Application of the River Habitat Survey Method in the Assessment of the Human Pressure Within the Lowland River Catchment: The Mollusc Biodiversity Versus Habitat Features
Abstract
1. Introduction
2. Materials and Methods
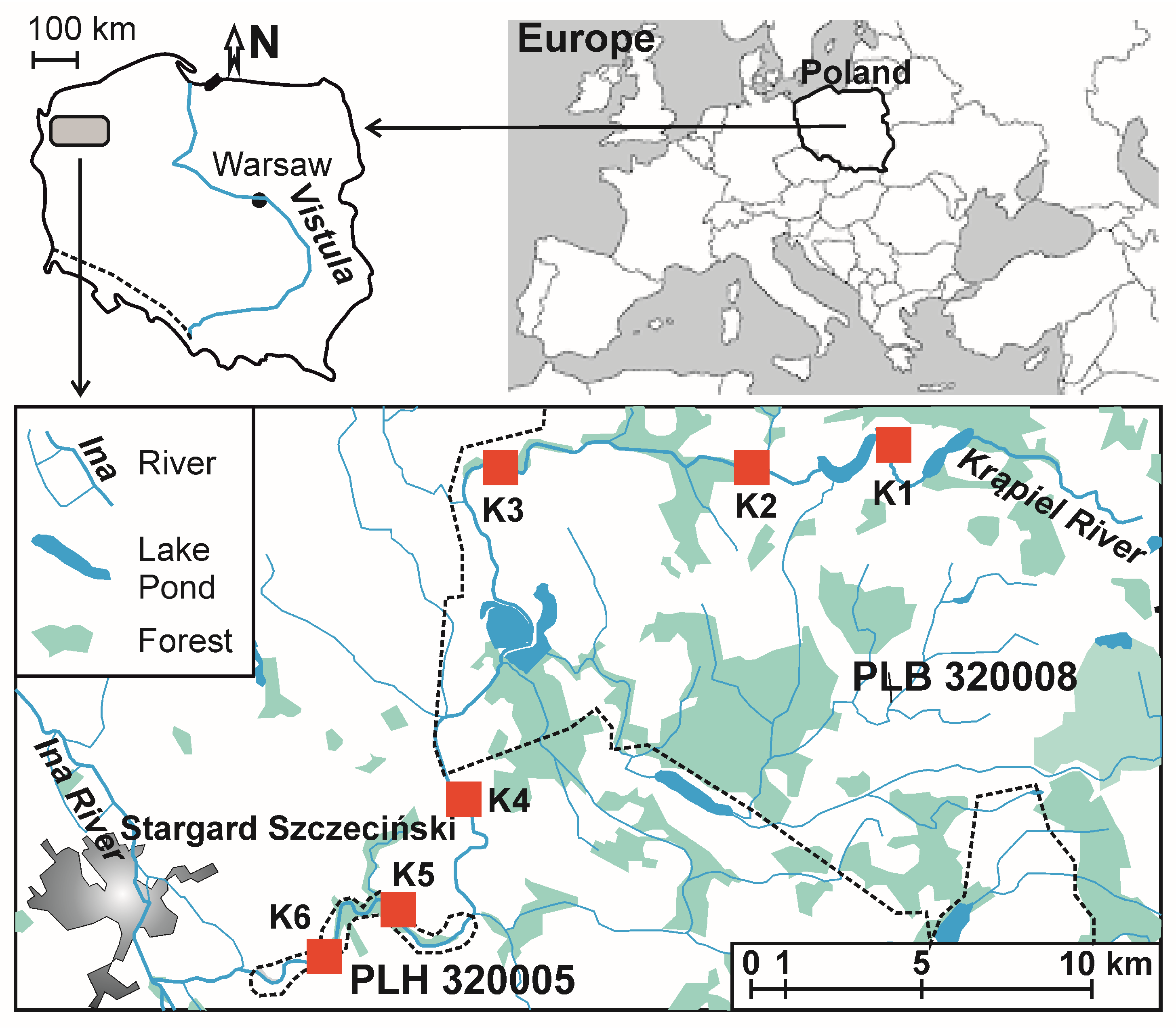
2.1. Study Area
2.2. Field and Laboratory Methods
- The Habitat Quality Assessment (HQA)
- The Habitat Modification Score (HMS)
- The River Habitat Quality (RHQ)
- The River Habitat Modification (RHM)
2.3. Statistical Analyses
3. Results
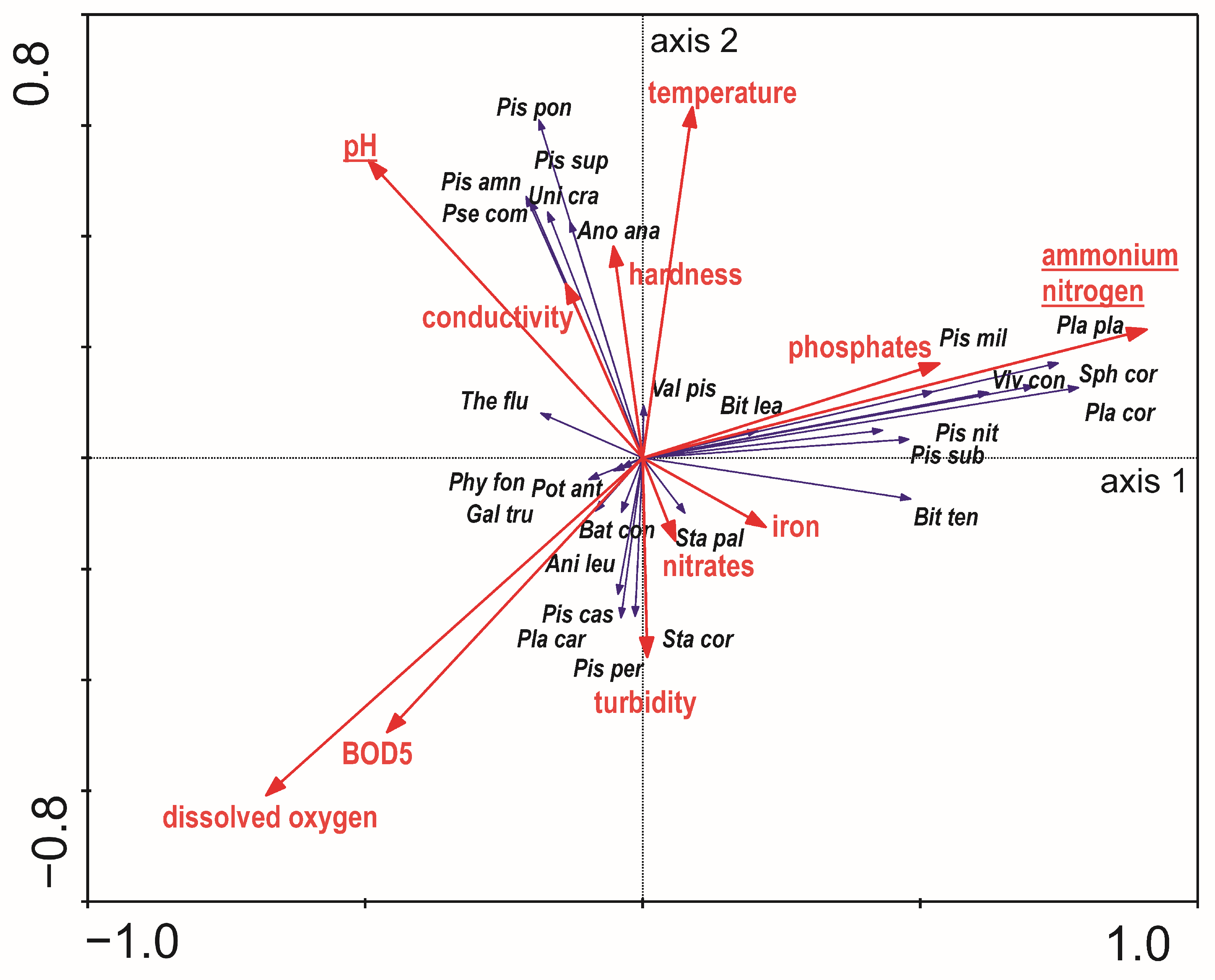
3.1. The Environmental Factors
3.2. The Structure of Mollusc Communities
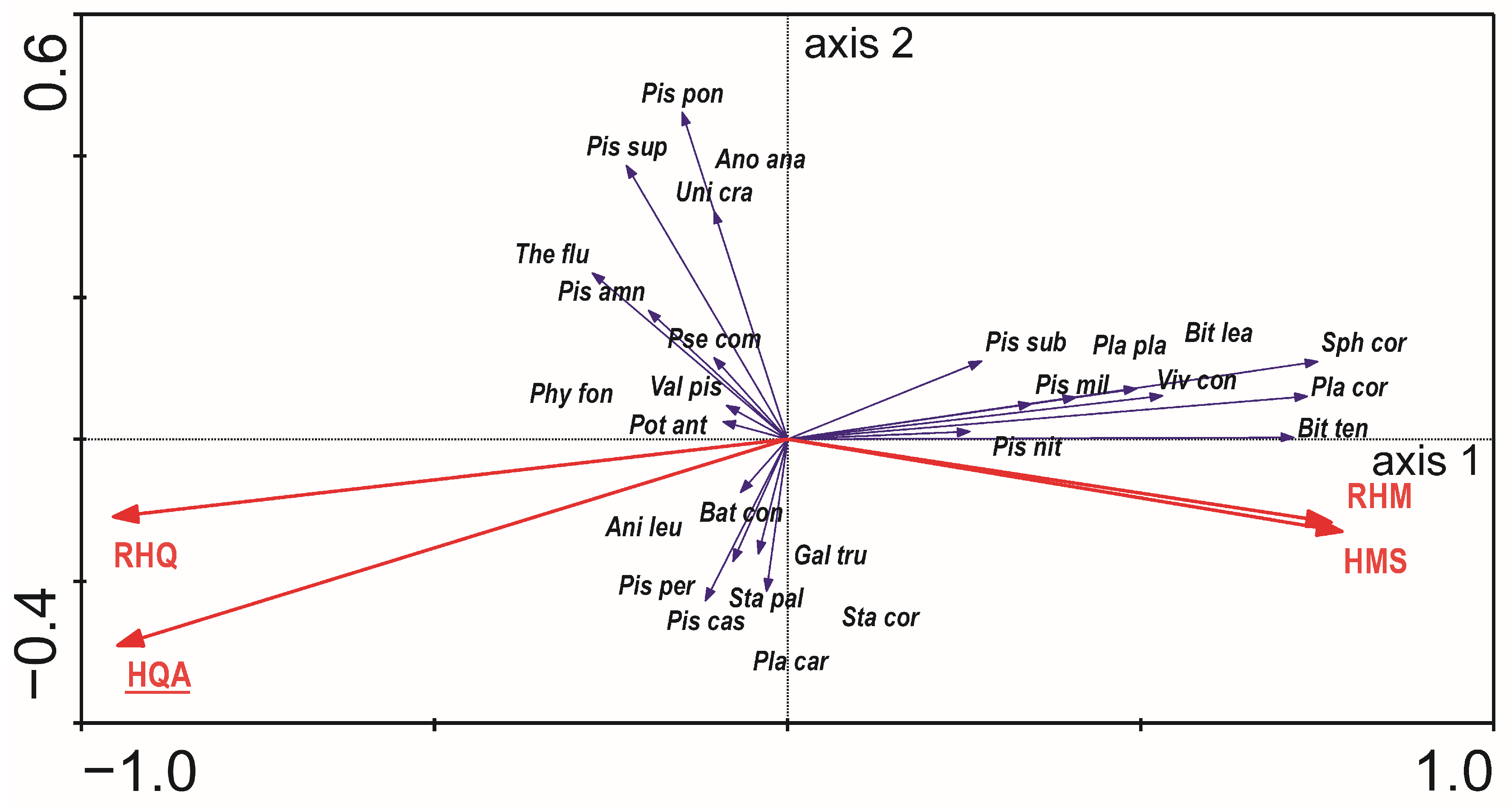
3.3. The Mollusc Communities Versus Habitat Features
4. Discussion
4.1. The Mollusc Communities: Rare and Threatened Species
4.2. The Mollusc Communities Versus Physical and Chemical Parameters of the Water
4.3. The Mollusc Communities Versus Habitat Features
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WWF. Living Planet Report 2022—Building a Nature-Positive Society; Almond, R.E.A., Grooten, M., Juffe Bignoli, D., Petersen, T., Eds.; WWF: Gland, Switzerland, 2022. [Google Scholar]
- Biodiversity Strategy 2030 Barrier Removal for River Restoration; European Commission, Publications Office of the European Union: Luxembourg, 2021.
- The IUCN Red List of Threatened Species, Version 2024-1. Available online: https://www.iucnredlist.org/ (accessed on 12 September 2024).
- Haag, W.R.; Williams, J.D. Biodiversity on the brink: An assessment of conservation strategies for North American freshwater mussels. Hydrobiologia 2014, 735, 45–60. [Google Scholar] [CrossRef]
- Hopper, G.W.; Bucholz, J.R.; DuBose, T.P.; Fogelman, K.J.; Keogh, S.M.; Kubala, M.E.; Lodato, M.B.; Nichols, D.H.; Sánchez González, I.; Pfeiffer, J.M.; et al. A trait dataset for freshwater mussels of the United States of America. Sci. Data 2023, 10, 745. [Google Scholar] [CrossRef] [PubMed]
- Ollard, I.; Aldridge, D.C. Declines in freshwater mussel density, size and productivity in the River Thames over the past half century. J. Anim. Ecol. 2023, 92, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, D.C.; Ollard, I.S.; Bespalaya, Y.V.; Bolotov, I.N.; Douda, K.; Geist, J.; Haag, W.R.; Klunzinger, M.W.; Lopes-Lima, M.; Mlambo, M.C.; et al. Freshwater mussel conservation: A global horizon scan of emerging threats and opportunities. Global Chang. Biol. 2023, 29, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Lima, M.; Reis, J.; Alvarez, M.G.; Anastácio, P.M.; Banha, F.; Beja, P.; Castro, P.; Gama, M.; Gil, M.G.; Gomes-dos-Santos, A.; et al. The silent extinction of freshwater mussels in Portugal. Biol. Conserv. 2023, 285, 110244. [Google Scholar] [CrossRef]
- Torres, S.H.; de Lucía, M.; Gutiérrez Gregoric, D.E.; Darrigran, G. Freshwater mussel conservation in southern South America: Update on distribution range and current threats. Aquat. Sci. 2024, 86, 38. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaya, Y.; Bódis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biol. Rev. 2017, 92, 572–607. [Google Scholar] [CrossRef]
- Szlauer-Łukaszewska, A.; Ławicki, Ł.; Engel, J.; Drewniak, E.; Ciężak, K.; Marchowski, D. Quantifying a mass mortality event in freshwater wildlife within the Lower Odra River: Insights from a large European river. Sci. Total Environ. 2024, 907, 167898. [Google Scholar] [CrossRef]
- Vaughn, C.C. Ecosystem services provided by freshwater mussels. Hydrobiologia 2018, 810, 15–27. [Google Scholar] [CrossRef]
- Zieritz, A.; Sousa, R.; Aldridge, D.C.; Douda, K.; Esteves, E.; Ferreira-Rodríguez, N.; Mageroy, J.H.; Nizzoli, D.; Osterling, M.; Reis, J.; et al. A global synthesis of ecosystem services provided and disrupted by freshwater bivalve molluscs. Biol. Rev. 2022, 97, 1967–1998. [Google Scholar] [CrossRef]
- Atkinson, C.L.; Hopper, G.W.; Kreeger, D.A.; Lopez, J.W.; Maine, A.N.; Sansom, B.J.; Schwalb, A.; Vaughn, C.C. Gains and gaps in knowledge surrounding freshwater mollusk ecosystem services. Freshw. Moll. Biol. Conserv. 2023, 26, 20–31. [Google Scholar] [CrossRef]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy; Official Journal of the European Communities (L327); Publications Office of the European Union: Luxembourg, 2000; pp. 1–72.
- Raven, P.J.; Holmes, N.T.H.; Dawson, F.H.; Everard, M. Quality assessment using River Habitat Survey data. Aquat. Conserv. 1998, 8, 477–499. [Google Scholar] [CrossRef]
- Raven, P.J.; Holmes, N.T.H.; Charrier, P.; Dawson, F.H.; Naura, M.; Boon, P.J. Towards a harmonized approach for hydromorphological assessment of rivers in Europe: A qualitative comparison of three survey methods. Aquat. Conserv. 2002, 12, 405–424. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Zgoła, T.; Jusik, S.; Hryc-Jusik, B.; Davson, F.H.; Raven, P. Hydromorfologiczna Ocean Wód Płynących. Podręcznik do Badan Terenowych Według Metody River Habitat Survey w Warunkach Polskich; Environment Agency, Poznań-Warrington: Poland, UK, 2012. [Google Scholar]
- Song, Y.; Song, S. Assessment of habitats on typical low hills in the Yitong River in Northeast China. J. Freshwater Ecol. 2017, 32, 591–599. [Google Scholar] [CrossRef]
- Costa, F.; Vieira, A. Decision Support Tools for River Restoration: The Implementation of the “River Habitat Survey” Methodology on the River Selho (Guimarães Municipality, Northwest Portugal). Hydrology 2021, 8, 69. [Google Scholar] [CrossRef]
- Directive 2009/147/EC of the European Parliament and of the Council of 30 November 2009 on the Conservation of Wild Birds (Codified Version); Official Journal of the European Union (L20/7); Publications Office of the European Union: Luxembourg, 2009; pp. 1–19.
- Piechocki, A. Mięczaki (Mollusca). Ślimaki (Gastropoda). Fauna Słodkowodna Polski, Zeszyt 7; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1979. [Google Scholar]
- Piechocki, A.; Dyduch-Falniowska, A. Mięczaki (Mollusca). Małże (Bivalvia). Fauna Słodkowodna Polski 7A; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1993. [Google Scholar]
- Glöer, P.; Meier-Brook, C. Süsswassermollusken. Ein Bestimmungsschlüssel für die Bundesrepublik Deutschland; Deutscher Jugendbund für Naturbeobachtung DJN: Hamburg, Germany, 1998. [Google Scholar]
- Glöer, P.; Mollusca, I. Süsswassergastropoden. Nord- und Mitteleuropas Bestimmungsschlüssel, Lebensweise, Verbreitung; ConchBooks: Hackenheim, Germany, 2002. [Google Scholar]
- Piechocki, A.; Wawrzyniak-Wydrowska, B. Guide to Freshwater and Marine Mollusca of Poland; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2016. [Google Scholar]
- Hermanowicz, W.; Dojlido, J.; Dożańska, W.; Koziorowski, B.; Zerbe, J. Fizyczno-Chemiczne Badanie Wody i Ścieków; Wydawnictwo Arkady: Warszawa, Poland, 1999. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde. Zweite, Umgearbeitete und Vermehrte Auflage; Springer-Verlag: Wien, NY, USA, 1964. [Google Scholar]
- Myślińska, E. Grunty Organiczne i Laboratoryjne Metody ich Badania; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2001. [Google Scholar]
- Environment Agency. River Habitat Survey in Britain and Ireland. In Field Survey Guidance Manual: 2003 Version; Environment Agency: Bristol, UK, 2003. [Google Scholar]
- Environment Agency. Hydromorfologiczna Ocena wód Płynących (River Habitat Survey); Environment Agency: Bristol, UK, 2007.
- Szoszkiewicz, K.; Gebler, D. Ocena warunków hydromorfologicznych rzek w Polsce metodą River Habitat Survey. Ochr. Sr. Zasobów Nat. 2011, 47, 70–81. [Google Scholar]
- Dziennik Ustaw. Journal of Laws of the Republic of Poland, Item 1475. Regulation of the Minister of Infrastructure of 25 June 2021 on the Classification Of Ecological Status, Ecological Potential and Chemical Status and the Method of Classifying the Status of Surface Water Bodies, as Well as Environmental Quality Standards for Priority Substances. 2021. Available online: https://dziennikustaw.gov.pl/DU/rok/2021/pozycja/1475 (accessed on 12 September 2024).
- Stefanidis, K.; Naura, M.; Kouvarda, T.; Latsiou, A.; Gritzalis, K.; Dimitriou, E. Expanding the habitat quality assessment of rivers in Greece using an updated River Habitat Survey toolbox. Ecohydrol. Hydrobiol. 2024, in press. [Google Scholar] [CrossRef]
- Tavzes, B.; Urbanič, G. New indices for assessment of hydromorphological alteration of rivers and their evaluation with benthic invertebrate communities; Alpine case study. Rev. Hydrobiol. 2009, 2, 133–161. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5), 2nd ed.; Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Lopes-Lima, M.; Prié, V.; Aldridge, D.C.; Urbańska, M. Pseudanodonta complanata. The IUCN Red List of Threatened Species. 2024: e.T211999791A212006932. Available online: https://www.iucnredlist.org/fr/species/211999791/212006932 (accessed on 2 September 2024).
- Lopes-Lima, M.; Prié, V.; Österling, M.; Zając, T.A. Unio crassus. The IUCN Red List of Threatened Species. 2024: e.T210291828A215467836. Available online: https://www.iucnredlist.org/species/210291828/215467836 (accessed on 2 September 2024).
- Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and Wild Fauna and Flora; Council of the European Communities: Brussels, Belgium, 1992.
- Hus, M.; Śmiałek, M.; Zając, K.; Zając, T. Occurrence of Unio crassus (Bivalvia, Unionidae) depending on water chemistry in the foreland of the Polish Carpathians. Pol. J. Environ. Stud. 2006, 15, 169–172. [Google Scholar]
- Denic, M.; Stoeckl, K.; Gum, B.; Geist, J. Physicochemical assessment of Unio crassus habitat quality in a small upland stream and implications for conservation. Hydrobiologia 2014, 735, 111–122. [Google Scholar] [CrossRef]
- Lewin, I. Mollusc communities of lowland rivers and oxbow lakes in agricultural areas with anthropogenically elevated nutrient concentration. Folia Malacol. 2014, 22, 87–159. [Google Scholar] [CrossRef]
- Vaessen, Q.; Houbrechts, G.; Campenhout, V.; Hambuckers, A. Which environmental factors influence the distribution patterns of an endangered freshwater mussel (Unio crassus)? Geomorphology 2024, 454, 109180. [Google Scholar] [CrossRef]
- Halabowski, D.; Sousa, R.; Lopes-Lima, M.; Killeen, I.; Aldridge, D.C.; Zając, K.; Mageroy, J.H.; Cossey, D.A.; Urbańska, M.; Österling, M.; et al. Off the conservation radar: The hidden story of Europe’s tiny pea clams (Bivalvia: Sphaeriidae). Biodivers. Conserv. 2024, 33, 3567–3581. [Google Scholar] [CrossRef]
- Piechocki, A. Gastropoda aquatica. Ślimaki wodne. In Czerwona Lista Zwierząt Ginących i Zagrożonych w Polsce. Red List of Threatened Animals in Poland; Głowaciński, Z., Ed.; Polish Academy of Sciences, Institute of Nature Conservation: Cracow, Poland, 2002; pp. 34–37. [Google Scholar]
- Beran, L.; Horsák, M. Distribution of Bithynia leachii (Sheppard, 1823) and Bithynia troschelii (Paasch, 1842) (Gastropoda: Bithyniidae) in the Czech Republic. Malacol. Bohemosl. 2009, 8, 19–23. [Google Scholar] [CrossRef]
- Radulović, S.; Laketić, D.; Vukov, D. A riverside tale: Assessment of altered habitat effects on macrophyte assemblage on the River Tamiš, Serbia. Arch. Biol. Sci. 2010, 62, 1163–1174. [Google Scholar] [CrossRef]
- Mouthon, J. Molluscs and biodegradable pollution in rivers: Proposal for a scale of sensitivity of species. Hydrobiologia 1996, 317, 221–229. [Google Scholar] [CrossRef]
- Økland, J. Factors regulating the distribution of fresh-water snails (Gastropoda) in Norway. Malacologia 1983, 24, 227–288. [Google Scholar]
- Ewald, M.L.; Feminella, J.W.; Lenertz, K.K.; Henry, R.P. Acute physiological responses of the freshwater snail Elimia flava (Mollusca: Pleuroceridae) to environmental pH and calcium. Comp. Biochem. Phys. C 2009, 150, 237–245. [Google Scholar] [CrossRef]
- Weber, E. Population size and structure of three mussel species (Bivalvia: Unionidae) in a northeastern German river with special regard to influences of environmental factors. Hydrobiologia 2005, 537, 169–183. [Google Scholar] [CrossRef]
- Douda, K. The Occurrence and Growth of Unio crassus (Mollusca: Bivalvia: Unionidae) in Lužnice River Basin in Respect to Water Quality. Acta Univ. Carol. Environ. 2007, 21, 57–63. [Google Scholar]
- Meier-Brook, C. Substrate relations in some Pisidium species (Eulamellibranchiata: Sphaeriidae). Malacologia 1969, 9, 121–125. [Google Scholar]
- Strayer, D.L. Understanding how nutrient cycles and freshwater mussels (Unionoida) affect one another. Hydrobiologia 2013, 1, 277–292. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Buffagni, A.; Davy-Bowker, J.; Lesny, J.; Chojnacki, B.H.; Zbierska, J.; Staniszewski, R.; Zgoła, T. Occurrence and variability of River Habitat Survey features across Europe and the consequences for data collection and evaluation. Hydrobiologia 2006, 566, 267–280. [Google Scholar] [CrossRef]
- Halabowski, D.; Lewin, I. Triggers for the Impoverishment of the Macroinvertebrate Communities in the Human-Impacted Rivers of Two Central European Ecoregions. Water Air Soil Poll. 2021, 232, 55. [Google Scholar] [CrossRef]
- Kiraga, M.J.; Markiewicz, A. A proposed quantitative method for assessing the impact of river regulation on its hydromorphological status. J. Water Land Develop. 2023, 57, 98–106. [Google Scholar] [CrossRef]
- Lewin, I.; Jusik, S.; Szoszkiewicz, K.; Czerniawska-Kusza, I.; Ławniczak, A.E. Application of the new multimetric MMI PL index for biological water quality assessment in reference and human-impacted streams (Poland, the Slovak Republic). Limnologica 2014, 49, 42–51. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Jusik, S.; Lewin, I.; Czerniawska-Kusza, I.; Kupiec, J.M.; Szostak, M. Macrophyte and macroinvertebrate patterns in unimpacted mountain rivers of two European ecoregions. Hydrobiologia 2018, 808, 327–342. [Google Scholar] [CrossRef]
- Kiraga, M.J. The Diversification of River Habitat Survey Output During the Four Seasons: Case Studies of Three Lowland Rivers in Poland. J. Ecol. Eng. 2020, 21, 116–126. [Google Scholar] [CrossRef]




| Parameter/ Unit | K1 | K2 | K3 | K4 | K5 | K6 | H-Value | p-Value |
|---|---|---|---|---|---|---|---|---|
| Temperature [°C] | 10.3–21.3 | 9.8–20.4 | 11.3–22.6 | 12.1–22.8 | 12.2–21.6 | 12.4–19.0 | 7.04 | 0.2173 |
| Conductivity [µS cm−1] | 142–188 *K5,6 | 158–208 *K6 | 157–242 *K6 | 188–242 | 195–246 *K1 | 205–345 *K1,2,3 | 25.62 | 0.0001 |
| Turbidity [mg dm−3] | 9.5–42.4 | 9.5–38.3 | 17.2–61.0 | 6.0–85.0 | 21.3–68.2 | 0.0–44.6 | 9.19 | 0.1016 |
| Dissolved oxygen [mg O2 dm−3] | 6.5–10.4 *K2 | 1.6–6.3 *K1,5,6 | 6.3–8.4 *K6 | 5.1–8.6 *K6 | 6.2–9.8 *K2 | 7.3–9.7 *K2,3,4 | 35.54 | 0.0001 |
| pH | 6.84–12.0 *K2 | 5.98–7.0 *K1,4,6 | 6.0–10.85 | 6.46–12.80 *K2 | 6.78–12.60 | 7.14–8.45 *K2 | 15.82 | 0.0074 |
| Ammonium nitrogen [mg N–NH4+ dm−3] | 0.10–0.56 | 0.18–2.48 | 0.28–0.70 *K5,6 | 0.25–0.41 | 0.18–0.34 *K3 | 0.18–0.40 *K3 | 17.94 | 0.0030 |
| Nitrates [mg NO3− dm–3] | 0.93–5.76 *K5 | 3.31–5.76 | 0.10–5.94 | 0.75–7.46 | 3.49–7.13 *K1 | 0.46–6.70 | 12.87 | 0.0247 |
| Phosphates [mg PO43– dm−3] | 0.02–0.25 *K2 | 0.24–0.75 *K1 | 0.13–0.71 | 0.26–0.49 | 0.04–1.30 | 0.04–0.31 | 15.03 | 0.0103 |
| Iron [mg Fe dm−3] | 0.06–0.20 | 0.10–0.29 | 0.14–0.41 *K4,6 | 0.02–0.10 *K3 | 0.09–0.35 | 0.08–0.37 *K3 | 23.56 | 0.0003 |
| Hardness [mg CaCO3 dm−3] | 44–217 | 103–208 | 103–256 | 118–231 | 90–171 | 127–288 | 13.56 | 0.0186 |
| BOD5 [mg O2 dm−3] | 0.0–8.1 *K2,3,4,5 | 0.0–5.6 *K1 | 2.3–5.1 *K1 | 3.7–5.5 *K1 | 1.4–6.1 *K1 | 3.7–6.2 | 19.24 | 0.0017 |
| Velocity [m s−1] | 0.003–0.397 | 0.011–0.250 | 0.001–0.589 | 0.124–0.358 | 0.004–0.672 | 0.047–0.541 | 5.21 | 0.3911 |
| Insolation [%] | 1.37–45.42 *K2 | 100.0–100.01 *K1,3,5 | 3.60–86.67 *K2 | 3.93–81.21 | 1.41–35.17 *K2 | 0.62–99.92 | 25.96 | 0.0001 |
| Organic [%] | 0.35–2.50 | 1.07–32.75 | 0.64–25.99 | 0.59–9.39 | 0.61–1.75 | 0.28–3.63 | 7.54 | 0.1835 |
| Mineral [%] | 97.51–99.65 | 67.25–98.93 | 74.01–99.36 | 90.61–99.41 | 98.25–99.39 | 96.37–99.72 | 7.54 | 0.1835 |
| Macrophytes cover | 0 | 0–5 | 0–5 | 0–3 | 0 | 0–2 | 10.96 | 0.0522 |
| Mean grain size of sediment M [mm] | 0.44–3.60 | 0.03–2.65 | 0.14–3.32 | 0.14–2.93 | 0.44–3.32 | 0.14–3.32 | 6.56 | 0.2551 |
| Sediment sorting W [%] | 0.30–1.75 | 0.94–1.75 | 0.53–1.75 | 0.94–1.38 | 0.37–1.38 | 0.13–1.27 | 10.29 | 0.0673 |
| Sampling Sections of the Krąpiel River | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa/Species | K1 | K2 | K3 | K4 | K5 | K6 | ||||||
| R | P | R | P | R | P | R | P | R | P | R | P | |
| Theodoxus fluviatilis | 50.68 | 77.50 | 30.99 | 46.15 | 6.25 | 57.14 | ||||||
| Viviparus contectus | 3.55 | 0.51 | ||||||||||
| Bithynia leachii | 1.14 | 0.35 | 8.23 | |||||||||
| Bithynia tentaculata | 51.14 | 24.82 | 29.31 | 7.69 | 6.25 | 21.05 | ||||||
| Potamopyrgus antipodarum | 5.83 | 6.25 | ||||||||||
| Valvata piscinalis | 2.74 | |||||||||||
| Galba truncatula | 15.38 | |||||||||||
| Radix juv. | 15.79 | |||||||||||
| Stagnicola corvus | 1.14 | |||||||||||
| Stagnicola palustris | 1.14 | |||||||||||
| Stagnicola sp. | 2.27 | 5.00 | ||||||||||
| Physa fontinalis | 2.74 | |||||||||||
| Anisus leucostoma | 47.37 | |||||||||||
| Bathyomphalus contortus | 7.14 | |||||||||||
| Gyraulus sp. | 1.41 | |||||||||||
| Planorbarius corneus | 5.68 | 3.19 | 10.28 | 1.37 | ||||||||
| Planorbis carinatus | 2.27 | |||||||||||
| Planorbis planorbis | 1.03 | |||||||||||
| Anodonta anatina | 1.41 | |||||||||||
| Pseudanodonta complanata | 2.82 | 7.69 | ||||||||||
| Unio crassus | 4.23 | |||||||||||
| Unio sp. | 1.41 | |||||||||||
| Pisidium amnicum | 2.74 | 2.50 | 1.41 | 30.77 | 18.75 | 15.38 | 7.14 | 5.26 | ||||
| Pisidium casertanum | 10.23 | 25.00 | 3.33 | 2.82 | 4.76 | |||||||
| Pisidium milium | 0.26 | |||||||||||
| Pisidium nitidum | 6.82 | 15.00 | 5.14 | 9.59 | 4.23 | 4.76 | ||||||
| Pisidium personatum | 35.00 | 5.48 | 2.38 | |||||||||
| Pisidium ponderosum | 2.82 | |||||||||||
| Pisidium subtruncatum | ||||||||||||
| Pisidium supinum | 1.14 | 5.00 | 9.59 | 6.67 | 26.76 | 12.50 | 7.14 | |||||
| Pisidium sp. | 9.09 | 15.00 | 5.66 | 5.48 | 8.45 | 7.69 | 10.53 | |||||
| Sphaerium corneum | 7.95 | 68.09 | 39.59 | 9.59 | 4.17 | 11.27 | 15.38 | 50.00 | 53.85 | 9.52 | ||
| Ʃ specimens | 88 | 20 | 282 | 389 | 73 | 120 | 71 | 13 | 16 | 13 | 42 | 19 |
| Ʃ taxa | 12 | 6 | 5 | 9 | 10 | 6 | 13 | 4 | 6 | 5 | 8 | 5 |
| The Shannon-Wiener index H′ | 2.48 | 2.28 | 1.24 | 2.27 | 2.44 | 1.27 | 2.86 | 1.74 | 2.08 | 1.88 | 2.15 | 1.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewin, I.; Śmietana, P.; Pakulnicka, J.; Stryjecki, R.; Stępień-Zawal, E.; Pešić, V.; Bańkowska, A.; Szlauer-Łukaszewska, A.; Michoński, G.; Achrem, M.; et al. Application of the River Habitat Survey Method in the Assessment of the Human Pressure Within the Lowland River Catchment: The Mollusc Biodiversity Versus Habitat Features. Water 2024, 16, 3448. https://doi.org/10.3390/w16233448
Lewin I, Śmietana P, Pakulnicka J, Stryjecki R, Stępień-Zawal E, Pešić V, Bańkowska A, Szlauer-Łukaszewska A, Michoński G, Achrem M, et al. Application of the River Habitat Survey Method in the Assessment of the Human Pressure Within the Lowland River Catchment: The Mollusc Biodiversity Versus Habitat Features. Water. 2024; 16(23):3448. https://doi.org/10.3390/w16233448
Chicago/Turabian StyleLewin, Iga, Przemysław Śmietana, Joanna Pakulnicka, Robert Stryjecki, Edyta Stępień-Zawal, Vladimir Pešić, Aleksandra Bańkowska, Agnieszka Szlauer-Łukaszewska, Grzegorz Michoński, Magdalena Achrem, and et al. 2024. "Application of the River Habitat Survey Method in the Assessment of the Human Pressure Within the Lowland River Catchment: The Mollusc Biodiversity Versus Habitat Features" Water 16, no. 23: 3448. https://doi.org/10.3390/w16233448
APA StyleLewin, I., Śmietana, P., Pakulnicka, J., Stryjecki, R., Stępień-Zawal, E., Pešić, V., Bańkowska, A., Szlauer-Łukaszewska, A., Michoński, G., Achrem, M., Krakowiak, M., Zawadzki, D., Chatterjee, T., & Zawal, A. (2024). Application of the River Habitat Survey Method in the Assessment of the Human Pressure Within the Lowland River Catchment: The Mollusc Biodiversity Versus Habitat Features. Water, 16(23), 3448. https://doi.org/10.3390/w16233448









