Adsorption of Tricyclazole and 2,4-Dichlorophenoxyacetic Acid onto Biochar Produced from Anaerobically Digested Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Biochar Derived from Anaerobically Digested Sludge
2.3. Characterization of Biochar Derived from Anaerobically Digested Sludge
2.4. Batch Experiments
2.5. Experimental Analysis Model
2.5.1. Isotherm Models
2.5.2. Kinetics Models
3. Results and Discussion
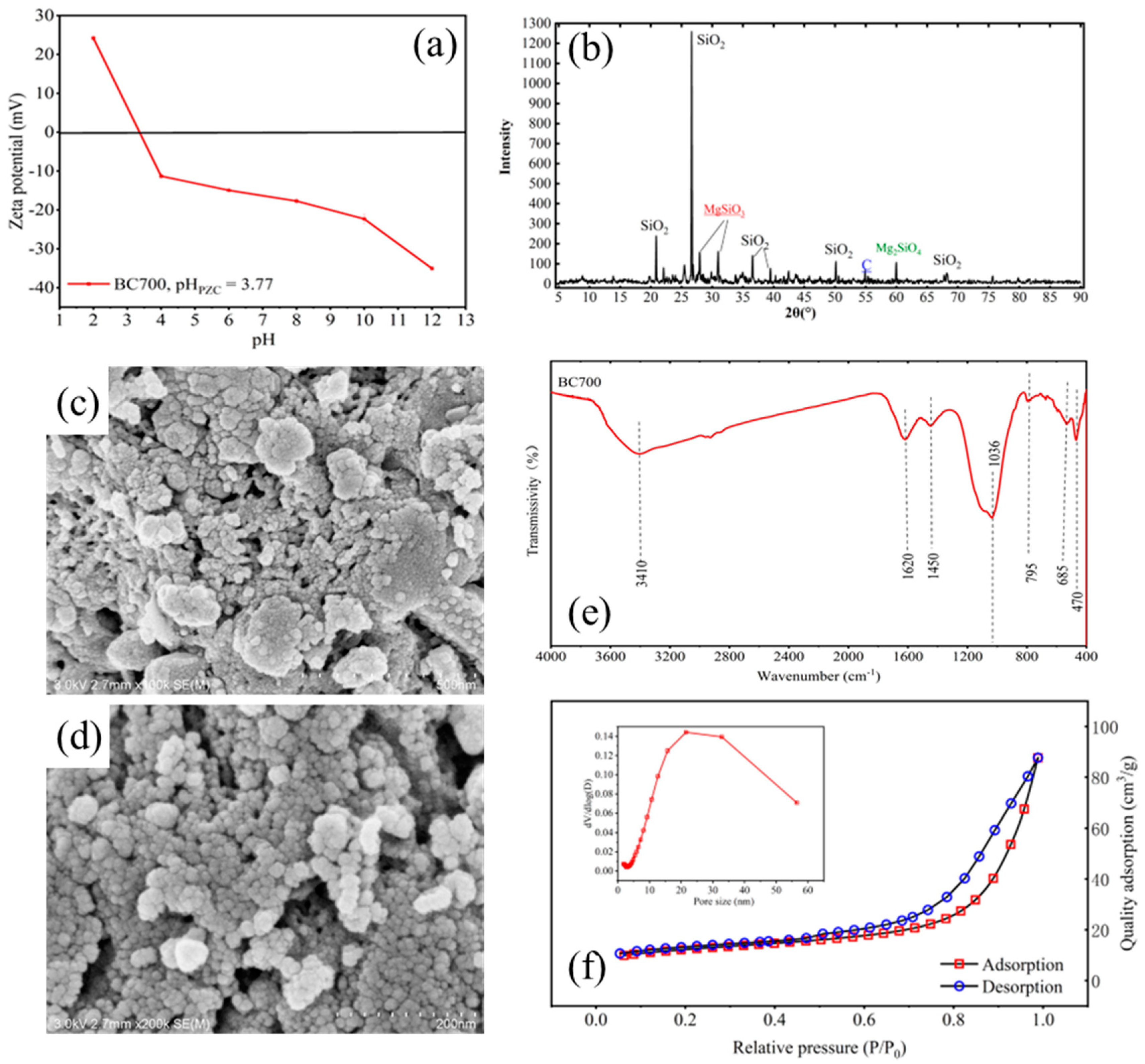
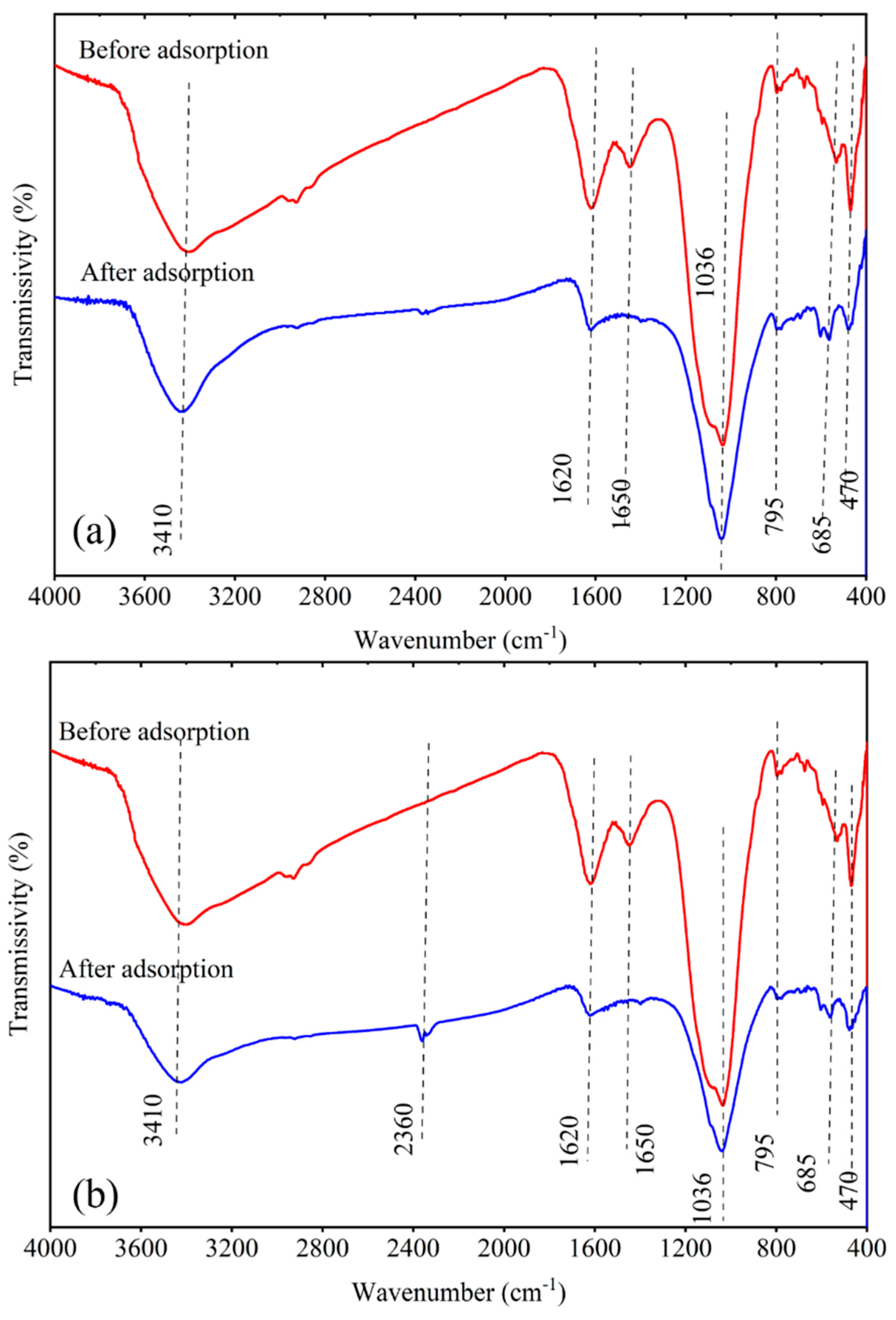
3.1. Characterization of BC700
3.2. Effect of Solution pH on the Adsorption of Tricyclazole and 2,4-D
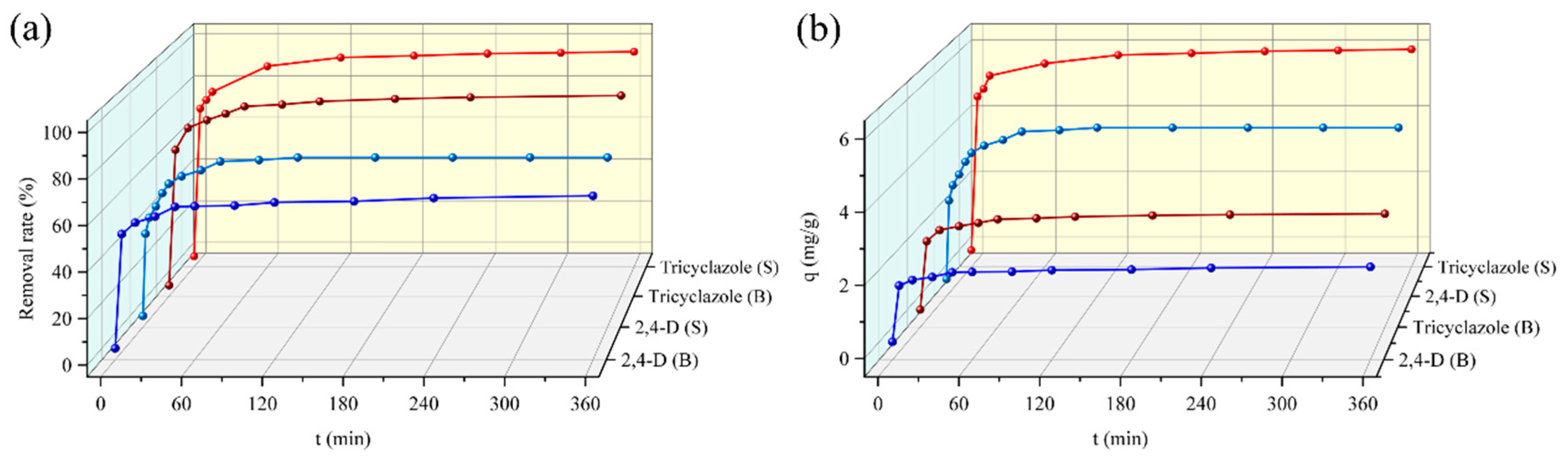
3.3. Effect of Contact Time
3.3.1. Single System
3.3.2. Binary System
3.4. Adsorption Isotherm
3.4.1. Single System
3.4.2. Binary System
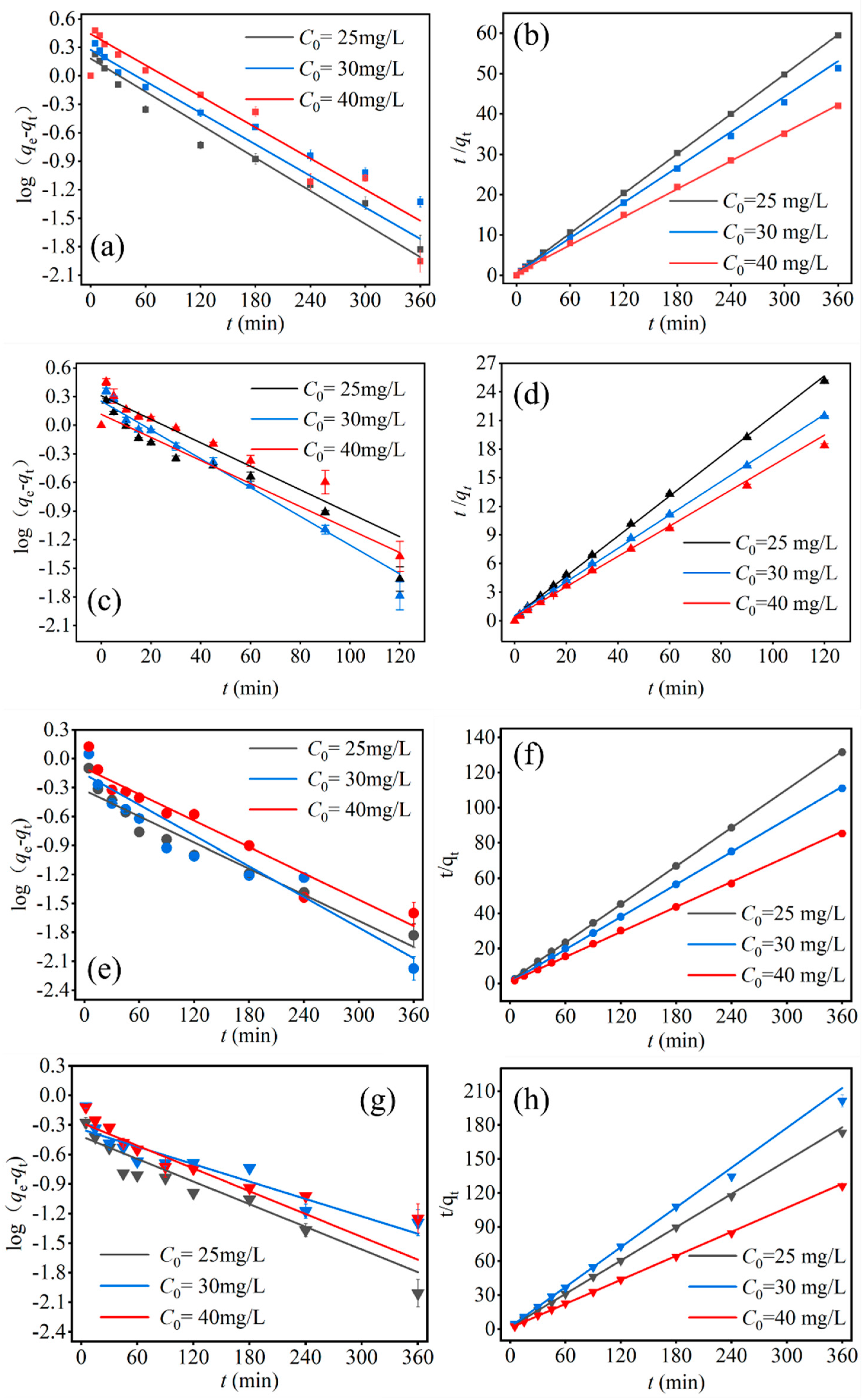
3.5. Adsorption Kinetic
3.5.1. Single System
3.5.2. Binary System
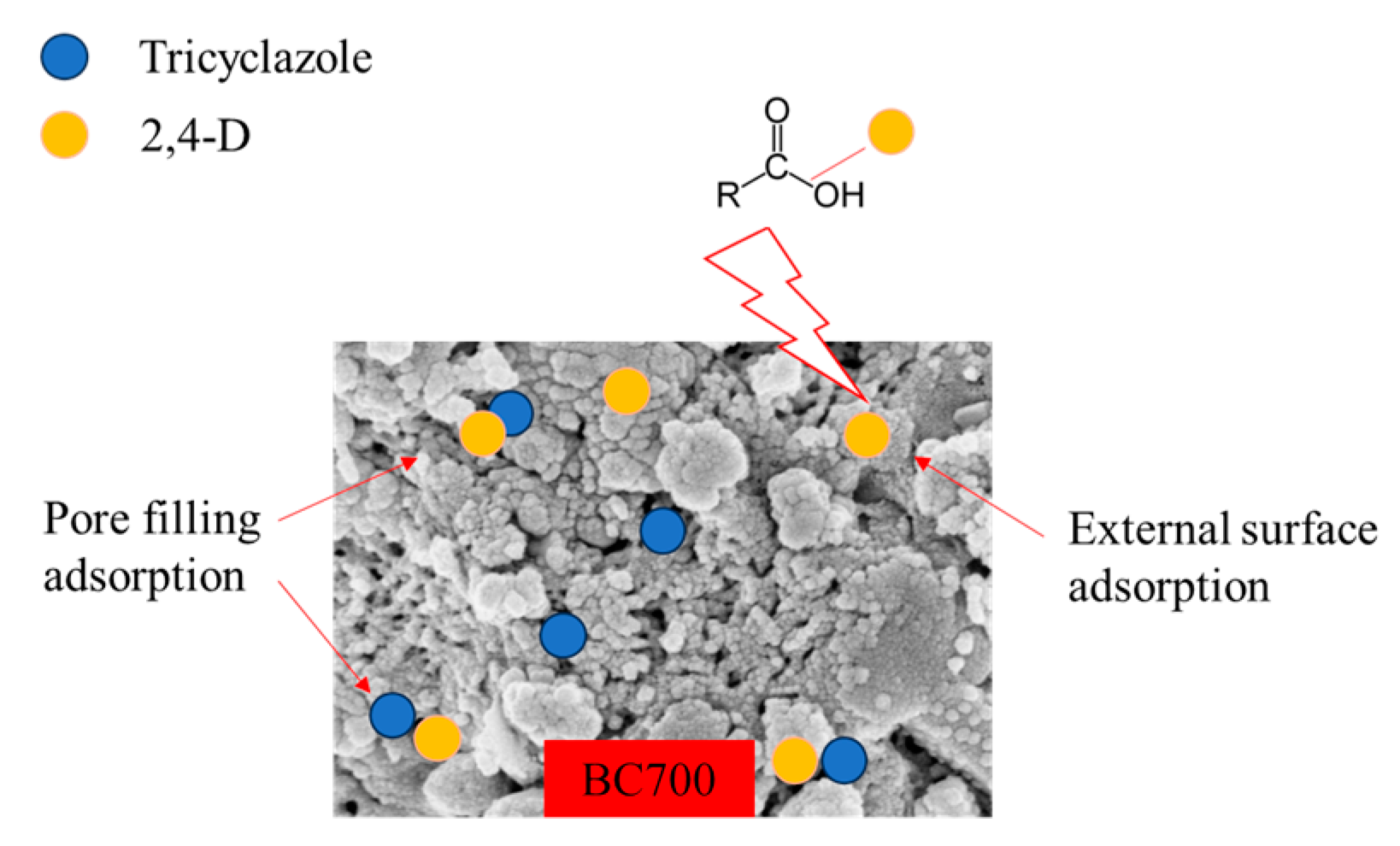
3.6. Possible Adsorption Mechanism of Tricyclazole and 2,4-Dichlorophenoxyacetic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nie, J.; Sun, Y.; Zhou, Y.; Kumar, M.; Usman, M.; Li, J.; Shao, J.; Wang, L.; Tsang, D.C.W. Bioremediation of water containing pesticides by microalgae: Mechanisms, methods, and prospects for future research. Sci. Total Environ. 2020, 707, 136080. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, R.M. Impact of farm size on intensity of pesticide use: Evidence from China. Sci. Total Environ. 2021, 753, 141696. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Jiang, H.N. Risk cognition, agricultural cooperatives training, and farmers’ pesticide overuse: Evidence from Shandong Province, China. Front. Public Health 2022, 10, 1032862. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Chen, Y.J.; Huang, L.; Zhang, Y.T.; Cao, N.N.; Guo, X.J.; Yao, C.L.; Li, X.F.; Duan, L.S.; Pang, S. Potential toxicity and dietary risk of tricyclazole to Chinese mitten crab (Eriocheir sinensis) in the rice-crab co-culture model. Environ. Pollut. 2023, 316, 120514. [Google Scholar] [CrossRef]
- Corvaro, M.; Gollapudi, B.B.; Mehta, J. A Critical Assessment of the Genotoxicity Profile of the Fungicide Tricyclazole. Environ. Mol. Mutagen. 2020, 61, 300–315. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Schadeck Netto, M.; Allasia, D.; Foletto, E.L.; Oliveira, L.F.S.; Dotto, G.L. Transforming shrub waste into a high-efficiency adsorbent: Application of Physalis peruvian chalice treated with strong acid to remove the 2,4-dichlorophenoxyacetic acid herbicide. J. Environ. Chem. Eng. 2021, 9, 104574. [Google Scholar] [CrossRef]
- Anton, B.J.; Cornelius Ruhs, E.; White, A.M.; Dehnert, G.K. Elucidating the effects of acute and chronic exposure to 2,4-Dichlorophenoxyacetic acid on fathead minnow (Pimephales promelas) innate immunity. Aquat. Toxicol. 2023, 260, 106571. [Google Scholar] [CrossRef]
- Tayyab, S.; Francis, J.A.; Kabir, M.Z.; Ghani, H.; Mohamad, S.B. Probing the interaction of 2,4-dichlorophenoxyacetic acid with human serum albumin as studied by experimental and computational approaches. Spectrochim. Acta A 2019, 207, 284–293. [Google Scholar] [CrossRef]
- Varjani, S.; Kumar, G.; Rene, E.R. Developments in biochar application for pesticide remediation: Current knowledge and future research directions. J. Environ. Manag. 2019, 232, 505–513. [Google Scholar] [CrossRef]
- Pinheiro, R.F.; Grimm, A.; Oliveira, M.L.S.; Vieillard, J.; Silva, L.F.O.; De Brum, I.A.S.; Lima, É.C.; Naushad, M.; Sellaoui, L.; Dotto Guilherme, L.; et al. Adsorptive behavior of the rare earth elements Ce and La on a soybean pod derived activated carbon: Application in synthetic solutions, real leachate and mechanistic insights by statistical physics modeling. Chem. Eng. J. 2023, 471, 144484. [Google Scholar] [CrossRef]
- Grimm, A.; dos Reis, G.S.; Dinh, V.M.; Larsson, S.H.; Mikkola, J.; Lima, E.C.; Xiong, S. Hardwood spent mushroom substrate–based activated biochar as a sustainable bioresource for removal of emerging pollutants from wastewater. Biomass Convers. Biorefin. 2024, 14, 2293–2309. [Google Scholar] [CrossRef]
- Chen, L.; Yang, H.; Hong, R.; Xie, X.; Zuo, R.; Zhang, X.; Chen, S.; Xu, D.; Zhang, Q. Tetracycline adsorption on sludge-bamboo biochar prepared by gradient modification and co-pyrolysis: Performance evaluation and mechanism insight. J. Environ. Chem. Eng. 2024, 12, 114121. [Google Scholar] [CrossRef]
- Lee, G.; Kim, C.; Park, C.; Ryu, B.; Hong, H. High-carbon-content biochar from chemical manufacturing plant sludge for effective removal of ciprofloxacin from aqueous media. Chemosphere 2024, 364, 143118. [Google Scholar] [CrossRef]
- Minaei, S.; Zoroufchi, B.K.; McPhedran, K.N.; Soltan, J. Adsorption of sulfamethoxazole and lincomycin from single and binary aqueous systems using acid-modified biochar from activated sludge biomass. J. Environ. Manag. 2024, 358, 120742. [Google Scholar] [CrossRef]
- Duan, S.; He, J.; Xin, X.; Li, L.; Zou, X.; Zhong, Y.; Zhang, J.; Cui, X. Characteristics of digested sludge-derived biochar for promoting methane production during anaerobic digestion of waste activated sludge. Bioresour. Technol. 2023, 384, 129245. [Google Scholar] [CrossRef]
- Li, Z.; Chen, K.; Zhai, S.; Yang, L.; Yu, T.; Yuan, H.; Zeng, B.; Yang, L.; Qi, F.; Zhu, H. Preparation of biochar from anaerobic digested sludge for enhancement of sludge dewatering. Chemosphere 2024, 362, 142687. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, G.; Zeng, Y.; Bao, X.; Liu, B.; Cheng, L. Endogenous iron-enriched biochar derived from steel mill wastewater sludge for tetracycline removal: Heavy metals stabilization, adsorption performance and mechanism. Chemosphere 2024, 359, 142263. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, Y.; Tan, X.; Li, L.; Cao, S.; Dou, J.; Chen, R.; Hu, X.; Qiu, Z.; Li, M.; et al. Sludge-based biochar preparation: Pyrolysis and co-pyrolysis methods, improvements, and environmental applications. Fuel 2024, 373, 132265. [Google Scholar] [CrossRef]
- Clurman, A.M.; Rodríguez-Narvaez, O.M.; Jayarathne, A.; De Silva, G.; Ranasinghe, M.I.; Goonetilleke, A.; Bandala, E.R. Influence of surface hydrophobicity/hydrophilicity of biochar on the removal of emerging contaminants. Chem. Eng. J. 2020, 402, 126277. [Google Scholar] [CrossRef]
- Shahryari, T.; Singh, P.; Raizada, P.; Davidyants, A.; Thangavelu, L.; Sivamani, S.; Naseri, A.; Vahidipour, F.; Ivanets, A.; Hosseini-Bandegharaei, A. Adsorption properties of Danthron-impregnated carbon nanotubes and their usage for solid phase extraction of heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128528. [Google Scholar] [CrossRef]
- Ezzati, R. Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J. 2020, 392, 123705. [Google Scholar] [CrossRef]
- Hai Nguyen, T.; Lima, E.C.; Juang, R.-S.; Bollinger, J.-C.; Chao, H.-P. Thermodynamic parameters of liquid-phase adsorption process calculated from different equilibrium constants related to adsorption isotherms: A comparison study. Environ. Chem. Eng. 2021, 9, 106674. [Google Scholar] [CrossRef]
- Ezzati, R.; Azizi, M.; Ezzati, S. A theoretical approach for evaluating the contributions of pseudo-first-order and pseudo-second-order kinetics models in the Langmuir rate equation. Vacuum 2024, 222, 113018. [Google Scholar] [CrossRef]
- Hong, M.; Zhang, L.; Tan, Z.; Huang, Q. Effect mechanism of biochar’s zeta potential on farmland soil’s cadmium immobilization. Environ. Sci. Pollut. Res. 2019, 26, 19738–19748. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Huang, H.; Zhao, D.; Kobayashi, N.; Chen, Y. Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge. J. Anal. Appl. Pyrolysis 2015, 112, 284–289. [Google Scholar] [CrossRef]
- Chen, J.; Fang, D.; Duan, F. Pore characteristics and fractal properties of biochar obtained from the pyrolysis of coarse wood in a fluidized-bed reactor. Appl. Energy 2018, 218, 54–65. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Pinto, D.; Lima, É.C.; Knani, S.; Grimm, A.; Silva, L.F.O.; Cadaval, T.R.S.; Dotto, G.L. Lanthanum uptake from water using chitosan with different configurations. React. Funct. Polym. 2022, 180, 105309. [Google Scholar] [CrossRef]
- Tong, D.; Zhuang, J.; Lee, J.; Buchanan, J.; Chen, X. Concurrent transport and removal of nitrate, phosphate and pesticides in low-cost metal- and carbon-based materials. Chemosphere 2019, 230, 84–91. [Google Scholar] [CrossRef]
- Zolfi Bavariani, M.; Ronaghi, A.; Ghasemi, R. Influence of Pyrolysis Temperatures on FTIR Analysis, Nutrient Bioavailability, and Agricultural use of Poultry Manure Biochars. Commun. Soil. Sci. Plant Anal. 2019, 50, 402–411. [Google Scholar] [CrossRef]
- Meng, F.; Wang, D. Effects of vacuum freeze drying pretreatment on biomass and biochar properties. Renew. Energy 2020, 155, 1–9. [Google Scholar] [CrossRef]
- Lalchawimawia, B.; Banerjee, T.; Dutta, A.; Choudhury, P.P.; Singh, N.; Mukhopadhyay, R.; Sahoo, D.; Dixit, M.; Mandal, A. Removal of tricyclazole residues from water by sorption on metal organic frameworks (MOFs): A theoretical insight of the experimental data. Surf. Interfaces 2024, 54, 105080. [Google Scholar] [CrossRef]
- Selvaraj, R.; Iyer, R.V.; Murugesan, G.; Goveas, L.C.; Varadavenkatesan, T.; Samanth, A.; Vinayagam, R. Modeling 2,4-dichlorophenoxyacetic acid adsorption on candle bush pod-derived activated carbon: Insights from advanced statistical physics models. J. Water Process Eng. 2024, 66, 106027. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, B. Influence of organic composition of biomass waste on biochar yield, calorific value, and specific surface area. J. Renew. Sustain. Energy 2018, 10, 013109. [Google Scholar] [CrossRef]
- Azarkan, S.; Peña, A.; Draoui, K.; Sainz-Díaz, C.I. Adsorption of two fungicides on natural clays of Morocco. Appl. Clay Sci. 2016, 123, 37–46. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Blachnio, M.; Marczewski, A.W.; Seczkowska, M.; Tarasiuk, B. Phenoxyacid pesticide adsorption on activated carbon—Equilibrium and kinetics. Chemosphere 2019, 214, 349–360. [Google Scholar] [CrossRef]
- Essandoh, M.; Wolgemuth, D.; Pittman, C.U.; Mohan, D.; Mlsna, T. Phenoxy herbicide removal from aqueous solutions using fast pyrolysis switchgrass biochar. Chemosphere 2017, 174, 49–57. [Google Scholar] [CrossRef]
- Zheng, W.; Guo, M.; Chow, T.; Bennett, D.N.; Rajagopalan, N. Sorption properties of greenwaste biochar for two triazine pesticides. J. Hazard. Mater. 2010, 181, 121–126. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, J.; Liu, J.; Liu, B.; Chen, G. Preparation and application of magnetic biochar in water treatment: A critical review. Sci. Total Environ. 2020, 711, 134847. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Yu, L.; Sun, T. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars. J. Hazard. Mater. 2013, 244, 217–224. [Google Scholar] [CrossRef]
- Zhang, B.; Xiong, S.; Xiao, B.; Yu, D.; Jia, X. Mechanism of wet sewage sludge pyrolysis in a tubular furnace. Int. J. Hydrogen Energy. 2011, 36, 355–363. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, M.; Amiri, M.J.; Beigzadeh, B. Adsorption of 2,4-dichlorophenoxyacetic acid using rice husk biochar, granular activated carbon, and multi-walled carbon nanotubes in a fixed bed column system. Water Sci. Technol. 2018, 78, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, N.; Tong, L.; Lv, Y.; Li, G. Characterization and evaluation of surface modified materials based on porous biochar and its adsorption properties for 2,4-dichlorophenoxyacetic acid. Chemosphere 2018, 210, 734–744. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, G.S.; Conrad, S.; Lima, E.C.; Naushad, M.; Manavalan, G.; Gentili, F.G.; Dotto, G.L.; Grimm, A. Synthesis of Highly Porous Lignin-Sulfonate Sulfur-Doped Carbon for Efficient Adsorption of Sodium Diclofenac and Synthetic Effluents. Nanomaterials 2024, 14, 1374. [Google Scholar] [CrossRef] [PubMed]

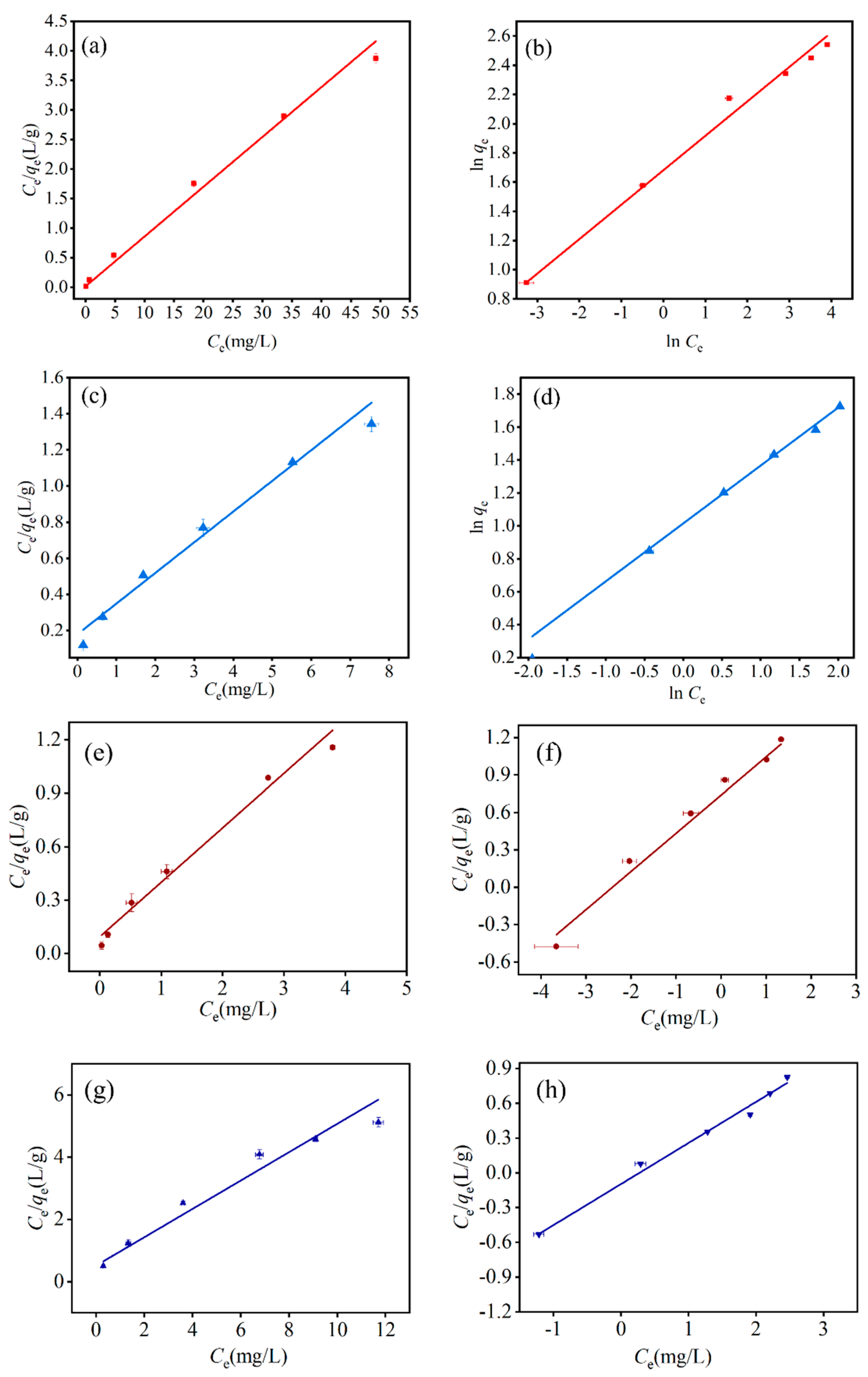
| Adsorbate | System Type | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF [L/(mg·g)] | 1/n | R2 | ||
| Tricyclazole | Single | 11.86 | 4.91 | 0.988 | 5.38 | 0.236 | 0.998 |
| Binary | 5.27 | 3.210 | 0.970 | 2.097 | 0.306 | 0.985 | |
| 2,4-D | Single | 7.89 | 0.945 | 0.985 | 2.76 | 0.352 | 0.998 |
| Binary | 3.20 | 0.874 | 0.969 | 0.909 | 0.355 | 0.997 | |
| Adsorbate | System Type | Pseudo-First-Order | Pseudo-Second-Order | C0(mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe, exp (mg/g) | qe, cal (mg/g) | kf | R2 | qe, exp (mg/g) | qe, cal (mg/g) | ks | R2 | |||
| Tricyclazole | Single | 6.06 | 1.520 | 0.0134 | 0.96 | 6.06 | 6.1 | 0.0474 | 0.9999 | 25 |
| Binary | 2.75 | 0.721 | 0.0104 | 0.975 | 2.75 | 2.757 | 0.0812 | 0.9999 | ||
| Single | 7.08 | 1.890 | 0.0128 | 0.921 | 7.08 | 6.85 | 0.0435 | 0.9988 | 30 | |
| Binary | 3.25 | 0.852 | 0.0122 | 0.839 | 3.25 | 3.239 | 0.1033 | 0.9999 | ||
| Single | 8.63 | 2.770 | 0.0126 | 0.963 | 8.63 | 8.63 | 0.0241 | 0.9995 | 40 | |
| Binary | 4.25 | 0.908 | 0.0105 | 0.936 | 4.25 | 4.205 | 0.0625 | 0.9995 | ||
| 2,4-D | Single | 4.79 | 4.700 | 0.0283 | 0.914 | 4.79 | 4.76 | 0.0911 | 0.999 | 25 |
| Binary | 2.09 | 0.661 | 0.0088 | 0.927 | 2.09 | 2.042 | 0.1318 | 0.9995 | ||
| Single | 5.61 | 4.140 | 0.0348 | 0.975 | 5.61 | 5.7 | 0.0539 | 0.999 | 30 | |
| Binary | 1.85 | 0.709 | 0.0068 | 0.677 | 1.85 | 1.709 | 0.1597 | 0.9988 | ||
| Single | 6.61 | 3.000 | 0.0278 | 0.947 | 6.61 | 6.25 | 0.0641 | 0.998 | 40 | |
| Binary | 2.93 | 0.758 | 0.0089 | 0.891 | 2.93 | 2.831 | 0.1085 | 0.9993 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Hou, Y. Adsorption of Tricyclazole and 2,4-Dichlorophenoxyacetic Acid onto Biochar Produced from Anaerobically Digested Sludge. Water 2024, 16, 2697. https://doi.org/10.3390/w16182697
Wang F, Hou Y. Adsorption of Tricyclazole and 2,4-Dichlorophenoxyacetic Acid onto Biochar Produced from Anaerobically Digested Sludge. Water. 2024; 16(18):2697. https://doi.org/10.3390/w16182697
Chicago/Turabian StyleWang, Fen, and Yingjian Hou. 2024. "Adsorption of Tricyclazole and 2,4-Dichlorophenoxyacetic Acid onto Biochar Produced from Anaerobically Digested Sludge" Water 16, no. 18: 2697. https://doi.org/10.3390/w16182697






