Phosphorus Sources and Transport Pathways in the North Chaohu Lake Catchment of China
Abstract
:1. Introduction
- (a)
- The main factors affecting the groundwater quality in the North Chaohu Lake Catchment were revealed using the factor analysis by employing the statistical software SPSS (version 19).
- (b)
- The P dissolution and diffusion were illustrated by analyzing the release of P from phosphate rock.
- (c)
- The risk to the ambient aquatic environment posed by the dissolution of P in this region was discussed by fitting the experimental data to the Langmuir kinetic equation.
2. Study Area
3. Methods and Results
3.1. Sampling and Experimental Procedure
3.2. Analysis Method
3.2.1. Fit of the Cumulative Content of P versus 1D Diffusion Equation
3.2.2. Fit of the P Release Rate Data versus the Derivative of the Diffusion Equation
3.2.3. Fit of the Released P Concentration versus the Langmuir Kinetic Equation
4. Analysis and Discussion
4.1. Groundwater Analysis
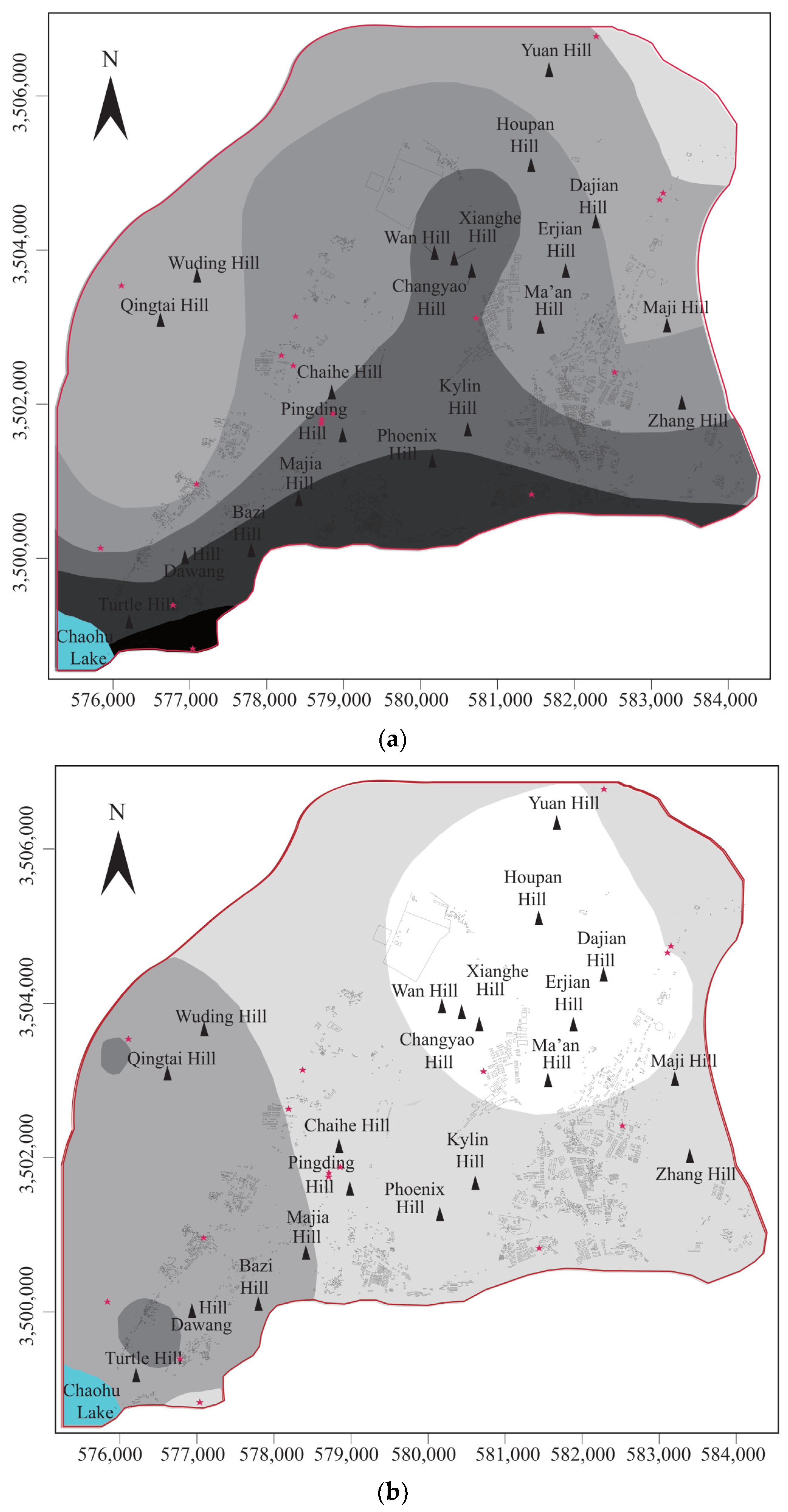
4.1.1. Spatial Analysis of Groundwater Total P
4.1.2. Factor Analysis
4.2. Analysis of the P Dissolution
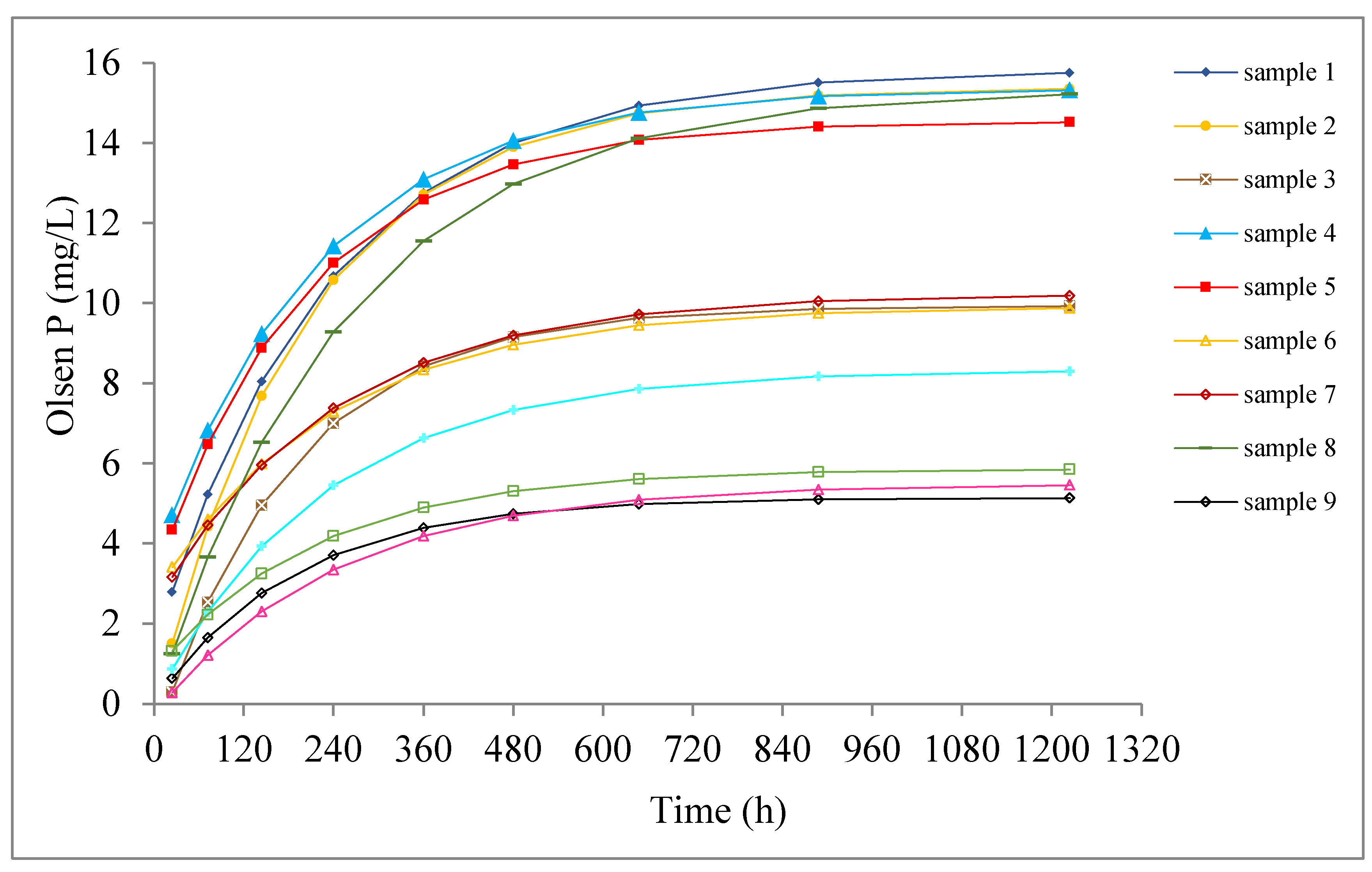
4.2.1. Cumulative Concentration of P
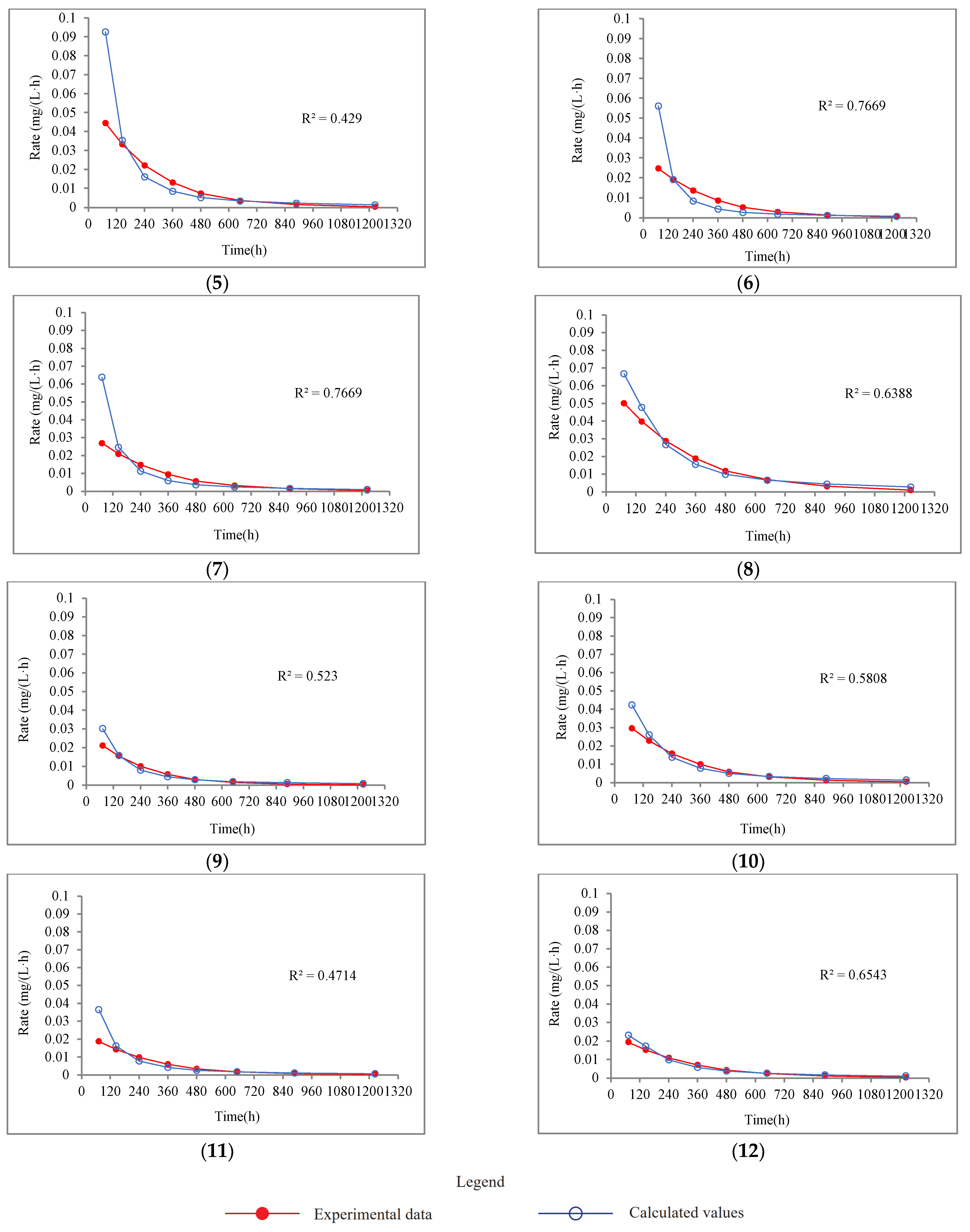
4.2.2. Release Rate of P
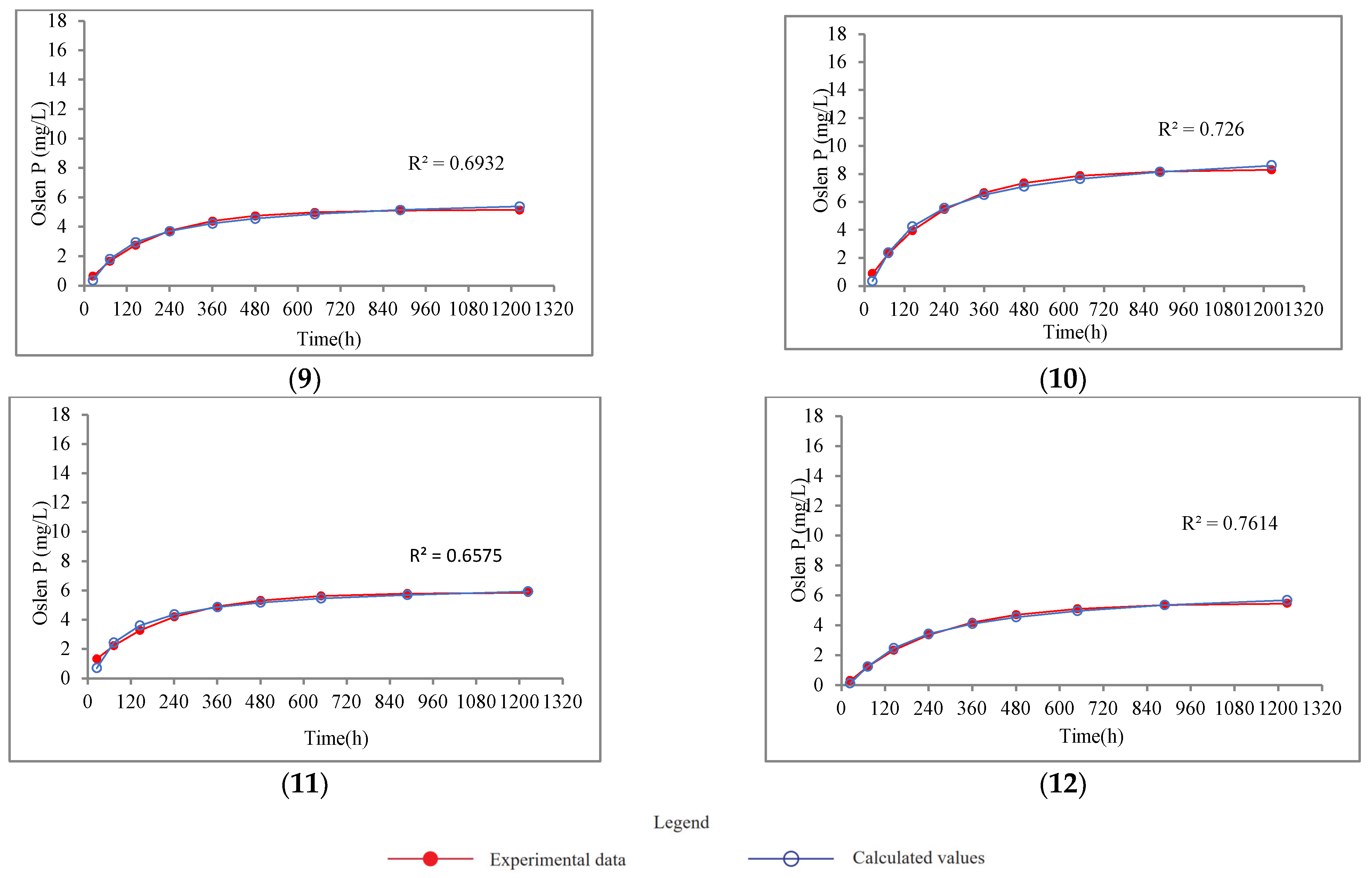
4.2.3. Potential Maximum Dissolution of P
5. Implications for the Source of P
6. Limitations and Future Works
7. Conclusions
- (1)
- The groundwater total P content in the North Chaohu Lake Catchment was high in the west and low in the east, with the highest values around the Chaohu Lake (greater than 2.0 mg/L).
- (2)
- The factor analysis revealed that the anthropogenic activities (especially the agricultural and mining activities) were the major factors affecting groundwater quality. In contrast, the interactions between groundwater and carbonate rock, the dissolution of the P-bearing minerals, and the fluorine-bearing minerals were the second, third, and fourth factors affecting the groundwater quality, in the order of decreasing effectiveness.
- (3)
- There were three periods for the dissolution of P, evident from the experimental breakthrough curves. The dissolution of P can be well described by a 1D diffusion equation and the Langmuir kinetic equation. The geometric factor A and diffusion coefficient Dx were estimated by fitting the data against the 1D diffusion equation. Samples with high P contents (sample 9~12) had smaller geometric factors, while the opposite was true for samples with low P contents (sample 1~8).
- (4)
- The low ambient temperature was probably responsible for the relatively lower diffusion coefficient compared to that of the previous studies. The constant k and the potential maximum dissolution of P, Cmax, were also obtained from fitting data with the Langmuir kinetic equation. According to the Cmax values, the dissolution of P could pose a risk to the ambient water body, and the release of P was independent of the total phosphorus content in the rock at the study area.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Gu, W.; Liu, X.; Liu, H.; Tang, G.; Yang, C. Combined impacts of algae-induced variations in water soluble organic matter and heavy metals on bacterial community structure in sediment from Chaohu Lake, a eutrophic shallow lake. Sci. Total Environ. 2023, 874, 162481. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hua, Z.; Wang, L. Sensitivity analysis of the Chaohu Lake eutrophication model with a new index based on the morris method. Water Sci. Technol. Water Supply 2018, 18, 1375–1387. [Google Scholar] [CrossRef]
- Ding, W.; Zhu, R.; Hou, L.; Wang, Q. Matrix-bound phosphine, phosphorus fractions and phosphatase activity through sediment profiles in Lake Chahu, China. Environ. Sci. Process. Impacts 2014, 16, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Chang, L. Transportation Traits of N and P in Chaohu Lake P-Rich Basin and the Consequent High-Risk Identification. Master’s Thesis, Nanjing Agriculture University, Nanjing, China, 2012. (In Chinese). [Google Scholar]
- Wang, X.; Wang, X.; Feng, Y.; Liu, H. Distribution of total phosphorus in the sediments in Chaohu Lake and its geological origin. J. Anhui Norm. Univ. (Nat. Sci.) 2007, 30, 496–499. (In Chinese) [Google Scholar]
- Qian, J.; Chu, Q.; Zhang, R.; Liu, Y.; Wan, W. Influence of recurrent rainfall and oxalic acid on phosphorus releasing from rocks phosphate in the Chaohu watershed, China. Chemosphere 2019, 215, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.R.; Townsend, A.R.; Farmer, G.L. Unexpected dominance of parent-material strontium in a tropical forest on highly weathered soils. Ecology 2005, 86, 626–632. [Google Scholar] [CrossRef]
- Porder, S.; Paytan, A.; Vitousek, P.M. Erosion and landscape development affect plant nutrient status in the Hawaiian Islands. Oecologia 2005, 142, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Filippelli, G.M.; Souch, C.; Horn, S.P.; Newkirk, D. The pre-Columbian footprint on terrestrial nutrient cycling in Costa Rica: Insights from phosphorus in a lake sediment record. J. Paleolimnol. 2010, 43, 843–856. [Google Scholar] [CrossRef]
- Gaur, A.C.; Ostwall, K.P. Influence of phosphate dissolving bacilli on yield and phosphate uptake of wheat crop. Indian J. Exp. Biol. 1972, 10, 393–394. [Google Scholar]
- Jurinak, J.J.; Dudley, L.M.; Allen, M.F.; Knight, W.G. The role of calcium oxalate in the availability of phosphorus in soils of semiarid regions: A thermodynamic study. Soil Sci. 1986, 142, 255–261. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Bernhardt, E.S. Biogeochemistry: An Analysis of Global Change, 4th ed.; Academic Press: San Diego, CA, USA, 2020. [Google Scholar]
- Aydin, I.; Imamoglu, S.; Aydin, F.; Saydut, A.; Hamamci, C. Determination of mineral phosphate species in sedimentary phosphate rock in Mardin, SE Anatolia, Turkey by sequential extraction. Microchem. J. 2009, 91, 63–69. [Google Scholar] [CrossRef]
- Kang, W.J.; Kolasa, K.V.; Rials, M.W. Groundwater inflow and associated transport of phosphorus to a hypereutrophic lake. Environ. Geol. 2005, 47, 565–575. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R.; McClellan, G.H. Kinetics of dissolution of phosphate rocks in soils. Soil Sci. Soc. Am. J. 1980, 44, 260–264. [Google Scholar] [CrossRef]
- Sikora, F.J.; Copeland, J.P.; Mullins, G.L.; Bartos, J.M. Phosphorus dissolution kinetics and bioavailability of water-insoluble fractions from monoammonium phosphate fertilizers. Soil Sci. Soc. Am. J. 1991, 55, 362–368. [Google Scholar] [CrossRef]
- Shen, Y.H. Study on the Existing Form and Release Rule of Rock Phosphorus in Eastern Section of the North Shore of Chaohu Lake Area, Hefei; Hefei University and Technology: Hefei, China, 2015; pp. 34–35, (In Chinese). [Google Scholar] [CrossRef]
- Hirmas, D.R.; Graham, R.C. Pedogenesis and Soil-Geomorphic Relationships in an arid mountain range, Mojave Desert, California. Soil Sci. Soc. Am. J. 2011, 75, 192–206. [Google Scholar] [CrossRef]
- Drewry, J.; Taylor, M.; Curran-Cournane, F.; Gray, C.; McDwell, R. Olsen P methods and soil quality monitoring: Are we comparing “apples” with “apples”? In Tools for Nutrient and Pollutant Management: Applications to Agriculture and Environmental Quality; Proceedings of a workshop. Occasional report No.17; Currie, L.D., Loganathan, P., Eds.; Fertiliser and Lime Research Centre, Massey University: Palmerston North, New Zealand, 2013; pp. 1–2. [Google Scholar]
- Jin, X.C.; Tu, Q.Y. Standard for the Investigation and Evaluation on the Eutrophication of Lakes; China Environmental Science Press: Beijing, China, 1990; pp. 15–16. (In Chinese) [Google Scholar]
- Spiteri, C.; Slomp, C.P.; Regnier, P.; Meile, C.; van Cappellen, P. Modelling the geochemical fate and transport of wastewater-derived phosphorus in contrasting groundwater systems. J. Contam. Hydrol. 2007, 92, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.C.; van Genuchten, M.T. Flux-averaged and volume-averaged concentrations in continuum approaches to solute transport. Water Resour. Res. 1984, 20, 866–872. [Google Scholar] [CrossRef]
- Fetter, C.W. Contaminant Transport, 2nd ed.; Waveland Press: Long Grove, IL, USA, 1999. [Google Scholar]
- You, K.H.; Zhan, H.B. New solutions for solute transport in a finite column with distance-dependent dispersivities and time-dependent solute sources. J. Hydrol. 2013, 487, 87–97. [Google Scholar] [CrossRef]
- Ogata, A.; Bank, R.B. A Solution of the Differential Equation of Longitudinal Dispersion in Porous Media; Geological Survey Professional Paper, No. 411-A; United States Government Printing Office, Washington: Washington, DC, USA, 1961. [Google Scholar]
- He, Z.L.; Baligar, V.C.; Martens, D.C.; Ritchey, K.D.; Kemper, W.D. Kinetics of phosphate rock dissolution in acidic soil amended with liming materials and cellulose. Soil Sci. Am. J. 1996, 60, 1589–1595. [Google Scholar] [CrossRef]
- MPCA. Procedures for Implementing River Eutrophication Standards in NPDES Wastewater Permits in Minnesota (Version 1.0); Minnesota Pollution Control Agency: College Road, MN, USA, 2015. [Google Scholar]
- Shang, G.P.; Shang, J.C. Causes and control countermeasures of eutrophication in Chaohu Lake, China. Chin. Geogr. Sci. 2005, 15, 348–354. [Google Scholar] [CrossRef]
- Charfi, S.; Zouari, K.; Feki, S.; Mami, E. Study of variation in groundwater quality in a coastal aquifer in north-eastern Tunisia using multivariate factor analysis. Quatern. Int. 2013, 302, 199–209. [Google Scholar] [CrossRef]
- Bahar, M.; Reza, S. Hydrochemical characteristics and quality assessment of shallow groundwater in a coastal area of Southwest Bangladesh. Environ. Earth Sci. 2010, 61, 1065–1073. [Google Scholar] [CrossRef]
- Edet, A.; Ukpong, A.; Nganje, T. Hydrochemical studies of Cross River Basin (southeastern Nigeria) river systems using cross plots, statistics and water quality index. Environ. Earth Sci. 2013, 70, 3043–3056. [Google Scholar] [CrossRef]
- Montcoudiol, N.; Molson, J.; Lemieux, J.M. Groundwater geochemistry of the Outaouais region (Quebec, Canada): A regional-scale study. Hydrogeol. J. 2015, 23, 377–396. [Google Scholar] [CrossRef]
- Garcia, J.H.; Li, W.W.; Arimoto, R.; Okrasinski, R.; Greenlee, J.; Walton, J.; Schloesslin, C.; Sage, S. Characterization and implication of potential fugitive dust sources in the Paso del Norte region. Sci. Total Environ. 2004, 325, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Lin, K.H.; Kuo, Y.M. Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci. Total Environ. 2003, 313, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Dragon, K. Application of factor analysis to study contamination of a semi-confined aquifer (Wielkopolska Buried Valley aquifer, Poland). J. Hydrol. 2006, 331, 272–279. [Google Scholar] [CrossRef]
- Jeong, C.H. Effect of land use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Republic of Korea. J. Hydrol. 2001, 253, 194–210. [Google Scholar] [CrossRef]
- Cronin, A.A.; Taylor, R.G.; Powell, K.L.; Barrett, M.H.; Trowsdale, S.A.; Lerner, D.N. Temporal variations in the depth-specific hydrochemistry and sewage-related microbiology of an urban sandstone aquifer, Nottingham, United Kingdom. Hydrogeol. J. 2003, 11, 205–216. [Google Scholar] [CrossRef]
- Choi, B.Y.; Yun, S.T.; Yu, S.Y.; Lee, P.K.; Park, S.S.; Chae, G.T.; Mayer, B. Hydrochemistry of urban groundwater in Seoul, Republic of Korea: Effects of land-use and pollutant recharge. Environ. Geol. 2005, 48, 979–990. [Google Scholar] [CrossRef]
- Han, D. Foundation of Chemical Kinetics; Beijing University Press: Beijing, China, 1987; pp. 1–8. (In Chinese) [Google Scholar]
- Hu, H.X. Study on the Transportation Process and Regularity of Soil and Water in Middle-Eastern Section of the North Shore of Lake Chaohu; Hefei University and Technology: Hefei, China, 2008; pp. 18–22. (In Chinese) [Google Scholar]
- Neal, C.; Heathwaite, A.L. Nutrient mobility within river basins: A European perspective. J. Hydrol. 2005, 304, 477–490. [Google Scholar] [CrossRef]
- Neal, C.; Jarvie, H.P. Agriculture, community, river eutrophication and the water framework directive. Hydrol. Process 2005, 19, 1895–1901. [Google Scholar] [CrossRef]
- Qian, J.Z.; Wang, L.L.; Zhan, H.B.; Chen, Z. Urban land-use effects on groundwater phosphate distribution in a shallow aquifer, Nanfei River basin, China. Hydrogeol. J. 2011, 19, 1431–1442. [Google Scholar] [CrossRef]
- Li, S.N. The Influence of Phosphorus in the Rock on Eutrophication in Chaohu Lake in the Phoenix Area; Hefei University and Technology: Hefei, China, 2012; pp. 19–20. (In Chinese) [Google Scholar]
- Yang, Y.; Guo, T.; Jiao, W. Destruction processes of mining on water environment in the mining area combining isotopic and hydrochemical tracer. Environ. Pollut. 2018, 237, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Meng, Z.; Jiao, W. Hydrological and pollution processes in mining area of Fenhe River Basin in China. Environ. Pollut. 2018, 234, 743–750. [Google Scholar] [CrossRef] [PubMed]
- He, Z.L.; Baligar, V.C.; Martens, D.C.; Ritchey, K.D.; Elrashidi, M. Effect of byproduct, nitrogen fertilizer, and zeolite on phosphate rock dissolution and extractable phosphorus in acid soil. Plant Soil 1999, 208, 199–207. [Google Scholar] [CrossRef]
- Katsev, S.; Tsandev, I.; L’Heureux, I.; Rancourt, D.G. Factors controlling long-term phosphorus efflux from lake sediments: Exploratory reactive-transport modeling. Chem. Geol. 2006, 234, 127–147. [Google Scholar] [CrossRef]
- Kulabako, N.R.; Nalubega, M.; Thunvik, R. Phosphorus transport in shallow groundwater in peri-urban Kampala, Uganda: Results from field and laboratory measurements. Environ. Geol. 2008, 53, 1535–1551. [Google Scholar] [CrossRef]
- Bedore, P.D.; David, M.B.; Stucki, J.W. Mechanisms of phosphorus control in urban streams receiving sewage effluent. Water Air Soil Poll. 2008, 191, 217–229. [Google Scholar] [CrossRef]
- Solim, S.U.; Wanganeo, A. Factors influencing release of phosphorus from sediments in a high productive polymictic lake system. Water Sci. Technol. 2009, 60, 1013–1023. [Google Scholar] [CrossRef]
- Juo, A.S.R.; Adams, F.F. Chemistry of LAC soils. In Soil Management Support Services Technical Monograph No. 14. Soil Conservation Service, Proceedings of the Symposium in Low Activity Clay (LAC) Soils, Las Vegas, NV, USA, 26–30 November 1984; United Nations: Washington, DC, USA, 1986. [Google Scholar]
- Van der Grift, B.; Rozemeijer, J.C.; Griffioen, J.; van der Velde, Y. Iron oxidation kinetics and phosphate immobilization along the flow-path from groundwater into surface water. Hydrol. Earth Syst. Sci. 2014, 18, 4687–4702. [Google Scholar] [CrossRef]
- Moller, J.; Mogensen, T. Use of an ion-exchanger for determining available phosphorus in soils. Soil Sci. 1953, 76, 297–306. [Google Scholar] [CrossRef]
- Chien, S.H.; Hammond, L.L. A simple chemical method for evaluating the agronomic potential of granulated phosphate rock. Soil Sci. Soc. Am. J. 1978, 42, 615–617. [Google Scholar] [CrossRef]
- Lai, T.M.; Eberl, D.D. Controlled and renewable release of phosphorus in soils from mixtures of phosphate rock and NH4-exchanged clinoptilolite. Zeolites 1986, 6, 129–132. [Google Scholar] [CrossRef]
- Li, Y.H.; Gregory, S. Diffusion of ions in sea water and in deep-sea sediments. Geochim. Cosmochim. Acta 1974, 38, 703–714. [Google Scholar]
- Pett-Ridge Julie, C. Contributions of dust to phosphorus cycling in tropical forests of the Luquillo Mountains, Purto Rico. Biogeochemistry 2009, 94, 63–80. [Google Scholar] [CrossRef]






| Parameter | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| TDS | 0.954 | 0.112 | 0.046 | −0.104 |
| NO3− | 0.949 | −0.031 | 0.145 | 0.005 |
| SO42− | 0.939 | −0.087 | 0.036 | 0.049 |
| Cl− | 0.697 | 0.379 | −0.056 | −0.001 |
| Mg2+ | −0.032 | 0.945 | 0.093 | −0.130 |
| Na+ | −0.033 | 0.935 | 0.092 | −0.123 |
| Ca2+ | 0.297 | 0.853 | 0.035 | 0.052 |
| TP | 0.028 | 0.008 | 0.958 | 0.068 |
| K+ | 0.103 | 0.169 | 0.933 | 0.009 |
| F− | −0.053 | 0.057 | 0.060 | 0.914 |
| pH | 0.021 | −0.237 | 0.014 | 0.873 |
| Eigenvalues | 3.281 | 2.747 | 1.837 | 1.650 |
| % of variance | 29.83 | 24.98 | 16.70 | 15.00 |
| Parameters | TP | NO3− | SO42− | Na+ | K+ | Mg2+ | Ca2+ | TDS | Cl− | F− | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | 1.000 | ||||||||||
| NO3− | 0.185 | 1.000 | |||||||||
| SO42− | 0.049 | 0.885 | 1.000 | ||||||||
| Na+ | 0.087 | −0.028 | −0.066 | 1.000 | |||||||
| K+ | 0.819 | 0.188 | 0.117 | 0.222 | 1.000 | ||||||
| Mg2+ | 0.055 | −0.039 | −0.100 | 0.907 | 0.265 | 1.000 | |||||
| Ca2+ | 0.102 | 0.272 | 0.196 | 0.726 | 0.158 | 0.727 | 1.000 | ||||
| TDS | 0.049 | 0.906 | 0.871 | 0.097 | 0.165 | 0.112 | 0.369 | 1.000 | |||
| Cl− | −0.031 | 0.558 | 0.517 | 0.252 | 0.138 | 0.300 | 0.455 | 0.634 | 1.000 | ||
| F− | 0.112 | −0.030 | −0.012 | −0.062 | 0.071 | −0.076 | 0.049 | −0.135 | −0.001 | 1.000 | |
| pH | 0.064 | 0.026 | 0.091 | −0.303 | −0.010 | −0.298 | −0.148 | −0.086 | −0.110 | 0.621 | 1.000 |
| Sample ID | TP (mg/kg) | ρb (kg/L) | A | Dx (cm2/s) | k | Cmax (mg/L) | r1 | r2 | r3 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 85 | 2.58 | 9.26 × 10−2 | 1.58 × 10−4 | 5.84 × 10−3 | 18.49 | 0.990 | 0.943 | 0.997 |
| 2 | 86 | 2.37 | 10.23 × 10−2 | 1.31 × 10−4 | 5.15 × 10−3 | 18.71 | 0.995 | 0.972 | 0.995 |
| 3 | 73 | 2.61 | 7.32 × 10−2 | 1.20 × 10−4 | 4.83 × 10−3 | 12.40 | 0.994 | 0.987 | 0.990 |
| 4 | 99 | 2.47 | 7.37 × 10−2 | 3.02 × 10−4 | 10.21 × 10−3 | 16.65 | 0.978 | 0.904 | 0.990 |
| 5 | 81 | 2.68 | 7.94 × 10−2 | 2.99 × 10−4 | 10.28 × 10−3 | 15.87 | 0.981 | 0.912 | 0.992 |
| 6 | 91 | 2.21 | 5.24 × 10−2 | 4.48 × 10−4 | 11.40 × 10−3 | 10.50 | 0.957 | 0.860 | 0.983 |
| 7 | 91 | 2.39 | 5.47 × 10−2 | 2.94 × 10−4 | 9.70 × 10−3 | 11.04 | 0.974 | 0.894 | 0.988 |
| 8 | 113 | 2.49 | 7.56 × 10−2 | 1.00 × 10−4 | 3.83 × 10−3 | 19.27 | 0.997 | 0.980 | 0.997 |
| 9 | 35,390 | 2.38 | 0.81 × 10−4 | 1.54 × 10−4 | 6.02 × 10−3 | 6.13 | 0.994 | 0.965 | 0.995 |
| 10 | 34,390 | 2.23 | 1.47 × 10−4 | 1.21 × 10−4 | 4.70 × 10−3 | 10.19 | 0.995 | 0.971 | 0.997 |
| 11 | 17,590 | 2.31 | 1.79 × 10−4 | 2.07 × 10−4 | 7.40 × 10−3 | 6.65 | 0.986 | 0.928 | 0.996 |
| 12 | 13,990 | 2.22 | 2.5 × 10−4 | 0.96 × 10−4 | 3.69 × 10−3 | 7.01 | 0.997 | 0.987 | 0.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhan, H.; Qian, J.; Zhang, R.; Zhang, Q.; Guan, H. Phosphorus Sources and Transport Pathways in the North Chaohu Lake Catchment of China. Water 2024, 16, 244. https://doi.org/10.3390/w16020244
Wang L, Zhan H, Qian J, Zhang R, Zhang Q, Guan H. Phosphorus Sources and Transport Pathways in the North Chaohu Lake Catchment of China. Water. 2024; 16(2):244. https://doi.org/10.3390/w16020244
Chicago/Turabian StyleWang, Lulu, Hongbin Zhan, Jiazhong Qian, Ruigang Zhang, Qing Zhang, and Houchun Guan. 2024. "Phosphorus Sources and Transport Pathways in the North Chaohu Lake Catchment of China" Water 16, no. 2: 244. https://doi.org/10.3390/w16020244
APA StyleWang, L., Zhan, H., Qian, J., Zhang, R., Zhang, Q., & Guan, H. (2024). Phosphorus Sources and Transport Pathways in the North Chaohu Lake Catchment of China. Water, 16(2), 244. https://doi.org/10.3390/w16020244







