Abstract
Modified sludge biochar, recognized for its notable economic and environmental benefits, demonstrates potential as an effective catalyst for peroxydisulfate (PDS) activation. Nevertheless, the specific mechanisms underlying its catalytic performance require more comprehensive investigation. In this study, a modified biochar (TSBC) doped with oxygen (O) and nitrogen (N) atoms was synthesized from sewage sludge and tannin extract, which significantly enhanced the activation of PDS for the degradation of sulfamethoxazole (SMX). The TSBC/PDS system demonstrated robust performance for SMX degradation, achieving over 90% efficiency over a wide pH range (3–10). Subsequent quenching experiments demonstrated that TSBC predominantly catalyzed PDS to generate , which effectively degraded SMX via a non-radical pathway. The O- and N-containing functional groups in TSBC were identified as the primary catalytic sites. Besides, density functional theory (DFT) calculations revealed that the incorporation of graphitic N significantly improved the adsorption capacity of PDS on the TSBC surface. Furthermore, based on the identification of intermediates and theoretical calculations, SMX was degraded mainly by two different pathways: S-N cleavage and oxidation. This study offers a foundational framework for the targeted modification of sludge biochar, thereby expanding its applications.
1. Introduction
Sulfamethoxazole (SMX), a sulfonamide antibiotic, is widely used in a multitude of therapeutic contexts in human and veterinary medicine, highlighting its pivotal role in modern medical practice [1]. However, the recalcitrant and strong antibacterial nature of SMX makes conventional physical and biological wastewater treatment methods inadequate to remove it [2,3]. Moreover, existing wastewater treatment systems lack effective antibiotic monitoring, leading to substantial concentrations of SMX remaining in treated wastewater [4]. As a result, the residual SMX accumulates in the environment, evidenced by its detection in various matrices, including surface water (especially rivers and lakes), groundwater, sediment, and even soil [5,6,7]. The accumulation of SMX in the environment not only increases the risk of bacterial resistance, but also stimulates the production and spread of antibiotic resistance genes (ARGs) through the food chain, presenting a significant threat to ecological integrity and human health [8,9].
Recent studies have conclusively demonstrated the efficacy of peroxydisulfate (PDS)-based advanced oxidation processes (AOPs) in the degradation of SMX. The growing interest in these processes stemmed from their exceptional chemical stability and adaptability across diverse environmental applications [10,11]. It is imperative to acknowledge that PDS remains inert in its native state and necessitates external energy activation. Consequently, the role of catalysts becomes paramount in improving the efficiency of PDS-based AOPs [12]. Recent evidence has illustrated that metal-free carbonaceous materials, such as graphene and biochar, exhibit effective activation of PDS, providing sustainable and environmentally advantageous alternatives [13,14]. As of recent, biochar derived from sewage sludge presents a viable choice for PDS-AOPs, attributed to its cost efficiency, biocompatibility, and sustainability [15].
In recent decades, rapid urbanization has led to a significant increase in wastewater volumes, resulting in substantial quantities of difficult-to-treat sludge that pose new environmental challenges [16]. Traditional sludge treatment methods, such as ocean dumping, incineration, and landfills, pose significant environmental risks due to the potential presence of toxic substances and heavy metals in the sludge [17]. Therefore, the advancing sludge resource recovery technology is now a critical necessity. Pyrolysis, which transforms sludge into bio-oil, biochar, and other valuable by-products, has emerged as a promising and high-performing solution in recent research [18]. Sludge-derived biochar, with its distinctive and carbon configuration, effectively activates PDS in both radical and non-radical ways, which enhances the versatility of the system [19]. Yin et al. [20] demonstrated that the incorporation of sludge biochar into PDS-based degradation systems significantly improved the catalytic efficiency via both radical and non-radical pathways, resulting in a substantial 48.3-fold acceleration in reaction rate compared to the separate use of PDS and sludge biochar. Nevertheless, the catalytic efficiency of sludge biochar is potentially constrained by its limited surface area, which restricts interactions with catalytic targets [21]. In addition, the potential leaching of heavy metals from sludge biochar raises concerns about its applicability in environmental pollution remediation, rendering its use controversial.
In response to these challenges, extensive research has focused on the co-pyrolysis of sludge with biomass, demonstrating considerable promise in improving the properties of sludge biochar [22,23]. It has been shown to improve the immobilization of heavy metals in sludge and facilitate the use of synthetic biochar for environmental purposes [24]. Co-pyrolysis also facilitates the integration of foreign atoms, offering the sludge biochar specific properties, which increases the interaction between PDS and organic pollutants [25]. Yin et al. [26] reported a notable increase in biochar surface area from 31.35 to 122.89 by incorporating walnut shells into sludge pyrolysis. Our previous study revealed that biochar produced from co-pyrolysis of sludge and tannin extract exhibited a highly porous structure and increased surface area, making it an effective PDS activator [27]. Notably, X-ray photoelectron spectroscopy (XPS) analysis of the synthesized biochar revealed that the heavy metal signals, which could be associated with catalytic efficiency, were weakened. Instead, it contained predominantly the heteroatoms of nitrogen (N) and oxygen (O) that promote catalysis. Nevertheless, the specific mechanisms underlying the PDS activation process remain to be fully elucidated.
Besides the role of surface area in determining the catalytic efficiency of biochar, the functional groups on its surface are also critical in influencing its properties. Further detailed analyses of the catalytic mechanism have revealed a notable influence of N- and O-containing functional groups on the catalytic efficiency of sludge biochar. Tan et al. [28] identified graphitic C, pyridine N, and graphitic N as the primary active sites in biochar, which are crucial for facilitating electron transfer and thus promoting PDS activation. Contrarily, Mian et al. [29] showed that pyridine N was the predominant active species in their research, facilitating the degradation of pollutants via non-radical pathways. Additionally, Yang et al. [30] emphasized the importance of O-functional containing groups, specifically hydroxyl (-OH), carbonyl (C=O), and ether (C-O) groups, in enhancing catalytic performance. However, in the current discourse on PDS activation by sludge biochar, the specific roles of different N and O species remain unclear and controversial, highlighting the need for further investigation. Therefore, identifying the precise forms of N and O doping that enhance catalytic efficiency is critical to advancing the development of sludge biochar as an effective catalyst.
In this study, biochar (TSBC) prepared by co-pyrolysis of sludge and tannin extract was investigated as a catalyst for PDS to effectively degrade SMX in aquatic environments. The active species within the TSBC/PDS system and the catalytic sites on TSBC were systematically investigated by quenching experiments and advanced characterization techniques. Additionally, the enhancement of PDS activation by various N- and O-containing functional groups on the TSBC surface was analyzed by Density Functional Theory (DFT) calculations, providing an in-depth investigation of the adsorption dynamics of PDS on TSBC and revealing the role of these functional groups. Moreover, the identification of SMX degradation intermediates, coupled with theoretical calculations, shed light on the degradation pathways of SMX. This study aims to clarify the role of functional groups on sludge biochar in facilitating PDS activation. It can contribute to a deeper understanding of the mechanical aspects and provide guidance for targeted modification of sludge biochar to improve its effectiveness as a catalyst.
2. Materials and Methods
2.1. Materials and Chemicals
Digested sludge with a moisture content of 70% was obtained from a sewage treatment facility in Longyan, Fujian. Industrial-grade wattle bark tannin extract was sourced from a commercial supplier in Jinan, Shandong. Sulfamethoxazole (SMX), Sodium persulfate , tertiary butanol (TBA), p-benzoquinone (p-BQ), furfuryl alcohol (FFA), and 2,2,6,6-tetramethylpiperidine (TEMP) were acquired from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). In addition, methanol (MeOH), ethanol (EtOH), hydrochloric acid (HCl), sodium chloride (NaCl), sodium hydroxide (NaOH), sodium nitrate , and sodium bicarbonate were obtained from Sinopharm chemical reagent Co., Ltd. (Shanghai, China). All chemicals employed in this study were of analytical grade. TSBC and conventional sludge biochar (SDBC) were synthesized employing the methodology delineated by Yang et al. [27], with detailed procedural steps provided in Text S1.
2.2. Experimental Procedures
The experiment investigating the catalytic degradation of SMX was conducted in a 150 mL Erlenmeyer flask. Initially, 25 mg of TSBC was added to 100 mL of an SMX solution with a concentration of 20 [31]. This mixture was placed in a constant-temperature shaking incubator for 30 min to establish adsorption-desorption. Once equilibrium was reached, 11.9 mg of was added to initiate the catalytic degradation process. To monitor the degradation, 1 mL samples of the reaction mixture were extracted at 15 min intervals, and 1 mL of a quenching agent was immediately added to halt the reaction. The samples were then filtered through a 0.22 m glass fiber filter. The residual mass concentration of SMX in these samples was quantified using high-performance liquid chromatography (HPLC), enabling the calculation of the SMX degradation rate. All experimental procedures were carried out at a consistent temperature of 25 °C and a shaking speed of 160 . The experimental methods detailing the effect of environmental variables on SMX degradation are outlined in Text S2.
The quenching experiment followed the same procedure as the degradation experiment. Upon achieving adsorption-desorption equilibrium, a quenching agent (methanol (MeOH), tertiary butanol (TBA), furfuryl alcohol (FFA), and p-benzoquinone (p-BQ)) was introduced. Samples were then taken for analysis at 15 min intervals.
2.3. Analytical Methods
The quantification of SMX in the samples was performed using high-performance liquid chromatography (HPLC, LC-20AT, Shimudzu, Kyoto, Japan) equipped with a C18 chromatographic column (Eclipse Plus C18, Agilent, Santa Clara, CA, USA) and a UV detector, ensuring accurate and efficient analysis [32]. The analytical conditions were optimized as follows: the detection wavelength was set at 269 nm, and the mobile phase consisted of 70% methanol and 30% ultrapure water (). The flow rate was maintained at 1 , and the injection volume was standardized to 20 L. The oven temperature was set to 30 °C. The detailed test procedure is supplemented in Text S3. Furthermore, the qualitative analysis of SMX degradation intermediates was conducted using liquid chromatography coupled with a quadrupole time-of-flight tandem mass spectrometry (LC-QTOF-MS, G6520B, Agilent, California, USA), employing a C18 chromatographic column (Eclipse Plus C18, Agilent, California, USA).
The kinetic degradation of SMX adheres to a pseudo-first-order kinetic model, as described by Equation (1),
where represents the mass concentration of SMX at time t (in ), denotes the initial mass concentration of SMX (in ), is the apparent reaction rate constant (), and t signifies reaction time (in minutes).
The crystal structure, surface functional groups, and zeta potential of TSBC are elaborated in Text S3, which also includes detailed methodologies for analyzing active species within the catalytic system.
2.4. Theoretical Calculation
The structure of biochar models and SMX was optimized using the Gaussian 09 software, employing the B3LYP/6-31G basis set [33]. The energies of the adsorbed models were computed utilizing the M062x functional combined with the 6-311G basis set in a solvation model density (SMD) environment, specifically water. All calculation processes were extended with dispersion correction (DFT-D3). The optimized adsorbed configurations, along with their electrostatic potential (ESP) maps, were visualized and analyzed by the CYLview20 and Multiwfn3.8 software, respectively [34,35]. The adsorption energy () of PDS on the constructed models was determined using the following equation [36]:
where , and represent the total energies (in eV) of the PDS adsorbed system, biochar models, and free PDS , respectively.
3. Results and Discussion
3.1. Degradation of SMX in the TSBC/PDS System
3.1.1. SMX Degradation Efficiency
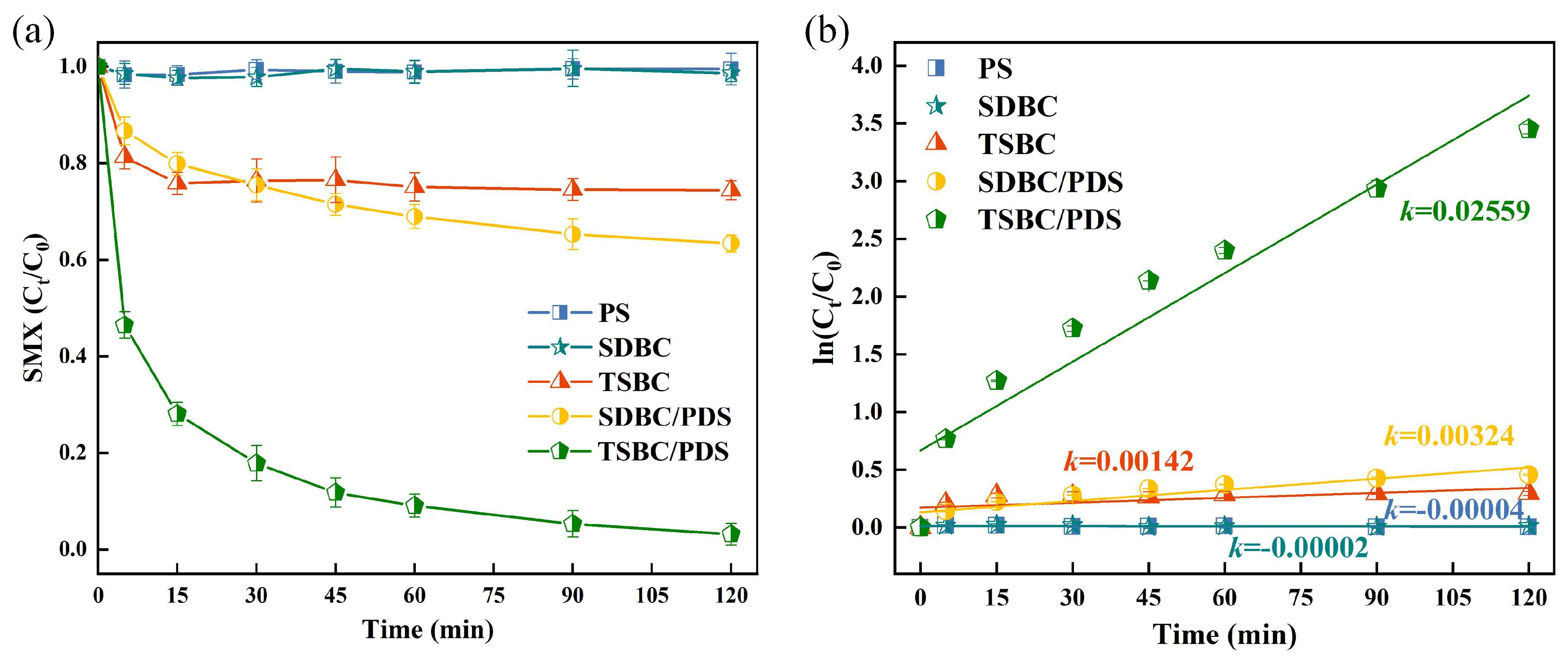
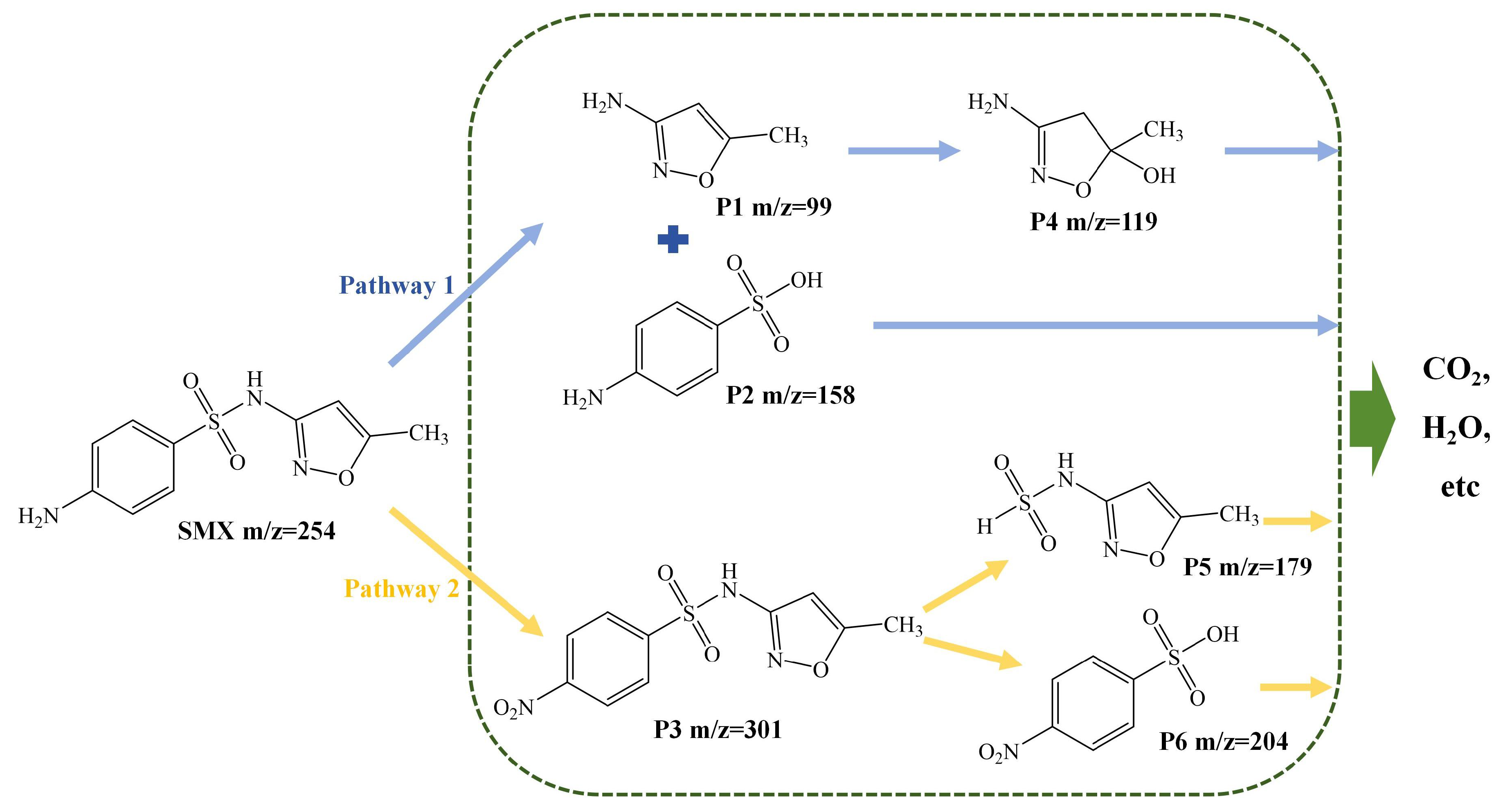
We evaluated the catalytic efficiency of TSBC by studying five different systems: PDS, SDBC, TSBC, SDBC/PDS, and TSBC/PDS. The comparative analysis focused on the degradation performance of SMX within these systems, as shown in Figure 1a. In the absence of a catalyst, the degradation of SMX in a PDS-only system was minimal, achieving a mere 2% removal over 120 min. This result highlighted the ineffectiveness of PDS alone in oxidatively degrading SMX. Conversely, TSBC notably improved PDS activation over SDBC, achieving a notable SMX degradation rate of 96.83%. Correspondingly, the observed reaction rate () for the TSBC/PDS system was seven times higher than that of the SDBC/PDS system, reaching 0.0256 (Figure 1b). The improved catalytic efficiency of TSBC was probably due to its porous structure (Figure S2) and heteroatom doping (Table S1). The porous structure of TSBC would promote closer contact between the pollutant and the catalyst, thereby enhancing PDS activation and pollutant degradation [37]. Furthermore, N doping would facilitate electron transfer between TSBC and , increasing the generation and thereby boosting the efficiency of the non-radical pathway in SMX degradation [28]. In summary, the TSBC demonstrated exceptional catalytic performance for PDS, significantly enhancing the degradation efficiency of SMX.
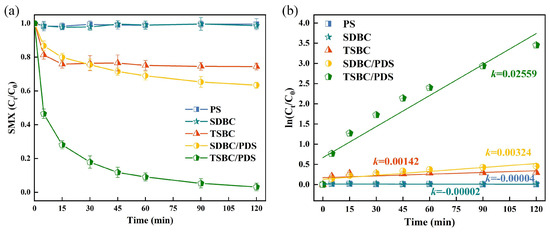
Figure 1.
(a) Performance of TSBC in activating PDS for SMX degradation. (b) SMX degradation kinetics fitted by pseudo-first-order reaction. (Reaction conditions: (SMX) = 20 , (PDS) = 240 , (TSBC) = 250 , pH = 5.76, T = 25 °C.)
3.1.2. Effect of pH on SMX Degradation
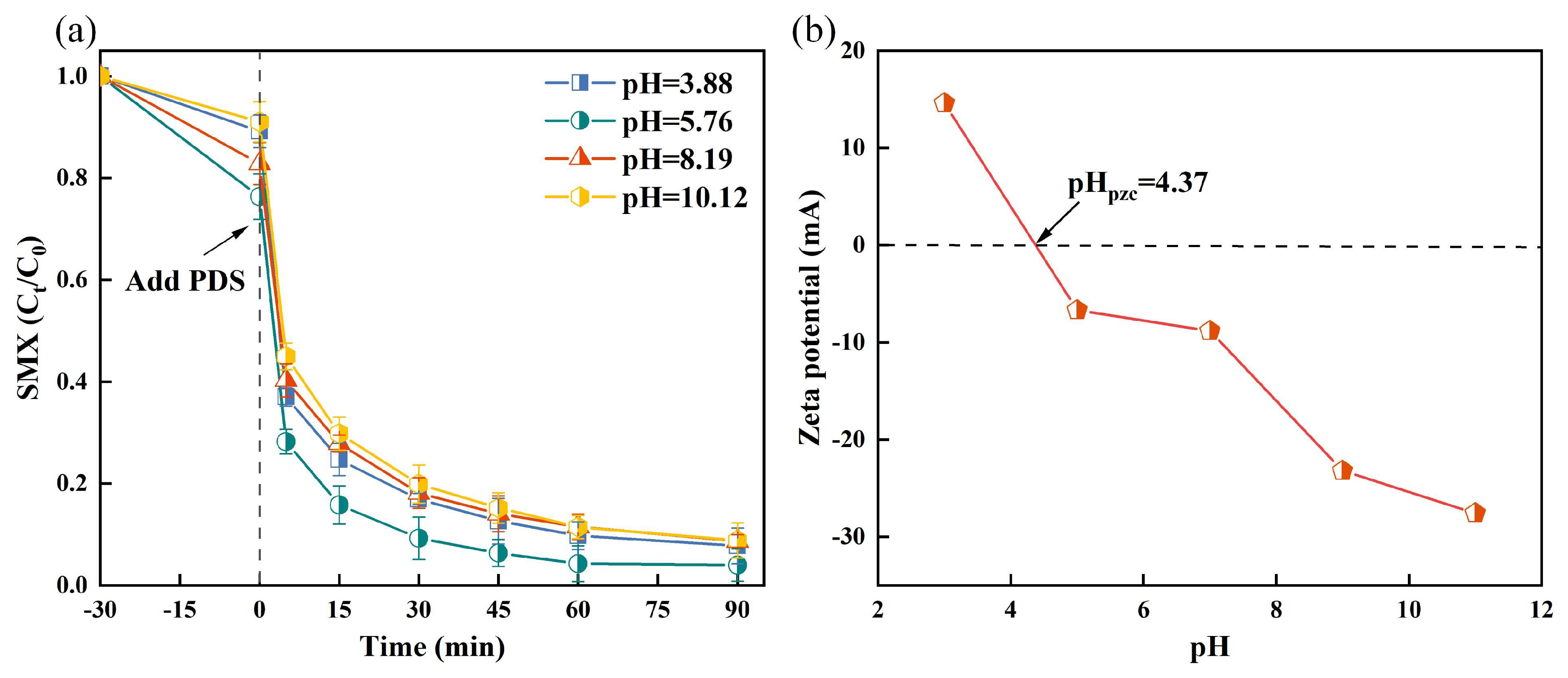
The pH of the solution critically affects the efficacy of PDS-based AOP systems. Therefore, we investigated the effect of pH on SMX removal in the TSBC/PDS system at pH values of 3.88, 5.76, 8.19, and 10.12. Figure 2a demonstrates that the effective SMX removal of the TSBC/PDS system over initial pH ranged from 3.88 to 10.12. Below pH 5.76, the degradation efficiency of SMX increased proportionally with rising pH, peaking at a degradation rate of 96.01% at pH 5.76. In contrast, an alkaline environment negatively affected the degradation of SMX within the TSBC/PDS system. The zero-point charge of TSBC was tested as 4.37 (Figure 2b), signifying a positively charged surface when the pH fell below 4.37, which enhanced its interaction with PDS ions [38]. Contrary to expectations, the maximum degradation rate of SMX was not observed at a pH of 3.88, which may be attributed to the prevalence of neutral SMX in solution, resulting in reduced interaction with the positively charged TSBC [39]. Therefore, the reduced interaction between TSBC, PDS, and SMX at pH 3.88 adversely affected the degradation rate of SMX. Additionally, the elevated concentration of hydrogen ions in a strongly acidic environment may form hydrogen bonds with the O-O bonds in PDS, hindering the effective contact between PDS and TSBC [38]. In general, test results revealed consistent degradation rates of more than 90% over the pH range of 3–10, highlighting the substantial adaptability of the TSBC/PDS system.
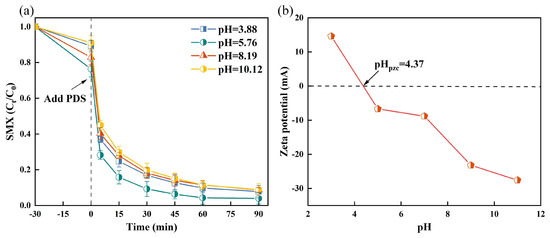
Figure 2.
(a) Effects of solution pH value on the SMX degradation in the TSBC/PDS system. (b) Zeta potential of TSBC terms of pH. (Reaction conditions: (SMX) = 20 , (PDS) = 120 , (TSBC) = 250 , T = 25 °C).
3.1.3. Effect of Temperature on SMX Degradation
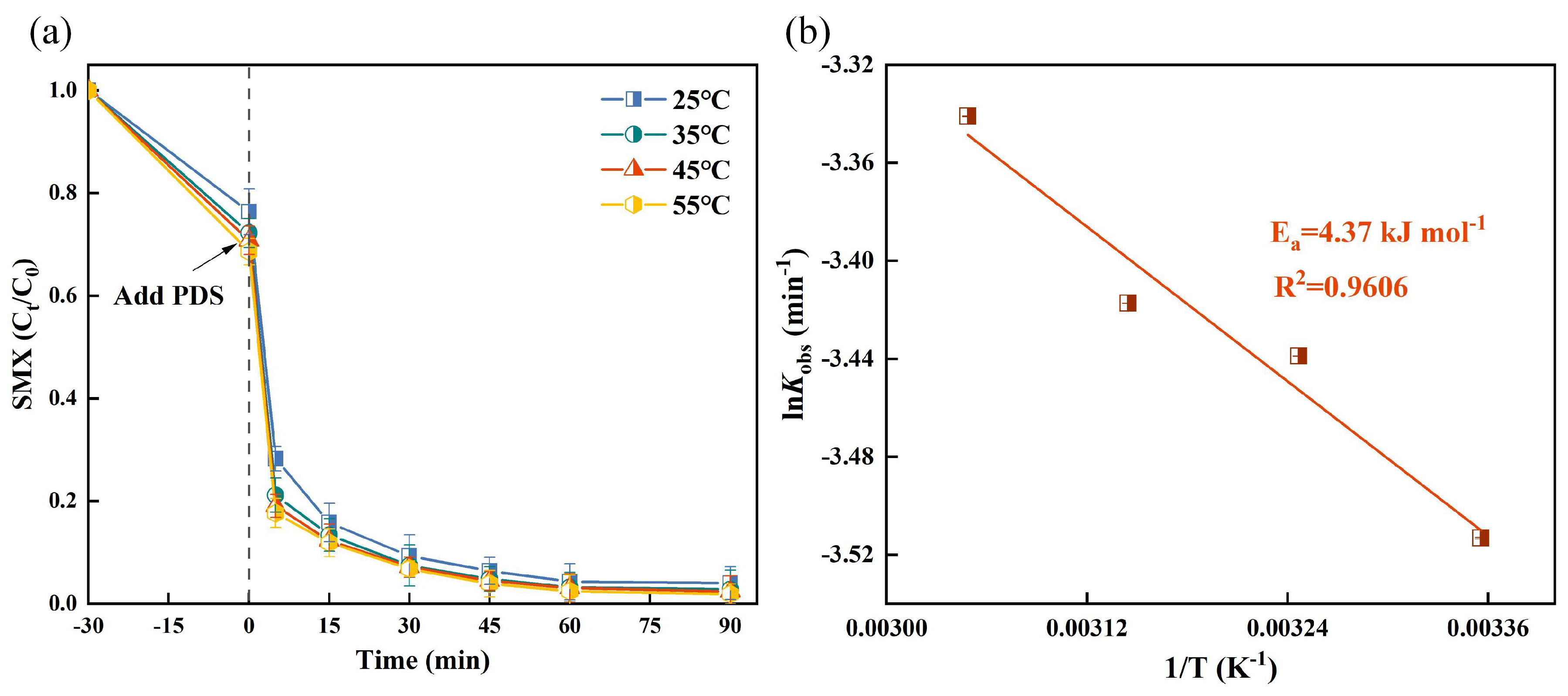
Increasing the reaction temperature has been demonstrated to enhance PDS decomposition and formation, thereby effectively facilitating pollutant degradation [40]. In the TSBC/PDS system, a notable improvement in degradation rate was observed as temperature increased from 25 °C to 55 °C (Figure 3a), peaking at 98.12% at 55 °C. The activation energy required for pollutant degradation varies significantly in heterogeneous catalytic degradation systems. The Arrhenius equation was used in the TSBC/PDS system to model the correlation between reaction rate constants and temperature [41]. In Figure 3b, results from the Arrhenius equation revealed that the TSBC/PDS system degraded SMX with an activation energy of 4.37 , which is considerably lower than reported values for analogous oxidation processes, highlighting the superior efficiency of the TSBC/PDS system in degrading SMX [42].
Figure 3.
(a) Effects of reaction temperature on the SMX degradation in the TSBC/PDS system. (b) Relationship between reaction rate constant and temperature in TSBC/PDS system. (Reaction conditions: (SMX) = 20 , (PDS) = 120 , (TSBC) = 250 , pH = 5.76.)
3.1.4. Effect of Natural Water Constituents on SMX Degradation
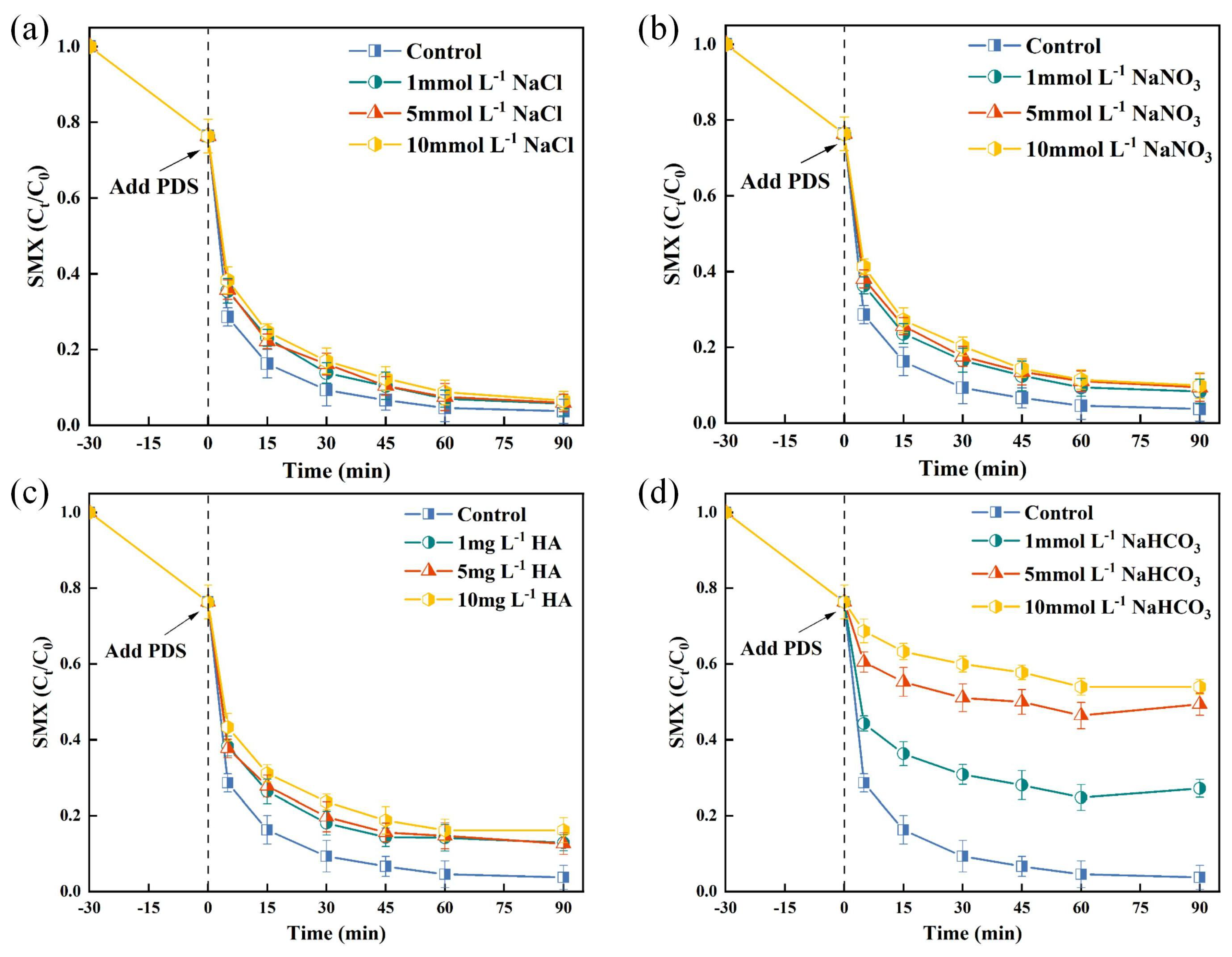
In wastewater treatment, the widespread presence of inorganic anions (e.g., , , ) and natural organic matter, notably humic acids (HA) leads to non-selective scavenging of free radicals [43]. Figure 4a demonstrates that minimally affected SMX degradation. Notably, even at elevated concentrations of 10 mmol/L, the reduction in SMX degradation rate was limited to only 3%. In the TSBC/PDS system, the degradation rate of SMX remained consistently above 90% within 120 min, demonstrating significant resistance to interference from , even as the concentration increased from 1 to 10 (Figure 4b). Previous studies have shown that the interaction of and ions with and radicals results in the generation of radicals possessing diminished oxidative capabilities [44]. Therefore, it is plausible to infer that the degradation of SMX in the TSBC/PDS system was not predominantly facilitated by and radicals. A slight decrease in SMX degradation was observed at a HA concentration of 1 in Figure 4c, which further decreased to 83.83% with 10 of HA. This decrease in degradation efficiency may be attributed to the competitive adsorption of HA and PDS, hindering the formation of reactive species [45]. Notably, the introduction of 10 significantly reduced the SMX degradation rate to 46.07% within 120 min in the TSBC/PDS system (Figure 4d). This decrease may be due to the interaction of with , thereby inhibiting the non-radical pathway [46]. Additionally, the presence of promotes an alkaline environment, which may influence the formation of radicals. In conclusion, TSBC/PDS demonstrated considerable adaptability for practical water treatment applications.
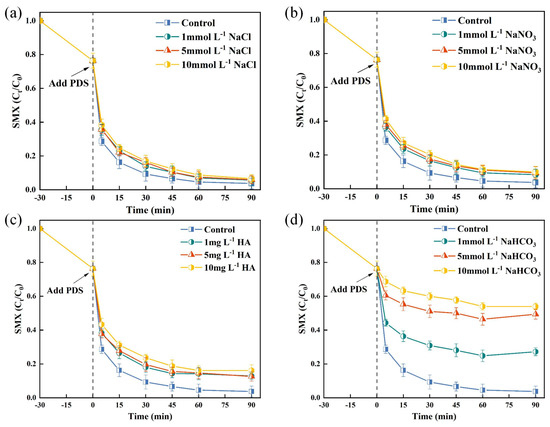
Figure 4.
Effects of anions and HA on SMX degradation in TSBC/PDS system: (a) , (b) , (c) HA, and (d) . (Reaction conditions: (SMX) = 20 , (PDS) = 120 , (TSBC) = 250 , pH = 5.76, T = 25 °C.)
3.2. Degradation Mechanism Analysis
3.2.1. Identification in the Reactive Oxygen Species
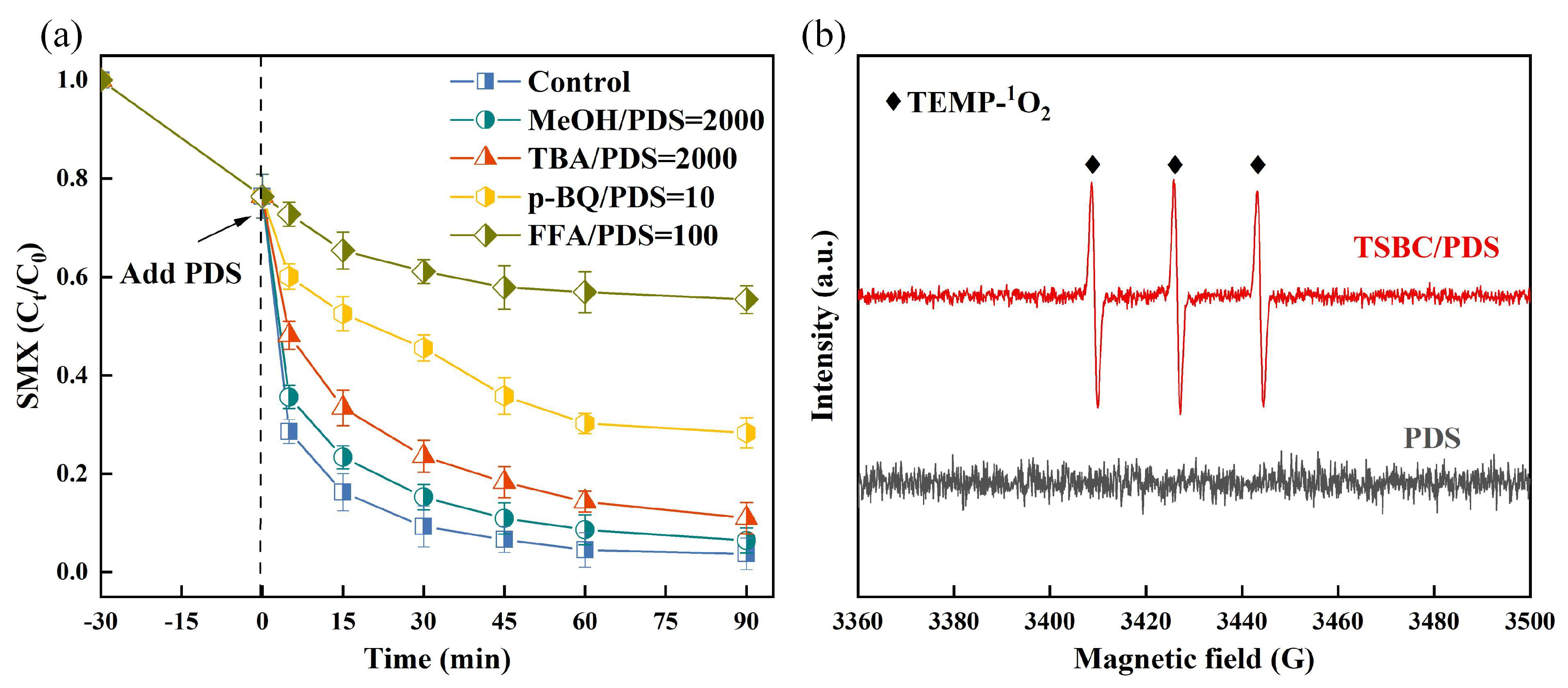
In investigating the degradation mechanism of SMX in the TSBC/PDS system, we used scavengers, including methanol (MeOH), tertiary butanol (TBA), furfuryl alcohol (FFA), and p-benzoquinone (p-BQ) to evaluate the individual contributions of active species. Methanol exhibits notable scavenging capabilities for both ( = 1.2–2.8) and ( = 1.6–7.7). When MeOH was introduced into the TSBC/PDS system, the degradation of SMX showed no significant inhibition, with removal rates consistently above 90% (Figure 5a). Additionally, the degradation of SMX exhibited minimal inhibition with the introduction of the scavenger TBA ( = 3.8–7.6) into the system [47]. Therefore, and were not the primary active species in the TSBC/PDS system. The introduction of p-BQ resulted in a decrease in SMX degradation to 71.69%, indicating the potential presence of in the system, with a limited effect on SMX degradation. Notably, FFA demonstrated a pronounced effect on the degradation of SMX in the TSBC/PDS system, resulting in a reduction in SMX degradation to 32.80%. This observation implied the potential presence of in the system, indicating its crucial involvement in the degradation process of SMX [48]. The EPR technique using TEMP as a spin trap further proved the produced in the TSBC/PDS system. No signal was observed in the individual PDS (Figure 5b). In contrast, the TSBC/PDS system exhibited distinct equidistant three-line peaks, characteristic of TEMP- (TEMPO), highlighting the generation of upon TSBC activation of PDS [49]. This observation illustrated that was generated upon TSBC activation of PDS and played an important role in degrading SMX.
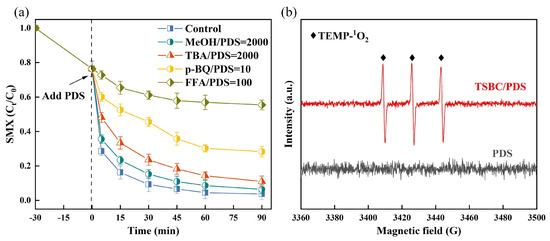
Figure 5.
(a) SMX degradation quenchers including MeOH, TBA, p-BQ, and FAA. (b) EPR spectra of the PDS system and the TSBC/PDS system. (Reaction conditions: (SMX) = 20 , (PDS) = 120 , (catalyst) = 250 , pH = 5.76, T = 25 °C.)
3.2.2. Identification in the Active Sites
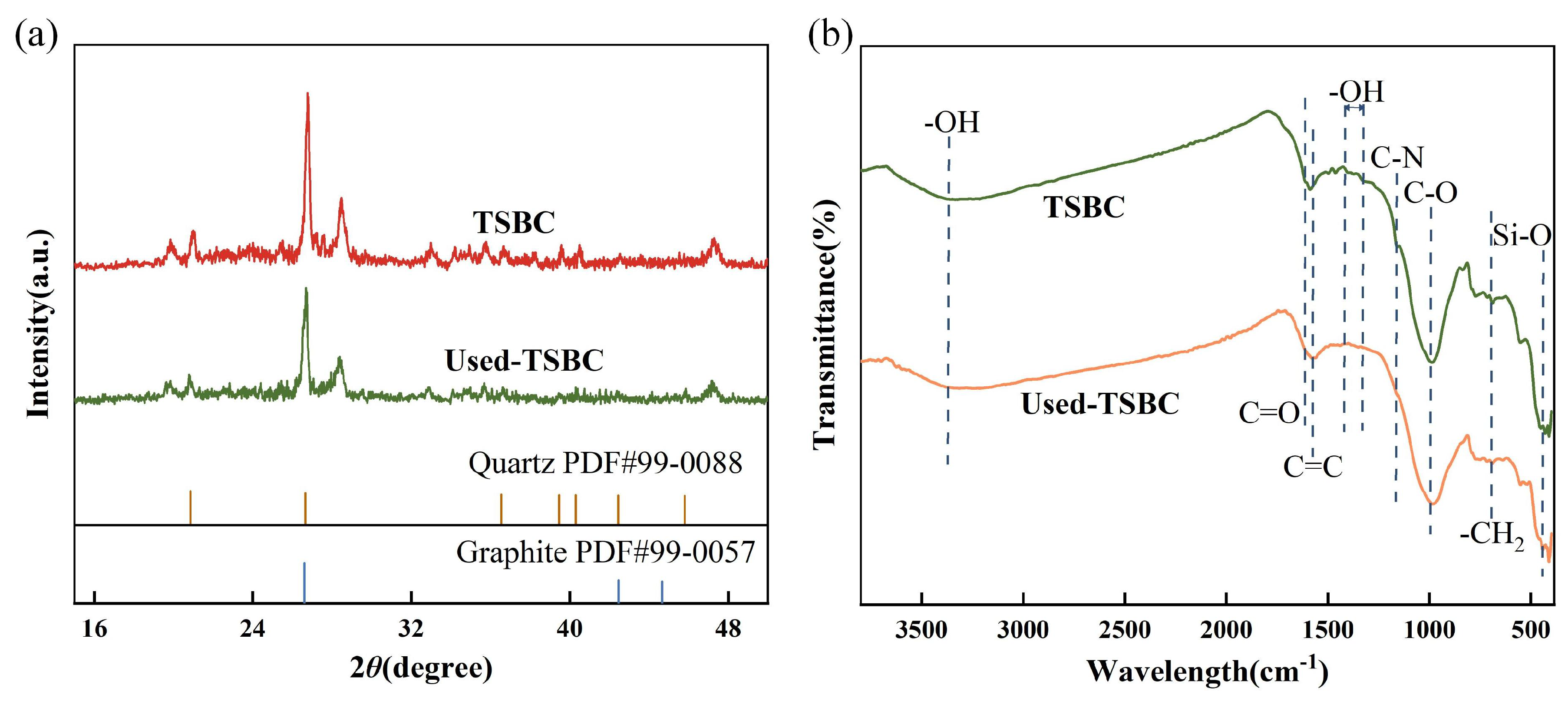
In order to elucidate the activation mechanism of PDS by TSBC, the characterization of TSBC was carried out both before and after the reaction. As shown by the XRD spectra in Figure 6a, no significant change was observed in TSBC crystal structure before and after degradation, indicating its inherent stability throughout the degradation process. Furthermore, the surface functional groups of TSBC before and after the reaction were determined by FT-IR spectroscopy, as depicted in Figure 6b. The characteristic peaks around 3370 , 690 , and 440 correspond to -OH, -, as well as Si-O groups, respectively, with no observable changes [50,51,52]. The notable shift in the weakened C=C bond peak (1580 ) towards a lower wavenumber indicated the adsorption of SMX and PDS, facilitated by - interactions with benzene ring groups in TSBC [53]. On the other hand, N atoms in TSBC promoted electron migration from adjacent C atoms, resulting in the formation of positively charged carbon sites. These sites demonstrated heightened selectivity for the negatively charged . This interaction could facilitate the direct activation of the O-O bond in PDS through a non-radical mechanism, leading to the generation of [54]. Moreover, recent studies have established that the C=O groups play a crucial role in PDS activation, acting primarily as an electron transfer and oxidation site [15]. It was also instrumental in the generation of during PDS decomposition. Consistent with previous studies, the signal intensities of the characteristic peaks associated with C=O (1615 ) and C-O (1020 ) were substantially reduced in the TSBC used [51,55]. This observation suggested the active participation of O-containing groups in TSBC in facilitating the PDS activation process for SMX degradation. In addition, the notable absence of the characteristic peaks at 1310–1390 and 1160 in the used TSBC suggested a reduced presence of -OH on the benzene ring as well as C-N functional groups on the benzene ring [56,57]. Numerous studies have demonstrated that N-containing groups functional groups significantly enhance non-radical processes for oxidizing organic pollutants [29]. In light of the characterization results presented, it was evident that the presence of O- and N-containing functional groups in TSBC enhanced the activation of PDS via a non-radical pathway.
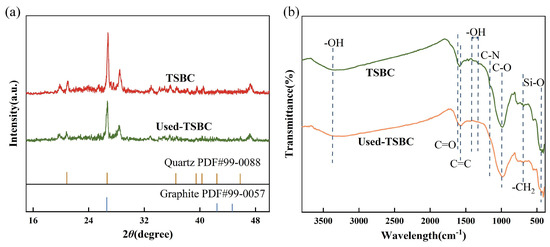
Figure 6.
(a) XRD and (b) FT-IR spectra of TSBC before and after reaction.
3.2.3. Theoretical Calculation
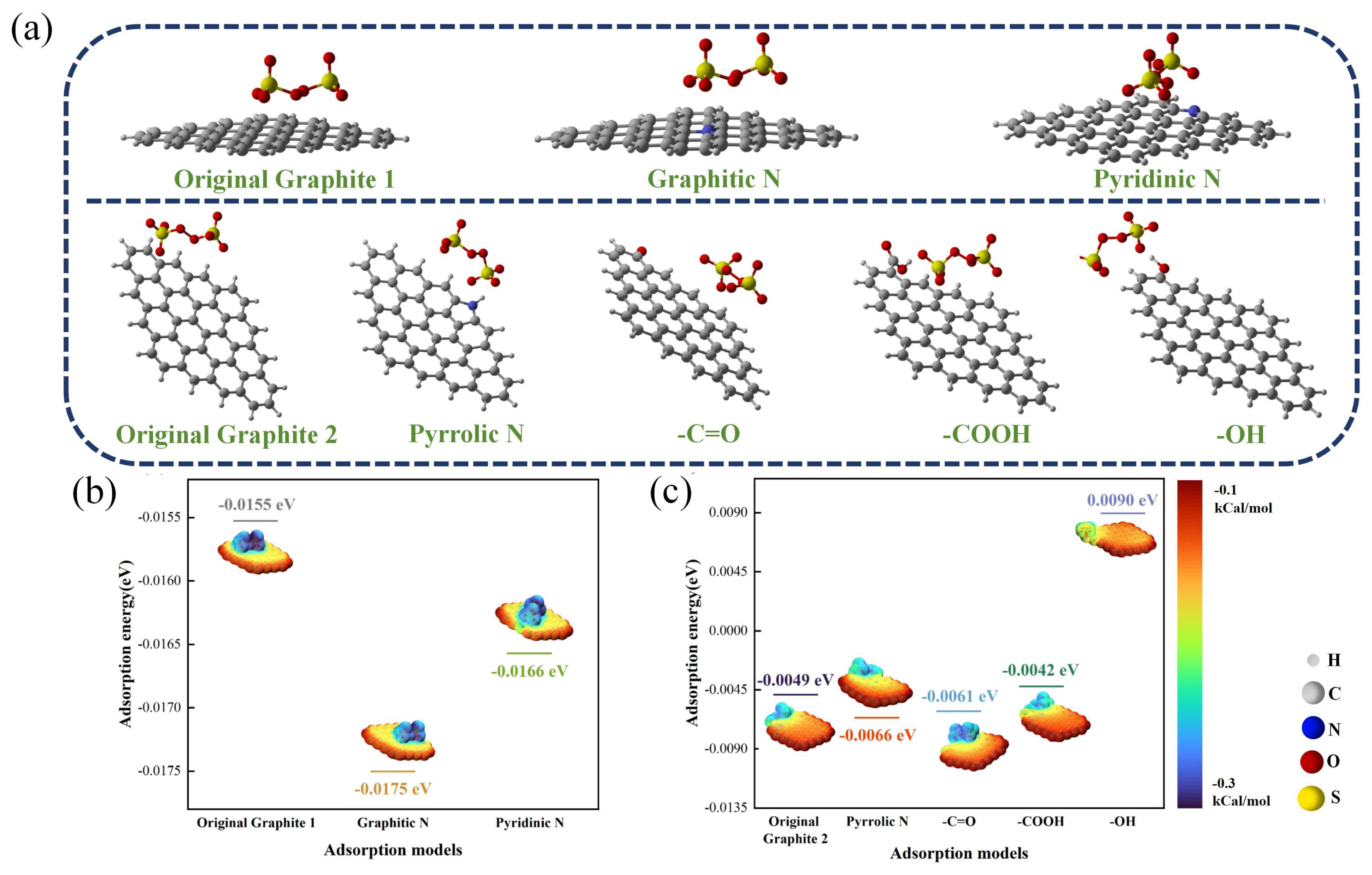
To investigate the impact of biochar functional groups on PDS activation, we conducted DFT calculations with Gaussian 09 software to delve into the microscopic interactions between different functional groups of biochar and PDS [58]. According to the above results, the O- and N-containing functional groups in TSBC were identified as potential catalytic sites. Consequently, seven models of N- or O-doped graphene were developed to elucidate the effect of different conformations of these functional groups. In the catalytic activation of PDS, the adsorption of PDS onto the catalyst surface is a critical and fundamental process [59]. Therefore, the adsorption configurations of PDS with different graphene models were intensively analyzed, as shown in Figure 7a. In aquatic settings, doped graphene models exhibited notable variability in their interactions with PDS. In the pursuit of refining comparative analytical precision, we computationally modeled two distinct adsorption configurations of pristine graphene with PDS. In Figure 7b, the adsorption energy comparative analysis revealed that graphitic and pyridinic N-doped graphene exhibited adsorption energies of −0.0175 eV and −0.0166 eV, respectively, which were markedly lower than that of pristine graphene (−0.0155 eV). Furthermore, the adsorption energies for pyridine N-containing groups (−0.0066 eV) and -C=O (−0.0061 eV) models were found to be lower compared to those of the original graphene model (−0.0049 eV) (Figure 7c). Extensive studies have indicated that the -C=O groups in biochar, possessing a lone pair of electrons, facilitate the cleavage of O-O bonds in PDS, consequently leading to the formation of [60]. Additionally, N in biochar has been reported to enhance the activation of PDS, facilitating its conversion to a non-radical pathway [20]. In particular, the graphite model incorporating graphitic N exhibited an enhanced affinity for PDS. Research by Yin et al. [61] clarified the central role of graphitic N in biochar and demonstrated its importance as a primary catalytic site for PDS activation, subsequently facilitating the degradation of tetracycline (TC). Due to its electron affinity, graphitic N effectively attracts electrons from adjacent C atoms, thereby enhancing the electron acceptor properties of these atoms. This allows for biochar to act as an efficient medium for electron transfer, promoting the adsorption and subsequent activation of PDS [28]. The results of the theoretical calculation highlighted the critical importance of graphitic N in PDS activation, corroborated by FT-IR findings showing a post-reaction reduction in C-N.
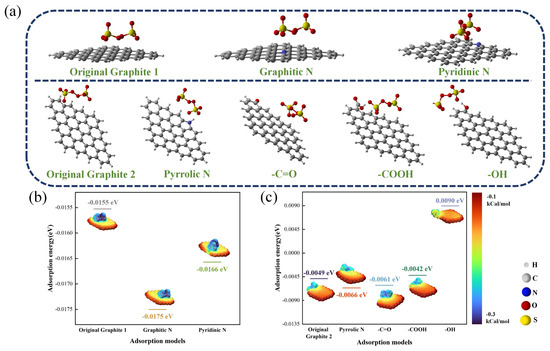
Figure 7.
(a) Adsorption configurations of different graphene models and PDS. (b,c) Adsorption energy and electrostatic potential (ESP) of different adsorption configurations.
3.3. Degradation Pathways of SMX
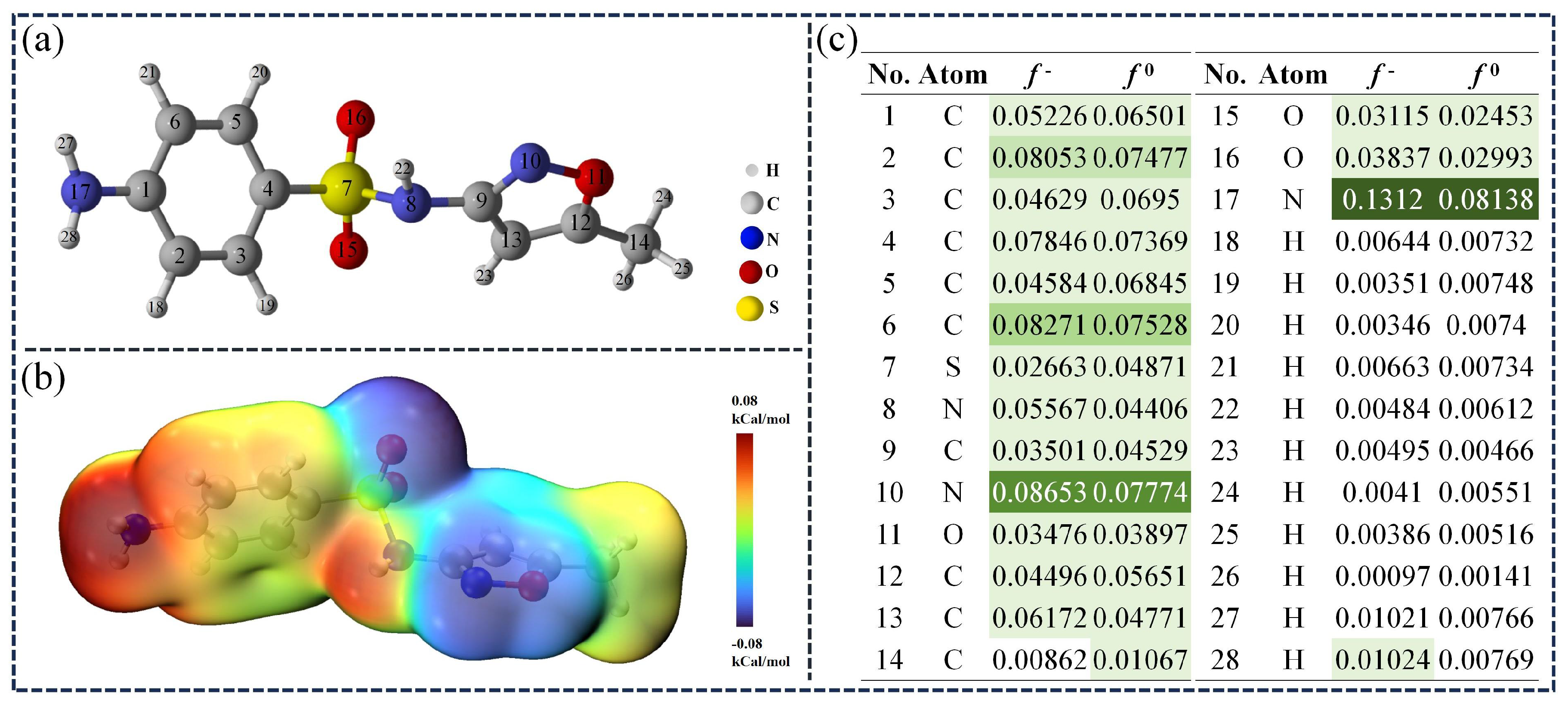
In order to better understand the degradation mechanism of SMX in the TSBC/PDS system, we applied DFT calculations to determine the electrostatic potential (ESP) of SMX. Additionally, the Fukui function was utilized to pinpoint probable reaction sites. As shown in Figure 8b, the group bonded to the benzene ring showed a significantly high charge density, as indicated by the prominent red area. Contrastingly, the area around the S atom exhibited significantly lower charge density, evidenced by the pronounced blue region. In previous studies, SMX has been shown to possess significant electron-donating characteristics, making it susceptible to electrophilic reactions with [62]. Consequently, the index demonstrates the reactive site of with SMX and the illustrates the selectivity of the radical towards SMX [59]. Theoretical calculations showed that atoms N17, N10, and C6 in SMX had higher and values (Figure 8c), indicating increased susceptibility to oxidative reactions initiated by and radicals.
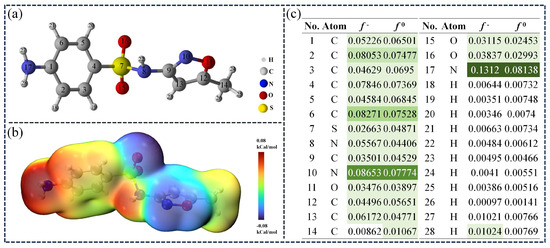
Figure 8.
(a) Optimized structure of SMX. (b) the ESP of SMX. (c) Fukui function of SMX (Larger values are marked with darker colors.).
The LC-MS/MS analysis revealed five intermediates (Figure S3). These intermediates led to two potential degradation pathways, as shown in Figure 9. In Pathway 1, the cleavage of the S-N bond in SMX led to the formation of products P1 (m/z = 99) and P2 (m/z = 158). This phenomenon could be attributed to the low ionization energy of the S-N bond in SMX, rendering it highly susceptible to attack and subsequent cleavage by reactive oxygen species (ROS) [62]. As the reaction progressed, P1 underwent hydroxylation to form P4 (m/z = 119). In Pathway 2, the N17 atom in SMX, which was susceptible to electrophilic reactions, was oxidized by ROS, especially . Thus, the group of SMX was oxidized to nitro-SMX (P3, m/z = 301), in agreement with previous research [63]. As the reaction progressed, P3 was continually broken down by the action of ROS, leading to the formation of smaller molecules, identified as P5 (m/z = 179) and P6 (m/z = 204). During the reaction, the identified intermediates were subject to consecutive ROS-mediated attacks, resulting in their fragmentation into smaller molecules, ultimately yielding and , among other by-products [64].
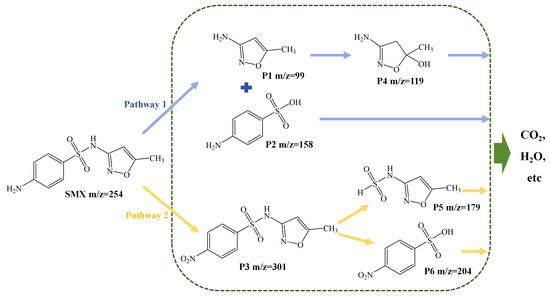
Figure 9.
The possible degradation pathways of SMX in TSBC/PDS system.
4. Conclusions
In summary, we focused on exploring the impact of O- and N-containing functional groups on augmenting the catalytic efficiency of modified sludge biochar (TSBC) to activate PDS for SMX degradation. The TSBC/PDS system exhibited exceptional efficiency in degrading SMX, achieving a removal rate of 96.83% within 120 min. Notably, the reaction rate of SMX degradation via TSBC-catalyzed PDS was seven times higher than that achieved with conventional sludge biochar (SDBC). In addition, this system demonstrated remarkable stability and efficiency, maintaining over 90% degradation efficiency across a broad pH range (3–10), making it a promising solution for varied applications. The quenching experiment and characterization results indicate that the C=O and C-N groups in TSBC primarily served as catalytic sites, mainly facilitating the non-radical pathway degradation of SMX by activating PDS to produce . Further theoretical analysis demonstrated that, among various N- and O-containing functional groups, graphitic N predominantly enhanced the integration of biochar with PDS, consequently facilitating the activation of PDS. SMX exhibited pronounced electrophilic properties, facilitating its reactivity with . Theoretical calculations and experimental evidence suggested that SMX degraded by two mechanisms: S-N cleavage and direct oxidation by . This research investigated the influence of various N and O species on PDS activation, emphasizing the significant role of graphitic N in enhancing PDS adsorption by biochar. These findings provide a fundamental framework for subsequent advances in improving the catalytic efficiency of sludge-derived biochar through specific modifications. The proposed improvements will streamline the application of sludge biochar in AOPs, thereby reducing operating costs. Furthermore, considering the similarities between certain industrial and sewage sludges, this approach could potentially extend to treating industrial sludges, particularly in the tannery and paper industries with low heavy metal concentrations, pending further validation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16030505/s1, Text S1: Materials and chemicals. Text S2: Experimental procedures. Text S3: Analytical methods. Table S1: Element composition of SDBC and TSBC. Figure S1: Calibration curve of SMX. Figure S2: SEM images of SDBC and TSBC, and Figure S3: SMX intermediates detected in TSBC/PDS system.
Author Contributions
Conceptualization, M.L., Y.H. and J.L.; methodology, J.L.; validation, Y.H. and J.L.; formal analysis, Y.H.; investigation, J.L., Y.H. and Y.Y.; data curation, Y.H. and J.L.; writing—original draft preparation, Y.H.; visualization, Y.H. and J.L.; supervision, Y.L.; project administration, M.L. and Y.L.; funding acquisition, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Science and Technology Projects in Fujian Province, grant number 2021G02001, and the Science and Technology Project of Innovation Platform in Fuzhou City, grant number 2022-P-024.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to Hangzhou Research Interest Information Technology Co., Ltd. for providing testing services.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, B.; He, Y.; Shi, W.; Liu, L.; Li, L.; Liu, C.; Lens, P.N. Biotransformation of sulfamethoxazole (SMX) by aerobic granular sludge: Removal performance, degradation mechanism and microbial response. Sci. Total Environ. 2023, 858, 159771. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; He, L.; Shen, S.; Li, Y.; He, Y.; Chen, Z.; Zhang, D.; Chen, Z.; Chen, X.; Wu, L.; et al. Unveiling the mechanisms of peracetic acid activation by iron-rich sludge biochar for sulfamethoxazole degradation with wide adaptability. J. Environ. Manag. 2023, 347, 119119. [Google Scholar] [CrossRef] [PubMed]
- Shang, K.; Morent, R.; Wang, N.; Wang, Y.; Peng, B.; Jiang, N.; Lu, N.; Li, J. Degradation of sulfamethoxazole (SMX) by water falling film DBD Plasma/Persulfate: Reactive species identification and their role in SMX degradation. Chem. Eng. J. 2022, 431, 133916. [Google Scholar] [CrossRef]
- Szymańska, U.; Wiergowski, M.; Sołtyszewski, I.; Kuzemko, J.; Wiergowska, G.; Woźniak, M.K. Presence of antibiotics in the aquatic environment in Europe and their analytical monitoring: Recent trends and perspectives. Microchem. J. 2019, 147, 729–740. [Google Scholar] [CrossRef]
- Guan, C.; Jiang, J.; Pang, S.; Ma, J.; Chen, X.; Lim, T.T. Nonradical transformation of sulfamethoxazole by carbon nanotube activated peroxydisulfate: Kinetics, mechanism and product toxicity. Chem. Eng. J. 2019, 378, 122147. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Qu, Y.; Zhang, X.; Zhang, Q.; Yang, D.; Wang, Q.; Dong, Z.; Zhao, J. Intestinal microbiota perturbations in the gastropod Trochus niloticus concurrently exposed to ocean acidification and environmentally relevant concentrations of sulfamethoxazole. Chemosphere 2023, 311, 137115. [Google Scholar] [CrossRef]
- Bai, Y.; Ruan, X.; Wang, F.; Antoine, G.; van der Hoek, J.P. Sulfonamides removal under different redox conditions and microbial response to sulfonamides stress during riverbank filtration: A laboratory column study. Chemosphere 2019, 220, 668–677. [Google Scholar] [CrossRef]
- Lu, W.; Chen, N.; Feng, C.; An, N.; Dong, Y. Peracetic acid-based electrochemical treatment of sulfamethoxazole and real antibiotic wastewater: Different role of anode and cathode. J. Hazard. Mater. 2024, 463, 132819. [Google Scholar] [CrossRef]
- Yu, X.; Jin, X.; Li, M.; Yu, Y.; Liu, H.; Zhou, R.; Yin, A.; Shi, J.; Sun, J.; Zhu, L. Mechanism and security of UV driven sodium percarbonate for sulfamethoxazole degradation using DFT and metabolomic analysis. Environ. Pollut. 2023, 323, 121352. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chu, K.; Sun, S.; Chen, H.; Song, B.; Wang, J.; Liu, Z.; Zhu, L. Synthesis of magnetic core-shell Fe3O4-Mn3O4 composite for degradation of sulfadiazine via peroxymonosulfate activation: Characterization, mechanism and toxicity analysis. J. Environ. Chem. Eng. 2023, 11, 109230. [Google Scholar] [CrossRef]
- Wang, L.L.; Jiang, S.F.; Huang, J.; Jiang, H. Oxygen-doped biochar for the activation of ferrate for the highly efficient degradation of sulfadiazine with a distinct pathway. J. Environ. Chem. Eng. 2022, 10, 108537. [Google Scholar] [CrossRef]
- Xiao, G.; Xu, T.; Faheem, M.; Xi, Y.; Zhou, T.; Moryani, H.T.; Bao, J.; Du, J. Evolution of singlet oxygen by activating peroxydisulfate and peroxymonosulfate: A review. Int. J. Environ. Res. Public Health 2021, 18, 3344. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, X.; Xi, S.; Miao, S.; Ding, J.; Cai, W.; Liu, S.; Yang, X.; Yang, H.; Gao, J.; et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient Fenton-like catalysis. J. Am. Chem. Soc. 2018, 140, 12469–12475. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J. Kinetics of PMS activation by graphene oxide and biochar. Chemosphere 2020, 239, 124812. [Google Scholar] [CrossRef]
- Fang, Z.; Zhou, Z.; Xue, G.; Yu, Y.; Wang, Q.; Cheng, B.; Ge, Y.; Qian, Y. Application of sludge biochar combined with peroxydisulfate to degrade fluoroquinolones: Efficiency, mechanisms and implication for ISCO. J. Hazard. Mater. 2022, 426, 128081. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, C.; Wang, S.; Ren, N.Q.; Ho, S.H. Graphitic biochar catalysts from anaerobic digestion sludge for nonradical degradation of micropollutants and disinfection. Chem. Eng. J. 2020, 384, 123244. [Google Scholar]
- Wang, T.; Zhou, Y.; Xue, Y.; Sang, T.; Ren, L.; Chen, S.; Liu, J.; Mei, M.; Li, J. Pyrolysis of hydrothermally dewatering sewage sludge: Highly efficient peroxydisulfate activation of derived biochar to degrade diclofenac. Environ. Pollut. 2022, 313, 120176. [Google Scholar] [CrossRef]
- Hung, C.M.; Chen, C.W.; Huang, C.P.; Lam, S.S.; Dong, C.D. Peroxymonosulfate activation by a metal-free biochar for sulfonamide antibiotic removal in water and associated bacterial community composition. Bioresour. Technol. 2022, 343, 126082. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Wang, J.; Deng, Y.; Liu, Y.; Feng, H.; Chen, S.; Ren, X. Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: Internal electron transfer mechanism. Chem. Eng. J. 2019, 364, 146–159. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Wu, Q.; Chang, J.S.; Ren, N. Singlet oxygen-dominated peroxydisulfate activation by sludge-derived biochar for sulfamethoxazole degradation through a nonradical oxidation pathway: Performance and mechanism. Chem. Eng. J. 2019, 357, 589–599. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, H.; Murugananthan, M.; Sun, P.; Dionysiou, D.D.; Zhang, K.; Khan, A.; Zhang, Y. Glucose and melamine derived nitrogen-doped carbonaceous catalyst for nonradical peroxymonosulfate activation. Carbon 2020, 156, 399–409. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Li, J.; Xue, Y.; Liu, J.; Mei, M.; Hou, H.; Chen, S. Co-pyrolysis behavior of sewage sludge and rice husk by TG-MS and residue analysis. J. Clean. Prod. 2020, 250, 119557. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Wang, M.; Naidu, R.; Liu, Y.; Man, Y.B.; Liang, X.; Wong, M.H.; Christie, P.; Zhang, Y.; et al. Co-pyrolysis of sewage sludge and rice husk/bamboo sawdust for biochar with high aromaticity and low metal mobility. Environ. Res. 2020, 191, 110034. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Liu, Q.; Zhang, X.; Cao, L.; Hu, B.; Shao, J.; Ding, F.; Guo, X.; Gao, B. Synergetic effect of co-pyrolysis of sewage sludge and lignin on biochar production and adsorption of methylene blue. Fuel 2022, 324, 124587. [Google Scholar] [CrossRef]
- Wang, R.; Duan, X.; Wang, S.; Ren, N.Q.; Ho, S.H. Production, properties, and catalytic applications of sludge derived biochar for environmental remediation. Water Res. 2020, 187, 116390. [Google Scholar]
- Yin, Q.; Liu, M.; Ren, H. Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, J.; Liu, Z.; Liu, M. Degradation of tetracycline by peroxydisulfate activation on sludge-derived biochar modified with tannin extract. Process. Saf. Environ. Prot. 2023, 173, 472–484. [Google Scholar] [CrossRef]
- Tan, J.; Chen, X.; Shang, M.; Cui, J.; Li, D.; Yang, F.; Zhang, Z.; Zhang, H.; Wu, Q.; Li, Y.; et al. N-doped biochar mediated peroxydisulfate activation for selective degradation of bisphenol A: The key role of potential difference-driven electron transfer mechanism. Chem. Eng. J. 2023, 468, 143476. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Zhou, H. Preparation of N-doped biochar from sewage sludge and melamine for peroxymonosulfate activation: N-functionality and catalytic mechanisms. Sci. Total Environ. 2020, 744, 140862. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Z.; Chen, W.; Chen, Y.; Wang, X.; Chen, H. Role of porous structure and active O-containing groups of activated biochar catalyst during biomass catalytic pyrolysis. Energy 2020, 210, 118646. [Google Scholar] [CrossRef]
- Zhao, C.; Meng, L.; Chu, H.; Wang, J.F.; Wang, T.; Ma, Y.; Wang, C.C. Ultrafast degradation of emerging organic pollutants via activation of peroxymonosulfate over Fe3C/Fe@ NCx: Singlet oxygen evolution and electron-transfer mechanisms. Appl. Catal. B Environ. 2023, 321, 122034. [Google Scholar] [CrossRef]
- Xu, K.; Lin, Q.; Fan, X.; Zheng, J.; Liu, Y.; Ma, Y.; He, J. Enhanced degradation of sulfamethoxazole by activation of peroxodisulfate with red mud modified biochar: Synergistic effect between adsorption and nonradical activation. Chem. Eng. J. 2023, 460, 141578. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Si, Q.; Luo, H.; Zhao, Q.; Ren, N. Sludge-derived biochar as efficient persulfate activators: Sulfurization-induced electronic structure modulation and disparate nonradical mechanisms. Appl. Catal. B Environ. 2020, 279, 119361. [Google Scholar] [CrossRef]
- CYLview20. Available online: https://www.cylview.org/index.html (accessed on 15 December 2023).
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Zhang, K.; Min, X.; Zhang, T.; Xie, M.; Si, M.; Chai, L.; Shi, Y. Selenium and nitrogen co-doped biochar as a new metal-free catalyst for adsorption of phenol and activation of peroxymonosulfate: Elucidating the enhanced catalytic performance and stability. J. Hazard. Mater. 2021, 413, 125294. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, Y.; Shi, J.; Xu, L.; Wang, Y.; Jin, P. A new application pattern for sludge-derived biochar adsorbent: Ideal persulfate activator for the high-efficiency mineralization of pollutants. J. Hazard. Mater. 2021, 419, 126343. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, D.; Wang, S.; Zhang, R.; Wang, Y.; Liu, M. Activation of peroxymonosulfate by nitrogen-doped porous carbon for efficient degradation of organic pollutants in water: Performance and mechanism. Sep. Purif. Technol. 2022, 280, 119791. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Functionalization, pH, and ionic strength influenced sorption of sulfamethoxazole on graphene. J. Environ. Chem. Eng. 2014, 2, 310–315. [Google Scholar] [CrossRef]
- Shi, Q.; Li, A.; Qing, Z.; Li, Y. Oxidative degradation of Orange G by persulfate activated with iron-immobilized resin chars. J. Ind. Eng. Chem. 2015, 25, 308–313. [Google Scholar] [CrossRef]
- Fan, Y.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q. Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process. J. Hazard. Mater. 2015, 300, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Berruti, I.; López, M.I.P.; Oller, I.; Laurenti, E.; Minella, M.; Calza, P. The reactivity of peroxymonosulfate towards sulfamethoxazole. Catal. Today 2023, 413, 113975. [Google Scholar] [CrossRef]
- Du, A.; Fu, H.; Wang, P.; Zhao, C.; Wang, C.C. Enhanced catalytic peroxymonosulfate activation for sulfonamide antibiotics degradation over the supported CoSx-CuSx derived from ZIF-L (Co) immobilized on copper foam. J. Hazard. Mater. 2022, 426, 128134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zou, X.; Mao, J.; Wu, X. Decomposition of sulfadiazine in a sonochemical Fe0-catalyzed persulfate system: Parameters optimizing and interferences of wastewater matrix. Appl. Catal. B Environ. 2016, 185, 31–41. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. B-doped graphitic porous biochar with enhanced surface affinity and electron transfer for efficient peroxydisulfate activation. Chem. Eng. J. 2020, 396, 125119. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, X.; Liu, S.; Liu, Y.; Zeng, G.; Ye, S.; Yin, Z.; Hu, X.; Liu, N. Catalytic degradation of estrogen by persulfate activated with iron-doped graphitic biochar: Process variables effects and matrix effects. Chem. Eng. J. 2019, 378, 122141. [Google Scholar] [CrossRef]
- Yu, Y.; Li, N.; Lu, X.; Yan, B.; Chen, G.; Wang, Y.; Duan, X.; Cheng, Z.; Wang, S. Co/N co-doped carbonized wood sponge with 3D porous framework for efficient peroxymonosulfate activation: Performance and internal mechanism. J. Hazard. Mater. 2022, 421, 126735. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, P.; Chen, T.; Gao, K.; Xiong, Y.; Lu, Y.; Dionysiou, D.D.; Wang, D. Efficient 1O2 production from H2O2 over lattice distortion controlled spinel ferrites. Appl. Catal. B Environ. 2024, 343, 123468. [Google Scholar] [CrossRef]
- Pei, X.; Peng, X.; Jia, X.; Wong, P.K. N-doped biochar from sewage sludge for catalytic peroxydisulfate activation toward sulfadiazine: Efficiency, mechanism, and stability. J. Hazard. Mater. 2021, 419, 126446. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, B.; Shen, J.; Yan, P.; Kang, J.; Wang, W.; Bi, L.; Zhu, X.; Li, Y.; Wang, S.; et al. Preparation of novel N-doped biochar and its high adsorption capacity for atrazine based on π–π electron donor-acceptor interaction. J. Hazard. Mater. 2022, 432, 128757. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, L.; Wang, R.; Li, G.; Rao, P.; Ju, M.; Jian, L.; Guo, X.; Che, L. Recyclable nitrogen-doped biochar via low-temperature pyrolysis for enhanced lead (II) removal. Chemosphere 2022, 286, 131666. [Google Scholar] [CrossRef] [PubMed]
- Sang, F.; Yin, Z.; Wang, W.; Almatrafi, E.; Wang, Y.; Zhao, B.; Gong, J.; Zhou, C.; Zhang, C.; Zeng, G.; et al. Degradation of ciprofloxacin using heterogeneous Fenton catalysts derived from natural pyrite and rice straw biochar. J. Clean. Prod. 2022, 378, 134459. [Google Scholar] [CrossRef]
- Yang, F.; Jin, C.; Wang, S.; Wang, Y.; Wei, L.; Zheng, L.; Gu, H.; Lam, S.S.; Naushad, M.; Li, C.; et al. Bamboo-based magnetic activated carbon for efficient removal of sulfadiazine: Application and adsorption mechanism. Chemosphere 2023, 323, 138245. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Feng, M.; Guo, L.; Zhai, Z.; Fang, Y.; Zhang, X.; Sharma, V.K. Nitrogen-sulfur co-doped industrial graphene as an efficient peroxymonosulfate activator: Singlet oxygen-dominated catalytic degradation of organic contaminants. Appl. Catal. B Environ. 2019, 251, 335–345. [Google Scholar] [CrossRef]
- Meghani, R.; Lahane, V.; Kotian, S.Y.; Lata, S.; Tripathi, S.; Ansari, K.M.; Yadav, A.K. Valorization of Ginger Waste-Derived Biochar for Simultaneous Multiclass Antibiotics Remediation in Aqueous Medium. ACS Omega 2023, 8, 11065–11075. [Google Scholar] [CrossRef]
- Bamdad, H.; Hawboldt, K.; MacQuarrie, S. Nitrogen functionalized biochar as a renewable adsorbent for efficient CO2 removal. Energy Fuels 2018, 32, 11742–11748. [Google Scholar] [CrossRef]
- Li, W.; Nie, C.; Wang, X.; Ye, H.; Li, D.; Ao, Z. Alkaline lignin-derived N-doped biochars as peroxymonosulfate activators for acetaminophen degradation: Performance and catalytic bridging mediated Electron-Transfer mechanism. Sep. Purif. Technol. 2023, 323, 124418. [Google Scholar] [CrossRef]
- Wu, W.; Wang, R.; Chang, H.; Zhong, N.; Zhang, T.; Wang, K.; Ren, N.; Ho, S.H. Rational electron tunning of magnetic biochar via N, S co-doping for intense tetracycline degradation: Efficiency improvement and toxicity alleviation. Chem. Eng. J. 2023, 458, 141470. [Google Scholar] [CrossRef]
- Wang, A.; Ni, J.; Wang, W.; Liu, D.; Zhu, Q.; Xue, B.; Chang, C.C.; Ma, J.; Zhao, Y. MOF Derived Co- Fe nitrogen doped graphite carbon@ crosslinked magnetic chitosan Micro- nanoreactor for environmental applications: Synergy enhancement effect of adsorption- PMS activation. Appl. Catal. Environ. 2022, 319, 121926. [Google Scholar] [CrossRef]
- Byambaa, B.; Seid, M.G.; Song, K.G.; Kim, E.J.; Lee, D.; Lee, C. Insight into disparate nonradical mechanisms of peroxymonosulfate and peroxydisulfate activation by N-doped oxygen-rich biochar: Unraveling the role of active sites. Chemosphere 2024, 346, 140563. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Yan, H.; Liang, Y.; Wang, H.; Duan, P.; Lai, B. Nitrogen doped biochar derived from algae as persulfate activator for the degradation of tetracycline: Role of exogenous N doping and electron transfer pathway. Sep. Purif. Technol. 2023, 318, 123970. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Liu, F.; Fu, W.; Lin, L.; Li, B. Comparison of chemical and biological degradation of sulfonamides: Solving the mystery of sulfonamide transformation. J. Hazard. Mater. 2022, 424, 127661. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Peng, W.; Liao, P.; Xiao, Y.; Xie, W.; Qian, A.; Ning, Y.; Zhang, N.; Niyuhire, E.; Cui, H.J. Natural sediments activating peroxydisulfate for in situ degradation of antibiotics under anoxic conditions: The critical role of surface-adsorbed and structural Fe (II). Chem. Eng. J. 2023, 472, 144938. [Google Scholar] [CrossRef]
- Li, J.Q.; Zhou, Z.W.; Li, X.; Yang, Y.L.; Gao, J.F.; Yu, R.; Wang, H.P.; Wang, N. Synergistically boosting sulfamerazine degradation via activation of peroxydisulfate by photocatalysis of Bi2O3-TiO2/PAC under visible light irradiation. Chem. Eng. J. 2022, 428, 132613. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).