A New Chemiluminescence Assay for Hypochlorite Detection in Water: A Synergistic Combination of WS2 Quantum Dots and Luminol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Chemiluminescence Analysis
2.2.1. Reactive Chlorine Species: Hypochlorite
2.2.2. Reactive Oxygen Species: Hydrogen Peroxide
2.2.3. Reactive Oxygen Species: Hydrogen Peroxide and Fe(II)
2.3. Statistics
3. Results and Discussion
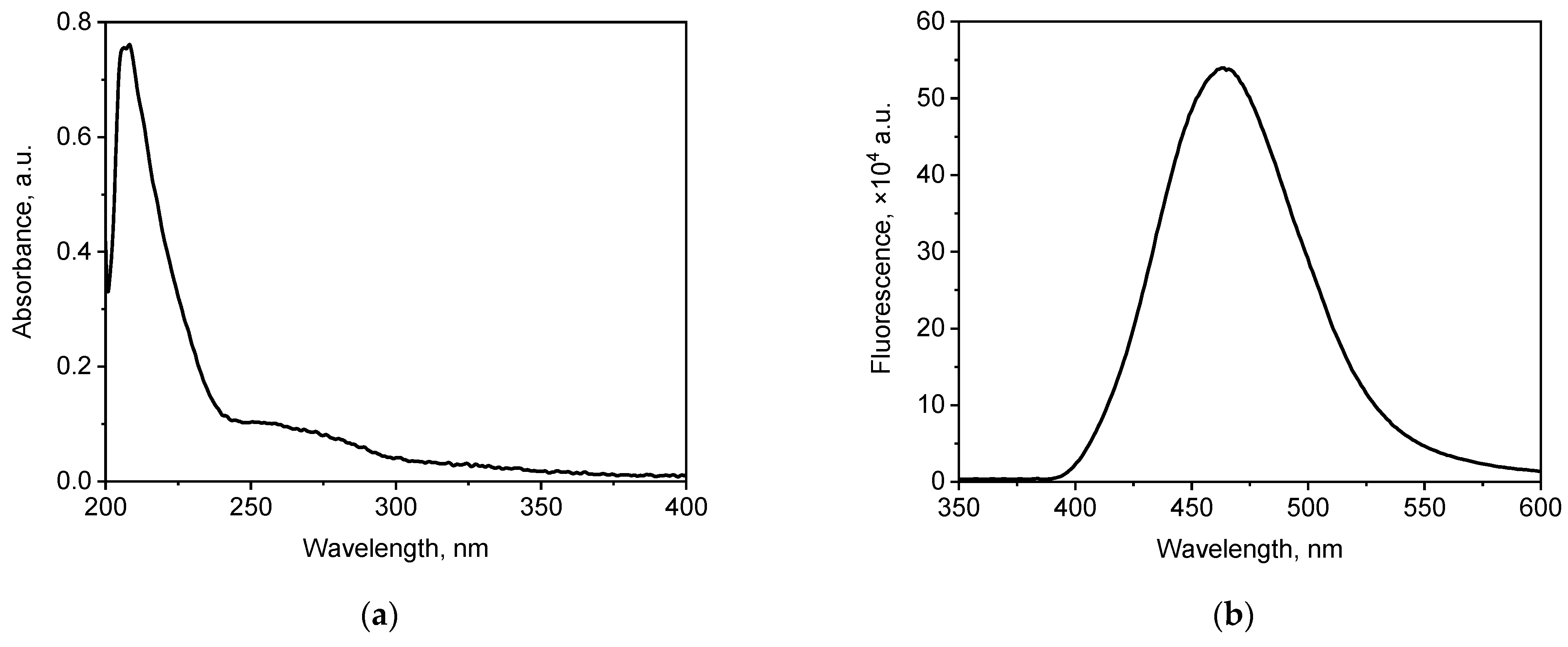
3.1. Characterisation of WS2 QDs
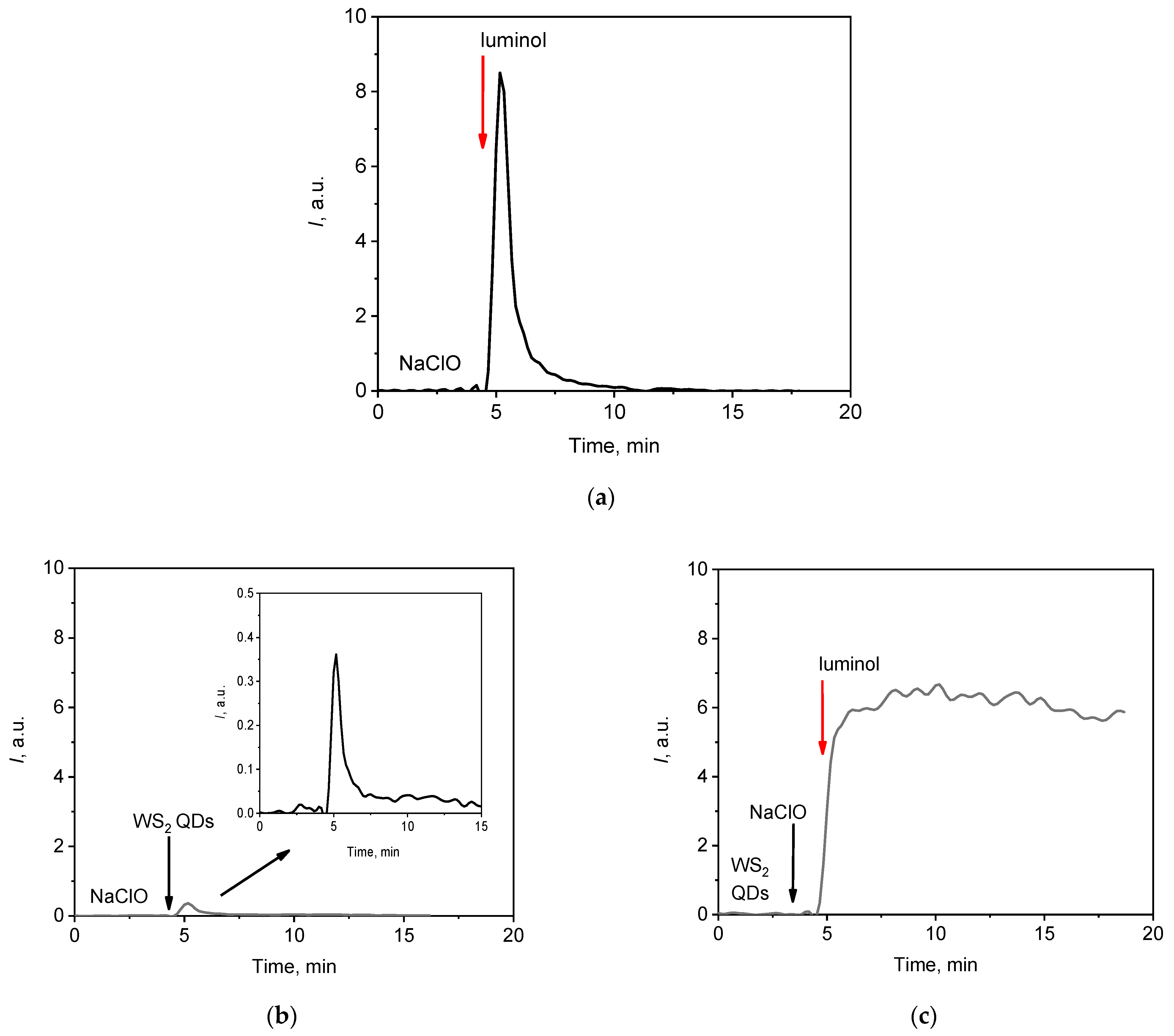
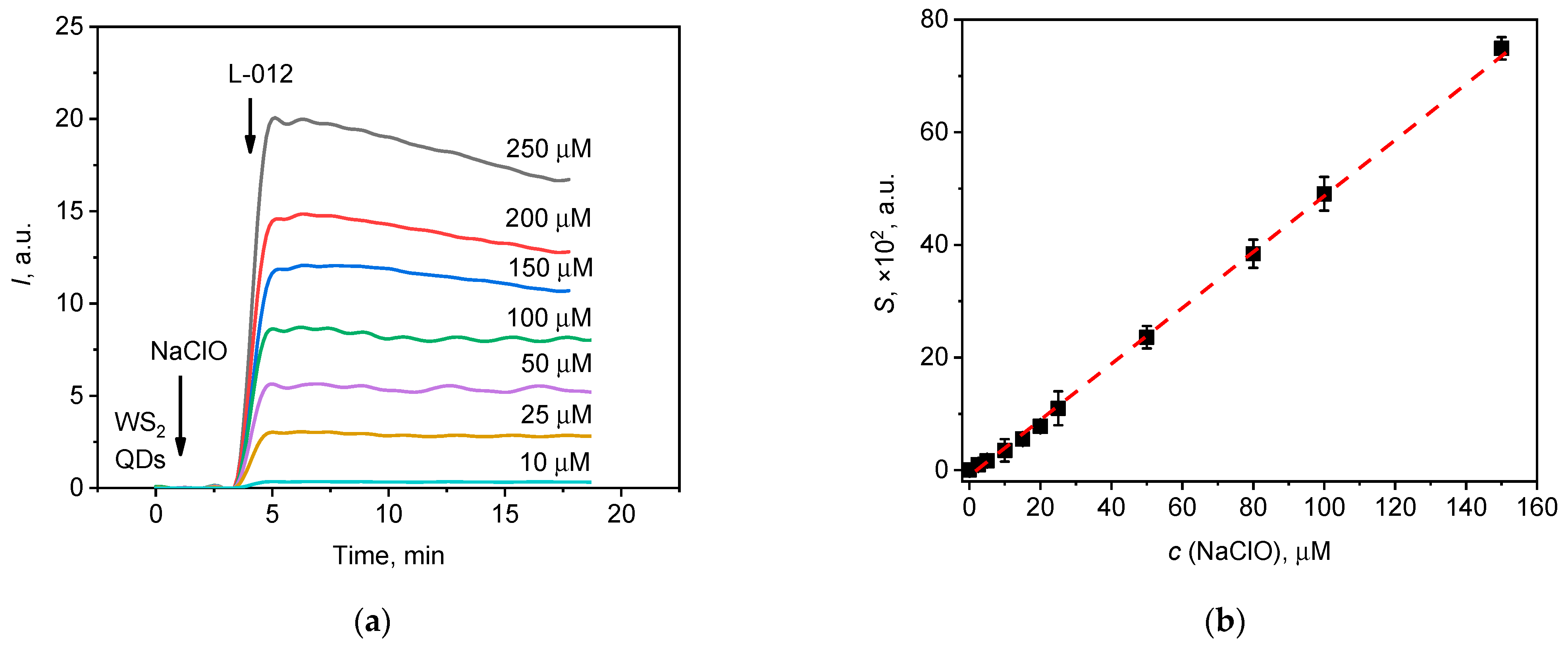
3.2. WS2 QDs as a Chemiluminescence Probe for the Detection of Hypochlorite
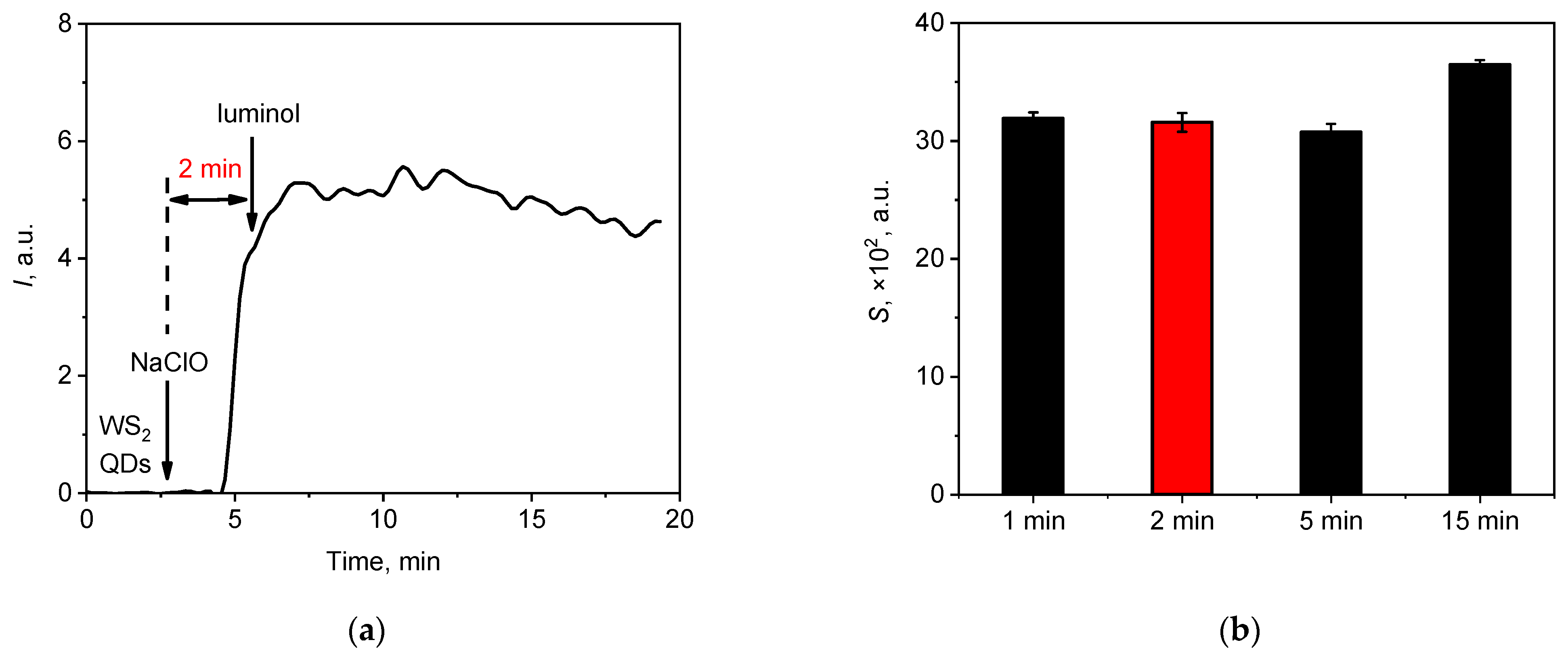
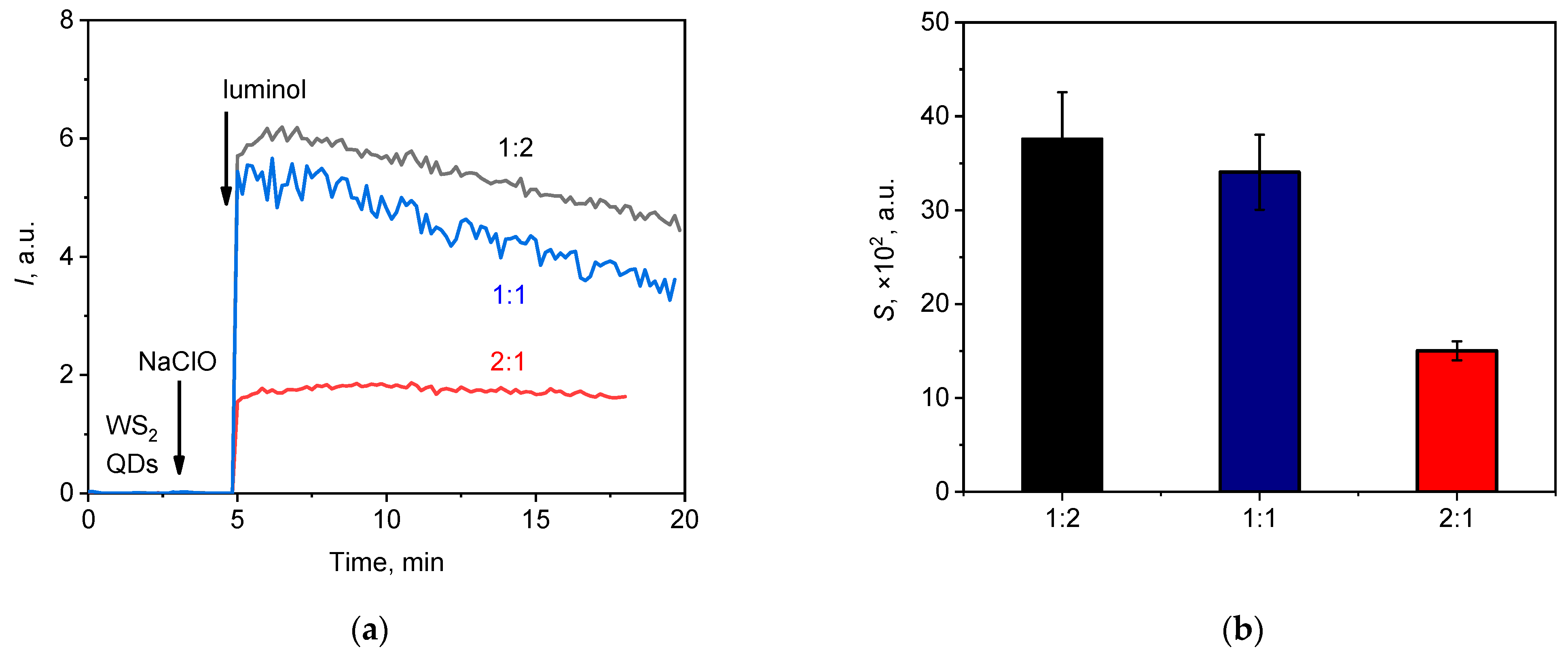
3.2.1. The Kinetic Profile of Chemiluminescence: Effects of Variables
3.2.2. The Possible Mechanism of Chemiluminescent Reactions Between NaClO and Luminol/WS2 QDs
3.2.3. Analytical Performance of NaClO/WS2 QDs/Luminol and NaClO/WS2 QDs/L-012 Probes
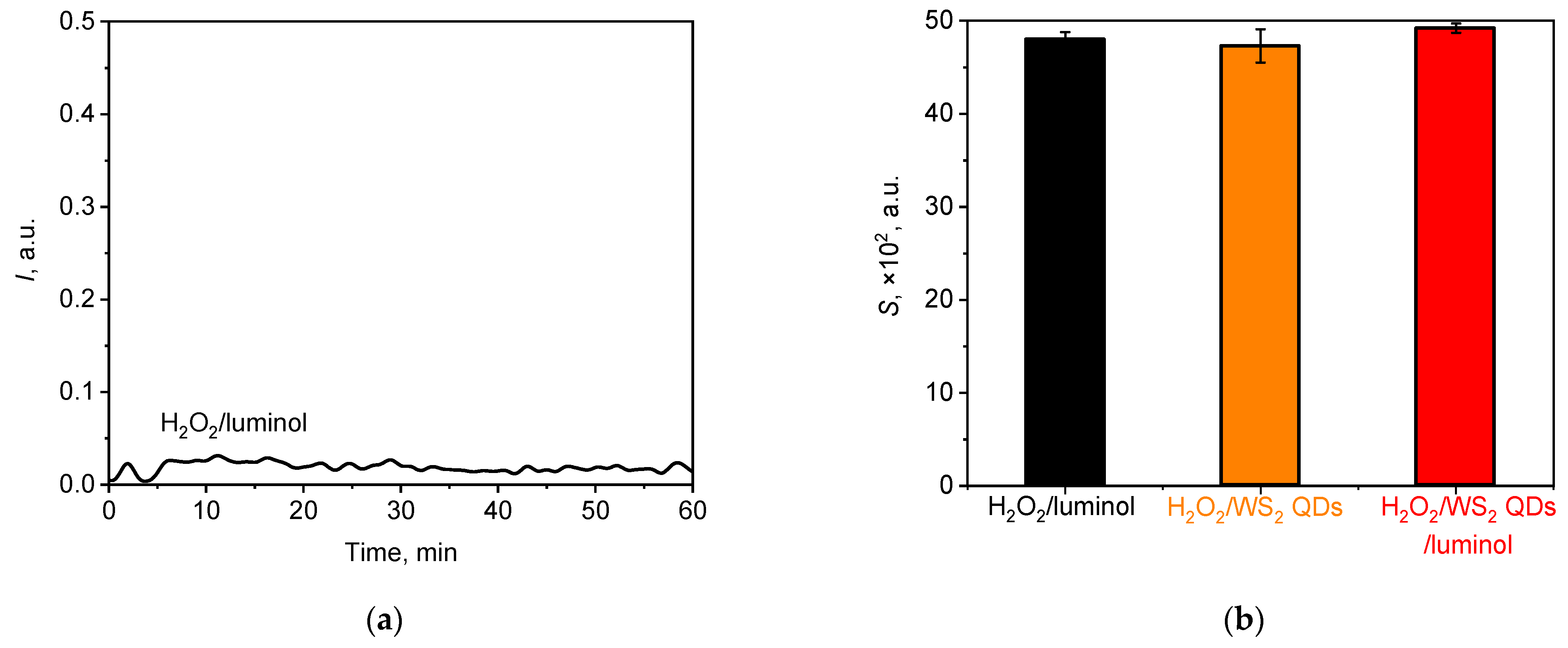
3.3. Analysis of the Interference of Hydrogen Peroxide
3.3.1. Hydrogen Peroxide
3.3.2. Hydrogen Peroxide in the Presence of Ferrous Ions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A comprehensive review on reactive oxygen species (ROS) in advanced oxidation processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, M.; Zhang, Y.; Fang, F.; Li, M.; An, F.; Zhao, D.; Zhang, J. Free radical as a double-edged sword in disease: Deriving strategic opportunities for nanotherapeutics. Coord. Chem. Rev. 2023, 475, 214875. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced oxidation processes for the removal of antibiotics from water. An overview. Water 2019, 12, 102. [Google Scholar] [CrossRef]
- Dong, H.; Chen, J.; Feng, L.; Zhang, W.; Guan, X.; Strathmann, T.J. Degradation of organic contaminants through activating bisulfite by cerium (IV): A sulfate radical-predominant oxidation process. Chem. Eng. J. 2019, 357, 328–336. [Google Scholar] [CrossRef]
- Dong, C.; Ji, J.; Shen, B.; Xing, M.; Zhang, J. Enhancement of H2O2 decomposition by the co-catalytic effect of WS2 on the Fenton reaction for the synchronous reduction of Cr(VI) and remediation of phenol. Environ. Sci. Technol. 2018, 52, 11297–11308. [Google Scholar] [CrossRef]
- Zhu, L.; Ji, J.; Liu, J.; Mine, S.; Matsuoka, M.; Zhang, J.; Xing, M. Designing 3D-MoS2 sponge as excellent cocatalysts in advanced oxidation processes for pollutant control. Angew. Chem. 2020, 132, 14072–14080. [Google Scholar] [CrossRef]
- Abramov, V.O.; Abramova, A.V.; Cravotto, G.; Nikonov, R.V.; Fedulov, I.S.; Ivanov, V.K. Flow-mode water treatment under simultaneous hydrodynamic cavitation and plasma. Ultrason. Sonochem. 2021, 70, 105323. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Martinez, M.C.; Andriantsitohaina, R. Reactive nitrogen species: Molecular mechanisms and potential significance in health and disease. Antioxid. Redox Signal. 2009, 11, 669–702. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Vladimirov, Y.A.; Proskurnina, E.V.; Izmajlov, D.Y. Kinetic chemiluminescence as a method for study of free radical reactions. Biophysics 2011, 56, 1055–1062. [Google Scholar] [CrossRef]
- Vladimirov, Y.A.; Proskurnina, E.V. Free radicals and cell chemiluminescence. Biochemistry 2009, 74, 1545–1566. [Google Scholar] [CrossRef]
- Halliwell, B. Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: Measurement, mechanism and the effects of nutrition. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 443, 37–52. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar]
- Zhao, Z. Iron and oxidizing species in oxidative stress and Alzheimer’s disease. Aging Med. 2019, 2, 82–87. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Torres, V.E. Reactive oxygen species and redox signaling in chronic kidney disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P. Evolution of the knowledge of free radicals and other oxidants. Oxid. Med. Cell. Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef]
- Ma, L.; Sun, S.; Wang, Y.; Jiang, K.; Zhu, J.; Li, J.; Lin, H. A graphene quantum dot-based fluorescent nanoprobe for hypochlorite detection in water and in living cells. Microchim. Acta 2017, 184, 3833–3840. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, M.; Yuan, Z. Methods for the detection of reactive oxygen species. Anal. Methods 2018, 10, 4625–4638. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Harrison, D.G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal. 2014, 20, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Gil-Guzman, E.; Mahran, A.M.; Sharma, R.K.; Nelson, D.R.; Agarwa, A. Quality control of reactive oxygen species measurement by luminol-dependent chemiluminescence assay. J. Androl. 2001, 22, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Kishikawa, N.; Ohyama, K.; Ohba, Y.; Kohno, M.; Masuda, T.; Takadate, A.; Nakashima, K.; Kuroda, N. Evaluation of chemiluminescence reagents for selective detection of reactive oxygen species. Anal. Chim. Acta 2010, 665, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.; Idrees, D.; Moxley, M.A.; Corbett, J.A.; Ahmad, F.; von Figura, G.; Sly, W.S.; Waheed, A.; Hassan, M.I. Luminol-based chemiluminescent signals: Clinical and non-clinical application and future uses. Appl. Biochem. Biotechnol. 2014, 173, 333–355. [Google Scholar] [CrossRef]
- Marquette, C.A.; Blum, L.J. Applications of the luminol chemiluminescent reaction in analytical chemistry. Anal. Bioanal. Chem. 2006, 385, 546–554. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, L.; Huang, J.; Xia, L.; Cui, M.; Zhang, X.; Gu, Y.; Wang, P. Chemiluminescence chitosan hydrogels based on the luminol analog L-012 for highly sensitive detection of ROS. Talanta 2019, 201, 455–459. [Google Scholar] [CrossRef]
- Daiber, A.; August, M.; Baldus, S.; Wendt, M.; Oelze, M.; Sydow, K.; Kleschyov, A.L.; Munzel, T. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic. Biol. Med. 2004, 36, 101–111. [Google Scholar] [CrossRef]
- Zielonka, J.; Lambeth, J.D.; Kalyanaraman, B. On the use of L-012, a luminol-based chemiluminescent probe, for detecting superoxide and identifying inhibitors of NADPH oxidase: A reevaluation. Free Radic. Biol. Med. 2013, 65, 1310–1314. [Google Scholar] [CrossRef]
- Chang, T.; Wu, L. Methylglyoxal, oxidative stress, and hypertension. Can. J. Physiol. Pharmacol. 2006, 84, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Aliaga, A.; Carcamo, J.; Gómez-Jeria, J.S.; Sanchez-Cortes, S.; Campos-Vallette, M.; Garcia-Ramos, J. Functionalization of Ag nanoparticles with the bis-acridinium lucigenin as a chemical assembler in the detection of persistent organic pollutants by surface-enhanced Raman scattering. Anal. Chim. Acta 2008, 624, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, X.-y.; Hai, X.; Song, W.; Ding, C.; Cao, J.; Bi, S. Chemiluminescence resonance energy transfer: From mechanisms to analytical applications. TrAC Trends Anal. Chem. 2020, 123, 115755. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum dots and their multimodal applications: A review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Chowdhury, T.; Sadler, E.C.; Kempa, T.J. Progress and prospects in transition-metal dichalcogenide research beyond 2D. Chem. Rev. 2020, 120, 12563–12591. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wu, J.; Zhang, Z.; Liao, Q.; Kang, Z.; Zhang, Y. Single-atom engineering to ignite 2D transition metal dichalcogenide based catalysis: Fundamentals, progress, and beyond. Chem. Rev. 2021, 122, 1273–1348. [Google Scholar] [CrossRef]
- Niknam, S.; Dehdast, S.A.; Pourdakan, O.; Shabani, M.; Koohi, M.K. Tungsten disulfide nanomaterials (WS2 NM) application in biosensors and nanomedicine: A review. Nanomed. Res. J. 2022, 7, 214–226. [Google Scholar]
- Ding, J.; Feng, A.; Li, X.; Ding, S.; Liu, L.; Ren, W. Properties, preparation, and application of tungsten disulfide: A review. J. Phys. D Appl. Phys. 2021, 54, 173002. [Google Scholar] [CrossRef]
- Yong, Y.; Cheng, X.; Bao, T.; Zu, M.; Yan, L.; Yin, W.; Ge, C.; Wang, D.; Gu, Z.; Zhao, Y. Tungsten sulfide quantum dots as multifunctional nanotheranostics for in vivo dual-modal image-guided photothermal/radiotherapy synergistic therapy. ACS Nano 2015, 9, 12451–12463. [Google Scholar] [CrossRef]
- He, Z.; Sheng, Y.; Rong, Y.; Lee, G.-D.; Li, J.; Warner, J.H. Layer-dependent modulation of tungsten disulfide photoluminescence by lateral electric fields. ACS Nano 2015, 9, 2740–2748. [Google Scholar] [CrossRef]
- Yan, Z.; Poh, E.T.; Zhang, Z.; Chua, S.T.; Wang, X.; Wu, X.; Chen, Z.; Yang, J.; Xu, Q.-H.; Goh, K.E.J. Band Nesting Bypass in WS2 Monolayers via Forster Resonance Energy Transfer. ACS Nano 2020, 14, 5946–5955. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jin, X.; Vdovenko, M.; Zhang, L.; Sakharov, I.Y.; Zhao, S. A WS2 nanosheet based chemiluminescence resonance energy transfer platform for sensing biomolecules. Chem. Commun. 2015, 51, 11092–11095. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Su, Y.; Sun, M.; Lv, Y. Homologous chemiluminescence resonance energy transfer on the interface of WS2 quantum dots for monitoring photocatalytic H2O2 evaluation. Microchem. J. 2021, 168, 106344. [Google Scholar] [CrossRef]
- Sun, T.; Su, Y.; Song, H.; Lv, Y. New advanced oxidation progress with chemiluminescence behavior based on NaClO triggered by WS2 nanosheets. J. Hazard. Mater. 2022, 429, 128329. [Google Scholar] [CrossRef] [PubMed]
- Ulfig, A.; Leichert, L.I. The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell. Mol. Life Sci. 2021, 78, 385–414. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic. Biol. Med. 2000, 29, 403–409. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Spickett, C.; Jerlich, A.; Panasenko, O.; Arnhold, J.; Pitt, A.; Stelmaszyńska, T.; Schaur, R. The reactions of hypochlorous acid, the reactive oxygen species produced by myeloperoxidase, with lipids. Acta Biochim. Pol. 2000, 47, 889–899. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Vissers, M.C.; Kettle, A.J. Myeloperoxidase. Curr. Opin. Hematol. 2000, 7, 53–58. [Google Scholar] [CrossRef]
- Khor, Y.; Chong, S.S.; Raman, A.A.A. Recent developments and sustainability in monitoring chlorine residuals for water quality control: A critical review. RSC Sustain. 2024, 2, 2468–2485. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, S.; Huang, W.; Wang, F.; Fang, S.; Ge, R.; Zhang, Q.; Zhang, L.; Du, W.; Fang, F. Current status of hypochlorite technology on the wastewater treatment and sludge disposal: Performance, principals and prospects. Sci. Total Environ. 2022, 803, 150085. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.-G.; Yuan, Q.; Lv, P.; Chen, K. Research progress of small molecule fluorescent probes for detecting hypochlorite. Sensors 2021, 21, 6326. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Q.; Cao, F.; Zhao, Q.; Ji, X. Colorimetric detection of hypochlorite in tap water based on the oxidation of 3, 3′, 5, 5′-tetramethyl benzidine. Anal. Methods 2015, 7, 4055–4058. [Google Scholar] [CrossRef]
- How, Z.T.; Linge, K.L.; Busetti, F.; Joll, C.A. Organic chloramines in drinking water: An assessment of formation, stability, reactivity and risk. Water Res. 2016, 93, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Yang, X. Colorimetric determination of hypochlorite with unmodified gold nanoparticles through the oxidation of a stabilizer thiol compound. Analyst 2012, 137, 2806–2812. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Zen, J.-M. Free chlorine detection based on EC’mechanism at an electroactive polymelamine-modified electrode. Electrochem. Commun. 2014, 46, 87–90. [Google Scholar] [CrossRef]
- Hallaj, T.; Amjadi, M.; Manzoori, J.L.; Shokri, R. Chemiluminescence reaction of glucose-derived graphene quantum dots with hypochlorite, and its application to the determination of free chlorine. Microchim. Acta 2015, 182, 789–796. [Google Scholar] [CrossRef]
- Ding, Y.; Ling, J.; Cai, J.; Wang, S.; Li, X.; Yang, M.; Zha, L.; Yan, J. A carbon dot-based hybrid fluorescent sensor for detecting free chlorine in water medium. Anal. Methods 2016, 8, 1157–1161. [Google Scholar] [CrossRef]
- Ashton, T.D.; Jolliffe, K.A.; Pfeffer, F.M. Luminescent probes for the bioimaging of small anionic species in vitro and in vivo. Chem. Soc. Rev. 2015, 44, 4547–4595. [Google Scholar] [CrossRef]
- Gadgil, A. Drinking water in developing countries. Annu. Rev. Energy 1998, 23, 253–286. [Google Scholar] [CrossRef]
- Montgomery, M.A.; Elimelech, M. Water and sanitation in developing countries: Including health in the equation. Environ. Sci. Technol. 2007, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Arnold, B.F.; Colford Jr, J.M. Treating water with chlorine at point-of-use to improve water quality and reduce child diarrhea in developing countries: A systematic review and meta-analysis. Am. J. Trop. Med. Hyg. 2007, 76, 354–364. [Google Scholar] [CrossRef]
- Sobsey, M.; Handzel, T.; Venczel, L. Chlorination and safe storage of household drinking water in developing countries to reduce waterborne disease. Water Sci. Technol. 2003, 47, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Szili, M.; Kasik, I.; Matejec, V.; Nagy, G.; Kovacs, B. Poly (luminol) based sensor array for determination of dissolved chlorine in water. Sens. Actuators B Chem. 2014, 192, 92–98. [Google Scholar] [CrossRef]
- Irons, G.P.; Greenway, G.M. Investigation into the detection of chlorine species by Rhodamine 6G chemiluminescence with electrochemical modification. Analyst 1995, 120, 477–483. [Google Scholar] [CrossRef]
- Claver, J.B.; Mirón, M.V.; Capitán-Vallvey, L. Determination of hypochlorite in water using a chemiluminescent test strip. Anal. Chim. Acta 2004, 522, 267–273. [Google Scholar] [CrossRef]
- Ishimaru, N.; Lin, J.-M.; Yamada, M. Luminol-free chlorine chemiluminescence in an oil-in-water microemulsion medium. Anal. Commun. 1998, 35, 67–69. [Google Scholar] [CrossRef]
- Nakamura, M.M.; Coichev, N.; Lin, J.-M.; Yamada, M. Flow-injection investigation of the chemiluminescent reaction of bis (2, 4, 6-(trichlorophenyl) oxalate) with free chlorine. Anal. Chim. Acta 2003, 484, 101–109. [Google Scholar] [CrossRef]
- Francis, P.S.; Barnett, N.W.; Lewis, S.W.; Lim, K.F. Hypohalites and related oxidants as chemiluminescence reagents: A review. Luminescence 2004, 19, 94–115. [Google Scholar] [CrossRef]
- Sozarukova, M.M.; Proskurnina, E.V.; Ivanov, V.K. Prooxidant potential of CeO2 nanoparticles towards hydrogen peroxide. Nanosyst. Phys. Chem. Math. 2021, 12, 283–290. [Google Scholar] [CrossRef]
- Irani-nezhad, M.H.; Khataee, A.; Hassanzadeh, J.; Orooji, Y. A chemiluminescent method for the detection of H2O2 and glucose based on intrinsic peroxidase-like activity of WS2 quantum dots. Molecules 2019, 24, 689. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Liu, Q.; Su, X. Fluorometric determination of the activity of alkaline phosphatase based on a system composed of WS2 quantum dots and MnO2 nanosheets. Microchim. Acta 2019, 186, 839. [Google Scholar] [CrossRef] [PubMed]
- Proskurnina, E.V.; Izmailov, D.Y.; Sozarukova, M.M.; Zhuravleva, T.A.; Leneva, I.A.; Poromov, A.A. Antioxidant potential of antiviral drug umifenovir. Molecules 2020, 25, 1577. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jin, M.; Du, P.; Zhang, C.; Cui, X.; Zhang, Y.; Wang, J.; Jin, F.; She, Y.; Shao, H. A review of enhancers for chemiluminescence enzyme immunoassay. Food Agr. Immunol. 2017, 28, 315–327. [Google Scholar] [CrossRef]
- Kaczmarek, M. Types of enhancers of chemiluminescent reaction systems in the liquid phase. Recent advances, perspectives, mechanisms and analytical application; a review. J. Lumin. 2024, 275, 120800. [Google Scholar] [CrossRef]
- Kandi, D.; Martha, S.; Parida, K. Quantum dots as enhancer in photocatalytic hydrogen evolution: A review. Int. J. Hydrogen Energy 2017, 42, 9467–9481. [Google Scholar] [CrossRef]
- Chen, H.; Lin, L.; Li, H.; Lin, J.-M. Quantum dots-enhanced chemiluminescence: Mechanism and application. Coord. Chem. Rev. 2014, 263, 86–100. [Google Scholar] [CrossRef]
- Vahid, B.; Hassanzadeh, J.; Khodakarami, B. CdSe quantum dots-sensitized chemiluminescence system and quenching effect of gold nanoclusters for cyanide detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 212, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Zhang, G.; Xu, W.; Han, Y. Chemiluminescence resonance energy transfer in the luminol–CdTe quantum dots conjugates. J. Lumin. 2010, 130, 995–999. [Google Scholar] [CrossRef]
- Shah, S.N.A.; Zheng, Y.; Li, H.; Lin, J.-M. Chemiluminescence character of ZnS quantum dots with bisulphite-hydrogen peroxide system in acidic medium. J. Phys. Chem. C 2016, 120, 9308–9316. [Google Scholar] [CrossRef]
- Zou, C.; Chen, M.; Luo, X.; Zhou, H.; Yu, T.; Yuan, C. Enhanced photoluminescence of WS2/WO3 heterostructural QDs. J. Alloys Compd. 2020, 834, 155066. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.-Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D.A. The antioxidant glutathione. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2023; Volume 121, pp. 109–141. [Google Scholar]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K.; Bishen, S.M.; Mishra, H.; Khurana, A. Molecular and therapeutic insights of alpha-lipoic acid as a potential molecule for disease prevention. Rev. Bras. Farmacogn. 2023, 33, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.K.; Polezhaeva, O.S.; Tret’yakov, Y.D. Nanocrystalline ceria: Synthesis, structure-sensitive properties, and promising applications. Russ. J. Gen. Chem. 2010, 80, 604–617. [Google Scholar] [CrossRef]
- Zholobak, N.M.; Ivanov, V.K.; Shcherbakov, A.B. Interaction of nanoceria with microorganisms. In Nanobiomaterials in Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 419–450. [Google Scholar]
- Ou, X.; Karmakar, B.; Awwad, N.S.; Ibrahium, H.A.; Osman, H.-E.H.; El-Kott, A.F.; Abdel-Daim, M.M. Au nanoparticles adorned chitosan-modified magnetic nanocomposite: An investigation towards its antioxidant and anti-hepatocarcinoma activity in vitro. Inorg. Chem. Commun. 2022, 137, 109221. [Google Scholar] [CrossRef]
- Boomi, P.; Ganesan, R.; Prabu Poorani, G.; Jegatheeswaran, S.; Balakumar, C.; Gurumallesh Prabu, H.; Anand, K.; Marimuthu Prabhu, N.; Jeyakanthan, J.; Saravanan, M. Phyto-engineered gold nanoparticles (AuNPs) with potential antibacterial, antioxidant, and wound healing activities under in vitro and in vivo conditions. Int. J. Nanomed. 2020, 2020, 7553–7568. [Google Scholar] [CrossRef]
- Nakagama, T.; Yamada, M.; Hobo, T. Chemiluminescence sensor with uranine immobilized on an anion-exchange resin for monitoring free chlorine in tap water. Anal. Chim. Acta 1990, 231, 7–12. [Google Scholar] [CrossRef]
- Deepa, S.; Venkatesan, R.; Jayalakshmi, S.; Priya, M.; Kim, S.-C. Recent advances in catalyst-enhanced luminol chemiluminescence system and its environmental and chemical applications. J. Environ. Chem. Eng. 2023, 11, 109853. [Google Scholar] [CrossRef]
- Lee, J.; Seliger, H. Quantum yields of the luminol chemiluminescence reaction in aqueous and aprotic solvents. Photochem. Photobiol. 1972, 15, 227–237. [Google Scholar] [CrossRef]
- Guo, J.-Z.; Cui, H.; Zhou, W.; Wang, W. Ag nanoparticle-catalyzed chemiluminescent reaction between luminol and hydrogen peroxide. J. Photochem. Photobiol. A Chem. 2008, 193, 89–96. [Google Scholar] [CrossRef]
- Klopf, L.L.; Nieman, T.A. Effect of iron(II), cobalt(II), copper(II), and manganese(II) on the chemiluminescence of luminol in the absence of hydrogen peroxide. Anal. Chem. 1983, 55, 1080–1083. [Google Scholar] [CrossRef]
- Held, A.; Halko, D.; Hurst, J. Mechanisms of chlorine oxidation of hydrogen peroxide. J. Am. Chem. Soc. 1978, 100, 5732–5740. [Google Scholar] [CrossRef]
- Qin, X.; Zhuang, Y.; Shi, B.; Li, Y.; Shi, Y. Effect of residual chlorine on iron particle formation considering drinking water conditions. J. Environ. Chem. Eng. 2021, 9, 106377. [Google Scholar] [CrossRef]
- Huang, J.; Jones, A.; Waite, T.D.; Chen, Y.; Huang, X.; Rosso, K.M.; Kappler, A.; Mansor, M.; Tratnyek, P.G.; Zhang, H. Fe(II) redox chemistry in the environment. Chem. Rev. 2021, 121, 8161–8233. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Hanna, P.M.; Mason, R.P. The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic. Biol. Med. 1997, 22, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.E.; Tarr, M.A. Quantitation of hydroxyl radical during Fenton oxidation following a single addition of iron and peroxide. Chemosphere 2000, 41, 409–417. [Google Scholar] [CrossRef]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef]
- Huang, M.; Wang, X.; Liu, C.; Fang, G.; Gao, J.; Wang, Y.; Zhou, D. Mechanism of metal sulfides accelerating Fe(II)/Fe(III) redox cycling to enhance pollutant degradation by persulfate: Metallic active sites vs. reducing sulfur species. J. Hazard. Mater. 2021, 404, 124175. [Google Scholar] [CrossRef]
- Barinov, A.K.; Sugakov, G.K. Access to clean water in Africa. Herald. Russ. Acad. Sci. 2024, 94, 550–559. [Google Scholar] [CrossRef]




| Reaction Mixture | Detection Limit (M) | Linear Range (M) | pH | Reference |
|---|---|---|---|---|
| Graphene quantum dots/ClO− | 3 × 10−7 | 5.0 × 10−7–1 × 10−3 | 8.0 | [58] |
| Poly(luminol)/ClO−/H2O2 | 5 × 10−7 | 5 × 10−7–4 × 10−3 | 8.0 | [65] |
| Rhodamine 6G/ClO− | 1.2 × 10−6 | – | 8.7 | [66] |
| bis(2,4,6(trichlorophenyl)oxalate)/ClO− | 2 × 10−6 | 2 × 10−6–3 × 10−5 | 6.5 | [69] |
| Uranine/ClO− | 3 × 10−6 | 2 × 10−6–1 × 10−3 | 6.0 | [90] |
| Fluoresceinate/ClO− | 5.3 × 10−6 | 1.5 × 10−4–6.9 × 10−4 | 9.5 | [67] |
| WS2 QDs/luminol/ClO− | 8 × 10−5 | 2.5 × 10−4–1.8 × 10−3 | 7.4 | This work |
| WS2 QDs/L-012/ClO− | 2 × 10−6 | 2.5 × 10−6–1.5 × 10−4 | 7.4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sozarukova, M.M.; Proskurnina, E.V.; Kochneva, E.M.; Barinov, A.K.; Baranchikov, A.E.; Ivanov, V.K. A New Chemiluminescence Assay for Hypochlorite Detection in Water: A Synergistic Combination of WS2 Quantum Dots and Luminol. Water 2024, 16, 3044. https://doi.org/10.3390/w16213044
Sozarukova MM, Proskurnina EV, Kochneva EM, Barinov AK, Baranchikov AE, Ivanov VK. A New Chemiluminescence Assay for Hypochlorite Detection in Water: A Synergistic Combination of WS2 Quantum Dots and Luminol. Water. 2024; 16(21):3044. https://doi.org/10.3390/w16213044
Chicago/Turabian StyleSozarukova, Madina M., Elena V. Proskurnina, Ekaterina M. Kochneva, Andrey K. Barinov, Alexander E. Baranchikov, and Vladimir K. Ivanov. 2024. "A New Chemiluminescence Assay for Hypochlorite Detection in Water: A Synergistic Combination of WS2 Quantum Dots and Luminol" Water 16, no. 21: 3044. https://doi.org/10.3390/w16213044
APA StyleSozarukova, M. M., Proskurnina, E. V., Kochneva, E. M., Barinov, A. K., Baranchikov, A. E., & Ivanov, V. K. (2024). A New Chemiluminescence Assay for Hypochlorite Detection in Water: A Synergistic Combination of WS2 Quantum Dots and Luminol. Water, 16(21), 3044. https://doi.org/10.3390/w16213044







