Interaction between Microplastics and Pathogens in Subsurface System: What We Know So Far
Abstract
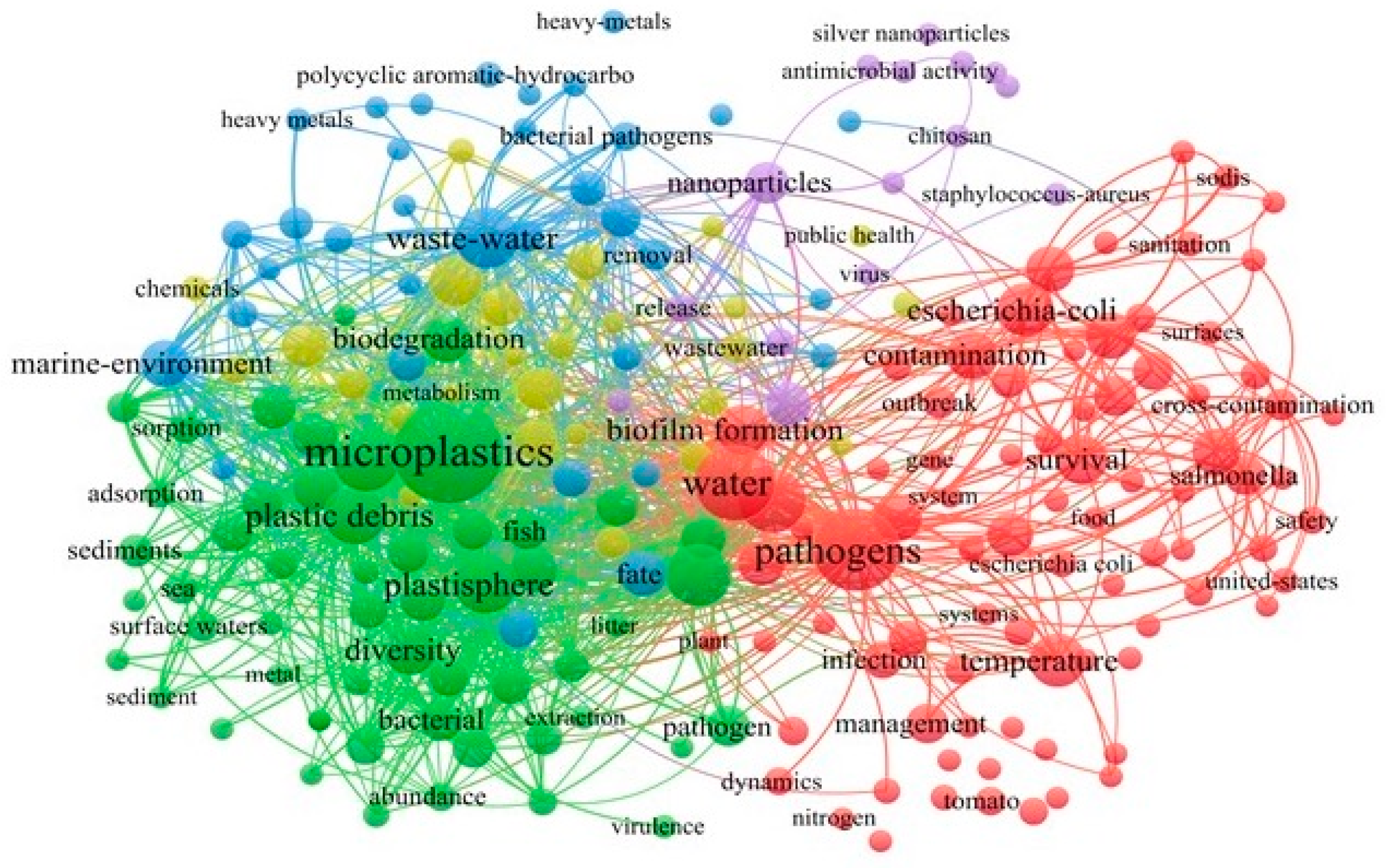
:1. Introduction
2. Sources and Features of Groundwater Microplastics
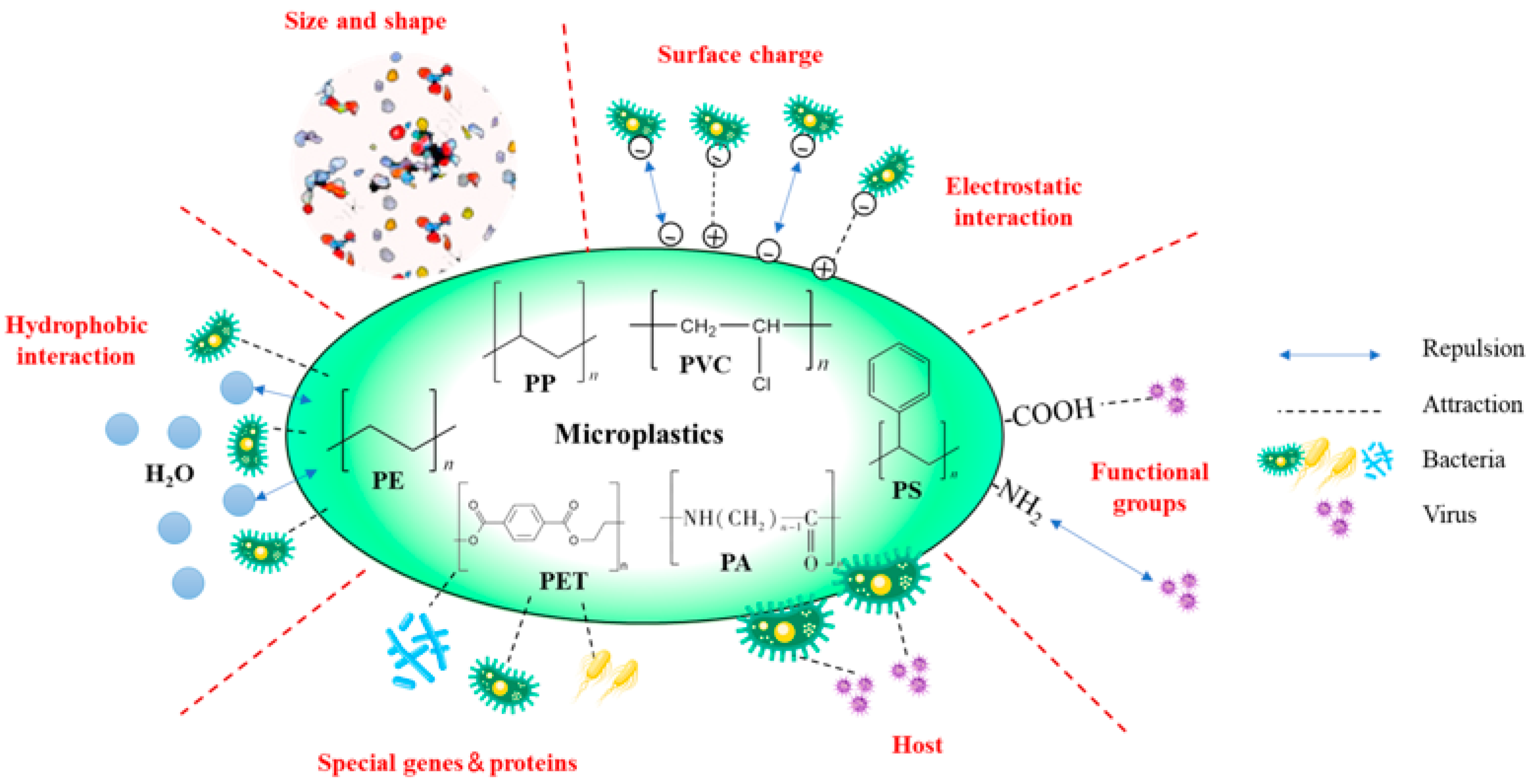
3. Interactions between Pathogens and Microplastics
3.1. Effects from the Physical Properties of Microplastics
3.2. Effects from Chemical Characteristics of Microplastics
3.3. Effects from Characteristics of Pathogens
3.3.1. Hydrophobicity
3.3.2. Surface Charge
3.3.3. Specific Properties
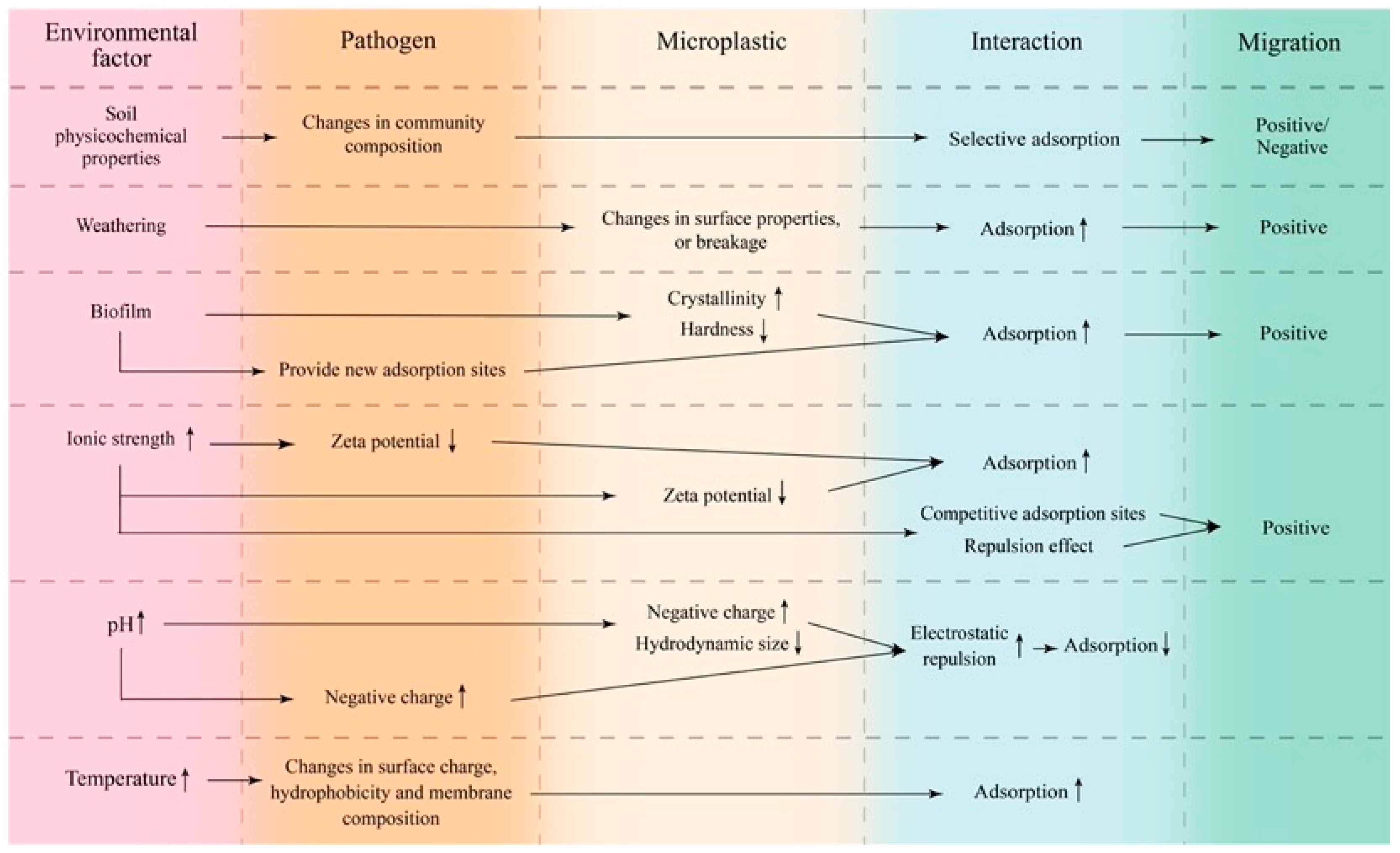
4. Effects of Environmental Factors on the Interactions between Microplastics and Pathogens
4.1. Soil Physicochemical Properties
4.2. Weathering
4.3. Biofilm
4.4. Ionic Strength
4.5. pH
4.6. Temperature
5. Effects of Combined Exposure to MPs and Pathogens on Organisms
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.; Baker, J.E.; Bamford, H.A. International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris. In Proceedings of the Conference Proceedings, Tacoma, WA, USA, 9–11 September 2008; University of Washington Tacoma: Tacoma, WA, USA, 2009. [Google Scholar]
- Collignon, A.; Hecq, J.-H.; Galgani, F.; Collard, F.; Goffart, A. Annual variation in neustonic micro- and meso-plastic particles and zooplankton in the Bay of Calvi (Mediterranean–Corsica). Mar. Pollut. Bull. 2014, 79, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- .He, L.; Wu, D.; Rong, H.; Li, M.; Tong, M.; Kim, H. Influence of Nano- and Microplastic Particles on the Transport and Deposition Behaviors of Bacteria in Quartz Sand. Environ. Sci. Technol. 2018, 52, 11555–11563. [Google Scholar] [CrossRef]
- Auta, H.; Emenike, C.; Fauziah, S. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, Z.; Wu, D.; Zhan, L.; Shi, H.; Xie, B. Occurrence of microplastics in landfill systems and their fate with landfill age. Water Res. 2019, 164, 114968. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017. [Google Scholar]
- Yurtsever, M. Glitters as a Source of Primary Microplastics: An Approach to Environmental Responsibility and Ethics. J. Agric. Environ. Ethics 2019, 32, 459–478. [Google Scholar] [CrossRef]
- Wang, L.; Wu, W.-M.; Bolan, N.S.; Tsang, D.C.; Li, Y.; Qin, M.; Hou, D. Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: Current status and future perspectives. J. Hazard. Mater. 2021, 401, 123415. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Hollman, P.C.H.; Peters, R.J.B. Potential Health Impact of Environmentally Released Micro- and Nanoplastics in the Human Food Production Chain: Experiences from Nanotoxicology. Environ. Sci. Technol. 2015, 49, 8932–8947. [Google Scholar] [CrossRef]
- da Costa, J.P.; Santos, P.S.; Duarte, A.C.; Rocha-Santos, T. (Nano)plastics in the environment–Sources, fates and effects. Sci. Total Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef]
- Birch, Q.T.; Potter, P.M.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Sources, transport, measurement and impact of nano and microplastics in urban watersheds. Rev. Environ. Sci. Bio/Technol. 2020, 19, 275–336. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Cai, L.; Sun, F.; Li, G.; Che, Y. Public attitudes towards microplastics: Perceptions, behaviors and policy implications. Resour. Conserv. Recycl. 2020, 163, 105096. [Google Scholar] [CrossRef]
- Chia, R.W.; Lee, J.-Y.; Kim, H.; Jang, J. Microplastic pollution in soil and groundwater: A review. Environ. Chem. Lett. 2021, 19, 4211–4224. [Google Scholar] [CrossRef]
- Chatziparaskeva, G.; Papamichael, I.; Zorpas, A.A. Microplastics in the coastal environment of Mediterranean and the impact on sustainability level. Sustain. Chem. Pharm. 2022, 29, 100768. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.L.; Zamparas, M.G.; Kapsalis, V.C. Investigating the Human Impacts and the Environmental Consequences of Microplastics Disposal into Water Resources. Sustainability 2022, 14, 828. [Google Scholar] [CrossRef]
- Gerolin, C.R.; Pupim, F.N.; Sawakuchi, A.O.; Grohmann, C.H.; Labuto, G.; Semensatto, D. Microplastics in sediments from Amazon rivers, Brazil. Sci. Total Environ. 2020, 749, 141604. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.; Jesuraja, K.; Venkatramanan, S.; Roy, P.D.; Kumari, V.J. Hazardous microplastic characteristics and its role as a vector of heavy metal in groundwater and surface water of coastal south India. J. Hazard. Mater. 2021, 402, 123786. [Google Scholar] [CrossRef]
- Yan, M.; Nie, H.; Xu, K.; He, Y.; Hu, Y.; Huang, Y.; Wang, J. Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere 2019, 217, 879–886. [Google Scholar] [CrossRef]
- Samandra, S.; Johnston, J.M.; Jaeger, J.E.; Symons, B.; Xie, S.; Currell, M.; Ellis, A.V.; Clarke, B.O. Microplastic contamination of an unconfined groundwater aquifer in Victoria, Australia. Sci. Total Environ. 2022, 802, 149727. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Z.; Li, G.; Wang, K.; Cai, X.; Xiong, X.; Wu, C. Factors affecting microplastic accumulation by wild fish: A case study in the Nandu River, South China. Sci. Total Environ. 2022, 847, 157486. [Google Scholar] [CrossRef]
- Bošković, N.; Joksimović, D.; Bajt, O. Microplastics in mussels from the Boka Kotorska Bay (Adriatic Sea) and impact on human health. Food Chem. Toxicol. 2023, 173, 113641. [Google Scholar] [CrossRef] [PubMed]
- Carlin, J.; Craig, C.; Little, S.; Donnelly, M.; Fox, D.; Zhai, L.; Walters, L. Microplastic accumulation in the gastrointestinal tracts in birds of prey in central Florida, USA. Environ. Pollut. 2020, 264, 114633. [Google Scholar] [CrossRef]
- Muhib, I.; Uddin, K.; Rahman, M.; Malafaia, G. Occurrence of microplastics in tap and bottled water, and food packaging: A narrative review on current knowledge. Sci. Total Environ. 2023, 865, 161274. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Hashem, M.; Mostafa, Y.S.; Alhaithloul, H.A.S.; Alghanem, S.M. Linking effects of microplastics to ecological impacts in marine environments. Chemosphere 2021, 264, 128541. [Google Scholar] [CrossRef]
- Chai, B.; Li, X.; Liu, H.; Lu, G.; Dang, Z.; Yin, H. Bacterial communities on soil microplastic at Guiyu, an E-Waste dismantling zone of China. Ecotoxicol. Environ. Saf. 2020, 195, 110521. [Google Scholar] [CrossRef]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an Abundant and Distinct Microbial Habitat in an Urban River. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef]
- Zhang, E.; Kim, M.; Rueda, L.; Rochman, C.; VanWormer, E.; Moore, J.; Shapiro, K. Association of zoonotic protozoan parasites with microplastics in seawater and implications for human and wildlife health. Sci. Rep. 2022, 12, 6532. [Google Scholar] [CrossRef]
- Rodrigues, A.; Oliver, D.M.; McCarron, A.; Quilliam, R.S. Colonisation of plastic pellets (nurdles) by E. coli at public bathing beaches. Mar. Pollut. Bull. 2019, 139, 376–380. [Google Scholar] [CrossRef]
- Fabra, M.; Williams, L.; Watts, J.E.; Hale, M.S.; Couceiro, F.; Preston, J. The plastic Trojan horse: Biofilms increase microplastic uptake in marine filter feeders impacting microbial transfer and organism health. Sci. Total Environ. 2021, 797, 149217. [Google Scholar] [CrossRef]
- Jiang, P.; Zhao, S.; Zhu, L.; Li, D. Microplastic-associated bacterial assemblages in the intertidal zone of the Yangtze Estuary. Sci. Total Environ. 2018, 624, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.R.; Abu Hasan, H.; Muhamad, M.H.; Ismail, N.; Abdullah, S.R.S. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Nizzetto, L.; Bussi, G.; Futter, M.N.; Butterfield, D.; Whitehead, P.G. A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ. Sci. Process. Impacts 2016, 18, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Pan, S.; Shen, Z.; Song, Y.; Jin, Y.; Wu, W.M.; Hou, D. Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environ. Pollut. 2019, 249, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Kumar, D.; Yoo, C.G.; Gitsov, I.; Majumder, E.L.-W. Conversion and removal strategies for microplastics in wastewater treatment plants and landfills. Chem. Eng. J. 2021, 406, 126715. [Google Scholar] [CrossRef]
- Li, N.; Han, Z.; Guo, N.; Zhou, Z.; Liu, Y.; Tang, Q. Microplastics spatiotemporal distribution and plastic-degrading bacteria identification in the sanitary and non-sanitary municipal solid waste landfills. J. Hazard. Mater. 2022, 438, 129452. [Google Scholar] [CrossRef]
- Yu, F.; Wu, Z.; Wang, J.; Li, Y.; Chu, R.; Pei, Y.; Ma, J. Effect of landfill age on the physical and chemical characteristics of waste plastics/microplastics in a waste landfill sites. Environ. Pollut. 2022, 306, 119366. [Google Scholar] [CrossRef]
- Liang, H.; Ji, Y.; Ge, W.; Wu, J.; Song, N.; Yin, Z.; Chai, C. Release kinetics of microplastics from disposable face masks into the aqueous environment. Sci. Total Environ. 2022, 816, 151650. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Silveira, I.T.; Chua, A.; Leusch, F.D. An audit of microplastic abundance throughout three Australian wastewater treatment plants. Chemosphere 2021, 263, 128294. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhu, Z.-R.; Li, W.-H.; Yan, X.; Wang, L.-K.; Zhang, L.; Jin, J.; Dai, X.; Ni, B.-J. Revisiting Microplastics in Landfill Leachate: Unnoticed Tiny Microplastics and Their Fate in Treatment Works. Water Res. 2021, 190, 116784. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Tang, J.; Lyu, H.; Wang, L.; Gillmore, A.B.; Schaeffer, S.M. Activities of Microplastics (MPs) in Agricultural Soil: A Review of MPs Pollution from the Perspective of Agricultural Ecosystems. J. Agric. Food Chem. 2022, 70, 4182–4201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, Y.; Liu, J.; Zhong, S.; Qian, Y.; Gao, P. An Overlooked Entry Pathway of Microplastics into Agricultural Soils from Application of Sludge-Based Fertilizers. Environ. Sci. Technol. 2020, 54, 4248–4255. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef] [PubMed]
- van Den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage sludge application as a vehicle for microplastics in eastern Spanish agricultural soils. Environ. Pollut. 2020, 261, 114198. [Google Scholar] [CrossRef]
- Wright, S.; Ulke, J.; Font, A.; Chan, K.; Kelly, F. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Zhou, D.; Cai, Y.; Yang, Z. Key factors controlling transport of micro- and nanoplastic in porous media and its effect on coexisting pollutants. Environ. Pollut. 2022, 293, 118503. [Google Scholar] [CrossRef]
- Pang, Z.; Huang, T.; Chen, Y. Diminished groundwater recharge and circulation relative to degrading riparian vegetation in the middle Tarim River, Xinjiang Uygur, Western China. Hydrol. Process. 2010, 24, 147–159. [Google Scholar] [CrossRef]
- Yeh, H.-F.; Lin, H.-I.; Lee, C.-H.; Hsu, K.-C.; Wu, C.-S. Identifying Seasonal Groundwater Recharge Using Environmental Stable Isotopes. Water 2014, 6, 2849–2861. [Google Scholar] [CrossRef]
- He, B.; Wijesiri, B.; Ayoko, G.A.; Egodawatta, P.; Rintoul, L.; Goonetilleke, A. Influential factors on microplastics occurrence in river sediments. Sci. Total Environ. 2020, 738, 139901. [Google Scholar] [CrossRef] [PubMed]
- Bian, P.; Liu, Y.; Zhao, K.; Hu, Y.; Zhang, J.; Kang, L.; Shen, W. Spatial variability of microplastic pollution on surface of rivers in a mountain-plain transitional area: A case study in the Chin Ling-Wei River Plain, China. Ecotoxicol. Environ. Saf. 2022, 232, 113298. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Sun, C.; Yang, F.; Wang, Y.; Hu, L.; Wang, L.; Li, M.; Gao, L. Microplastics in 48 wastewater treatment plants reveal regional differences in physical characteristics and shape-dependent removal in the transition zone between North and South China. Sci. Total Environ. 2022, 834, 155320. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, Y.; Kou, D.; Yang, W.; Tang, W.; Chen, Q.; Que, S.; Zhao, X. Factors Impacting Microplastic Biofilm Community and Biological Risks Posed by Microplastics in Drinking Water Sources. Water Air Soil Pollut. 2022, 233, 179. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Zhen, Z.; Yan, Y.; Yan, C.; Ma, X.; Sun, L.; Wang, M.; Zhou, X.; Hu, A. Bacterial community colonization on tire microplastics in typical urban water environments and associated impacting factors. Environ. Pollut. 2020, 265, 114922. [Google Scholar] [CrossRef] [PubMed]
- Ballent, A.; Corcoran, P.L.; Madden, O.; Helm, P.A.; Longstaffe, F.J. Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar. Pollut. Bull. 2016, 110, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Quik, J.T.; Sun, M.; Koelmans, A.A. Fate of nano- and microplastic in freshwater systems: A modeling study. Environ. Pollut. 2017, 220, 540–548. [Google Scholar] [CrossRef]
- Zuo, L.-Z.; Li, H.-X.; Lin, L.; Sun, Y.-X.; Diao, Z.-H.; Liu, S.; Zhang, Z.-Y.; Xu, X.-R. Sorption and desorption of phenanthrene on biodegradable poly(butylene adipate co-terephtalate) microplastics. Chemosphere 2019, 215, 25–32. [Google Scholar] [CrossRef]
- Rühs, P.; Böcker, L.; Inglis, R.; Fischer, P. Studying bacterial hydrophobicity and biofilm formation at liquid–liquid interfaces through interfacial rheology and pendant drop tensiometry. Colloids Surf. B Biointerfaces 2014, 117, 174–184. [Google Scholar] [CrossRef]
- Liu, H.; Yue, L.; Zhao, Y.; Li, J.; Fu, Y.; Deng, H.; Feng, D.; Li, Q.; Yu, H.; Zhang, Y.; et al. Changes in bacterial community structures in soil caused by migration and aging of microplastics. Sci. Total Environ. 2022, 848, 157790. [Google Scholar] [CrossRef]
- Helmberger, M.S.; Tiemann, L.K.; Grieshop, M.J. Towards an ecology of soil microplastics. Funct. Ecol. 2020, 34, 550–560. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, S.; Wang, X.; Yang, X.; Zhang, C.; Qi, Y.; Guo, X. The occurrence and distribution characteristics of microplastics in the agricultural soils of Shaanxi Province, in north-western China. Sci. Total Environ. 2020, 720, 137525. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ouyang, Z.; Liu, P.; Zhao, X.; Wu, R.; Zhang, C.; Lin, C.; Li, Y.; Guo, X. Distribution and characteristics of microplastics in the basin of Chishui River in Renhuai, China. Sci. Total Environ. 2021, 773, 145591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, D.; Yang, X.; Teng, J.; Liu, Y.; Zhang, C.; Zhao, J.; Yin, X.; You, L.; Liu, Y.; et al. Microplastic pollution in the surface sediments collected from Sishili Bay, North Yellow Sea, China. Mar. Pollut. Bull. 2019, 141, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bradford, S.A.; Kim, H.; Shen, C.; Sasidharan, S.; Shang, J. Contributions of Nanoscale Roughness to Anomalous Colloid Retention and Stability Behavior. Langmuir 2017, 33, 10094–10105. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Rong, H.; Li, M.; Zhang, M.; Liu, S.; Yang, M.; Tong, M. Bacteria have different effects on the transport behaviors of positively and negatively charged microplastics in porous media. J. Hazard. Mater. 2021, 415, 125550. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wu, D.; Xia, J.; Shi, H.; Kim, H. Influence of physicochemical surface properties on the adhesion of bacteria onto four types of plastics. Sci. Total Environ. 2019, 671, 1101–1107. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.G.; Muñoz-Tabares, J.A.; Godoy-Gallardo, M.; Juárez, A.; Gil, F.-J. S. sanguinis adhesion on rough titanium surfaces: Effect of culture media. Mater. Sci. Eng. C 2013, 33, 714–720. [Google Scholar] [CrossRef]
- Cohen, N.; Radian, A. Microplastic Textile Fibers Accumulate in Sand and Are Potential Sources of Micro(nano)plastic Pollution. Environ. Sci. Technol. 2022, 56, 17635–17642. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-D.; Feng, W.; Gallup, M.; Kim, J.-H.; Gum, J.; Kim, Y.; Basbaum, C. Activation of NF-κB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa -induced mucin overproduction in epithelial cells. Proc. Natl. Acad. Sci. USA 1998, 95, 5718–5723. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, S.E.; Neal, J.T.; Mittge, E.; Seredick, B.M.; Guillemin, K. Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proc. Natl. Acad. Sci. USA 2010, 108, 4570–4577. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.M.; Mittge, E.; Kuhlman, J.; Baden, K.N.; Cheesman, S.E.; Guillemin, K. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev. Biol. 2006, 297, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Drzyzga, O. The strengths and weaknesses of Gordonia: A review of an emerging genus with increasing biotechnological potential. Crit. Rev. Microbiol. 2012, 38, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Arkatkar, A.; Juwarkar, A.A.; Bhaduri, S.; Uppara, P.V.; Doble, M. Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface. Int. Biodeterior. Biodegrad. 2010, 64, 530–536. [Google Scholar] [CrossRef]
- Ren, L.; Lin, Z.; Liu, H.; Hu, H. Bacteria-mediated phthalic acid esters degradation and related molecular mechanisms. Appl. Microbiol. Biotechnol. 2018, 102, 1085–1096. [Google Scholar] [CrossRef]
- Zhang, W.; Chai, J.; Li, S.; Wang, X.; Wu, S.; Liang, Z.; Baloch, M.Y.J.; Silva, L.F.; Zhang, D. Physiological characteristics, geochemical properties and hydrological variables influencing pathogen migration in subsurface system: What we know or not? Geosci. Front. 2022, 13, 101346. [Google Scholar] [CrossRef]
- Bao, R.; Pu, J.; Xie, C.; Mehmood, T.; Chen, W.; Gao, L.; Lin, W.; Su, Y.; Lin, X.; Peng, L. Aging of biodegradable blended plastic generates microplastics and attached bacterial communities in air and aqueous environments. J. Hazard. Mater. 2022, 434, 128891. [Google Scholar] [CrossRef]
- Das, M.P. Effect of cell surface hydrophobicity in microbial biofilm formation. Eur. J. Exp. Biol. 2014, 4, 254–256. [Google Scholar]
- Zhang, F.; Wang, Z.; Vijver, M.G.; Peijnenburg, W.J. Theoretical investigation on the interactions of microplastics with a SARS-CoV-2 RNA fragment and their potential impacts on viral transport and exposure. Sci. Total Environ. 2022, 842, 156812. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yu, Z.; Ngiam, L.; Guo, J. Microplastics as potential carriers of viruses could prolong virus survival and infectivity. Water Res. 2022, 225, 119115. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Fang, Z.; Zhong, H.; Shi, L.; Yang, X.; Liu, Z. Transport of Pseudomonas aeruginosa in Porous Media Mediated by Low-Concentration Surfactants: The Critical Role of Surfactant to Change Cell Surface Hydrophobicity. Water Resour. Res. 2020, 56, 26103. [Google Scholar] [CrossRef]
- Armanious, A.; Aeppli, M.; Jacak, R.; Refardt, D.; Sigstam, T.; Kohn, T.; Sander, M. Viruses at Solid–Water Interfaces: A Systematic Assessment of Interactions Driving Adsorption. Environ. Sci. Technol. 2016, 50, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Jiang, Y.; Zeng, G.; Liu, Z.; Liu, L.; Liu, Y.; Yang, X.; Lai, M.; He, Y. Effect of low-concentration rhamnolipid on adsorption of Pseudomonas aeruginosa ATCC 9027 on hydrophilic and hydrophobic surfaces. J. Hazard. Mater. 2015, 285, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, J.; Bao, M.; Zhao, L.; Li, Y. The proliferation and colonization of functional bacteria on amorphous polyethylene terephthalate: Key role of ultraviolet irradiation and nonionic surfactant polysorbate 80 addition. Chemosphere 2022, 291, 132940. [Google Scholar] [CrossRef] [PubMed]
- Angelini, T.E.; Roper, M.; Kolter, R.; Weitz, D.A.; Brenner, M.P. Bacillus subtilis spreads by surfing on waves of surfactant. Proc. Natl. Acad. Sci. USA 2009, 106, 18109–18113. [Google Scholar] [CrossRef]
- Gunning, P.; Mackie, A.; Gunning, A.; Wilde, P.; Woodward, N.; Morris, V. The effect of surfactant type on protein displacement from the air–water interface. Food Hydrocoll. 2004, 18, 509–515. [Google Scholar] [CrossRef]
- Klein, B.; Bouriat, P.; Goulas, P.; Grimaud, R. Behavior of Marinobacter hydrocarbonoclasticus SP17 cells during initiation of biofilm formation at the alkane–water interface. Biotechnol. Bioeng. 2010, 105, 461–468. [Google Scholar] [CrossRef]
- Wu, H.; Fang, H.; Xu, C.; Ye, J.; Cai, Q.; Shi, J. Transport and retention of copper oxide nanoparticles under unfavorable deposition conditions caused by repulsive van der Waals force in saturated porous media. Environ. Pollut. 2020, 256, 113400. [Google Scholar] [CrossRef]
- Dika, C.; Duval, J.F.; Francius, G.; Perrin, A.; Gantzer, C. Isoelectric point is an inadequate descriptor of MS2, Phi X 174 and PRD1 phages adhesion on abiotic surfaces. J. Colloid Interface Sci. 2015, 446, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Y.; Qin, X.; Jia, W.; Chai, L.; Huang, M.; Huang, Y. Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Sci. Total Environ. 2019, 688, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Sabatini, L.; Giorgi, L.; Pettinari, G.; Valentini, L.; Gobbi, P. A Multidisciplinary Approach in Examining the Susceptibility to Microbial Attack of Polyacrylic and Polyurethane Resins Used in Art Restoration. Int. J. Mol. Sci. 2022, 23, 11725. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhao, S.; Chen, Y.; Li, Y.; Zou, G.; Tao, Y.; Zhang, W.; Wang, L.; Zhang, H. Diversity and potential functional characteristics of phage communities colonizing microplastic biofilms. Environ. Res. 2023, 219, 115103. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Bordoloi, N.K.; Rai, S.K.; Mukherjee, A.K.; Karak, N. Biodegradable and biocompatible epoxidized vegetable oil modified thermostable poly(vinyl chloride): Thermal and performance characteristics post biodegradation with Pseudomonas aeruginosa and Achromobacter sp. J. Hazard. Mater. 2012, 209–210, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.J.; Sekhar, V.C.; Bhaskar, T.; Nampoothiri, K.M. Microbial assisted High Impact Polystyrene (HIPS) degradation. Bioresour. Technol. 2016, 213, 204–207. [Google Scholar] [CrossRef]
- Sekhar, V.C.; Nampoothiri, K.M.; Mohan, A.J.; Nair, N.R.; Bhaskar, T.; Pandey, A. Microbial degradation of high impact polystyrene (HIPS), an e-plastic with decabromodiphenyl oxide and antimony trioxide. J. Hazard. Mater. 2016, 318, 347–354. [Google Scholar] [CrossRef]
- El Othmany, R.; Zahir, H.; Ellouali, M.; Latrache, H. Current Understanding on Adhesion and Biofilm Development in Actinobacteria. Int. J. Microbiol. 2021, 2021, 6637438. [Google Scholar] [CrossRef] [PubMed]
- Simmons, E.L.; Drescher, K.; Nadell, C.D.; Bucci, V. Phage mobility is a core determinant of phage–bacteria coexistence in biofilms. ISME J. 2018, 12, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Penrod, S.L.; Olson, T.M.; Grant, S.B. Deposition Kinetics of Two Viruses in Packed Beds of Quartz Granular Media. Langmuir 1996, 12, 5576–5587. [Google Scholar] [CrossRef]
- Liu, J.; He, X.-X.; Lin, X.-R.; Chen, W.-C.; Zhou, Q.-X.; Shu, W.-S.; Huang, L.-N. Ecological Effects of Combined Pollution Associated with E-Waste Recycling on the Composition and Diversity of Soil Microbial Communities. Environ. Sci. Technol. 2015, 49, 6438–6447. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Dong, C.; Wu, J.; Liu, X.; Wu, Y.; Chen, X.; Yu, S. Ecological effects of soil properties and metal concentrations on the composition and diversity of microbial communities associated with land use patterns in an electronic waste recycling region. Sci. Total Environ. 2017, 601–602, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Zamparas, M.; Kyriakopoulos, G.L.; Kapsalis, V.C.; Drosos, M.; Kalavrouziotis, I.K. Application of novel composite materials as sediment capping agents: Column experiments and modelling. Desalination Water Treat. 2019, 170, 111–118. [Google Scholar] [CrossRef]
- Jiang, B.; Adebayo, A.; Jia, J.; Xing, Y.; Deng, S.; Guo, L.; Liang, Y.; Zhang, D. Impacts of heavy metals and soil properties at a Nigerian e-waste site on soil microbial community. J. Hazard. Mater. 2019, 362, 187–195. [Google Scholar] [CrossRef]
- Duan, J.; Bolan, N.; Li, Y.; Ding, S.; Atugoda, T.; Vithanage, M.; Sarkar, B.; Tsang, D.C.; Kirkham, M. Weathering of microplastics and interaction with other coexisting constituents in terrestrial and aquatic environments. Water Res. 2021, 196, 117011. [Google Scholar] [CrossRef]
- Purwiyanto, A.I.S.; Suteja, Y.; Trisno; Ningrum, P.S.; Putri, W.A.E.; Rozirwan; Agustriani, F.; Fauziyah; Cordova, M.R.; Koropitan, A.F. Concentration and adsorption of Pb and Cu in microplastics: Case study in aquatic environment. Mar. Pollut. Bull. 2020, 158, 111380. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, R.; Li, Z.; Yan, B. Adsorption properties and influencing factors of Cu(II) on polystyrene and polyethylene terephthalate microplastics in seawater. Sci. Total Environ. 2022, 812, 152573. [Google Scholar] [CrossRef]
- Park, Y.-W.; Lee, G.-S.; Kwak, J.-S. A study on compound contents for plastic injection molding products of metallic resin pigment. J. Mech. Sci. Technol. 2016, 30, 5673–5677. [Google Scholar] [CrossRef]
- Cabrera, G.; Pérez, R.; Gómez, J.; Ábalos, A.; Cantero, D. Toxic effects of dissolved heavy metals on Desulfovibrio vulgaris and Desulfovibrio sp. strains. J. Hazard. Mater. 2006, 135, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Zhang, M.; Liu, G.; Lu, T.; Qu, Q.; Du, B.; Pan, X. Effects of Soil Residual Plastic Film on Soil Microbial Community Structure and Fertility. Water Air Soil Pollut. 2018, 229, 261. [Google Scholar] [CrossRef]
- Lengeler, J.W.; Drews, G.; Schlegel, H.G. Biology of the Prokaryotes; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Yuan, Q.; Sun, R.; Yu, P.; Cheng, Y.; Wu, W.; Bao, J.; Alvarez, P.J. UV-aging of microplastics increases proximal ARG donor-recipient adsorption and leaching of chemicals that synergistically enhance antibiotic resistance propagation. J. Hazard. Mater. 2022, 427, 127895. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Du, H.; Huang, Y.; Yin, X.; Liu, Y.; Li, Y.; Liu, H.; Wang, X. Selective adsorption of antibiotics on aged microplastics originating from mariculture benefits the colonization of opportunistic pathogenic bacteria. Environ. Pollut. 2022, 313, 120157. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, I.; Voukkali, I.; Jeguirim, M.; Argirusis, N.; Jellali, S.; Sourkouni, G.; Argirusis, C.; Zorpas, A.A. End-of-Life Management and Recycling on PV Solar Energy Production. Energies 2022, 15, 6430. [Google Scholar] [CrossRef]
- He, L.; Rong, H.; Wu, D.; Li, M.; Wang, C.; Tong, M. Influence of biofilm on the transport and deposition behaviors of nano- and micro-plastic particles in quartz sand. Water Res. 2020, 178, 115808. [Google Scholar] [CrossRef]
- Lichter, J.A.; Thompson, M.T.; Delgadillo, M.; Nishikawa, T.; Rubner, M.F.; Van Vliet, K.J. Substrata Mechanical Stiffness Can Regulate Adhesion of Viable Bacteria. Biomacromolecules 2008, 9, 1571–1578. [Google Scholar] [CrossRef]
- McGivney, E.; Cederholm, L.; Barth, A.; Hakkarainen, M.; Hamacher-Barth, E.; Ogonowski, M.; Gorokhova, E. Rapid Physicochemical Changes in Microplastic Induced by Biofilm Formation. Front. Bioeng. Biotechnol. 2020, 8, 205. [Google Scholar] [CrossRef]
- Moresco, V.; Oliver, D.M.; Weidmann, M.; Matallana-Surget, S.; Quilliam, R.S. Survival of human enteric and respiratory viruses on plastics in soil, freshwater, and marine environments. Environ. Res. 2021, 199, 111367. [Google Scholar] [CrossRef]
- Moresco, V.; Charatzidou, A.; Oliver, D.M.; Weidmann, M.; Matallana-Surget, S.; Quilliam, R.S. Binding, recovery, and infectiousness of enveloped and non-enveloped viruses associated with plastic pollution in surface water. Environ. Pollut. 2022, 308, 119594. [Google Scholar] [CrossRef]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.-T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process. Impacts 2013, 15, 1949–1956. [Google Scholar] [CrossRef]
- Chatziparaskeva, G.; Papamichael, I.; Voukkali, I.; Loizia, P.; Sourkouni, G.; Argirusis, C.; Zorpas, A.A. End-of-Life of Composite Materials in the Framework of the Circular Economy. Microplastics 2022, 1, 377–392. [Google Scholar] [CrossRef]
- Agans, R.T.; Gordon, A.; Hussain, S.; Paliy, O. Titanium Dioxide Nanoparticles Elicit Lower Direct Inhibitory Effect on Human Gut Microbiota Than Silver Nanoparticles. Toxicol. Sci. 2019, 172, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Milner, J.; Boudreau, M.D.; Gokulan, K.; Cerniglia, C.E.; Khare, S. Effects of subchronic exposure of silver nanoparticles on intestinal microbiota and gut-associated immune responses in the ileum of Sprague-Dawley rats. Nanotoxicology 2015, 9, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.-Z.; Busetti, F.; Langsa, M.; Croué, J.-P. Roles of singlet oxygen and dissolved organic matter in self-sensitized photo-oxidation of antibiotic norfloxacin under sunlight irradiation. Water Res. 2016, 106, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Nanaboina, V.; Korshin, G.V.; Jiang, W. Spectroscopic study of degradation products of ciprofloxacin, norfloxacin and lomefloxacin formed in ozonated wastewater. Water Res. 2012, 46, 5235–5246. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Fu, Q.S.; Gao, D.; Criddle, C.S.; Leckie, J.O. Effect of solution chemistry on the adsorption of perfluorooctane sulfonate onto mineral surfaces. Water Res. 2010, 44, 2654–2662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L.; Chen, H.; Zhang, M. Retention and transport behavior of microplastic particles in water-saturated porous media. Sci. Total Environ. 2022, 808, 152154. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, D.; Ren, W.; Liu, B. Transport of Enterococcus faecalis in granular activated carbon column: Potential energy, migration, and release. Colloids Surf. B Biointerfaces 2019, 183, 110415. [Google Scholar] [CrossRef]
- Schinner, T.; Letzner, A.; Liedtke, S.; Castro, F.D.; Eydelnant, I.A.; Tufenkji, N. Transport of selected bacterial pathogens in agricultural soil and quartz sand. Water Res. 2010, 44, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Chrysikopoulos, C.V.; Aravantinou, A.F. Virus attachment onto quartz sand: Role of grain size and temperature. J. Environ. Chem. Eng. 2014, 2, 796–801. [Google Scholar] [CrossRef]
- Sasidharan, S.; Torkzaban, S.; Bradford, S.A.; Cook, P.G.; Gupta, V.V. Temperature dependency of virus and nanoparticle transport and retention in saturated porous media. J. Contam. Hydrol. 2017, 196, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Hong, M.; Wang, Y.; Dong, P.; Cheng, H.; Yan, H.; Yao, Z.; Li, D.; Wang, K.; Zhang, D. Assessing the Risks of Potential Bacterial Pathogens Attaching to Different Microplastics during the Summer–Autumn Period in a Mariculture Cage. Microorganisms 2021, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, L.J.; Turner, J.W. Temporal changes in water temperature and salinity drive the formation of a reversible plastic-specific microbial community. FEMS Microbiol. Ecol. 2020, 96, fiaa230. [Google Scholar] [CrossRef] [PubMed]
- Cappello, S.; Guglielmino, S.P. Effects of growth temperature on polystyrene adhesion of Pseudomonas aeruginosa ATCC 27853. Braz. J. Microbiol. 2006, 37, 205–207. [Google Scholar] [CrossRef]
- Bales, R.C.; Hinkle, S.R.; Kroeger, T.W.; Stocking, K.; Gerba, C.P. Bacteriophage adsorption during transport through porous media: Chemical perturbations and reversibility. Environ. Sci. Technol. 1991, 25, 2088–2095. [Google Scholar] [CrossRef]
- Briandet, R.; Meylheuc, T.; Maher, C.; Bellon-Fontaine, M.N. Listeria monocytogenes Scott A: Cell Surface Charge, Hydrophobicity, and Electron Donor and Acceptor Characteristics under Different Environmental Growth Conditions. Appl. Environ. Microbiol. 1999, 65, 5328–5333. [Google Scholar] [CrossRef]
- Ajaj, A.; J’bari, S.; Ononogbo, A.; Buonocore, F.; Bear, J.C.; Mayes, A.G.; Morgan, H. An Insight into the Growing Concerns of Styrene Monomer and Poly(Styrene) Fragment Migration into Food and Drink Simulants from Poly(Styrene) Packaging. Foods 2021, 10, 1136. [Google Scholar] [CrossRef]
- Ye, X.; Wang, P.; Wu, Y.; Zhou, Y.; Sheng, Y.; Lao, K. Microplastic acts as a vector for contaminants: The release behavior of dibutyl phthalate from polyvinyl chloride pipe fragments in water phase. Environ. Sci. Pollut. Res. 2020, 27, 42082–42091. [Google Scholar] [CrossRef]
- Murano, C.; Donnarumma, V.; Corsi, I.; Casotti, R.; Palumbo, A. Impact of Microbial Colonization of Polystyrene Microbeads on the Toxicological Responses in the Sea Urchin Paracentrotus lividus. Environ. Sci. Technol. 2021, 55, 7990–8000. [Google Scholar] [CrossRef] [PubMed]
- Vroom, R.J.; Koelmans, A.A.; Besseling, E.; Halsband, C. Aging of microplastics promotes their ingestion by marine zooplankton. Environ. Pollut. 2017, 231, 987–996. [Google Scholar] [CrossRef]
- Hansen, P.; von Bargen, K.; Jünger-Leif, A.; Haas, A. Laboratory Plasticware Induces Expression of a Bacterial Virulence Factor. mSphere 2022, 7, e0031122. [Google Scholar] [CrossRef]
- Sun, C.; Teng, J.; Wang, D.; Zhao, J.; Shan, E.; Wang, Q. The adverse impact of microplastics and their attached pathogen on hemocyte function and antioxidative response in the mussel Mytilus galloprovincialis. Chemosphere 2023, 325, 138381. [Google Scholar] [CrossRef]
- Al Akhrass, F.; Al Wohoush, I.; Chaftari, A.-M.; Reitzel, R.; Jiang, Y.; Ghannoum, M.; Tarrand, J.; Hachem, R.; Raad, I. Rhodococcus Bacteremia in Cancer Patients Is Mostly Catheter Related and Associated with Biofilm Formation. PLoS ONE 2012, 7, e32945. [Google Scholar] [CrossRef] [PubMed]

| Type | Molecular Structure | Classification | Brief |
|---|---|---|---|
| Polyethylene terephthalate (PET) | 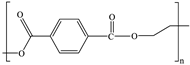 ((C10H8O4)n) |  | Polyethylene terephthalate, often abbreviated as PET, is the fourth most produced polymer in the world and is commonly used in producing synthetic fibers, food, and liquid containers. |
| High-density polyethylene |  ((C2H4)n) |  | HDPE, or high-density polyethylene, is recognized for its high strength-to-density ratio and is often used to produce plastic bottles and corrosion-resistant products. |
| Polyvinyl chloride (PVC) | 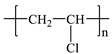 ((C2H3Cl)n) |  | Polyvinyl chloride, often abbreviated as PVC, is the third largest synthetic polymer produced in the world. Rigid PVC is commonly used in profile applications such as doors and windows, while flexible PVC is used for insulating cables, rainwear, and inflatable products. |
| Low-density polyethylene | 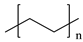 ((C2H4)n) |  | Low-density polyethylene is one of the world’s most widely produced plastics with low tensile strength and high elasticity. Its most common use is in plastic bags and films. |
| Polypropylene(PP) |  ((C3H6)n) |  | Polypropylene is a chemically resistant material and is the second most produced polymer after polyethylene. It is used in a wide range of applications including medical, packaging, and industrial. |
| Polystyrene (PS) | 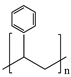 ((C8H8)n) |  | Polystyrene is one of the most frequently used polymers, with an annual production capacity of millions of tons. Its primary applications include protective packaging, disposable dinnerware, and model construction kits. |
| Nylon |  |  | Nylon, also known as polyamide, was the first synthetic fiber in the world to have extraordinarily high abrasion resistance. It works in several applications including fabrics and wear-resistant components. |
| Styrene block copolymers |  |  | SBCs are a thermoplastic elastomer family. It has qualities comparable to natural rubber and offers high elongation, processability, and environmental stability, making it an important raw material for toys, furniture, medical, and automotive parts. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Hong, X.; Chai, J.; Wan, B.; Zhao, K.; Han, C.; Zhang, W.; Huan, H. Interaction between Microplastics and Pathogens in Subsurface System: What We Know So Far. Water 2024, 16, 499. https://doi.org/10.3390/w16030499
Zhao H, Hong X, Chai J, Wan B, Zhao K, Han C, Zhang W, Huan H. Interaction between Microplastics and Pathogens in Subsurface System: What We Know So Far. Water. 2024; 16(3):499. https://doi.org/10.3390/w16030499
Chicago/Turabian StyleZhao, Hongyu, Xiaotao Hong, Juanfen Chai, Bo Wan, Kaichao Zhao, Cuihong Han, Wenjing Zhang, and Huan Huan. 2024. "Interaction between Microplastics and Pathogens in Subsurface System: What We Know So Far" Water 16, no. 3: 499. https://doi.org/10.3390/w16030499
APA StyleZhao, H., Hong, X., Chai, J., Wan, B., Zhao, K., Han, C., Zhang, W., & Huan, H. (2024). Interaction between Microplastics and Pathogens in Subsurface System: What We Know So Far. Water, 16(3), 499. https://doi.org/10.3390/w16030499










