Assessing the Benthic Ecological Quality in the Intertidal Zone of Cheonsu Bay, Korea, Using Multiple Biotic Indices
Abstract
:1. Introduction
2. Materials and Methods
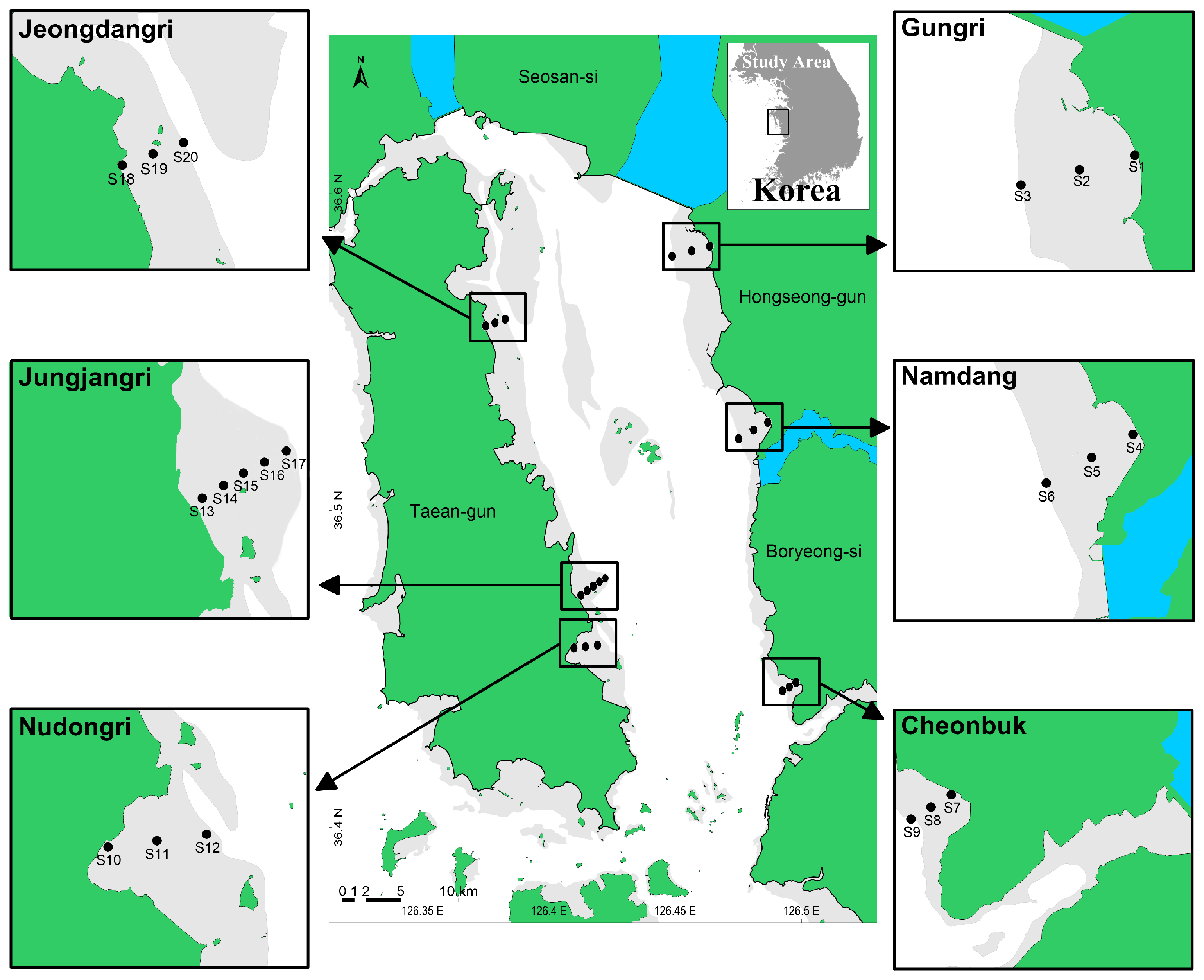
2.1. Study Area
2.2. Sample Collection and Analysis
2.3. Biotic Data Analysis
2.3.1. Dominance Index
2.3.2. Biotic Indices
| Indices | Algorithm | Method | Index Values | EcoQs | Acceptable or Unacceptable | Reference | Note |
|---|---|---|---|---|---|---|---|
| AMBI | Relative susceptibility of macrobenthic assemblages to pollution | 0.0–1.2 1.2–3.3 3.3–5.0 5.0–6.0 >6.0 | High Good Moderate Poor Bad | Acceptable Acceptable Unacceptable Unacceptable Unacceptable | [8] | EGI: disturbance-sensitive species; EGII: disturbance-indifferent species; EGIII: disturbance-tolerant species; EGIV: second-order opportunistic species; EGV: first-order opportunistic species. | |
| BENTIX | Relative susceptibility of macrobenthic assemblages to pollution | 6–4.5 4.5–3.5 3.5–2.5 2.5–2.0 0.0 | High Good Moderate Poor Bad | Acceptable Acceptable Unacceptable Unacceptable Unacceptable | [9] | GI = EGI + EGII; GII = EGIII + EGIV; GIII = EGV. | |
| BOPA | Relative abundance of amphipods and opportunistic polychaetes | 0–0.045 0.045–0.139 0.139–0.193 0.193–0.267 0.267–0.301 | High Good Moderate Poor Bad | Acceptable Acceptable Unacceptable Unacceptable Unacceptable | [10] | fp: opportunistic polychaetes frequency; fa: amphipods frequency | |
| BPI | Consider type of feeding and life history | 60–100 40–60 30–40 20–30 0–20 | High Good Moderate Poor Bad | Acceptable Acceptable Unacceptable Unacceptable Unacceptable | [33] | N1: filter feeders or large carnivores; N2: surface deposit feeders or small carnivores; N3: subterranean deposit feeders; N4: opportunistic species | |
| M-AMBI | Relative susceptibility of macrobenthic assemblages to pollution; richness and diversity(H’) | >0.77 0.53–0.77 0.38–0.53 0.20–0.38 ≤0.2 | High Good Moderate Poor Bad | Acceptable Acceptable Unacceptable Unacceptable Unacceptable | [12] | H’: Shannon diversity index; S: number of species | |
| W-values | Abundances of species and biomass of species | >0.50 0.15–0.49 −0.14–0.14 −0.49–−0.15 −1–−0.5 | High Good Moderate Poor Bad | Acceptable Acceptable Unacceptable Unacceptable Unacceptable | [35] | S: The number of species; Bi: the biomass of the species i; Ai: the abundance of the species i | |
| H’(log2) | Relative abundances of species | >4 4–3 3–2 2–1 <1 | High Good Moderate Poor Bad | Acceptable Acceptable Unacceptable Unacceptable Unacceptable | [15] | Ni: Number of individuals belonging to the ith species; N: Total number of individuals. |
2.4. Statistical Analysis
3. Results
3.1. Environment Factors
3.2. Macrobenthic Structure Characteristics and Dominant Species
3.3. Biotic Indices Values and Ecological Quality
3.4. Correlation Analysis
3.5. Kappa Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liao, J.; Feng, H.; Yan, S.; Cui, X.; Tang, S.; Liu, X. Comprehensive Distribution Characteristics and Risks of Heavy Metals in Typical Intertidal Zones and Their Relationship with Urban Economic Indicators. Ecol. Indic. 2023, 148, 110112. [Google Scholar] [CrossRef]
- Nordlund, L.M.; De La Torre-Castro, M.; Erlandsson, J.; Conand, C.; Muthiga, N.; Jiddawi, N.; Gullström, M. Intertidal Zone Management in the Western Indian Ocean: Assessing Current Status and Future Possibilities Using Expert Opinions. Ambio J. Environ. Soc. 2014, 43, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yoon, S.J.; Kim, T.; Ryu, J.; Kang, S.-G.; Khim, J.S. Response to Oiled Wildlife in the Management and Evaluation of Marine Oil Spills in South Korea: A Review. Reg. Stud. Mar. Sci. 2020, 40, 101542. [Google Scholar] [CrossRef]
- Nazeer, Z.; Khan, S.A.; Manikandan, K.; Manokaran, S.; Hsu, H.H.; Joydas, T.; Lyla, P.S. Macrofaunal Assemblage in the Intertidal Area of Saudi Arabian Gulf Coast. Reg. Stud. Mar. Sci. 2021, 47, 101954. [Google Scholar] [CrossRef]
- Pearson, T.H.; Rosenberg, R. Macrobenthic Succession in Relation to Organic Enrichment and Pollution of the Marine Environment. Oceanogr. Mar. Biol. Ann. Rev. 1978, 16, 229–311. [Google Scholar]
- Luo, X.; Lin, S.; Yang, J.; Shen, J.; Fan, Y.; Zhang, L. Benthic Habitat Quality Assessment Based on Biological Indices in Xiaoqing River Estuary and Its Adjacent Sea of Laizhou Bay, China. J. Ocean. Univ. China 2017, 16, 537–546. [Google Scholar] [CrossRef]
- Liu, L.; Li, A.; Zhu, L.; Xue, S.; Li, J.; Zhang, C.; Yu, W.; Ma, Z.; Zhuang, H.; Jiang, Z.; et al. The Application of the Generalized Additive Model to Represent Macrobenthos near Xiaoqing Estuary, Laizhou Bay. Biology 2023, 12, 1146. [Google Scholar] [CrossRef]
- Borja, A.; Franco, J.; Pérez, V. A Marine Biotic Index to Establish the Ecological Quality of Soft-Bottom Benthos Within European Estuarine and Coastal Environments. Mar. Pollut. Bull. 2000, 40, 1100–1114. [Google Scholar] [CrossRef]
- Simboura, N.; Zenetos, A. Benthic Indicators to Use in Ecological Quality Classification of Mediterranean Soft Bottom Marine Ecosystems, Including a New Biotic Index. Medit. Mar. Sci. 2002, 3, 77–111. [Google Scholar] [CrossRef]
- Dauvin, J.C.; Ruellet, T. Polychaete/Amphipod Ratio Revisited. Mar. Pollut. Bull. 2007, 55, 215–224. [Google Scholar] [CrossRef]
- KORDI (Korea Ocean Research and Development Institute). Marine Environmental Assessment Based on the Benthic Faunal Communities; Report of Management Technique for Marine Environmental Protection Last Year; KORDI: Ansan, Republic of Korea, 1995. [Google Scholar]
- Muxika, I.; Borja, Á.; Bald, J. Using Historical Data, Expert Judgement and Multivariate Analysis in Assessing Reference Conditions and Benthic Ecological Status, According to the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 16–29. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R. Relearning the ABC: Taxonomic Changes and Abundance/Biomass Relationships in Disturbed Benthic Communities. Mar. Biol. 1994, 118, 739–744. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication, 1st ed.; The University of Illinois Press: Urbana, IL, USA, 1964; pp. 1–131. [Google Scholar]
- Reiss, H.; Kröncke, I. Seasonal Variability of Benthic Indices: An Approach to Test the Applicability of Different Indices for Ecosystem Quality Assessment. Mar. Pollut. Bull. 2005, 50, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Zettler, M.L.; Schiedek, D.; Bobertz, B. Benthic Biodiversity Indices versus Salinity Gradient in the Southern Baltic Sea. Mar. Pollut. Bull. 2007, 55, 258–270. [Google Scholar] [CrossRef]
- Magni, P.; Vesal, S.E.; Giampaoletti, J.; Como, S.; Gravina, M.F. Joint Use of Biological Traits, Diversity and Biotic Indices to Assess the Ecological Quality Status of a Mediterranean Transitional System. Ecol. Indic. 2023, 147, 109939. [Google Scholar] [CrossRef]
- Yan, J.; Sui, J.; Xu, Y.; Li, X.; Wang, H.; Zhang, B. Assessment of the Benthic Ecological Status in Adjacent Areas of the Yangtze River Estuary, China, Using AMBI, M-AMBI and BOPA Biotic Indices. Mar. Pollut. Bull. 2020, 153, 111020. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fan, B.; Huang, Y.; Yu, P.; Li, Y.; Chen, M.; Cai, M.; Lv, W.; Jiang, Q.; Zhao, Y. Assessing the Ecological Health of the Chongming Dongtan Nature Reserve, China, Using Different Benthic Biotic Indices. Mar. Pollut. Bull. 2019, 146, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Khedhri, I.; Afli, A.; Aleya, L. Structuring Factors of the Spatio-Temporal Variability of Macrozoobenthos Assemblages in a Southern Mediterranean Lagoon: How Useful for Bioindication Is a Multi-Biotic Indices Approach? Mar. Pollut. Bull. 2017, 114, 515–527. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Esposito, V.; Giacobbe, S.; Renzi, M.; Mangano, M.C.; Vivona, P.; Consoli, P.; Scotti, G.; Andaloro, F.; Romeo, T. Ecological Assessment of a Heavily Human-Stressed Area in the Gulf of Milazzo, Central Mediterranean Sea: An Integrated Study of Biological, Physical and Chemical Indicators. Mar. Pollut. Bull. 2016, 106, 260–273. [Google Scholar] [CrossRef]
- Ryu, J.; Hong, S.; Chang, W.K.; Khim, J.S. Performance Evaluation and Validation of Ecological Indices toward Site-Specific Application for Varying Benthic Conditions in Korean Coasts. Sci. Total Environ. 2016, 541, 1161–1171. [Google Scholar] [CrossRef]
- Yao, K.-S.; He, Y.-J.; Van Den Brink, P.J.; Dai, Y.-N.; Yang, Y.; Cui, Y.-D. Multivariate Approaches to Assess the Drivers of Benthic Macroinvertebrate Communities and Biotic Indices in a Highly Urbanized Fluvial-Estuarine System. Ecol. Indic. 2022, 139, 108956. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Wang, X.; Bidegain, G.; Sun, X.; Bian, X.; Zhang, X. Assessment of the Benthic Ecological Quality Status (EcoQs) of Laizhou Bay (China) with an Integrated AMBI, M−AMBI, BENTIX, BO2A and Feeding Evenness Index. Ecol. Indic. 2023, 153, 110456. [Google Scholar] [CrossRef]
- Jung, R.-H.; Seo, I.-S.; Lee, W.-C.; Kim, H.-C.; Park, S.-R.; Kim, J.-B.; Oh, C.-W.; Choi, B.-M. Community Structure and Health Assessment of Macrobenthic Assemblages at Spring and Summer in Cheonsu Bay, West Coast of Korea. Sea J. Korean Soc. Oceanogr. 2014, 19, 272–286. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, K.H.; Shim, J.; Han, J.H.; Choi, Y.H.; Khang, B.-J. Massive Sedimentation of Fine Sediment with Organic Matter and Enhanced Benthic–Pelagic Coupling by an Artificial Dyke in Semi-Enclosed Chonsu Bay, Korea. Mar. Pollut. Bull. 2012, 64, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Choi, M.S.; Yang, S.L.; Ma, C.W.; Park, S.C. Final Report on the Environmental Survey and Fishery Utilization Study of Cheonsu Bay. Hongseong, Republic of Korea, 2011; p. 95. [Google Scholar]
- Kim, S.K. Assessment of Long-Term Variation in Macrobenthic Community and Ecological Quality Status in the Sutbital Zone of Cheonsu Bay. Master’s Thesis, Soonchunhyang University, Asan, Republic of Korea, 2023. [Google Scholar]
- National Institute of Fisheries Science. National Institute of Fisheries Science Notification of Marine Environmental Process Test Standards 2010; National Institute of Fisheries Science: Busan, Republic of Korea, 2010; Available online: https://www.nifs.go.kr/board/actionBoard0052List.do?BBS_CL_CD=ALL (accessed on 19 November 2023).
- Xu, Z.L.; Cheng, Y.Y. Aggregated Intensity of Dominant Species of Zooplankton in Autumn in the East China Sea and Yellow Sea. J. Ecol. 1989, 8, 13–15. [Google Scholar]
- Borja, A.; Dauer, D.M.; Díaz, R.; Llansó, R.J.; Muxika, I.; Rodríguez, J.G.; Schaffner, L. Assessing Estuarine Benthic Quality Conditions in Chesapeake Bay: A Comparison of Three Indices. Ecol. Indic. 2008, 8, 395–403. [Google Scholar] [CrossRef]
- Borja, A.; Tunberg, B.G. Assessing Benthic Health in Stressed Subtropical Estuaries, Eastern Florida, USA Using AMBI and M-AMBI. Ecol. Indic. 2011, 11, 295–303. [Google Scholar] [CrossRef]
- Seo, J.-Y.; Lim, H.-S.; Choi, J.-W. Threshold Value of Benthic Pollution Index (BPI) for a Muddy Healthy Benthic Faunal Community and Its Application to Jinhae Bay in the Southern Coast of Korea. Ocean Sci. J. 2014, 49, 313–328. [Google Scholar] [CrossRef]
- Blanchet, H.; Lavesque, N.; Ruellet, T.; Dauvin, J.C.; Sauriau, P.G.; Desroy, N.; Desclaux, C.; Leconte, M.; Bachelet, G.; Janson, A.-L.; et al. Use of Biotic Indices in Semi-Enclosed Coastal Ecosystems and Transitional Waters Habitats—Implications for the Implementation of the European Water Framework Directive. Ecol. Indic. 2008, 8, 360–372. [Google Scholar] [CrossRef]
- Marín-Guirao, L.; Cesar, A.; Marín, A.; Lloret, J.; Vita, R. Establishing the Ecological Quality Status of Soft-Bottom Mining-Impacted Coastal Water Bodies in the Scope of the Water Framework Directive. Mar. Pollut. Bull. 2005, 50, 374–387. [Google Scholar] [CrossRef]
- Monserud, R.A.; Leemans, R. Comparing Global Vegetation Maps with the Kappa Statistic. Ecol. Modell. 1992, 62, 275–293. [Google Scholar] [CrossRef]
- Medeiros, J.P.; Chaves, M.L.; Silva, G.; Azeda, C.; Costa, J.L.; Marques, J.C.; Costa, M.J.; Chainho, P. Benthic Condition in Low Salinity Areas of the Mira Estuary (Portugal): Lessons Learnt from Freshwater and Marine Assessment Tools. Ecol. Indic. 2012, 19, 79–88. [Google Scholar] [CrossRef]
- Luo, X.X.; Yang, J.Q. Progress in Researches on Benthic Indices of Assessing Marine Ecosystem Health. Mari. Sci. Bull. 2009, 28, 106–112. [Google Scholar]
- Simboura, N.; Reizopoulou, S. An Intercalibration of Classification Metrics of Benthic Macroinvertebrates in Coastal and Transitional Ecosystems of the Eastern Mediterranean Ecoregion (Greece). Mar. Pollut. Bull. 2008, 56, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Simboura, N.; Papathanassiou, E.; Sakellariou, D. The Use of a Biotic Index (Bentix) in Assessing Long-Term Effects of Dumping Coarse Metalliferous Waste on Soft Bottom Benthic Communities. Ecol. Indic. 2007, 7, 164–180. [Google Scholar] [CrossRef]
- Lee, J.Y. Changes in the Water Quality (Nutrients, Organic Materials and Trace Metals) by Freshwater Discharge from Artificial Lake in Cheonsu Bay. Master’s Thesis, Chungnam National University, Daejeon, Republic of Korea, 2020. [Google Scholar]
- Wu, H.-Y.; Fu, S.-F.; Hu, W.-J.; Chen, F.-G.; Cai, X.-Q.; Chen, Q.-H.; Wu, Y.-B. Response of Different Benthic Biotic Indices to Eutrophication and Sediment Heavy Metal Pollution, in Fujian Coastal Water, East China Sea. Chemosphere 2022, 307, 135653. [Google Scholar] [CrossRef] [PubMed]
- Mulik, J.; Sukumaran, S.; Dias, H.Q. Can the Ecological Status of Three Differentially Impacted Monsoonal Tropical Estuaries in NW India, Be Adequately Assessed by a Common Estuarine Benthic Index? Ecol. Indic. 2020, 119, 106807. [Google Scholar] [CrossRef]
- Kim, J.H. Spatiotemporal Distribution of Benthic Polychaetous Community in Gwangyng Bay, Southern Coast of Korea Late-2000′s -Focused on Investigated Data from December 2007 and April 2008. Master’s Thesis, Chonnam National University, Gwangju, Republic of Korea, 2010. [Google Scholar]
- Park, S.W. Seasonal and Spatial Distribution of Benthic Polychaetous Community on the South-Eastern Coastal Region, Korea. Master’s Thesis, Chonnam National University, Gwangju, Republic of Korea, 2014. [Google Scholar]
- Jo, J.G. Spatial Distribution of Benthic Polychaetous Communities on the South-Eastern Continental Shelf, South Sea of Korea. Master’s Thesis, Chonnam National University, Gwangju, Republic of Korea, 2016. [Google Scholar]
- Son, C.R. Spatio-Temporal Variation of Macrobenthic Community on Muchangpo Tidal Flat of the West Coast of Korea. Master’s Thesis, Chonnam National University, Gwangju, Republic of Korea, 2023. [Google Scholar]
- Kim, J.C. Benthic Environment and Community Structure of Macrobenthos at the Tidal Flats in Chungnam, Korea. Ph.D. Thesis, Soonchunhyang University, Asan, Republic of Korea, 2010. [Google Scholar]
- Park, S.H. Macrobenthic Community of Tidal Flat in Cheonsu Bay, South Korea. Master’s Thesis, Soonchunhyang University, Asan, Republic of Korea, 2021. [Google Scholar]
- Seo, J.Y. A Study on the Determination of Threshold Value of Benthic Community Health and Application of Benthic Pollution Index (BPI) to Special Management Areas the Southern Coasts of Korea. Ph.D. Thesis, Pusan National University, Pusan, Republic of Korea, 2016. [Google Scholar]
- Jeon, S.R.; Ong, G.; Koo, J.-H.; Park, J.-W.; Kim, Y.C.; Jeung, H.D.; Cho, J.K. Comparison of the Seawater-Sediment Environment and Habitat Properties with Variable Mud Shrimp Upogebia major Burrow Hole Density and Its Influence on Recruitment and Settlement in the Cheonsu Bay Tidal Flats. Korean J. Fish. Aquat. Sci. 2022, 55, 171–182. [Google Scholar] [CrossRef]
- Borja, Á.; Marín, S.L.; Muxika, I.; Pino, L.; Rodríguez, J.G. Is there a possibility of ranking benthic quality assessment indices to select the most responsive to different human pressures? Mar. Pollut. Bull. 2015, 97, 85–94. [Google Scholar] [CrossRef]





| Environmental Factors | Max | Min | Mean | SD |
|---|---|---|---|---|
| AVS, mg/g | 0.11 | 0 | 0.022 | 0.03 |
| COD, mg/g | 17.75 | 3.77 | 9.30 | 3.74 |
| IL, % | 4.84 | 1.51 | 2.84 | 0.90 |
| Gravel, % | 2.18 | 0 | 0.22 | 0.53 |
| Sand, % | 77.4 | 0 | 31.88 | 26.22 |
| Silt, % | 14.5 | 88.93 | 49.47 | 27.35 |
| Clay, % | 42.23 | 7.66 | 18.42 | 9.28 |
| Mean grain, ∅ | 7.37 | 2.53 | 5.38 | 1.24 |
| Season | Taxa | Specie | Dominant Value |
|---|---|---|---|
| Summer | Eumalacostraca | Upogebia major | 0.185 |
| Sedentaria | Heteromastus filiformis | 0.078 | |
| Eumalacostraca | Macrophthalmus japonicus | 0.023 | |
| Autumn | Eumalacostraca | Macrophthalmus japonicus | 0.096 |
| Sedentaria | Heteromastus filiformis | 0.042 | |
| Nemertea | Nemertea | 0.032 | |
| Caenogastropoda | Nassarius sp. | 0.021 |
| Indices | Kappa Value | %Match | Level of Agreement |
|---|---|---|---|
| AMBI/M-AMBI | 0.307 | 82.5 | Low |
| AMBI/BENTIX | 0.538 | 92.5 | Moderate |
| AMBI/BOPA | 0.787 | 97.5 | Very good |
| AMBI/BPI | 0.655 | 65.5 | Good |
| AMBI/H’ | 0.061 | 42.5 | Very Low |
| AMBI/W-value | 0.032 | 65 | Null |
| M-AMBI/BENTIX | 0.489 | 85 | Moderate |
| M-AMBI/BOPA | 0.437 | 85 | Moderate |
| M-AMBI/BPI | 0.162 | 80 | Very Low |
| M-AMBI/H’ | 0.297 | 60 | Low |
| M-AMBI/W-value | 0.304 | 70 | Low |
| BENTIX/BOPA | 0.734 | 95 | Very good |
| BENTIX/BPI | 0.304 | 90 | Low |
| BENTIX/H’ | 0.158 | 50 | Very low |
| BENTIX/W-value | 0.015 | 60 | Null |
| BOPA/BPI | 0.481 | 95 | Moderate |
| BOPA/H’ | 0.093 | 45 | Very low |
| BOPA/W-value | −0.016 | 60 | Null |
| BPI/H’ | 0.03 | 40 | Null |
| BPI/W-value | 0.082 | 65 | Very low |
| H’/W-value | 0.435 | 70 | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Ma, C.-W.; Kim, S.-K.; Park, S.-H. Assessing the Benthic Ecological Quality in the Intertidal Zone of Cheonsu Bay, Korea, Using Multiple Biotic Indices. Water 2024, 16, 272. https://doi.org/10.3390/w16020272
Liang J, Ma C-W, Kim S-K, Park S-H. Assessing the Benthic Ecological Quality in the Intertidal Zone of Cheonsu Bay, Korea, Using Multiple Biotic Indices. Water. 2024; 16(2):272. https://doi.org/10.3390/w16020272
Chicago/Turabian StyleLiang, Jian, Chae-Woo Ma, Seon-Kyu Kim, and Sang-Hyeok Park. 2024. "Assessing the Benthic Ecological Quality in the Intertidal Zone of Cheonsu Bay, Korea, Using Multiple Biotic Indices" Water 16, no. 2: 272. https://doi.org/10.3390/w16020272





