Metabolic Rates of Rainbow Trout Eggs in Reconstructed Salmonid Egg Pockets
Abstract
:1. Introduction
2. Methodology
2.1. Microcosm Setup
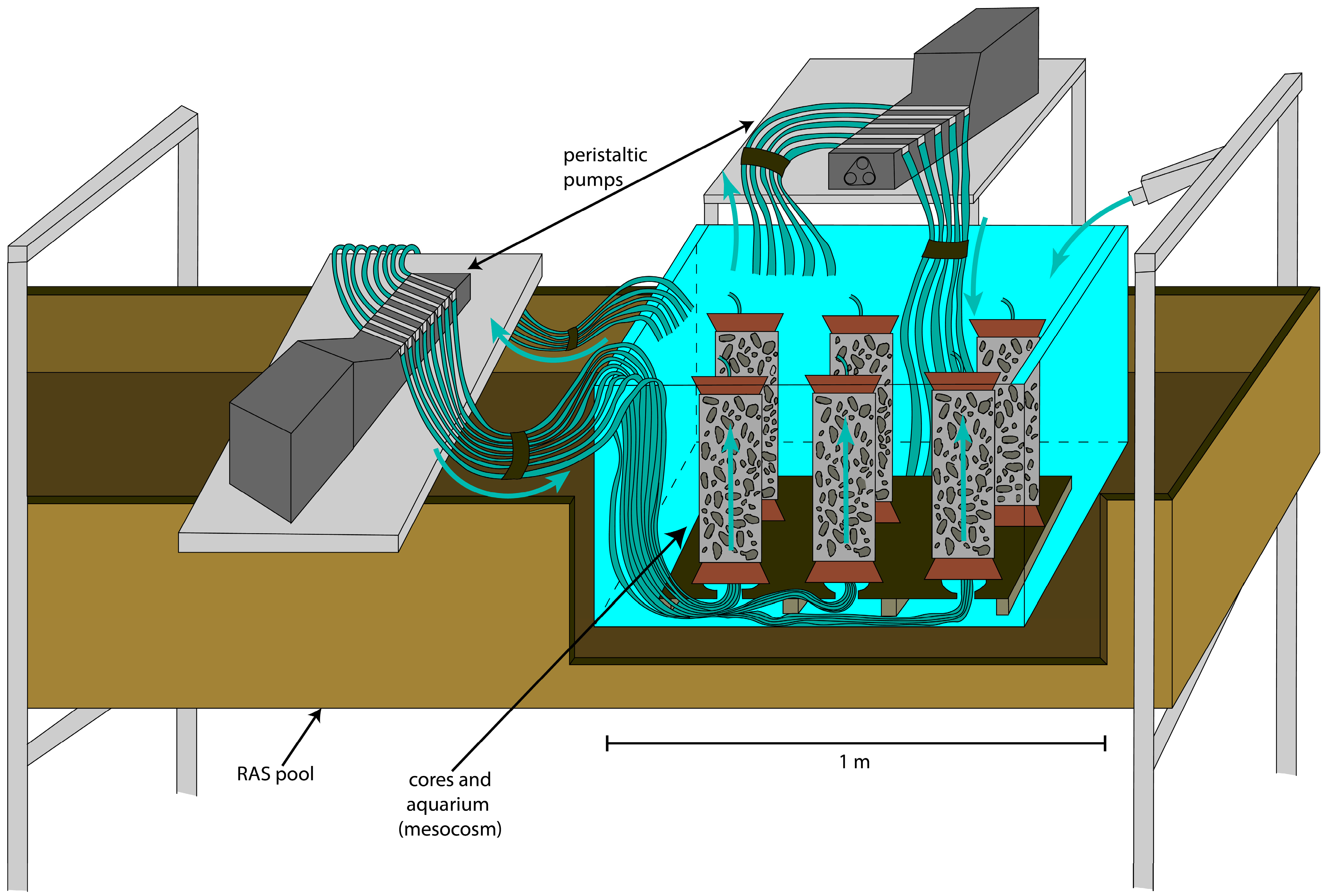
2.2. Mesocosm Setup
2.3. Data Analysis
3. Results and Discussion
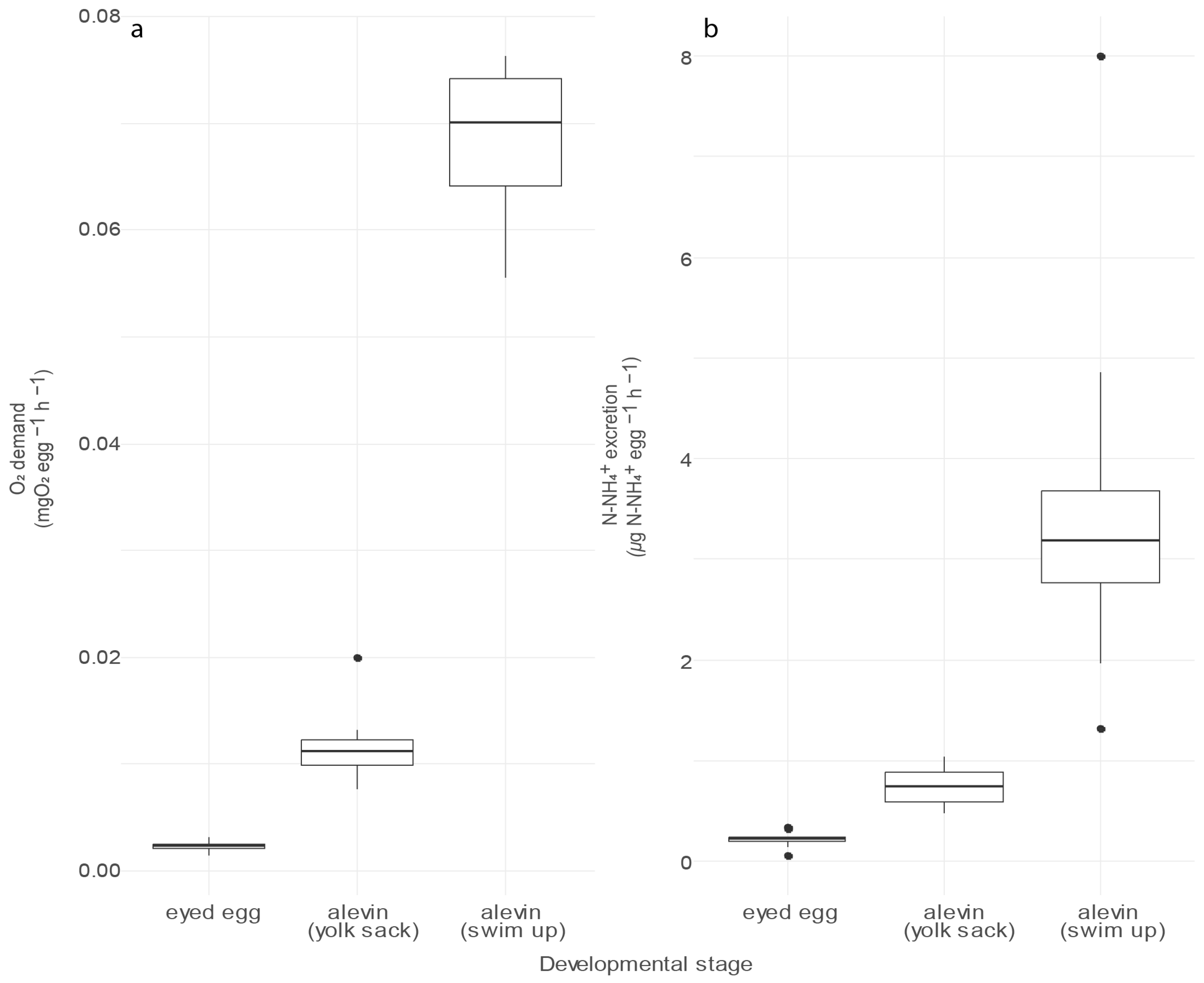
3.1. Egg Respiration and Excretion Rates Measured in Microcosms
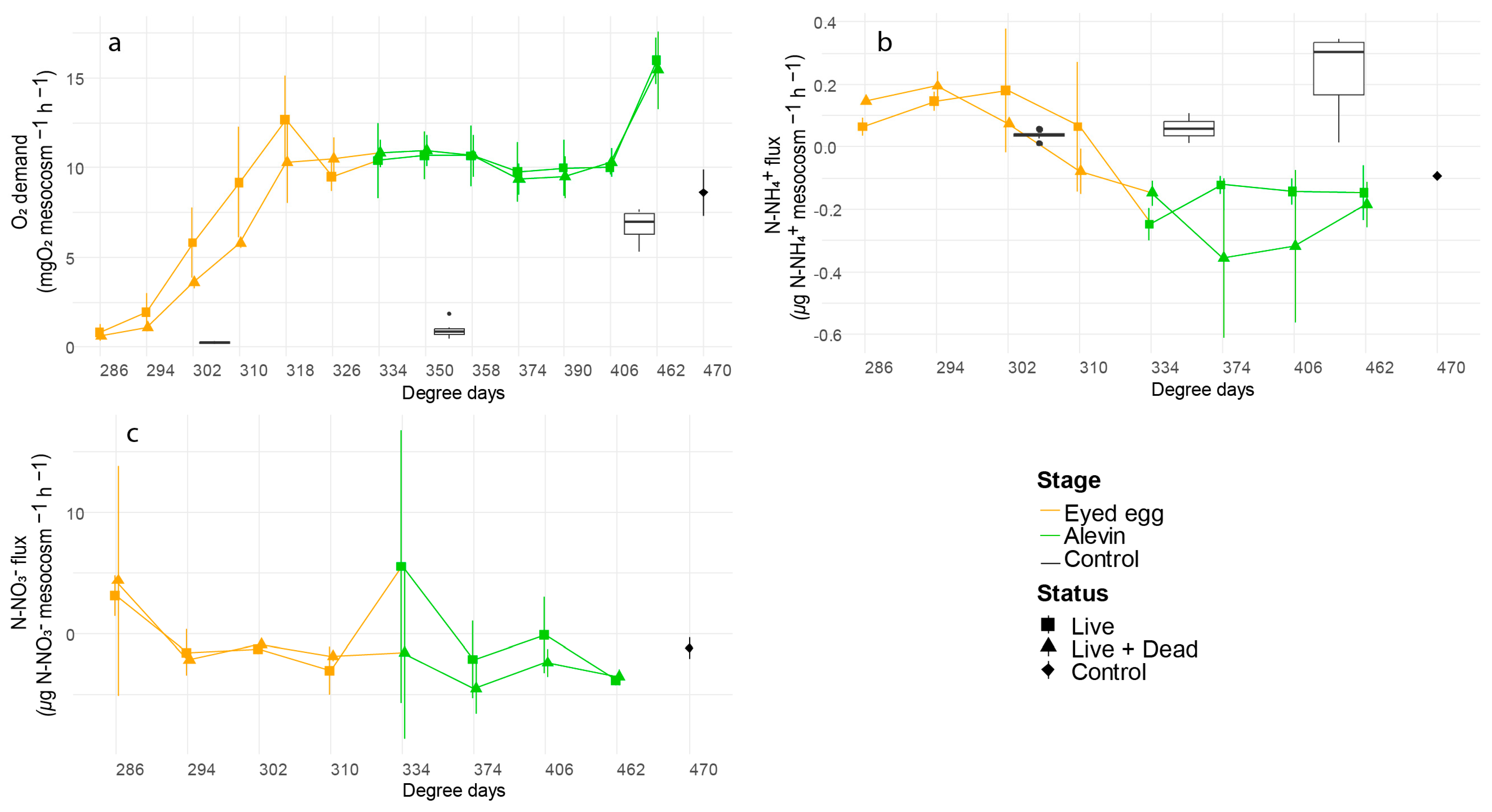
3.2. Mesocosms
3.2.1. Setups
3.2.2. Survival and Substratum Composition
3.2.3. Stages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crisp, T. Trout and Salmon: Ecology, Conservation and Rehabilitation; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Cope, R.S. Responses of Sockeye Salmon (Oncorhynchus nerka) Embryos to Intragravel Incubation Environments in Selected Streams within the Stuart-Takla Watershed; University of British Columbia: Vancouver, BC, Canada, 1996. [Google Scholar]
- Malcolm, I.A.; Youngson, A.F.; Soulsby, C. Survival of salmonid eggs in a degraded gravel-bed stream: Effects of groundwater-surface water interactions. River Res. Appl. 2003, 19, 303–316. [Google Scholar] [CrossRef]
- Greig, S.; Sear, D.; Carling, P. A field-based assessment of oxygen supply to incubating Atlantic salmon (Salmo salar) embryos. Hydrol. Process. Int. J. 2007, 21, 3087–3100. [Google Scholar] [CrossRef]
- Pulg, U.; Barlaup, B.T.; Sternecker, K.; Trepl, L.; Unfer, G. Restoration of spawning habitats of brown trout (Salmo trutta) in a regulated chalk stream. River Res. Appl. 2013, 29, 172–182. [Google Scholar] [CrossRef]
- Rubin, J.F.; Glimsäter, C. Egg-to-fry survival of the sea trout in some streams of Gotland. J. Fish Biol. 1996, 48, 585–606. [Google Scholar]
- Malcolm, I.; Middlemas, C.; Soulsby, C.; Middlemas, S.; Youngson, A. Hyporheic zone processes in a canalised agricultural stream: Implications for salmonid embryo survival. Fundam. Appl. Limnol. 2010, 176, 319. [Google Scholar] [CrossRef]
- Ingendahl, D. Dissolved oxygen concentration and emergence of sea trout fry from natural redds in tributaries of the River Rhine. J. Fish Biol. 2001, 58, 325–341. [Google Scholar] [CrossRef]
- Casas-Mulet, R.; Saltveit, S.J.; Alfredsen, K. The survival of Atlantic salmon (Salmo salar) eggs during dewatering in a river subjected to hydropeaking. River Res. Appl. 2015, 31, 433–446. [Google Scholar] [CrossRef]
- Shokri, M.; Cozzoli, F.; Vignes, F.; Bertoli, M.; Pizzul, E.; Basset, A. Metabolic rate and climate change across latitudes: Evidence of mass-dependent responses in aquatic amphipods. J. Exp. Biol. 2022, 225, jeb244842. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Martin, B.T.; Dudley, P.N.; Kashef, N.S.; Stafford, D.M.; Reeder, W.J.; Tonina, D.; Del Rio, A.M.; Scott Foott, J.; Danner, E.M. The biophysical basis of thermal tolerance in fish eggs. Proc. R. Soc. B 2020, 287, 20201550. [Google Scholar] [CrossRef] [PubMed]
- Greig, S.; Sear, D.; Carling, P. The impact of fine sediment accumulation on the survival of incubating salmon progeny: Implications for sediment management. Sci. Total Environ. 2005, 344, 241–258. [Google Scholar] [CrossRef]
- Chapman, G. Ambient Water Quality Criteria for Dissolved Oxygen; US Environmental Protection Agency, Office of Water Regulations and Standards: Washington, DC, USA, 1986.
- Bjornn, T.C.; Reiser, D.W. Habitat requirements of salmonids in streams. Am. Fish. Soc. Spec. Publ. 1991, 19, 138. [Google Scholar]
- Tonina, D.; Buffington, J.M. A three-dimensional model for analyzing the effects of salmon redds on hyporheic exchange and egg pocket habitat. Can. J. Fish. Aquat. Sci. 2009, 66, 2157–2173. [Google Scholar] [CrossRef]
- Cardenas, M.B.; Ford, A.E.; Kaufman, M.H.; Kessler, A.J.; Cook, P.L. Hyporheic flow and dissolved oxygen distribution in fish nests: The effects of open channel velocity, permeability patterns, and groundwater upwelling. J. Geophys. Res. Biogeosci. 2016, 121, 3113–3130. [Google Scholar] [CrossRef]
- Politi, T.; Zilius, M.; Bartoli, M.; Cardini, U.; Marzocchi, U.; Bonaglia, S. Direct contribution of invertebrate holobionts to methane release from coastal sediments. Limnol. Oceanogr. Lett. 2023, 8, 876–884. [Google Scholar] [CrossRef]
- Alderdice, D.; Wickett, W.; Brett, J. Some effects of temporary exposure to low dissolved oxygen levels on Pacific salmon eggs. J. Fish. Board Can. 1958, 15, 229–250. [Google Scholar] [CrossRef]
- Ehrhardt, M. Methods of Seawater Analysis; VCH Publishers: Hoboken, NJ, USA, 1999. [Google Scholar]
- Nika, N. Reproductive Ecology and Success of Sea Trout Salmo trutta L. in a Small Lowland Stream of Western Lithuania. Doctoral Dissertation, Klaipeda University, Coastal Research and Planning Institute, Klaipeda, Lithuania, 2011. [Google Scholar]
- R Core Team R. R: A Language and Environment for Statistical Computing; R Core Team R: Vienna, Austria, 2023. [Google Scholar]
- Kassambara, A. Package ‘rstatix’: Pipe-Friendly Framework for Basic Statistical Tests (0.6.0) [Computer Software]. 2020. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 10 January 2024).
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Wickett, W.P. The oxygen supply to salmon eggs in spawning beds. J. Fish. Board Can. 1954, 11, 933–953. [Google Scholar] [CrossRef]
- Rombough, P.J. Growth, aerobic metabolism, and dissolved oxygen requirements of embryos and alevins of steelhead, Salmo gairdneri. Can. J. Zool. 1988, 66, 651–660. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Kaushik, S. Nitrogen and energy metabolism during the early ontogeny of diploid and triploid rainbow trout (Salmo gairdneri R.). Comp. Biochem. Physiol. Part A Physiol. 1987, 87, 157–160. [Google Scholar] [CrossRef]
- Lindroth, A. Sauerstoffverbrauch der Fische. II. Verschiedene Entwicklungs und Altersstadien vom Lachs und Hecht. Z. Für Vgl. Physiol. 1942, 29, 583–594. [Google Scholar] [CrossRef]
- Hayes, F.; Wilmot, I.; Livingstone, D. The oxygen consumption of the salmon egg in relation to development and activity. J. Exp. Zool. 1951, 116, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Smith, S. Studies in the Development of the Rainbow Trout (Salmo Irideus) I. The Heat Production and Nitrogenous Excretion. J. Exp. Biol. 1947, 23, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Fry, F. The effect of environmental factors on the physiology of fish. Fish Physiol. 1971, 6, 1–98. [Google Scholar]
- Fry, F. The aquatic respiration of fish. In Physiology of Fishes; Academic Press: New York, NY, USA, 1957; Volume I, pp. 1–63. [Google Scholar]
- Nika, N.; Zilius, M.; Ruginis, T.; Giordani, G.; Bagdonas, K.; Benelli, S.; Bartoli, M. Benthic metabolism in fluvial sediments with larvae of Lampetra sp. Water 2021, 13, 1002. [Google Scholar] [CrossRef]
- Burgner, R.L. Life history of sockeye salmon (Oncorhynchus nerka). In Pacific Salmon Life Histories; Groot, C., Margolis, L., Eds.; University of British Columbia Press: Vancouver, BC, Canada, 1991; pp. 1–117. [Google Scholar]
- Healey, M. Life history of chinook salmon (Oncorhynchus tshawytscha). Pac. Salmon Life Hist. 1991, 311–394. [Google Scholar]
- Wright, P.; Fyhn, H. Ontogeny of nitrogen metabolism and excretion. Fish Physiol. 2001, 20, 149–200. [Google Scholar]
- Rahaman-Noronha, E.; O’Donnell, M.; Pilley, C.; Wright, P. Excretion and distribution of ammonia and the influence of boundary layer acidification in embryonic rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 1996, 199, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Pitts, R. Production and excretion of ammonia in relation to acid-base regulation. Handb. Physiol. Sect. 1973, 8, 455–496. [Google Scholar]
- Burke, N. Physical Controls on Salmon Spawning Habitat Quality and Embryo Fitness: An Integrated Analysis; University of Southampton: Southampton, UK, 2011. [Google Scholar]
- McGurk, M.D.; Landry, F.; Tang, A.; Hanks, C.C. Acute and chronic toxicity of nitrate to early life stages of lake trout (Salvelinus namaycush) and lake whitefish (Coregonus clupeaformis). Environ. Toxicol. Chem. Int. J. 2006, 25, 2187–2196. [Google Scholar] [CrossRef]
- Kincheloe, J.W.; Wedemeyer, G.A.; Koch, D.L. Tolerance of developing salmonid eggs and fry to nitrate exposure. Bull. Environ. Contam. Toxicol. 1979, 23, 575–578. [Google Scholar] [CrossRef]
- Malcolm, I.; Soulsby, C.; Youngson, A.; Hannah, D.; McLaren, I.; Thorne, A. Hydrological influences on hyporheic water quality: Implications for salmon egg survival. Hydrol. Process. 2004, 18, 1543–1560. [Google Scholar] [CrossRef]
- Dumas, J.; Bassenave, J.; Jarry, M.; Barriere, L.; Glise, S. Effects of fish farm effluents on egg-to-fry development and survival of brown trout in artificial redds. J. Fish Biol. 2007, 70, 1734–1758. [Google Scholar] [CrossRef]
- Chapman, D. Critical review of variables used to define effects of fines in redds of large salmonids. Trans. Am. Fish. Soc. 1988, 117, 1–21. [Google Scholar] [CrossRef]
- Lapointe, M.F.; Bergeron, N.E.; Bérubé, F.; Pouliot, M.-A.; Johnston, P. Interactive effects of substrate sand and silt contents, redd-scale hydraulic gradients, and interstitial velocities on egg-to-emergence survival of Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 2004, 61, 2271–2277. [Google Scholar] [CrossRef]
- Chevalier, B. Report of Engineering and Biological Literature Pertaining to the Aquatic Environment: With Special Emphasis on Dissolved Oxygen and Sediment Effects on Salmonid Habitat; Colorado State University, Department of Agriculture and Chemical Engineering: Fort Collins, CO, USA, 1984. [Google Scholar]
- Eddy, F. Ammonia in estuaries and effects on fish. J. Fish Biol. 2005, 67, 1495–1513. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y. Numerical modeling of biofilm growth at the pore scale. In Proceedings of the 1999 Conference on Hazardous Waste Research, St. Louis, MO, USA, 24–27 May 1999; pp. 215–226. [Google Scholar]
- Battin, T.J.; Kaplan, L.A.; Newbold, J.D.; Cheng, X.; Hansen, C. Effects of current velocity on the nascent architecture of stream microbial biofilms. Appl. Environ. Microbiol. 2003, 69, 5443–5452. [Google Scholar] [CrossRef]
- Battin, T.; Sengschmitt, D. Linking sediment biofilms, hydrodynamics, and river bed clogging: Evidence from a large river. Microb. Ecol. 1999, 37, 185–196. [Google Scholar] [CrossRef]
- Finn, R.N. The physiology and toxicology of salmonid eggs and larvae in relation to water quality criteria. Aquat. Toxicol. 2007, 81, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Hedin, L.O.; Von Fischer, J.C.; Ostrom, N.E.; Kennedy, B.P.; Brown, M.G.; Robertson, G.P. Thermodynamic constraints on nitrogentransformations and other biogeochemicalprocesses at soil–stream interfaces. Ecology 1998, 79, 684–703. [Google Scholar] [CrossRef]
- Dahm, C.N.; Grimm, N.B.; Marmonier, P.; Valett, H.M.; Vervier, P. Nutrient dynamics at the interface between surface waters and groundwaters. Freshw. Biol. 1998, 40, 427–451. [Google Scholar] [CrossRef]
- Benelli, S.; Bartoli, M. Worms and submersed macrophytes reduce methane release and increase nutrient removal in organic sediments. Limnol. Oceanogr. Lett. 2021, 6, 329–338. [Google Scholar] [CrossRef]
- Naldi, M.; Nizzoli, D.; Bartoli, M.; Viaroli, P.; Viaroli, P. Effect of filter-feeding mollusks on growth of green macroalgae and nutrient cycling in a heavily exploited coastal lagoon. Estuar. Coast. Shelf Sci. 2020, 239, 106679. [Google Scholar] [CrossRef]
- Bonaglia, S.; Bartoli, M.; Gunnarsson, J.S.; Rahm, L.; Raymond, C.; Svensson, O.; Yekta, S.S.; Brüchert, V. Effect of reoxygenation and Marenzelleria spp. bioturbation on Baltic Sea sediment metabolism. Mar. Ecol. Prog. Ser. 2013, 482, 43–55. [Google Scholar] [CrossRef]
- García-Robledo, E.; Corzo, A. Effects of macroalgal blooms on carbon and nitrogen biogeochemical cycling in photoautotrophic sediments: An experimental mesocosm. Mar. Pollut. Bull. 2011, 62, 1550–1556. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benetti, R.; Politi, T.; Bartoli, M.; Nika, N. Metabolic Rates of Rainbow Trout Eggs in Reconstructed Salmonid Egg Pockets. Water 2024, 16, 612. https://doi.org/10.3390/w16040612
Benetti R, Politi T, Bartoli M, Nika N. Metabolic Rates of Rainbow Trout Eggs in Reconstructed Salmonid Egg Pockets. Water. 2024; 16(4):612. https://doi.org/10.3390/w16040612
Chicago/Turabian StyleBenetti, Rudy, Tobia Politi, Marco Bartoli, and Nerijus Nika. 2024. "Metabolic Rates of Rainbow Trout Eggs in Reconstructed Salmonid Egg Pockets" Water 16, no. 4: 612. https://doi.org/10.3390/w16040612
APA StyleBenetti, R., Politi, T., Bartoli, M., & Nika, N. (2024). Metabolic Rates of Rainbow Trout Eggs in Reconstructed Salmonid Egg Pockets. Water, 16(4), 612. https://doi.org/10.3390/w16040612






