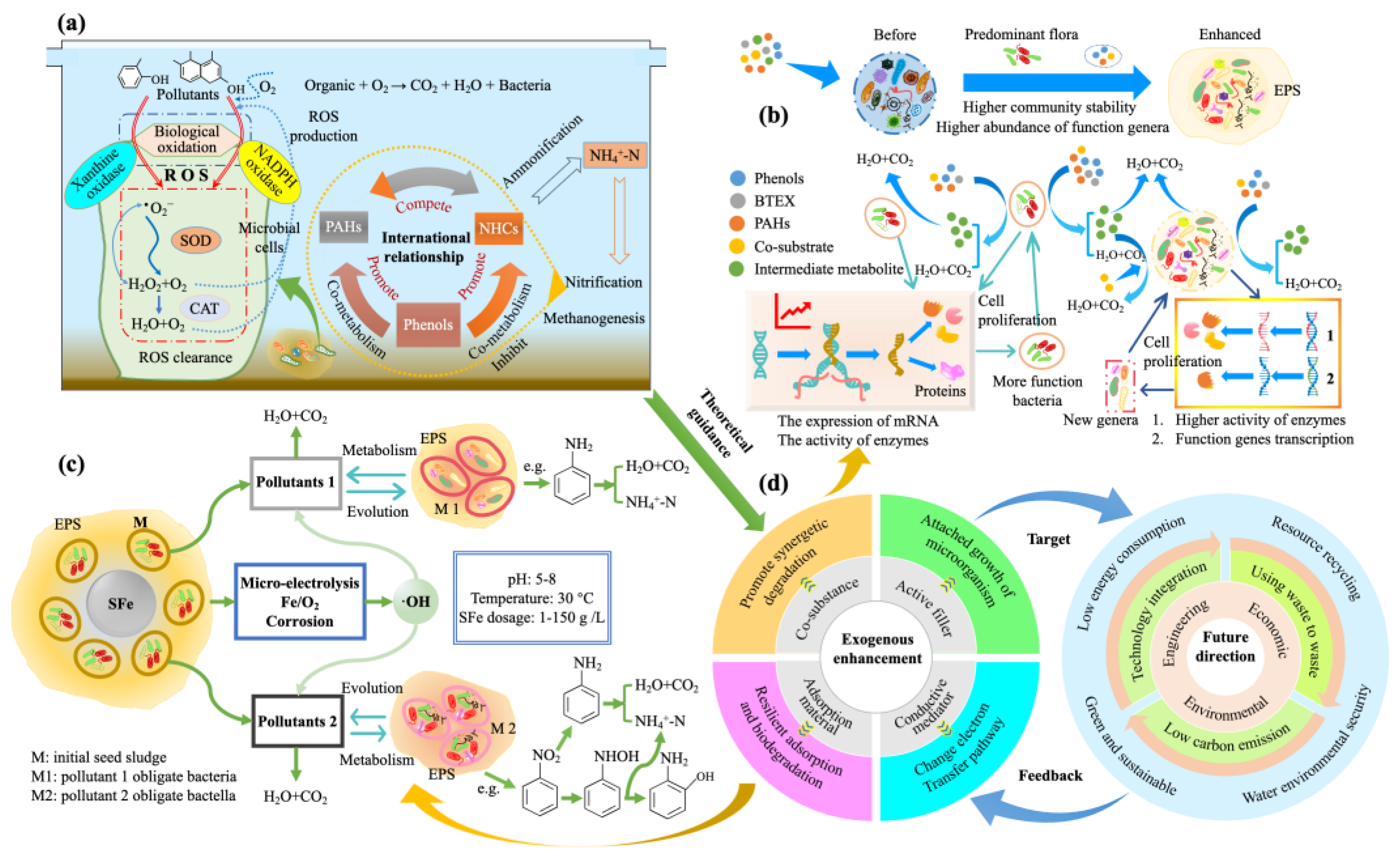
3.3. Bio-Enhancement
3.4. Development Tactics towards NZD
4. Advanced Treatment Processes
4.1. Advanced Oxidation Processes
4.2. Combined Processes
5. Resource Utilization
5.1. Tar
5.2. Phenol and Ammonia
| Process | Main Feature | Recovery Effect | Challenges | Application Examples |
|---|---|---|---|---|
| Lurgi Phenosolvan-CLL process | Extraction sequence: acidification–phenol extraction–acid gas stripping–ammonia recovery; DIPE as extractant; five-stage mixed-clarification tank for continuous countercurrent extraction. | Effluent: monophenol content < 20 μg/g; polyphenol extraction rate 85%; total phenol removal > 99%; free ammonia < 50 μg/g; COD < 3000 mg/L. | Effective energy conservation; verification needed for dephenolization under high acid gas concentrations; higher costs; no successful industrial examples in China. | Sasol Project, South Africa [156]; Great Plains Coal Gasification Project, USA [157]. |
| Sedin process | Phenol recovery precedes ammonia recovery; extraction sequence: acid gas removal, extraction, ammonia removal under alkaline conditions; DIPE as extractant. | Effluent: v total phenol < 900 mg/L; total ammonia < 300 mg/L; COD < 4000 mg/L. Recovery rate: phenol about 84%; ammonia about 97%. | Extraction before ammonia distillation leads to alkaline extraction water impacting phenol removal; inefficient acid removal tower; and challenges with acidic gases and ammonia in CGW causing equipment fouling. | Yima, Henan Province, China [158]. |
| Desulfurization–ammonia removal (double-tower)–extraction dephenolation process | Includes acid gas stripping tower, extraction tower, amine stripping tower, amine distillation tower, solvent distillation tower; DIPE as extractant. | Effluent: total phenol < 900 mg/L; total ammonia < 300 mg/L; COD < 4000 mg/L. Recovery rate: phenol ~84%; ammonia ~98%. | Acidic gas elimination leads to high ammonia concentrations and pH issues; and inadequate removal of phenolic compounds. | |
| Desulfurization–ammonia removal (single-tower)–extraction deadenylation process | Ammonia recovery before phenol; acidic extraction; DIPE or MIBK as extractant. | Effluent (DIPE): COD < 4000 mg/L; total phenol < 600 mg/L; ammonia nitrogen < 300 mg/L. Effluent (MIBK): COD < 2000 mg/L; total phenol < 300 mg/L; ammonia nitrogen < 300 mg/L. Recovery rate: phenol 86–95%; ammonia ~99.8%. | Widely applied in the coal chemical industry and wastewater treatment in China; variations in extraction solvent affect treatment efficiency. | Harbin, Heilongjiang Province, China [159]; Ordos, China [159]. |
5.3. Recycled Water
5.4. Salt
5.5. Sludge
6. Concluding Remarks and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lyu, L.; Fang, L. A Study on E-C Translation of BP Statistical Review of World Energy 2022 from the Perspective of Schema Theory. J. Linguist. Commun. Stud. 2023, 2, 10–14. [Google Scholar] [CrossRef]
- National Bureau of Statistics. Statistical Bulletin of the People's Republic of China on National Economic and Social Development for 2023; National Bureau of Statistics: Beijing, China, 2023.
- Wang, Y. Developing prospect for production of semi coke, coal tar and retort oven gas. Fuel Chem. Process. 2010, 41, 9–11. [Google Scholar] [CrossRef]
- Cao, Q. Technical Basis and Process Development for Extractive Separation of Emulsified Tar and Polyphenol from Semi-Coking Wastewater; South China University of Technology: Guangzhou, China, 2023. [Google Scholar]
- China Industry Research Network. Comprehensive Survey and Development Prospect Forecast Report of China's Semi-Coke Industry (2023–2029); China Industry Research Network, 2023; Available online: https://www.cir.cn/0/03/LanTanDeFaZhanQianJing.html (accessed on 11 August 2024).
- Shi, J.; Wan, N.; Li, L.; Li, Z.; Han, H. Review on treatment technologies of coal gasification wastewater in China. J. Clean. Prod. 2022, 333, 130166. [Google Scholar] [CrossRef]
- Kwiecińska, A.A.; Lajnert, R.; Bigda, R. Coke oven wastewater—Formation, treatment and utilization methods—A review. Proc. ECOpole 2017, 11, 19–28. [Google Scholar] [CrossRef]
- Luo, J.; Sheng, K. Comments on the treatment technologies of semi-coking wastewater. Ind. Water Treat. 2017, 37, 15–19. [Google Scholar]
- Liu, Y. Studies on the Removal Performance of Organic Pollutants in Semi-Coking Wastewater and the Biotransformation Characteristics of Quinoline Organic Compounds. Ph.D. Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2019. [Google Scholar]
- Liu, Y.; Liu, Y.J.; Liu, J. Study on the Removal Effects and Genotoxicity Evaluation of Phenols in a Semi-Coking Wastewater Treatment Stages. J. Water Chem. Technol. 2020, 42, 297–304. [Google Scholar] [CrossRef]
- Kwarciak-Kozlowska, A.; Worwag, M. The Impact of an Ultrasonic Field on the Efficiency of Coke Wastewater Treatment in a Sequencing Batch Reactor. Energies 2021, 14, 963. [Google Scholar] [CrossRef]
- Xu, L. Study on Optimization of Phenol—Containing Wastewater Treatment Process in the Semi-Coking. Master’s Thesis, Yanan University, Yan’an, China, 2020. [Google Scholar]
- Zhang, L.; Zhang, Z.; Liu, S.; An, L.; Wang, Y.; Meng, Q. Research Progress of Semi-coking Wastewater Pretreatment Technology. Coal Chem. Ind. 2018, 46, 53–57. [Google Scholar]
- Wu, Z.; Li, R.; Teng, J. Reformation practice of phenol-ammonia recovery unit for treating semi-coking wastewater. Ind. Water Treat. 2023, 43, 197–202. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Yan, L.; Li, J.; Xie, G.; Chen, S.; Wu, J. From pollutant to valuable phenolic resin: A novel reutilization strategy of industrial semi-coking wastewater. J. Clean. Prod. 2022, 377, 134477. [Google Scholar] [CrossRef]
- Tan, X.; Zheng, H.; Zhang, H.; Wang, Y.; Yue, T.; Yuan, L. Progress in the pretreatment methods for semi-coking wastewater and its current treatment conditions. Ind. Water Treat. 2014, 34, 13–16+21. [Google Scholar]
- Shu, J.; Zhang, Y.; Yang, Y.; Zhang, Z.; Zhang, Q.; Gao, W.; Feng, L.; Wei, X. A new eco-friendly method for efficient recovery and reuse of phenols in a semi-coke wastewater. Environ. Technol. Innov. 2022, 28, 102951. [Google Scholar] [CrossRef]
- Cheng, B.; Qu, X.; Zhang, R.; Bi, J. Research progress on resourceful pretreatment technologies of coal pyrolysis wastewater. Coal Process. Compr. Util. 2023, 10, 91–101. [Google Scholar] [CrossRef]
- Chen, B.; Yang, S.; Wu, Y.; Suo, M.; Qian, Y. Intensified phenols extraction and oil removal for industrial semi-coking wastewater: A novel economic pretreatment process design. J. Clean. Prod. 2020, 242, 118453. [Google Scholar] [CrossRef]
- Bai, X.; Nie, M.; Diwu, Z.; Wang, L.; Nie, H.; Wang, Y.; Yin, Q.; Zhang, B. Simultaneous biodegradation of phenolics and petroleum hydrocarbons from semi-coking wastewater: Construction of bacterial consortium and their metabolic division of labor. Bioresour. Technol. 2022, 347, 126377. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.; Li, P.; Zhang, T.; Sun, X. Research advances on recovery of oil, phenols, and ammonia in coal chemical wastewater. Chem. Ind. Eng. Process 2021, 40, 1048–1057. [Google Scholar] [CrossRef]
- Wei, L.; Li, M.; Gao, F.; Zhang, Y.; Li, C.; Zhang, Q. A novel integrated sequential air flotation in cold-rolling emulsion wastewater (CREW) treatment: Satisfying oil droplets growth laws at various stages. J. Water Process Eng. 2022, 48, 102852. [Google Scholar] [CrossRef]
- Gai, H.; Zhang, X.; Chen, S.; Wang, C.; Xiao, M.; Huang, T.; Wang, J.; Song, H. An improved tar-water separation process of low-rank coal conversion wastewater for increasing the tar yield and reducing the oil content in wastewater. Chem. Eng. J. 2020, 383, 123229. [Google Scholar] [CrossRef]
- Molaei, S.; Saleh, A.; Ghoulipour, V.; Seidi, S. Dissolved carbon dioxide flotation: An effective way for phase separation in emulsification microextraction method. J. Chromatogr. A 2015, 1388, 280–285. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhou, H.; Zhao, Q.; Xiao, Y. Pilot-scale trail of a novel integrated process towards promoting polymer-flooding sewage treatment by reducing back-mixed feedstocks. Chem. Eng. Process.-Process Intensif. 2023, 183, 109255. [Google Scholar] [CrossRef]
- Li, D. Research on Improvement of Treatment Effectiveness of Coal Chemical Wastewater Based on Nitrogen Flotation Degreasing. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2014. [Google Scholar]
- Zhao, M.; Jing, L.; Zhang, A.; Liu, X.; Liu, S.; Ma, Z. Expermental study on pretreatment of semi-coking wastewater by demulsification- coagulation-electrocoagulationg. Appl. Chem. Ind. 2023, 52, 2358–2361. [Google Scholar] [CrossRef]
- Joshi, P.K. A Microwave Acssisted, Chemical Free Approach for Separation of Crude Oil–Water Emulsions. Master’s Thesis, Lamar University-Beaumont, Beaumont, TX, USA, 2021. [Google Scholar]
- Kang, Z.-H.; Zhou, L.; Jiang, Q.; Zhang, Z.-Y.; Men, H.-K. Combination of microwave demulsification, ozone oxidation and biological aerated filter for advanced treatment of oilfield wastewater with low biodegradability. J. Water Reuse Desalination 2015, 5, 465–472. [Google Scholar] [CrossRef]
- Elmobarak, W.F.; Almomani, F. Application of magnetic nanoparticles for the removal of oil from oil-in-water emulsion: Regeneration/reuse of spent particles. J. Pet. Sci. Eng. 2021, 203, 108591. [Google Scholar] [CrossRef]
- Feng, D.; Yu, Z.; Chen, Y.; Qian, Y. Novel single stripper with side-draw to remove ammonia and sour gas simultaneously for coal-gasification wastewater treatment and the industrial implementation. Ind. Eng. Chem. Res. 2009, 48, 5816–5823. [Google Scholar] [CrossRef]
- Wu, D.; Liu, W.; Zhang, Q.; LU, S.; Chai, S.; Zhang, Y.; Huang, X. Research progress on high concentration ammonia nitrogen wastewater treatment methods. J. Salt Sci. Chem. Ind. 2020, 49, 10–15+19. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Luo, M.; Yu, G. Research on natural zeolite for the removal of ammonia nitrogen from coal chemical wastewater. Ind. Water Treat. 2018, 38, 46–49. [Google Scholar]
- Wang, W.; Ren, X.; Yang, K.; Hu, Z.; Yuan, S. Inhibition of ammonia on anaerobic digestion of synthetic coal gasification wastewater and recovery using struvite precipitation. J. Hazard. Mater. 2017, 340, 152–159. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, W.; Shan, Y.; Lv, H.; Song, Y. Treatment of high concentrated ammonia-nitrogen wastewater in coal gasification process by air stripping. Chem. Ind. Eng. 2010, 27, 486–489. [Google Scholar]
- Yu, F.; Song, Q.; Zheng, B.; Huang, L.; Shan, Y. A typical process of refinery sour water stripper unit and its design parameters. Petrochem. Technol. 2014, 43, 555–560. [Google Scholar]
- Gai, H.J.; Song, H.B.; Xiao, M.; Feng, Y.R.; Wu, Y.M.; Zhou, H.; Chen, B.H. Conceptual design of a modified phenol and ammonia recovery process for the treatment of coal gasification wastewater. Chem. Eng. J. 2016, 304, 621–628. [Google Scholar] [CrossRef]
- Greminger, D.C. Solvent extraction of phenols from water. Ind. Eng. Chem. Process Des. Dev. 1980, 21, 51–54. [Google Scholar] [CrossRef]
- Guo, C.; Cao, Q.; Chen, B.; Yang, S.; Qian, Y. Development of synergistic extraction process for highly efficient removal of phenols from coal gasification wastewater. J. Clean. Prod. 2019, 211, 380–386. [Google Scholar] [CrossRef]
- Yang, C.F.; Yu, Q.A.; Zhang, L.J.; Feng, J.Z. Solvent extraction process development and on-site trial-plant for phenol removal from industrial coal-gasification wastewater. Chem. Eng. J. 2006, 117, 179–185. [Google Scholar] [CrossRef]
- Liang, B.; Han, J. Experimental study on technological parameters optimization for extraction and removal of phenol from coal chemical wastewater. Shanxi Chem. Ind. 2018, 38, 150–152. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, Z.; Chen, Z.; Yu, Z. Process retrofit and industrial implementation of phenol and ammonia recovery from coal gasification wastewater. CIESC J. 2010, 61, 1821–1828. [Google Scholar]
- Cui, Z. A Study on Nearly Zero Liquid Discharge of High Concentrated Organic Wastewater of Fixed-Bed Coal Gasification. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2018. [Google Scholar]
- Chen, B.; Wu, Y.; Suo, M.; Yang, S. Liquid liquid equilibrium and data Correlation for quaternary (methyl isobutyl ketone plus n-pentanol plus phenol plus water) system at 101 kPa and 298.2 K: Phenol coextraction with synergistic solvents. J. Chem. Eng. Data 2020, 65, 4567–4574. [Google Scholar] [CrossRef]
- Feng, Y.; Song, H.; Xiao, M.; Lin, K.; Guo, K.; Gai, H. Development of Phenols Recovery process from coal gasification wastewater with mesityl oxide as a novel extractant. J. Clean. Prod. 2017, 166, 1314–1322. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, J.; Wei, H.; Song, X.; Ma, X. Study on high efficiency extraction of phenolic-containing wastewater from coal chemical industry. Coal Sci. Technol. 2019, 47, 219–224. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, K.; Jiang, M.; Lu, R. Phase equilibrium measurement, thermodynamics modeling and process simulation for extraction of phenols from coal chemical wastewater with methyl propyl ketone. Chem. Eng. Res. Des. 2019, 147, 587–596. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.; Dai, J.; Liu, W.; Yang, H.; Bai, Z. Efficient extraction of phenol from wastewater by ionic micro-emulsion method: Anionic and cationic. Chin. J. Chem. Eng. 2023, 58, 137–145. [Google Scholar] [CrossRef]
- Wang, L.; Song, S.; Jiang, S.; Wang, L. Adsorption process optimization for phenolic wastewater treatment with macroporous resin. Desalination Water Treat. 2019, 143, 192–196. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, R.; Duan, Z.; Wang, X. Comparative study of five different macroporous resins as separators and purifiers of apple polyphenols. In Proceedings of the 2nd Asian Horticultural Congress (AHC), Chengdu, China, 26–28 September 2016; pp. 347–353. [Google Scholar]
- Sriramoju, S.K.; Dash, P.S.; Majumdar, S. Meso-porous activated carbon from lignite waste and its application in methylene Blue adsorption and coke plant effluent treatment. J. Environ. Chem. Eng. 2021, 9, 104784. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, J.; Zhang, Y.; Zhang, L.; Zhang, G. Preatment of cetyltrimethy ammonium bromide-modified zeolite and its performance in adsorption of phenol from wastewater. Environ. Pollut. Control 2018, 40, 907–912. [Google Scholar] [CrossRef]
- Wei, R.; Pang, R.; Li, Y. Resource recycle of phenols by resin adsorption in coking wastewater. Ind. Water Treat. 2008, 28, 65–69. [Google Scholar]
- Yang, L.Y.; Liu, Y.J.; Zhang, A.N.; Liu, Z.; Yang, Z.Z.; Li, X.W.; Li, Z.H. Construction of aldehyde-based, ester-based hyper-cross-linked polar resin and its selective adsorption mechanism for phenol in coal chemical wastewater. Environ. Res. 2024, 246, 118140. [Google Scholar] [CrossRef]
- Lang, C. Separation and Regeneration of Phenolic Organic Ccompounds from Coal Chemical Wastewater Based on Resin Adsorption Method. Master's Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2022. [Google Scholar]
- Sun, W.; Xie, S.; Sun, Y.; Qiu, X.; Zhou, J. Preparation of Mn/Zn@PG catalyst for catalytic oxidation treatment of coal chemical wastewater. Int. J. Environ. Res. Public Health 2022, 19, 10812. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhou, S.; Sun, Y.; Xu, Y.; Zheng, H. W-Ag-Ti@γ-Al2O3 particle electrodes for enhanced electrocatalytic pretreatment of coal chemical wastewater. J. Environ. Chem. Eng. 2021, 9, 104681. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Q.; Zhang, X.; Shi, B.; Qin, D.; Wang, J.; Zhang, A.; Liu, Y. Degradation of phenol by Cu-Ni bimetallic-doped sludge biochar as a fenton-like catalyst: Mechanistic study and practical application. Sep. Purif. Technol. 2024, 347, 127554. [Google Scholar] [CrossRef]
- Liu, Z.; Teng, Y.; Xu, Y.; Zheng, Y.; Zhang, Y.; Zhu, M.; Sun, Y. Ozone catalytic oxidation of biologically pretreated semi-coking wastewater (BPSCW) by spinel-type MnFe2O4 magnetic nanoparticles. Sep. Purif. Technol. 2022, 278, 118277. [Google Scholar] [CrossRef]
- Xu, P.; Chu, X.; Li, Y.; Zhang, F. Research on coal chemical wastewater treatment by nano-TiO2 powder photocatalytic oxidation process. In Proceedings of the 2nd International Conference on Chemical Engineering and Advanced Materials (CEAM 2012), Guangzhou, China, 13–15 July 2012; pp. 2232–2236. [Google Scholar]
- Lee, C.; Kim, H.-H.; Park, N.-B. Chemistry of persulfates for the oxidation of organic contaminants in water. Membr. Water Treat. 2018, 9, 405–419. [Google Scholar] [CrossRef]
- Anotai, J.; Masomboon, N.; Chuang, C.; Lu, M. Persulfate oxidation for the aniline degradation in aqueous systems. Water Sci. Technol. 2011, 63, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Liu, M. The Selection of Oxidation Techniques on Pre-Treatment and Advanced Treatment of Coking Wastewater. Master’s Thesis, South China University of Technology, Guangzhou, China, 2018. [Google Scholar]
- Ma, W.; Zhang, X.; Han, H.; Shi, X.; Kong, Q.; Yu, T.; Zhao, F. Overview of enhancing biological treatment of coal chemical wastewater: New strategies and future directions. J. Environ. Sci. 2024, 135, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, B. A Study on Zero Liquid Discharge Technology from Coal Chemical Industry and Development of Treatment Process of High Concentrated Phenolic Ammonia Wastewater. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2020. [Google Scholar]
- Institute of Process Engineering, Chinese Academy of Sciences. Integrated Technology and Equipment for Whole-Process Pollution Control of Semi-Coking Wastewater. Available online: https://ipe.cas.cn/cgzh/jscg/gcsf/202111/t20211104_6244785.html (accessed on 19 July 2024).
- Cheng, X.; Qiao, H.; Ji, Q. Treatment of Wastewater after Ammonia and Phenol Recovery from Coal-to-SNG Plant by Biochemical + Ozone Catalytic Oxidation + MBR Process. Coal Chem. Ind. 2020, 48, 57–60. [Google Scholar] [CrossRef]
- Hou, Z.; Zhou, X.; Zhao, Z.; Dong, W.; Wang, H.; Liu, H.; Zeng, Z.; Xie, J. Advanced aromatic organic compounds removal from refractory coking wastewater in a step-feed three-stage integrated A/O bio-filter: Spectrum characterization and biodegradation mechanism. J. Environ. Manag. 2022, 322, 116140. [Google Scholar] [CrossRef]
- Chen, Z.; Li, D.; Wen, Q. Investigation of hydrolysis acidification process during anaerobic treatment of coal gasification wastewater (CGW): Evolution of dissolved organic matter and biotoxicity. Sci. Total Environ. 2020, 723, 137995. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, X.; Han, H.; Shi, X.; Kong, Q.; Yu, T.; Zhao, F. Biotoxicity dynamic change and key toxic organics identification of coal chemical wastewater along a novel full-scale treatment process. J. Environ. Sci. 2024, 138, 277–287. [Google Scholar] [CrossRef]
- Bao, H.; Li, Y.; Zhao, Y.; Zhang, L.; Pan, Y. Mechanism and experimental research on treatment of coal chemical wastewater by external circular anaerobic reactor. In Proceedings of the International Conference on Energy, Environment and Sustainable Development (EESD 2012), Jilin, China, 12–14 October 2013; pp. 2109–2114. [Google Scholar]
- Wan, N.; Shi, J.; Zhou, P.; Zhang, X.; Zhang, X.; Huang, Y.; Liu, J. Efficiency of submerged ceramic flat membrane bioreactor in the treatment of coal chemical wastewater. J. Water Process Eng. 2023, 53, 103638. [Google Scholar] [CrossRef]
- Hou, B.; Li, Z.; Deng, R.; Ren, B. Advanced treatment of coal chemical industry wastewater by expansive flow biological aerated filter. Fresenius Environ. Bull. 2017, 26, 4517–4521. [Google Scholar]
- Guo, J.; Chen, W.; Wang, L.; Ma, F. SBR co-metabolism process for the advanced treatment of petrochemical wastewater. Chem. Ind. Eng. Process 2013, 32, 2768–2772+2777. [Google Scholar]
- Kong, W.; Li, Y.; Zhang, Y.; Mei, Y.; Tabassum, S. Biological treatment of refractory organic compounds in coal gasification wastewater: A review. J. Water Process Eng. 2024, 60, 105255. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, H.; Wu, C.; Qiu, G.; Feng, C.; Wei, C. Structure and function of microbial community involved in a novel full-scale prefix oxic coking wastewater treatment O/H/O system. Water Res. 2019, 164, 114963. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tabassum, S.; Chu, C.; Zhang, Z. Inhibitory effect of high phenol concentration in treating coal gasification wastewater in anaerobic biofilter. J. Environ. Sci. 2018, 64, 207–215. [Google Scholar] [CrossRef]
- Fan, Y.; Yan, D.; Chen, X.; Ran, X.; Cao, W.; Li, H.; Wan, J. Novel insights into the co-metabolism of pyridine with different carbon substrates: Performance, metabolism pathway and microbial community. J. Hazard. Mater. 2023, 465, 133396. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Y.; Cao, H.; Sheng, Y.; Li, H.; Li, Y.; Zhao, H.; Gui, X. Metagenomic insights into the microbiota profiles and bioaugmentation mechanism of organics removal in coal gasification wastewater in an anaerobic/anoxic/oxic system by methanol. Bioresour. Technol. 2018, 264, 106–115. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Yang, F.; Tan, Z.; Chen, J. Biodegradability of some nitrogenous heterocyclic compounds and co-degradationwith phenol by denitrifiers in anoxic sludge reactor. Water Sci. Technol. 2015, 72, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Wang, Q.; Wu, J.; Liu, T.; Hong, Y.; Liu, W.; Mei, J. The effect of anaerobic co-substrate on removal of COD, phenol and methane production in coal gasification wastewater treatment. Pol. J. Environ. Stud. 2020, 29, 4175–4181. [Google Scholar] [CrossRef]
- Rea, V.S.G.; Bueno, B.E.; Cerqueda-Garcia, D.; Munoz Sierra, J.D.; Spanjers, H.; van Lier, J.B. Degradation of p-cresol, resorcinol, and phenol in anaerobic membrane bioreactors under saline conditions. Chem. Eng. J. 2022, 430, 132672. [Google Scholar] [CrossRef]
- Zhao, Q.; Han, H.; Hou, B.; Zhuang, H.; Jia, S.; Fang, F. Nitrogen removal from coal gasification wastewater by activated carbon technologies combined with short-cut nitrogen removal process. J. Environ. Sci. 2014, 26, 2231–2239. [Google Scholar] [CrossRef]
- Zheng, M.; Zhu, H.; Han, Y.; Xu, C.; Zhang, Z.; Han, H. Comparative investigation on carbon-based moving bed biofilm reactor (MBBR) for synchronous removal of phenols and ammonia in treating coal pyrolysis wastewater at pilot-scale. Bioresour. Technol. 2019, 288, 121590. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Wang, Q.; Wu, J.; Liu, T.; Liu, H.; Hong, Y.; Huang, T. Enhanced anaerobic co-metabolism of coal gasification wastewater via the assistance of zero-valent iron. J. Water Process Eng. 2021, 40, 101817. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Zhang, S.; Liu, Z.; Hu, T.; Wang, X.; Peng, X.; Dai, H.; Wu, J.; Hu, F. Iron-carbon micro-electrolysis material enhanced high-solid anaerobic digestion: Performance and microbial mechanism. Biochem. Eng. J. 2024, 201, 109132. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Liu, L.; Tabassum, S.; Sun, J.; Hong, Y. Enhanced phenols removal and methane production with the assistance of graphene under anaerobic co-digestion conditions. Sci. Total Environ. 2021, 759, 143523. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Shi, J.; Xu, C.; Ma, W.; Zhang, Z.; Zhu, H.; Han, H. Ecological and functional research into microbiomes for targeted phenolic removal in anoxic carbon-based fluidized bed reactor (CBFBR) treating coal pyrolysis wastewater (CPW). Bioresour. Technol. 2020, 308, 123308. [Google Scholar] [CrossRef]
- Zheng, M.; Xu, C.; Zhong, D.; Han, Y.; Zhang, Z.; Zhu, H.; Han, H. Synergistic degradation on aromatic cyclic organics of coal pyrolysis wastewater by lignite activated coke-active sludge process. Chem. Eng. J. 2019, 364, 410–419. [Google Scholar] [CrossRef]
- Shi, J.; Han, Y.; Xu, C.; Han, H. Enhanced anaerobic degradation of selected nitrogen heterocyclic compounds with the assistance of carboxymethyl cellulose. Sci. Total Environ. 2019, 689, 781–788. [Google Scholar] [CrossRef]
- Zheng, M.; Shi, J.; Xu, C.; Han, Y.; Zhang, Z.; Han, H. Insights into electroactive biofilms for enhanced phenolic degradation of coal pyrolysis wastewater (CPW) by magnetic activated coke (MAC): Metagenomic analysis in attached biofilm and suspended sludge. J. Hazard. Mater. 2020, 395, 122688. [Google Scholar] [CrossRef]
- Zhu, H.; Han, Y.; Ma, W.; Han, H.; Ma, W.; Xu, C. New insights into enhanced anaerobic degradation of coal gasification wastewater (CGW) with the assistance of graphene. Bioresour. Technol. 2018, 262, 302–309. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhu, H.; Shan, S.; Zhang, L.; Fang, C.; Shi, Y. Potential enhancement of direct interspecies electron transfer for anaerobic degradation of coal gasification wastewater using up-flow anaerobic sludge blanket (UASB) with nitrogen doped sewage sludge carbon assisted. Bioresour. Technol. 2018, 270, 230–235. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Y.; Liu, X.; Zhang, A.; Yang, R.; Han, Y.; Liu, P.; He, H.b.; Li, Z. Regulation methods and enhanced mechanism on the efficient degradation of aromatics in biochemical treatment system of coal chemical wastewater. J. Environ. Manag. 2023, 348, 119358. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, W.; Li, J.; Li, J. Degradation of different wastewater by a biological sponge iron system: Microbial growth and influencing factors. RSC Adv. 2024, 14, 17318–17325. [Google Scholar] [CrossRef]
- Mueller, C.; da Silveira Silveira, S.F.; Daloso, D.d.M.; Mendes, G.C.; Merchant, A.; Kuki, K.N.; Oliva, M.A.; Loureiro, M.E.; Miyasaka Almeida, A. Ecophysiological responses to excess iron in lowland and upland rice cultivars. Chemosphere 2017, 189, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, B.R.; Neto, W.B.; Machado, A.E.H.; Trovo, A.G. Biodiesel wastewater treatment by coagulation-flocculation: Evaluation and optimization of operational parameters. J. Braz. Chem. Soc. 2017, 28, 800–807. [Google Scholar] [CrossRef]
- Gai, H.; Guo, K.; Zhang, N.; Feng, Y.; Lin, K.; Song, H.; Xiao, M. Application of resin in advanced treatment of coal chemical industry wastewater. In Proceedings of the International Conference on Energy Development and Environmental Protection (EDEP), Guilin, China, 18–20 August 2017; pp. 383–388. [Google Scholar]
- Zhao, F.; Mu, B.; Zhang, T.; Dong, C.; Zhu, Y.; Zong, L.; Wang, A. Synthesis of biochar/clay mineral nanocomposites using oil shale semi-coke waste for removal of organic pollutants. Biochar 2023, 5, 7. [Google Scholar] [CrossRef]
- Ji, Q.; Tabassum, S.; Hena, S.; Silva, C.G.; Yu, G.; Zhang, Z. A review on the coal gasification wastewater treatment technologies: Past, present and future outlook. J. Clean. Prod. 2016, 126, 38–55. [Google Scholar] [CrossRef]
- Manna, M.; Sen, S. Advanced oxidation process: A sustainable technology for treating refractory organic compounds present in industrial wastewater. Environ. Sci. Pollut. Res. 2023, 30, 25477–25505. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, P.; Jiang, Y.; Sun, L.; Sun, X. Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods. Chemosphere 2023, 311, 136993. [Google Scholar] [CrossRef]
- Peng, R.; Yu, P.; Luo, Y. Coke plant wastewater posttreatment by fenton and electro-fenton processes. Environ. Eng. Sci. 2017, 34, 89–95. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J.; Dong, J.; Liu, H.; Sun, X. Treatment of coking wastewater by an advanced Fenton oxidation process using iron powder and hydrogen peroxide. Chemosphere 2012, 86, 409–414. [Google Scholar] [CrossRef]
- Meng, G.; Jiang, N.; Wang, Y.; Zhang, H.; Tang, Y.; Lv, Y.; Bai, J. Treatment of coking wastewater in a heterogeneous electro-Fenton system: Optimization of treatment parameters, characterization, and removal mechanism. J. Water Process Eng. 2022, 45, 102482. [Google Scholar] [CrossRef]
- Tan, L.; Lu, S.; Fang, Z.; Cheng, W.; Tsang, E.P. Enhanced reductive debromination and subsequent oxidative ring-opening of decabromodiphenyl ether by integrated catalyst of nZVI supported on magnetic Fe3O4 nanoparticles. Appl. Catal. B—Environ. 2017, 200, 200–210. [Google Scholar] [CrossRef]
- Wang, G.; Tang, K.; Hambly, A.C.; Zhang, Y.; Andersen, H.R. Sustainable and reagentless fenton treatment of complex wastewater. Environ. Sci. Technol. 2023, 57, 626–634. [Google Scholar] [CrossRef]
- Xing, M.; Xu, W.; Dong, C.; Bai, Y.; Zeng, J.; Zhou, Y.; Zhang, J.; Yin, Y. Metal Sulfides as Excellent Co-Catalysts for H2O2 Decomposition in Advanced Oxidation Processes. Chem 2018, 4, 1359–1372. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, F.; Hu, Y.; Feng, C.; Wu, H. Ozonation in water treatment: The generation, basic properties of ozone and its practical application. Rev. Chem. Eng. 2017, 33, 49–89. [Google Scholar] [CrossRef]
- Yu, G.; Wu, Y.; Cao, H.; Ge, Q.; Dai, Q.; Sun, S.; Xie, Y. Insights into the Mechanism of Ozone Activation and Singlet Oxygen Generation on N-Doped Defective Nanocarbons: A DFT and Machine Learning Study. Environ. Sci. Technol. 2022, 56, 7853–7863. [Google Scholar] [CrossRef]
- Yu, G.; Wang, Y.; Cao, H.; Zhao, H.; Xie, Y. Reactive Oxygen Species and Catalytic Active Sites in Heterogeneous Catalytic Ozonation for Water Purification. Environ. Sci. Technol. 2020, 54, 5931–5946. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, J.; Chen, Z. Combination of ozonation and biological aerated filter (BAF) for bio-treated coking wastewater. Sep. Purif. Technol. 2014, 132, 610–615. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, C.; Hu, Y.; Wu, H. Zinc ferrite catalysts for ozonation of aqueous organic contaminants: Phenol and bio-treated coking wastewater. Sep. Purif. Technol. 2015, 156, 625–635. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Li, B.; Liu, X.; Zhou, X.; Zhang, C.; Qin, L.; Li, L.; Zhang, M.; Yi, H.; et al. Heteroatom doping in metal-free carbonaceous materials for the enhancement of persulfate activation. Chem. Eng. J. 2022, 427, 131655. [Google Scholar] [CrossRef]
- Zhang, Z.; Yue, X.; Duan, Y.; Zhang, X.; Gao, Y.; Zhu, R.; Cui, X. Sulfate radical oxidation combined with iron flocculation for upgrading biological effluent of coking wastewater. RSC Adv. 2018, 8, 38765–38772. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, S.; Chen, Y.; Zhong, D.; Du, Q.; Li, J.; Li, R.; Du, X.; Zhang, J. Fe3O4-CuO@Lignite activated coke activated persulfate advanced treatment of phenolic wastewater from coal chemical industry. Environ. Res. 2022, 213, 113601. [Google Scholar] [CrossRef]
- Dong, P.; Song, Y.; Wu, L.; Wang, Y.; Yin, N.; Wang, Y.; Li, Y. Synergistic removal of organics from semi-coke wastewater by persulfate and cyanide tailings. Sep. Purif. Technol. 2024, 333, 126056. [Google Scholar] [CrossRef]
- Wang, H.; Quan, B.; Bo, G.; Zhang, Y.; Liu, L.; Zhang, J.; Zhang, X.; Zhang, C. Advanced oxidation treatment of dissolved organic matter from wastewater treatment plant secondary effluent using scattering electrical reactor. J. Clean. Prod. 2020, 267, 122258. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, J.; Zhao, G.; Tang, Y.; Li, J.; Li, F.; Zhuang, H.; Chen, J.; Lin, H.; Zhang, Y. Investigation on an electrochemical pilot equipment for water softening with an automatic descaling system: Parameter optimization and energy consumption analysis. J. Clean. Prod. 2020, 276, 123178. [Google Scholar] [CrossRef]
- Anglada, A.; Urtiaga, A.; Ortiz, I. Contributions of electrochemical oxidation to waste-water treatment: Fundamentals and review of applications. J. Chem. Technol. Biotechnol. 2009, 84, 1747–1755. [Google Scholar] [CrossRef]
- Ghime, D.; Ghosh, P. Removal of organic compounds found in the wastewater through electrochemical advanced oxidation processes: A Review. Russ. J. Electrochem. 2019, 55, 591–620. [Google Scholar] [CrossRef]
- Shestakova, M.; Sillanpaa, M. Electrode materials used for electrochemical oxidation of organic compounds in wastewater. Rev. Environ. Sci. Bio-Technol. 2017, 16, 223–238. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Wang, J. Iron-based dual active site-mediated peroxymonosulfate activation for the degradation of emerging organic pollutants. Environ. Sci. Technol. 2021, 55, 15412–15422. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Wang, C.; Ma, J.; Wang, J. Preparation of Ti4O7 Reactive Electrochemical Membrane for Electrochemical Oxidation of Coking Wastewater. Sustainability 2023, 15, 15488. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Radiation-induced degradation of sulfamethoxazole in the presence of various inorganic anions. Chem. Eng. J. 2018, 351, 688–696. [Google Scholar] [CrossRef]
- Trojanowicz, M. Removal of persistent organic pollutants (POPs) from waters and wastewaters by the use of ionizing radiation. Sci. Total Environ. 2020, 718, 134425. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Hu, C. Advanced treatment of coking wastewater: Recent advances and prospects. Chemosphere 2024, 349, 140923. [Google Scholar] [CrossRef]
- Jin, X.; Li, E.; Lu, S.; Qiu, Z.; Sui, Q. Coking wastewater treatment for industrial reuse purpose: Combining biological processes with ultrafiltration, nanofiltration and reverse osmosis. J. Environ. Sci. 2013, 25, 1565–1574. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Sun, H.; Cheng, F.; Liu, Y. Advanced treatment of biologically treated coking wastewater by membrane distillation coupled with pre-coagulation. Desalination 2016, 380, 43–51. [Google Scholar] [CrossRef]
- Xia, Y.; Li, W.; He, X.; Liu, D.; Sun, Y.; Chang, J.; Liu, J. Efficient Removal of Organic Matter from Biotreated Coking Wastewater by Coagulation Combined with Sludge-Based Activated Carbon Adsorption. Water 2022, 14, 2446. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, W.; Li, B.; Duan, J.; Lv, Y.; Liu, W.; Ying, W. Combined fenton oxidation and biological activated carbon process for recycling of coking plant effluent. J. Hazard. Mater. 2011, 189, 308–314. [Google Scholar] [CrossRef]
- Wei, Q.; Qiao, S.; Sun, B.; Zou, H.; Chen, J.; Shao, L. Study on the treatment of simulated coking wastewater by O3 and O3/Fenton processes in a rotating packed bed. RSC Adv. 2015, 5, 93386–93393. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Li, C.; Liu, W.; Lichtfouse, E. High increase in biodegradability of coking wastewater enhanced by Mn ore tailings in Fenton/O3 combined processes. Int. J. Environ. Sci. Technol. 2020, 18, 173–184. [Google Scholar] [CrossRef]
- Qin, Q.; Yang, H.; Xu, H.; Deng, J.; Zhao, R.; Huang, G.; Wang, P.; Wang, J. Experiment study on the separation of bituminous coal adsorption and the synergism of ultraviolet and electrochemistry in the pretreatment of coal chemical wastewater. Fuel 2021, 288, 119712. [Google Scholar] [CrossRef]
- Xie, L.; Liu, J.; Lu, X.; Wang, H.; Yue, X. Advanced treatment of coking wastewater by electrocatalytic oxidation-activated carbon adsorption. Ind. Water Treat. 2021, 41, 69–74. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Yang, L.; Li, W.; Wang, W.; Liu, P. Ti-Sn-Ce/bamboo biochar particle electrodes for enhanced electrocatalytic treatment of coking wastewater in a three-dimensional electrochemical reaction system. J. Clean. Prod. 2020, 258, 120273. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Li, J.; Yin, N.; Dong, P.; Wang, Y.; Long, J.; Li, W. Treatment of semi-coking wastewater by synergistic process of flocculation-electrolytic oxidation. China Environ. Sci. 2024, 44, 2063–2072. [Google Scholar] [CrossRef]
- Bi, Q.; Xue, J.; Guo, Y.; Li, G.; Lei, M. COD removal from real semi-coke wastewater by electro-fenton technology. Chin. J. Environ. Eng. 2012, 6, 4310–4314. [Google Scholar]
- Wang, B.; Chang, X.; Ma, H. Electrochemical Oxidation of Refractory Organics in the Coking Wastewater and Chemical Oxygen Demand (COD) Removal under Extremely Mild Conditions. Ind. Eng. Chem. Res. 2008, 47, 8478–8483. [Google Scholar] [CrossRef]
- Hou, Z.; Zhou, J.; Wu, L.; Zhang, Q.; Song, Y.; Tian, Y. Study on synergetic-photoelectrocatalytic degradation of phenol by nano-TiO2 Three-dimensional electrode. Nonferrous Met. Eng. 2019, 9, 1–6+28. [Google Scholar]
- Fu, L.; Li, M.; Li, Y.; Xi, H.; Yu, Y.; Wu, C.; Zhou, Y. Characterization of ozone dosage reduction mechanism in catalytic ozonation process coupled with coagulation and flocculation. Sep. Purif. Technol. 2021, 279, 119761. [Google Scholar] [CrossRef]
- Jing, Y.; Chaplin, B.P. Electrochemical impedance spectroscopy study of membrane fouling characterization at a conductive sub-stoichiometric TiO2 reactive electrochemical membrane: Transmission line model development. J. Membr. Sci. 2016, 511, 238–249. [Google Scholar] [CrossRef]
- Urtiaga, A. Electrochemical technologies combined with membrane filtration. Curr. Opin. Electrochem. 2021, 27, 100691. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, S.; Jin, R.; Zhou, J.; Quan, X. A novel aerobic electrochemical membrane bioreactor with CNTs hollow fiber membrane by electrochemical oxidation to improve water quality and mitigate membrane fouling. Water Res. 2019, 151, 54–63. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, C.; Quan, B.; Tang, Y.; Zhang, Y.; Su, C.; Zhao, G. Electrocoagulation coupled with conductive ceramic membrane filtration for wastewater treatment: Toward membrane modification, characterization, and application. Water Res. 2022, 220, 118612. [Google Scholar] [CrossRef]
- Nabgan, W.; Saeed, M.; Jalil, A.A.; Nabgan, B.; Gambo, Y.; Ali, M.W.; Ikram, M.; Fauzi, A.A.; Owgi, A.H.K.; Hussain, I.; et al. A state of the art review on electrochemical technique for the remediation of pharmaceuticals containing wastewater. Environ. Res. 2022, 210, 112975. [Google Scholar] [CrossRef]
- Misal, S.N.; Lin, M.-H.; Mehraeen, S.; Chaplin, B.P. Modeling electrochemical oxidation and reduction of sulfamethoxazole using electrocatalytic reactive electrochemical membranes. J. Hazard. Mater. 2020, 384, 121420. [Google Scholar] [CrossRef]
- Zhi, D.; Wang, J.; Zhou, Y.; Luo, Z.; Sun, Y.; Wan, Z.; Luo, L.; Tsang, D.C.W.; Dionysiou, D.D. Development of ozonation and reactive electrochemical membrane coupled process: Enhanced tetracycline mineralization and toxicity reduction. Chem. Eng. J. 2020, 383, 123149. [Google Scholar] [CrossRef]
- Giwa, A.; Dindi, A.; Kujawa, J. Membrane bioreactors and electrochemical processes for treatment of wastewaters containing heavy metal ions, organics, micropollutants and dyes: Recent developments. J. Hazard. Mater. 2019, 370, 172–195. [Google Scholar] [CrossRef]
- Mou, Y.; Xia, Y.; Zhang, S.; He, Y.; Shen, W.; Li, J. Aniline removed from simulated wastewater by electro-Fenton process using electric energy from photovoltaic modules. Desalination Water Treat. 2022, 247, 173–183. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, W.; Zhang, J.; Xie, L.; Zeng, X.; Zhong, J.; Zhao, O.; Yang, K.; Li, Z.; Zou, R.; et al. Carbon reduction measures-based life cycle assessment of the photovoltaic-supported sewage treatment system. Sustain. Cities Soc. 2024, 101, 105074. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, C.; Gong, J.; Liu, K.; Cao, Y. Development of coalescence coupled hydrocyclone assembly. China Pet. Mach. 2018, 46, 83–87. [Google Scholar] [CrossRef]
- He, M.; Zhang, R.; Zhang, K.; Liu, Y.; Su, Y.; Jiang, Z. Reduced graphene oxide aerogel membranes fabricated through hydrogen bond mediation for highly efficient oil/water separation. J. Mater. Chem. A 2019, 7, 11468–11477. [Google Scholar] [CrossRef]
- Ikhsan, S.N.W.; Yusof, N.; Aziz, F.; Misdan, N.; Ismail, A.F.; Lau, W.-J.; Jaafar, J.; Salleh, W.N.W.; Hairom, N.H.H. Efficient separation of oily wastewater using polyethersulfone mixed matrix membrane incorporated with halloysite nanotube-hydrous ferric oxide nanoparticle. Sep. Purif. Technol. 2018, 199, 161–169. [Google Scholar] [CrossRef]
- Cao, H.; Xu, G.; Ning, P.; Shi, S. Co-extraction and synergistic detoxification technology and its application in high-concentration wastewater from coal chemical industry. Chin. J. Process Eng. 2019, 19, 81–92. [Google Scholar]
- He, X.; Wang, C. Zero discharge technology and solution idea of waste water from new coal chemistry. Coal Sci. Technol. 2015, 43, 120–124. [Google Scholar] [CrossRef]
- van Dyk, J.C.; Keyser, M.J.; Coertzen, A. Syngas production from South African coal sources using Sasol-Lurgi gasifiers. Int. J. Coal Geol. 2006, 65, 243–253. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Yang, Z. Research progresses on phenols and ammonia nitrogen recovery and treatment methods in coal chemical wastewater (CCW). Environ. Technol. Rev. 2022, 11, 226–242. [Google Scholar] [CrossRef]
- Wu, X. Research on Problems Facing and Technology Optimization of Coal Chemical Industry Wastewater Treatment Technology. Master's Thesis, Harbin Institute of Technology, Harbin, China, 2016. [Google Scholar]
- Yu, Z.; Chen, Y.; Feng, D.; Qian, Y. Process development, simulation, and industrial implementation of a new coal-gasification wastewater treatment installation for phenol and ammonia removal. Ind. Eng. Chem. Res. 2010, 49, 2874–2881. [Google Scholar] [CrossRef]
- Wang, L. Research of Reuse of Coking Wastewater. Master's Thesis, East China University of Science and Technology, Shanghai, China, 2017. [Google Scholar]
- Ma, X.; An, D.; Kou, Y.; Tan, G. Design and application of the combined process of membrane method for the advanced treatment of coking wastewater. Ind. Water Treat. 2017, 37, 102–105. [Google Scholar]
- Chen, F.; Zhang, Z.; Zeng, F.; Yang, Y.; Li, X. Pilot-scale treatment of hypersaline coal chemical wastewater with zero liquid discharge. Desalination 2021, 518, 115303. [Google Scholar] [CrossRef]
- Chen, J.; Tu, Y.; Shao, G.; Zhang, F.; Zhou, Z.; Tian, S.; Ren, Z. Catalytic ozonation performance of calcium-loaded catalyst (Ca-C/Al2O3) for effective treatment of high salt organic wastewater. Sep. Purif. Technol. 2022, 301, 121937. [Google Scholar] [CrossRef]
- Liu, B.; Govindan, R.; Muthuchamy, M.; Cheng, S.; Li, X.; Ye, L.; Wang, L.; Guo, S.; Li, W.; Alharbi, N.S.; et al. Halophilic archaea and their extracellular polymeric compounds in the treatment of high salt wastewater containing phenol. Chemosphere 2022, 294, 133732. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, Y.; Su, N.; Lu, H.; Li, J.; Li, J.; Hao, H. Research on fractional crysallization technologies for recovering salts from high salinity wastewater. Chem. Ind. Eng. 2019, 36, 10–23. [Google Scholar] [CrossRef]
- Dong, J.; Liu, C.; Xu, S.; Qi, Z.; Gao, Q.; Dong, Y.; Shen, J. Influencing factors of bipolar membrane electrodialysis applied to regeneration of acid and base from high-salt wastewater. Membr. Sci. Technol. 2023, 43, 116–126. [Google Scholar] [CrossRef]
- Du, H. Investigation on Factional Crystallization of High Salinity.Wastewater from Coal Industry. Master's Thesis, Zhengzhou University, Zhengzhou, China, 2021. [Google Scholar]
- Ma, H.; Wang, H.; Tian, C.; Chang, Y.; Yuan, W.; Qi, Y.; Chao, Z.; Chen, W.; Lv, W. An optimized design for zero liquid discharge from coal chemical industry: A case study in China. J. Clean. Prod. 2021, 319, 128572. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Ye, G.; Wei, H.; Wei, J.; Li, F.; Jiang, C.; Liu, X.; Fu, Y.; Zhu, Z. Formation and treatment methods evaluation of solid-phase substances in coking wastewater treatment. Acta Sci. Circumstantiae 2020, 40, 2548–2556. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, H.; Guo, Y.; Cheng, F. Effect of addition of coking sludge on the preparation of activated coke and its adsorption performance of pb2+. Coal Convers. 2020, 43, 65–72. [Google Scholar] [CrossRef]







| Adsorbent | Removal Efficiency | Advantage | Limitations | Regeneration Ability | Application |
|---|---|---|---|---|---|
| Macroporous Resins [49,50] | High (up to 95%) | High selectivity for phenolic compounds, flexible, long lifespan, narrow pore distribution | Sensitive to fouling (e.g., oils, colored substances), expensive regeneration agents | Good, possible chemical regeneration | Ideal for biochemical pretreatment and post-treatment; suitable for coal chemical wastewater (COW, SCOW) |
| Activated Carbon [51] | Moderate to High (60–90%) | Large surface area, effective for various pollutants, relatively low cost | Prone to saturation and fouling, difficult regeneration | Moderate, possible thermal regeneration | Widely used in industrial wastewater treatment, general use for phenolic waste removal |
| Zeolites [52] | Moderate (50–80%) | High thermal and chemical stability, reusable, low-cost | Lower adsorption capacity for organic pollutants compared to resins | Good, reusable after heat treatment | Suitable for initial treatment and multi-stage adsorption in conjunction with other methods |
| Process | Principle | Advantage | Challenges for SCOW |
|---|---|---|---|
| Hydrolysis Acidification (HA) [69] | Hydrolysis is the process where microorganisms complete biocatalytic oxidation reactions by releasing extracellular free enzymes or fixing enzymes. Acidification is a typical fermentation process where microorganisms produce mainly organic acids. | Converts difficult-to-biodegrade substances into easily biodegradable substances; improves wastewater biodegradability; widely used as a pretreatment for low-concentration, refractory wastewater; superior impact load resistance. | Inadequate removal of toxic pollutants in short-term processes; requires refinement of parameters or implementation of biological enhancement. |
| Anoxic/Oxic (A/O) [70] | In the anaerobic phase, heterotrophic bacteria degrade complex organic compounds into simpler ones, facilitating ammonification to break down proteins into free ammonia. During the aerobic phase, autotrophic bacteria metabolize and convert ammonia into nitrates and remove organic pollutants. Under anoxic conditions, denitrification occurs, reducing nitrates to nitrogen gas. | Widely used; simple process and low investment cost. | Poor removal of nitrogen, phosphorus, and refractory organic matter; not suitable for direct treatment of SCOW. |
| Anaerobic/Anoxic/Oxic process (A/A/O) [70] | Consistent with the A/O process. | Widely used; better nitrogen and phosphorus removal efficiency than A/O; robust against abrupt load changes. | Unsatisfactory removal efficiency for refractory organic compounds; inhibitory effect of toxic substances on nitrification and denitrification bacteria. |
| Sequencing Batch Reactor (SBR) [70] | A synchronized technology for nitrogen and carbon removal in activated sludge wastewater treatment, operating in an intermittent aeration mode. | Reduced number of processing equipment; simplified structure; easy operation and maintenance; process adjustable to water quality and quantity; impact load resistance. | Without a primary sedimentation tank, it is prone to floating sludge; and requires combination with other processes like co-digestion acidification to treat SCOW. |
| Up-flow Anaerobic Sludge Blanket (UASB) [71] | Forms good settling sludge floc and combines it with a sludge sedimentation system in the reactor to separate gas, liquid, and solid phases. | Widely used for CCW; high organic loading; long hydraulic retention time (HRT); and no need for stirring equipment or sedimentation tanks. | It is sensitive to sudden changes in water quality and load, and its shock resistance is slightly poor. It is necessary to enhance the tolerance of microorganisms to toxic pollutants. |
| Membrane Bioreactor (MBR) [72] | Combines high-efficiency membrane separation technology with the traditional activated sludge process; retains active sludge and large molecular organic substances in the biological reaction pool, eliminating the need for a secondary sedimentation tank. | Excellent organic matter removal efficiency, especially for phenolic substances and difficult-to-degrade organic matter; superior effluent quality compared to traditional sedimentation tanks. | Limited anti-pollution ability; prone to membrane pollution; suitable for deep treatment of effluent from biochemical treatment. |
| Biological Aerated Filter (BAF) [73] | Oxidation and decomposition of pollutants by microorganisms on filter media; adsorption and retention by filter media and microbial film; internal denitrification by microbial film microenvironment. | Simple process; good effluent quality; strong resistance to load increases; high oxygen transmission efficiency; reasonable bacterial community structure; effective denitrification. | Suitable for advanced treatment of effluent from biochemical treatment. |
| Microbial Co-Metabolism [74,75] | The addition of co-substrates provides a rich source of carbon and energy, enhancing microorganism activity and promoting the synthesis of corresponding enzymes, thereby facilitating the biodegradation of difficult-to-degrade substrates. | Significantly enhances microorganism activity; improves the efficiency of degrading difficult-to-degrade organic matter. | Tremendous potential for development. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, B.; Tang, Y.; Li, T.; Yu, H.; Cui, T.; Zhang, C.; Zhang, L.; Su, P.; Zhang, R. Technological Advancements and Prospects for Near-Zero-Discharge Treatment of Semi-Coking Wastewater. Water 2024, 16, 2614. https://doi.org/10.3390/w16182614
Quan B, Tang Y, Li T, Yu H, Cui T, Zhang C, Zhang L, Su P, Zhang R. Technological Advancements and Prospects for Near-Zero-Discharge Treatment of Semi-Coking Wastewater. Water. 2024; 16(18):2614. https://doi.org/10.3390/w16182614
Chicago/Turabian StyleQuan, Bingxu, Yuanhui Tang, Tingting Li, Huifang Yu, Tingting Cui, Chunhui Zhang, Lei Zhang, Peidong Su, and Rui Zhang. 2024. "Technological Advancements and Prospects for Near-Zero-Discharge Treatment of Semi-Coking Wastewater" Water 16, no. 18: 2614. https://doi.org/10.3390/w16182614








