Abstract
Algae communities as primary producers are essential elements of aquatic ecosystems and contribute significantly to oxygen production, carbon dioxide fixation, and nutrient transport processes in water bodies. The use of algae-based carbon capture and storage technologies does not produce harmful by-products that require disposal, and the resulting algal biomass can be valuable across various industrial sectors. In this study, model experiments were conducted to develop sequential absorption–microalgae hybrid CO2-capture methods. To facilitate CO2 capture from flue gases, wood biomass ash (WBA), an agricultural by-product, was utilized for its alkaline properties, while the flue gas scrubbing medium was regenerated by algae that restored alkalinity during their growth. In our experiments, one of our goals was to determine the optimal conditions for achieving maximum algal biomass growth in the shortest possible time. The suitability of WBA for flue gas cleaning was tested via simulation of CO2 introduction. Moreover, a method was developed to determine the dissolved inorganic carbon content with the use of an OxiTop device monitoring the changes in pressure. The applied device was a closed, static, and pressure-based respirometer originally designed to determine the biological activity of microorganisms in both solid and liquid samples. In addition, the effects of CO2-enriched WBA extract on algae cultivation were also analyzed, confirming that it imposed no growth inhibition and identifying the concentration (10% WBA) that optimally promoted algal growth. The optimal initial algal concentration and nutrient conditions for maximum growth were also determined.
1. Introduction
Global carbon dioxide (CO2) emissions have been a critical environmental issue for decades, driven largely by human activities such as the burning of fossil fuels (e.g., coal, oil, and natural gas) used for energy production, transportation, and industrial processes. Additional sources include agriculture, waste disposal, garbage incineration, residential sector, and mining [1]. The mean global concentration of CO2 in the atmosphere had increased from 280 ppm in the 1700s to 380 ppm in 2005 [2] and to 427 ppm by the year 2023 [3]. CO2 has a long atmospheric lifetime of 50 to 200 years [4]. However, only about 45% of emitted CO2 remains in the atmosphere [5], while the rest is absorbed by the ocean (25%) [6] and by terrestrial ecosystems such as forests (30%) [7]. This balance can fluctuate annually due to natural climate cycles and other influencing factors [8]. Deforestation also contributes to increased CO2 levels in the atmosphere, since forests are significant factors in CO2 absorption [9,10]. The emission of primary greenhouse gases (CO2, CH4, and N2O), is a key driver of climate change, since greenhouse gases can trap and retain thermal radiation in the Earth’s atmosphere, leading to global warming [1].
The wide scale of emission sources highlights the importance of adopting strategies to reduce greenhouse gas emissions. These strategies are essential for addressing climate change and advancing sustainable development. International efforts to reduce emissions, especially the Kyoto Protocol in 1997 and the Paris Agreement in 2015, play a decisive role in forming a collective front against climate change [11]. Meanwhile, the European Union has adopted a net emissions reduction target of 55% by 2030. This objective sets the foundation for the EU to attain climate neutrality by 2050 [12]. While some countries and sectors have made efforts to reduce their carbon footprint through renewable energy (including wind, solar, and hydropower), energy efficiency, carbon capture technologies, and reforestation, global CO2 emissions continue to grow, particularly in developing economies, posing a major challenge for climate action. Total emissions amounted to 38.52 gigatons of CO2 equivalents (GtCO2eq) in 2022, highlighting the urgent need for collective efforts to mitigate the adverse effects of climate change [1]. Global CO2 emissions from industry and fossil fuels were 37.15 billion metric tons (GtCO2) in 2022. It is expected that emissions will increase by 1.1% in 2023 to a record high of 37.55 GtCO2 [13]. According to the Emissions Database for Global Atmospheric Research, the power industry is the highest contributor, with 38.1% of overall emissions, followed by transport (20.7%) and industrial combustion (17%) [1,14]. From the early 1970s to 2022, there was a consistent trend of increasing emissions of greenhouse gases (CO2 represents 73.5% of the total emissions). However, a temporary reduction in CO2 emissions was observed due to the COVID-19 pandemic in 2020, although a new increase was observed from 2021 [15,16,17]. The ongoing increase in atmospheric CO2 levels presents serious risks including biodiversity loss, rising sea levels, and more extreme weather events. The observed continuously increasing tendency indicates the urgent need for global collaboration and effective measures to decrease emissions of CO2 and other greenhouse gases, to mitigate the adverse effects of anthropogenic activities on the global climate and environment [1].
Based on the previous ‘European Green Deal’ and the ‘European Climate Law’, the production and utilization of renewable energy sources have also been emphasized [12,18,19,20]. Notably, bioenergy remains the predominant source of renewable energy within the EU, despite the significant growth of wind and solar energy in the past decade. Biomass for energy purposes accounts for nearly 60% of renewable energy sources in the EU. In 2017, forest biomass accounted for 69% of the total biomass used for energy production [21,22]. According to global estimates, 900–1000 million tons of energy waste are generated annually, with 476 million tons of ash from biomass combustion. In the EU, this amount reaches 100 million tons per year. Consequently, it seems reasonable to reuse the ash from biomass combustion for various applications, including agriculture, remediation of degraded land, environmental protection, zeolite synthesis, recovery of rare earth metals, and plastic production [23]. The utilization of cement kiln dust (CKD) in carbon capture technologies has long been a focus of research [24,25,26], due to the well-documented and significant contribution of the cement industry to global CO2 emissions. However, the quality of CKD can vary significantly based on the combusted materials. Therefore, the application of wood biomass ash (WBA), which can be more precisely qualitatively characterized, has emerged as a favorable alternative [27]. This by-product serves as a valuable fertilizer component, as WBA is rich in essential nutrients (e.g., calcium (Ca), potassium (K), and microelements). However, it may also contain environmentally harmful substances, including heavy metals and combustion-derived substances, such as polycyclic aromatic hydrocarbons (PAHs) and volatile organic compounds (VOCs) [23,28].
Our approach promotes the use of WBA as agricultural waste to facilitate CO2 capture through its alkaline properties, while regenerating the flue gas scrubber medium via algae, which re-alkalizes the medium during its growth. The increase in pH is attributed to the external conversion of HCO3− into CO2 and OH− during photosynthesis, facilitated by algal cells with a high affinity for CO2 uptake [29]. Microalgae are highly efficient at CO2 fixation, absorbing considerably more CO2 per area than terrestrial plants, which are limited by soil, water, and climate requirements. Controlled microalgae cultures can minimize moisture loss, leading to higher photosynthetic efficiency and enhanced CO2 absorption [30]. Carbon rarely limits algal growth under natural conditions [31], but the low atmospheric CO2 concentration (~0.042%) is insufficient for intensive algal cultivation. To overcome this limitation, artificial carbon sources such as NaHCO3 or compressed CO2 are usually added, although these can be costly [32]. Therefore, the current research focuses on cost-effective, sustainable sources of CO2, such as power plant flue gas, which contains up to 15% CO2 [33,34,35], and biogas from anaerobic digestion [36,37,38].
Algal communities in aquatic ecosystems serve as primary producers, constitute the majority of biomass, and play an important role in the carbon cycle and oxygen production. Moreover, they are essential elements of aquatic food webs and nutrient transport processes [30,31]. Due to their fast growth and sensitivity to changes in water quality, algae are often used as indicator organisms during ecotoxicological testing to evaluate the possible environmental risks arising from water contaminants. In addition to the crucial importance of algae in surface waters, algal communities play a multifaceted role in the sustainable management of water resources. Algae contribute significantly to the maintenance and improvement of water quality. They are natural biofilters capable of absorbing, removing, and accumulating nutrients and certain water pollutants (e.g., phenols, pesticide active ingredients, and hydrocarbons). Therefore, algal species offer a sustainable approach to water remediation. However, the massive proliferation of certain species of algae can lead to eutrophication of surface waters, resulting in the deterioration of water quality. The potential of algae in the water–energy nexus is increasingly being recognized, as they offer a renewable resource for biofuel production. Algae play a crucial role in carbon sequestration by absorbing carbon dioxide from the atmosphere and incorporating it into their biomass [39]. Thus, the application of microalgae-based carbon capture and storage (CCS) technology can contribute to mitigating climate change.
The effects of elevated CO2 concentrations on the growth and viability of several microalgae strains, including Arthrospira platensis, Chlorella ellipsoidea, C. vulgaris, Gloeotila pulchra, and Elliptochloris subsphaerica, were examined. Cultures were grown at constant CO2 levels (0.04%, 3%, 6%, and 9%), and the highest biomass growth rate (0.37 g L−1 day−1) was observed for Chlorella vulgaris at 3% CO2 [39]. The impact of elevated CO2 levels on algal growth varied. A 95% increase in dry weight was observed for C. vulgaris with 1% CO2-enriched air compared with the control, supporting the application of high CO2 levels for maximum productivity [40]. However, under extreme CO2 levels (40%), extracellular carbonic anhydrase (CA) activity decreased significantly, with almost no intracellular activity [41]. CO2 fixation rates of 0.93 and 0.69 g L−1 day−1 were achieved with 2 and 1 g L−1 NaHCO3 in 100 mL flasks, respectively [42]. CO2 bubbling resulted in significantly higher growth rates, while aerated cultures reached a value of 0.045–0.075 g L−1. The application of model flue gas with 15% CO2 resulted in a growth rate that was nearly ten times higher (0.624 g L−1 day−1) [43]. Nevertheless, lipid content and biomass productivity decreased as CO2 concentration increased (2–15% CO2) [44]. Conversely, C. vulgaris adapted rapidly to 5–12% CO2 levels with no effects on photosynthesis [45], while growth of C. vulgaris was indicated at 18% CO2, suggesting its potential for CO2 mitigation in flue gas emissions [46]. Additionally, CO2 supplementation can prevent pH inhibition in carbon-limited cultures [31,47]. According to another study, a membrane photobioreactor fixed CO2 0.95–5.40 times more efficiently than conventional bioreactors [48]. The proposed system used a sequential method, where the CO2 scrubbing and solution regeneration through the algal carbon fixation phase were separated.
Microalgae-based CCS technology presents a promising solution, as microalgae are estimated to produce about half of atmospheric oxygen while simultaneously using CO2 for photoautotrophic growth [49]. Microalgae-based CCS strategies offer several advantages. Microalgae grow faster and fix CO2 more effectively than conventional plants, and completely recycle CO2 by converting it into chemical energy via photosynthesis [50,51]. Energy production from microalgal biomass through biochemical, thermochemical, chemical, and direct combustion processes yields a variety of end products, such as bioethanol from fermentation, biomethane from anaerobic digestion, syngas from gasification, biodiesel, bio-oil, and charcoal from pyrolysis [43,52]. Numerous studies, especially on biodiesel production, have focused on developing cost-effective, large-scale production methods capable of competing with petroleum-based fuels. Although third-generation fuels derived from algae offer significant advantages over traditional crop-based fuels, the release of CO2 back into the atmosphere during combustion means that biomass energy cannot be considered a form of carbon storage technology [53]. Microalgae provide a sustainable feed alternative for livestock. In addition, microalgae help to balance the needs of food, feed, and biofuel while lowering environmental impact [54]. Biotechnological advancements have optimized microalgal strains to efficiently produce nutrients and sequester carbon, enhancing their roles as “cell factories” [55]. However, further research on risk assessment is essential for the safe utilization of microalgal biomass derived from industrial flue gases [56]. Although the CO2 captured by microalgae is eventually released back into the atmosphere when used in fuel or feed, microalgae-based chemical production can contribute to negative emissions. Moreover, land freed from conventional agriculture could be used for afforestation, further reducing CO2 emissions [57].
Summarizing our objectives, our approach advocates the use of WBA as agricultural waste to facilitate CO2 capture through its alkaline properties while regenerating the flue gas scrubber medium via algae, which re-alkalizes the medium during its growth. Our primary objective was to contribute to the implementation of this industrial technology through laboratory-scale model experiments. In these experiments, we aimed to identify the most optimal settings for maximizing algal biomass growth in the shortest possible time. Firstly, the suitability of WBA for flue gas cleaning was tested with a simulation of CO2 introduction with a gas cylinder dispersing fine gas bubbles into the WBA extract via a diffuser. Then, the total dissolved inorganic carbon content of the extract was determined using OxiTop respirometer devices (see Section 2.3) to monitor the change in pressure. Furthermore, the potential of CO2-enriched WBA extract for algal cultivation was also analyzed. The possible inhibitory effects of the washing solution on the algae, as well as the WBA concentration, initial algal concentration, and nutrient conditions that most optimally supported growth, were identified.
2. Materials and Methods
2.1. Characterization of the Applied Strain of Algae and the Culturing Conditions
During our modeling experiments, a stock culture of C. vulgaris was used as a test organism. The applied unicellular green algal strain (Chlorella vulgaris Beijerinck—CCAP 211/11b) was sourced from the Culture Collection of Algae at Charles University in Prague (Prague, Czech Republic) and cultured in Scharlau Algae Broth, a nutrient salt medium (Scharlab group, Barcelona, Spain) [58]. The application of C. vulgaris is advantageous due to its resilience to environmental fluctuations, tolerance to high CO2 levels during cultivation, and ease of handling. Additionally, C. vulgaris was selected because of its extensive scientific documentation and high market demand [59]. Following the manufacturer’s instructions (Scharlab group, Barcelona, Spain), 1.87 g of the nutrient mixture was added to 1 L of the algal suspension. C. vulgaris was cultured in Erlenmeyer flasks at a controlled temperature (25 ± 1 °C) and under continuous illumination provided by four Osram Biolux full-spectrum fluorescent tubes (T8 L30W/965 Daylight 6500K, 26 × 895 mm) emitting 2665 lux (59.22 µmol/m2/s) at the surface of the glass shelf [58,60].
2.2. Extraction of Wood Biomass Ash
The WBA sample used in the experiment was collected from fly ash derived from a biomass power plant located in Szakoly, Hungary. The power plant uses locally sourced wood-based biomass that is stored and chopped on site for combustion. The fuel enters the 50 m3 bunker in front of the boiler through a screen and an iron magnet. The capacity of the unit is 19.8 MW, its efficiency is 30.7%, and the annual operating time is 7900 h. The composition of the biomass changes seasonally, which affects the properties of the ash depending on the raw material. The properties of the ash are also influenced by the duration of combustion, the temperature, the cooling time, and the nature of the initial material [61,62]. In the laboratory, a 1:10 distilled water extract was prepared from the WBA sample in three replicates (Table 1).

Table 1.
Composition of the 1:10 distilled water extract of wood biomass ash (WBA).
The selected elements in the extracts were determined with an atomic emission spectrometer (MP AES, Agilent 4210, Santa Clara, CA, USA). The high contents of potassium (K) and calcium (Ca) measured in the extract were consistent with previously reported high concentration values [23,28]. The elemental analysis was performed in triplicate.
After 24 h of mixing, the solution was filtered through filter paper (Whatman No. 42, pore size = 0.25 μm). The total alkalinity of the extract was determined using an automatic potentiometric titrator (Auto Titrator SCHOTT Instrument TitroLine easy Germany), in three replicates. The transition point was determined using Origin software (version number 6.0) and differential function fitting (Figure 1).
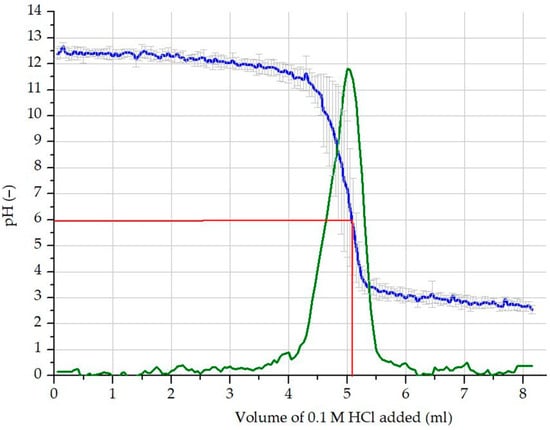
Figure 1.
Determination of total alkalinity by automatic potentiometric titration: average measured pH (blue line); the first derivate curve of the measured pH (green line); volume and pH values corresponding to the inflection point of the measured pH and the maximum of the first derivative curve, pH = 6.01, added acid = 5.1 mL (red lines).
For the experiment, 5.1 mL of acid was added to 10 mL extract. The concentration of [OH−] in the WBA was therefore calculated to be 0.51 mol kg−1, equivalent to 0.051 mol L−1 in the 1:10 water extract, with a pH value of 12.71.
2.3. Model Experiment for the Optimization of Wood Biomass Ash Extract
After applying the extraction method described in the previous chapter (Section 2.2), the effects of different treatments with various rates of WBA (0%, 10%, 25%, and 50%) were assessed along with a blank unit. The exact compositions of the treatment and the blank are summarized in Table 2. To ensure that the growth results could be compared with the optimal growth, treatments and control experiments were conducted under the same laboratory conditions using conventional nutrient media (Scharlau Algae Broth, Scharlab group, Barcelona, Spain). The bubbled CO2 gas from a cylinder passed through the samples with the use of a diffuser (ISTA Ceramic UFO CO2 Diffuser, 25 mm Suction Cup). After the CO2 scrubbing process, the pH of all samples was adjusted from their initial pH to 6±0.05 (presented in Table 2). The samples were placed in 500 mL Erlenmeyer flasks on the illuminated glass shelf, where the pH was monitored every 12 h during the cultivation using a digital pH/mV meter (OP-211/2, Radelkis Kft., Budapest, Hungary) [63]. The effects of the treatments as well as the control conditions were investigated in three repetitions. Algal biomass was assessed by measuring the optical density at 440 nm using a spectrophotometer (Jenway 6405 UV/VIS, Cole-Parmer Instrument Co., Neots, UK). Then, the concentration of biomass was calculated according to the following formula (1) [58]:
Algal biomass in mg (dry matter) = ln(1 − OD(440 nm)/3.29)/(−0.0022)

Table 2.
Experimental design and composition of the treatments and blank units during the model experiment for wood biomass ash extract optimization.
Total dissolved inorganic carbon (DIC) is usually measured by acidifying a seawater sample to convert HCO3− and CO32− into undissociated CO2 for extraction and titration [64]. In our study, we utilized OxiTop devices to determine DIC based on pressure measurements, the developed method not having previously been documented in the literature. An OxiTop device (OxiTop-IDS B6M-2.5 WTW, Weilheim, Germany) is a closed, static, pressure-based respirometer originally designed to assess the biological activity of microorganisms in both solid and liquid samples [65]. During the measurement, a CO2-enriched algal suspension was sealed in the OxiTop chamber with a beaker containing 50 mL of 1 M HCl. After measuring the base pressure for 10 min, the acid was mixed into the suspension by shaking; thus, CO2 was released. The final pressure stabilized over the next 20 min, and the results were then obtained. Before the model experiment, the CO2-diffuse solution containing algae and ash extract was divided: half was used for the OxiTop measurement, and the other half was used for the algal cultivation model. After the experiment was completed, the DIC values of the solutions were also measured in triplicate, providing both initial and final DIC values for each treatment. Based on the measured pressure, the molar quantity of CO2 was calculated via the ideal gas law to determine DIC values according to the mass of carbon in a molar quantity of CO2 (12 g mol−1) and the volume of the suspension (200 mL).
Our method was adapted from the Scheibler calcimeter according to the Hungarian Standard MSZ-08 0206-2:1978 [66], which measures soil carbonate content by generating CO2 gas through the addition of acid, resulting in displacement in a measuring cylinder [67]. In contrast to this volume-based approach [68], our OxiTop device detected pressure changes in a thermostatic setup maintained at a constant temperature of 20 °C. If a standardized acid mixing method is developed, this device could be also used to investigate the kinetics of CO2 release.
2.4. Experiment for the Optimization of the Initial Algal Biomass and Nutrients
During the optimization of initial algal biomass and nutrient content, the experimental settings were similar to those for the WBA extract optimization (Section 2.3). The exact compositions of the applied treatments and the blank are summarized in Table 3. The effects of the treatments and also the control conditions were investigated in three repetitions. At the beginning and end of the test, the solutions were centrifuged at 5000 rpm for 15 min (Hermle Z 206 A centrifuge, Hermle Labortechnik GmbH, Wehingen, Germany), followed by filtration through 0.45 µm filter paper to measure nutrient content. The N content was measured by Parnas–Wagner steam distillation, determining NH4+-N and NO3−-N values [69]. The P content was extracted using an ammonium lactate solution and determined with a spectrophotometer (Photometer Spekol 221, Iskra Elektronik, Stuttgart, Germany) [70]. The K content was determined with the use of a flame photometer (Flamom-B, Medicor Zrt., Budapest, Hungary) [71].

Table 3.
Experimental design and composition of the treatments and blank units during the model experiment for initial algal biomass and nutrient optimization.
2.5. Statistical Analysis
During our model experiments, the effects of the treatments according to the different experimental compositions were investigated in three repetitions. In addition, the control conditions were tested also in triplicate. The statistical analyses were performed with the use of the R Statistical program 4.2.1. (R Development Core Team, Vienna, Austria). Before the statistical analysis, the normality of the data and the homogeneity of variance were checked by Shapiro–Wilk and Levene’s or Bartlett’s tests at a significance level of 0.050. The effects of the treatments were evaluated using one-way ANOVA during the model experiment to optimize the WBA extract. During the experiments for the optimization of the initial algal biomass and Algae Broth nutrient level, the effects of the two factors (initial level of algal concentration and Algae Broth nutrients) and their possible interaction were investigated with the use of two-way ANOVA analysis. Tukey’s honest significant difference (HSD) testing was used for post hoc analysis to assess the significant differences between groups. The data were evaluated using the Kruskal–Wallis test if the conditions for ANOVA were not met, with the use of Dunn’s test for the comparison of the different groups at a significance level of 0.050. Where the initial and final values were available, the statistical analysis of those parameters (algal concentration, DIC, N, P, and K concentrations) was also carried out with the use of the R Statistical program 4.2.1, according to the applied treatments. For each treatment, the differences between the initial and final values were evaluated by two-sample t-tests.
3. Results
3.1. Results of the Wood Biomass Ash Extract Optimization
During our experiment, the applied culturing media were originally basic due to the addition of WBA. With the addition of a higher rate of WBA, the media became more alkaline, and thus, more CO2 was captured. The highest final pH value was observed for the 10% WBA treatment (Figure 2). A sigmoidal curve was fitted to the average values of measured biomass data across all treatments (R2 ≥ 0.992), as shown in Figure 3. The analysis of these functions enabled us to determine the theoretical maximum producible biomass under these conditions (A2) as well as the time required for that growth (dx) (Table 4). It was noted that the maximum biomass was higher in the 25% treatment (176 mg L−1 dry mass), while the time required for growth was more favorable in the 10% treatment.
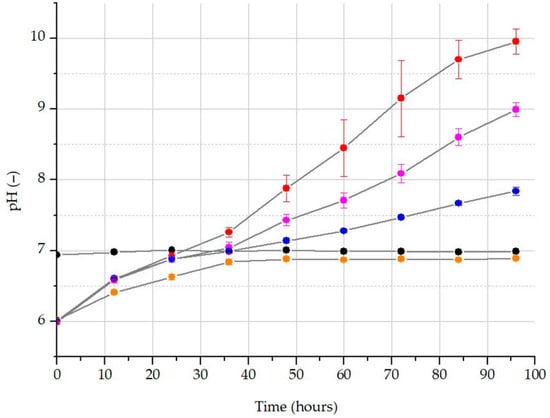
Figure 2.
Changes in the pH value of differently carbonated treatments: blank treatment without CO2 diffusion (black); 0% WBA addition with CO2 scrubbing (orange); 10% WBA addition with CO2 scrubbing (red); 25% WBA addition with CO2 scrubbing (purple); 50% WBA addition with CO2 scrubbing (blue).
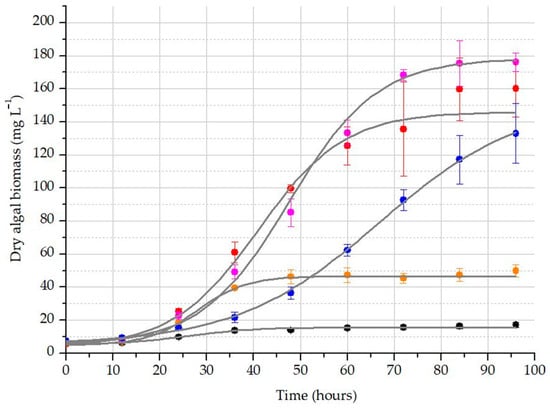
Figure 3.
The growth of algal biomass in the differently carbonized treatments: blank treatment without CO2 diffusion (black); 0% WBA addition with CO2 scrubbing (orange); 10% WBA addition with CO2 scrubbing (red); 25% WBA addition with CO2 scrubbing (purple); 50% WBA addition with CO2 scrubbing (blue).

Table 4.
The determined parameters based on the fitted sigmoidal curves during the wood biomass ash extract optimization.
The average pressure results from the OxiTop system, along with standard deviations, are shown in Figure 4. To calculate the CO2 released following each treatment, the initial basic values (after 5 min) were subtracted from the values after acidification (after 30 min), according to the known volume of air space in the bottles and the constant temperature of 20 °C provided by the thermostat. Our findings demonstrate that DIC can be measured using this device. Based on the calculated DIC results, expressed as mg of carbon per liter of solution (mg C L−1) (Figure 5, Table 5), the 10% WBA treatment proved to be the most effective, with 93% of the dissolved carbon utilized by the algae. This indicates that, in this treatment, the algae nearly completely regenerated the CO2-scrubbing medium.
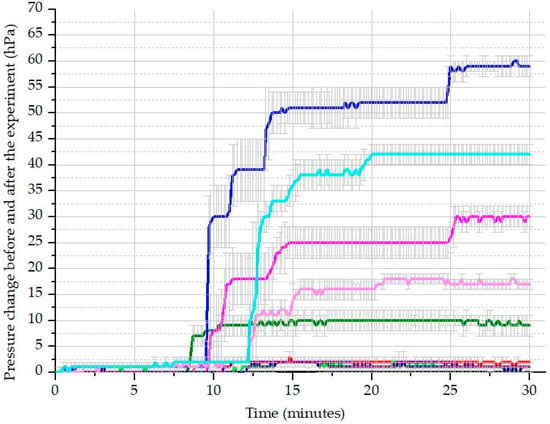
Figure 4.
The measured pressure values after acidification before and after the algae-cultivating model experiment: blank initial (black), blank final (dark grey); 0% WBA initial (red), 0% WBA final (purple); 10% WBA initial (olive), 10% WBA final (green); 25% WBA initial (magenta, 25% WBA final (light magenta); 50% WBA initial (blue), 50% WBA final (light blue).
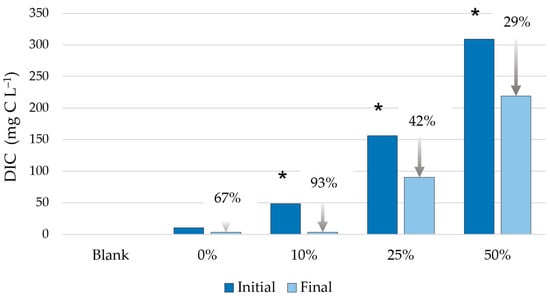
Figure 5.
The measured total dissolved inorganic carbon (DIC) contents at the beginning and the end of the algae-cultivating model experiment (the percentages on the x-axis represent the concentration of the added WBA extract; * indicates significant difference between the initial and final DIC values at a significance level of 0.050).

Table 5.
Summarized results of wood biomass ash treatments.
According to the statistical analysis of the results for the WBA treatments, higher final algal concentrations, biomass changes, and specific growth rates were detected for the WBA treatments compared with the control (p ≤ 0.038). The highest final algal concentrations, biomass change, and specific growth rates were determined for 10% and 25% WBA treatments compared with the control (p < 0.001). Significant differences were demonstrated between the initial and final algal concentrations for each treatment (p ≤ 0.006). Compared with the control, significantly higher pH values were measured for the 10%, 25%, and 50% WBA treatments (p < 0.001), while a significantly different pH change was demonstrated in each group (p < 0.050). The initial DIC values were significantly different in each treated group, while the highest final DIC values were measured for the treatment with 50% WBA (p < 0.001).
Significant differences were observed in the initial and final DIC values determined for treatments with 10%, 25%, and 50% WBA (p ≤ 0.003). In contrast, there was no significant difference between the initial and final DIC values measured in the treatment with 0% WBA (p = 0.057) (Figure 5). The highest DIC removal was observed for 10% WBA treatment (p ≤ 0.014), although the difference was not significant compared with the 0% WBA treatment (p = 0.223). Based on the parameters relating to algal growth, the 10% and 25% WBA treatments were found to be the most appropriate, while the highest rate of DIC removal was detected for 0% and 10% WBA treatments (Table 5). Based on the results and tested parameters, the 10% WBA treatment can be considered the most effective.
3.2. Results for the Initial Algal Biomass and Nutrient Optimization
In the control sample, the increase in biomass led to a rapid rise in pH (Figure 6). Due to the lack of WBA extract, a significant amount of CO2 was not captured in this sample, resulting in limited carbon available for algal growth. In the control, a very low buffering capacity was demonstrated. Consequently, the rapid formation of hydroxide ions associated with algal growth apparently caused a quick increase in pH in these samples. In contrast, WBA acted as a buffering system in the other treatments, rendering them significantly less responsive to algal activity. Regarding pH, three treatments (A8N100, A5N50, and A2N100) achieved similarly high levels.
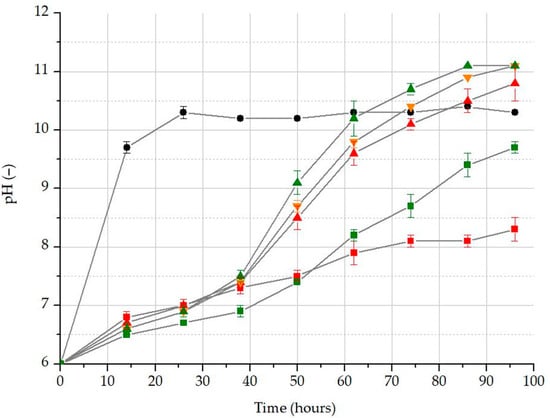
Figure 6.
Changes in the pH values according to the initial algae biomass and nutrient levels: blank treatment (black dot); A2N0 (red square); A2N100 (red up triangle); A5N50 (orange down triangle); A8N0 (green square); A8N100 (green triangle).
3.3. Results for Biomass Growth in the Differently Inoculated and Nutrient-Supplied Treatments
As shown in Table 6, A5N50 and A8N100 treatments were expected to reach their maximum around 270 mg L−1 (A2), while the lower dx value of the latter meant that it reached this maximum in a shorter time. The relatively high x0 value for the A8N0 treatment suggests that it has greater reserves of available resources, as can also be deduced from the slightly flattened shape of its sigmoid curve (Figure 7). Interestingly, A8N100 treatment was associated with significantly faster growth than A2N100, suggesting that initial algal concentration may have had a stronger influence on biomass growth than nutrient supply.

Table 6.
The determined parameters based on the fitted sigmoidal curves during the initial algal biomass and nutrient optimization.
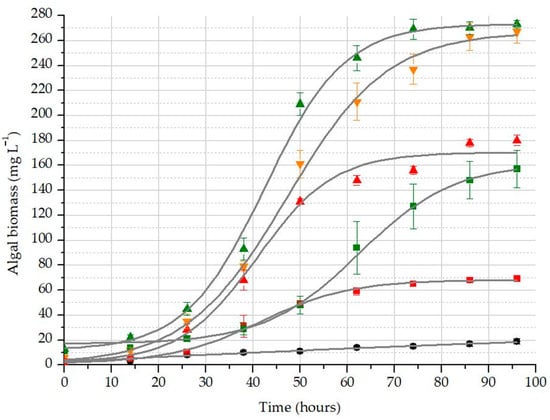
Figure 7.
Growth of algal biomass in the differently inoculated and nutrient-supplied treatments: blank treatment (black dot); A2N0 (red square); A2N100 (red up triangle); A5N50 (orange down triangle); A8N0 (green square); A8N100 (green triangle).
3.4. Observed Changes in the Nutrient Content of the Media
The primary aim of analyzing each nutrient element was to evaluate the potential purification efficiency of algae when wastewater was used as a nutrient source. Interestingly, in the blank, A2N0, and A8N0 samples, the initial N content was almost minimal (Table 7), although biomass growth was generally observed (Figure 7, Table 8). However, the linear regression indicated that the correlation of the measured biomass increased with the consumption of N, P, and K nutrients. Relatively low correlation values were observed for P (R2 = 0.5569), K (R2 = 0.5755), and N (R2 = 0.7584). The highest initial N level was detected for the A8N100 treatment (p ≤ 0.007). Similarly, the lowest final N concentrations were also observed for the blank, A2N0, and A8N0 treatments. Additionally, the highest final N levels compared with the control were measured for A2N100 and A8N100 (p < 0.001). The statistical analysis suggested that the values of the initial and final N levels were significantly affected by the initial algal biomass and nutrient level (p < 0.001). In addition, the interaction between the two factors (initial algal biomass and nutrient level) was also significant with regard to the initial N values (p = 0.003). Compared with the initial N concentrations, the level of N was significantly reduced in treatments where the initial level of Algae Broth nutrient was ≥50% (A2N100, A5N50, and A8N100) (p < 0.020) (Table 7).

Table 7.
The measured initial and final levels of nitrogen, phosphorus, and potassium in the media of the different treatments.

Table 8.
Summarized results of initial algal inoculation and nutrient optimization in the various treatments.
Similar to the N levels, the initial P levels were also low in the blank, A2N0, and A8N0 treatments. Compared with the blank samples, the highest initial P concentrations were detected for A2N100 and A8N100 (p < 0.001). Compared with the control, the highest final P levels were measured for the A2N100 and A5N50 treatments (p < 0.025). In the other samples, significant differences compared with the blank were not detected in the final P levels (p ≥ 0.068). The values of initial and final P levels were also significantly affected by the initial algal biomass and Algae Broth nutrient level (p ≤ 0.044). Moreover, the interaction between the two factors also significantly affected the final P values (p < 0.001). A significant decrease between the initial and final measured P levels was observed for all treatments (p ≤ 0.029), except the A8N0 treatment (p = 0.061) (Table 7).
Compared with the control, the highest initial and final K values were also detected for the A8N100 treatment (p < 0.001). Based on the results, the lowest initial and final K levels were determined in the blank samples (p < 0.001). In the samples of A2N0 and A8N0, there were no differences in the initial and final K concentrations (p ≥ 0.161). The initial and final K levels were also significantly affected by the initial algal biomass and Algae Broth nutrient level (p ≤ 0.038). However, the interaction between the two factors was not proved based on the statistical analysis of initial and final K levels (p ≥ 0.241). Significant reductions in final K levels compared with initial K levels were not observed for the treatments where the initial level of Algae Broth nutrients was 0% (A2N0 and A8N0) (p ≥ 0.856). In the other treatments, significantly decreased final K levels compared with the corresponding initial levels were detected (p ≤ 0.024) (Table 7).
Compared with the initial algal concentrations, the final algal concentrations were significantly higher in each treatment (p ≤ 0.003). Additionally, significantly higher final algal concentrations, biomass change, specific growth rates, and doubling times were detected for the different WBA treatments compared with the control samples (p < 0.001). The highest final algal concentrations and biomass change compared with the control were determined for the A5N50 and A8N100 treatments (p < 0.001). Additionally, the highest specific growth rates and the largest increase in biomass compared with the control were observed for the A2N100 and A5N50 treatments (p < 0.001) (Table 8), although the specific growth rates in these treatments ranked only within the top three for net biomass increase. The observed pH changes were not significantly different in those treatments where the initial level of Algae Broth nutrient was ≥50% (A2N100, A5N50, and A8N100) (p ≥ 0.124). In the other treatments, the pH change compared with the control was different in each group (p ≤ 0.020). According to the statistical analysis, the measured and calculated parameters were significantly affected by the initial level of the Algae Broth nutrient (p ≤ 0.011). The significant effect of initial algal concentration on the tested parameters was proven, except for the doubling time (p = 0.134). Moreover, the interaction between initial algal concentration and Algae Broth nutrient level was proven to be significant in the case of biomass multiplication (p = 0.026).
Comparing of the algal concentration and variable nutrient supplies with the resulting biomass increase (Figure 8), none of the parameters caused a significant growth above the average value. This phenomenon might be attributed to the depletion of another environmental resource, such as dissolved carbon, which could have acted as a limiting factor for growth. If this were not the case, the growth under the average parameter setting would ideally fall midway between the biomass levels observed under the two extreme parameter settings. If these two parameters were acting in opposition, nutrient availability appeared to exert a stronger influence on biomass. However, it is important to note that although both parameters can be scaled within the same dimension, comparability issues arise due to differences in scaling dimensions.
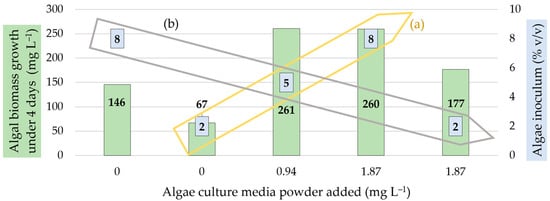
Figure 8.
Trends indicated between the algal biomass growth obtained with increasing levels of nutrient media and the (a) directly and (b) inversely proportional initial algal inoculation treatments.
4. Discussion
Alkalization of the medium during algal biomass growth has been documented in various studies [29,74,75,76]. In our experiment, the culturing medium was initially basic due to the addition of WBA. As the WBA rate increased, the medium became more alkaline, leading to greater capture of CO2. The highest final pH value was detected in the 10% WBA treatment (Figure 2), suggesting that algal growth in these groups was capable of fully regenerating the pH. One of the major findings of our research is the improvement of the low inhibitory effect of pH on the growth of Chlorella vulgaris compared with the effect of carbon limitation within the medium. According to Ihnken et al., Chlorella spp. typically thrive at a pH range of 6 to 6.5 [77], while C. vulgaris can also grow effectively at an alkaline pH of 10.5. Additionally, the ability of these algae to tolerate extremely low pH is well documented, as their resilience underlies small-scale zooplankton control methods [32,78,79].
The applied OxiTop system is suitable for the measurement of DIC. During the WBA extract optimization, the 10% and 25% WBA treatments were identified as the most suitable according to the parameters for algal growth, whereas the highest DIC removal was observed in the 0% and 10% WBA treatments (Table 5). Overall, based on the results and evaluated parameters, the 10% WBA treatment emerges as the most effective option. According to the assessment of the initial algal biomass and nutrient optimization, the increase in biomass caused a rapid rise in pH in the control group (Figure 8). Due to the absence of significant CO2 sequestration, limited carbon sources were available for algal growth. As a result, the control demonstrated very low buffering capacity. The rapid formation of hydroxide ions associated with algal growth is likely to have contributed to the quick increase in pH in these samples. In contrast, the presence of WBA in the other treatments acted as a buffering system, making them significantly less responsive to algal activity. The results of biomass growth in the differently inoculated and nutrient-supplied treatments suggest that initial algal concentration may have a greater impact on biomass growth than the availability of nutrients. According to the results, the values of biomass change, mass multiplication, specific growth rate, and doubling time measured for 10% WBA and A2N100 were consistent.
The main purpose of analyzing each nutrient element was to assess the potential purification efficiency of algae when utilizing wastewater as a nutrient source. Interestingly, in the blank, A2N0, and A8N0 samples, the initial N content was nearly negligible (Table 7), yet, biomass growth was generally observed. This phenomenon can possibly be attributed to the ability of certain algal species, such as Chlorella, to adjust their biochemical composition in N-deficient environments. Since N is primarily necessary for the construction of amino acids that form proteins, it is probable that the algae increased their lipid content at the expense of protein content [80,81]. This also explains why N deficiency did not inhibit overall mass growth. Notably, P is often considered as a limiting factor for the onset of eutrophication [82,83,84], but it was not depleted in any of the treatments, indicating that it was not a limiting factor in this experiment. It is important to note that this method combined with ash extract was not effective for the removal of K, as the K content of the WBA extract significantly increased the K levels within the system compared with the blank without added WBA extract (Table 7). Our results suggest that carbon availability is the primary driver of algal biomass growth, followed by N and P, which is consistent with the elemental composition of Chlorella reported in the literature (CO0.48H1.83N0.11P0.01) or the commonly used Redfield ratio for phytoplankton (C–N–P ratio of 106:16:1) [53,85,86,87]. During the correlation of the measured biomass increase with the consumption of the measured nutrients, relatively low correlation values were demonstrated (P: R2 = 0.5569, K: R2 = 0.5755, N: R2 = 0.7584), which suggests that N availability affected the algal growth across the treatments. However, other factors probably had a significantly greater impact.
Based on the statistical analysis of the initial algal inoculation and nutrient optimization, significantly higher final algal concentrations, biomass change, and specific growth rates were detected in the different WBA treatments compared with the control. The highest specific growth rates and the largest increase in biomass were observed for the A2N100 and A5N50 treatments (p < 0.001) (Table 8). Although the specific growth rate in these treatments ranked only within the top three for net biomass increase, this observation suggests that specific growth rate more effectively reflected the efficiency of each treatment, as a useful metric to optimize and determine the appropriate initial parameters. However, the equation for this derived parameter is fundamentally based on the initial algal quantity and the value obtained is inversely proportional to this initial amount [72]. Consequently, specific growth rates were not sequential in relation to the measured parameters in the various treatments, and thus cannot be used for final decision making.
According to our results, the WBA extract effectively absorbed CO2 without exhibiting any toxic effects on algal growth. However, at higher concentrations, it inhibited growth, resulting in an extended lag phase. Algae can utilize the CO2 from the WBA extract as a carbon source, leading to CO2 depletion, increased biomass, and a rise in pH levels. Furthermore, the ash extract can be partially regenerated, so the pH drops from 12 to 6, and then stabilizes around 10 with 10% treatment. Notably, the highest CO2 removal efficiency (93%) was also observed for the 10% treatment, with a 29-fold increase in biomass, a specific growth rate of 0.84, and pH restoration all accomplished within a 4-day period. Therefore, the 10% WBA addition treatment was selected for further research.
However, the composition of the WBA can vary due to several factors (e.g., duration of combustion, temperature, and the characteristics of the raw material) [61,62]. Our results indicate that WBA can serve as an effective tool for CO2 sequestration, eliminating the need for inorganic alkalis such as KOH, Ca(OH)2, and NaOH in waste management applications. During utilization in flue gas cleaning to capture CO2, algae can regenerate the solution from a pH of 6 to around 11, starting from an initial pH of 12. The quantity of initial algal biomass significantly affects the growth rate and pH changes, offering a more precise measure of growth dynamics than optical density. After the uptake of CO2 from bicarbonate ions (HCO3−), residual hydroxide ions (OH−) remain, which increasingly inhibit algal growth as pH approaches 10. The maximum growth threshold was 273 mg dry weight per liter, probably determined by the amount of carbon sequestered by the ash extract. This relationship remained consistent across different treatment conditions. Based on the elemental composition of Chlorella, approximately 50% of the mass of dry biomass consists of carbon, which facilitates a straightforward estimation of the amount of carbon captured from the solution. Our findings relate to a broader environmental context; if the acidification of seas is not caused by CO2 dissolving in the water, then algae will not be able to restore the pH of the water to neutral through biomass production and the associated release of hydroxide ions. The presence of aquatic pollutants in the oceans can contribute to acidification, not only limiting the ability of oceans to absorb carbon dioxide but also reducing the activity of the algae in these ecosystems.
The growth of algae is primarily limited by carbon availability. However, growth dynamics are also significantly affected by nutrient availability and the initial biomass of the algae. In addition, the quantity of the nutrient solution is crucial for the determination of the growth rate although it does not dictate the maximum growth capacity, as evidenced by the flattening of the growth curves despite the significant residual nutrients. K was present in excess due to the presence of the ash. Thus, the algae could only absorb a fraction of this excess. The measured P indicated higher consumption rates at higher initial concentrations, probably due to the presence of excess N in these scenarios. At low initial concentrations, N was almost completely depleted, while at higher initial values, the rate of consumption was proportionally lower. Nevertheless, this N loss appeared disproportionate when quantified and does not explain the observed increase in biomass calculated by Mandalam and Palsson (6.5% N) [86]. It is probable that while N ultimately limited growth, the algae managed to partially compensate for this deficiency by utilizing other nutrients.
The comparison of experimental results on microalgal growth with the literature data is often challenging. It is very difficult to compare the results of tests performed under varying conditions. For such tests, it is essential to present results in comparable units and standardized metrics. However, the literature lacks a unified approach: some studies report biomass growth per unit area [88,89,90,91,92], while others use per-unit-volume metrics [93,94,95,96,97,98]. Area-based growth metrics are inherently less comparable as they fail to account for culture depth [99]. As a result, this study utilizes volumetric-specific metrics to express biomass growth. Nevertheless, the objective comparability of results is still limited by the duration of the experiment. Although this may seem manageable using a time-specific metric, such as mg L−1 day−1, this approach is significantly influenced by the chosen time interval. For example, the sigmoidal growth curves in Figure 5 demonstrate that excluding the saturation phase after day 3 or the initial lag phase could result in significantly higher volumetric daily productivity values, reflecting only the exponential growth phase. Some authors have reported productivity values for specific days without including average values, potentially resulting in overly optimistic projections when used in literature-based assessments of microalgae’s potential for industrial applications. Since our proposed technique could realistically be applied in batch systems at an operational scale, it is crucial to report realistic values reflecting the average productivity over the entire growth cycle.
During the 4-day experiment, the highest volumetric productivity was recorded in the A5N50 and A8N100 treatments, with values of 65.3 ± 2.2 mg L−1 day−1 and 65.0 ± 1.0 mg L−1 day−1, respectively. These observed values are statistically indistinguishable. The maximum productivity was detected after 50 h (2.08 days) for A8N100 and 62 h (2.58 days) for A5N50, with maximum values of 93.9 ± 4.5 mg L−1 day−1 and 79.3 ± 5.7 mg L−1 day−1, respectively. Although the duration of the experiment was 4 days, this approach also allowed the determination of the optimal culturing duration under the specific treatment conditions applied. On an annual scale, these results correspond to 34.28 kg and 28.95 kg of dry algal biomass per cubic meter, respectively. High variability was observed in the reported values of biomass productivity, while the reported values for Chlorella species range from 2.98 to 5370 mg L−1 day−1 [100]. According to our prior study, C. vulgaris suspensions at maximum density (the determined algal biomass was 770 and 960 mg L−1 after filtration and evaporation, respectively) exhibit optical properties that limit precise quantification [58]. Our previous findings suggest that studies reporting values exceeding 1 g L−1 based on optical density measurements may include methodological inconsistencies or errors in biomass conversion. The reported values in the present study are consistent with the ranges reported in several studies [95,101], where the range of the observed biomass productivity was 35.83–81.67 mg L−1 day−1. Our results are also in good agreement with the results of a study where C. vulgaris cultured in wastewater with 10% CO2 injection achieved 381.16–499.52 mg L−1 productivity over 23 days, corresponding to daily averages of 16.5–21.7 mg L−1 day−1 [101]. Additionally, cultures maintained at constant CO2 levels (0.04%, 3%, 6%, and 9%) showed the highest biomass growth rate (0.37 g L−1 day−1) for C. vulgaris at 3% CO2 [39].
Several approaches are available for quantifying CO2 sequestration during various experiments. The most straightforward method derives from the measured decrease in DIC. In the initial model experiment, where the 10% WBA extract treatment was applied, a DIC reduction of 45.2 ± 7.96 mg L−1 was observed over 4 days (Table 5), corresponding to an average sequestration rate of 41.4 ± 7.3 mg CO2 L−1 day−1. This rate is expected to be higher in the second model experiment, where biomass growth was further optimized. Another method calculates CO2 sequestration based on biomass growth and its carbon content. According to Mandalam and Palsson, the carbon content of C. vulgaris is 62 ± 15% of biomass dry weight [86]. Using this approach, the CO2 fixation rate for the A8N100 treatment was determined to be 213 ± 52 mg CO2 L−1 day−1. In contrast, the reported maximal fixation rates ranged from 4.51–14.26 mg CO2 L−1 day−1 to 56.26–85.72 mg CO2 L−1 day−1 under varying conditions [101]. CO2 fixation rates of 0.93 and 0.69 g L−1 day−1 were achieved using 2 and 1 g L−1 NaHCO3 in 100 mL flasks, respectively [42]. In another study, Chlorella sp. cultured in an airlift photobioreactor produced a biomass of 109–264 mg L−1 day−1 [102]. Using a third approach, biomass productivity can be converted into CO2 fixation rates based on the literature values [103], resulting in 1.6–2 kg CO2 fixed per kg (d.m.) year−1. Across the three approaches, annual CO2 sequestration rates of 15.1 ± 2.7, 77.9 ± 18.8, and 54.4–68.6 kg CO2 m−3 year−1 were calculated. The lower rates observed in the first model experiment were attributed to suboptimal growth conditions. Based on the calculations, our results indicate that scaling up the model experiment to capture 1 ton of CO2 per year would require 13–66 m3 of algal culture.
However, the economic feasibility of such systems is unattainable without valorization of the resulting algal biomass. Moreover, the efficiency of carbon sequestration is closely linked to the downstream utilization of the algal biomass, highlighting the necessity of an integrated approach to evaluating the potential of carbon capture and storage. The technology demonstrated and optimized through the model experiments is uniquely suitable for operation in closed photobioreactor systems. In open pond systems, injected CO2 gas bubbles through the water medium (which must be shallow for adequate illumination) and escapes into the atmosphere. Consequently, open systems cannot serve as effective CCS techniques [104,105,106,107]. Our proposed method employs a sequential approach, where the CO2 absorption and solution regeneration phases are spatially separated from the carbon sequestration phase involving algal biomass cultivation. As a result, CO2 diffusion is unnecessary during the cultivation phase, though oxygen produced via photosynthesis must be vented through a valve. This design improves the efficiency of carbon capture by minimizing gas losses and maintaining controlled cultivation conditions.
5. Conclusions
The involvement of algae-based solutions in water resource management and CCS technologies can contribute to climate change mitigation and support sustainable development goals by preserving aquatic ecosystems and ensuring appropriate water quality. Based on our results, DIC can be measured with the use of the OxiTop device. Although the developed method requires further standardization, OxiTop is also suitable for the detection of the dynamics of gas evolution, which could help to distinguish different carbonate forms. Based on the measurements of the DIC content in the different treatments with different concentrations of WBA solutions after bubbling CO2 through them (compared with freshly boiled distilled water), WBA could potentially be used in CCS technologies to capture CO2. However, during the possible application of WBA, we have to take into consideration the possible variability of WBA composition, as well as the additional challenges associated with scaling up microalgae culture systems. Our approach also provides an opportunity to utilize agricultural waste, reducing the need for landfilling and supporting the circular transition. Our model experiments showed that CO2-enriched WBA was not toxic to the growth of algae. Since they play a crucial role in water quality and the carbon cycle of aquatic ecosystems, algae are able to absorb the CO2 content from the washing solution, effectively regenerating it, which allows it to be re-alkalized and reused for further CO2 capture. Among the WBA water extracts tested at different concentrations, a 10% v/v concentration was optimal for algae. The addition of 10 WBA promoted the fastest growth, restored pH to initial levels, and achieved the highest DIC consumption. During a subsequent experiment, the optimal initial algal density and the amount of nutrients were determined. We observed that starting with a higher density of algae supported rapid growth but did not require the maximum treatment level. Additionally, using half the amount of nutrients recommended by the manufacturer still resulted in robust growth.
Author Contributions
Conceptualization, G.F.; methodology, G.F. and I.C.; validation, G.F., S.K., I.C., and A.S. (András Székács); formal analysis, G.F. and S.K.; investigation, G.F., I.C., A.B.D., A.T., and M.G.; writing—original draft preparation, G.F., S.K., and A.B.D.; writing—review and editing, I.C., A.S. (András Székács), and L.A.; visualization, A.S. (András Sebők) and I.C.; supervision, I.C., A.S. (András Székács), and L.A.; funding acquisition, C.G. and L.A. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the project “The feasibility of the circular economy during national defense activities” of the 2021 Thematic Excellence Programme of the National Research, Development and Innovation Office (grant no. TKP2021-NVA-22), led by the Centre for Circular Economy Analysis. Our work was also supported by the Research Excellence Programme 2024 and by the 2024 Flagship Research Groups Programme of the Hungarian University of Agriculture and Life Sciences.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the Centre for Circular Economy Analysis established by the Hungarian University of Agriculture and Life Sciences for integrating this international collaboration into its educational profile.
Conflicts of Interest
Author László Aleksza has been employed by ProfiKomp Environmental Technologies Inc. The remaining authors declare that the research was conducted without any commercial or financial relationships that could be interpreted as potential conflicts of interest.
References
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef] [PubMed]
- Raupach, M.R.; Marland, G.; Ciais, P.; Le Quere, C.; Canadell, J.G.; Klepper, G.; Field, C.B. Global and regional drivers of accelerating CO2 emissions. Proc. Natl. Acad. Sci. USA 2007, 104, 10288–10293. [Google Scholar] [CrossRef] [PubMed]
- National Oceanic and Atmospheric Administration. During a Year of Extremes, Carbon Dioxide Levels Surge Faster than Ever. 2024. Available online: https://www.noaa.gov/news-release/during-year-of-extremes-carbon-dioxide-levels-surge-faster-than-ever (accessed on 6 November 2024).
- Inman, M. Carbon is forever. Nat. Clim. Change 2008, 1, 156–158. [Google Scholar] [CrossRef]
- Raupach, M.R.; Gloor, M.; Sarmiento, J.L.; Canadell, J.G.; Frölicher, T.L.; Gasser, T.; Trudinger, C.M. The declining uptake rate of atmospheric CO2 by land and ocean sinks. Biogeosciences 2014, 11, 3453–3475. [Google Scholar] [CrossRef]
- Watson, A.J.; Schuster, U.; Shutler, J.D.; Holding, T.; Ashton, I.G.; Landschützer, P.; Goddijn-Murphy, L. Revised estimates of ocean-atmosphere CO2 flux are consistent with ocean carbon inventory. Nat. Commun. 2020, 11, 4422. [Google Scholar] [CrossRef] [PubMed]
- Duffy, K.A.; Schwalm, C.R.; Arcus, V.L.; Koch, G.W.; Liang, L.L.; Schipper, L.A. How close are we to the temperature tipping point of the terrestrial biosphere? Sci. Adv. 2021, 7, eaay1052. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.P.; Le Quéré, C.; Andrew, R.M.; Canadell, J.G.; Friedlingstein, P.; Ilyina, T.; Tans, P. Towards real-time verification of CO2 emissions. Nat. Clim. Change 2017, 7, 848–850. [Google Scholar] [CrossRef]
- Koerner, B.; Klopatek, J. Anthropogenic and natural CO2 emission sources in an arid urban environment. Environ. Pollut. 2002, 116, 45–51. [Google Scholar] [CrossRef]
- Raihan, A.; Tuspekova, A. Dynamic impacts of economic growth, energy use, urbanization, tourism, agricultural value-added, and forested area on carbon dioxide emissions in Brazil. J. Environ. Stud. Sci. 2022, 12, 794–814. [Google Scholar] [CrossRef]
- Lau, L.C.; Lee, K.T.; Mohamed, A.R. Global warming mitigation and renewable energy policy development from the Kyoto protocol to the Copenhagen accord—A comment. Renew. Sustain. Energy Rev. 2012, 16, 5280–5284. [Google Scholar] [CrossRef]
- European Parliament and of the Council. Regulation (EU) 2021/1119 of the European Parliament and of the Council of 30 June 2021 establishing the framework for achieving climate neutrality and amending Regulations (EC) No 401/2009 and (EU) 2018/1999 (‘European Climate Law’). OJ EU 2021, L 243, 1–17. [Google Scholar]
- STATISTA. Annual Carbon Dioxide (CO2) Emissions Worldwide from 1940 to 2023. 2023. Available online: https://www.statista.com/statistics/276629/global-co2-emissions/ (accessed on 6 November 2024).
- Crippa, M.; Guizzardi, D.; Muntean, M.; Schaaf, E.; Dentener, F.; Van Aardenne, J.A.; Janssens-Maenhout, G. Gridded emissions of air pollutants for the period 1970–2012 within EDGAR v4. 3.2. Earth Syst. Sci. Data 2018, 10, 1987–2013. [Google Scholar] [CrossRef]
- Liu, Z.; Ciais, P.; Deng, Z.; Lei, R.; Davis, S.J.; Feng, S.; Schellnhuber, H.J. Near-real-time monitoring of global CO2 emissions reveals the effects of the COVID-19 pandemic. Nat. Commun. 2020, 11, 5172. [Google Scholar] [CrossRef] [PubMed]
- Le Quéré, C.; Jackson, R.B.; Jones, M.W.; Smith, A.J.; Abernethy, S.; Andrew, R.M.; Peters, G.P. Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nat. Сlim. Сhange 2020, 10, 647–653. [Google Scholar] [CrossRef]
- Ray, R.L.; Singh, V.P.; Singh, S.K.; Acharya, B.S.; He, Y. What is the impact of COVID-19 pandemic on global carbon emissions? Sci. Total Environ. 2022, 816, 151503. [Google Scholar] [CrossRef]
- Rivas, S.; Urraca, R.; Bertoldi, P.; Thiel, C. Towards the EU Green Deal: Local key factors to achieve ambitious 2030 climate targets. J. Clean. Prod. 2021, 320, 128878. [Google Scholar] [CrossRef]
- Tutak, M.; Brodny, J.; Bindzár, P. Assessing the level of energy and climate sustainability in the European Union countries in the context of the European green deal strategy and agenda 2030. Energies 2021, 14, 1767. [Google Scholar] [CrossRef]
- Zlateva, P.; Terziev, A.; Mileva, N.M. Sustainable solutions for energy production from biomass materials. Sustainability 2024, 16, 7732. [Google Scholar] [CrossRef]
- Ali, S.; Akter, S.; Ymeri, P.; Fogarassy, C. How the use of biomass for green energy and waste incineration practice will affect GDP growth in the less developed countries of the EU (A case study with Visegrad and Balkan countries). Energies 2022, 15, 2308. [Google Scholar] [CrossRef]
- Kożuch, A.; Cywicka, D.; Adamowicz, K.; Wieruszewski, M.; Wysocka-Fijorek, E.; Kiełbasa, P. The use of forest biomass for energy purposes in selected European countries. Energies 2023, 16, 5776. [Google Scholar] [CrossRef]
- Odzijewicz, J.I.; Wołejko, E.; Wydro, U.; Wasil, M.; Jabłońska-Trypuć, A. Utilization of ashes from biomass combustion. Energies 2022, 15, 9653. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Gierke, J.S.; Kawatra, S.K.; Eisele, T.C.; Sutter, L.L. Carbon dioxide sequestration in cement kiln dust through mineral carbonation. Environ. Sci. Technol. 2009, 43, 1986–1992. [Google Scholar] [CrossRef]
- Li, L.; Yu, H.; Puxty, G.; Zhou, S.; Conway, W.; Feron, P. Integrated CO2 capture and mineralization based on monoethanolamine and Lime Kiln Dust. Ind. Eng. Chem. Res. 2024, 63, 16019–16028. [Google Scholar] [CrossRef]
- Uliasz-Bocheńczyk, A.; Deja, J. Potential application of cement kiln dust in carbon capture, utilisation, and storage technology. Energy 2024, 292, 130412. [Google Scholar] [CrossRef]
- Koch, R.; Sailer, G.; Paczkowski, S.; Pelz, S.; Poetsch, J.; Müller, J. Lab-scale carbonation of wood ash for CO2-sequestration. Energies 2021, 14, 7371. [Google Scholar] [CrossRef]
- Adamczyk, J.; Smołka-Danielowska, D.; Krzątała, A.; Krzykawski, T. Chemical and mineral composition of bottom ash from agri-food biomass produced under low combustion conditions. Int. J. Environ. Sci. Technol. 2024, 21, 4025–4036. [Google Scholar] [CrossRef]
- Shiraiwa, Y.; Goyal, A.; Tolbert, N.E. Alkalization of the medium by unicellular green algae during uptake dissolved inorganic carbon. Plant Cell Physiol. 1993, 34, 649–657. [Google Scholar] [CrossRef]
- Brown, L.M.; Zeiler, K.G. Aquatic biomass and carbon dioxide trapping. Energy Convers. Manag. 1993, 34, 1005–1013. [Google Scholar] [CrossRef]
- Goldman, J.C.; Porcella, D.B.; Joe Middlebrooks, E.; Toerien, D.F. The effect of carbon on algal growth -its relationship to eutrophication. Water Res. 1972, 6, 637–679. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Ho, S.; Chen, C.; Lee, D.; Chang, J. Perspectives on microalgal CO2-emission mitigation systems—A review. Biotechnol. Adv. 2011, 29, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Vunjak-Novakovic, G.; Kim, Y.; Wu, X.; Berzin, I.; Merchuk, J.C. Air-lift bioreactors for algal growth on flue gas: Mathematical modeling and pilot-plant studies. Ind. Eng. Chem. Res. 2005, 44, 6154–6163. [Google Scholar] [CrossRef]
- Yoo, C.; Jun, S.; Lee, J.; Ahn, C.; Oh, H. Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 2010, 101, S71–S74. [Google Scholar] [CrossRef]
- Kao, C.; Chiu, S.; Huang, T.; Dai, L.; Hsu, L.; Lin, C. Ability of a mutant strain of the microalga Chlorella sp. to capture carbon dioxide for biogas upgrading. Appl. Energy 2012, 93, 176–183. [Google Scholar] [CrossRef]
- Mann, G.; Schlegel, M.; Schumann, R.; Sakalauskas, A. Biogas-conditioning with microalgae. Agron. Res. 2009, 7, 33–38. [Google Scholar]
- Fekete, G. Impact of Biogas Injection on Growth of Algal Biomass. Master’s Thesis, Cranfield University, Cranfield, UK, 2013. [Google Scholar]
- Chunzhuk, E.A.; Grigorenko, A.V.; Kiseleva, S.V.; Chernova, N.I.; Ryndin, K.G.; Kumar, V.; Vlaskin, M.S. The influence of elevated CO2 concentrations on the growth of various microalgae strains. Plants 2023, 12, 2470. [Google Scholar] [CrossRef] [PubMed]
- Azov, Y. Effect of pH on inorganic carbon uptake in algal cultures. Appl. Environ. Microbiol. 1982, 43, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Pesheva, I.; Kodama, M.; Dionisio-Sese, M.L.; Miyachi, S. Changes in photosynthetic characteristics induced by transferring air-grown cells of Chlorococcum littorale to high-CO2 conditions. Plant Cell Physiol. 1994, 35, 379–387. [Google Scholar]
- Ratomski, P.; Hawrot-Paw, M.; Koniuszy, A. Utilisation of CO2 from sodium bicarbonate to produce Chlorella vulgaris biomass in tubular photobioreactors for biofuel purposes. Sustainability 2021, 13, 9118. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Wu, N.; Lan, C.Q. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Kao, C.; Tsai, M.; Ong, S.; Chen, C.; Lin, C. Lipid accumulation and CO2 utilization of Nannochloropsis oculate in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef]
- Nielsen, E.S.; Willemoes, M. The influence of CO2 concentration and pH on two Chlorella species grown in continuous light. Physiol. Plant. 1966, 19, 279–293. [Google Scholar] [CrossRef]
- de Morais, M.G.; Costa, J.A.V. Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnol. Lett. 2007, 29, 1349–1352. [Google Scholar] [CrossRef]
- Heubeck, S.; Craggs, R.; Shilton, A. Influence of CO2 scrubbing from biogas on the treatment performance of a high rate algal pond. Water Sci. Technol. 2007, 55, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, Y.; Cheng, L.; Zhang, L.; Tang, D.; Chen, H. Optimization of carbon dioxide fixation by Chlorella vulgaris cultivated in a membrane-photobioreactor. Chem. Eng. Technol. 2007, 30, 1094–1099. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Tohidfar, M.; Jouzani, G.S.; Safarnejad, M.; Pazouki, M. Biodiesel production from genetically engineered microalgae: Future of bioenergy in Iran. Renew. Sustain. Energy Rev. 2011, 15, 1918–1927. [Google Scholar] [CrossRef]
- Demirbas, A. Current technologies for the thermo-conversion of biomass into fuels and chemicals. Energy Sources 2004, 26, 715–730. [Google Scholar] [CrossRef]
- Bonenfant, D.; Mimeault, M.; Hausler, R. Determination of the structural features of distinct amines important for the absorption of CO2 and regeneration in aqueous solution. Ind. Eng. Chem. Res. 2003, 42, 3179–3184. [Google Scholar] [CrossRef]
- Fekete, G. Industrial use of microalgal biomass. Biohulladék 2017, 11, 13–19. (In Hungarian) [Google Scholar]
- Dragone, G.; Fernandes, B.; Vicente, A.A.; Teixeira, J.A. Third generation biofuels from microalgae. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; Volume 2, pp. 1355–1366. [Google Scholar]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- Ahmad, A.; Hassan, S.W.; Banat, F. An overview of microalgae biomass as a sustainable aquaculture feed ingredient: Food security and circular economy. Bioengineered 2022, 13, 9521–9547. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, D.J.; Everard, C.D.; Fagan, C.C.; McDonnell, K.P. Carbon sequestration and the role of biological carbon mitigation: A review. Renew. Sustain. Energy Rev. 2013, 21, 712–727. [Google Scholar] [CrossRef]
- Greene, C.H.; Huntley, M.E.; Archibald, I.; Gerber, L.N.; Sills, D.L.; Granados, J.; Tester, J.W.; Beal, C.M.; Walsh, M.J.; Bidigare, R.R.; et al. Marine microalgae: Climate, energy, and food security from the sea. Oceanography 2016, 29, 10–15. [Google Scholar] [CrossRef]
- Fekete, G.; Sebők, A.; Klátyik, S.; Varga, Z.I.; Grósz, J.; Czinkota, I.; Székács, A.; Aleksza, L. Comparative analysis of laboratory-based and spectroscopic methods used to estimate the algal density of Chlorella vulgaris. Microorganisms 2024, 12, 1050. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; Harrison, S.T. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Psychol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Ördög, V. Contributions to the standardization of laboratory alga tests. Bot. Közlemények—Bot. Publ. 1981, 68, 85–93. [Google Scholar]
- Mokka. Szakolyi Pernye. 2018. Available online: https://mokkka.hu/node/1021 (accessed on 6 November 2024). (In Hungarian).
- Klebercz, O.; Böröndi, T.; Magos, Z.; Feigl, V.; Uzinger, N.; Gruiz, K. Session: E. 3-Sustainable Use of the Subsurface Scale-Up Experiments for Composing Cultivation Media from Degraded Soils and Waste Amendments. 2013. Available online: https://xn--krinfo-wxa.hu/sites/default/files/2345paper_long._Klebercz.pdf (accessed on 6 November 2024).
- Mirzahosseini, A.; Noszál, B. Species-specific standard redox potential of thiol-disulfide systems: A key parameter to develop agents against oxidative stress. Sci. Rep. 2016, 6, 37596. [Google Scholar] [CrossRef]
- Johnson, K.M.; Sieburth, J.M.; leB Williams, P.J.; Brändström, L. Coulometric total carbon dioxide analysis for marine studies: Automation and calibration. Mar. Chem. 1987, 21, 117–133. [Google Scholar] [CrossRef]
- Strotmann, U.; Reuschenbach, P.; Schwarz, H.; Pagga, U. Development and evaluation of an online CO2 evolution test and a multicomponent biodegradation test system. Appl. Environ. Microbiol. 2004, 70, 4621–4628. [Google Scholar] [CrossRef]
- MSZ 08-0206-2:1978; A talaj egyes kémiai tulajdonságainak vizsgálata. Laboratóriumi vizsgálatok. (pH-érték, szódában kifejezett fenolftalein lúgosság, vízben oldható összes só, hidrolitos (yˇ1^-érték) és kicserélődési aciditás (yˇ2^-érték)). Magyar Szabványügyi Testület: Budapest, Hungary, 1978. (In Hungarian)
- Kádár, I. Kármentesítési Kézikönyv 2. A Szennyezett Talajok Vizsgálatáról; Környezetvédelmi Minisztérium: Budapest, Hungary, 1998. (In Hungarian) [Google Scholar]
- Filep, T.; Rékási, M. Factors controlling dissolved organic carbon (DOC), dissolved organic nitrogen (DON) and DOC/DON ratio in arable soils based on a dataset from Hungary. Geoderma 2011, 162, 312–318. [Google Scholar] [CrossRef]
- Parnas, J.K.; Wagner, R. Über die Ausführung von Bestimmungen kleiner Stickstoffmengen nach KJELDAHL. Biochem. Z. 1921, 125, 253. [Google Scholar] [CrossRef]
- Amery, F.; Vandecasteele, B.; D’Hose, T.; Nawara, S.; Elsen, A.; Odeurs, W.; Vandendriessche, H.; Arlotti, D.; Mcgrath, S.P.; Cougnon, M.; et al. Dynamics of soil phosphorus measured by ammonium lactate extraction as a function of the soil phosphorus balance and soil properties. Geoderma. 2021, 385, 114855. [Google Scholar] [CrossRef]
- Gerchev, G.; Mihaylova, G. Milk yield and chemical composition of sheep milk in Srednostaroplaninska and Tetevenska breeds. Biotechnol. Anim. Husb. 2012, 28, 241–251. [Google Scholar] [CrossRef]
- Koçer, A.T.; İnan, B.; Özçimen, D.; Gökalp, İ. A study of microalgae cultivation in hydrothermal carbonization process water: Nutrient recycling, characterization and process design. Environ. Technol. Innov. 2023, 30, 103048. [Google Scholar] [CrossRef]
- Algae Research and Supply. Measuring Growth. 2024. Available online: https://algaeresearchsupply.com/pages/measuring-growth (accessed on 6 November 2024).
- Adamec, L.; Ondok, J.P. Water alkalization due to photosynthesis of aquatic plants: The dependence on total alkalinity. Aquat. Bot. 1992, 43, 93–98. [Google Scholar] [CrossRef]
- Kuo, C.M.; Lin, T.H.; Yang, Y.C.; Zhang, W.X.; Lai, J.T.; Wu, H.T.; Chang, J.S.; Lin, C.S. Ability of an alkali-tolerant mutant strain of the microalga Chlorella sp. AT1 to capture carbon dioxide for increasing carbon dioxide utilization efficiency. Bioresour. Technol. 2017, 244, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Zerveas, S.; Mente, M.S.; Tsakiri, D.; Kotzabasis, K. Microalgal photosynthesis induces alkalization of aquatic environment as a result of H+ uptake independently from CO2 concentration–New perspectives for environmental applications. J. Environ. Manag. 2021, 289, 112546. [Google Scholar] [CrossRef]
- Ihnken, S.; Beardall, J.; Kromkamp, J.C.; Serrano, C.G.; Torres, M.A.; Masojídek, J.; Malpartida, I.; Abdala, R.; Gil Jerez, C.; Malapascua, J.R.F.; et al. Light acclimation and pH perturbations affect photosynthetic performance in Chlorella mass culture. Aquat. Biol. 2014, 22, 95–110. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Elsevier Academic Press: Cambridge, UK, 2005; Volume 578. [Google Scholar]
- Montemezzani, V.; Duggan, I.C.; Hogg, I.D.; Craggs, R.J. A review of potential methods for zooplankton control in wastewater treatment High Rate Algal Ponds and algal production raceways. Algal Res. 2015, 11, 211–226. [Google Scholar] [CrossRef]
- Barker, H.A. Photosynthesis in diatoms. Arch. Microbiol. 1935, 6, 141–156. [Google Scholar] [CrossRef]
- Piorreck, M.; Baasch, K.H.; Pohl, P. Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry 1984, 23, 207–216. [Google Scholar] [CrossRef]
- Schindler, D.W. Eutrophication and recovery in experimental lakes: Implications for lake management. Science 1974, 184, 897–899. [Google Scholar] [CrossRef]
- Correll, D.L. The role of phosphorus in the eutrophication of receiving waters: A review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef]
- Carpenter, S.R. Phosphorus control is critical to mitigating eutrophication. Proc. Natl. Acad. Sci. USA 2008, 105, 11039–11040. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Mandalam, R.K.; Palsson, B. Elemental balancing of biomass and medium composition enhances growth capacity in high-density Chlorella vulgaris cultures. Biotechnol. Bioeng. 1998, 59, 605–611. [Google Scholar] [CrossRef]
- Tyrrell, T. Redfield Ratio. In Encyclopedia of Ocean Sciences; Steele, J.H., Thorpe, A.A., Turekian, K.K., Eds.; Elsevier Academic Press: Cambridge, UK, 2001; pp. 2377–2387. [Google Scholar]
- Matos, Â.P.; Ferreira, W.B.; Morioka, L.R.I.; Moecke, E.H.S.; França, K.B.; Sant’Anna, E.S. Cultivation of Chlorella vulgaris in medium supplemented with desalination concentrate grown in a pilot-scale open raceway. Braz. J. Chem. Eng. 2018, 35, 1183–1192. [Google Scholar] [CrossRef]
- Huang, Y.; Xiong, W.; Liao, Q.; Fu, Q.; Xia, A.; Zhu, X.; Sun, Y. Comparison of Chlorella vulgaris biomass productivity cultivated in biofilm and suspension from the aspect of light transmission and microalgae affinity to carbon dioxide. Bioresour. Technol. 2016, 222, 367–373. [Google Scholar] [CrossRef]
- Ramírez-López, C.; Perales-Vela, H.V.; Fernández-Linares, L. Biomass and lipid production from Chlorella vulgaris UTEX 26 cultivated in 2 m3 raceway ponds under semicontinuous mode during the spring season. Bioresour. Technol. 2019, 274, 252–260. [Google Scholar] [CrossRef]
- Melo, M.; Fernandes, S.; Caetano, N.; Borges, M.T. Chlorella vulgaris (SAG 211-12) biofilm formation capacity and proposal of a rotating flat plate photobioreactor for more sustainable biomass production. J. Appl. Phycol. 2018, 30, 887–899. [Google Scholar] [CrossRef]
- Valdovinos-García, E.M.; Petriz-Prieto, M.A.; Olán-Acosta, M.D.L.Á.; Barajas-Fernández, J.; Guzmán-López, A.; Bravo-Sánchez, M.G. Production of microalgal biomass in photobioreactors as feedstock for bioenergy and other uses: A techno-economic study of harvesting stage. Appl. Sci. 2021, 11, 4386. [Google Scholar] [CrossRef]
- Ammar, S.H. Cultivation of microalgae Chlorella vulgaris in airlift photobioreactor for biomass production using commercial NPK nutrients. Khwarizmi Eng. J. 2016, 12, 90–99. [Google Scholar]
- Ren, H.; Tuo, J.; Addy, M.M.; Zhang, R.; Lu, Q.; Anderson, E.; Chen, P.; Ruan, R. Cultivation of Chlorella vulgaris in a pilot-scale photobioreactor using real centrate wastewater with waste glycerol for improving microalgae biomass production and wastewater nutrients removal. Bioresour. Technol. 2017, 245, 1130–1138. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Lozano, P.; Adjé, F.; Ouattara, A.; Vian, M.A.; Tranchant, C.; Lozano, Y. Effects of temperature and other operational parameters on Chlorella vulgaris mass cultivation in a simple and low-cost column photobioreactor. Appl. Biochem. Biotechnol. 2015, 177, 389–406. [Google Scholar] [CrossRef]
- Razzak, S.A.; Ali, S.A.M.; Hossain, M.M.; Mouanda, A.N. Biological CO2 fixation using Chlorella vulgaris and its thermal characteristics through thermogravimetric analysis. Bioprocess Biosyst. Eng. 2016, 39, 1651–1658. [Google Scholar] [CrossRef]
- Ashour, M.; Mansour, A.T.; Alkhamis, Y.A.; Elshobary, M. Usage of Chlorella and diverse microalgae for CO2 capture-towards a bioenergy revolution. Front. Bioeng. Biotechnol. 2024, 12, 1387519. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.X.; Huang, Y.; Fu, Q.; Liao, Q.; Zhu, X. Kinetic characteristics and modeling of microalgae Chlorella vulgaris growth and CO2 biofixation considering the coupled effects of light intensity and dissolved inorganic carbon. Bioresour. Technol. 2016, 206, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Wen, X.; Geng, Y.; Ouyang, Z.; Luo, L.; Li, Y. Growth rate and biomass productivity of Chlorella as affected by culture depth and cell density in an open circular photobioreactor. J. Microbiol. Biotechnol. 2013, 23, 539–544. [Google Scholar] [CrossRef]
- Je, S.; Yamaoka, Y. Biotechnological approaches for biomass and lipid production using microalgae Chlorella and its future perspectives. J. Microbiol. Biotechnol. 2022, 32, 1357. [Google Scholar] [CrossRef]
- Kong, W.; Kong, J.; Ma, J.; Lyu, H.; Feng, S.; Wang, Z.; Yuan, P.; Shen, B. Chlorella vulgaris cultivation in simulated wastewater for the biomass production, nutrients removal and CO2 fixation simultaneously. J. Environ. Manag. 2021, 284, 112070. [Google Scholar] [CrossRef] [PubMed]
- James, C.M.; Al-Khars, A.M. An intensive continuous culture system using tubular photobio-reactors for producing microalgae. Aquaculture 1990, 87, 381–393. [Google Scholar] [CrossRef]
- Bai, A.; Popp, J.; Pető, K.; Szőke, I.; Harangi-Rákos, M.; Gabnai, Z. The significance of forests and algae in CO2 balance: A Hungarian case study. Sustainability 2017, 9, 857. [Google Scholar] [CrossRef]
- Narala, R.R.; Garg, S.; Sharma, K.K.; Thomas-Hall, S.R.; Deme, M.; Li, Y.; Schenk, P.M. Comparison of microalgae cultivation in photobioreactor, open raceway pond, and a two-stage hybrid system. Front. Energy Res. 2016, 4, 29. [Google Scholar] [CrossRef]
- Abou-Shanab, R. Green renewable Energy for Sustainable socio-economic development. In Proceedings of the 14th International Conference on Environmental Science and Technology, Rhodes, Greece, 3–5 September 2015; pp. 3–5. [Google Scholar]
- Bošnjaković, M.; Sinaga, N. The perspective of large-scale production of algae biodiesel. Appl. Sci. 2020, 10, 8181. [Google Scholar] [CrossRef]
- Egbo, M.K.; Okoani, A.O.; Okoh, I.E. Photobioreactors for microalgae cultivation—An overview. Int. J. Sci. Eng. Res. 2018, 9, 65–74. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).