Abstract
This study was carried out to elucidate the causes of massive outbreaks of Aurelia coerulea in Geoje Bay, Korea, from November 2022 to October 2023. Adult medusae consistently spawn with planulae, and the populations of A. coerulea in Geoje Bay could be categorized into current-year and overwintering populations. The current-year population began with the emergence of ephyrae in February and grew until October, while the overwintering population comprised a mixture of surviving current-year population and additional individuals that joined during the warm season. The size of the planulae are significantly larger than the annual average during the cold season. These results appear to be the energy accumulation of planulae for polyp formation under low water temperatures. Planulae form polyps within a temperature range of 5–25 °C, suggesting the possibility of year-round polyp recruitment. In Geoje Bay, the highest appearance rate of A. coerulea was in April (8.71 ± 12.5 ind. m−3), with ephyrae experiencing higher growth rates up to the young medusa stage. However, from April, a decline in zooplankton biomass resulted in reduced growth rates in adults, indicating that jellyfish growth was primarily regulated by food availability. Additionally, submersed oyster shells in oyster farms served as the main habitat for jellyfish polyps. A. coerulea populations were also characterized by the continuous spawning of planulae throughout the year. In conclusion, this study suggests that stable polyp habitats, abundant food supply during the initial developmental period of the population, and suitable ranges of water temperature were significant factors inducing the massive outbreak of A. coerulea in Geoje Bay, Korea.
Keywords:
Aurelia coerulea; jellyfish bloom; massive outbreak; population dynamics; planulae; polyp; habitat 1. Introduction
Jellyfish play various ecological roles in marine ecosystems, both as predators and prey for various marine organisms, significantly influencing marine food webs [1]. Over the past few decades, several studies have highlighted the critical role of their chemical properties in advancing biomedical research and biotechnology [2,3,4,5]. Jellyfish used in food and medicinal products can generate significant economic benefits [6]. However, during large local outbreaks, some jellyfish are known to directly reduce the biomass of fishery resources, including fish eggs, larvae, and juveniles, by preying on them [7]. They also decrease zooplankton populations and disrupt ecosystem structures, thereby decreasing food availability for fish [8,9,10,11]. Mass jellyfish occurrences can severely impact the fishing and aquaculture industries by damaging nets during operations and degrading catch quality. Additionally, they can block cooling water intakes at coastal power plants, leading to significant power losses and economic damage to cities [12,13]. Furthermore, jellyfish stings can result in beach closures, negatively impacting the coastal tourism industry [14].
Aurelia coerulea is a species that causes significant fishery damage along the Korean coasts. The genus Aurelia is distributed across a broad range of climatic zones, from tropical to boreal regions, and is known to proliferate in large numbers in semi-enclosed bays and coastal waters [15]. Recent phylogenetic analyses have indicated that occurrences of the genus Aurelia in East Asian coastal waters, including those of Korea, China, and Japan, are as attributed to A. coerulea [16]. Massive outbreaks of jellyfish are associated with various factors, including increases in artificial structures, eutrophication [7], environmental degradation, global warming and climate change, overfishing [10,17], and invasions of alien species [18]. These factors often act simultaneously, leading to significant increases in jellyfish populations in East Asian coastal areas as a result of rapid development [7].
This study was conducted to determine the cause of the mass outbreak of A. coerulea in Geoje Bay, Korea, using a combination of laboratory experiments and a field survey. To understand jellyfish mass outbreaks, it is essential to have a comprehensive understanding of their population recruitment and development processes. The former can be regulated by the production of planulae (sexual production) and ephyrae (asexual production). Specifically, ephyra production is influenced by the availability of adequate polyp habitats within the natural ecosystem. Additionally, the understanding of population development characteristics is crucial for elucidating the causes of mass jellyfish outbreaks. This study aimed to (1) investigate the habitat of the polyp stage in the life cycle of A. coerulea, which lives attached to the benthos and reproduces asexually; (2) examine the population dynamics of ephyrae, young medusae, and adult medusae produced through strobilation; and (3) describe the mechanism of A. coerulea medusa mass production in the study area by studying the characteristics of planulae spawned by mature medusae though sexual reproduction.
2. Materials and Methods
2.1. Study Area
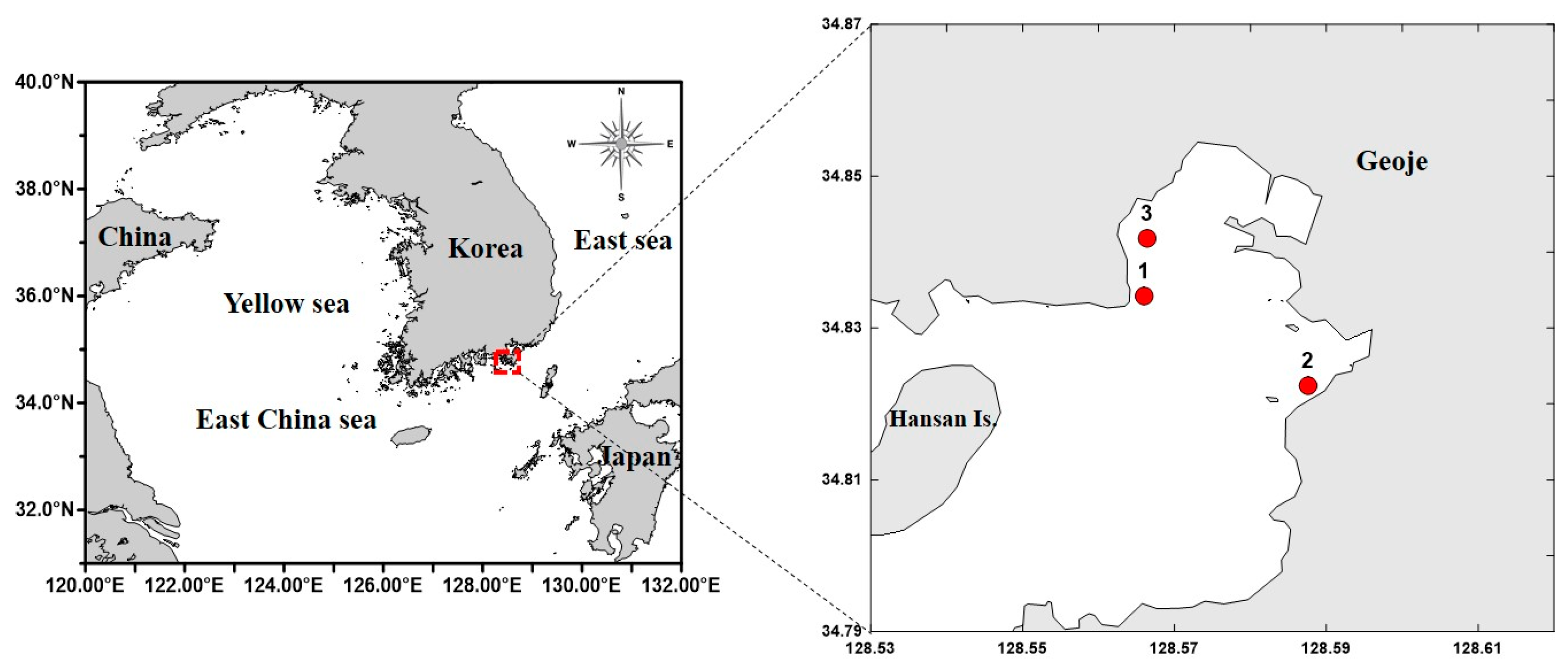
Geoje Bay (34°8′ N, 128°57′ E), located on the southern coast of Korea, was selected as the study area, and the target species was A. coerulea. The study period spanned from November 2022 to October 2023, with surveys conducted at three fixed sites every 30 days (Figure 1).
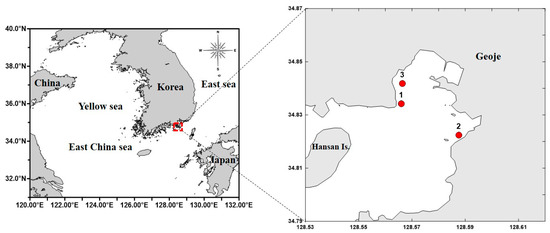
Figure 1.
Map of the sampling stations in Geoje Bay, Korea (Red dots: Sample and survey locations).
2.2. Sample Collection and Processing
For the population dynamics study, the jellyfish samples were divided into ephyrae (bell diameter < 10 mm), young medusae (BD < 30 mm), and medusae (BD > 30 mm). The water temperature and salinity were measured using a conductivity, temperature, and depth (CTD) device (Concerto3 model, RBR, Ltd., Kanata, ON, Canada) from the surface to the bottom. Water samples were collected from surface waters (1–2 m) using a Niskin water sampler. The average water depth was 8.0 m. After filtering the sampled water through Whatman GF/F filters, the chlorophyll-a (Chl-a) concentration was determined using a Turner Designs 10 AU fluorometer, San Jose, CA, USA. Nutrients, including dissolved inorganic nitrogen (DIN) and phosphorus (DIP), were analyzed using an auto-analyzer (QuAAtro, Seal Analytical, Norderstedt, Germany). The abundances of A. coerulea and zooplankton were determined using a conical net with a 60 cm diameter and a 200 µm mesh, equipped with a flow meter (model 2030R, General Oceanics, Inc, Miami, FL, USA). An oblique tow was conducted from the bottom to the surface over approximately 3–5 min at a towing speed of 2 knots using a small boat. Medusae were collected and counted on site. The collected samples of zooplankton, including ephyrae, were fixed in 5% buffered formalin and counted under a stereo microscope (Nikon DS-Ri2 camera, 16 MP, Tokyo, Japan). Zooplankton were identified to the species level whenever possible, and their abundance, along with that of jellyfish, was expressed as individuals per cubic meter (ind. m−3).
For monthly studies of size–frequency distribution, more than 100 medusae were randomly collected from small fishing boats and pontoons using hand nets. The bell diameter (mm) and wet weight (g) of the medusae were measured on site using calipers and a scale. Since jellyfish have different body weights even if they are of similar size, the values of size and weight used to obtain the size–weight regression were calculated using the average weight value in the 5 mm interval of umbrella sizes. The growth rate was calculated using the method of Toyokawa et al. [19], expressed as G = (ln Wi-t − ln Wt)/t, where G is expressed in d−1, W is the wet weight, t is the time between two sampling dates, and Wi is the estimated wet weight of medusae from the average diameter between day i and day i + t.
To measure the size of the planulae, more than 100 medusae were randomly collected each month, and planulae were collected from a minimum of 30 mature individuals that visibly contained them. Planulae attached to the brood pouch at the base of the oral arm of the female medusae’s subumbrellas were filtered through a 50 µm sieve using running seawater and transferred to 20 µm filtered seawater. The collected planulae were rinsed with filtered seawater (20 µm) through a 50 µm sieve to remove mucus, after which they were fixed in 5% formalin. The planulae were photographed using a digital camera mounted on a stereo microscope (Nikon DS-Ri2, Japan), and their longitudinal and transverse lengths were measured using NIS-Elements (Nikon Instruments Inc., Melville, NY, USA) version 3.0 software. Additionally, for the polyp formation experiments, the planulae were kept alive and transported to the laboratory in filtered seawater (salinity 30–32 ppt maintained at field water temperature and kept in an incubator.
2.3. Polyp Habitat Survey
The survey of polyp habitats was conducted in the study waters from March to April in both 2023 and 2024. An underwater dive survey was performed to assess polyp habitat distribution and polyp density in the field. Polyp habitats were documented through photography (Canon EOS 200D and Nikon D5, Tokyo, Japan) and videos, and samples were collected for the identification of A. coerulea polyps.
2.4. Polyp Formation Experiment
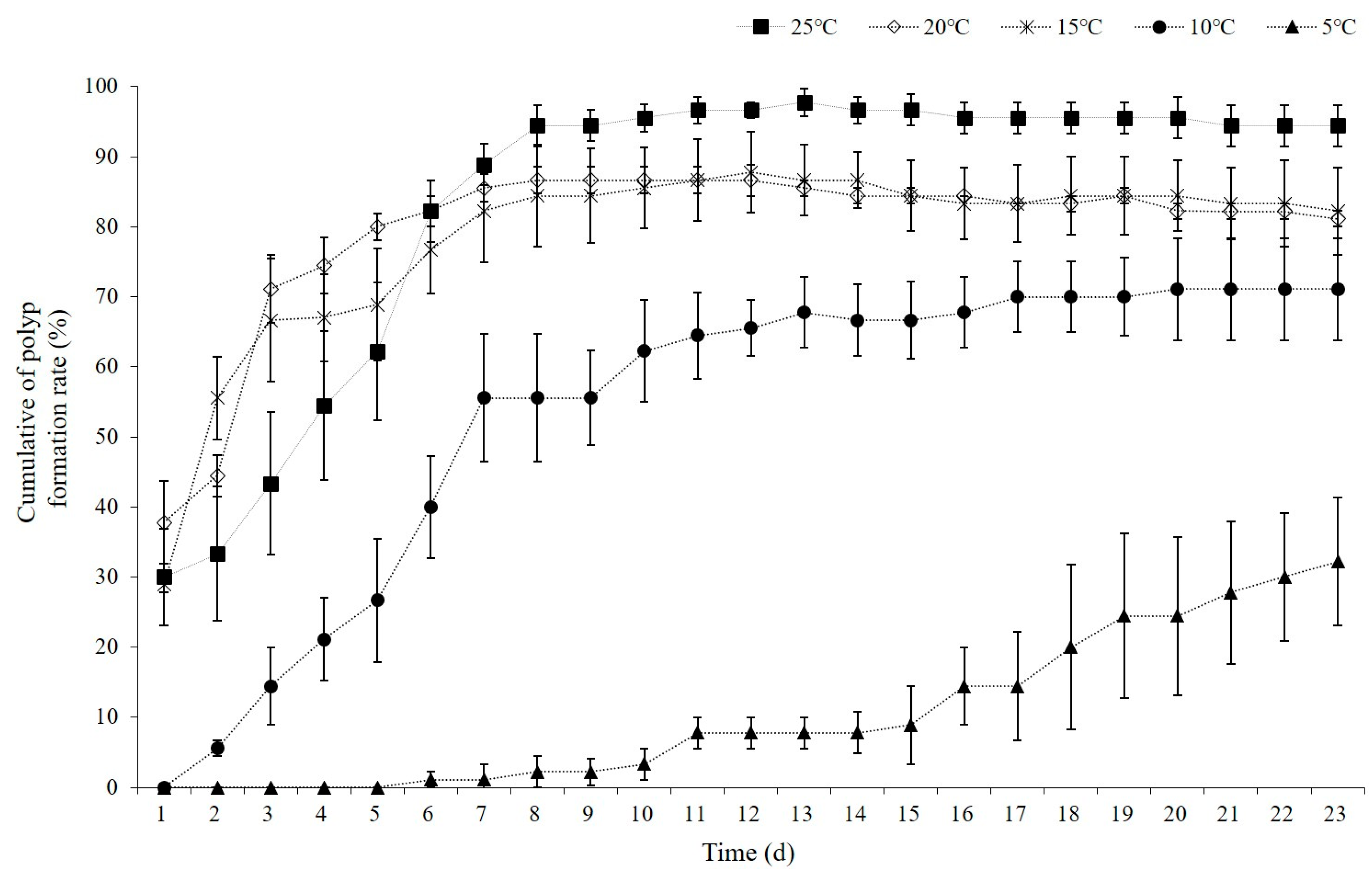
We used field surveys and laboratory experiments to analyze the characteristics of the planulae. The experiment to measure the rate of polyp formation at different temperatures involved filtering seawater with a salinity of 32 ppt through a 20 µm filter into square Petri dishes (125 mm × 125 mm × 17.8 mm), each containing 100 mL of water. Thirty live planulae were randomly selected and added to each dish using a pipette. Considering the water temperatures of the study area, the experiments were divided into five temperature intervals (5, 10, 15, 20, and 25 °C) and conducted in an incubator for 23 days. Each experiment was repeated three times. The seawater was partially refreshed every three days. The rate of polyp formation was assessed by counting the cumulative number of polyps formed.
2.5. Molecular Analysis
Following the protocol described in Park et al. [20], mtCOI and 16S rRNA partial sequences were obtained from 18 polyps for molecular analysis. A BLAST [21] search calculated the percent of identity between the sequences from this study and the NCBI database. Sequence information from this study was deposited in GenBank. Genetic analysis confirmed that the polyps collected in this study were indeed A. coerulea.
2.6. Statistical Analysis
All statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL), with a significance level set at p < 0.05. t-tests were conducted to compare the mean growth rates between two different time periods. Power regression analysis was used to assess the relationship between the wet weight of individual A. coerulea specimens and their corresponding bell diameter. ANOVA was used to assess the seasonal variations in planulae size across different months from November 2022 to October 2023. Additionally, it was used to evaluate the differences in cumulative polyp formation rates across various temperature conditions (5, 10, 15, 20, and 25 °C).
3. Results
3.1. Habitat Conditions of A. coerulea
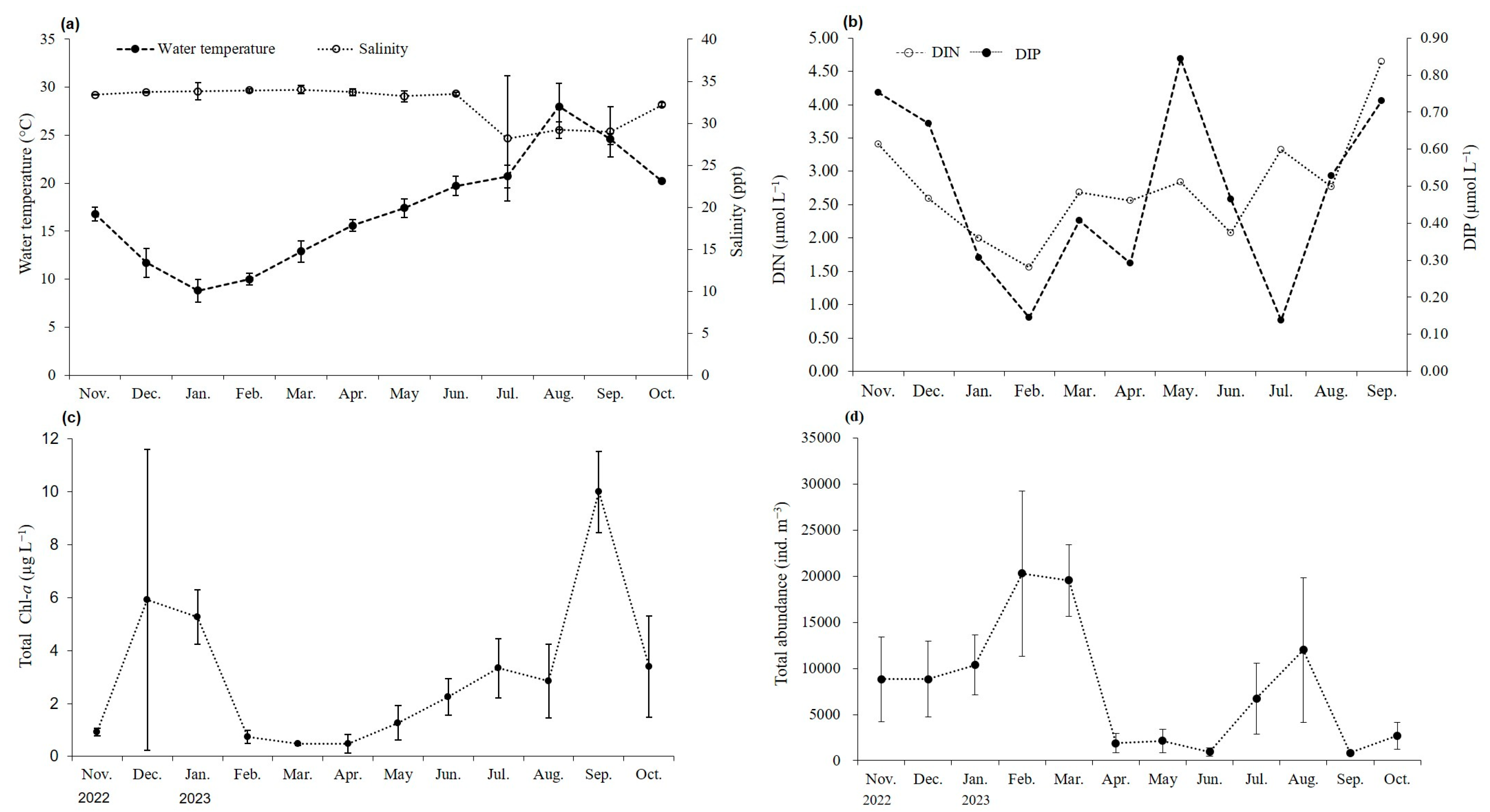
The study area was a semi-enclosed bay with a maximum depth of 10 m. From November 2022 to October 2023, the average monthly water temperature ranged from 8.8 °C in January to 28.0 °C in August. The average monthly salinity ranged from 28.2 to 34.0 ppt, being the lowest during the summer monsoon period in July. However, the salinity was maintained constant at 32.3 ppt (Figure 2). The concentrations of DIN and DIP ranged from 1.56 to 4.64 µmol L−1 and from 0.14 to 0.84 µmol L−1, respectively.
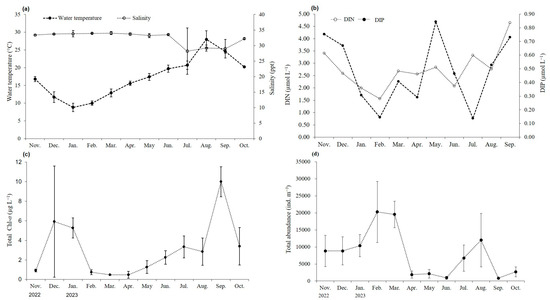
Figure 2.
Average monthly environmental data from November 2022 to October 2023: (a) water temperature and salinity, (b) nutrients (DIN and DIP), (c) total Chl-a concentration, and (d) total abundance of zooplankton.
Chlorophyll distribution, which can be used to estimate phytoplankton biomass, ranged from 0.5 to 10.0 µg L−1, with an annual average value of 3.1 ± 2.84 µg L−1. Additionally, monthly fluctuations in chlorophyll showed two peaks during the study period, one in December and another in September (Figure 2). The total abundance of zooplankton temporarily increased in July and August, followed by a sharp decline post September. The dominant zooplankton species with the highest density each month was Acartia spp., a small copepod commonly found in the southern coastal waters of Korea. From December to March of the following year, the brackish water copepod Eurytemora pacifica was the second most dominant zooplankton species, while cirriped larvae appeared in relatively large numbers from May to October.
3.2. Habitat of A. coerulea Polyps
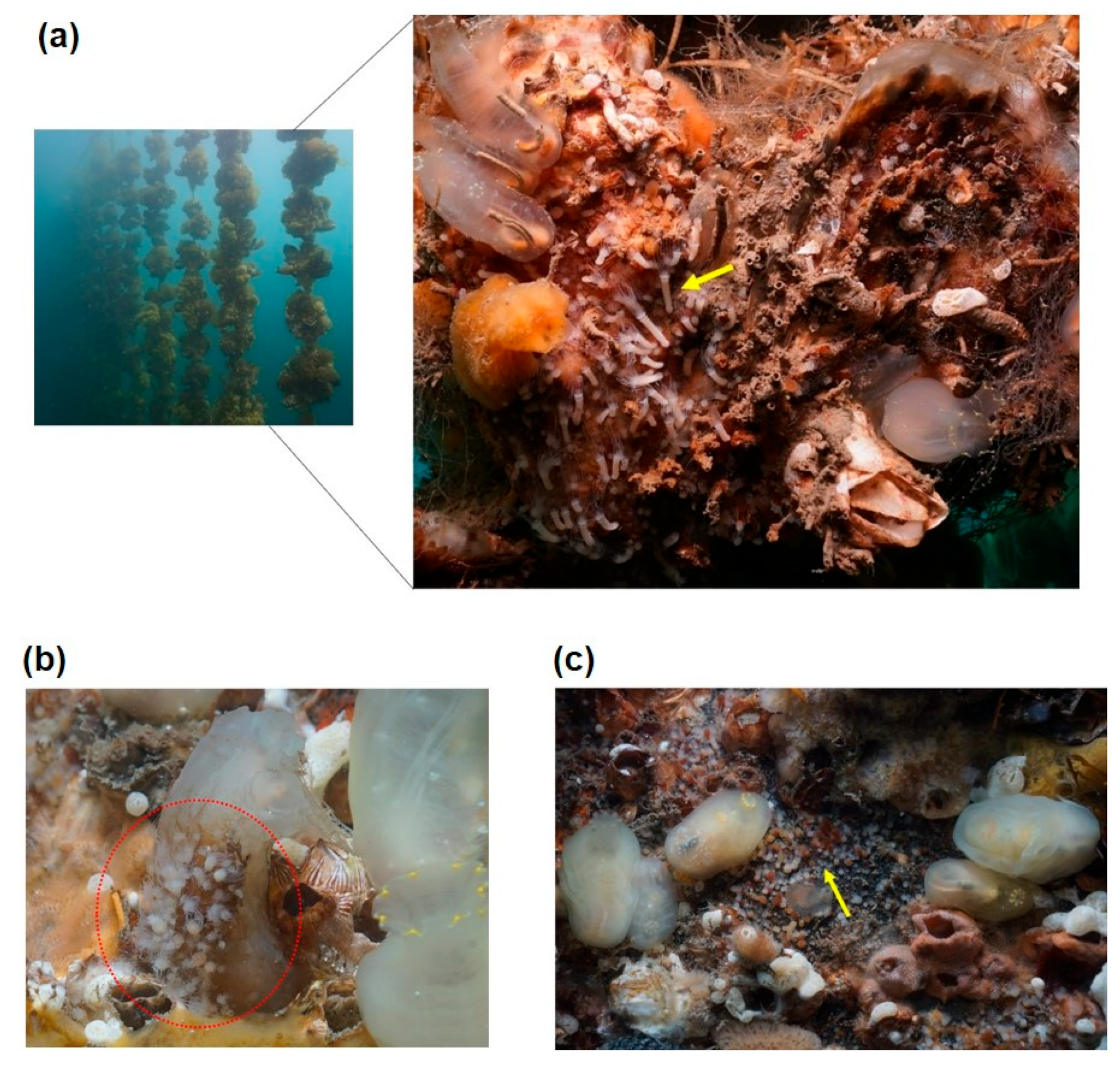
The underwater structures surveyed for A. coerulea polyps included breakwaters, harbor walls, subtidal rocks, small fishing boats, pontoons beneath barges, buoys, and oyster shells at submersible oyster farms (Figure 3). Polyps were found in varying densities on all of the surveyed structures except for harbor walls and subtidal rocks. These structures were colonized by a mixture of species, including ghost sea squirts (Ciona sp.), sea anemones, sponges, tube worms (Hydroides sp.), and juvenile barnacles, with polyps primarily located in empty spaces between these organisms or in areas without any attachments. Polyp densities were recorded at 152–205 ind./10 cm × 10 cm on the undersides of barges, 174–218 ind./10 cm × 10 cm on pontoons, and 100–142 ind./10 cm × 10 cm under small fishing boats. Oyster farms, especially those with oyster shells from submersible cultures older than three years, exhibited high concentrations of polyps. Polyps were mainly distributed on the overlapping and downward-facing sides of oyster shells, either colonizing entire shells or clustering in parts. Their densities were 172–384 ind./10 cm × 10 cm on shells over 10 cm in size and 13–14 ind./10 cm × 10 cm on 10 cm scallop shells used as substrates for spat production. In some cases, polyps > 80 ind./10 cm × 10 cm were also observed attached to the surfaces of ghost sea squirts (Figure 3). The survey conducted in March 2023 noted that 8–24% of the attached polyps were undergoing strobilation. A total of 36 new partial sequences were successfully obtained from 18 polyps collected in Geoje Bay using two genetic markers, mtCOI and 16S rRNA. BLAST searches revealed that these sequences exhibited the highest similarity to A. coerulea von Lendenfeld, 1884 (complete genome, accession no: NC046792) in the NCBI database, with identity values ranging from 99.53% to 99.88% for mtCOI and from 99.54% to 99.84% for 16S rRNA.
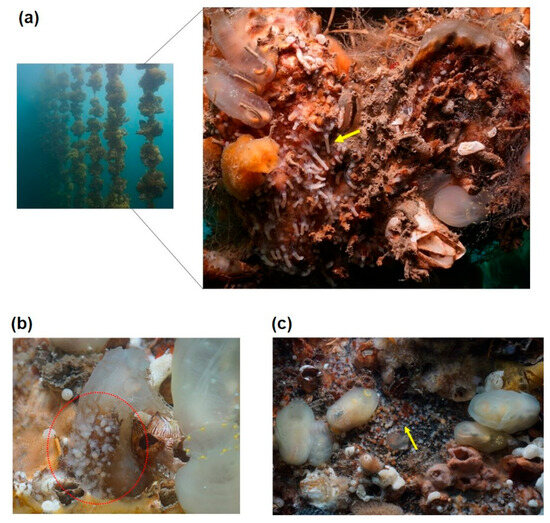
Figure 3.
Aurelia coerulea polyps residing on artificial structures. Polyps attached (a) to submerged oyster lines, where elongated polyps indicate ongoing strobilation; (b) to the surface of Ciona sp.; and (c) to the underside of a pontoon with red tips on polyps indicating strobilation or detecting ephyrae. Red circles are polyp colonies and yellow arrows indicate ongoing strobilation.
3.3. Population Dynamics and Growth Rates of A. coerulea
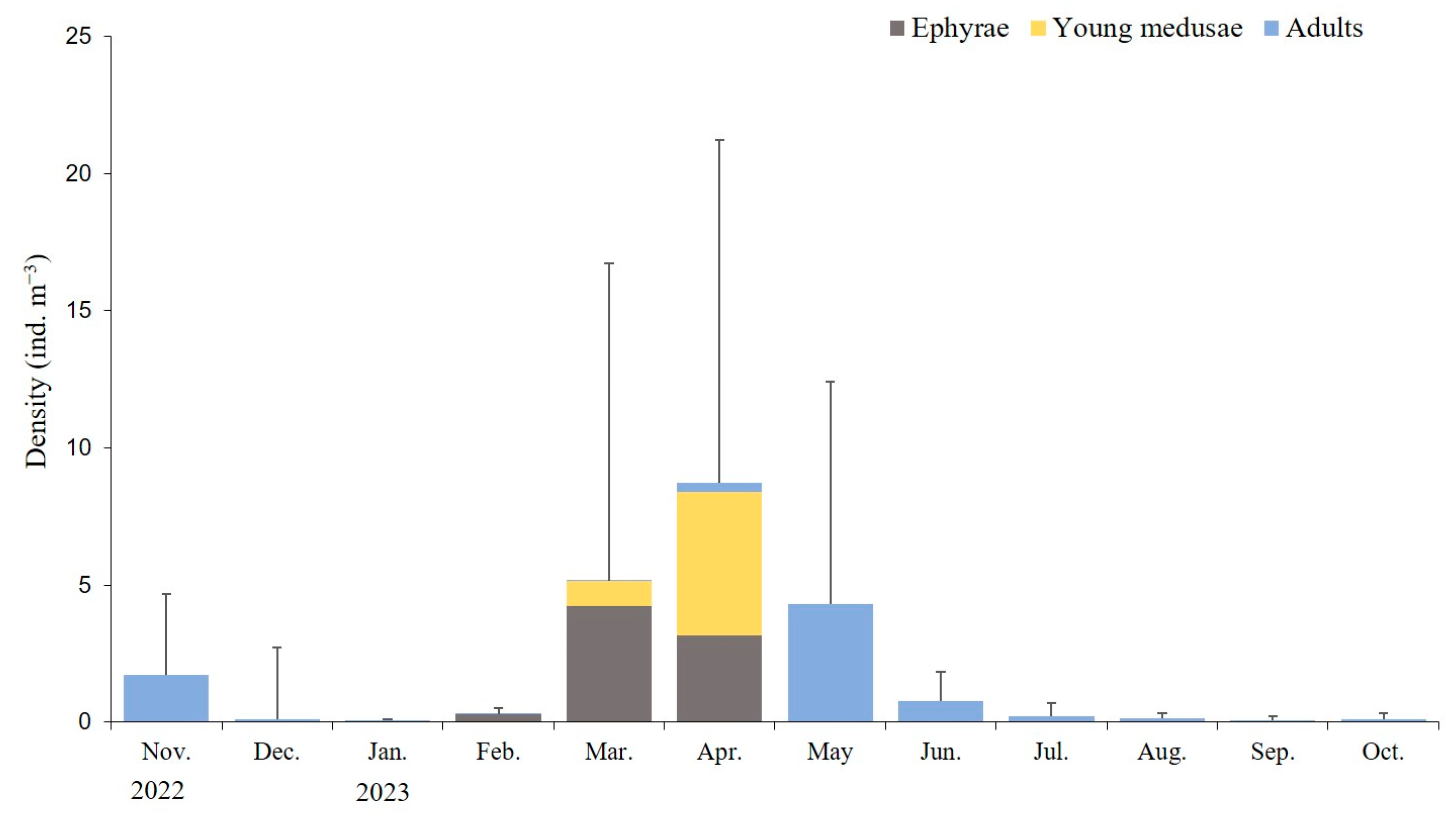
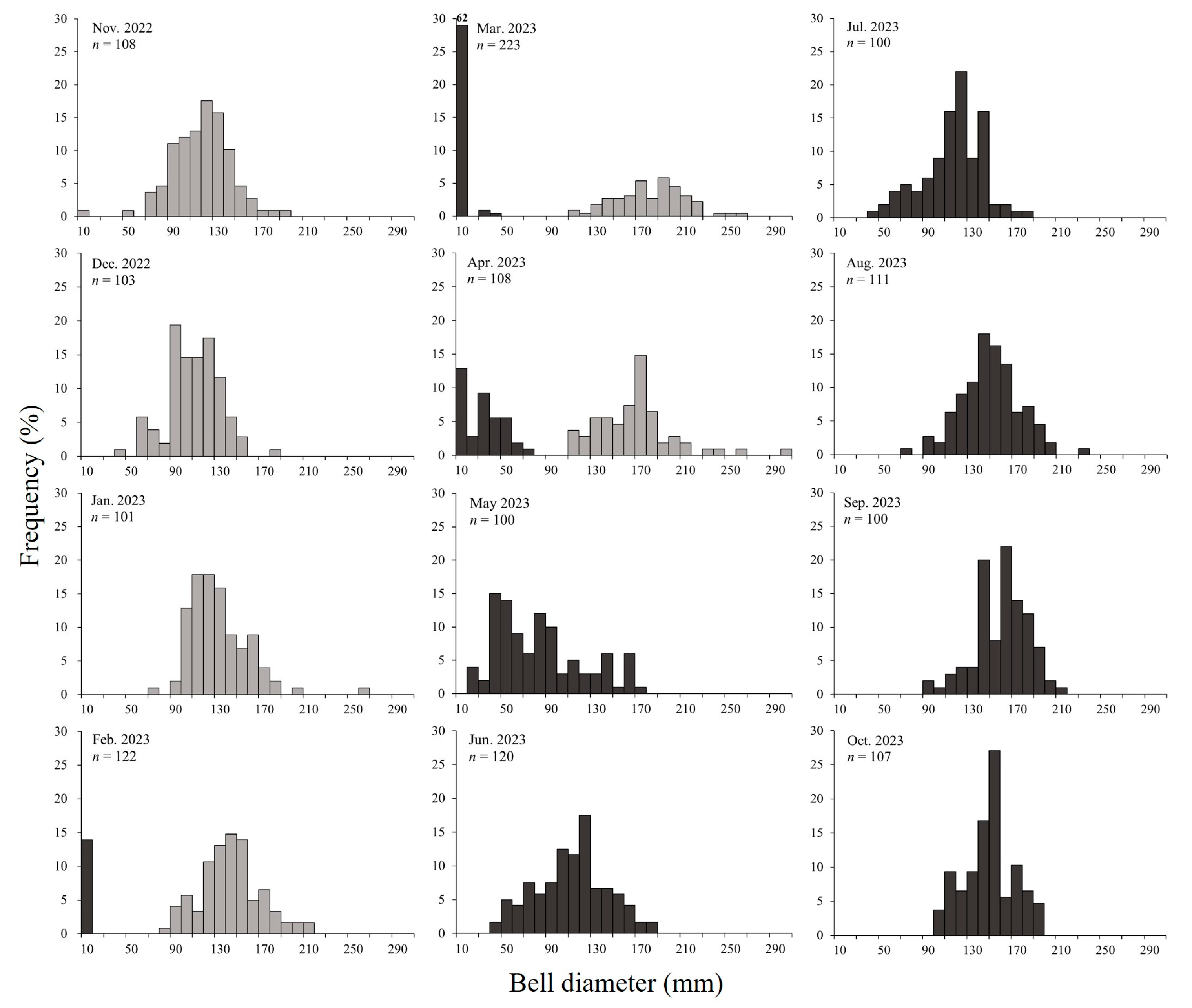
During the study period, the average monthly density of A. coerulea in Geoje Bay was 0.05–8.71 ind. m−3, with the highest density observed in April 2023 and the lowest in January 2023. Ephyrae densities ranged from 0.01 ± 0.02 ind. m−3 in November 2022 to 0.29–4.22 ind. m−3 in the period between February and April 2023, peaking in March. Young medusae under 30 mm exhibited densities of 0.93 ± 3.04 ind. m−3 in March and 5.23 ± 7.89 ind. m−3 in April, displaying the highest densities in April. Medusae (adults) were present throughout the year, with densities ranging from 0.01 to 4.32 ind. m−3, peaking in May (Figure 4).
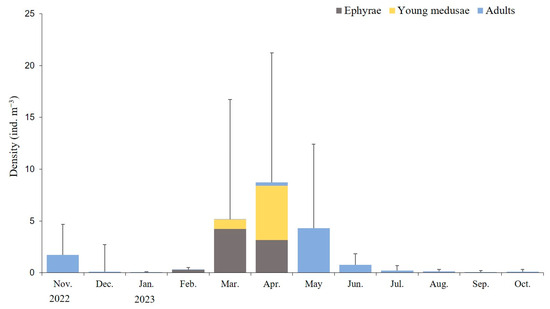
Figure 4.
Average monthly density of Aurelia coerulea at different developmental stages. Data are presented as the mean ± S.D.
Geoje Bay is characterized by the year-round presence of jellyfish, with A. coerulea populations forming distinct groups consisting of both overwintering individuals and newly recruited cohorts (Figure 5). Initially, the overwintering population formed before November 2022 and persisted until April 2023. The current-year population, generated from ephyrae between February and April 2023, continued until after October. The overwintering population appeared to be a mixture of survivors from the spring of 2022 that did not perish after autumn spawning, supplemented by additional young medusae through summer and autumn. The medusae of 50–60 mm between November and December 2022 had large amounts of planulae. Therefore, these individuals were not those that had shrunk after spawning in summer (July–August). Considering the summer water temperatures and growth rates in Geoje Bay, we estimated that the population originated in summer 2022.
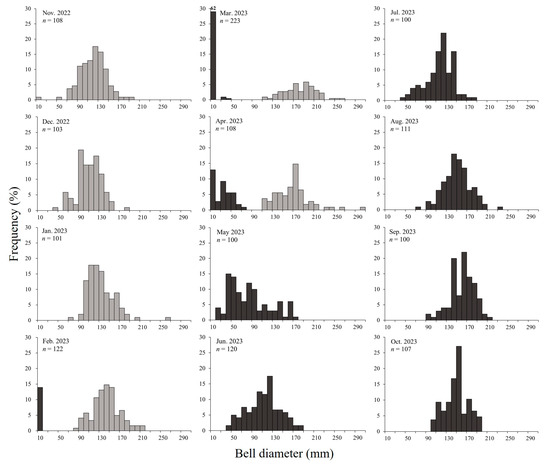
Figure 5.
Size frequency distribution of Aurelia coerulea in the study area (gray bars: overwintering population; black bars: current-year population).
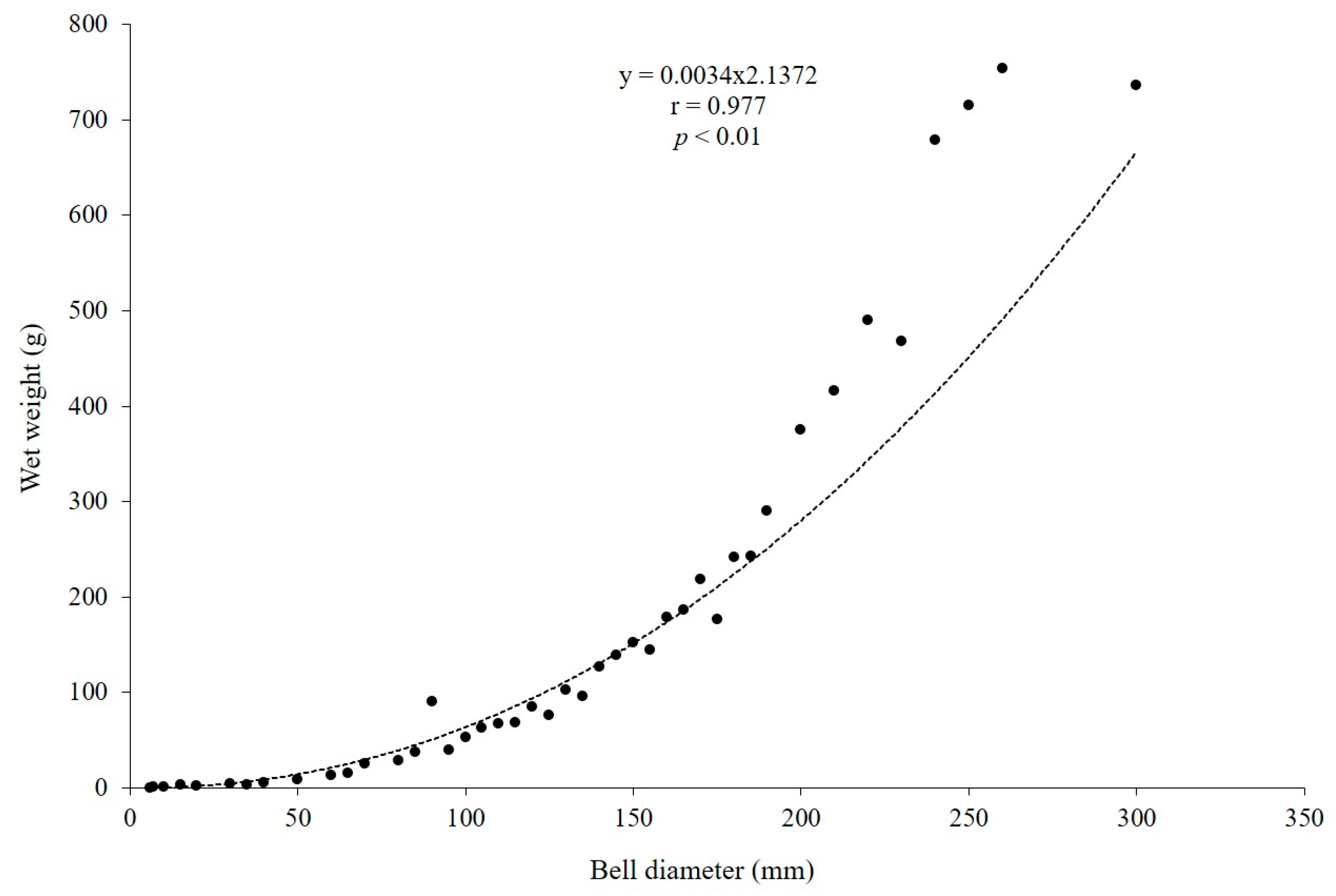
From November 2022 to October 2023, over 100 medusae with bell diameters of 6–300 mm and wet weights of 0.05–754 g were collected each month. The relationship between the wet weight of individual A. coerulea specimens (WT, g) and their corresponding size is shown in Figure 6 (r = 0.977, p < 0.01, n = 44; Figure 6).
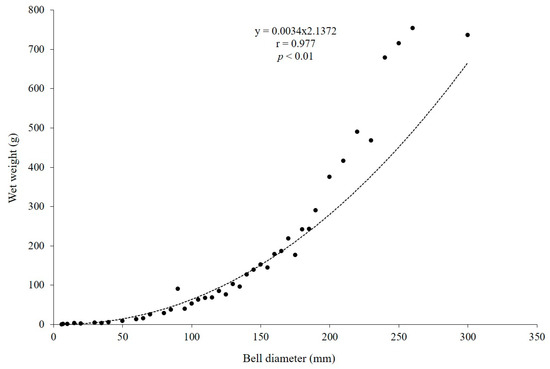
Figure 6.
Average size–weight relationship of Aurelia coerulea (n = 44).
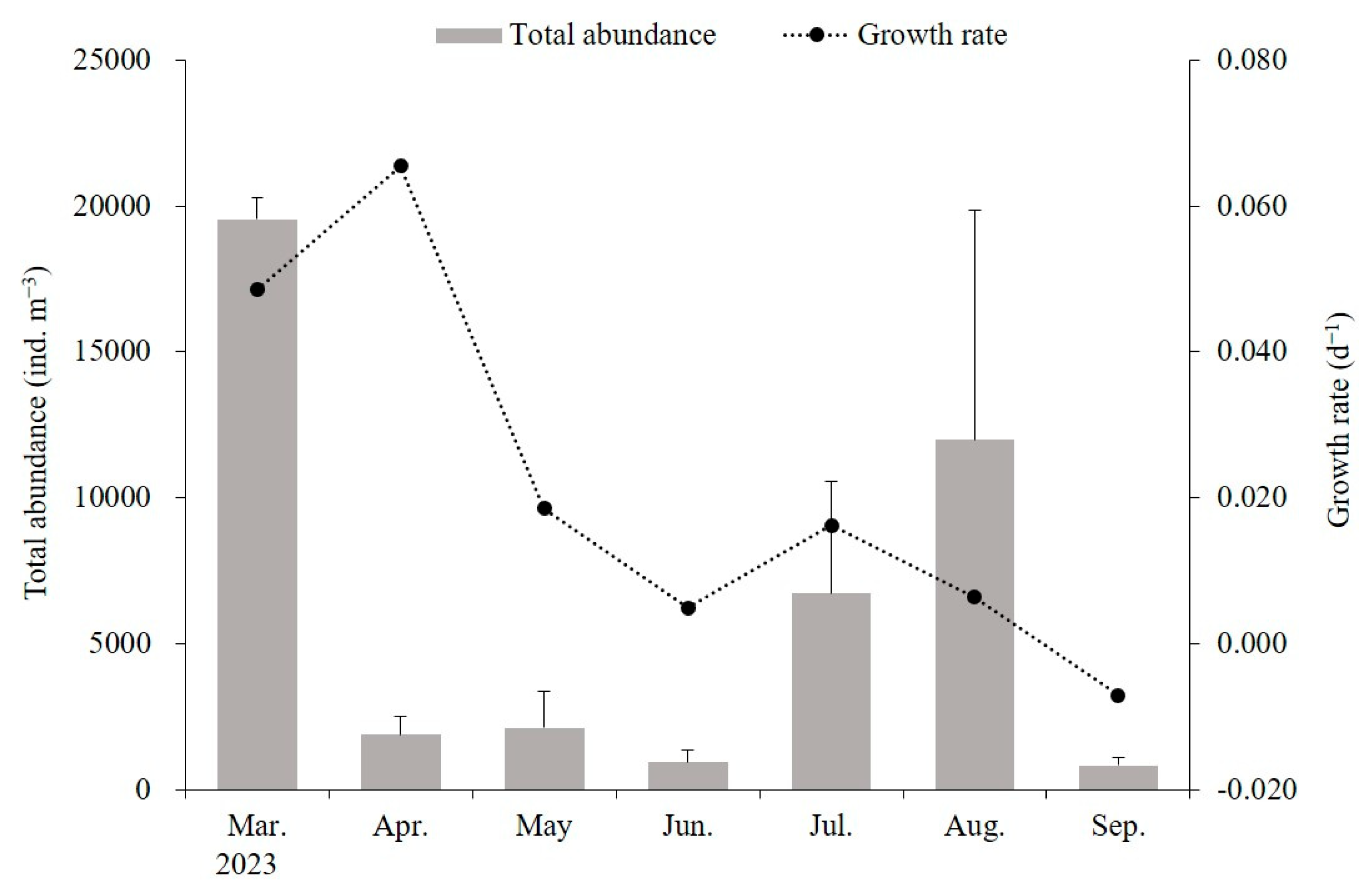
The average size of mature medusae with planulae was 149.2 ± 40.7 mm, although smaller medusae, around 60 mm, were also observed in December 2022. The A. coerulea population was established before November 2022, and from February 2023 onwards, new populations emerged with the appearance of ephyrae, continuing until after October. The daily growth rate ranged from −0.007 to 0.065 d−1, with higher growth rates of 0.049–0.065 d−1 observed during the initial growth period of ephyrae and young medusae between March and April. However, the growth rates gradually decreased, dropping to 0.005 d−1 by June and becoming negative in September (Figure 7).
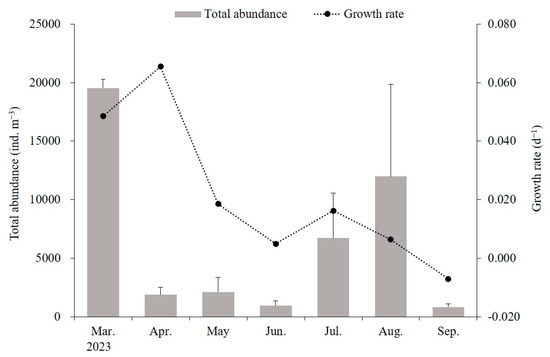
Figure 7.
Growth rates of Aurelia coerulea and zooplankton biomass. Zooplankton biomass represents the mean values from three sampling points. Data are presented as the mean ± S.D.
3.4. Characteristics of A. coerulea planulae
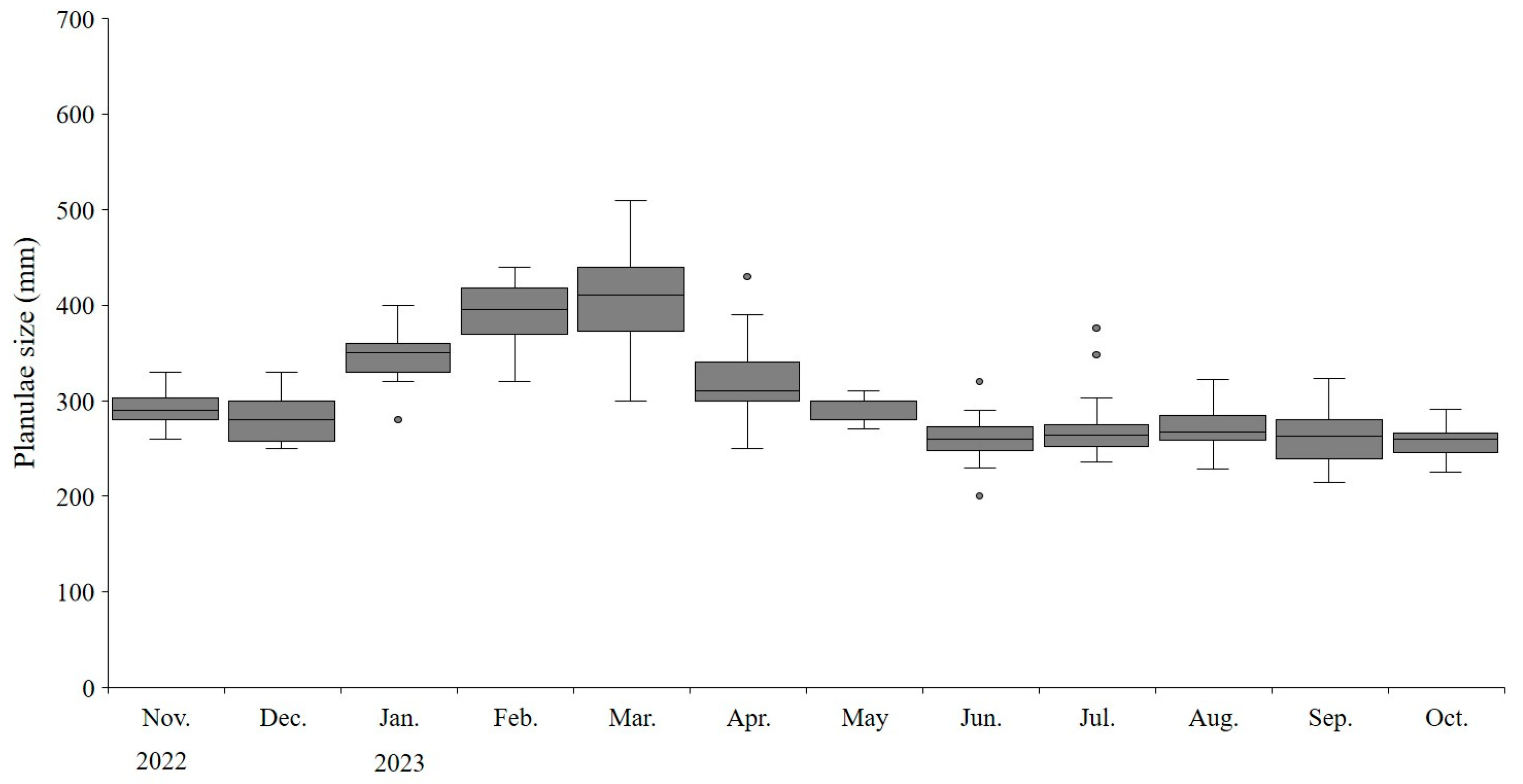
We observed that A. coerulea continuously harbors planulae throughout the year. From November 2022 to October 2023, the size of the planulae ranged from 200 to 510 µm, with an average size of 302µm. During the colder months from January to March, the planulae significantly increased in size (t-test, p < 0.05), measuring 388 ± 46.0 µm (Figure 8). Significant seasonal variations in planulae size were observed across different months (ANOVA, p < 0.05). The minimum and maximum sizes of medusae with planulae were 60 mm and 300 mm, respectively.
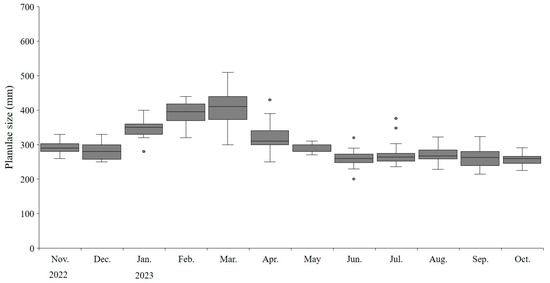
Figure 8.
Size distribution of Aurelia coerulea planulae. The boxplots represent the median (line within the box), interquartile range (IQR; box), and potential outliers (points outside the whiskers) of planulae size (µm). The whiskers extend to the lowest and highest values within 1.5 times the IQR from the lower and upper quartiles.
In experiments on polyp formation from planulae, the cumulative polyp formation rate reached over 50% within 5 days at temperatures of 15, 20, and 25 °C, and within 7 days at 10 °C. At 5 °C, the polyp formation rate was only 32.2 ± 15.8% even after the end of the experiment on day 23. Notably, at 15 °C, 55.6 ± 10.2% of the polyps formed within 2 days, and at 20 °C, 71.1 ± 8.4% of the polyps formed within 3 days (Figure 9). At 5 °C, some of the planulae that had been alive since day 19 were eaten by polyps. At temperatures between 15 and 25 °C, polyps typically developed more than eight tentacles within 2 days, while at 5 °C, they developed six or fewer. Some polyps exhibited abnormal forms without any tentacles. Stolons were formed on day 16 at 25 °C and day 13 at 20 °C; however, they did not develop in the other temperature groups.
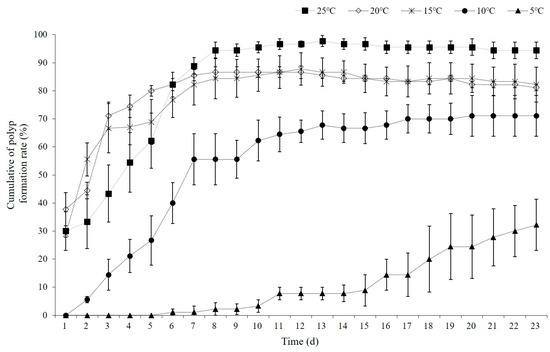
Figure 9.
Cumulative polyp formation rate (%) for Aurelia coerulea planulae at temperature ranging from 5 °C to 25 °C. Data are presented as the mean ± S.D.
4. Discussion
4.1. Habitat Environment of A. coerulea
Since 2010, outbreaks of A. coerulea have been occurring continuously along the southern coast of Korea during the warm season. In particular, Geoje Bay, where this study was conducted, is the most representative region, with a large number of A. coerulea. Aurelia species are known to inhabit semi-enclosed or enclosed shallow bays with limited tidal exchange more densely than open or deep waters [22,23]. Temperature, salinity, and food availability are the major environmental factors influencing the growth and abundance of polyps and jellyfish [24]. Temperature and salinity directly affect the asexual reproduction rates of jellyfish by affecting their metabolic processes and indirectly influence food consumption and capture [25,26]. If the environmental factors such as temperature and salinity are favorable during the polyp and ephyra stages, jellyfish outbreaks may occur. These environmental factors can directly influence the size and timing of jellyfish populations [7]. Notably, temperature is a major trigger for strobilation in polyps [27]; an increase in temperature accelerates the rate of strobilation and the quantity of ephyrae produced [28]. It also significantly affects the growth rate of ephyrae [29], and, along with salinity, limits the latitudinal distribution of polyps and affects the physiology of jellyfish [30,31].
The genus Aurelia comprises more than 13 species, including A. coerulea, which thrive under a wide range of environmental conditions worldwide [32]. According to a taxonomic study of the genus Aurelia [16], A. coerulea occurs in East Asia, including Korea, Japan, and China. Individuals previously reported as A. aurita in East Asia were likely misidentified A. coerulea individuals. Therefore, we considered that the previous reports of Aurelia species in East Asia were A. coerulea, and we compared them accordingly (Table S1 in Supplementary Files). The water temperature ranged from 8.8 °C to 28.0 °C in the study area, displaying a pattern typical for temperate waters, with low temperatures in winter and high temperatures in summer. During the study period, salinity remained constant at an average of 32.2 ppt without high seasonal fluctuations. The ranges of water temperature and salinity in A. coerulea habitats vary depending on each area; for instance, the temperature range in Japan ranges from 3.2 °C to 30.7 °C [33], whereas that in Tapong Bay, Taiwan, ranges from 17 °C to 33 °C [34]. In general, Aurelia species are known to adapt well to wide ranges of water temperature and salinity [35,36]. Hence, the temperature conditions in Geoje Bay are deemed suitable for A. coerulea.
During the study period, the variation in Chl-a concentration was relatively high between December and January and between September and October, and lower between February and May. Previous studies have reported that the southern coast of Korea is characterized by high phytoplankton biomass during the winter due to low depth, active water mass mixing (providing nutrients), and sufficient light [37,38]. Conversely, the summer phytoplankton bloom in the southern coast is known to occur due to the increase in nutrients from land following the seasonal monsoon [39]. However, it is important to note that our analysis primarily focused on the abundance of zooplankton, without detailed consideration of size or species composition. Previous studies have highlighted the critical role of microzooplankton such as planktonic ciliates and copepod nauplii as the food sources for A. coerulea at various life stages, with evidence showing that polyps utilize diverse groups of microzooplankton, achieving relatively high carbon ingestion rates from ciliates, dinoflagellates, mollusks, and copepod nauplii [40,41]. For example, several research works have demonstrated that planktonic ciliates significantly contribute to feeding activity and assimilation during the polyp stage of A. aurita [42,43]. Additionally, planktonic ciliates have been observed to be essential for the growth and feeding responses of A. coerulea ephyra and metephyra stages, suggesting that the availability of these smaller prey items could influence jellyfish population dynamics [41]. Because mesozooplankton communities are mainly composed of copepods such as Acartia spp. and Eurytemora pacifica in Geoje Bay, it is plausible that the increase in zooplankton abundance during the study period, coupled with an adequate supply of smaller microzooplankton such as copepod nauplius, contributed to the lower initial mortality rates and higher recruitment observed in the A. coerulea population.
4.2. A. coerulea Polyp Habitats
A. coerulea naturally inhabits the southern and western coasts of Korea, representing a native species. Polyps are perennial and play a crucial role in determining the biomass of adult jellyfish through asexual reproduction, which involves the release of numerous ephyrae via transverse fission [44,45]. Therefore, information about the ecological features of polyps is important for comprehending the mechanisms behind jellyfish outbreaks. However, identifying polyps in natural environments is challenging due to their small and inconspicuous appearance, making it difficult to determine the causes of massive jellyfish outbreaks [46]. Recent research has focused on identifying polyp habitats using eDNA techniques [47]. Toyokawa et al. [48] reported that ephyrae released from settled polyps are gradually dispersed by water movement, so the locations where ephyrae are found are typically close to polyp colonies.
In this study, we conducted underwater surveys from March to April (at approximately 8–10 °C), a period favorable for observing strobilation, to identify habitats where polyps readily attach, such as hard surfaces and areas with massive ephyrae outbreaks. During the survey period, polyps were readily found on various artificial structures, predominantly in shaded areas, forming patches or scattered in various forms. Interestingly, some of the polyps were also found living on the surfaces of sessile ascidians (Ciona sp.) (Figure 3); however, most were attached to nooks within sessile biota and oyster shells. During the same period, the Korean Marine Environment Management Corporation surveyed polyp habitats in Geoje Bay, covering 1553 m−2. Polyps were found on five floating plastic structures used as buoys in aquaculture sites and barges, covering an area of 303 m2 with 5.3 × 106 polyps [49]. Although the number of A. coerulea polyps in a limited space is not indicative of the total number of polyps in Geoje Bay, these results indicate that a sufficient population size is required to trigger massive jellyfish outbreaks.
Previous studies have shown that polyps naturally prefer the shaded, horizontal surfaces of clam shells and artificial substrates [46,50,51,52,53,54]. The aquaculture structures on which polyps were most commonly found provide shaded substrates suitable for polyp habitation [53]; this is particularly true for submersible oyster farms, which contain structures with restricted access to benthic predators [55]. According to the Organization for Economic Co-operation and Development (OECD) statistics, in 2019, Korea had the second highest oyster production worldwide, with 326,190 tons produced [56]. In 2022, Korea’s oyster production was 300,692 tons, of which 250,593 tons were produced in the southern coast, including Geoje, accounting for 83% of the total production. Thus, the study area is a representative site for oyster aquaculture.
Globally, the aquaculture of mollusks (such as mussels, scallops, and oysters) and marine fish has dramatically increased in recent decades, particularly in Asia [7]. This expansion can create favorable habitats for jellyfish polyps. Aquaculture can unintentionally benefit jellyfish populations in several ways. The eutrophicated conditions around aquafarms promote jellyfish growth [7]. Moreover, aquaculture structures provide additional substrates for polyps during their benthic stage. Lo et al. [53] reported that in Tapong Bay, Taiwan, A. aurita was abundant when extensive oyster and fish aquaculture was practiced; however, after the removal of aquaculture facilities, jellyfish numbers declined drastically.
Potential artificial habitats suitable for jellyfish polyps include seabed pillars, estuarine docks, floating docks, jetties, breakwaters, platforms supporting coastal wind turbines, bridges, urban artificial canals, artificial reefs, and coastal debris, all of which add to coastal structures and provide surfaces suitable for polyp habitation [46,54]. The increase in coastal structures provides habitats for jellyfish polyps, and the proliferation of polyp habitats is correlated with jellyfish occurrences [46,52,57].
4.3. Characteristics of A. coerulea Populations
Over the past 15 years, outbreaks of A. coerulea in the southern coast of Korea have begun with the appearance of ephyrae from February to April. As they grow, the abundance of medusae reaches seasonal peak between June and October [58]. This seasonal distribution pattern was also observed in this study, indicating that the growth characteristics of A. coerulea in Geoje Bay may represent the features of A. coerulea populations in the entire southern coast of Korea.
The populations of A. coerulea could be divided into current-year and overwintering populations in Geoje Bay. The current-year population began with the occurrence of ephyrae in February and continued until October, while the overwintering population comprised a mixture of some surviving individuals form current-year population and additional individuals that joined during the warm season. Typically, adult medusae are known to shrink and die after spawning [28]; however, as seen in the occurrence of small-sized adult medusae between November and December 2022, the maintenance of the adult population through winter and into the following spring suggested re-enlistment throughout the year. However, we did not identify ephyrae in the field from July to October. These results were presumed to be due to the scarce abundance of ephyrae and relatively short duration period of the ephyra stage under high water temperatures.
In Maizuru Bay, Japan, ephyrae occur from December to May; in Lake Nakaumi, Japan and Tapong Bay, Taiwan, they occur from December to April; and in Horsea Lake, UK, they are maintained for seven months, from December to June (Table S1 in Supplementary Files). These regions are characterized by a long duration of ephyra emergence, lasting five to eight months. However, in our study area, the occurrence of ephyrae in the water column was recorded only between February and April. In Tokyo Bay, Japan, and the Black Sea, the duration of ephyra emergence was similar to that in the study area; however, medusae occurred year-round. Laboratory experiments showed that A. coerulea polyps produced from the population in Geoje Bay release ephyrae continuously under sufficient food conditions at 8 °C and 16 °C. Additionally, Aurelia species can overwinter at relatively low temperatures, with a life span of 4 months to 2 years [22,33,59]. Previous studies have indicated that A. aurita produces planulae all year round, and large individuals appearing in the spring originated from groups that overwintered the previous year, suggesting that the lifespan of medusae in Tokyo Bay exceeds one year [19,60]. Differences in ephyra emergence duration and lifespan reflect the broad adaptive capacity of Aurelia species to varying environmental conditions [60].
During the study period, the bell size of A. coerulea in Geoje Bay ranged from 10 to 300 mm, with an average size of 124.6 ± 21.4 mm, and the wet weight ranged from 1 to 778 g, averaging 124.3 ± 70.7 g. The wet weight increased exponentially with the increase in bell diameter. The maximum bell diameter in Geoje Bay was 300 mm, which was smaller than that observed in certain regions of the Black Sea, Baltic Sea, and the Mediterranean, but similar to sizes reported in East Asia (Table S1 in Supplementary Files). Jellyfish size and weight could be influenced by growth, food quantity and quality, collection period, maturity stage, and ecological factors such as temperature, salinity, and seasonal differences [61]. Particularly, the size of Aurelia species varied significantly among the years, reflecting intra-species competition and food scarcity during high-temperature periods [62]. Food availability is a crucial limiting factor for jellyfish growth [36]. In habitats with unrestricted food, medusae can reach a maximum bell diameter of 300–400 mm, and mature individuals tend to be relatively large [63]. This phenomenon occurrs because sufficient food decreases the carbon content in the body, enabling rapid growth and increasing the body’s surface area to enhance the prey capture capabilities [28]. Hamner and Jenssen [64] showed that A. aurita cultivated in artificial conditions grew to larger final sizes than those in natural populations when fed consistently. Lucas [28] reported that food availability allowed A. aurita to grow beyond 400 mm. However, in Denmark’s Kertinge Nor, the maximum average bell diameter of food-restricted A. aurita populations was only 54 ± 12 mm [65].
The growth rate of jellyfish could be influenced by the availability of food, the size and quality of prey, and their behavior [66]. The genus Aurelia is known to consume all medium- to large-sized zooplankton and various particle sizes [41,60], with stomach contents primarily consisting of adult copepods and Balanid nauplii, which make up 31–34% of the total stomach content. This suggests that copepods and various larvae are significant food sources for jellyfish [67]. In the Black Sea, their diet includes mainly copepods, bivalve larvae, Rathkea octopunctata, and fish eggs [68]. Additionally, prey size and shape significantly affect the feeding response of Aurelia sp. [69]. Møller et al. [63] showed that growth rates increased with the increase in the variety of food types and concentrations, including Artemia nauplii, Balanus sp., Brachionus sp., and R. octopunctata; however, when fed only Acartia tonsa, the growth rate was low, even at high food concentrations.
In Geoje Bay, the zooplankton community was influenced by freshwater inflow from surrounding small rivers, resulting in a mix of brackish species, with the copepod Acartia spp. being the dominant species. Additionally, brackish copepods such as Eurytemora pacifica appeared in large quantities during the winter, while cirriped larvae were the dominant species in the summer. R. octopunctata also appeared at low densities in the study waters. A massive outbreak with relatively higher growth rates of ephyrae and young medusae was observed between February and April. This period coincided with the highest abundance of zooplankton as the main food source. This result indicated that food availability is a significant factor influencing the outbreaks of A. coerulea in Geoje Bay. In addition, the lowest growth rate of A. coerulea in June might be attributed to the relatively low abundance of zooplankton. Uye and Shimauchi [67] reported that high-density occurences of A. aurita could significantly reduce the biomass of the zooplankton community due to intense predation pressure. Thus, the variations in monthly growth rates, with initial higher rates followed by lower rates entering the warm season and ultimately negative growth, reflect the trends reported in previous studies [23,70].
Overall, this study suggests that the massive outbreak of jellyfish was driven by the sufficient ephyra abundance supplied from large polyp habitats such as oyster farms, as well as abundant food supply between February and April, which allowed the current-year population of ephyrae and young medusae to grow significantly in Geoje Bay.
4.4. Planulae Characteristics
The planulae of Aurelia species are known to be 200–300 µm in size [71]. However, previous studies have reported that larger planulae (up to 700 µm) bypass the polyp stage and directly develop into ephyrae in Urazoko and Maizuru Bay in Japan [72,73]. The average size of planulae during our study period was 303 µm. However, it was not possible to confirm whether the relatively large planulae, measuring over 400–510 µm between January and March in the study waters, directly developed into ephyrae as previously reported. Thatje and Hall [74] reported a negative correlation between the egg size of marine invertebrates and temperature. Schneider [75] reported that A. aurita is known to produce fewer planulae with higher organic content to reduce mortality and enhance settlement during the cold season. The results of the present study also showed that the relatively large size of the planulae was likely due to the accumulation of energy, enhancing their chances of survival at lower water temperatures.
Another significant characteristic of the investigated A. coerulea population in Geoje Bay was the year-round presence of medusae that continuously spawn planulae. This continuous spawning could be confirmed through the analysis of monthly collected zooplankton samples, and experiments conducted considering the water temperatures of the study waters helped assess their settlement into polyps. The planulae showed over 50% polyp formation within seven days at temperatures of 10–25 °C, and a 30% formation rate at 5 °C. Lucas et al. [76] noticed that the duration required for planulae settlement and polyp transformation could be prolonged in cold water. Schneider and Weisse [72] estimated that the theoretical maximum lifespan of A. aurita planulae could exceed ten days based on the metabolic rate at around 20 °C, suggesting that lower temperatures could extend their maximum lifespan due to reduced metabolic demands. In the present study, water temperature-dependent polyp formation experiments were performed with planulae collected from mature females in July 2022. The planulae size ranged from 240 to 320 µm, and once released into the water, they typically settled on suitable substrates within a week as their metabolic energy depleted. Successful settled planulae transformed into polyps, which then proliferated asexually, significantly influencing the population size of jellyfish. The continuous formation of polyps throughout the year is thought to significantly contribute to the maintenance of high polyp abundance essential for mass outbreaks of jellyfish in Geoje Bay.
5. Conclusions
This study has elucidated several crucial aspects of the ecology and life cycle of A. coerulea in Geoje Bay, focusing on environmental influences, life stages, and habitat interactions. The results particularly highlighted the significant impact of artificial oyster farms, water temperature, and diet on A. coerulea population dynamics. These conditions favor the asexual reproductive processes of Aurelia species, leading to frequent and sometimes massive jellyfish blooms. Our research revealed that A. coerulea populations in Geoje Bay are profoundly affected by seasonal changes, with distinct winter and current-year populations driven by environmental conditions and food availability. The overwintering population benefits from the lower water temperatures that prolong planulae viability, while the current-year population thrives due to increased food availability, particularly that of zooplankton, during warmer months. This dual-phase population cycle is critical for understanding the persistence and potential growth of jellyfish populations in temperate waters. Additionally, this study pointed out the effects of human activities, particularly aquaculture, on shaping the habitat and survival prospects of jellyfish polyps. Submerged structures, such as those used in oyster farming, provide stable substrates for polyp settlement, indirectly promoting higher jellyfish densities. This relationship reinforces the complex interactions between human activities and marine ecosystems, often with unintended ecological consequences. The life cycle of A. coerulea, marked by the ability of polyps to withstand diverse environmental conditions and produce multiple generations of medusae annually, suggests a resilient adaptation strategy that allows these organisms to rapidly capitalize on favorable conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16192846/s1, Table S1: Habitat and occurrence patterns of Aurelia species. Table S2: Total abundance of zooplankton species in the Geoje Bay. References [77,78,79,80,81,82] are cited in Supplementary Materials.
Author Contributions
Conceptualization, K.Y.K., S.H.Y., and W.P.; methodology, K.Y.K. and S.H.Y.; software, S.Y.C.; validation, K.Y.K. and S.Y.C.; formal analysis, K.Y.K., S.H.Y., and S.Y.C.; investigation, K.Y.K., S.H.Y., and S.Y.C.; resources, K.Y.K. and S.H.Y.; data curation, K.Y.K. and S.H.Y.; writing—original draft preparation, K.Y.K., S.H.Y., and W.P.; writing—review and editing, K.Y.K., S.H.Y., and W.P.; visualization, S.Y.C.; supervision, W.P.; project administration, S.H.Y.; funding acquisition, S.H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the National Institute of Fisheries Science, Korea (No. 2024040).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank G.H. Hong, Y.J. Jung, and S.Y. Oh for field assistance and laboratory support. We also appreciated the anonymous reviewers who greatly improved an earlier version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Milisenda, G.; Rosa, S.; Fuentes, V.L.; Boero, F.; Guglielmo, L.; Purcell, J.E.; Piraino, S. Jellyfish as Prey: Frequency of Predation and Selective Foraging of Boops boops (Vertebrata, Actinopterygii) on the Mauve Stinger Pelagia noctiluca (Cnidaria, Scyphozoa). PLoS ONE 2014, 9, e94600. [Google Scholar] [CrossRef]
- Båmstedt, U. Trophodynamics of the Scyphomedusae Aurelia aurita. Predation rate in relation to abundance, size and type of prey organism. J. Plankton Res. 1990, 12, 125–131. [Google Scholar] [CrossRef]
- Olesen, N.J. Clearance potential of jellyfish Aurelia aurita and predation impact on zooplankton in a shallow cove. Mar. Ecol. Prog. Ser. 1995, 124, 63–72. [Google Scholar] [CrossRef]
- Hansson, L.J.; Moeslund, O.; Kiørboe, T.; Riisgård, H.U. Clearance rates of jellyfish and their potential predation impact on zooplankton and fish larvae in a neritic ecosystem (Limfjorden, Denmark). Mar. Ecol. Prog. Ser. 2005, 304, 117–131. [Google Scholar] [CrossRef]
- Lucas, C.H.; Gelcich, S.; Uye, S.I. Living with jellyfish: Management and adaptation strategies. In Jellyfish Blooms; Springer: Dordretch, The Netherlands, 2014; pp. 129–150. [Google Scholar] [CrossRef]
- Hsieh, P.Y.H.; Leong, F.-M.; Rudloe, J. Jellyfish as food. Hydrobiologia 2001, 451, 11–17. [Google Scholar] [CrossRef]
- Purcell, J.E.; Uye, S.; Lo, W.-T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Purcell, J.E. Predation on fish eggs and larvae by pelagic cnidarians and ctenophores. Bull. Mar. Sci. 1985, 37, 739–755. [Google Scholar]
- Purcell, J.E.; Arai, M.N. Interactions of pelagic cnidarians and ctenophores with fish: A review. Hydrobiologia 2001, 451, 27–44. [Google Scholar] [CrossRef]
- Richardson, A.J.; Bakun, A.; Hays, G.C.; Gibbons, M.J. The jellyfish joyride: Causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol. 2009, 24, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Uye, S.I. Human forcing of the copepod–fish–jellyfish triangular trophic relationship. Hydrobiologia 2011, 666, 71–83. [Google Scholar] [CrossRef]
- Purcell, J.E. Environmental effects on asexual reproduction rates of the scyphozoan Aurelia labiata. Mar. Ecol. Prog. Ser. 2007, 348, 183–196. [Google Scholar] [CrossRef]
- Helm, R.R. Evolution and development of scyphozoan jellyfish. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1228–1250. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.A.; Loureiro, M.L.; Piñol, L.; Sastre, S.; Voltaire, L.; Canepa, A. Analyzing beach recreationists’ preferences for the reduction of jellyfish blooms: Economic results from a stated-choice experiment in Catalonia, Spain. PLoS ONE 2015, 10, 0126681. [Google Scholar] [CrossRef] [PubMed]
- Alenka, M.; Kogovšek, T.; Andreja, R.; Catenacci, L. Blooms and population dynamics of moon jellyfish in the northern Adriatic. Cah. Biol. Mar. 2012, 53, 337–342. [Google Scholar]
- Scorrano, S.; Aglieri, G.; Boero, F.; Dawson, M.N.; Piraino, S. Unmasking Aurelia species in the Mediterranean Sea: An integrative morphometric and molecular approach. Zool. J. Linn. Soc. 2017, 180, 243–267. [Google Scholar]
- Lynam, C.P.; Gibbons, M.J.; Axelsen, B.E.; Sparks, C.A.J.; Coetzee, J.; Heywood, B.G.; Brierley, A.S. Jellyfish overtake fish in a heavily fished ecosystem. Curr. Biol. 2006, 16, R492–R493. [Google Scholar] [CrossRef]
- Brodeur, R.D.; Sugisaki, H.; Hunt, G.L., Jr. Increases in jellyfish biomass in the Bering Sea: Implications for the ecosystem. Mar. Ecol. Prog. Ser. 2002, 233, 89–103. [Google Scholar] [CrossRef]
- Toyokawa, M.; Furota, T.; Terazaki, M. Life history and seasonal abundance of Aurelia aurita medusae in Tokyo Bay, Japan. Plankton Biol. Ecol. 2000, 47, 48–58. [Google Scholar]
- Park, N.; Yeom, J.; Jeong, R.; Lee, W. Novel attempt at discrimination of a bullet-shaped siphonophore (Family Diphyidae) using matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-ToF MS). Sci. Rep. 2021, 11, 19077. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Lucas, C.H. Population dynamics of Aurelia aurita (Scyphozoa) from an isolated brackish lake, with particular reference to sexual reproduction. J. Plankton Res. 1996, 18, 987–1007. [Google Scholar] [CrossRef]
- Ishii, H.; Båmstedt, U. Food regulation of growth and maturation in a natural population of Aurelia aurita (L.). J. Plankton Res. 1998, 20, 805–816. [Google Scholar] [CrossRef]
- Yongze, X.; Qian, L.; Mei, Z.; Yu, Z.; Tiezhu, M.I.; Zhigang, Y.U. Effects of Temperature and Salinity on the Asexual Reproduction of Aurelia coerulea Polyps. J. Oceanol. Limnol. 2020, 38, 133–142. [Google Scholar]
- Ma, X.; Purcell, J.E. Effects of temperature, salinity, and predators on mortality of and colonization by the invasive hydrozoan Moerisia lyonsi. Mar. Biol. 2005, 147, 215–224. [Google Scholar] [CrossRef]
- Purcell, J.E. Climate effects on formation of jellyfish and ctenophore blooms: A review. J. Mar. Biol. Assoc. UK 2005, 85, 461–476. [Google Scholar] [CrossRef]
- Holst, S. Effects of climate warming on strobilation and ephyra production of North Sea scyphozoan jellyfish. Hydrobiologia 2012, 690, 127–140. [Google Scholar] [CrossRef]
- Lucas, C.H. Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia 2001, 451, 229–246. [Google Scholar] [CrossRef]
- Fu, Z.; Li, J.; Wang, J.; Lai, J.; Liu, Y.; Sun, M. Combined effects of temperature and salinity on the growth and pulsation of moon jellyfish (Aurelia coerulea) ephyrae. Am. J. Life Sci. 2020, 8, 144–151. [Google Scholar] [CrossRef]
- Willcox, S.; Moltschaniwskyj, N.A.; Crawford, C.M. Population dynamics of natural colonies of Aurelia sp. scyphistomae in Tasmania, Australia. Mar. Biol. 2008, 154, 661–670. [Google Scholar] [CrossRef]
- Gambill, M.; Peck, M.A. Respiration rates of the polyps of four jellyfish species: Potential thermal triggers and limits. J. Exp. Mar. Biol. Ecol. 2014, 459, 17–22. [Google Scholar] [CrossRef]
- Lawley, J.W.; Gamero-Mora, E.; Maronna, M.M.; Chiaverano, L.M.; Stampar, S.N.; Hopcroft, R.R.; Collins, A.G.; Morandini, A.C. The importance of molecular characters when morphological variability hinders diagnosability: Systematics of the moon jellyfish genus Aurelia (Cnidaria: Scyphozoa). PeerJ 2021, 9, e11954. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-H.; Kawahara, M.; Uye, S. Seasonal variations in the trophic relationship between the scyphomedusa Aurelia aurita s. l. and mesozooplankton in a eutrophic brackish-water lake, Japan. Plankton Benthos Res. 2009, 4, 14–22. [Google Scholar] [CrossRef]
- Lo, W.T.; Chen, I.L. Population succession and feeding of Scyphomedusae, Aurelia aurita, in a eutrophic tropical lagoon in Taiwan. Estuarine Coast. Shelf Sci. 2008, 76, 227–238. [Google Scholar] [CrossRef]
- Miyake, H.; Iwao, K.; Kakinuma, Y. Life history and environment of Aurelia aurita. South Pac. Study. 1997, 17, 273–285. [Google Scholar]
- Kogovšek, T.; Molineiro, J.C.; Lučić, D.; Onofri, I.; Gangai, B.; Miloslavić, M.; Bonnet, D.; Malej, A. Interannual size changes of adult Aurelia sp. 5 medusae stage in the Marine Protected Area of Mljet Island South Adriatic. Acta Adriat. 2012, 53, 233–242. [Google Scholar]
- Yoon, Y.H. The Characteristics on the Spatio-temporal Distributions of Phytoplankton Communities in Deukryang Bay, Southwestern Korea. Korean Soc. Environ. Biol. 1999, 17, 481–492. [Google Scholar]
- Yoo, M.H.; Song, T.Y.; Kim, E.S.; Choi, J.K. The characteristics on the spatial and temporal distribution of phytoplankton in the western Jinhae bay, Korea. J. Korean Soc. Oceanogr. 2007, 12, 305–314. [Google Scholar]
- Lee, M.; Baek, S.H. Changes in marine environmental factors and phytoplankton community composition observed via short-term investigation in a harbor in the eastern part of the south sea of Korea. J. Korean Soc. Mar. Environ. Saf. 2017, 23, 669–676. [Google Scholar] [CrossRef]
- Kamiyama, T. Microzooplankton as a food source for the scyphozoan Aurelia coerulea: Growth and feeding responses of the polyp stage in field assemblages. Plankton Benthos Res. 2023, 18, 34–46. [Google Scholar] [CrossRef]
- Kamiyama, T. Planktonic ciliates as food for the scyphozoan Aurelia coerulea: Feeding and growth responses of ephyra and metephyra stages. J. Oceanogr. 2018, 74, 53–63. [Google Scholar] [CrossRef]
- Kamiyama, T. Planktonic ciliates as food for the scyphozoan Aurelia aurita (sl): Effects on asexual reproduction of the polyp stage. J. Exp. Mar. Bio. Ecol. 2013, 445, 21–28. [Google Scholar] [CrossRef]
- Kamiyama, T. Planktonic ciliates as a food source for the scyphozoan Aurelia aurita (sl): Feeding activity and assimilation of the polyp stage. J. Exp. Mar. Bio. Ecol. 2011, 407, 207–215. [Google Scholar] [CrossRef]
- Robinson, K.L.; Graham, W.M. Long-term change in the abundances of northern Gulf of Mexico Scyphomedusae Chrysaora sp. and Aurelia spp. with links to climate variability. Limnol. Oceanogr. 2013, 58, 235–253. [Google Scholar] [CrossRef]
- Wang, Y.T.; Sun, S. Population dynamics of Aurelia sp.1 ephyrae and medusae in Jiaozhou Bay, China. Hydrobiologia 2015, 754, 147–155. [Google Scholar] [CrossRef]
- Duarte, C.M.; Pitt, K.A.; Lucas, C.H.; Purcell, J.E.; Uye, S.I.; Robinson, K.; Brotz, L.; Decker, M.B.; Sutherland, K.R.; Malej, A.; et al. Is global ocean sprawl a cause of jellyfish blooms? Front. Ecol. Environ. 2013, 11, 91–97. [Google Scholar] [CrossRef]
- Scott, J.M.; Dean, R.J.; Michael, J.K. Use of eDNA to determine source location of deadly jellyfish (Cubozoa) in an open coastal system. Coasts 2024, 4, 198–212. [Google Scholar] [CrossRef]
- Toyokawa, M.; Aoki, K.; Yamada, S.; Yasuda, A.; Murata, Y.; Kikuchi, T. Distribution of ephyrae and polyps of jellyfish aurelia aurita (Linnaeus 1758) sensu lato in Mikawa Bay, Japan. J. Oceanogr. 2011, 67, 209–218. [Google Scholar] [CrossRef]
- MOF. Annual Report for the Restoration and Improvement of Marine Habitats in Korea, 39–49. Available online: http://www.mof.go.kr (accessed on 20 December 2023).
- Pitt, K. Life history and settlement preferences of the edible jellyfish Catostylus mosaicus (Scyphozoa: Rhizostomeae). Mar. Biol. 2000, 136, 269–279. [Google Scholar] [CrossRef]
- Miyake, H.; Terazaki, M.; Kakinuma, Y. On the polyps of the common jellyfish Aurelia aurita in Kagoshima Bay. J. Oceanogr. 2002, 58, 451–459. [Google Scholar] [CrossRef]
- Holst, S.; Jarms, G. Substrate choice and settlement preferences of planula larvae of five Scyphozoa (Cnidaria) from German Bight, North Sea. Mar. Biol. 2007, 151, 863–871. [Google Scholar] [CrossRef]
- Lo, W.T.; Purcell, J.E.; Hung, J.J.; Su, H.M.; Hsu, P.K. Enhancement of jellyfish (Aurelia aurita) populations by extensive aquaculture rafts in a coastal lagoon in Taiwan. ICES J. Mar. Sci. 2008, 65, 453–461. [Google Scholar] [CrossRef]
- Ishii, H.; Katsukoshi, K. Seasonal and vertical distribution of Aurelia aurita polyps on a pylon in the inner most part of Tokyo Bay. J. Oceanogr. 2010, 66, 329–336. [Google Scholar] [CrossRef]
- Condon, R.H.; Lukas, C.H.; Pitt, K.A.; Uye, S.I. Jellyfish blooms and ecological interactions. Mar. Ecol. Prog. Ser. 2014, 510, 109–110. [Google Scholar] [CrossRef]
- FAO-Food and Agriculture Organization of the United Nations. Fisheries and Aquaculture Department Website. 2007. Available online: http://www.fao.org (accessed on 13 May 2024).
- Hoover, R.A.; Purcell, J.E. Substrate preferences of scyphozoan Aurelia labiata polyps Namong common dock-building materials. Hydrobiologia 2009, 616, 259–267. [Google Scholar] [CrossRef]
- National Institute of Fisheries Science. Report on the Jellyfish Monitoring and Mitigation in 2020, Busan, Republic of Korea, National Institute of Fisheries Science Printing Office. 2020. Available online: http://nifs.go.kr/main.do (accessed on 25 March 2021).
- Yasuda, T. Ecological studies on the jellyfish, Aurelia aurita in Urazoko Bay, Fukui Prefecture—IV. Monthly change in the bell-length composition and breeding season. Bull. JPN Soc. Sci. Fish. 1971, 37, 364–370, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Omori, M.; Ishii, H.; Fujinaga, A. Life history strategy of Aurelia aurita (Cnidaria, Scyphomedusae) and its impact on the zooplankton community of Tokyo Bay. ICES J. Mar. Sci. 1995, 52, 597–603. [Google Scholar] [CrossRef]
- Özdemir, Z.B.; Özdemir, S.; Özsandıkçı, U.; Büyükdeveci, F.; Baykal, B. The seasonally determination of disc diameter-weight relationship of moon jellyfish Aurelia aurita in the Black Sea Coasts of Turkey. Turk. J. Marit. Mar. Sci. 2019, 5, 8–16. [Google Scholar]
- Schneider, G. The common jellyfish Aurelia aurita: Standing stock, excretion and nutrient regeneration in the Kiel Bight, western Baltic 1982~1984. Mar. Biol. 1989, 100, 507–514. [Google Scholar] [CrossRef]
- Møller, L.F.; Riisgård, H.U. Population dynamics, growth and predation impact of the common jellyfish Aurelia aurita and two hydromedusae, Sarsia tubulosa, and Aequorea vitrina in Limfjorden (Denmark). Mar. Ecol. Prog. Ser. 2007, 346, 153–165. [Google Scholar] [CrossRef]
- Hamner, W.M.; Jenssen, R.M. Growth, degrowth, and irreversible cell differentiation in Aurelia aurita. Am. Zool. 1974, 14, 833–849. [Google Scholar] [CrossRef]
- Olesen, N.J.; Frandsen, K.; Riisgard, H.H.U. Population dynamics, growth and energetics of jellyfish Aurelia aurita in a shallow fjord. Mar. Ecol. Prog. Ser. 1994, 105, 9–18. [Google Scholar] [CrossRef]
- Båmstedt, U.; Wild, B.; Martinussen, M.B. Significance of food type for growth of ephyrae Aurelia aurita (Scyphozoa). Mar. Biol. 2001, 139, 641–650. [Google Scholar] [CrossRef]
- Uye, S.; Shimauchi, H. Population biomass, feeding, respiration and growth rates, and carbon budget of the scyphomedusa Aurelia aurita in the Inland Sea of Japan. J. Plankton Res. 2005, 27, 237–248. [Google Scholar] [CrossRef]
- Mutlu, E. Distribution and abundance of moon jellyfish (Aurelia aurita) and its zooplankton food in the Black Sea. Mar. Biol. 2001, 138, 329–339. [Google Scholar] [CrossRef]
- Gröndahl, F. Interactions between polyps of Aurelia aurita and planktonic larvae of scyphozoans: An experimental study. Mar. Ecol. Prog. Ser. 1988, 45, 87–93. [Google Scholar] [CrossRef]
- Möller, H. Population dynamics of Aurelia aurita medusae in Kiel Bight, Germany (FRG). Mar. Biol. 1980, 60, 123–128. [Google Scholar] [CrossRef]
- Kakinuma, Y. An Experimental Study of the Life Cycle and Organ Differentiation of Aurelia aurita Lamarck. Bull. Mar. Bio. Stn. Asamushi. 1975, 101–112. [Google Scholar]
- Schneider, G.; Weisse, T. Metabolism measurements of Aurelia aurita planulae larvae, and calculation of maximal survival period of the free swimming stage. Helgol. Meeresunters 1985, 39, 43–47. [Google Scholar] [CrossRef]
- Yasuda, T. Ecological studies on the jellyfish, Aurelia aurita in Urazoko Bay, Fukui Prefecture—XI. An observation on ephyrae formation. Publ. Seto. Mar. Bio. Lab. 1975, 22, 75–80. [Google Scholar] [CrossRef]
- Thatje, S.; Hall, S. The effect of temperature on the evolution of per offspring investment in a globally distributed family of marine invertebrates (Crustacea: Decapoda: Lithodidae). Mar. Biol. 2016, 163, 48. [Google Scholar] [CrossRef]
- Schneider, G. Larvae production of the common jellyfish Aurelia aurita in the western Baltic. Kiel. Meersforsch. Sonderh. 1988, 6, 295–300. [Google Scholar]
- Lucas, C.H.; Graham, W.M.; Widmer, C. Jellyfish life histories: Role of polyps in forming and maintaining scyphomedusa populations. Adv. Mar. Biol. 2012, 63, 133–196. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.S.; Suzuki, K.W.; Kumakura, E.; Sato, K.; Oe, Y.; Sato, T.; Sawada, H.; Masuda, R.; Nogata, Y. Seasonal alternation of the ontogenetic development of the moon jellyfish Aurelia coerulea in Maizuru Bay, Japan. PLoS ONE 2019, 14, e0225513. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Yamada, S.; Toyokawa, M.; Yasuda, A.; Kikuchi, T. Horizontal distribution and growth of jellyfish, Aurelia aurita (Linnaeus 1758) sensu lato, in Mikawa Bay, Japan. Coast. Mar. Sci. 2012, 35, 103–111. [Google Scholar]
- Marques, R.; Albouy-Boyer, S.; Delpy, F.; Carre, C.; Le Floc’h, E.; Roques, C.; Molinero, J.C.; Bonnet, D. Pelagic population dynamics of Aurelia sp. in French Mediterranean lagoons. J. Plankton Res. 2015, 37, 1019–1035. [Google Scholar] [CrossRef]
- Benovic, A.; Lucic, D.; Onofri, V.; Pehardia, M.; Caric, M.; Jasprica, N.; Bobanovic-Colic, S. Ecological characteristics of the Mljet Islands seawater lakes (South Adriatic Sea) with special reference to their resident populations of medusae. Sci. Mar. 2000, 64, 197–206. [Google Scholar] [CrossRef]
- Hernroth, L.; Gröndahl, F. On the biology of Aurelia aurita (L.) 1. Release and growth of Aurelia aurita (L.) ephyrae in the Gullmar Fjord, western Sweden, 1982–1983. Ophelia 1983, 22, 189–199. [Google Scholar] [CrossRef]
- Lucas, C.H.; Williams, J.A. Population dynamics of the scyphomedusa Aurelia aurita in Southampton Water. J. Plankton Res. 1994, 16, 879–895. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).