Effects of Underwater Lighting Time on the Growth of Vallisneria spinulosa Yan and Its Water Restoration Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Monitoring Method
2.3.1. Water Sample Test
2.3.2. Physiological and Growth Characteristics of V. spinulosa
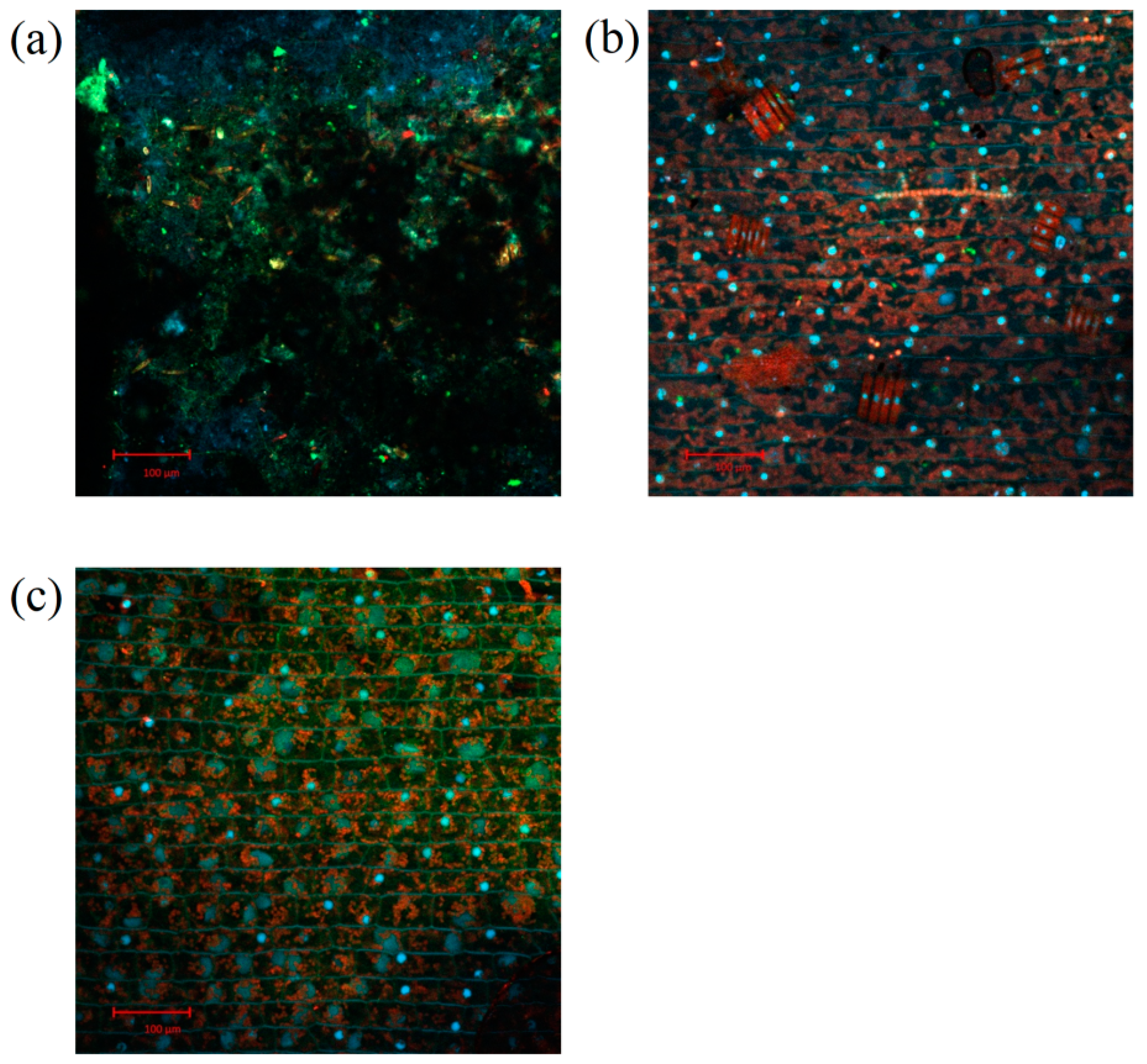
2.3.3. CLSM Determination
2.3.4. Microbial Sequencing
- DNA Extraction and PCR Amplification
- 2.
- Illumina Miseq sequencing
2.4. Data Analysis
3. Results and Discussion
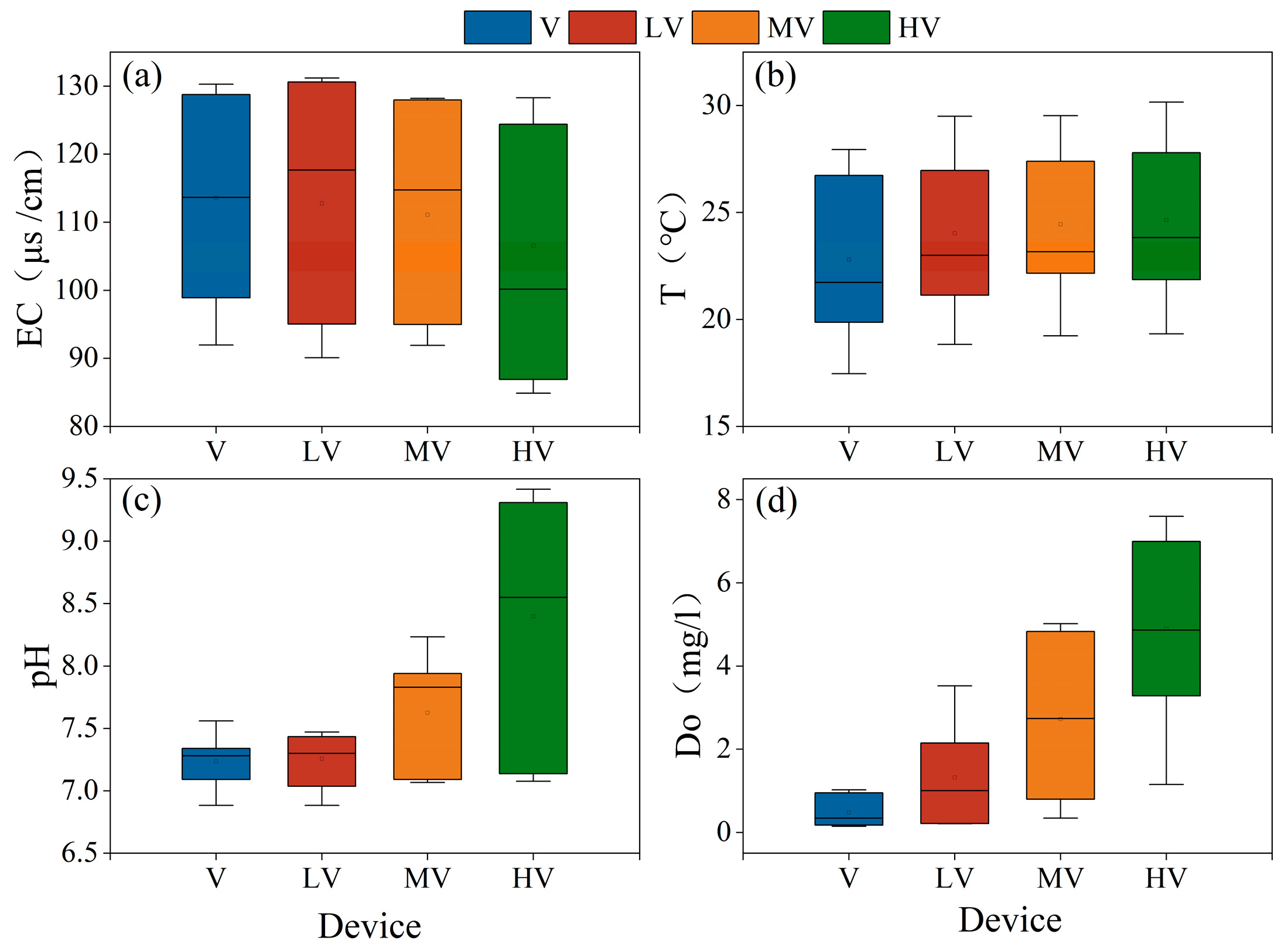
3.1. The Effect of the Light Duration Ratio on the Water Purification Effect of the System
3.1.1. Physical and Chemical Environment of Water Body
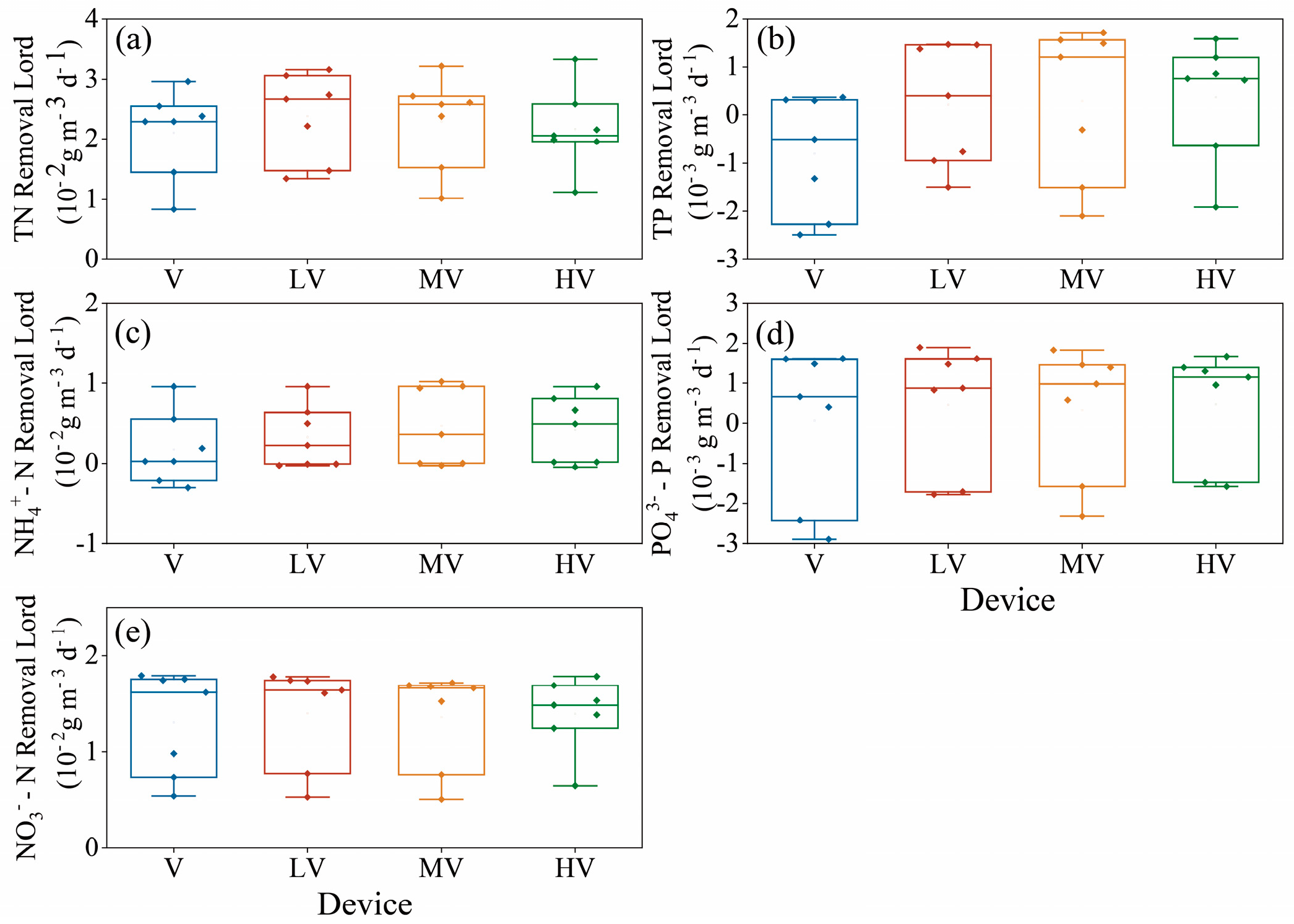
3.1.2. Removal of N and P
3.2. The Effect of the Light Duration Ratio on the Photosynthetic System of V. spinulosa
3.2.1. Fv/Fm and ETRmax of V. spinulosa
3.2.2. Chlorophyll Content in Leaves of V. spinulosa
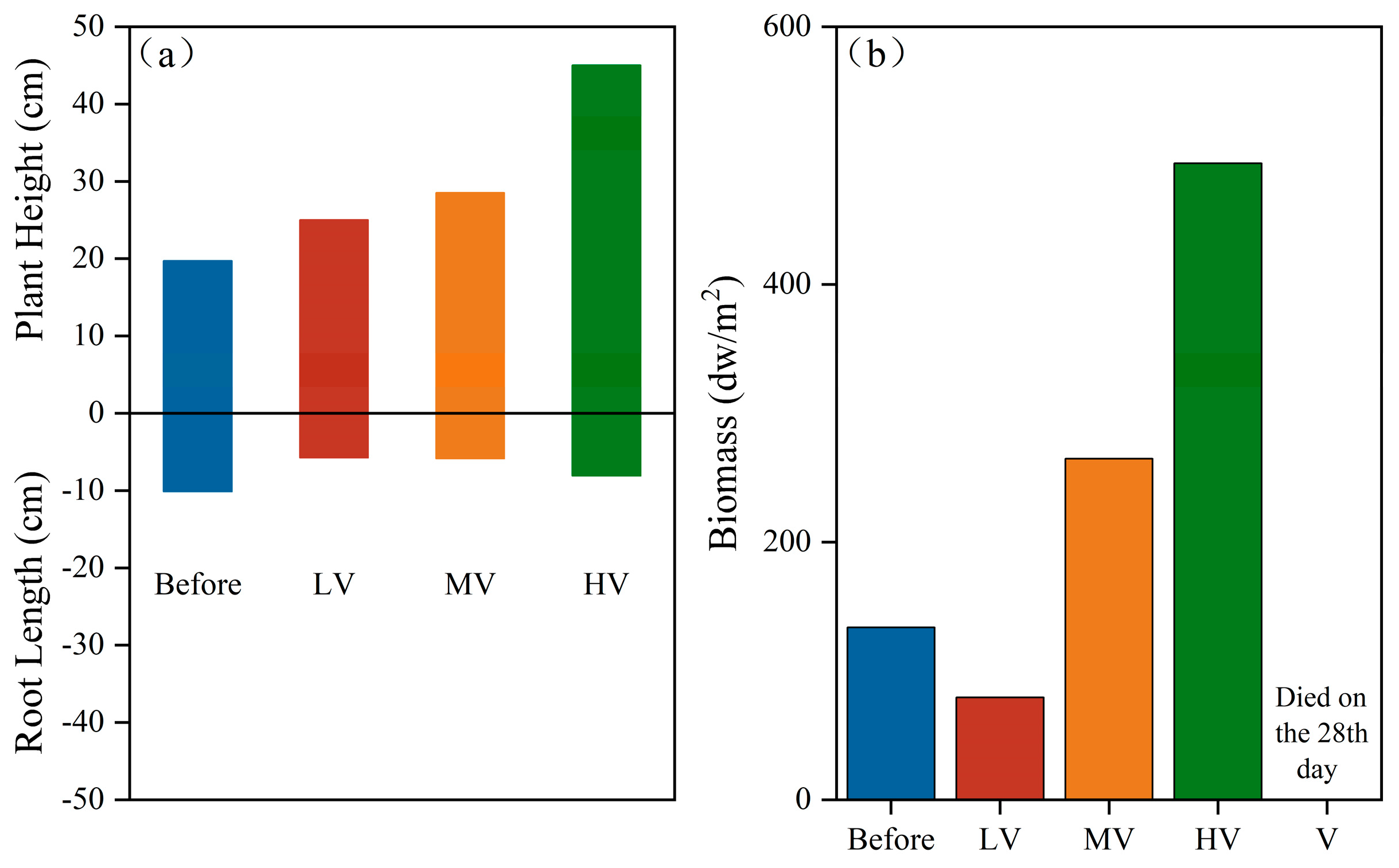
3.3. The Effect of the Light Duration Ratio on the Biomass of V. spinulosa
3.4. The Effect of the Light Duration Ratio on the Leaf Surface of V. spinulosa
3.5. The Difference in the Microbial Community in the System Under Different Light Duration Ratios
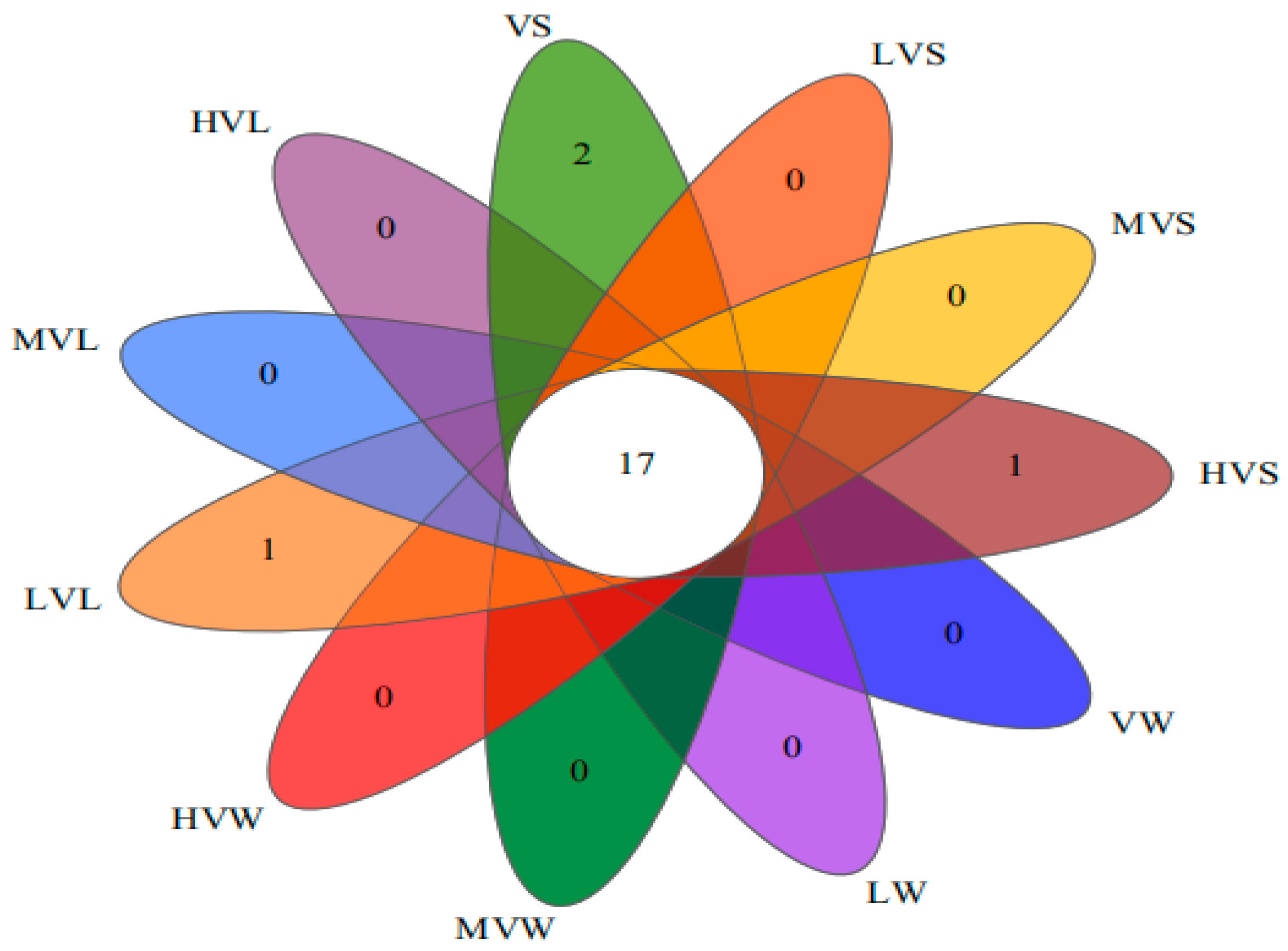
3.5.1. Changes in Microbial Community Abundance and Diversity
- α−Diversity Analysis
- 2.
- Classification distribution at phylum and genus levels
3.5.2. Changes and Differences in Microbial Community Structure
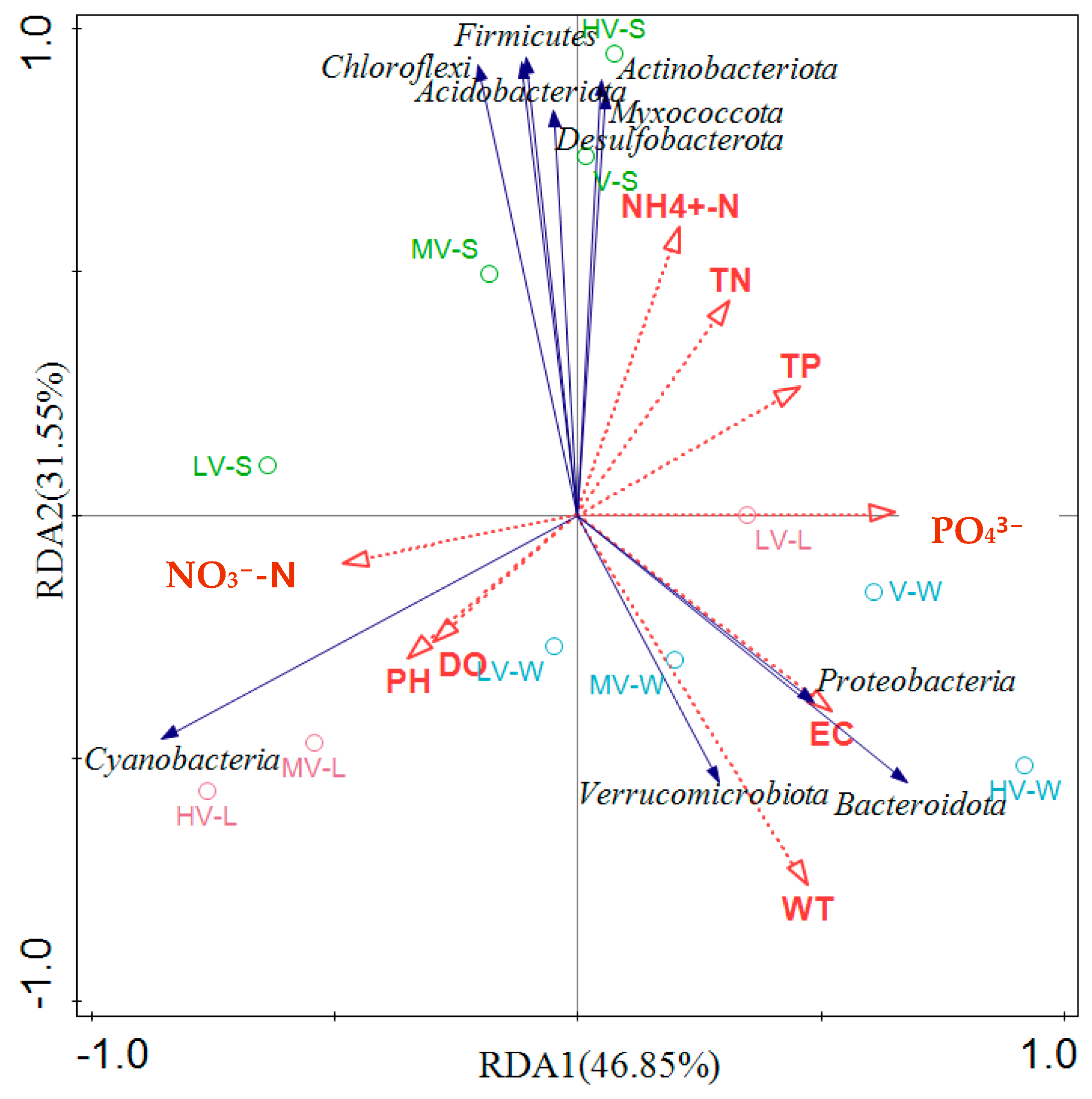
3.6. The Relationship Between Light Duration, Microbial Community, and Environmental Variables
4. Conclusions
- (1)
- There was no significant difference in T and EC between different light duration ratios of auxiliary light sources, but there was a significant difference in DO. A high light duration promoted more photosynthesis of plants, removed CO2 from the water, and increased the pH of the water. Although different light duration ratios had no significant effect on the purification capacity of the water quality in the system, low and medium light duration ratios could promote the removal of NH4+–N and TN, and TP had the highest removal load under the high light duration ratio. Compared with the group without an auxiliary light source (i.e., the light duration ratio is 0:24), the removal efficiency of each water quality index in the group with an auxiliary light source is better.
- (2)
- The different light duration ratios of the auxiliary light source have a significant effect on the growth of V. spinulosa. When the light duration ratio is 18:6, V. spinulosa has the highest Fv/Fm and ETRmax, the highest chlorophyll content, the highest plant height and biomass growth rate, and the lowest algae abundance.
- (3)
- Under different light duration ratios, the sediment area in the 18:6 light group had the highest bacterial abundance and diversity, while the water and leaf surface in the 6:18 light group had the highest bacterial abundance and diversity. There were significant differences in the bacterial communities between the group without additional light ratio (V group 0:24) and the groups with low, medium, and high light duration ratios (LV group 6:18, MV group 12:12, HV group 18:6). The response of various dominant phyla to different light duration ratios in different locations (water, leaf surface, and sediment area) was significantly different. The addition of high light duration resulted in significantly different taxa, including Flavobacteriaceae, Moraxellaceae, and Candidatus Paenicardinium, in the habitat of V. spinulosa. The number of differential microbial groups was the largest in the LV group, and the number of differential microorganisms was the least in the MV group, with only Silanimonas, which was a biomarker. The V. spinulosa without additional light ratio group had a more mature biofilm, and the relative abundances of Dechloromonas and Bdellovibrio were higher.
- (4)
- T and EC were the two main environmental factors affecting the bacterial community of the four light duration ratios. The representative species Proteobacteria, Bacteroidota, and Verrucomicrobia have a strong positive correlation, and T and EC have a strong positive correlation with the species abundance of these three phyla, that is, within a certain range, the species abundances of these three phyla increase with the increase in temperature and conductivity. Cyanobacteria was positively correlated with water quality parameters (NO3−–N, pH, and DO), while NH4+–N, TN, and TP were positively correlated with six phyla, such as Actinobacteriota and Chloroflexi.
- (5)
- Path analysis showed that different light duration ratios had a very significant correlation with the physical and chemical environment of water (p < 0.001) and had a significant correlation with the characteristics of V. spinulosa (p < 0.05). According to conjecture, the system mainly improves the photosynthesis ability of V. spinulosa leaves by adapting to different light duration ratios, removes CO2 in water, and increases the pH of water. After improving the physical and chemical environment of the water body, the purpose of promoting the clonal reproduction and biomass increase of V. spinulosa was achieved, which was also conducive to the removal of N and P in the water by the system. The change in water properties and the difference in the light duration ratio also affect the change in microorganisms in the system.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazur, R. Lakes Restoration: Analysis of terminology incorrectly used in the scientific literature. Acta Sci. Pol. Form. Circumiectus 2019, 18, 135–146. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, R.; Jeppesen, E.; Zhang, E. Submerged macrophytes can counterbalance the negative effects of rising temperature and eutrophication by inhibiting the photosynthetic activity of cyanobacteria and adjusting their morphology and physiology. Freshw. Biol. 2024, 69, 1842–1856. [Google Scholar] [CrossRef]
- Zhang, X.; Mei, X.; Gulati, R.D. Effects of Omnivorous tilapia on water turbidity and primary production dynamics in shallow lakes: Implications for ecosystem management. Rev. Fish. Biol. Fish. 2016, 27, 245–254. [Google Scholar] [CrossRef]
- Parra, L.; Rocher, J.; Escrivá, J.; Lloret, J. Design and development of low cost smart turbidity sensor for water quality monitoring in fish farms. Aquac. Eng. 2018, 81, 10–18. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, J.; Zhong, P.; Zhang, X.; Ning, J.; Chen, D.; Gao, Y.; He, H.; Jeppesen, E. Successful restoration of a tropical shallow eutrophic lake: Strong bottom−up but weak top−down effects recorded. Water Res. 2018, 146, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Qin, B.; Shi, K.; Deng, J.; Zhou, Y. Aquatic vegetation in response to increased eutrophication and degraded light climate in Eastern Lake Taihu: Implications for lake ecological restoration. Sci. Rep. 2016, 6, 23867. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Kozerski, H.P.; Pluntke, T.; Rinke, K. The influence of macrophytes on sedimentation and nutrient retention in the lower River Spree (Germany). Water Res. 2003, 37, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hua, Z. The effect of vegetation on sediment resuspension and phosphorus release under hydrodynamic disturbance in shallow lakes. Ecol. Eng. 2014, 69, 55–62. [Google Scholar] [CrossRef]
- Blindow, I.; Hargeby, A.; Hilt, S. Facilitation of clear−water conditions in shallow lakes by macrophytes: Differences between charophyte and angiosperm dominance. Hydrobiologia 2014, 737, 99–110. [Google Scholar] [CrossRef]
- Choudhury, M.I.; Urrutia−Corderoc, P.; HuanZhang Ekvall, M.K.; Medeiros, L.R.; Hanssona, L. Charophytes collapse beyond a critical warming and brownification threshold in shallow lake systems. Sci. Total Environ. 2019, 661, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, Y.; Li, Q.; Gao, J.; Zhai, S.; Zhou, Y.; Cheng, Y. Long−term and inter−monthly dynamics of aquatic vegetation and its relation with environmental factors in Taihu Lake, China. Sci. Total Environ. 2019, 651, 367–380. [Google Scholar] [CrossRef]
- Geist, J. Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 2011, 11, 1507–1516. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeppesen, E.; Liu, X.; Qin, B.; Shi, K.; Zhou, Y.; Thomaz, S.M.; Deng, J. Global loss of aquatic vegetation in lakes. Earth−Sci. Rev. 2017, 173, 259–265. [Google Scholar] [CrossRef]
- He, D.; Ren, L.; Wu, Q.L. Contrasting diversity of epibiotic bacteria and surrounding bacterioplankton of a common submerged macrophyte, Potamogeton crispus, in freshwater lakes. FEMS Microbiol. Ecol. 2014, 90, 551–562. [Google Scholar] [CrossRef]
- Yan, L.; Mu, X.; Han, B.; Zhang, S.; Qiu, C.; Ohore, O.E. Ammonium loading disturbed the microbial food webs in biofilms attached to submersed macrophyte Vallisneria natans. Sci. Total Environ. 2019, 659, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Lacoul, P.; Freedman, B. Environmental influences on aquatic plants in freshwater ecosystems. Environ. Rev. 2006, 14, 89–136. [Google Scholar] [CrossRef]
- Arthaud, F.; Mousset, M.; Vallod, D.; Robin, J.; Wezel, A. Gudrunbornette Effect of light stress from phytoplankton on the relationship between aquatic vegetation and the propagule bank in shallow lakes. Freshw. Biol. 2012, 57, 666–675. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Xiao, S.; Chen, R.; Luo, C.; Zhu, T.; Cao, T.; Ni, L.; Xie, P.; Su, H.; et al. Effects of benthivorous fish disturbance on chlorophyll a contents in water and the growth of two submersed macrophytes with different growth forms under two light regimes. Sci. Total Environ. 2020, 704, 135269. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Yu, D.; You, W.; Wang, L. Algae mediate submerged macrophyte response to nutrient and dissolved inorganic carbon loading: A mesocosm study on different species. Chemosphere 2013, 93, 1301–1308. [Google Scholar] [CrossRef]
- Chen, J.; Cao, T.; Zhang, X.; Xi, Y.; Ni, L.; Jeppesen, E. Differential photosynthetic and morphological adaptations to low light affect depth distribution of two submersed macrophytes in lakes. Sci. Rep. 2016, 6, 34028. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, W.; Li, X.; Chu, Q.; Tang, N.; Shu, B.; Liu, G.; Xing, W. How many submerged macrophyte species are needed to improve water clarity and quality in Yangtze floodplain lakes? Sci. Total Environ. 2020, 724, 138267. [Google Scholar] [CrossRef] [PubMed]
- Waiters, M.; Erken, M.; Majdi, N.; Arndt, H.; Norf, H.; Reinshagen, M.; Traunspurger, W.; Walterscheid, A.; Wey, J.K. The food web perspective on aquatic biofilms. Ecol. Monogr. 2018, 88, 543–559. [Google Scholar]
- Sorrell, B.K. Regulation of Root Anaerobiosis and Carbon Translocation by Light and Root Aeration in Isoetes Alpinus. Plant Cell Environ. 2004, 27, 1102–1111. [Google Scholar] [CrossRef]
- Xie, Y.; Luo, W.; Ren, B.; Li, F. Morphological and physiological responses to sediment type and light availability in roots of the submerged plant Myriophyllum spicatum. Ann. Bot. 2007, 100, 1517–1523. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, P.; Pan, S.; Li, Y.; Lin, L.; Niu, L.; Wang, L.; Zhang, H. The role of microbial communities on primary producers in aquatic ecosystems: Implications in turbidity stress resistance. Environ. Res. 2022, 215, 114353. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, U.; Cescatti, A.; Rodeghiero, M.; Tosens, T. Complex adjustments of photosynthetic potentials and internal diffusion conductance to current and previous light availabilities and leaf age in Mediterranean evergreen species Quercus ilex. Plant Cell Environ. 2006, 29, 1159–1178. [Google Scholar] [CrossRef]
- Thompson, W.A.; Huang, L.K.; Kriedemann, P.E. Photosynthetic Response to Light and Nutrients in Sun−tolerant and Shade−tolerant Rainforest Trees. II.* Leaf Gas Exchange and Component Processes of Photosynthesis. Funct. Plant Biol. 1992, 19, 19–42. [Google Scholar] [CrossRef]
- Hanelt, D.; Hawes, I.; Rae, R. Reduction of UV−B radiation causes an enhancement of photoinhibition in high light stressed aquatic plants from New Zealand lakes. J. Photochem. Photobiol. B 2006, 84, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Zefferman, E. Increasing canopy shading reduces growth but not establishment of Elodea nuttallii and Myriophyllum spicatum in stream channels. Hydrobiologia 2014, 734, 159–170. [Google Scholar] [CrossRef]
- Yuan, J.; Bai, Z.; Ye, S.; Liu, H.; Wang, Y.; Li, F.; Xie, Y.; Gao, A.; Wu, A. High−light inhibition of two submerged macrophytes in a shallow water experiment. AoB Plants 2022, 14, plac009. [Google Scholar] [CrossRef]
- Fu, H.; Yuan, G.; Cao, T.; Ni, L.; Zhang, M.; Wang, S. An alternative mechanism for shade adaptation: Implication of allometric responses of three submersed macrophytes to water depth. Ecol. Res. 2012, 27, 1087–1094. [Google Scholar] [CrossRef]
- Yuan, G.; Fu, H.; Zhong, J.; Lou, Q.; Ni, L.; Cao, T. Growth and C/N metabolism of three submersed macrophytes in response to water depths. Environ. Exp. Bot. 2016, 122, 94–99. [Google Scholar] [CrossRef]
- Chen, J.; Chou, Q.; Ren, W.; Su, H.; Zhang, M.; Cao, T.; Zhu, T.; Ni, L.; Liu, Z.; Xie, P. Growth, morphology and C/N metabolism responses of a model submersed macrophyte, Vallisneria natans, to various light regimes. Ecol. Indic. 2022, 136, 18652. [Google Scholar] [CrossRef]
- Yan, X.; Yu, D.; Li, Y. The effects of elevated CO2 on clonal growth and nutrient content of submerge plant Vallisneria spinulosa. Chemosphere 2006, 62, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ren, W.; Chou, Q.; Su, H.; Ni, L.; Zhang, M.; Liu, Z.; Xie, P. Alterations in biomass allocation indicate the adaptation of submersed macrophytes to low−light stress. Ecol. Indic. 2020, 113, 106235. [Google Scholar] [CrossRef]
- HJ 636−2012; Water Quality−Determination of Total Nitrogen−Alkaline Potassium Persulfate Digestion UV Spectrophotometric Method[S]. Ministry of Ecology and Environment: Beijing, China, 2012.
- GB 11893−89; Water Quality—Determination of Total Phosphorus–Ammonium Molybdate Spectrophotometric method[S]. Ministry of Ecology and Environment: Beijing, China, 1989.
- HJ/T 346−2007; Water Quality—Determination of Nitrate–Nitrogen–Ultraviolet Spectrophotometry[S]. Ministry of Ecology and Environment: Beijing, China, 2007.
- HJ 534—2009; Ambient Air—Determination of Ammonia—Sodium Hypochlorite–Salicylic Acid Spectrophotometry[S]. Ministry of Ecology and Environment: Beijing, China, 2009.
- Chae, S.S.; DuBien, J.L.; Warde, W.D. A method of predicting the number of clusters using Rand’s statistic. Comput. Stat. Data Anal. 2006, 50, 3531–3546. [Google Scholar] [CrossRef]
- Huang, L.; Wang, N.; Deng, C.; Liang, Y.; Wang, Q.; Liu, M.; Chen, Y. Interactive effect of carbon source with influent COD/N on nitrogen removal and microbial community structure in subsurface flow constructed wetlands. J. Environ. Manag. 2019, 250, 109491. [Google Scholar] [CrossRef]
- Fan, J.; Wang, W.; Zhang, B.; Guo, Y.; Ngo, H.H.; Guo, W.; Zhang, J.; Wu, H. Nitrogen removal in intermittently aerated vertical flow constructed wetlands: Impact of influent COD/N ratios. Bioresour. Technol. 2013, 143, 461–466. [Google Scholar] [CrossRef]
- Rajta, A.; Bhatia, R.; Setia, H.; Pathani, P. Role of heterotrophic aerobic denitrifying bacteria in nitrate removal from wastewater. J. Appl. Microbiol. 2020, 128, 1261–1278. [Google Scholar] [CrossRef] [PubMed]
- Copetti, D.; Valsecchi, L.; Tartari, G.; Mingazzini, M.; Palumbo, M.T. Phosphate adsorption by riverborne clay sediments in a southern−Italy Mediterranean reservoir: Insights from a “natural geo−engineering”experiment. Sci. Total Environ. 2023, 856, 159225. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi−Sarvestani, Z.; Modarres−Sanavy, S.A.M.; Nicola, S. Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiol. Biochem. 2016, 106, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pan, J.; Ren, W.; Yang, J.; Luo, L. The effects of water depth on the growth of two submerged macrophytes in an in situ experiment. J. Freshw. Ecol. 2021, 36, 271–284. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.; Yang, Q.; Gu, Y.; Wang, P.; Li, Z.; Li, L. Performance and mechanism of the in situ restoration effect on VHCs in the polluted river water based on the orthogonal experiment: Photosynthetic fluorescence characteristics and microbial community analysis. Environ. Sci. Pollut. Res. 2022, 29, 43004–43018. [Google Scholar] [CrossRef]
- Mishra, S.; Agrawal, S.B. Interactive effects between supplemental ultraviolet−B radiation and heavy metals on the growth and biochemical characteristics of Spinacia oleracea L. Braz. J. Plant Physiol. 2006, 18, 307–314. [Google Scholar]
- Zhao, X.; Jia, T.; Hu, X. HCAR Is a Limitation Factor for Chlorophyll Cycle and Chlorophyll b Degradation in Chlorophyll−b−Overproducing Plants. Biomolecules 2020, 10, 1639. [Google Scholar] [CrossRef] [PubMed]
- Conde−Álvarez, R.M.; Bañares−España, E.; Nieto−Caldera, J.M.; Flores−Moya, A.; Figueroa, F.L. Photosynthetic performance of the aquatic macrophyte Althenia orientalis to solar radiation along its vertical stems. Oecologia 2011, 166, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gu, P.; Luo, X.; Zhang, H.; Huang, S.; Zhang, J.; Zheng, Z. Pilot−scale study on the effects of cyanobacterial blooms on Vallisneria natans and biofilms at different phosphorus concentrations. Environ. Pollut. 2020, 265, 114996. [Google Scholar] [CrossRef] [PubMed]
- Gerbersdorf, S.U.; Koca, K.; de Beer, D.; Chennu, A.; Noss, C.; Risse−Buhl, U.; Weitere, M.; Eiff, O.; Wagner, M.; Aberle, J.; et al. Exploring flow−biofilm−sediment interactions: Assessment of current status and future challenges. Water Res. 2020, 185, 116182. [Google Scholar] [CrossRef]
- Shi, L.; Huang, Y.; Zhang, M.; Yu, Y.; Lu, Y.; Kong, F. Bacterial community dynamics and functional variation during the long−term decomposition of cyanobacterial blooms in−vitro. Sci. Total Environ. 2017, 598, 77–86. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, H.; Xiao, W.; Lu, J.; Di Lu Chen, X.; Zheng, X.; Jeppesen, E.; Zhang, W.; Wang, L. Ecotoxicological effects of sulfonamide on and its removal by the submerged plant Vallisneria natans (Lour.) Hara. Water Res. 2020, 170, 115354. [Google Scholar] [CrossRef] [PubMed]
- Kordahi, M.A.; Ayoub, G.M.; Zayyat, R.M. A critical review of current research on cyanobacterial cells and associated toxins in aquatic environments: Occurrence, impact, and treatment methods. J. Environ. Chem. Eng. 2024, 12, 113931. [Google Scholar] [CrossRef]
- Fang, S.; Cao, W.; Wu, Q.; Cheng, S.; Yang, Y.; Liu, J.; Wu, Y.; Fang, F.; Feng, Q.; Cao, J.; et al. Multifaceted roles of methylisothiazolinone intervention in sludge disintegration and acidogenic and methanogenic pathways for efficient carboxylate production during anaerobic fermentation. Chem. Eng. J. 2023, 472, 145022. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, B.; Yang, H.; Wang, B.; Zhang, N.; Dou, H.; Wei, G.; Sun, Y.; Zhang, L. Investigation of glycerol−derived binary and ternary systems in CO2 capture process. Fuel 2017, 210, 836–843. [Google Scholar] [CrossRef]
- Seo, H.; Kim, J.H.; Lee, S.M.; Lee, S.W. The plant−associated flavobacterium: A hidden helper for improving plant health. Plant Pathol. 2024, 40, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Duffy, B.; Nowak, J.; Clement, C.; Barka, E.A. Use of plant growth−promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Défago, G. Biological control of soil−borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van Wees, S.C.M.; Ton, J.; Van Pelt, J.A.; Van Loon, L.C. Signaling in rhizobacteria−induced systemic resistance in Arabidopsis thaliana. Plant Biol. 2002, 4, 535–544. [Google Scholar] [CrossRef]
- Li, B.; Tao, Y.; Mao, Z.; Gu, Q.; Han, Y.; Hu, B.; Wang, H.; Lai, A.; Xing, P.; Wu, Q.L. Iron oxides act as an alternative electron acceptor for aerobic methanotrophs in anoxic lake sediments. Water Res. 2023, 234, 119833. [Google Scholar] [CrossRef] [PubMed]
- Gerber, E.; Bernard, R.; Castang, S.; Chabot, N.; Coze, F.; Dreux−Zigha, A.; Hauser, E.; Hivin, P.; Joseph, P.; CLazarelli, G.L.J.O. Deinococcus as new chassis for industrial biotechnology: Biology, physiology and tools. J. Appl. Microbiol. 2015, 119, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Haller, L.; Tonolla, M.; Zopfi, J.; Peduzzi, R.; Wildi, W.; Poté, J. Composition of bacterial and archaeal communities in freshwater sediments with different contamination levels (Lake Geneva, Switzerland). Water Res. 2011, 45, 1213–1228. [Google Scholar] [CrossRef] [PubMed]






| Group | Setting Condition | Light Duration Ratio (h/h) |
|---|---|---|
| V | V. spinulosa without auxiliary light source | 0:24 |
| LV | V. spinulosa + low light duration ratio | 6:18 |
| MV | V. spinulosa + medium light duration ratio | 12:12 |
| HV | V. spinulosa + high light duration ratio | 18:16 |
| Number | Sample | Sobs | Ace | Chao | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|---|---|
| VW | V–Water | 975 | 1346.5769 | 1274.7556 | 3.9456 | 0.0573 | 0.9946 |
| LVW | LV–Water | 1364 | 1713.4491 | 1687.9471 | 4.6054 | 0.0248 | 0.9939 |
| MVW | MV–Water | 780 | 1019.8195 | 1005.6240 | 3.6098 | 0.0703 | 0.9959 |
| HVW | HV–Water | 715 | 1016.6226 | 970.0484 | 3.4843 | 0.0569 | 0.9959 |
| LVL | LV–Leaf surface | 2895 | 3983.0310 | 3753.0581 | 4.1703 | 0.1270 | 0.9812 |
| MVL | MV–Leaf surface | 2261 | 3189.3955 | 3043.4607 | 4.4975 | 0.0473 | 0.9864 |
| HVL | HV–Leaf surface | 863 | 1129.9715 | 1076.6892 | 3.6245 | 0.1206 | 0.9961 |
| VS | V–Sediment | 4973 | 6459.3254 | 6147.5857 | 6.1970 | 0.0235 | 0.9713 |
| LVS | LV–Sediment | 4173 | 5726.0228 | 5431.6128 | 4.4752 | 0.1259 | 0.9720 |
| MVS | MV–Sediment | 5315 | 6690.4853 | 6222.6734 | 6.6212 | 0.0166 | 0.9630 |
| HVS | HV–Sediment | 6519 | 7953.2989 | 7437.1660 | 7.3283 | 0.0049 | 0.9691 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M.; Zhao, J.; Zhou, X.; Li, F.; Zhao, M.; Zheng, X.; Tang, Y.; Yang, C.; Jin, Z.; Wu, S. Effects of Underwater Lighting Time on the Growth of Vallisneria spinulosa Yan and Its Water Restoration Process. Water 2024, 16, 3697. https://doi.org/10.3390/w16243697
Wei M, Zhao J, Zhou X, Li F, Zhao M, Zheng X, Tang Y, Yang C, Jin Z, Wu S. Effects of Underwater Lighting Time on the Growth of Vallisneria spinulosa Yan and Its Water Restoration Process. Water. 2024; 16(24):3697. https://doi.org/10.3390/w16243697
Chicago/Turabian StyleWei, Mengyi, Jinshan Zhao, Xiaolin Zhou, Fengdan Li, Min Zhao, Xiangyong Zheng, Ye Tang, Chang Yang, Zhenmin Jin, and Suqing Wu. 2024. "Effects of Underwater Lighting Time on the Growth of Vallisneria spinulosa Yan and Its Water Restoration Process" Water 16, no. 24: 3697. https://doi.org/10.3390/w16243697
APA StyleWei, M., Zhao, J., Zhou, X., Li, F., Zhao, M., Zheng, X., Tang, Y., Yang, C., Jin, Z., & Wu, S. (2024). Effects of Underwater Lighting Time on the Growth of Vallisneria spinulosa Yan and Its Water Restoration Process. Water, 16(24), 3697. https://doi.org/10.3390/w16243697






