Spatiotemporal Variation and Influences of Acidification in the North Pacific, 1995–2019
Abstract
:1. Introduction
2. Data and Methods
2.1. Data
2.2. Method
2.2.1. EOF
2.2.2. Geodetector
3. Results
3.1. Spatiotemporal Variability in the North Pacific Ocean
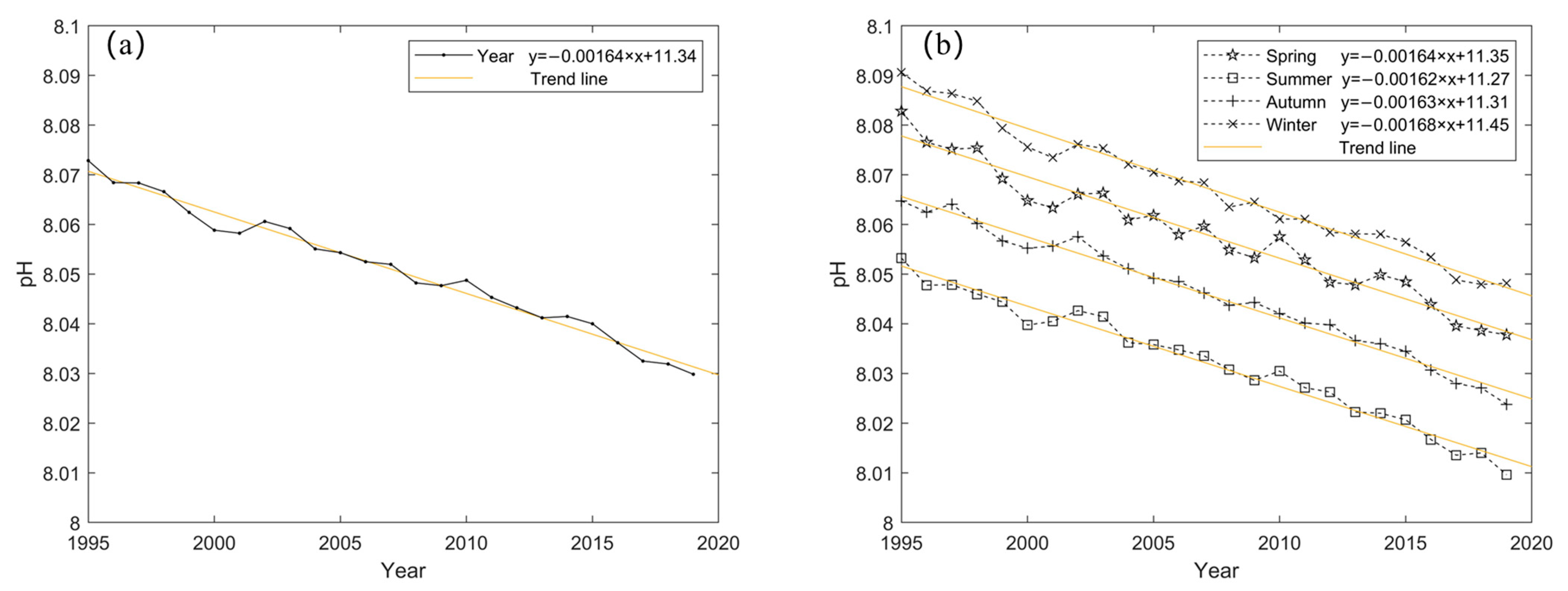
3.1.1. Annual and Seasonal Variation of pH
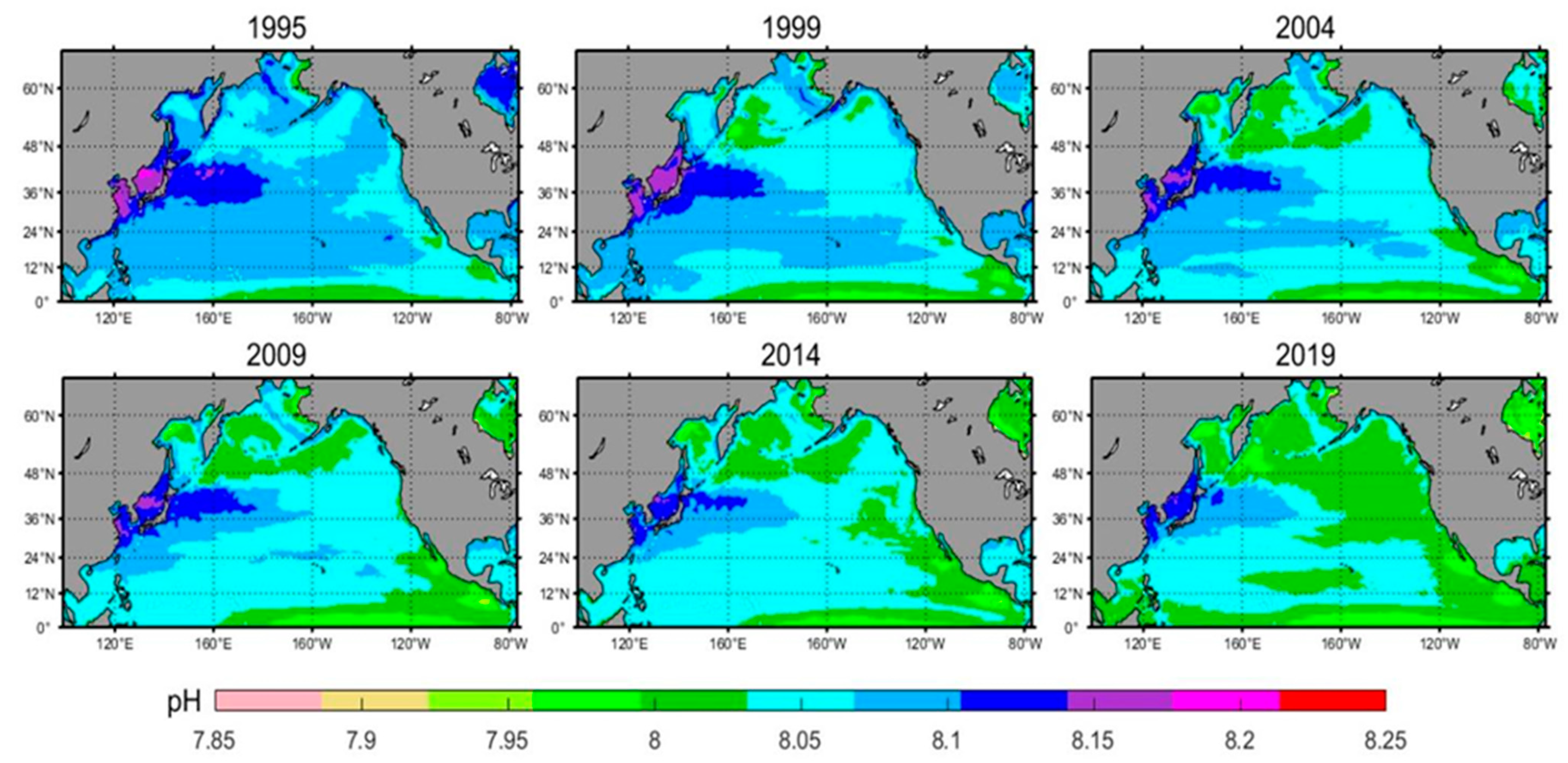
3.1.2. Spatial Distribution Characteristics of pH
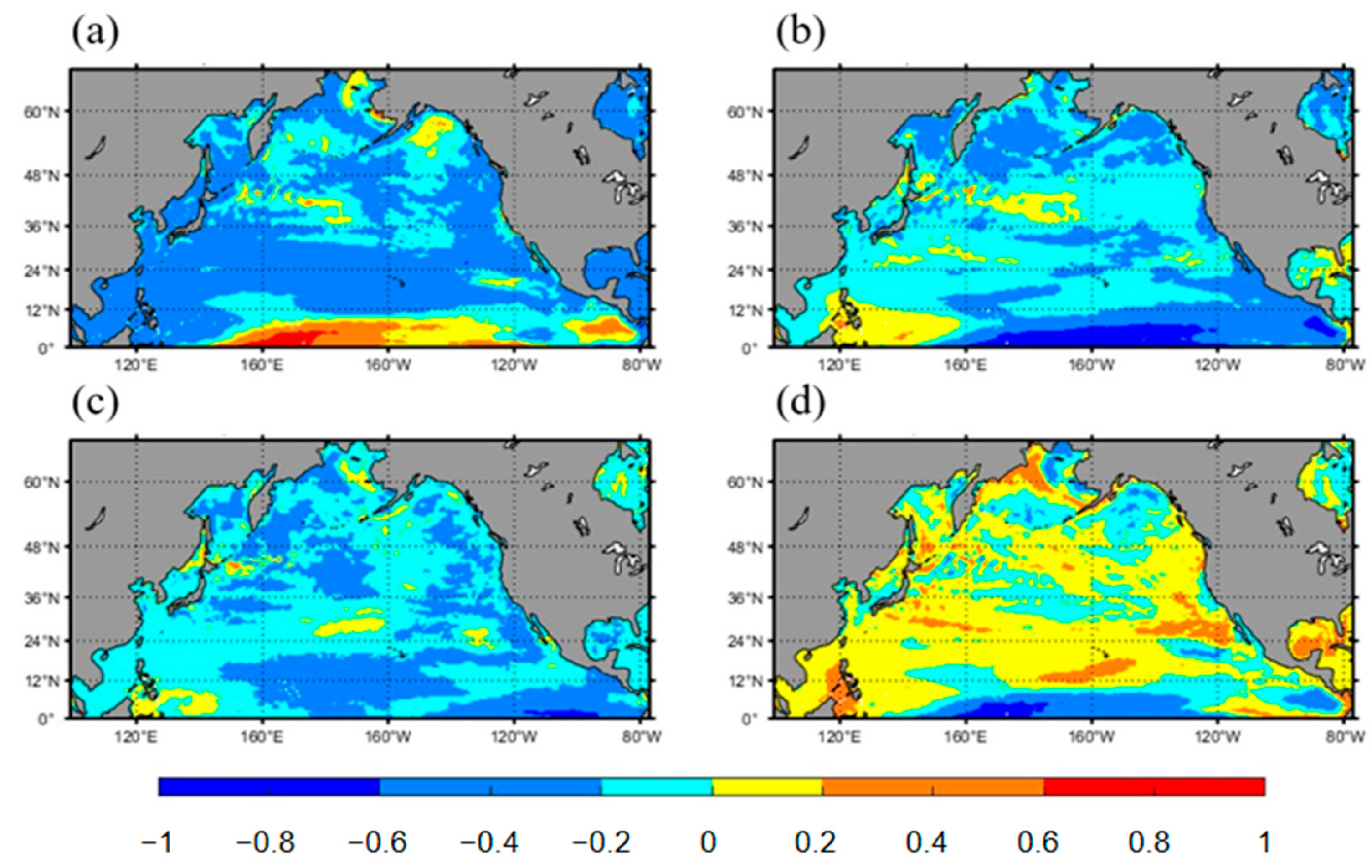
3.2. Influencing Factors of Surface Acidification in the North Pacific Ocean
3.2.1. SST
3.2.2. SSS
3.2.3. Chl a
3.2.4. DO
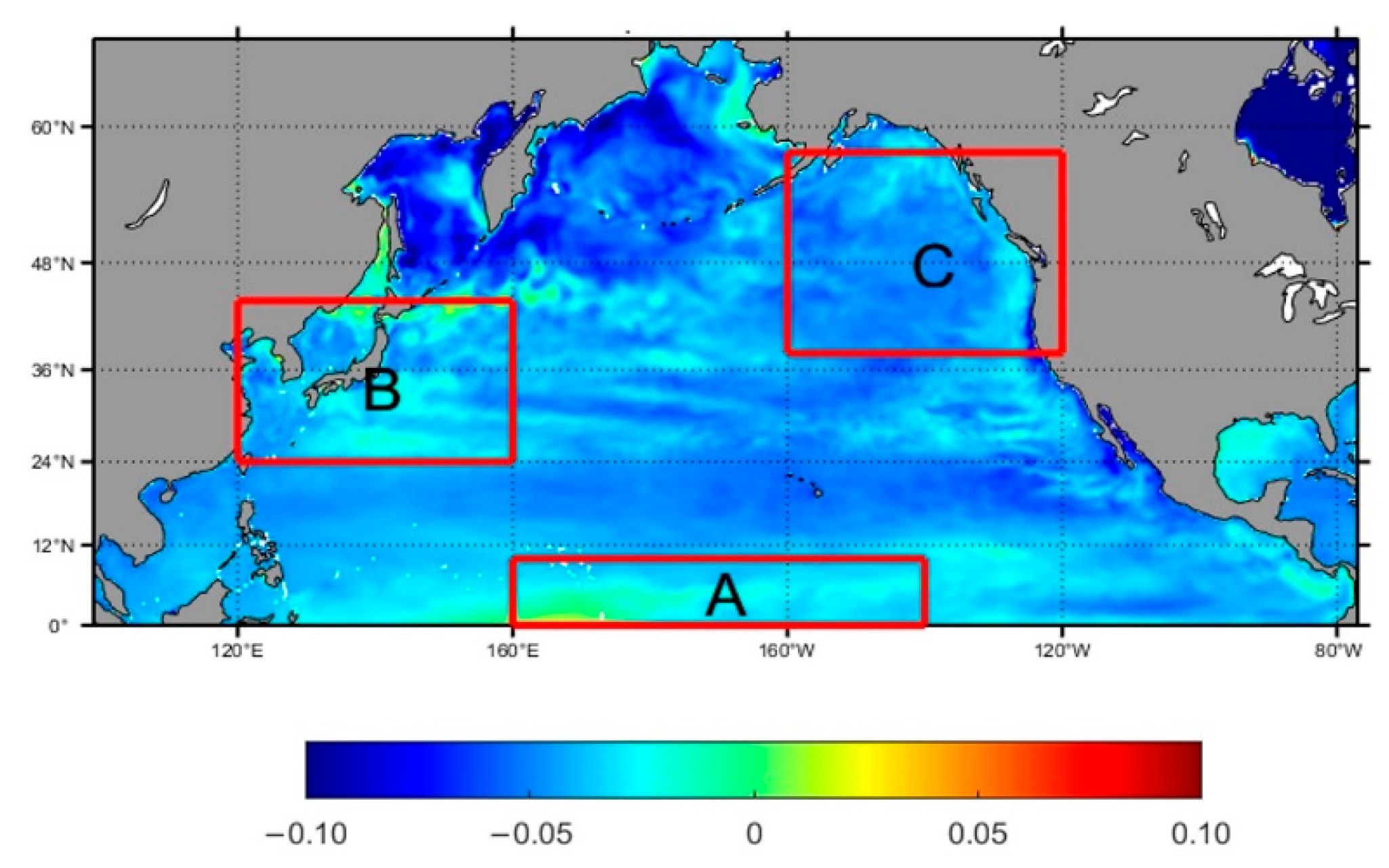
3.3. Spatiotemporal Variation of Typical Partitions
3.3.1. Equatorial Central Region
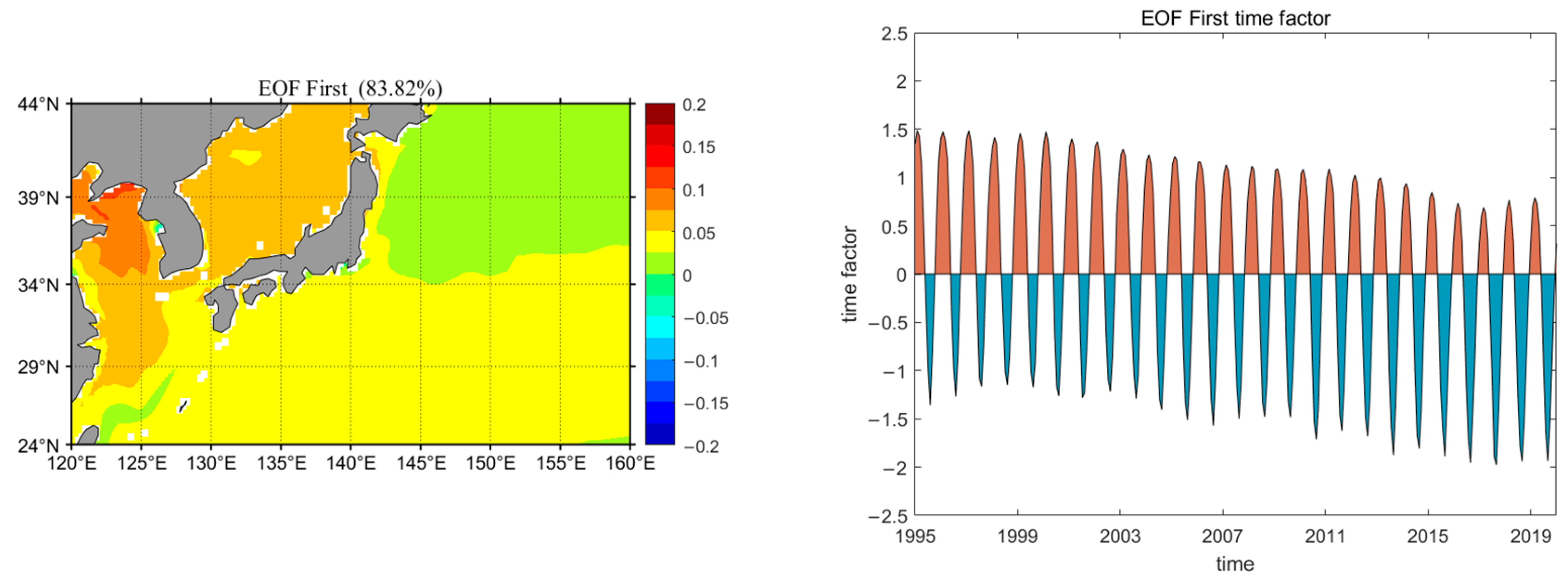
3.3.2. Eastern Regions of China and Japan
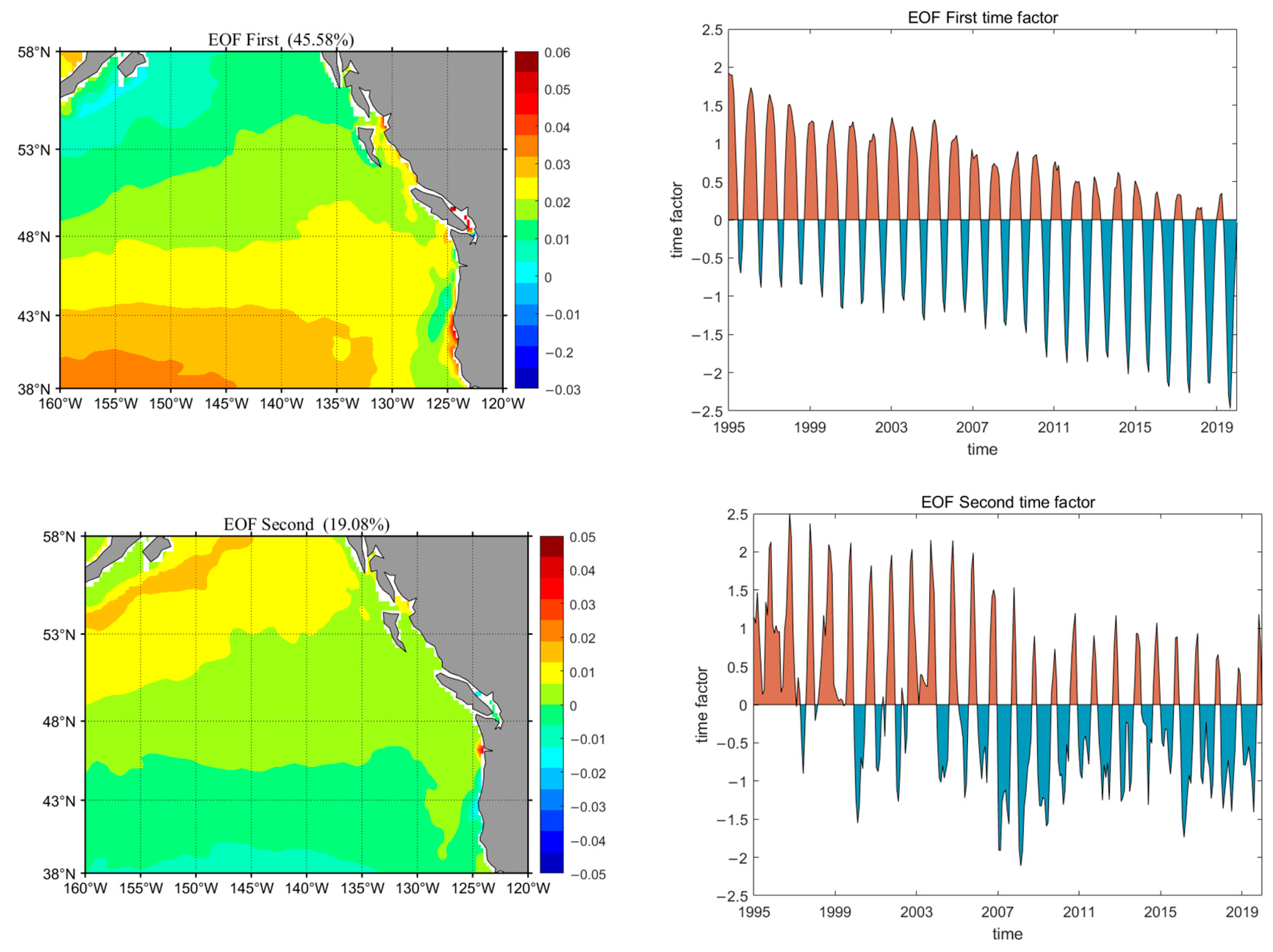
3.3.3. Western Region of Canada
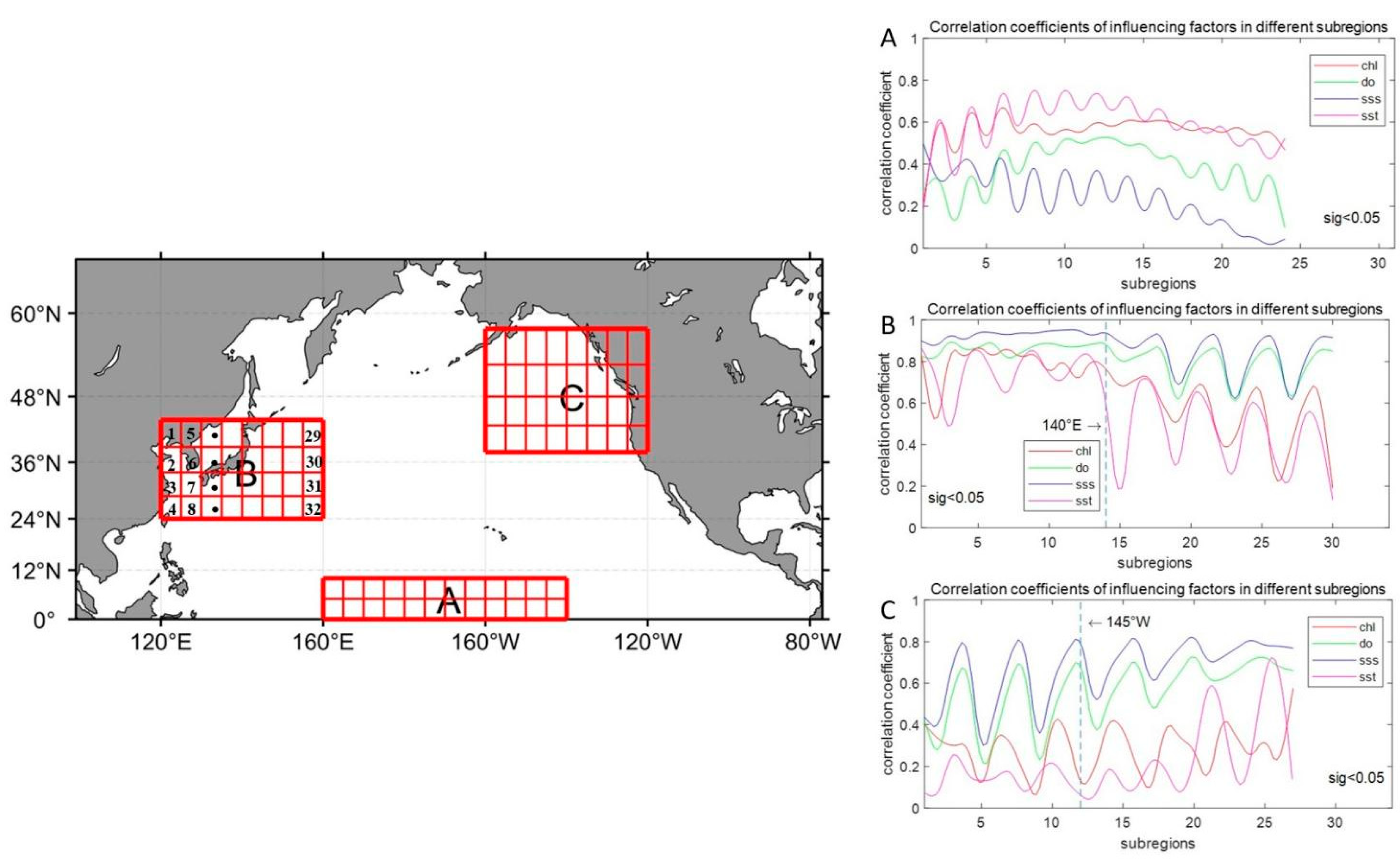
3.4. Discussion of the Main Controlling Factors of Typical Partitions
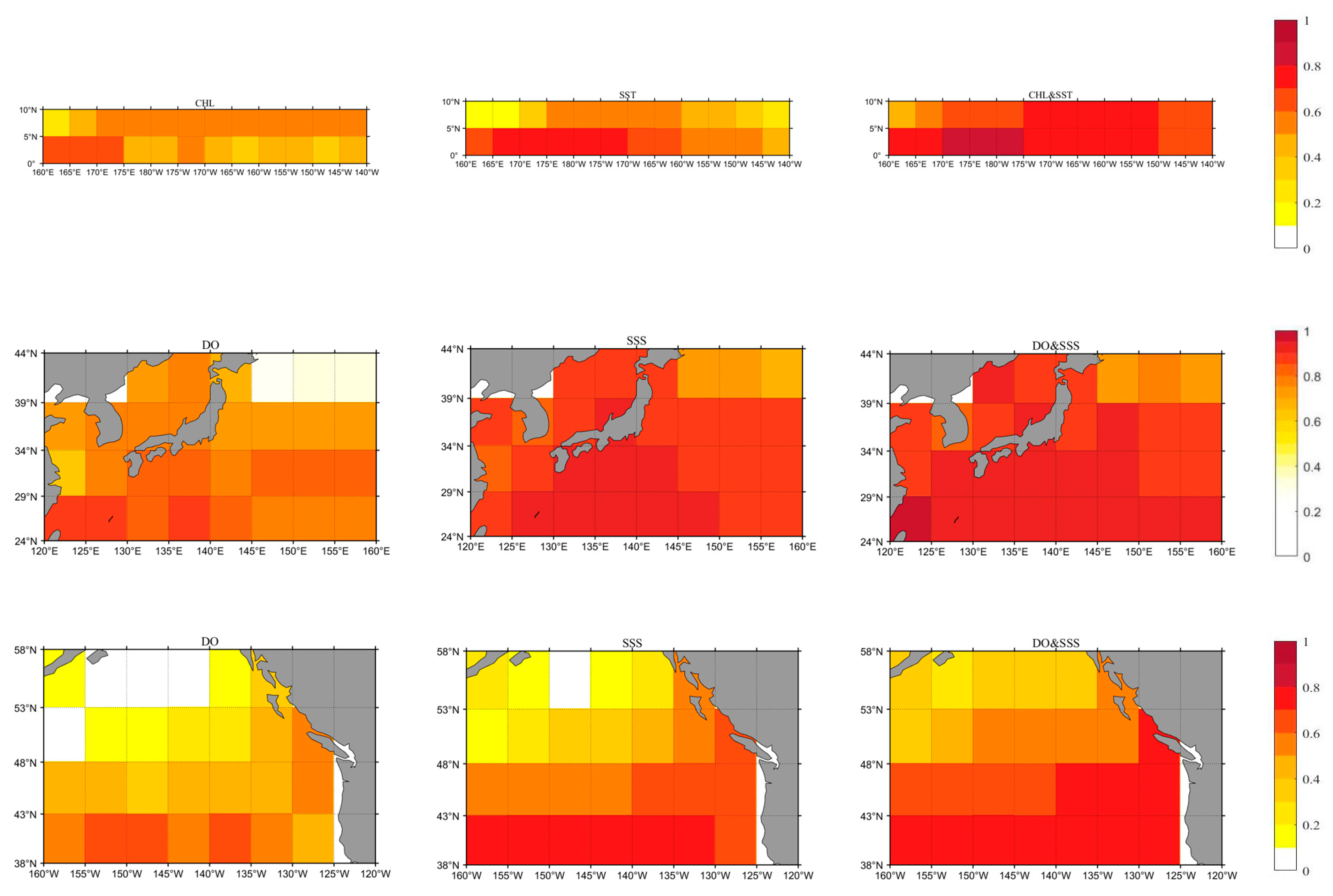
3.5. Quantitative Analysis of Main Control Factors
3.6. Discussion of A, B, and C Acidification Mechanisms
4. Conclusions
- (1)
- Surface water pH has decreased linearly by approximately 0.043 over the 25-year period. Seasonal variations reveal that pH levels peak in winter and reach their lowest point in summer. Spring and winter show the most significant changes, while summer and autumn exhibit more stable pH levels. The surface water pH displays a gradient from high in the northwest to low in the southeast. Over time, there is a noticeable stepwise decline in pH from west to east. Acidification is most severe in the central and western North Pacific, while other regions, particularly equatorial and high-latitude areas extending towards the central ocean, exhibit a more uniform level of acidification.
- (2)
- Beyond CO2 emissions, several environmental factors significantly impact surface water pH in the North Pacific. These include sea surface temperature (SST), salinity, chlorophyll-a concentration, and dissolved oxygen. The surface pH is negatively correlated with temperature, salinity, and chlorophyll-a concentration and positively correlated with dissolved oxygen. Among these factors, SST has the most pronounced effect on pH, followed by dissolved oxygen, salinity, and chlorophyll-a concentration.
- (3)
- The acidification levels in regions A, B, and C exhibit distinct characteristics. Based on the EOF analysis results and the specific conditions of each sea area, it is found that the acidification in region A is primarily influenced by the El Niño phenomenon, showing a periodic downward trend. In Region B, coastal acidification is more severe due to upwelling or eutrophication. Furthermore, summer marine biological activities, such as coral reef systems and human activities, have exacerbated this effect. The stepped distribution of acidification in Region C is mainly influenced by the North Pacific Current and the Alaska Current.
- (4)
- The influence of these factors varies by region and over time. In Region A, chlorophyll-a and SST are the primary drivers of acidification, accounting for more than 70% of the changes when their interactions are considered. In Regions B and C, dissolved oxygen (DO) and salinity (SSS) are more influential, contributing approximately 90% and 50% of the acidification, respectively, when interactions are factored in.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hauri, C.; Gruber, N.; Plattner, G.K.; Alin, S.; Feely, R.A.; Hales, B.; Wheeler, P.A. Ocean acidification in the California current system. Oceanography 2009, 22, 60–71. [Google Scholar] [CrossRef]
- Watanabe, Y.W.; Li, B.F.; Yamasaki, R.; Yunoki, S.; Imai, K.; Hosoda, S.; Nakano, Y. Spatiotemporal changes of ocean carbon species in the western North Pacific using parameterization technique. J. Oceanogr. 2020, 76, 155–167. [Google Scholar] [CrossRef]
- Fujii, M.; Chai, F.; Shi, L.; Inoue, H.Y.; Ishii, M. Seasonal and interannual variability of oceanic carbon cycling in the western and central tropical-subtropical pacific: A physical-biogeochemical modeling study. J. Oceanogr. 2009, 65, 689–701. [Google Scholar] [CrossRef]
- Jiang, M.; Chai, F. Physical control on the seasonal cycle of surface pCO2 in the equatorial Pacific. Geophys. Res. Lett. 2006, 33, 265–288. [Google Scholar] [CrossRef]
- Gao, G.; Wang, T.; Sun, J.; Zhao, X.; Wang, L.; Guo, X.; Gao, K. Contrasting responses of phytoplankton productivity between coastal and offshore surface waters in the Taiwan Strait and the South China Sea to short-term seawater acidification. Biogeosciences 2022, 19, 2795–2804. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, H.; Zang, K.; Zheng, N.; Huo, C.; Deng, X.; Wang, J. Contrasting effects of temperature and biological processes on aragonite saturation state at the A4HDYD station in the North Yellow Sea. Mar. Environ. Res. 2023, 192, 106187. [Google Scholar] [CrossRef]
- Dore, J.E.; Lukas, R.; Sadler, D.W.; Church, M.J.; Karl, D.M. Physical and biogeochemical modulation of ocean acidification in the central North Pacific. Proc. Natl. Acad. Sci. USA 2009, 106, 12235–12240. [Google Scholar] [CrossRef]
- Midorikawa, T.; Ishii, M.; Saito, S.; Sasano, D.; Kosugi, N.; Motoi, T.; Kamiya, H.; Nakadate, A.; Nemoto, K.; Inoue, H.Y. Decreasing pH trend estimated from 25-year time series of carbonate parameters in the western North Pacific. Tellus B Chem. Phys. Meteorol. 2010, 62, 649–659. [Google Scholar] [CrossRef]
- Takahashi, T.; Sutherland, S.C.; Chipman, D.W.; Goddard, J.G.; Ho, C.; Newberger, T.; Sweeney, C.; Munro, D.R. Climatological distributions of pH, pCO2, total CO2, alkalinity, and CaCO3 saturation in the global surface ocean, and temporal changes at selected locations. Mar. Chem. 2014, 164, 95–125. [Google Scholar] [CrossRef]
- Pelejero, C.; Calvo, E.; McCulloch, M.T.; Marshall, J.F.; Gagan, M.K.; Lough, J.M.; Opdyke, B.N. Preindustrial to modern interdecadal variability in coral reef pH. Science 2005, 309, 2204–2207. [Google Scholar] [CrossRef]
- Song, J.; Qu, B.; Li, X.; Yuan, H.; Li, N.; Duan, L. Carbon sinks/sources in the Yellow and East China Seas—Air-sea interface exchange, dissolution in seawater, and burial in sediments. Sci. China Earth Sci. 2018, 61, 1583–1593. [Google Scholar] [CrossRef]
- Zeebe, R.E. History of Seawater Carbonate Chemistry, atmospheric CO2, and Ocean Acidification. Annu. Rev. Earth Planet. Sci. 2012, 40, 141–165. [Google Scholar] [CrossRef]
- Kopáček, J.; Kaňa, J.; Bičárová, S.; Brahney, J.; Navrátil, T.; Norton, S.A.; Porcal, P.; Stuchlík, E. Climate change accelerates recovery of the Tatra Mountain lakes from acidification and increases their nutrient and chlorophyll a concentrations. Aquat. Sci. 2019, 81, 70. [Google Scholar] [CrossRef]
- Hao, Q. Temporal and Spatial Distribution Characteristics and Environmental Regulation Mechanism of Chlorophyll and Primary Productivity in Coastal China. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2010. [Google Scholar]
- Guo, J. Evolution of Surface pH and pCO2 in the Western Tropical Pacific over the Past 150,000 Years and Their Influencing Factors. Master’s Thesis, Institute of Oceanography, Chinese Academy of Sciences, Qingdao, China, 2015. [Google Scholar]
- Chan, F.; Barth, J.A.; Blanchette, C.A.; Byrne, R.H.; Chavez, F.; Cheriton, O.; Feely, R.A.; Friederich, G.; Gaylord, B.; Gouhier, T.; et al. Persistent spatial structuring of coastal ocean acidification in the California Current System. Sci. Rep. 2017, 7, 2526. [Google Scholar] [CrossRef] [PubMed]
- Hudson, C.J.; Agostini, S.; Wada, S.; Hall-Spencer, J.M.; Connell, S.D.; Harvey, B.P. Ocean acidification increases the impact of typhoons on algal communities. Sci. Total Environ. 2023, 865, 161269. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, G.C.; du Randt, A.; Seanego, K.G.; Tsanwani, M. Variability and controls of the ocean acidification metrics pH and pCO2 in a large embayment of an Eastern Boundary Upwelling System (EBUS). Estuar. Coast. Shelf Sci. 2023, 292, 108473. [Google Scholar] [CrossRef]
- George, R.; Job, S.; Semba, M.; Monga, E.; Lugendo, B.; Tuda, A.; Kimirei, I. High-frequency dynamics of pH, dissolved oxygen, and temperature in the coastal ecosystems of the Tanga-Pemba Seascape: Implications for upwelling-enhanced ocean acidification and deoxygenation. Front. Mar. Sci. 2024, 10, 1286870. [Google Scholar] [CrossRef]
- Sridevi, B.; Sarma, V.V.S.S. Role of river discharge and warming on ocean acidification and pCO2 levels in the Bay of Bengal. Tellus B Chem. Phys. Meteorol. 2021, 73, 1–20. [Google Scholar] [CrossRef]
- Monaco, C.L.; Metzl, N.; Fin, J.; Mignon, C.; Cuet, P.; Douville, E.; Gehlen, M.; Chau, T.T.T.; Tribollet, A. Distribution and long-term change of the sea surface carbonate system in the Mozambique Channel (1963–2019). Deep Sea Res. Part II Top. Stud. Oceanogr. 2021, 186, 104936. [Google Scholar] [CrossRef]
- Lorenz, E.N. Empirical Orthogonal Functions and Statistical Weather Prediction; Sci. Rep. No. 1, Statistical Forecasting Project; M.I.T.: Cambridge, MA, USA, 1956; Volume 48. [Google Scholar]
- Wang, J.; Xu, C. GeoDetectors: Principles and Prospects. Acta Geogr. Sin. 2017, 72, 116–134. [Google Scholar]
- Wang, J.; Zhu, S.; Liu, J.; Wang, X.; Wang, J.; Xu, J.; Yao, P.; Yang, Y. Frequency, Intensity and Influences of Tropical Cyclones in the Northwest Pacific and China, 1977–2018. Sustainability 2023, 15, 3933. [Google Scholar] [CrossRef]
- Haugan, P.M.; Drange, H. Effects of CO2 on the ocean environment. Energy Convers. Manag. 1996, 37, 1019–1022. [Google Scholar] [CrossRef]
- Feely, R.A.; Doney, S.C.; Cooley, S.R. Ocean acidification: Present conditions and future changes in a high-CO2 world. Oceanography 2009, 22, 36–47. [Google Scholar] [CrossRef]
- Jiang, L.-Q.; Carter, B.R.; Feely, R.A.; Lauvset, S.K.; Olsen, A. Surface ocean pH and buffer capacity: Past, present and future. Sci. Rep. 2019, 9, 18624. [Google Scholar] [CrossRef]
- Long, A.; Chen, S.; Zhou, W.; Xu, J.; Sun, C.; Zhang, F.; Zhang, J.; Xu, H. Distribution characteristics and correlation between nutrients, dissolved oxygen, pH value and chlorophyll in autumn in northern South China Sea. Mar. Sci. Technol. Bull. 2006, 25, 9–16. [Google Scholar]
- González-Dávila, M.; Santana-Casiano, J.M.; Rueda, M.-J.; Llinás, O.; González-Dávila, E.-F. Seasonal and interannual variability of sea-surface carbon dioxide species at the European Station for Time Series in the Ocean at the Canary Islands (ESTOC) between 1996 and 2000. Glob. Biogeochem. Cycles 2003, 17, 1076. [Google Scholar] [CrossRef]
- Raven, J.; Caldeira, K.; Elderfield, H.; Hoegh-Guldberg, O.; Liss, P.; Riebesell, U.; Shepherd, J.; Turley, C.; Watson, A.; Heap, R.; et al. Ocean Acidification Due to Increasing Atmospheric Carbon Dioxide; The Royal Society: London, UK, 2005. [Google Scholar]
- Nakano, Y.; Watanabe, Y.W. Reconstruction of pH in the surface seawater over the North Pacific basin for all seasons using temperature and chlorophyll-a. J. Oceanogr. 2005, 61, 673–680. [Google Scholar] [CrossRef]
- Li, F. Distribution characteristics and influencing factors of seawater pH in the adjacent sea area of the Yellow River Estuary in August 1985. Mar. Limnol. Bull. 1988, 4, 35–40. [Google Scholar]
- Ke, D. Interannual Variation of pH Value in the South China Sea and Its Relationship with Temperature and Salt. Mar. Sci. Technol. Bull. 1990, 9, 23–27. [Google Scholar]
- Liu, X.; Sun, D.; Huang, B.; Wang, J. Study on surface sea acidification trend and influencing factors in coastal waters of the East China Sea. Ocean. Limnol. 2017, 48, 398–405. [Google Scholar]
- Xiao, J. Study on Ocean Acidification in the Chukchi Sea and the Nordic Sea. Master’s Thesis, Third Institute of Oceanography, State Oceanic Administration, Xiamen, China, 2015. [Google Scholar]
- Björk, M.M.; Fransson, A.; Torstensson, A.; Chierici, M. Ocean acidification state in western Antarctic surface waters: Controls and interannual variability. Biogeosciences 2014, 11, 57–73. [Google Scholar] [CrossRef]
- Krumhardt, K.M.; Lovenduski, N.S.; Long, M.C.; Levy, M.; Lindsay, K.; Moore, J.K.; Nissen, C. Coccolithophore growth and calcification in an acidified ocean: Insights from community Earth system model simulations. J. Adv. Model. Earth Syst. 2019, 11, 1418–1437. [Google Scholar] [CrossRef]
- Li, J.; Chai, G.; Xiao, Y.; Li, Z. The impacts of ocean acidification, warming and their interactive effects on coral prokaryotic symbionts. Environ. Microbiome 2023, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Pettorelli, N.; Hartmann, A.C.; Quinn, R.A.; Plaisance, L.; O’Mahoney, M.; Meyer, C.P.; Fabricius, K.E.; Knowlton, N.; Ransome, E. Decline of a distinct coral reef holobiont community under ocean acidification. Microbiome 2024, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Tena, R.A.; López-Pérez, A.; Reyes-Bonilla, H.; Calderon-Aguilera, L.E.; Orión, M.-L.; Zaragoza, F.A.R.; Cupul-Magaña, A.L.; Rodríguez-Troncoso, A.P.; Ayala-Bocos, A. Calcification of coral assemblages in the eastern Pacific: Reshuffling calcification scenarios under climate change. Ecol. Indic. 2018, 95, 726–734. [Google Scholar] [CrossRef]
- Trotter, J.; Montagna, P.; McCulloch, M.; Silenzi, S.; Reynaud, S.; Mortimer, G.; Martin, S.; Ferrier-Pagès, C.; Gattuso, J.-P.; Rodolfo-Metalpa, R. Quantifying the pH ‘vital effect’ in the temperate zooxanthellate coral Cladocora caespitosa: Validation of the boron seawater pH proxy. Earth Planet. Sci. Lett. 2011, 303, 163–173. [Google Scholar] [CrossRef]
- Sun, J.-Z.; Zhang, D.; Yi, X.; Beardall, J.; Gao, K. Ocean deoxygenation dampens resistance of diatoms to ocean acidification in darkness. Front. Mar. Sci. 2024, 11, 1387552. [Google Scholar] [CrossRef]



| Parameter | Correlation Coefficient | Deviation | Measured Average-Simulated Average | RMSE |
|---|---|---|---|---|
| pH | 0.95 | 0.02 | / | 0.04 |
| DO/(µmol·L−1) | 0.96 | 7.44 | / | 16.61 |
| chl a/(µg·L−1) | 0.81 | / | / | 0.59 |
| SST/°C | / | / | 0.04 | 0.45 |
| SSS/‰ | / | / | −0.002 | 0.06 |
| Factor | Basis for Judgment | Interactions |
|---|---|---|
| X1, X2 | q(X1 ∩ X2) < Min(q(X1), q(X2)) | Nonlinear weakening |
| X1, X2 | Min(q(X1), q(X2)) < q(X1 ∩ X2) < Max(q(X1), q(X2)) | Single-factor nonlinearity weakens |
| X1, X2 | q(X1 ∩ X2) > Max(q(X1), q(X2)) | Two-factor enhancement |
| X1, X2 X1, X2 | q(X1 ∩ X2) = q(X1) + q(X2) q(X1 ∩ X2) > q(X1) + q(X2) | Independent Nonlinear enhancement |
| Environmental Factors | Correlation Coefficient | Dispersion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | Spring | Summer | Autumn | Winter | Year | Spring | Summer | Autumn | Winter | |
| SST | −0.721 ** | −0.658 ** | −0.714 ** | −0.699 ** | −0.702 ** | 0.011 | 0.010 | 0.011 | 0.012 | 0.010 |
| SSS | −0.425 * | −0.267 * | −0.412 * | −0.589 * | −0.401 * | 0.004 | 0.001 | 0.001 | 0.001 | 0.001 |
| chl a | −0.328 | −0.448 * | −0.344 | −0.167 | −0.174 | 0.030 | 0.037 | 0.070 | 0.037 | 0.021 |
| DO | 0.655 ** | 0.347 | 0.652 ** | 0.601 ** | 0.709 ** | 0.001 | 0.004 | 0.005 | 0.004 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, J.; Mao, J.; Liu, J. Spatiotemporal Variation and Influences of Acidification in the North Pacific, 1995–2019. Water 2024, 16, 2705. https://doi.org/10.3390/w16182705
Wang X, Wang J, Mao J, Liu J. Spatiotemporal Variation and Influences of Acidification in the North Pacific, 1995–2019. Water. 2024; 16(18):2705. https://doi.org/10.3390/w16182705
Chicago/Turabian StyleWang, Xun, Jie Wang, Jingjing Mao, and Jiaming Liu. 2024. "Spatiotemporal Variation and Influences of Acidification in the North Pacific, 1995–2019" Water 16, no. 18: 2705. https://doi.org/10.3390/w16182705






