Capacity and Mechanisms of Phosphate Adsorption on Lanthanum-Modified Dewatered Sludge-Based Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of La-SBBC
2.2. Characterization of La-SBBC
2.3. Batch Adsorption Tests
2.4. Fixed-Bed Column Experiments
3. Results and Discussion
3.1. Preparation Ratio of La-SBBC
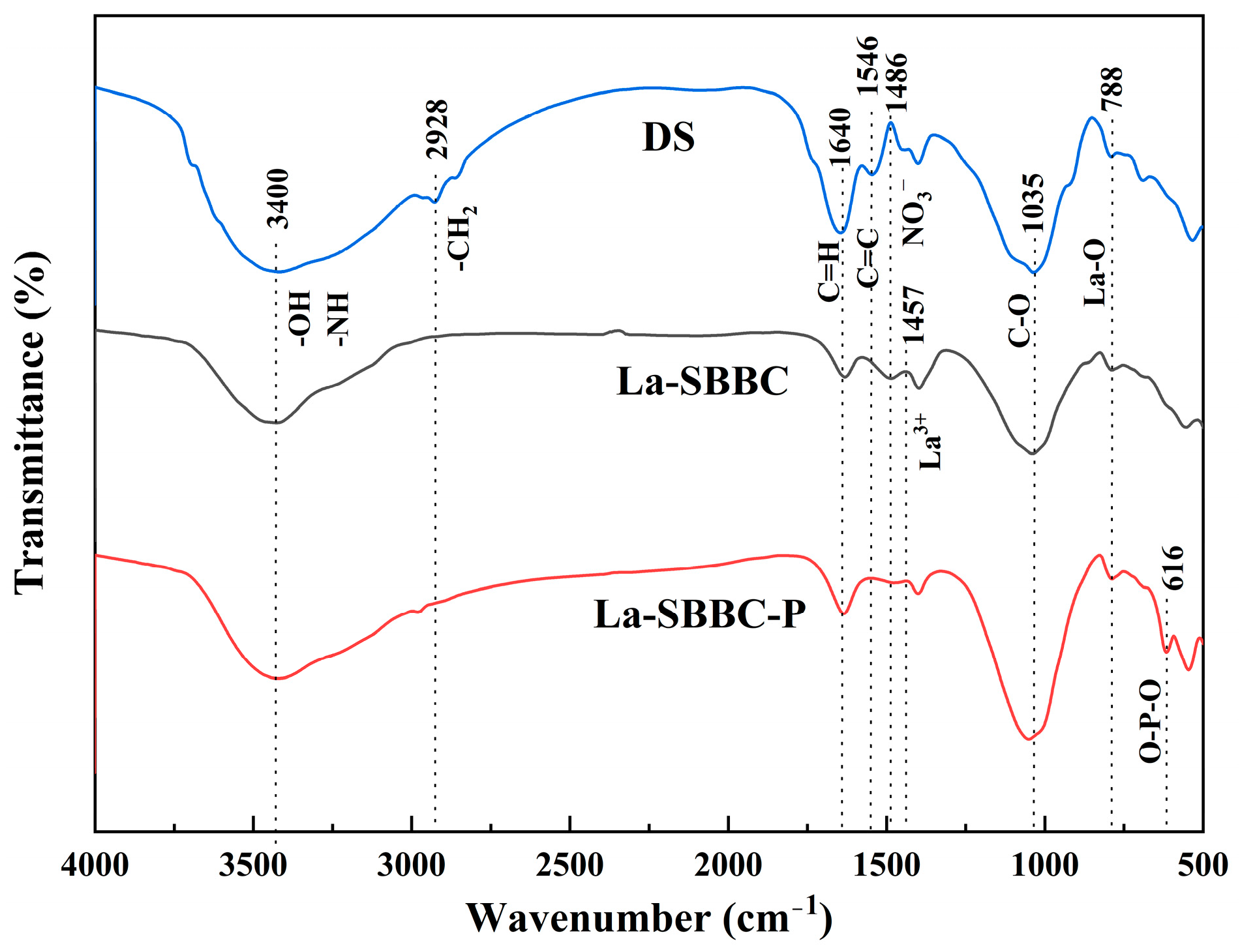
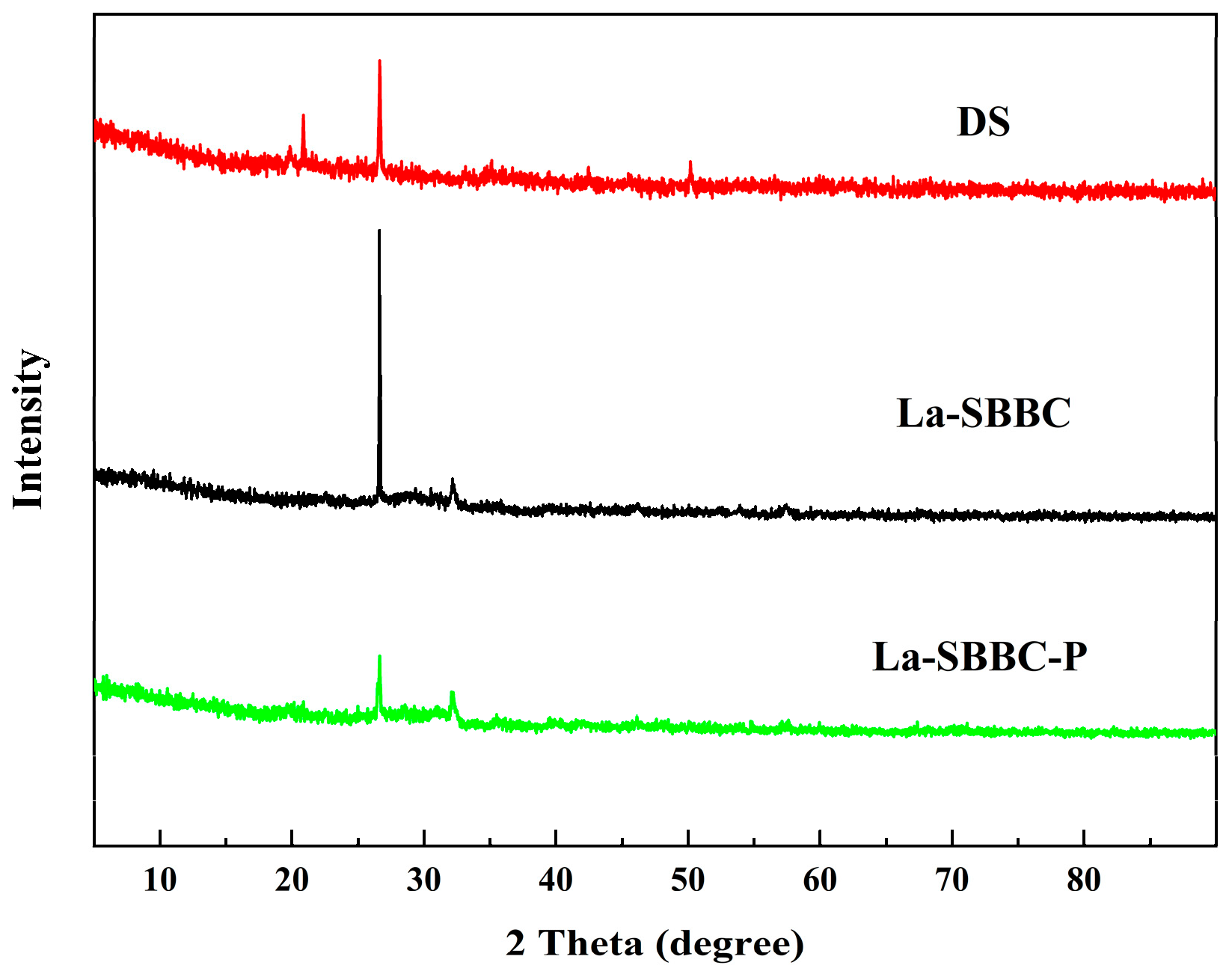
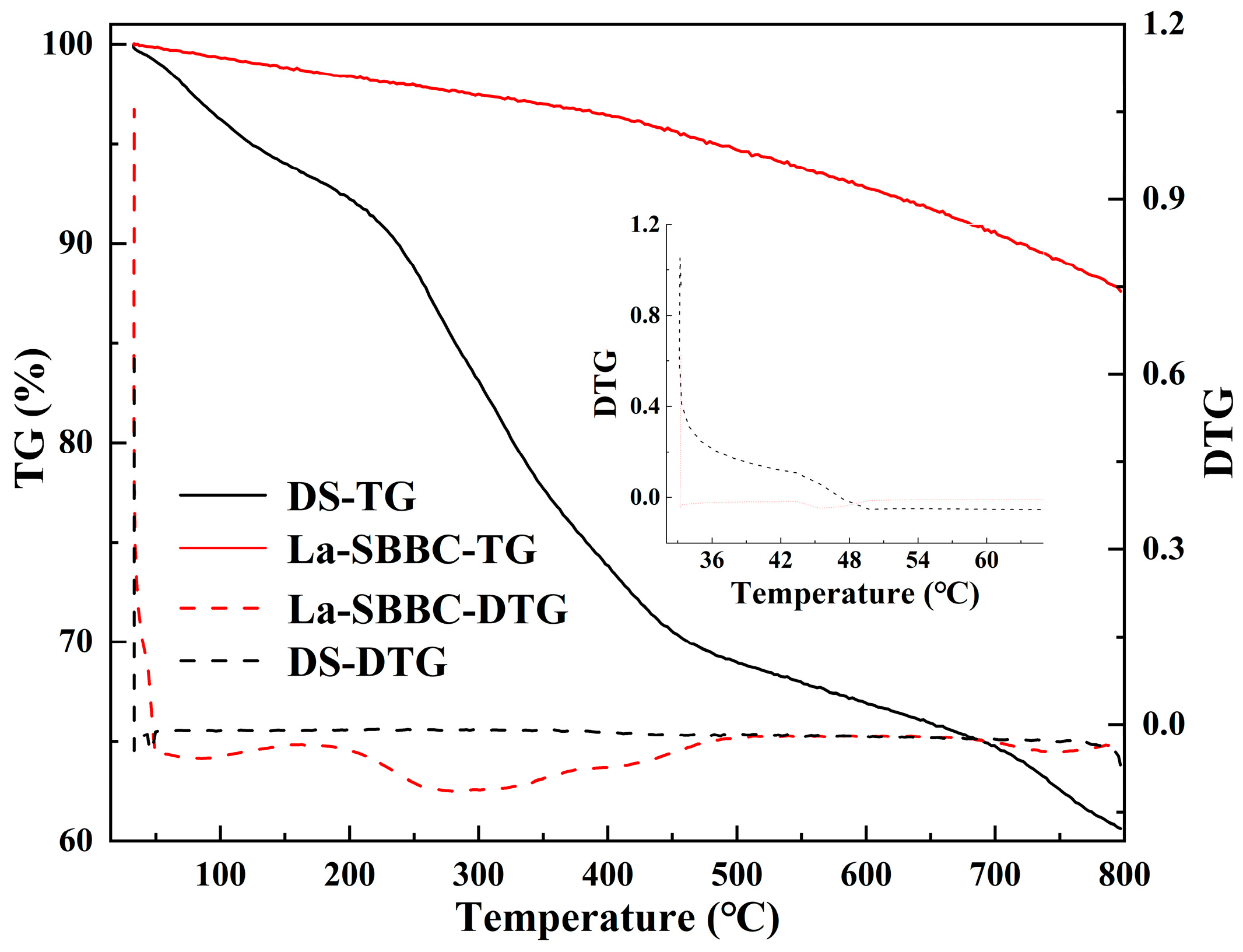
3.2. Characterization of La-SBBC
3.3. Phosphate Adsorption
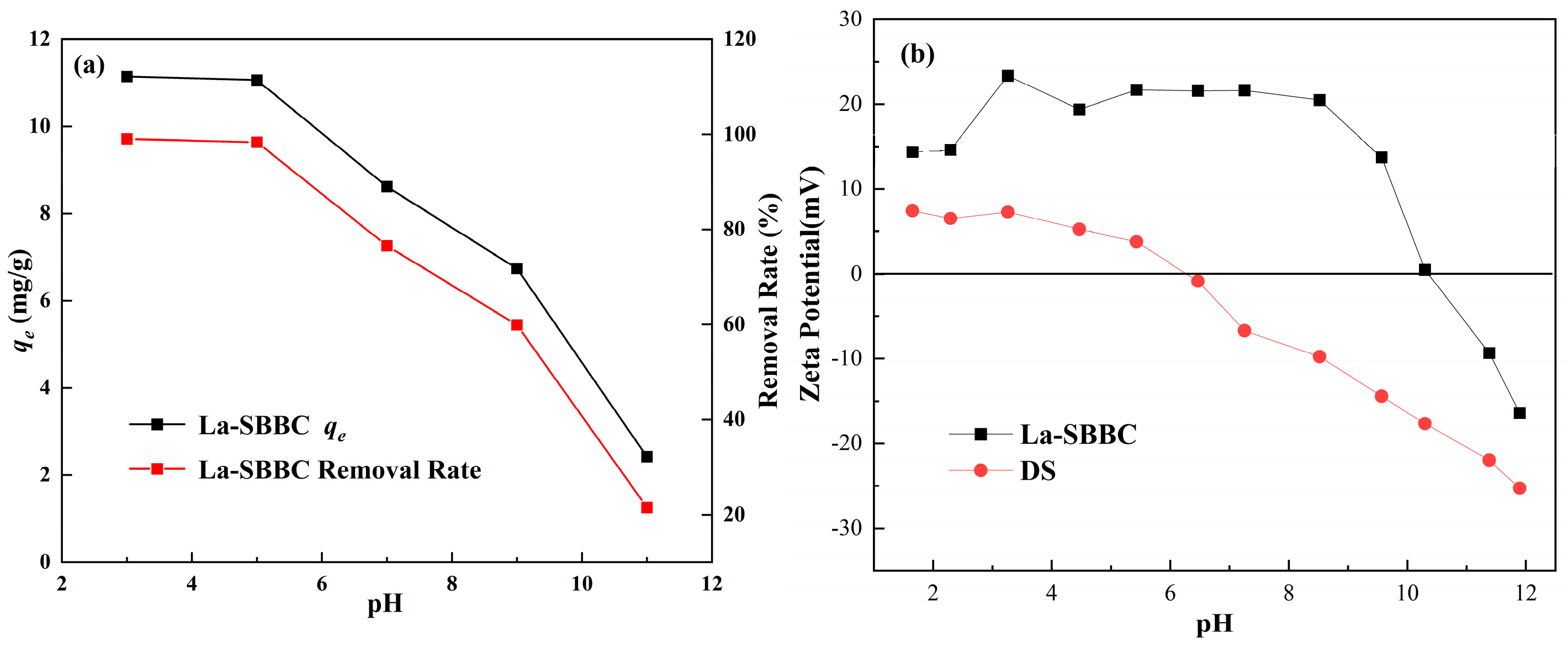
3.3.1. Effect of pH on Phosphate Adsorption
3.3.2. Effect of La-SBBC Dosage on Phosphate Adsorption
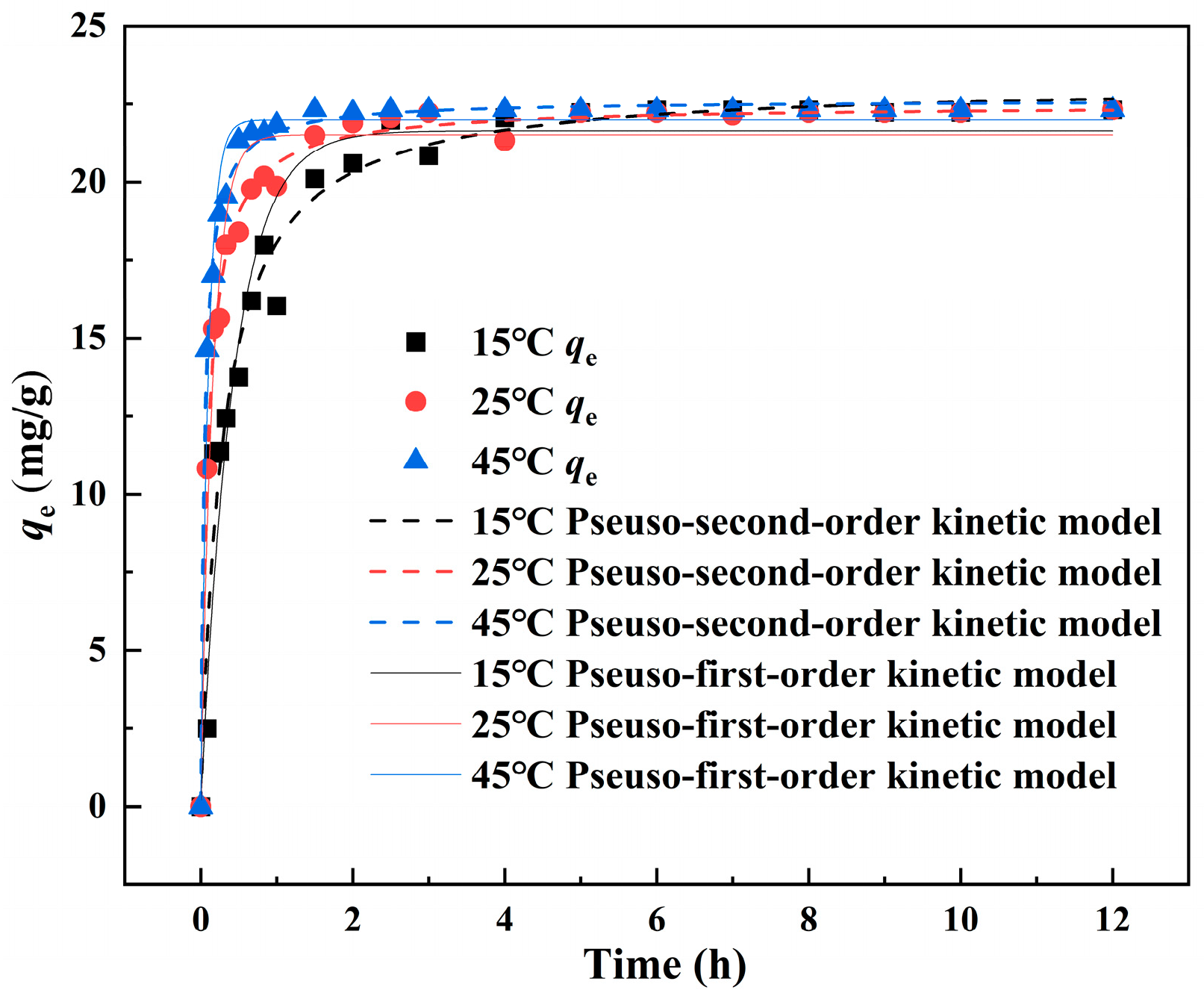
3.4. Adsorption Kinetics
3.5. Adsorption Isotherms
3.6. Adsorption Thermodynamics
3.7. Adsorption–Desorption Cycles
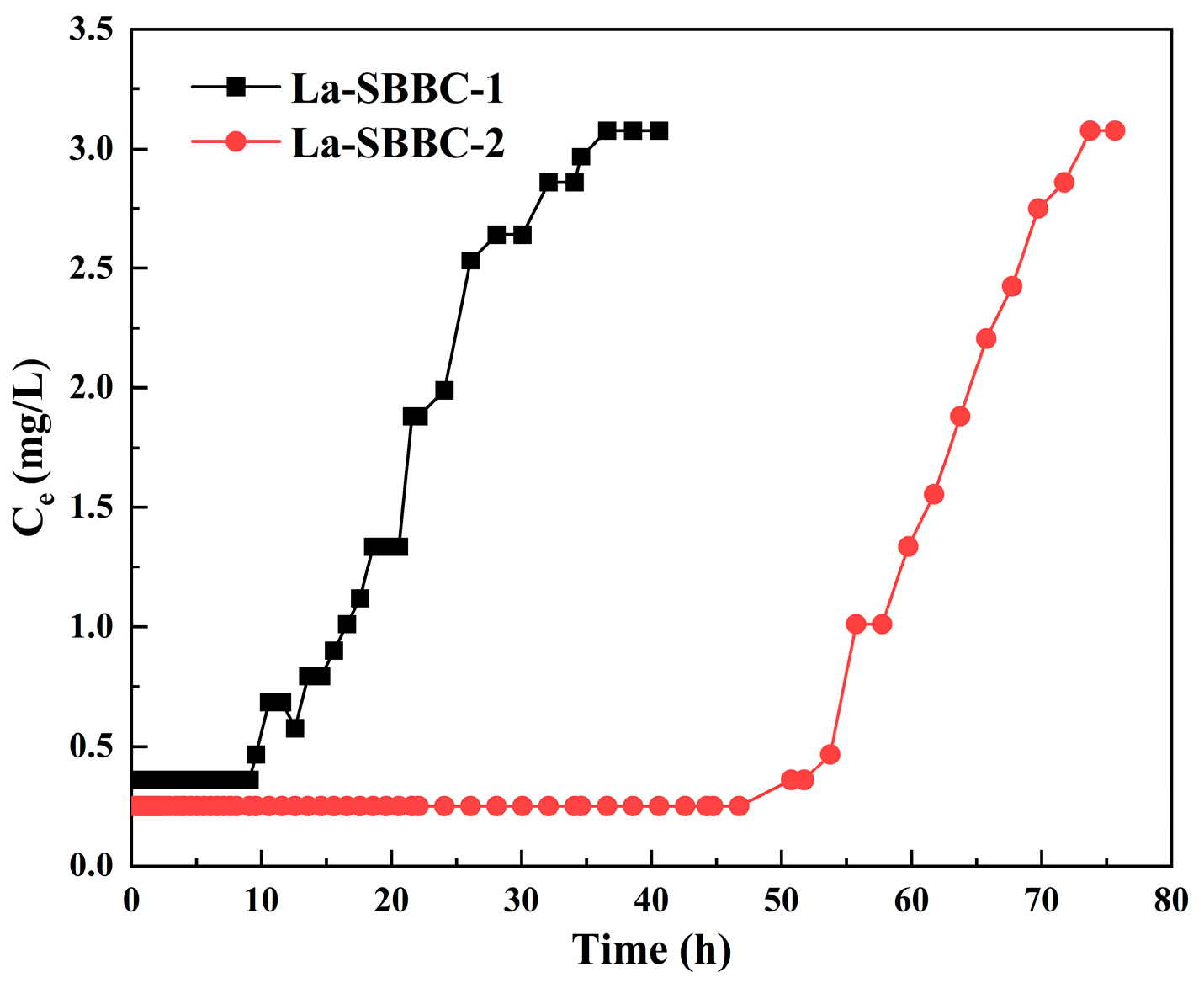
3.8. Fixed-Bed Column Dynamic Adsorption
3.9. Phosphate Adsorption Mechanism on La-SBBC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Xu, Q.; Liu, X.; Wang, D.; Wu, Y.; Wang, Q.; Liu, Y.; Li, X.; An, H.; Zhao, J.; Chen, F.; et al. Free Ammonia-Based Pretreatment Enhances Phosphorus Release and Recovery from Waste Activated Sludge. Chemosphere 2018, 213, 276–284. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Q.; Wang, D.; Wang, S.; Chen, F.; Zhong, Y.; Yi, K.; Yao, F.; Jiang, C.; Li, S.; et al. Nickel Toxicity to the Performance and Microbial Community of Enhanced Biological Phosphorus Removal System. Chem. Eng. J. 2017, 313, 415–423. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Yang, Q.; Zheng, W.; Wu, Y.; Zeng, T.; Zeng, G. Improved Biological Phosphorus Removal Performance Driven by the Aerobic/Extended-Idle Regime with Propionate as the Sole Carbon Source. Water Res. 2012, 46, 3868–3878. [Google Scholar] [CrossRef]
- Mayer, B.K.; Baker, L.A.; Boyer, T.H.; Drechsel, P.; Gifford, M.; Hanjra, M.A.; Parameswaran, P.; Stoltzfus, J.; Westerhoff, P.; Rittmann, B.E. Total Value of Phosphorus Recovery. Environ. Sci. Technol. 2016, 50, 6606–6620. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Xu, X.; Qi, S.; Zhao, Y.; Ren, Z.; Gao, B. Preferable Uptake of Phosphate by Hydrous Zirconium Oxide Nanoparticles Embedded in Quaternary-Ammonium Chinese Reed. J. Colloid Interface Sci. 2017, 496, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Lee, S.; Lee, Y.J. Synthesis of Novel Magnesium Ferrite (MgFe2O4)/Biochar Magnetic Composites and Its Adsorption Behavior for Phosphate in Aqueous Solutions. Bioresour. Technol. 2017, 245, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Mo, C.; Li, X.; Zhang, J.; An, H.; Yang, Q.; Wang, D.; Zhao, J.; Zhong, Y.; Zeng, G. Effects of Different Ratios of Glucose to Acetate on Phosphorus Removal and Microbial Community of Enhanced Biological Phosphorus Removal (EBPR) System. Environ. Sci. Pollut. Res. 2017, 24, 4494–4505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Masek, O.; Parikh, S.J.; Ok, Y.S. Evaluating Biochar and Its Modifications for the Removal of Ammonium, Nitrate, and Phosphate in Water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, Z.; Wu, Z.; Xu, F.; Yang, D.; He, Q.; Li, G.; Chen, Y. Novel Lanthanum Doped Biochars Derived from Lignocellulosic Wastes for Efficient Phosphate Removal and Regeneration. Bioresour. Technol. 2019, 289, 121600. [Google Scholar] [CrossRef]
- Yang, S.; Jin, P.; Wang, X.; Zhang, Q.; Chen, X. Phosphate Recovery through Adsorption Assisted Precipitation Using Novel Precipitation Material Developed from Building Waste: Behavior and Mechanism. Chem. Eng. J. 2016, 292, 246–254. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Lahori, A.H.; Mahar, A. Recovery of Phosphate from Aqueous Solution by Magnesium Oxide Decorated Magnetic Biochar and Its Potential as Phosphate-Based Fertilizer Substitute. Bioresour. Technol. 2016, 215, 209–214. [Google Scholar] [CrossRef]
- Qiu, H.; Liang, C.; Yu, J.; Zhang, Q.; Song, M.; Chen, F. Preferable Phosphate Sequestration by Nano-La(III) (Hydr)Oxides Modified Wheat Straw with Excellent Properties in Regeneration. Chem. Eng. J. 2017, 315, 345–354. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, R.; Duan, X.; Wang, S.; Ren, N.; Ho, S.-H. Production, Properties, and Catalytic Applications of Sludge Derived Biochar for Environmental Remediation. Water Res. 2020, 187, 116390. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium Chain Carboxylic Acids Production from Waste Biomass: Current Advances and Perspectives. Biotechnol. Adv. 2019, 37, 599–615. [Google Scholar] [CrossRef]
- Chen, Y.; Ho, S.-H.; Nagarajan, D.; Ren, N.; Chang, J.-S. Waste Biorefineries—Integrating Anaerobic Digestion and Microalgae Cultivation for Bioenergy Production. Curr. Opin. Biotechnol. 2018, 50, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, Y.; Yu, H.; Li, Y.; Yuan, Q.; Xiao, H.; Li, F.; Pan, B. Using Sewage Sludge with High Ash Content for Biochar Production and Cu (II) Sorption. Sci. Total Environ. 2020, 713, 136663. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Ye, J.; Shi, C.; Zhang, P.; Guo, J.; Zubair, M.; Chang, J.; Zhang, L. Pyrolysis Temperature Regulates Sludge-Derived Biochar Production, Phosphate Adsorption and Phosphate Retention in Soil. J. Environ. Chem. Eng. 2022, 10, 107744. [Google Scholar] [CrossRef]
- Wang, J.; Tong, X.; Wang, S. Zirconium-Modified Activated Sludge as a Low-Cost Adsorbent for Phosphate Removal in Aqueous Solution. Water Air Soil Pollut. 2018, 229, 47. [Google Scholar] [CrossRef]
- Pradel, M.; Aissani, L.; Villot, J.; Baudez, J.-C.; Laforest, V. From Waste to Added Value Product: Towards a Paradigm Shift in Life Cycle Assessment Applied to Wastewater Sludge—A Review. J. Clean. Prod. 2016, 131, 60–75. [Google Scholar] [CrossRef]
- Yang, X.; Xu, G.; Yu, H.; Zhang, Z. Preparation of Ferric-Activated Sludge-Based Adsorbent from Biological Sludge for Tetracycline Removal. Bioresour. Technol. 2016, 211, 566–573. [Google Scholar] [CrossRef]
- Liao, T.; Li, T.; Su, X.; Yu, X.; Song, H.; Zhu, Y.; Zhang, Y. La(OH)3-Modified Magnetic Pineapple Biochar as Novel Adsorbents for Efficient Phosphate Removal. Bioresour. Technol. 2018, 263, 207–213. [Google Scholar] [CrossRef]
- Yin, Q.; Ren, H.; Wang, R.; Zhao, Z. Evaluation of Nitrate and Phosphate Adsorption on Al-Modified Biochar: Influence of Al Content. Sci. Total Environ. 2018, 631–632, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Raei, E.; Talebbeydokhti, N. Enhanced Removal of Phosphate from Aqueous Solutions Using a Modified Sludge Derived Biochar: Comparative Study of Various Modifying Cations and RSM Based Optimization of Pyrolysis Parameters. J. Environ. Manag. 2018, 225, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, Y.; Huang, Y.; Song, L.; Chen, H.; Zhu, S.; Tang, C. Enhanced Adsorption of Phosphate on Orange Peel-Based Biochar Activated by Ca/Zn Composite: Adsorption Efficiency and Mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129728. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F.; Zhao, J.; Wang, S.; Yao, F.; Wang, D.; et al. Effectiveness and Mechanisms of Phosphate Adsorption on Iron-Modified Biochars Derived from Waste Activated Sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef]
- Fang, L.; Liu, R.; Li, J.; Xu, C.; Huang, L.-Z.; Wang, D. Magnetite/Lanthanum Hydroxide for Phosphate Sequestration and Recovery from Lake and the Attenuation Effects of Sediment Particles. Water Res. 2018, 130, 243–254. [Google Scholar] [CrossRef]
- Min, X.; Wu, X.; Shao, P.; Ren, Z.; Ding, L.; Luo, X. Ultra-High Capacity of Lanthanum-Doped UiO-66 for Phosphate Capture: Unusual Doping of Lanthanum by the Reduction of Coordination Number. Chem. Eng. J. 2019, 358, 321–330. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, S.; Wu, D.; Chen, F. Control of Internal Phosphorus Loading in Eutrophic Lakes Using Lanthanum-Modified Zeolite. Chem. Eng. J. 2017, 327, 505–513. [Google Scholar] [CrossRef]
- He, J.; Wang, W.; Sun, F.; Shi, W.; Qi, D.; Wang, K.; Shi, R.; Cui, F.; Wang, C.; Chen, X. Highly Efficient Phosphate Scavenger Based on Well-Dispersed La(OH)3 Nanorods in Polyacrylonitrile Nanofibers for Nutrient-Starvation Antibacteria. ACS Nano 2015, 9, 9292–9302. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y.; Zhu, R.; Liu, J.; Usman, M.; Chen, Q.; He, H. Superior Adsorption of Phosphate by Ferrihydrite-Coated and Lanthanum-Decorated Magnetite. J. Colloid Interface Sci. 2018, 530, 704–713. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Huang, H.; Zhao, N.; Zhang, M.; Cao, L. Investigation into Lanthanum-Coated Biochar Obtained from Urban Dewatered Sewage Sludge for Enhanced Phosphate Adsorption. Sci. Total Environ. 2020, 714, 136839. [Google Scholar] [CrossRef]
- He, L.; Chen, Y.; Sun, F.; Li, Y.; Huang, W.; Yang, S. Controlled Release of Phosphorus Using Lanthanum-Modified Hydrochar Synthesized from Water Treatment Sludge: Adsorption Behavior and Immobilization Mechanism. J. Water Process Eng. 2022, 50, 103319. [Google Scholar] [CrossRef]
- Li, J.; Cao, L.; Li, B.; Huang, H.; Yu, W.; Sun, C.; Long, K.; Young, B. Utilization of Activated Sludge and Shell Wastes for the Preparation of Ca-Loaded Biochar for Phosphate Removal and Recovery. J. Clean. Prod. 2023, 382, 135395. [Google Scholar] [CrossRef]
- Jia, Z.; Zeng, W.; Xu, H.; Li, S.; Peng, Y. Adsorption Removal and Reuse of Phosphate from Wastewater Using a Novel Adsorbent of Lanthanum-Modified Platanus Biochar. Process Saf. Environ. Protect. 2020, 140, 221–232. [Google Scholar] [CrossRef]
- Cao, H.; Wu, X.; Syed-Hassan, S.S.A.; Zhang, S.; Mood, S.H.; Milan, Y.J.; Garcia-Perez, M. Characteristics and Mechanisms of Phosphorous Adsorption by Rape Straw-Derived Biochar Functionalized with Calcium from Eggshell. Bioresour. Technol. 2020, 318, 124063. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Dai, X.; Khan, K.Y.; Li, T.; Yang, X.; He, Z. Removal of Phosphate from Aqueous Solution Using Magnesium-Alginate/Chitosan Modified Biochar Microspheres Derived from Thalia Dealbata. Bioresour. Technol. 2016, 218, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Q.; Liu, J.; Chang, N.; Wan, L.; Chen, J. Phosphate Adsorption on Lanthanum Hydroxide-Doped Activated Carbon Fiber. Chem. Eng. J. 2012, 185–186, 160–167. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Li, Q.; Xu, M.; Guo, Y.; Li, J.; Wu, T. pH Effect on Re(VII) and Se(IV) Diffusion in Compacted GMZ Bentonite. Appl. Geochem. 2016, 73, 1–7. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Yang, Q.; Wang, D.; Xu, Q.; Yao, F.; Chen, F.; Tao, Z.; Huang, X. Hydrated Lanthanum Oxide-Modified Diatomite as Highly Efficient Adsorbent for Low-Concentration Phosphate Removal from Secondary Effluents. J. Environ. Manag. 2019, 231, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.A.; Haridas, A. Removal of Phosphate from Aqueous Solutions and Sewage Using Natural and Surface Modified Coir Pith. J. Hazard. Mater. 2008, 152, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Kaljunen, J.U.; Yazdani, R.; Al-Juboori, R.A.; Zborowski, C.; Meinander, K.; Mikola, A. Adsorptive Behavior of Phosphorus onto Recycled Waste Biosolids after Being Acid Leached from Wastewater Sludge. Chem. Eng. J. Adv. 2022, 11, 100329. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar Produced from Oak Sawdust by Lanthanum (La)-Involved Pyrolysis for Adsorption of Ammonium (NH4+), Nitrate (NO3−), and Phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef]
- Aryee, A.A.; Mpatani, F.M.; Zhang, X.; Kani, A.N.; Dovi, E.; Han, R.; Li, Z.; Qu, L. Iron (III) and Iminodiacetic Acid Functionalized Magnetic Peanut Husk for the Removal of Phosphate from Solution: Characterization, Kinetic and Equilibrium Studies. J. Clean. Prod. 2020, 268, 122191. [Google Scholar] [CrossRef]
- Shi, W.; Fu, Y.; Jiang, W.; Ye, Y.; Kang, J.; Liu, D.; Ren, Y.; Li, D.; Luo, C.; Xu, Z. Enhanced Phosphate Removal by Zeolite Loaded with Mg–Al–La Ternary (Hydr)Oxides from Aqueous Solutions: Performance and Mechanism. Chem. Eng. J. 2019, 357, 33–44. [Google Scholar] [CrossRef]
- Lalley, J.; Han, C.; Li, X.; Dionysiou, D.D.; Nadagouda, M.N. Phosphate Adsorption Using Modified Iron Oxide-Based Sorbents in Lake Water: Kinetics, Equilibrium, and Column Tests. Chem. Eng. J. 2016, 284, 1386–1396. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-Modified Biochar for Simultaneous Removal of Ammonium, Nitrate, and Phosphate from Eutrophic Water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar] [CrossRef]
- Jang, H.M.; Yoo, S.; Choi, Y.-K.; Park, S.; Kan, E. Adsorption Isotherm, Kinetic Modeling and Mechanism of Tetracycline on Pinus Taeda-Derived Activated Biochar. Bioresour. Technol. 2018, 259, 24–31. [Google Scholar] [CrossRef]
- Qu, J.; Akindolie, M.S.; Feng, Y.; Jiang, Z.; Zhang, G.; Jiang, Q.; Deng, F.; Cao, B.; Zhang, Y. One-Pot Hydrothermal Synthesis of NaLa(CO3)2 Decorated Magnetic Biochar for Efficient Phosphate Removal from Water: Kinetics, Isotherms, Thermodynamics, Mechanisms and Reusability Exploration. Chem. Eng. J. 2020, 394, 124915. [Google Scholar] [CrossRef]
- Wang, S.; Kong, L.; Long, J.; Su, M.; Diao, Z.; Chang, X.; Chen, D.; Song, G.; Shih, K. Adsorption of Phosphorus by Calcium-Flour Biochar: Isotherm, Kinetic and Transformation Studies. Chemosphere 2018, 195, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Han, M.; Shih, K.; Su, M.; Diao, Z.; Long, J.; Chen, D.; Hou, L.; Peng, Y. Nano-Rod Ca-Decorated Sludge Derived Carbon for Removal of Phosphorus. Environ. Pollut. 2018, 233, 698–705. [Google Scholar] [CrossRef]
- Yang, H.I.; Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Anyia, A.O.; Chang, S.X. Adsorption of Ammonium in Aqueous Solutions by Pine Sawdust and Wheat Straw Biochars. Environ. Sci. Pollut. Res. 2018, 25, 25638–25647. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Shen, F.; Li, T. Phosphate Adsorption on Lanthanum Loaded Biochar. Chemosphere 2016, 150, 1–7. [Google Scholar] [CrossRef]
- Lǚ, J.; Liu, H.; Liu, R.; Zhao, X.; Sun, L.; Qu, J. Adsorptive Removal of Phosphate by a Nanostructured Fe–Al–Mn Trimetal Oxide Adsorbent. Powder Technol. 2013, 233, 146–154. [Google Scholar] [CrossRef]
- Van Truong, T.; Kim, Y.-J.; Kim, D.-J. Study of Biochar Impregnated with Al Recovered from Water Sludge for Phosphate Adsorption/Desorption. J. Clean. Prod. 2023, 383, 135507. [Google Scholar] [CrossRef]
- Song, X.; Pan, Y.; Wu, Q.; Cheng, Z.; Ma, W. Phosphate Removal from Aqueous Solutions by Adsorption Using Ferric Sludge. Desalination 2011, 280, 384–390. [Google Scholar] [CrossRef]
- Chen, A.; Lv, L.; Hu, R.; Wei, X.; Guan, J.; Meng, X. Achieving Win-Win Outcomes with Cerium-Loaded Porous Aluminum Sludge Hydrogel Microspheres for Enhanced Phosphate Removal. Sci. Total Environ. 2023, 867, 161530. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Yang, Y.; Lei, T.; Ye, Z.; Huang, S.; Fu, X.; Liu, P.; Li, H. Removal of Phosphate by a Novel Activated Sewage Sludge Biochar: Equilibrium, Kinetic and Mechanism Studies. Appl. Energy Combust. Sci. 2022, 9, 100056. [Google Scholar] [CrossRef]
- Karunanithi, R.; Ok, Y.S.; Dharmarajan, R.; Ahmad, M.; Seshadri, B.; Bolan, N.; Naidu, R. Sorption, Kinetics and Thermodynamics of Phosphate Sorption onto Soybean Stover Derived Biochar. Environ. Technol. Innov. 2017, 8, 113–125. [Google Scholar] [CrossRef]
- Nollet, H.; Roels, M.; Lutgen, P.; Van der Meeren, P.; Verstraete, W. Removal of PCBs from Wastewater Using Fly Ash. Chemosphere 2003, 53, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Liu, M.; Ren, H. Biochar Produced from the Co-Pyrolysis of Sewage Sludge and Walnut Shell for Ammonium and Phosphate Adsorption from Water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, L.; Zhao, L.; Zhang, H.; Yin, J.; Wei, G.; Qian, K.; Wang, Y.; Yu, C. A Designed Nanoporous Material for Phosphate Removal with High Efficiency. J. Mater. Chem. 2011, 21, 2489–2494. [Google Scholar] [CrossRef]
- Xiong, W.; Tong, J.; Yang, Z.; Zeng, G.; Zhou, Y.; Wang, D.; Song, P.; Xu, R.; Zhang, C.; Cheng, M. Adsorption of Phosphate from Aqueous Solution Using Iron-Zirconium Modified Activated Carbon Nanofiber: Performance and Mechanism. J. Colloid Interface Sci. 2017, 493, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Fang, L.; Fortner, J.D.; Guan, X.; Lo, I.M.C. Highly Efficient and Selective Phosphate Removal from Wastewater by Magnetically Recoverable La(OH)3/Fe3O4 Nanocomposites. Water Res. 2017, 126, 179–188. [Google Scholar] [CrossRef] [PubMed]







| Material | Specific Surface Area | Aperture | Pore Volume |
|---|---|---|---|
| DS | 7.92 | 20.96 | 0.6 |
| La-SBBC | 18.4 | 10.03 | 0.6 |
| Samples | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| Qe (mg/g) | k1 (h−1) | R2 | Qe (mg/g) | K2 (h−1) | R2 | |
| La-SBBC 15 °C | 21.645 | 2.335 | 0.949 | 22.315 | 0.151 | 0.973 |
| La-SBBC 25 °C | 21.515 | 6.286 | 0.958 | 22.256 | 0.488 | 0.994 |
| La-SBBC 45 °C | 21.985 | 10.161 | 0.977 | 22.467 | 0.938 | 0.997 |
| Samples | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | KL (L/g) | R2 | KF (L/g) | 1/n | R2 | |
| La-SBBC 15 °C | 95.829 | 0.247 | 0.815 | 19.569 | 0.340 | 0.928 |
| La-SBBC 25 °C | 108.954 | 0.165 | 0.861 | 22.958 | 0.325 | 0.956 |
| La-SBBC 35 °C | 136.634 | 0.067 | 0.855 | 25.458 | 0.325 | 0.950 |
| La-SBBC 45 °C | 123.177 | 0.202 | 0.874 | 29.816 | 0.300 | 0.953 |
| La-SBBC 65 °C | 136.999 | 0.142 | 0.884 | 31.076 | 0.305 | 0.948 |
| Adsorbents | Qm (mg/g) | References |
|---|---|---|
| La-modified sludge-based biochar (La-SBBC) | 136.999 | This study |
| La-coated sewage sludge biochar | 93.91 | [31] |
| La-modified water treatment sludge hydrochar | 72.69 | [32] |
| oyster shell-modified activated sludge biochar | 129.03 | [33] |
| sludge biochar modified with FeCl3 | 111.0 | [25] |
| Aluminum-impregnated sludge biochar | 11.9 | [54] |
| Ferric sludge biochar | 36.67 | [55] |
| Aluminum sludge biochar | 36.303 | [56] |
| Mg-modified sludge biochar | 97.45 | [57] |
| Samples | ΔG (kJ/mol) | ΔS (J/mol·K) | ΔH (kJ/mol) |
|---|---|---|---|
| La-SBBC 15 °C | −105.410 | 4.273 | 1059.370 |
| La-SBBC 25 °C | −334.641 | ||
| La-SBBC 35 °C | −222.890 | ||
| La-SBBC 45 °C | −283.026 | ||
| La-SBBC 65 °C | −382.348 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, J.; Li, Q.; Sun, X.; Zhang, H.; Xing, M.; Dong, B.; Zhu, H. Capacity and Mechanisms of Phosphate Adsorption on Lanthanum-Modified Dewatered Sludge-Based Biochar. Water 2024, 16, 418. https://doi.org/10.3390/w16030418
Mo J, Li Q, Sun X, Zhang H, Xing M, Dong B, Zhu H. Capacity and Mechanisms of Phosphate Adsorption on Lanthanum-Modified Dewatered Sludge-Based Biochar. Water. 2024; 16(3):418. https://doi.org/10.3390/w16030418
Chicago/Turabian StyleMo, Jingjing, Qian Li, Xiaojie Sun, Hongxia Zhang, Meiyan Xing, Bin Dong, and Hongxiang Zhu. 2024. "Capacity and Mechanisms of Phosphate Adsorption on Lanthanum-Modified Dewatered Sludge-Based Biochar" Water 16, no. 3: 418. https://doi.org/10.3390/w16030418





