Field Demonstration of In Situ Slow-Release Oxygen Chemicals Coupled with Microbial Agents for Injection to Remediate BTEX Contamination
Abstract
1. Introduction
2. Experimental Methodology
2.1. Experimental Materials
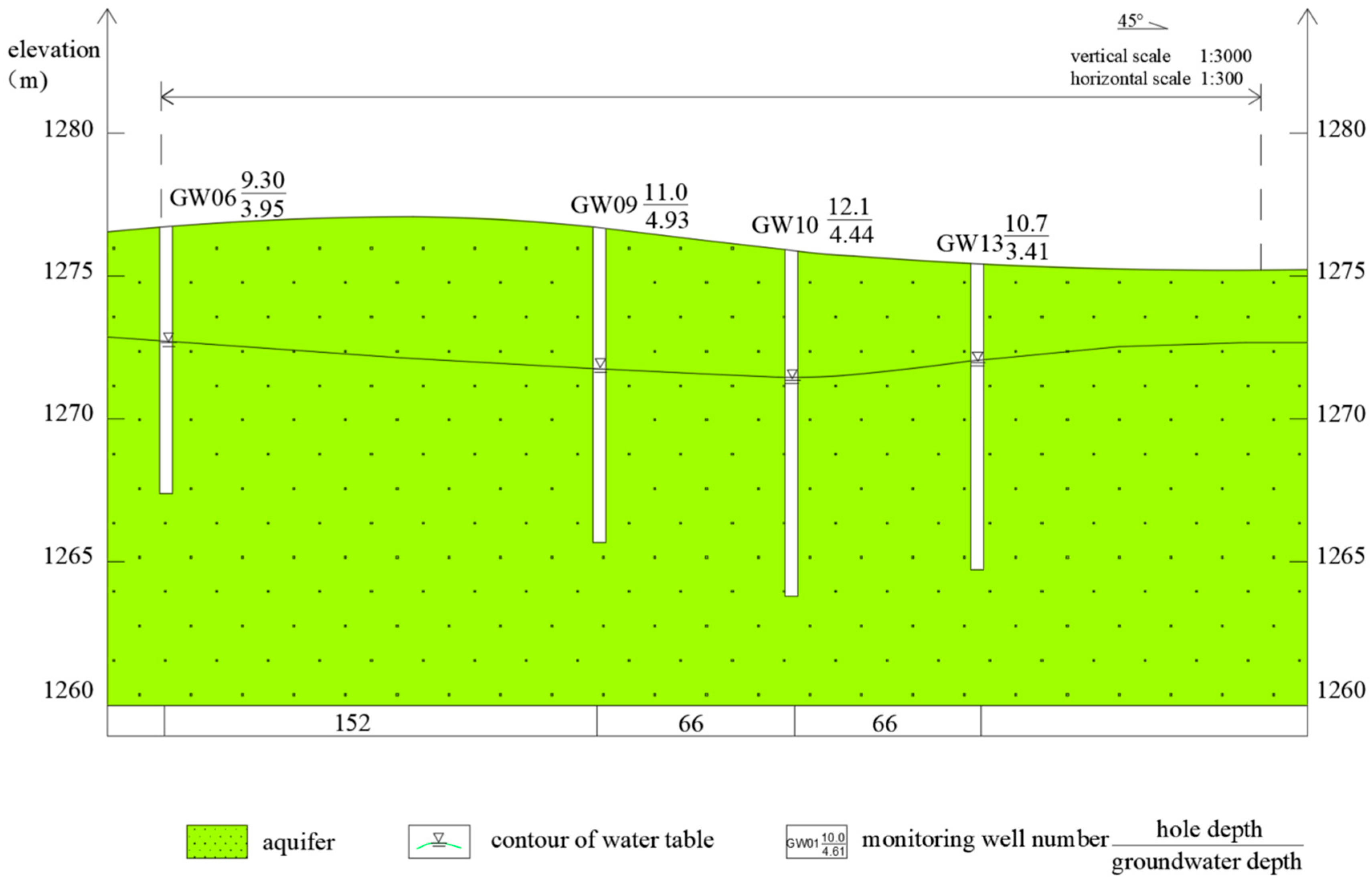
2.2. Hydrogeologic Conditions of the Test Site
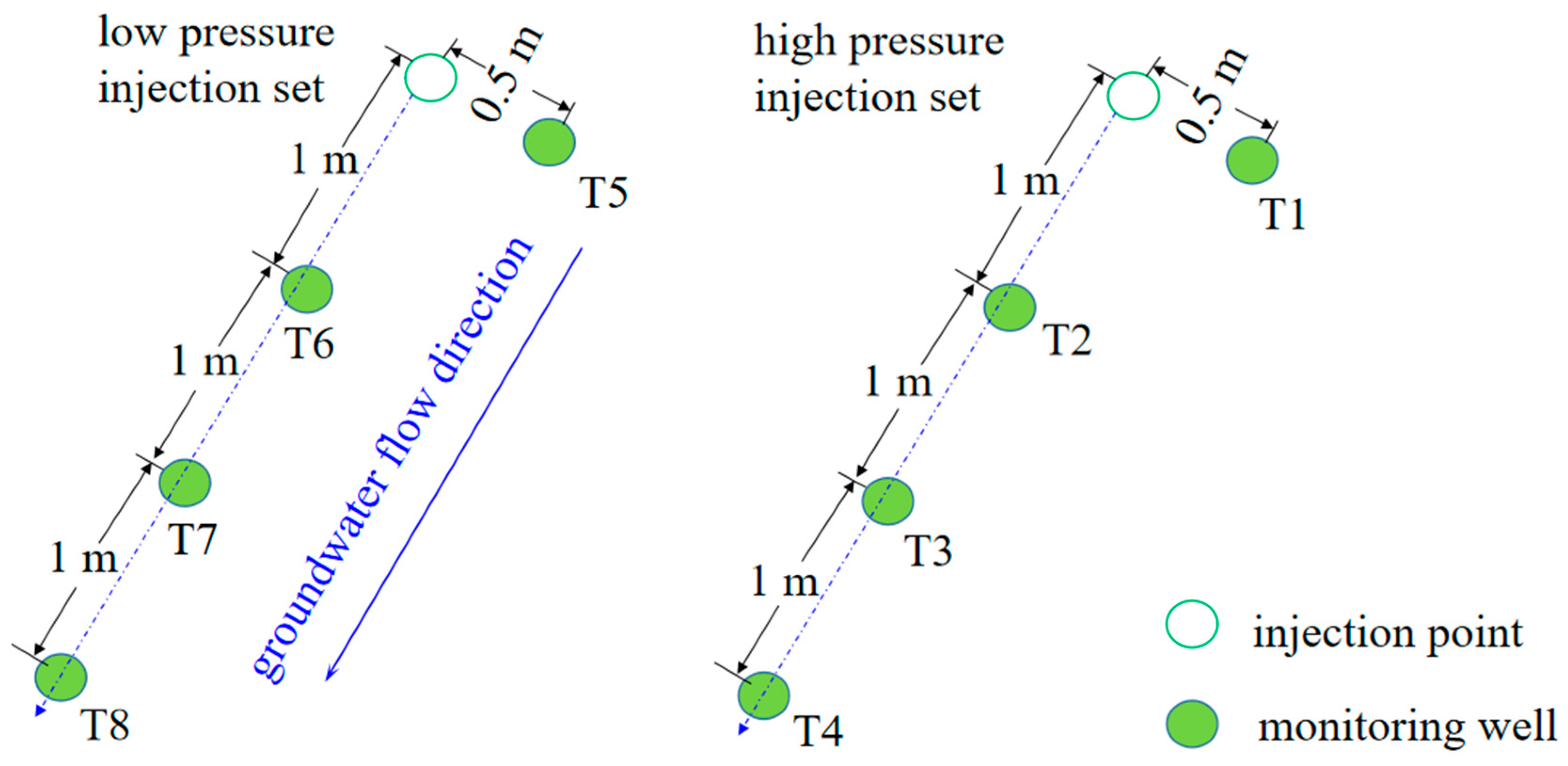
2.3. Injection Impact Radius Test
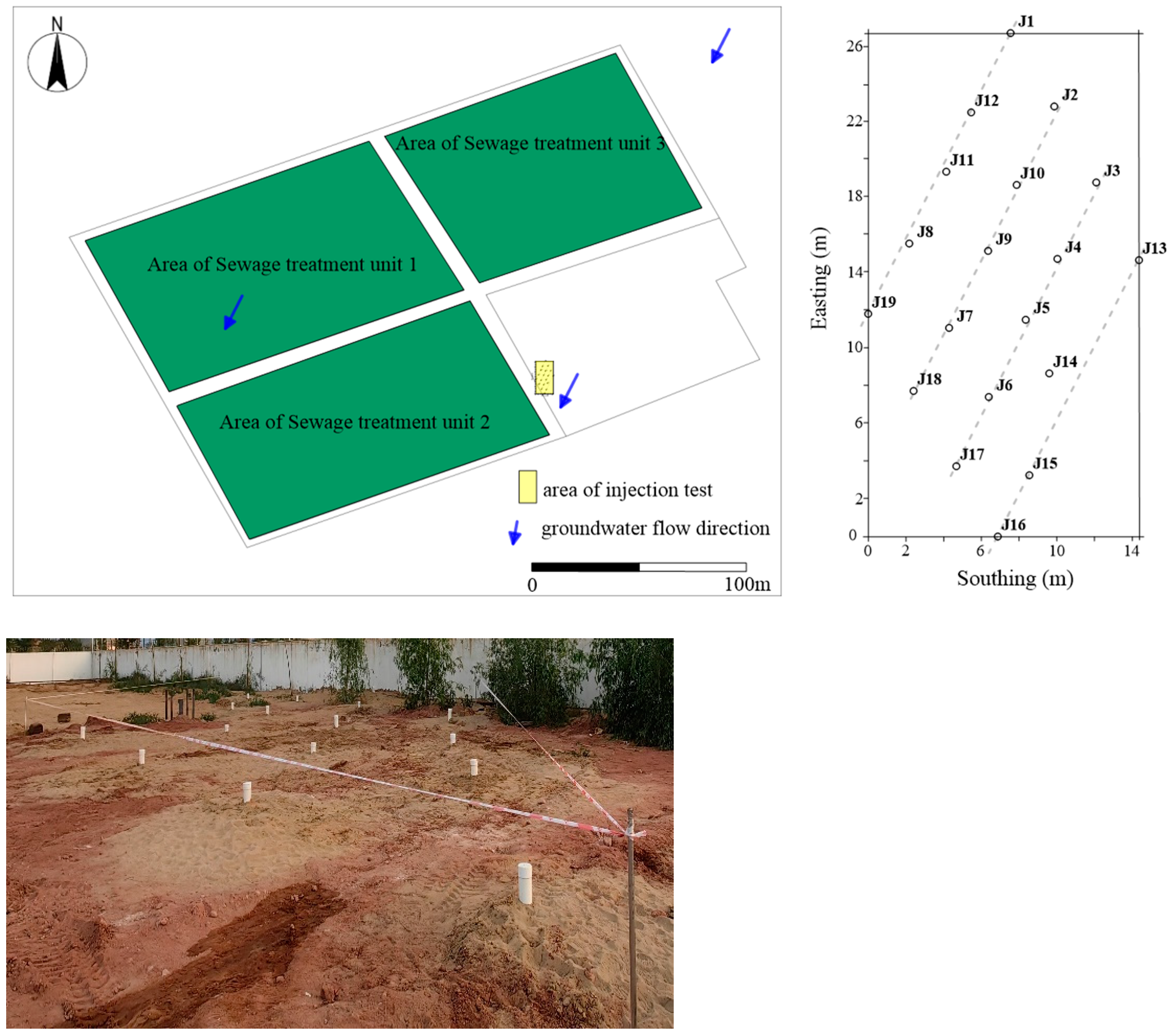
2.4. Monitoring Well Placement and Test Program
2.5. Initial Contamination Conditions at the Site
2.6. Coupling Injection Test
3. Results and Discussion
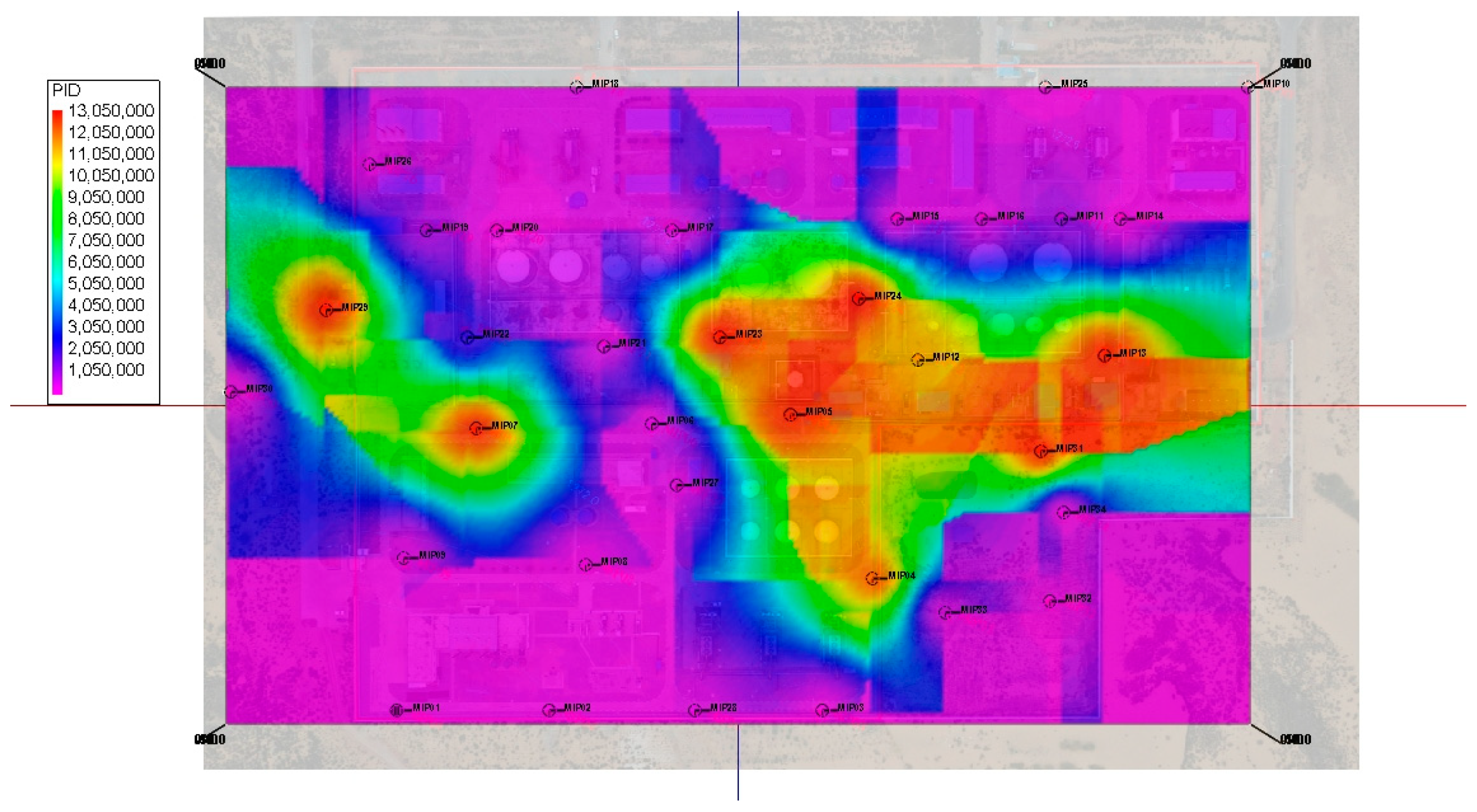
3.1. Injection Instantaneous Influence Radius
3.2. Changes in Groundwater Dissolved Oxygen Concentration after Injection of Slow-Release Oxygen Materials
3.3. Characterization of Spatial and Temporal Changes in Microbial Abundance after Coupled Injection
3.4. Characterization of Spatial and Temporal Changes in Pollution after Coupled Injection
3.5. Actual BTEX Degradation Efficiencies after Pollution Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, B.; Huang, F.; Zhang, C.; Huang, G.; Xue, Q.; Liu, F. Pollution characteristics of aromatic hydrocarbons in the groundwater of China. J. Contam. Hydrol. 2020, 233, 103676. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hurtado, A.; Eguilior, S.; Borrajo, J.F.L. Acute and chronic risk assessment of BTEX in the return water of hydraulic fracturing operations in Marcellus Shale. Sci. Total Environ. 2024, 906, 167638. [Google Scholar] [CrossRef] [PubMed]
- Khaled, I.; Saidi, I.; Ferjani, H.; Ahmed, R.B.; Alrezaki, A.; Guesmi, F.; Bouzenna, H.; Harrath, A.H. BTEX induces histopathological alterations, oxidative stress response and DNA damage in the testis of the freshwater leech Erpobdella johanssoni (Johansson, 1927). J. King Saud Univ.-Sci. 2022, 34, 102196. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, G.; Hao, C.; Xie, Q.; Zhang, Q. Microbial community structures in petroleum contaminated soils at an oil field, Hebei, China. CLEAN–Soil Air Water 2016, 44, 829–839. [Google Scholar] [CrossRef]
- Kaur, G.; Lecka, J.; Krol, M.; Brar, S.K. Novel BTEX-degrading strains from subsurface soil: Isolation, identification and growth evaluation. Environ. Pollut. 2023, 335, 122303. [Google Scholar] [CrossRef]
- Yu, B.; Yuan, Z.; Yu, Z.; Feng, X.-S. BTEX in the environment: An update on sources, fate, distribution, pretreatment, analysis, and removal techniques. Chem. Eng. J. 2022, 435, 134825. [Google Scholar] [CrossRef]
- Vaezihir, A.; Bayanlou, M.B.; Ahmadnezhad, Z.; Barzegari, G. Remediation of BTEX plume in a continuous flow model using zeolite-PRB. J. Contam. Hydrol. 2020, 230, 103604. [Google Scholar] [CrossRef] [PubMed]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, Y.; Zhao, J.; Li, S.; Schulz, S.; Deng, L. BTEX biodegradation is linked to bacterial community assembly patterns in contaminated groundwater ecosystem. J. Hazard. Mater. 2021, 419, 126205. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, G.; Ji, Y.; Li, P.; Yu, X.; Qiao, W.; Wang, B.; Shi, K.; Liu, W.; Liang, B.; et al. Electron acceptors determine the BTEX degradation capacity of anaerobic microbiota via regulating the microbial community. Environ. Res. 2022, 215, 114420. [Google Scholar] [CrossRef]
- Hauptfeld, E.; Pelkmans, J.; Huisman, T.T.; Anocic, A.; Snoek, B.L.; von Meijenfeldt, F.A.B.; Gerritse, J.; van Leeuwen, J.; Leurink, G.; van Lit, A.; et al. A metagenomic portrait of the microbial community responsible for two decades of bioremediation of poly-contaminated groundwater. Water Res. 2022, 221, 118767. [Google Scholar] [CrossRef]
- Dou, J.; Liu, X.; Hu, Z. Anaerobic BTEX degradation in soil bioaugmented with mixed consortia under nitrate reducing conditions. J. Environ. Sci. 2008, 20, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ye, J.; Jiang, K.; Wang, Y.; Li, Y. Oil contamination drives the transformation of soil microbial communities: Co-occurrence pattern, metabolic enzymes and culturable hydrocarbon-degrading bacteria. Ecotoxicol. Environ. Saf. 2021, 225, 112740. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Dong, H.; Kukkadapu, R.K.; Ni, S.; Zeng, Q.; Hu, J.; Coffin, E.; Zhao, S.; Sommer, A.J.; McCarrick, R.M.; et al. Lignin-enhanced reduction of structural Fe (III) in nontronite: Dual roles of lignin as electron shuttle and donor. Geochim. Cosmochim. Acta 2021, 307, 1–21. [Google Scholar] [CrossRef]
- Lin, C.-W.; Cheng, Y.-W.; Tsai, S.-L. Multi-substrate biodegradation kinetics of MTBE and BTEX mixtures by Pseudomonas aeruginosa. Process Biochem. 2007, 42, 1211–1217. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, D.; Yang, L.; Wei, J.; Kong, L.; Xie, W.; Ding, D.; Fan, T.; Deng, S. Natural attenuation of BTEX and chlorobenzenes in a formerly contaminated pesticide site in China: Examining kinetics, mechanisms, and isotopes analysis. Sci. Total Environ. 2024, 918, 170506. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Tian, X.; Wang, G.; Hao, C.; Liu, F. Bacterial diversity and biogeochemical processes of oil-contaminated groundwater, Baoding, North China. Geomicrobiol. J. 2016, 33, 537–551. [Google Scholar] [CrossRef]
- Sun, P.; Hua, Y.; Zhao, J.; Wang, C.; Tan, Q.; Shen, G. Insights into the mechanism of hydrogen peroxide activation with biochar produced from anaerobically digested residues at different pyrolysis temperatures for the degradation of BTEXS. Sci. Total Environ. 2021, 788, 147718. [Google Scholar] [CrossRef]
- Chen, X.; Sheng, Y.; Wang, G.; Guo, L.; Zhang, H.; Zhang, F.; Yang, T.; Huang, D.; Han, X.; Zhou, L. Microbial compositional and functional traits of BTEX and salinity co-contaminated shallow groundwater by produced water. Water Res. 2022, 215, 118277. [Google Scholar] [CrossRef]
- Tang, X.; Li, Z.; Wang, Z.; Yu, C.; Sun, H.; Wang, J.; Wang, C. Efficient remediation of PAHs contaminated site soil using the novel slow-release oxidant material. Chem. Eng. J. 2023, 472, 144713. [Google Scholar] [CrossRef]
- Yang, C.-F.; Liu, S.-H.; Su, Y.-M.; Chen, Y.-R.; Lin, C.-W.; Lin, K.-L. Bioremediation capability evaluation of benzene and sulfolane contaminated groundwater: Determination of bioremediation parameters. Sci. Total Environ. 2019, 648, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Song, X.; Wang, Q.; Zhang, Z.; Che, J.; Chen, X.; Tang, Z.; Liu, X. Mechanisms of biostimulant-enhanced biodegradation of PAHs and BTEX mixed contaminants in soil by native microbial consortium. Environ. Pollut. 2023, 318, 120831. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Maher, K.; Navarre-Sitchler, A.; Druhan, J.; Meile, C.; Lawrence, C.; Moore, J.; Perdrial, J.; Sullivan, P.; Thompson, A.; et al. Expanding the role of reactive transport models in critical zone processes. Earth-Sci. Rev. 2017, 165, 280–301. [Google Scholar] [CrossRef]
- Kapellos, G.E. Microbial strategies for oil biodegradation. In Modeling of Microscale Transport in Biological Processes; Academic Press: Cambridge, MA, USA, 2017; pp. 19–39. [Google Scholar]
- Scammacca, O.; Sauzet, O.; Michelin, J.; Choquet, P.; Garnier, P.; Gabrielle, B.; Baveye, P.C.; Montagne, D. Effect of spatial scale of soil data on estimates of soil ecosystem services: Case study in 100 km2 area in France. Eur. J. Soil Sci. 2023, 74, e13359. [Google Scholar] [CrossRef]
- Ellis, D.E.; Lutz, E.J.; Odom, J.M.; Buchanan, R.J.; Bartlett, C.L.; Lee, M.D.; Harkness, M.R.; DeWeerd, K.A. Bioaugmentation for accelerated in situ anaerobic bioremediation. Environ. Sci. Technol. 2000, 34, 2254–2260. [Google Scholar] [CrossRef]
- Mo, Y.; Dong, J.; Zhao, H. Field demonstration of in-situ microemulsion flushing for enhanced remediation of multiple chlorinated solvents contaminated aquifer. J. Hazard. Mater. 2024, 463, 132772. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Acio, J.A.; El Telib, A.E. Aerobic biodegradation of BTEX: Progresses and Prospects. J. Environ. Chem. Eng. 2014, 2, 1104–1122. [Google Scholar] [CrossRef]
- Wang, W.; Jia, J.; Zhang, B.; Xiao, B.; Yang, H.; Zhang, S.; Gao, X.; Han, Y.; Zhang, S.; Liu, Z.; et al. A review of Sustained release materials for remediation of organically contaminated groundwater: Material preparation, applications and prospects for practical application. J. Hazard. Mater. Adv. 2024, 13, 100393. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Zhang, L.; Xie, D.; Onwosi, C.O.; Muhammad, W.I.; Odoh, C.K.; Sam, K.; Idenyi, J.N. Bioaugmentation as a green technology for hydrocarbon pollution remediation. Problems and prospects. J. Environ. Manag. 2022, 304, 114313. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Baars, O.; Guo, D.; Whitham, J.; Srivastava, S.; Dong, H. Mineral-bound trace metals as cofactors for anaerobic biological nitrogen fixation. Environ. Sci. Technol. 2023, 57, 7206–7216. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Sheng, Y.; Guo, C.; Wang, S.; Yang, S.; Zhang, M. Incorporating the Soil Gas Gradient Method and Functional Genes to Assess the Natural Source Zone Depletion at a Petroleum-Hydrocarbon-Contaminated Site of a Purification Plant in Northwest China. Life 2022, 13, 114. [Google Scholar] [CrossRef]
- Sheng, Y.; Liu, Y.; Yang, J.; Dong, H.; Liu, B.; Zhang, H.; Li, A.; Wei, Y.; Li, G.; Zhang, D. History of petroleum disturbance triggering the depth-resolved assembly process of microbial communities in the vadose zone. J. Hazard. Mater. 2021, 402, 124060. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Sheng, Y.; Gan, S.; Guo, C.; Wang, S.; Cai, P.; Zhang, M. Metagenomic and Isotopic Insights into Carbon Fixation by Autotrophic Microorganisms in a Petroleum Hydrocarbon Impacted Red Clay Aquifer. Environ. Pollut. 2024, 361, 124824. [Google Scholar] [CrossRef]
- Zhang, M.; Ning, Z.; Guo, C.; Shi, C.; Zhang, S.; Sheng, Y.; Chen, Z. Using Compound Specific Isotope Analysis to decipher the 1, 2, 3-trichloropropane-to-Allyl chloride transformation by groundwater microbial communities. Environ. Pollut. 2023, 316, 120577. [Google Scholar] [CrossRef] [PubMed]
- Lhotský, O.; Kukačka, J.; Slunský, J.; Marková, K.; Němeček, J.; Knytl, V.; Cajthaml, T. The effects of hydraulic/pneumatic fracturing-enhanced remediation (FRAC-IN) at a site contaminated by chlorinated ethenes: A case study. J. Hazard. Mater. 2021, 417, 125883. [Google Scholar] [CrossRef] [PubMed]
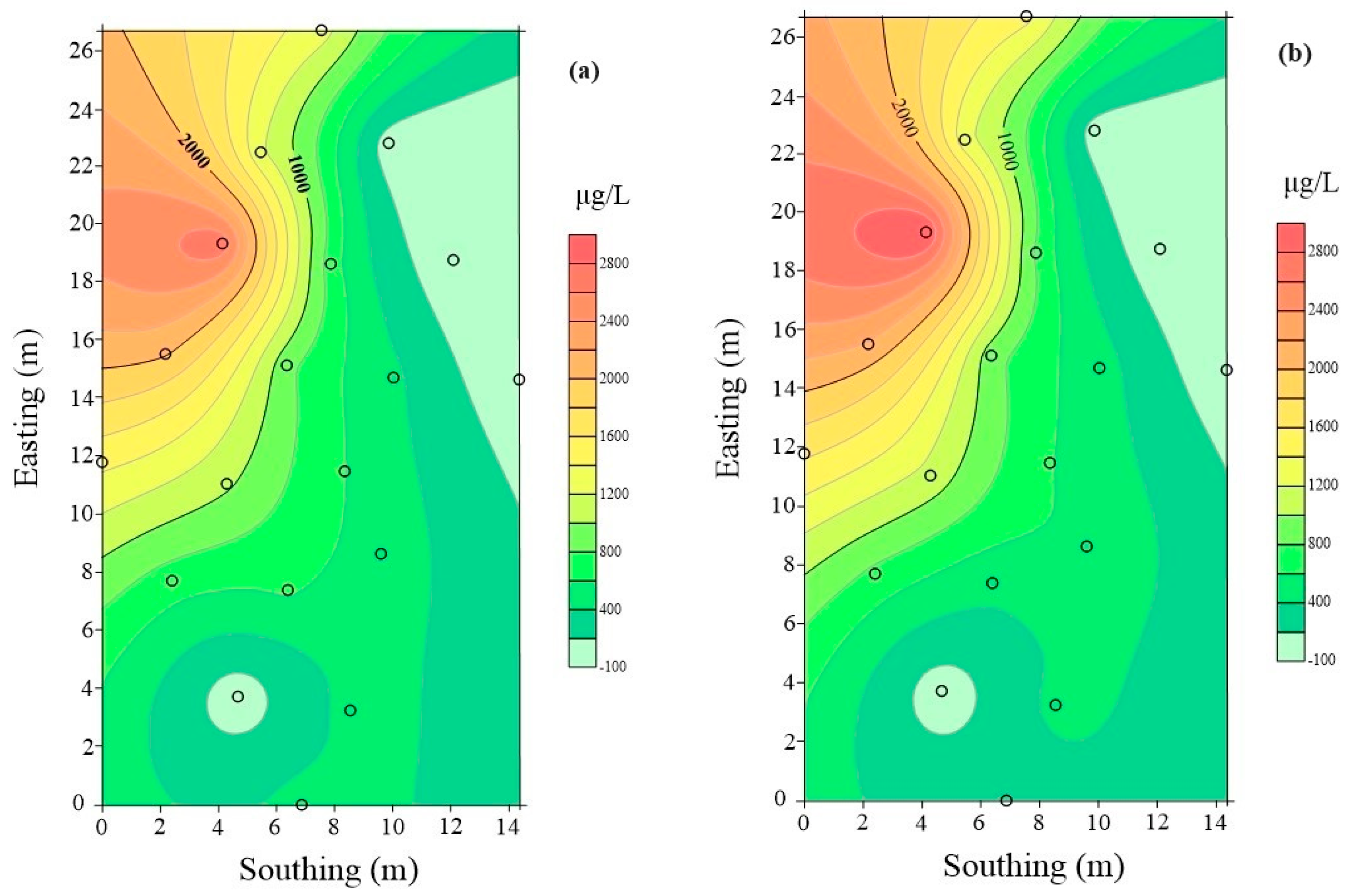
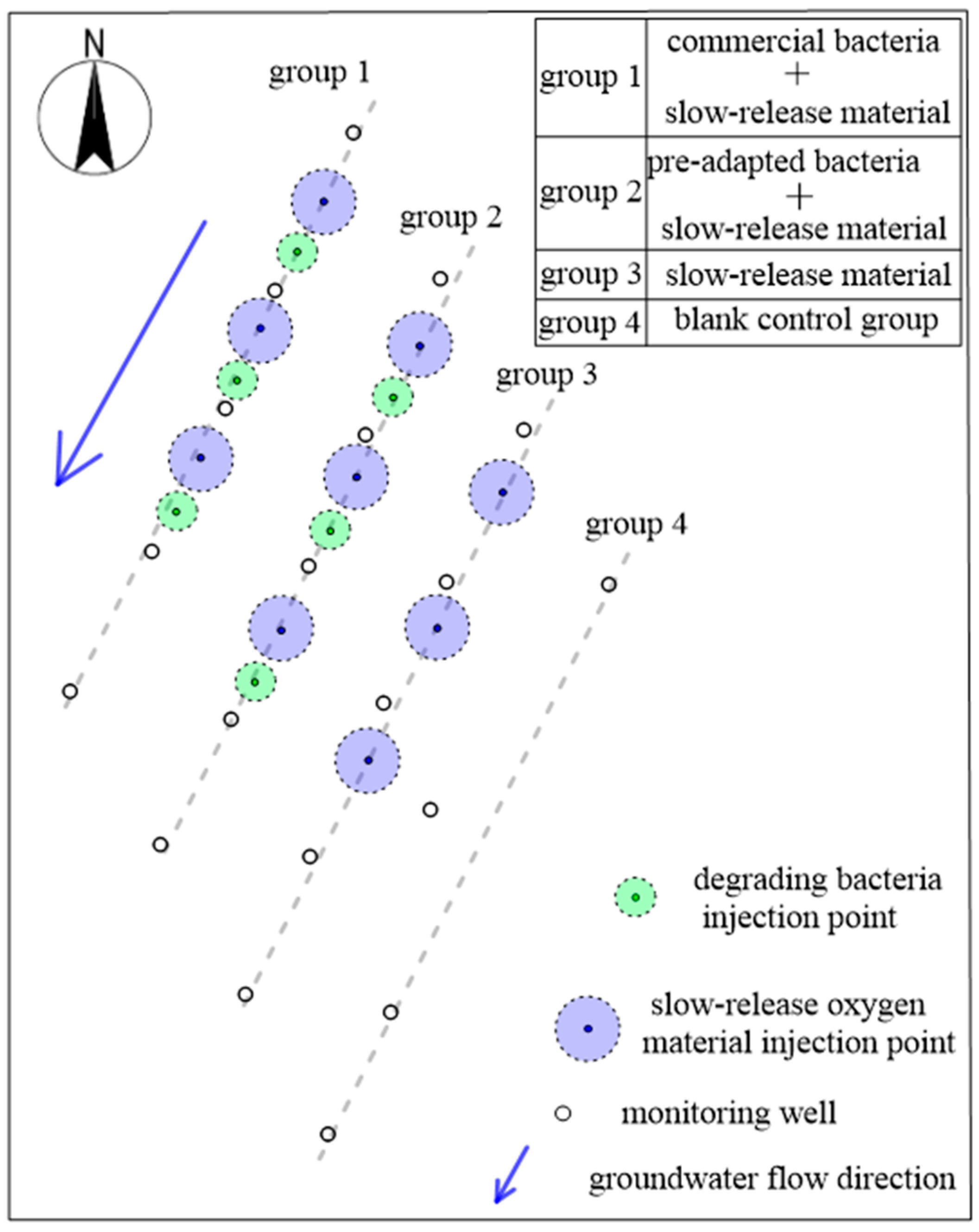
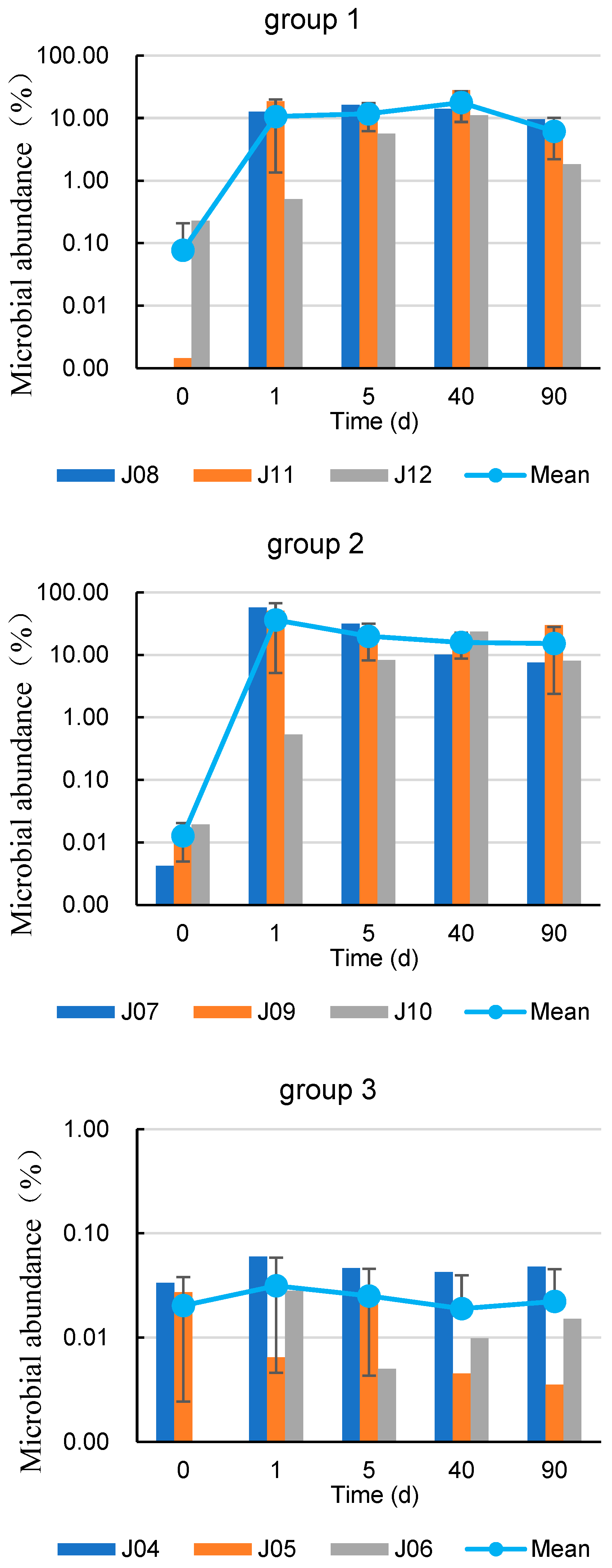
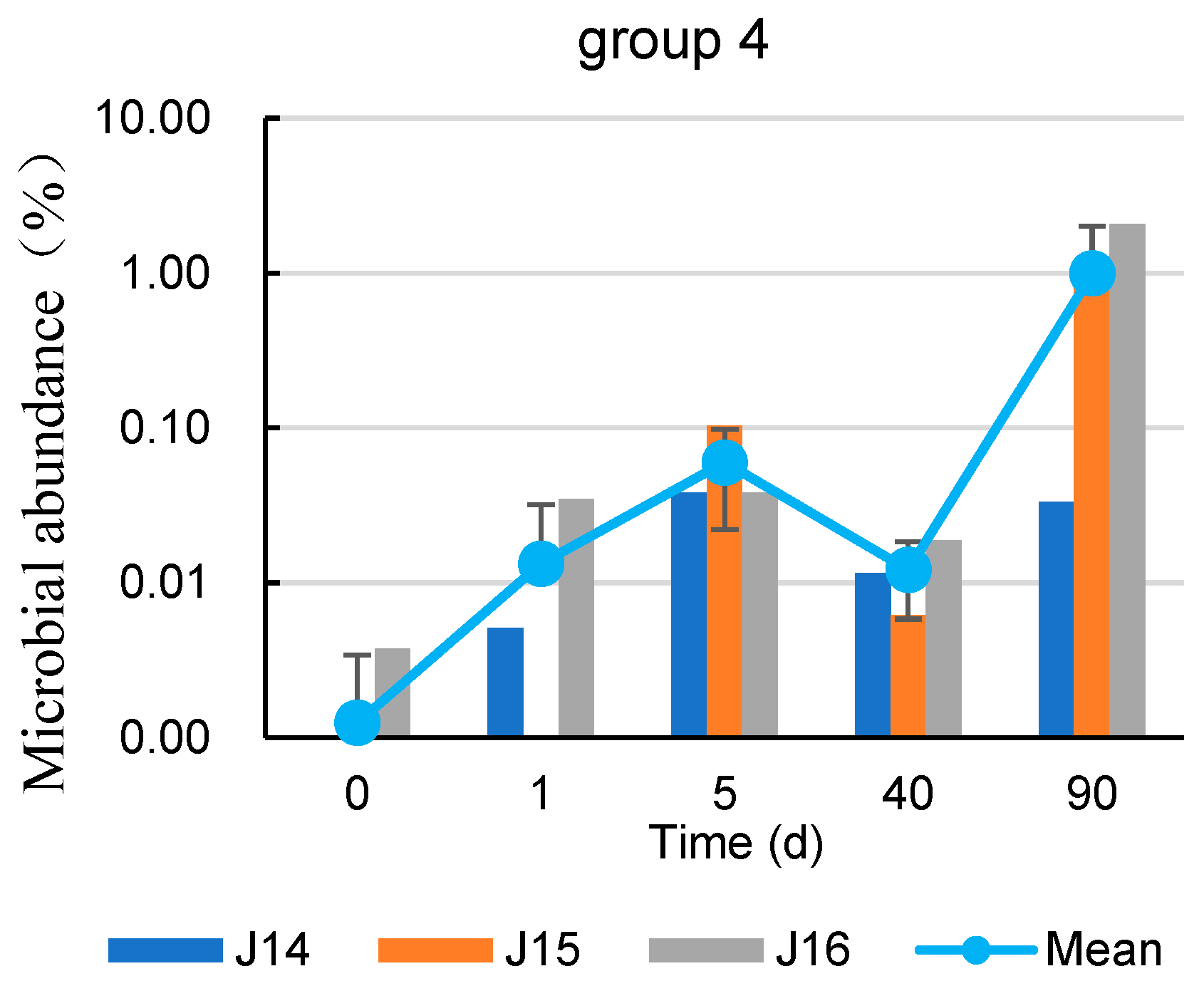
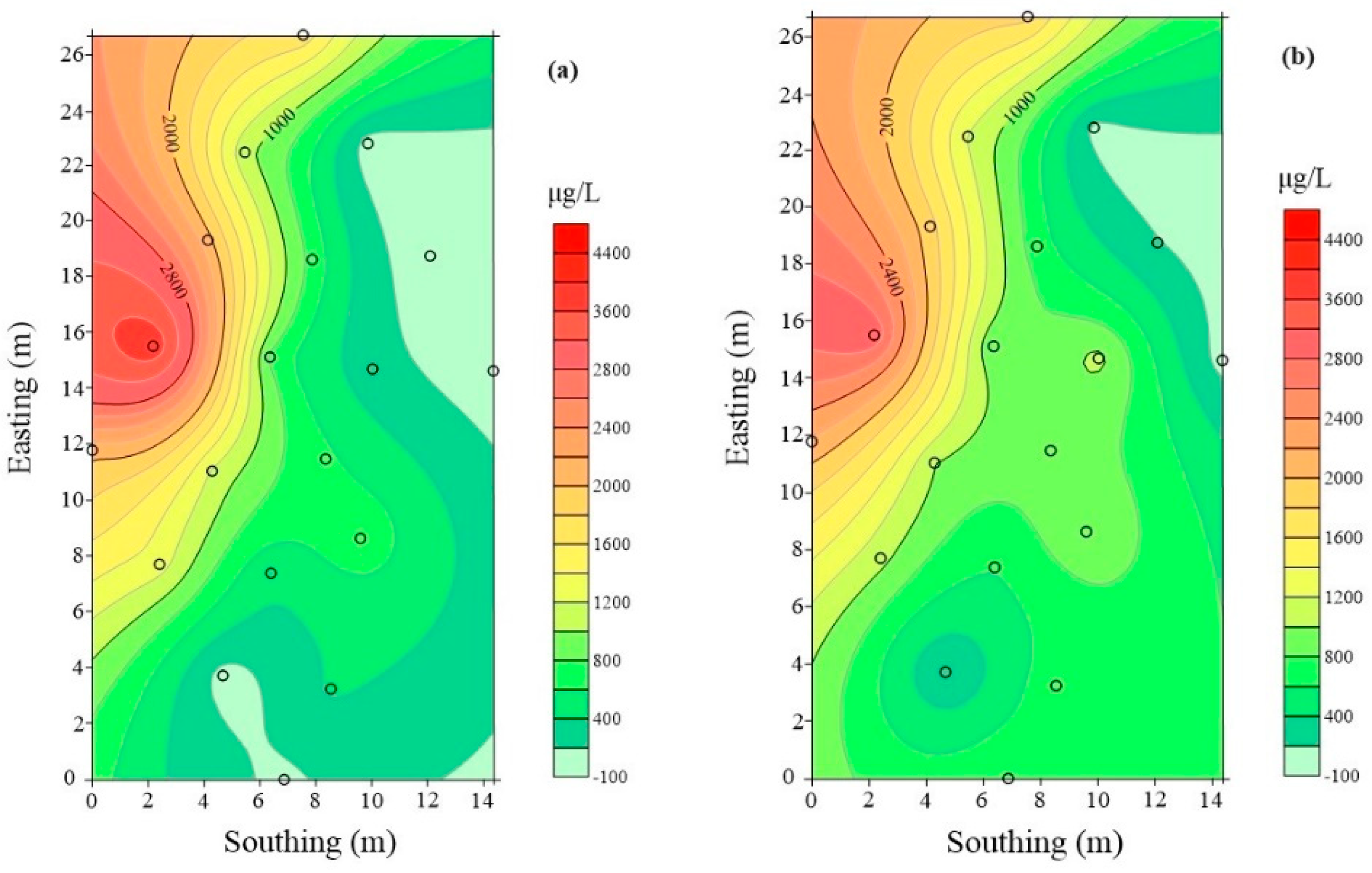

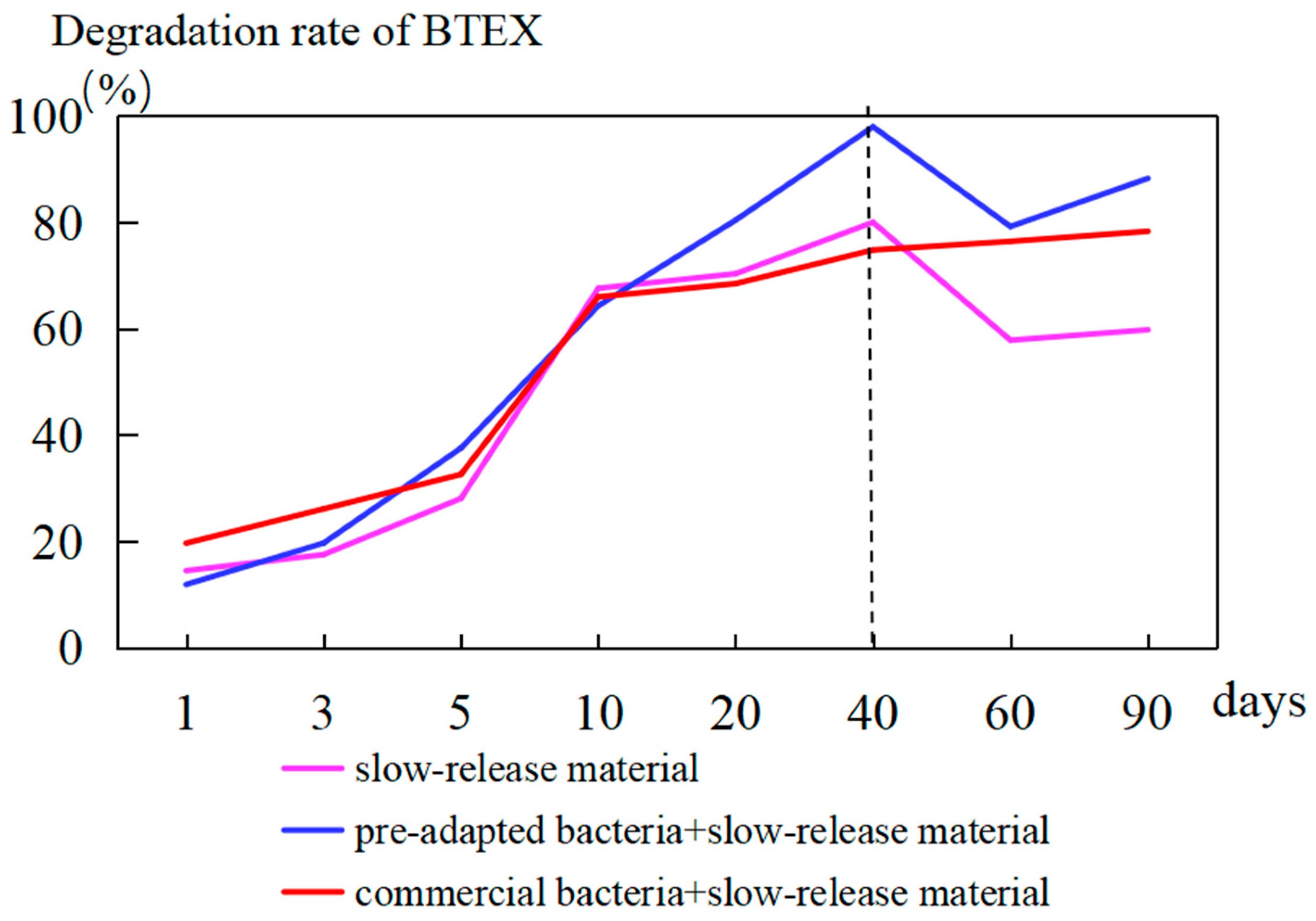
| Well | 7 d before Injection BTEX (μg/L) | 90 d after Injection BTEX (μg/L) | Growth Rate (%) |
|---|---|---|---|
| J14 | 514.72 | 2876.67 | 458.88 |
| J15 | 446.80 | 3098.78 | 593.55 |
| J16 | 474.46 | 3642.29 | 667.67 |
| Average value | 478.66 | 3205.91 | 573.37 |
| Group | Well | 7 d before Injection BTEX (μg/L) | 90 d after Injection BTEX Ratio Analysis Value (μg/L) | 90 d after Injection BTEX Detection Value (μg/L) | Calculated BTEX Degradation Efficiencies (%) |
|---|---|---|---|---|---|
| Group of slow-release materials | J04 | 445.15 | 2995.85 | 35.34 | 98.82 |
| J05 | 602.64 | 4055.77 | 3307.19 | 18.46 | |
| J06 | 624.96 | 4205.97 | 3792.03 | 9.84 | |
| Average value | 42.37 | ||||
| Group of slow-release materials and pre-adapted bacterial agents | J07 | 1033.84 | 6957.75 | 2228.64 | 67.97 |
| J09 | 850.20 | 5721.85 | 12.48 | 99.78 | |
| J10 | 690.10 | 4644.36 | 205.13 | 95.58 | |
| Average value | 87.78 | ||||
| Group of slow-release materials coupled with commercial fungicides | J08 | 2019.71 | 13,592.65 | 3080.80 | 77.33 |
| J11 | 2740.37 | 18,442.66 | 1102.06 | 94.02 | |
| J12 | 1231.42 | 8287.46 | 3452.41 | 58.34 | |
| Average value | 76.57 | ||||
| Group | Well | 90 d after Injection BTEX-Detection Value (μg/L) | 90 d after Injection BTEX-Simulation Value (μg/L) | Calculated BTEX Degradation Efficiencies (%) |
|---|---|---|---|---|
| Group of slow-release materials | J04 | 35.34 | 5376.23 | 99.34 |
| J05 | 3307.19 | 5794.89 | 42.93 | |
| J06 | 3792.03 | 6025.91 | 37.07 | |
| Average value | 59.78 | |||
| Group of slow-release materials and pre-adapted bacterial agents | J07 | 2228.64 | 6849.18 | 67.46 |
| J09 | 12.48 | 8780.12 | 99.86 | |
| J10 | 205.13 | 7952.25 | 97.42 | |
| Average value | 88.25 | |||
| Group of slow-release materials coupled with commercial fungicides | J08 | 3080.8 | 8428.11 | 63.45 |
| J11 | 1102.06 | 15,936.54 | 93.08 | |
| J12 | 3452.41 | 15,959.31 | 78.37 | |
| Average value | 78.29 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhang, S.; Ma, S.; Zhao, S.; Liu, Z. Field Demonstration of In Situ Slow-Release Oxygen Chemicals Coupled with Microbial Agents for Injection to Remediate BTEX Contamination. Water 2024, 16, 2815. https://doi.org/10.3390/w16192815
Yang S, Zhang S, Ma S, Zhao S, Liu Z. Field Demonstration of In Situ Slow-Release Oxygen Chemicals Coupled with Microbial Agents for Injection to Remediate BTEX Contamination. Water. 2024; 16(19):2815. https://doi.org/10.3390/w16192815
Chicago/Turabian StyleYang, Shuai, Shucai Zhang, Shici Ma, Sheng Zhao, and Zhengwei Liu. 2024. "Field Demonstration of In Situ Slow-Release Oxygen Chemicals Coupled with Microbial Agents for Injection to Remediate BTEX Contamination" Water 16, no. 19: 2815. https://doi.org/10.3390/w16192815
APA StyleYang, S., Zhang, S., Ma, S., Zhao, S., & Liu, Z. (2024). Field Demonstration of In Situ Slow-Release Oxygen Chemicals Coupled with Microbial Agents for Injection to Remediate BTEX Contamination. Water, 16(19), 2815. https://doi.org/10.3390/w16192815







