Abstract
Frequent surface water–groundwater interactions and prevalent anthropogenic inputs make karst water systems vulnerable to human disturbance. As a typical karst region in North China, the Jinan Spring Catchment has become increasingly threatened due to rapid population growth and urban expansion. In this study, the local river–spring interaction and its interference with the hydrogeochemical evolution of groundwater are evaluated based on water stable isotopes and hydrochemistry. Twenty-two karst groundwater, eleven Quaternary pore water, sixteen spring water, and thirty-two surface water samples were collected during low- and high-flow conditions over the course of a year. The isotopic signatures of four different water types display significant differences, reflecting the recharge–discharge relationship of the karst water system. Mountainous springs feature lighter isotopes, whereas urban springs have significantly heavier isotopes. The result of end-member mixing analysis shows that the surface–groundwater interaction varies spatially and temporally within the spring catchment. Urban springs receive considerable replenishment from the surface water, especially after rainy episodes (up to 50%), while mountainous springs show little hydraulic dependence on surface water leakage (4~6%). Local mineral dissolution (including calcite, dolomite, gypsum, and halite), CO2 dissolution/exsolution, and cation exchange are the main hydrogeochemical processes constraining water chemistry in the spring catchment. The deterioration of water quality can be attributed to anthropogenic influences involving the discharge of domestic effluents, agricultural activities, and irrigation return flow. The findings of this work can improve our understanding of the complex karst water system and serve as a reference for sustainable groundwater management in other karst areas of northern China.
1. Introduction
Karst regions cover about 15% of the Earth’s continental surface [1]. Karst groundwater is amongst the most vital water resources globally, supplying approximately 25% of the world’s population with portable water [2]. Karst aquifers are typically highly dynamic, with water level and hydrochemistry responding to precipitation, drought, and extraction on varying time scales [3]. Moreover, the high-permeability networks of fissures and conduits in karst formations render karst aquifers highly vulnerable to pollution and difficult to remediate once destroyed [4]. Hence, improving the knowledge of the hydrological and hydrogeochemical processes in karst water systems is essential for conserving and protecting this invaluable resource.
Research on karst hydrology and the water environment has provided insights into groundwater flow observation and simulation [5,6], contaminant transport processes [7,8], and groundwater flow and quality changes [9,10]. However, few studies are convincing enough to interpret the effect of global change on surface–groundwater interactions in karstic hydrological systems. Karst environments are intrinsically sensitive to external changes and susceptible to contamination because of the rapid groundwater flow rates and lack of filtration in karst features. Human pressure on karst systems is increasing dramatically with rapid population growth and urbanization, accompanied by increases in pumping, wastewater, landfills, and other sources of anthropogenic pollutants. A more detailed consideration of the surface–groundwater connection and its effect on the hydrogeochemical evolution of groundwater, accounting for the varying geological backgrounds and hydrological conditions, is warranted. Understanding how natural processes and human activities influence groundwater origin and hydrochemistry has implications for long-term groundwater management. Nevertheless, such work is challenging due to the geological heterogeneity of karst systems, the uneven distribution of groundwater, frequent surface–groundwater interaction, and a high degree of hydrological variability. Furthermore, the evolution of karstic hydrological systems is a complex issue that requires the application of proper methodologies.
Several methods have been applied in a simple or integrated way to explore the hydrological and hydrogeochemical processes in karst water systems during the past few decades [11]. Environmental tracers are useful, well-developed techniques for investigating water sources and fluxes in the hydrological cycle. Thereinto, water stable isotopes (δ18O and δ2H), mainly affected by meteorological inputs, behave conservatively in the water circulation and can capture the “fingerprints” of water origin and flow paths [12]. Recent investigations of δ18O and δ2H have made advances in ascertaining the interactions of surface water and groundwater in the stream/karst aquifer continuum [13], revealing the recharge sources of springs and streams [14,15], elucidating the relationships among rain, springs, and streams [16], as well as the effects of anthropogenic contaminant sources, ecological restoration, and engineering activities on karst environments [10,17]. The capability to characterize spatial and temporal variations makes stable isotopes a means of understanding the evolution of karstic hydrological systems [18]. The major ions in groundwater (e.g., Na+, K+, Ca2+, Mg2+, Cl−, SO42−, and HCO3−) are mainly controlled by water–rock interactions [19], thus documenting the geochemical information of different aquifers and providing evidence of hydrogeochemical interactions due to mixing processes [20]. Additionally, groundwater chemistry is affected by human interference (such as nonpoint source pollution from agricultural fertilizers and domestic sewage), with nitrates being one of the most common contaminants [21,22]. Therefore, tracing the hydrochemical changes in groundwater is critical for understanding the role of surface–groundwater connection in hydrogeochemical evolution. The integrated application of stable isotopes and hydrochemistry provides effective information for illuminating the formation mechanism of groundwater chemistry and evaluating surface–groundwater interactions [23,24,25,26].
The Jinan Spring Catchment (JSC) is among the most typical representatives of karst regions in northern China and is famous for its abundant and high-quality karst water [27]. For decades, karst groundwater has been the main source for domestic, agricultural, and industrial usage by local residents. Moreover, the diverse and spectacular springs scattered throughout the city have become an important tourism resource with priceless historical and cultural value. Some of the springs can even be dated back to the Spring and Autumn period of Chinese history, approximately 684 BC (Anonymous, Spring and Autumn Annals). And this is also the reason why Jinan is called the “City of Springs”. In brief, spring water is the spirit and backbone of this city, playing an irreplaceable role in the nature, culture, economy, ecology, livelihood, and social identity of Jinan. Nevertheless, rapid economic and population growth has resulted in the overexploitation and degradation of karst aquifers since the 1970s [28]. The most famous, Baotu Spring, first dried up in 1972 and has suffered from frequent interruptions since then [29]. Moreover, the mean levels of Cl−, NO3−, and SO42− in the Baotu Spring increased from 9.2, 8.8, and 6.0 mg/L in 1955 to 116.9, 70.8, and 43.5 mg/L in 2020, respectively [30]. According to the Jinan Statistical Yearbook, the GDP grew more than 63 times from 1989 to 2018, with the build-up area increasing from 890 to 1170 km2. Hence, the water environment problems in the spring catchment are expected to increase given future human activities and climate change, which may disturb the water cycle and hydrogeochemical evolution through the karst water system, thus affecting the availability of karst groundwater for different uses. This highlights the urgency of adequately protecting this resource, which has great implications for the sustainable utilization and management of groundwater [31]. The local government has implemented a series of measures to restore the karst aquifers and maintain the perennial outflow of the springs, including reasonable groundwater abstraction, artificial recharge, and importing water from the Yellow and Yangtze rivers. The JSC has been studied extensively, with the main focus on groundwater extraction strategy [32,33], groundwater quality variations [34,35,36], groundwater hydrodynamic responses to exploitation and rainfall [37,38], and geothermal water genesis and deep groundwater circulation [39,40]. However, there remains a lack of systematic research on the water sources and groundwater hydrogeochemistry across the entire spring catchment under the increasing impact of human activities. The surface–groundwater interaction and groundwater chemical evolution in the spring catchment under different hydrologic conditions are not yet clear. In order to further understand the interference of natural processes and anthropogenic activities in the hydrogeochemical evolution of groundwater, this study presents a comprehensive analysis of the JSC by incorporating stable isotopes, hydrochemistry, and data analysis methods.
The objectives of the present study were (i) to identify the origin of groundwater; (ii) to evaluate and quantify the interaction between surface water and karst groundwater during different hydrologic conditions; (iii) to elucidate the main natural and anthropogenic factors affecting the evolution of groundwater chemistry; and (iv) to propose a conceptual model of hydrological and hydrogeochemical processes for the JSC. Such knowledge would further the understanding of water circulation and hydrogeochemical evolution in the JSC and provide a sound basis for implementing sustainable water resource management in this area and other urbanizing karst regions.
2. Study Area
2.1. Background
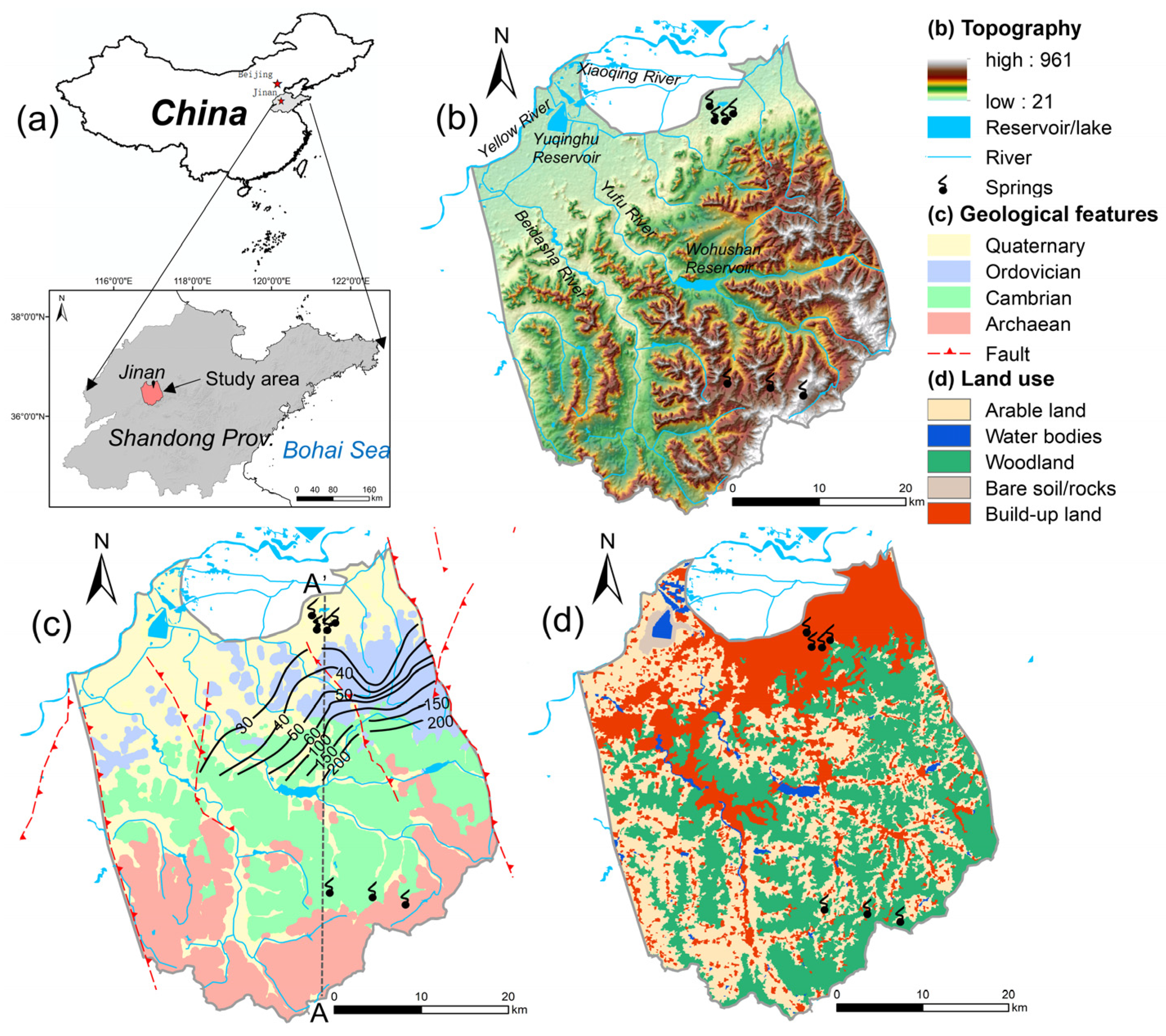
Jinan City is the political, economic, scientific, and technological center of Shandong Province in northern China, located in the middle and lower reaches of the Yellow River Basin (Figure 1). The Jinan Spring Catchment (JSC) is situated in the middle of the urban area, with about 5.54 million people living there, accounting for 84% of the total population of the city. It covers an area of approximately 1500 km2, stretching from 36°28′ to 36°46′ N and 116°40′ to 117°140′ E. The northern boundary of the catchment is the contact zone of metamorphism and magmatism along the Yellow River; the southern border is the groundwater divide lying in the mountainous area; and the eastern and western boundaries are the Dongwu Fault and Mashan Fault, respectively. The catchment is a typical monoclinic structure, with elevation descending from 961 m above sea level (m a.s.l.) in the south to 25 m a.s.l. in the north. And the landforms transform from steep, lower mountainous areas to continuous hilly land, to a piedmont-inclined plain, and further to the alluvial plain of the Yellow River. The JSC has featured agriculture cover since 1980, and now its dominant land use types are woodland (36.6%), agriculture (34.1%), and build-up area (26.6%) (Figure 1d).
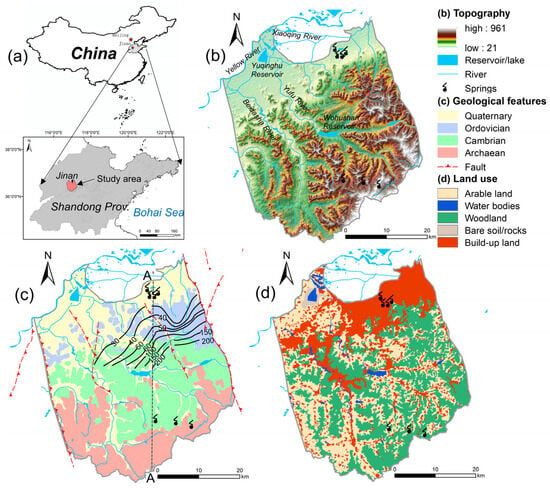
Figure 1.
(a) Location of the Jinan Spring Catchment in northern China; (b) topographic structure and surface water system; (c) geology; and (d) land uses. The land use and land cover data were provided by the Data Center for Resources and Environmental Sciences, China Academy of Sciences (RESDC, 2018).
2.2. Climate and Hydrology
The JSC has a temperate continental monsoon climate with noticeable temperature differences between winter and summer. On the basis of a 69-year record (1951–2019), the mean annual rainfall is approximately 692 mm, the average annual temperature is about 15 °C, and the mean annual evaporation is approximately 1475 mm. Additionally, more than 70% of the total annual rainfall falls from June to September, making a clear distinction between the wet season and the dry season within a year. The Yufuhe River, Beidashahe River, and Wohushan Reservoir are the major surface water bodies in the area. The Yufuhe River and Beidashahe River are important tributaries of the Yellow River, both of which originate in the northern piedmont of Mount Tai and flow northward to the urban area, and can supply the karst aquifers by river water leaking. Other streams flowing through the catchment generally belong to the Yufuhe River system and the Beidashahe River system, most of which are seasonal streams during flood seasons. The rivers fill up after heavy rain episodes and revert to minimum flows during the dry season, regulated to some extent by upstream reservoirs.
2.3. Geological and Hydrogeological Setting
The main outcropping strata in this area are Archean metamorphic rocks, Cambrian carbonate rocks, and Ordovician carbonate rocks, extending to the northwest and overlain by Neoproterozoic and Quaternary sediments. The Archean metamorphic rocks (Taishan group) form the basement of the spring catchment. The Cambrian carbonate strata are characterized by interbeds of limestone and shale, well-exposed from south to north. The Ordovician formation consists of thick-bedded limestone, mostly scattered in the middle of the catchment. In addition, solution sinkholes, fissures, fractures, and conduits are prevalent in the Cambrian–Ordovician carbonate strata, and are usually filled or semi-filled with calcite, dolomite, or gypsum and minor amounts of halite [41]. Carboniferous, Permian, and Cretaceous sandstone and shale are rarely exposed and are largely overlain by sediments. The Mesozoic intrusive rocks (gabbro and diorite) are distributed in the northern part of the catchment and are covered by Quaternary sediment. Several faults have developed throughout the JSC, such as the Dongwu Fault, the Mashan Fault, and the Qianfoshan Fault, most of which are NW-trending or NE-trending, facilitating the formation of the springs.
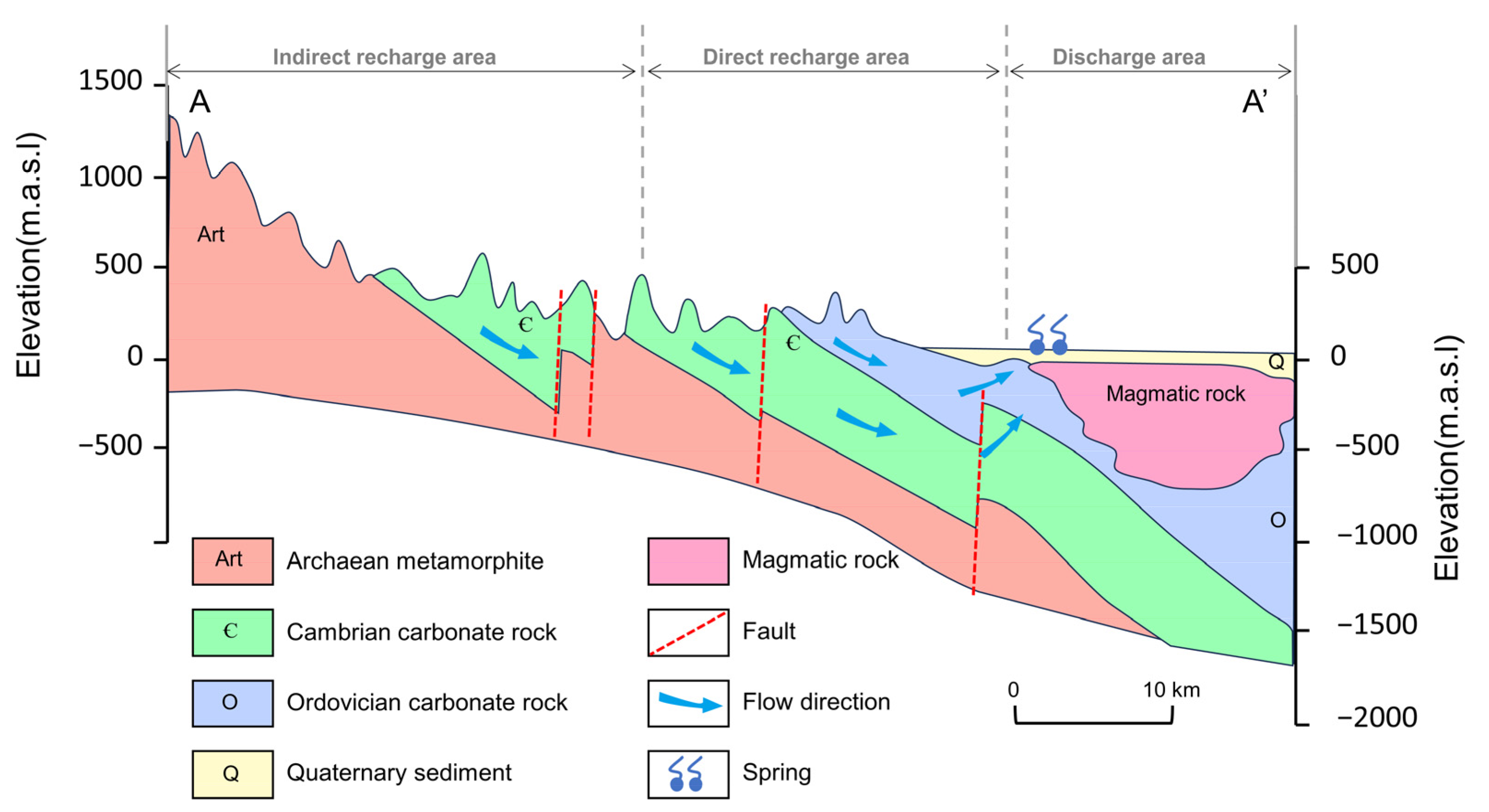
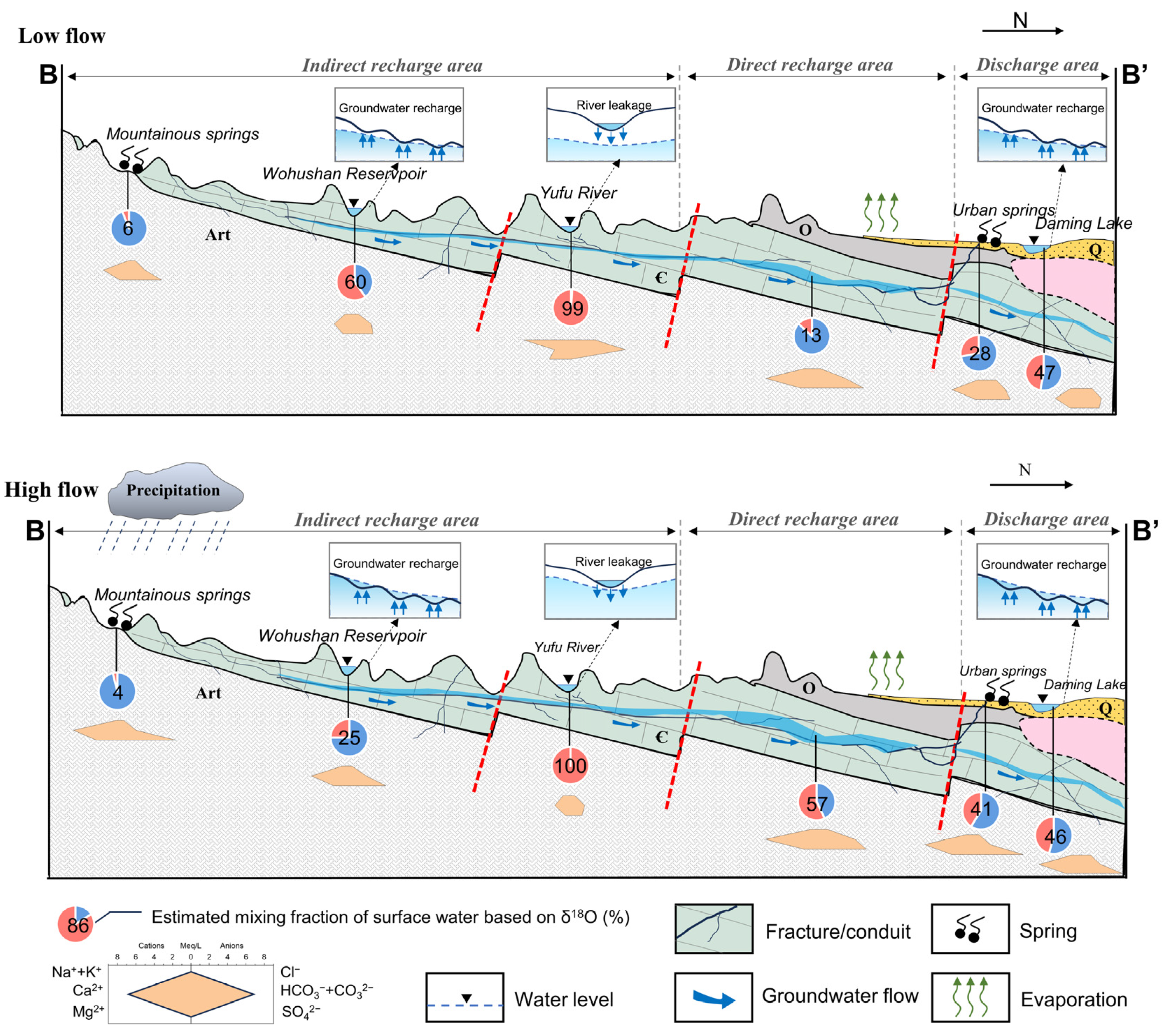
The JSC is a karst water system with high heterogeneity and well-developed karst fissures, fractures, and conduits in the carbonate rocks [31]. The aquifer system consists of three layers. The first layer is the Quaternary aquifer in loose sediments, which plays an important role in the percolation of precipitation and irrigation return flow. The Quaternary aquifer is approximately 5–40 m thick, and the exploitation potential for water supply is limited. The second layer is the karst aquifer in the Cambrian–Ordovician carbonate strata, in which the high-permeability networks of fissures and conduits function as large pathways for subsurface flow and surface water seepage. The third layer is fractured water in Archaean bedrock, which has limited fracture development and is commonly regarded as an aquitard. Quaternary and karst groundwater account for almost 80% of total groundwater discharge, while the Archaean aquifer only exists in specific structures and contributes little to local water resources. Karst groundwater is mainly recharged by atmospheric precipitation, with additional recharges including surface water leakage, irrigation return flow, and inflow from the overlying Quaternary aquifer [42]. Depending on hydrogeological conditions, the JSC is divided into three sections: the indirect recharge area (IRA) in the south, the direct recharge area (DRA) in the middle, and the discharge area (DA) in the north (Figure 2). Driven by topography, karst groundwater flows from south to north and overflows as springs in the DA due to blockage by Mesozoic igneous rocks. The most famous springs in the urban area are named the Four Famous Springs, i.e., Baotu Springs, Zhenzhu Springs, Heihu Springs, and Wulongtan Springs, which are of great aesthetic and cultural value. Apart from springs, artificial exploitation for different uses is currently the major form of groundwater discharge in this area.
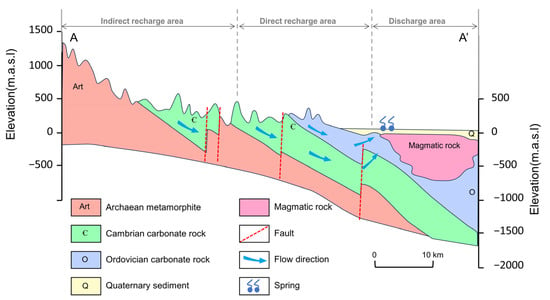
Figure 2.
Conceptual hydrogeological cross-section of the Jinan Spring Catchment. See profile orientation in Figure 1.
2.4. Groundwater Exploitation and History of Springs Drying Up
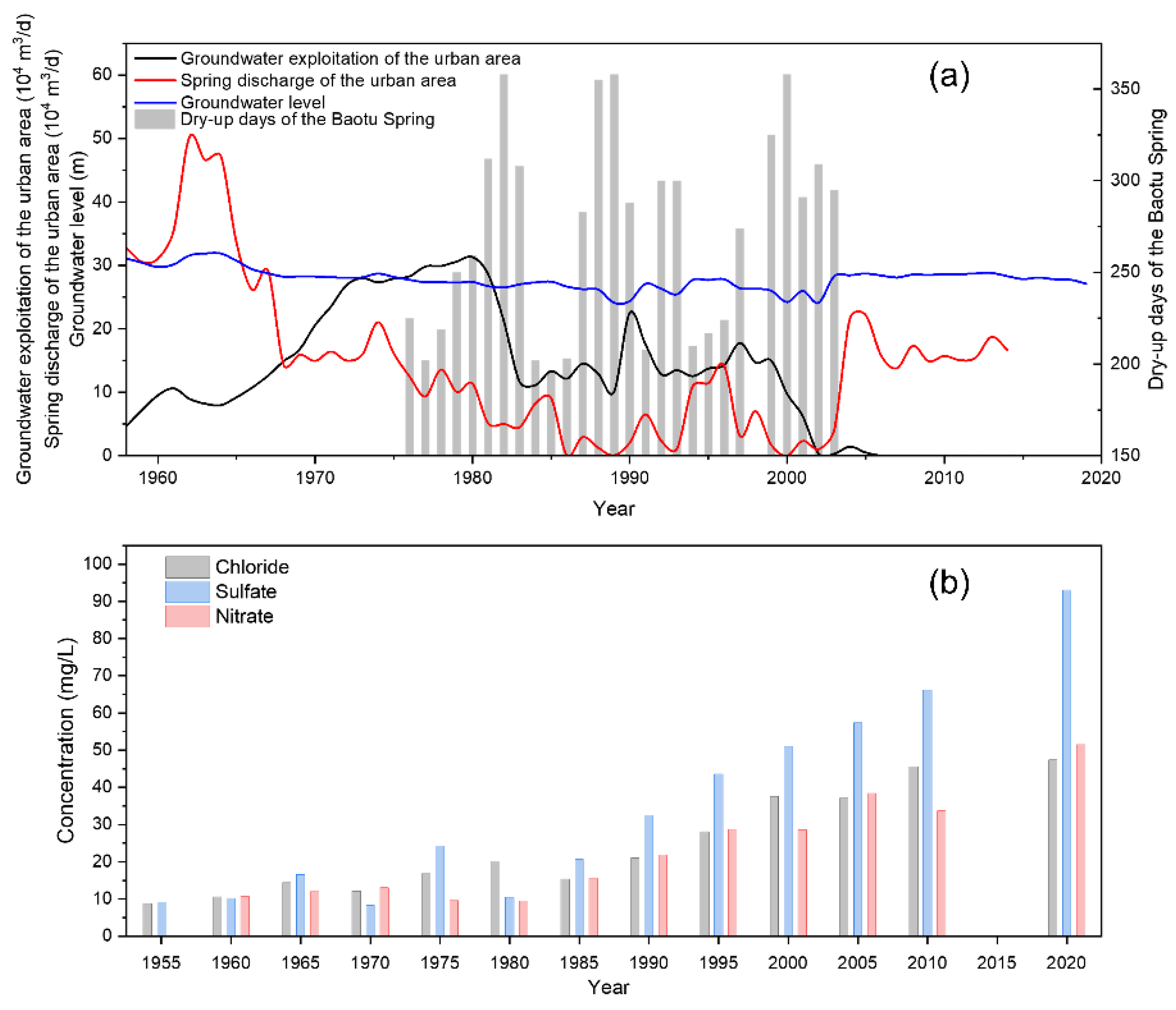
In the early stages, artificial exploitation of groundwater in Jinan was on a modest scale, and groundwater was mainly discharged into springs and rivers. As recorded, the total spring flow in the JSC ranged from 3 × 105 to 4 × 105 m3/d in the 1960s and reached a maximum of 5.1 × 105 m3/d in 1962. With the development of socio-economics and urbanization, the demand for groundwater has been increasing, and the pumping rate in this area increased remarkably from 10 × 104 m3/d in 1960 to 30 × 104 m3/d in 1980. Artificial pumping is generally concentrated from April to July and October to November each year, according to the seasonal tillage (spring plowing and winter wheat irrigation). Thus, the groundwater level usually declines gradually in March, reaches its lowest level in May before the start of the rainy season, and returns to the highest level of the year in September–October. Impacted by artificial pumping, the spring discharge has dropped dramatically since the 1960s, resulting in the loss of spring groups and the degradation of spring-dependent ecosystems [43]. For example, the most famous spring, Baotu Spring, experienced a break in 1972 and has often dried up during the dry season ever since (Figure 3). Between 1999 and 2002, it stopped overflowing for a total of approximately 926 days [44]. In addition, the groundwater quality in the area has become progressively more saline since the 1960s, with salinity and hardness increasing each year [45]. Remarkably, the average content of chloride, nitrate, and sulfate in the Baotu Spring changed from 9.2, 8.8, and 6.0 mg/L in 1955 to 116.9, 70.8, and 43.5 mg/L in 2020, respectively [30].
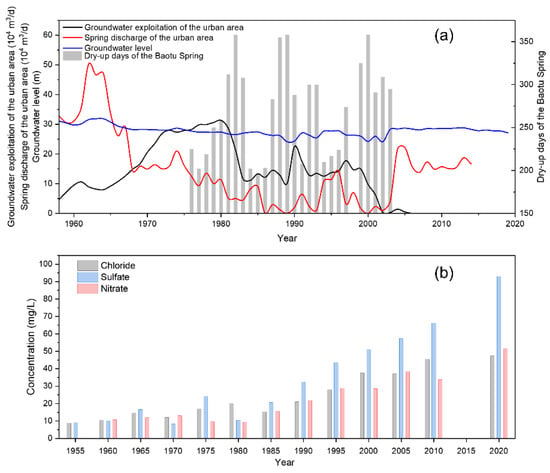
Figure 3.
(a) Groundwater level, groundwater exploitation, spring discharge in the urban area, and dry-up days of the Baotu Spring. (b) Changes in average concentrations of Cl−, SO42−, and NO3− in the Baotu Spring from 1955 to 2020.
A series of steps have been taken to maintain the continuous outflow of these springs. For instance, the local government started to reduce groundwater exploitation in this region in 2002 and transferred artificial pumping from urban to suburban areas. As the urban pumping rate dropped from 30 × 104 m3/d to 12 × 104 m3/d, the decline in groundwater level slowed and even recovered in some years, while the spring discharge remained relatively low. More powerful measures were subsequently implemented, such as importing water from the Yellow River and Yangtze River and artificial recharge. And the water transferred from other basins has become a major source of water supply in Jinan City, accounting for 44% of the total water use in 2017 [46]. The intensity of groundwater exploitation has been effectively narrowed after the cessation of pumping in the well field at Mount La and Dayang village in the area. The above approach has had some effect and contributed to the restoration of spring discharge and the water environment. However, the springs are still at risk of drying up and deteriorating water quality during the annual dry season under the impact of natural climatic and anthropogenic activities.
3. Materials and Methods
3.1. Water Sampling and Measurement
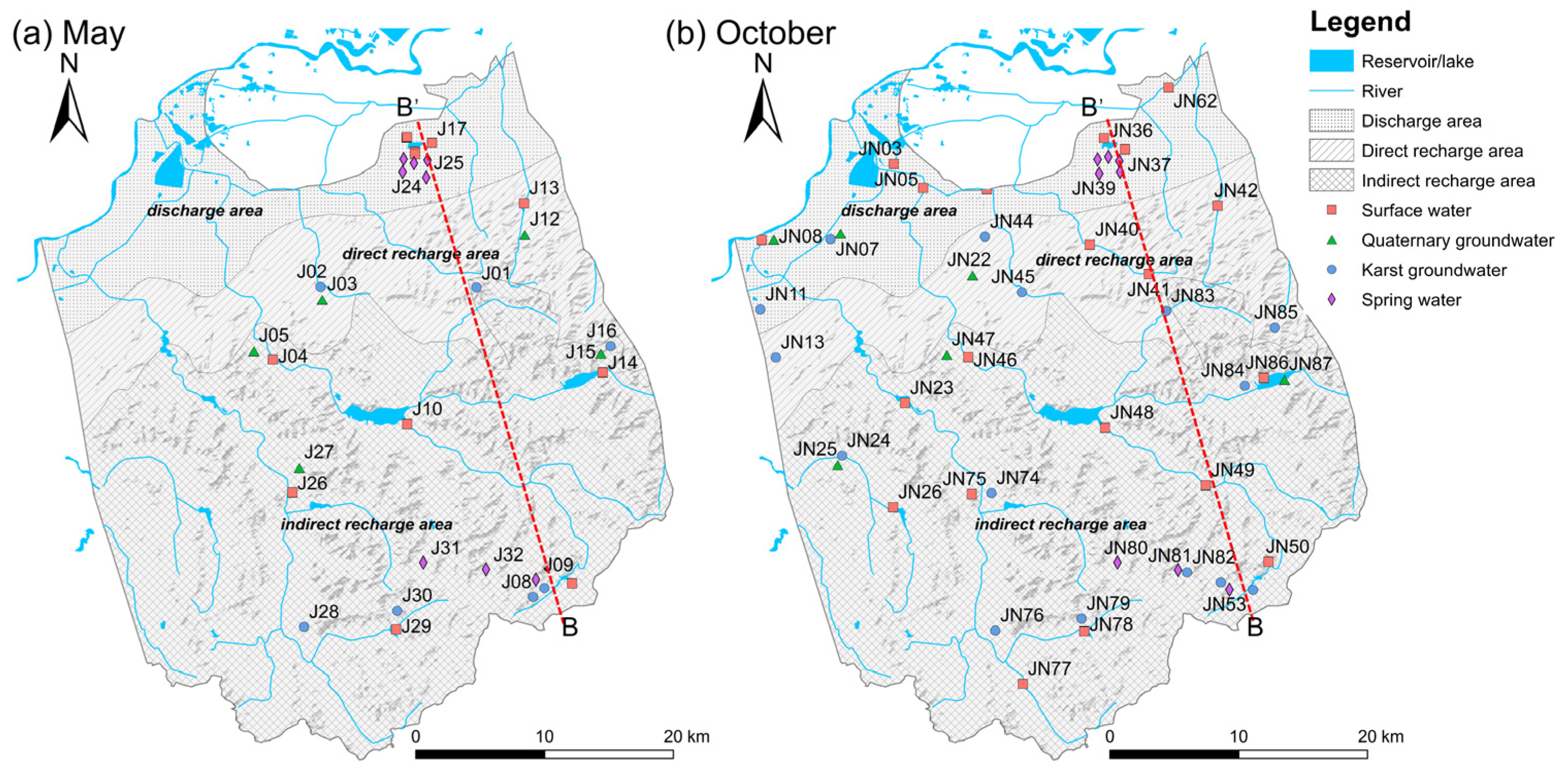
Two sampling campaigns were conducted in May 2020 and October 2020, corresponding to the low-flow and high-flow conditions in the study area, respectively. A total of 81 water samples were collected, including 49 groundwater samples (22 karst GW, 16 spring water, and 11 Quaternary GW) and 32 surface water samples. All sampling locations are shown in Figure 4. The primary principle of surface and groundwater sampling campaigns is to follow the rough flowing direction of the stream network and karst aquifer. Moreover, the sampling sites were selected to be representative of the indirect recharge area (IRA), direct recharge area (DRA), and discharge area (DA) of the Jinan Spring Catchment (JSC).
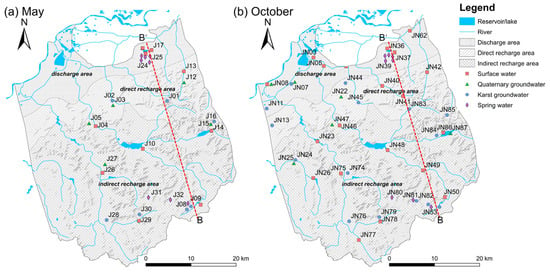
Figure 4.
Map showing the sampling sites in (a) May and (b) October.
Surface water sampling was carried out along the Yufuhe River and the Beidashahe River, with samples taken at a depth of 15 cm below the water surface. Karst groundwater samples were pumped from monitoring wells (well depths between 200 and 400 m) drilled in carbonate aquifers. Due to the lack of monitoring wells in some areas, certain production wells were also used. Quaternary GW samples were taken from irrigation and domestic supply wells (well depths ranging from 10 to 40 m) drilled in the Quaternary aquifer. Before sampling, the wells were pumped for at least 30 min to stabilize the physicochemical parameters (temperature, pH, electrical conductivity, and dissolved oxygen). Spring water samples were directly collected from the springs outcropped in the study area, in the urban area and the southern mountainous area. At each sampling site, triplicate water samples were collected for the analysis of cation, anion, and stable isotopes, respectively. Samples for hydrochemistry analysis were filtered on site through 0.45 μm membrane filters and immediately collected into 100 mL HDPE bottles (pre-washed at least three times with sampled water). The samples prepared for cation analysis were acidified to pH < 2 by adding high-purity H2SO4. The samples prepared for δ2H and δ18O analysis were preserved in 5 mL glass bottles without any air space. All water samples were overflowed, tightly capped, sealed with parafilm to isolate them from air contact, and stored at 4 °C until analysis.
Electrical conductivity (EC, μS/cm), pH, water temperature (T, °C), dissolved oxygen (DO, mg/L), and oxidation reduction potential (ORP, mV) were measured in situ using a portable multi-parameter digital meter (HQ40d, HACH, Loveland, CO, USA), which was calibrated beforehand. Most of the water samples had pH values between 6.5 and 8.9, indicating that the carbon species were mainly dissolved as HCO3−. The HCO3− was determined by titrating with 0.02 mol/L sulfuric acid within 24 h of sampling, and methyl orange was used as an indicator. The hydrochemistry analysis of the water samples was conducted in the physical and chemical analysis laboratory of the Institute of Geographic Sciences and Natural Resources Research (IGSNRR), Chinese Academy of Sciences (CAS). Major cations (Ca2+, Mg2+, Na+, and K+) were measured using inductively coupled plasma optical emission spectrometry (ICP-OES, Optima 5300DV, PerkinElmer, Waltham, MA, USA). Major anions (Cl−, SO42−, and NO3−) were analyzed by ion chromatography (IC, ICS-2100, Dionex, Sunnyvale, CA, USA). The limits of detection of ICP-OES and IC are both 1 mg/L, and the analytical precision for major ions was within 1%. For all water samples, charge balance errors were less than 8%. The saturation index (SI) values of minerals and the partial pressure values of CO2 (logPCO2) in water samples were calculated using PHREEQC 3.6.2 [47]. Stable isotope (18O and 2H) analysis was performed by a liquid water isotope analyzer (DLT100, Los Gatos Research, San Jose, CA, USA) at the Key Laboratory of Water Cycle and Related Land Surface Processes of IGSNRR, CAS, with precisions of ±1‰ and ±0.1‰ for δ2H and δ18O, respectively. The results were expressed in parts per thousand (‰) deviations relative to the international standard (V-SMOW, Vienna Standard Mean Ocean Water) and calibrated using IAEA standards.
3.2. Data Analysis Methods
The two-way ANOVA was performed to examine the influence of water types and hydrological conditions on the variations of physicochemical and hydrochemical parameters [48]. Normality and homogeneity of variances were evaluated using the one-sample Shapiro–Wilk and Levene’s tests, respectively. Logarithmic transformations were required for DO, Na+, K+, HCO3−, SIcalcite, SIdolomite, SIgypsum, and logPCO2 to obtain a normal distribution. For abnormally distributed or unequal variance variables (p < 0.05), the non-parametric Dunnett’s test was used. Multiple comparisons were conducted using Least Significant Difference (LSD) test.
3.3. End-Member Mixing Analysis
An end-member mixing analysis (EMMA) was carried out based on measured conservative parameters to assess the potential mixing between groundwater and surface water. The fraction of surface water (fSW) in each sample is calculated using the following formula [49]:
where Csam, CGW, and CSW denote the concentrations of a selected indicator in the considered sample, karst GW, and surface water, respectively. Application of EMMA requires that (i) the selected indicators for end-members be representative and distinctive, and (ii) the selected indicators be only sensitive to the mixing process and rarely influenced by other factors.
4. Results
4.1. Physical and Hydrochemical Characteristics of Water Samples
The statistical summary of the physicochemical and isotopic parameters of water samples is shown in Table 1. The results of a two-way ANOVA are presented in Table 2. Most of the physical and chemical indicators were found to vary across a large range in surface water, both during low- and high-flow conditions (Figure 5 and Figure S1). The pH showed statistically significant differences in terms of water types and hydrological conditions (p < 0.001, Table 2).

Table 1.
Statistical summary of physicochemical parameters of water samples collected during low- and high-flow conditions in the Jinan Spring Catchment.

Table 2.
Summary of a two-way ANOVA on the variations of physicochemical parameters and stable isotopes of water samples in the Jinan Spring Catchment.
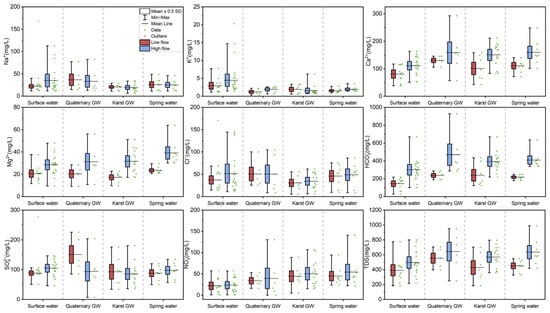
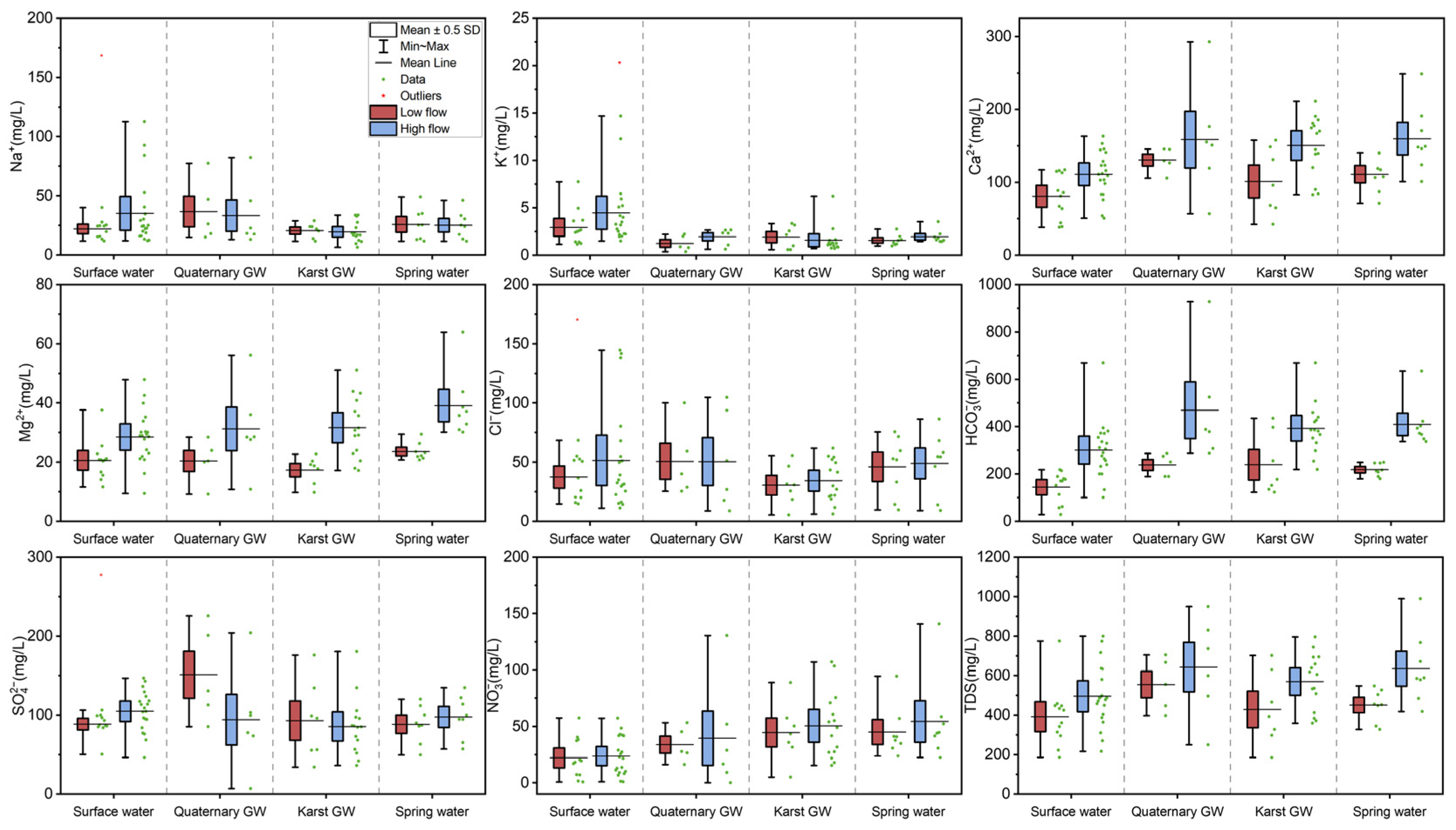
Figure 5.
Box plots for major ions and TDS in water samples during low-flow and high-flow conditions.
Surface water was generally alkaline throughout the study period, while groundwater (Quaternary GW, karst GW, and spring water) was slightly alkaline during low-flow conditions and weakly acidic during high-flow conditions. Moreover, the mean pH values in surface water were significantly higher than those of Quaternary GW, karst GW, and spring water, regardless of season (p < 0.05, Figure S1). This observation is consistent with the fact that groundwater is more aggressive than surface water, particularly in karst regions [50,51]. In addition, pH showed a significant decreasing trend from low- to high-flow with respect to all four types of water (Figure S1), which suggests the enhancement of water aggressiveness after rainy episodes. Water temperature (T) demonstrated significant differences in terms of both water types and hydrological conditions (p < 0.001, Table 2). The temperature was ranked as surface water > spring water > karst GW > Quaternary GW during low-flow conditions, and spring water > Quaternary GW > karst GW > surface water during high-flow conditions. Surface water temperature dropped significantly from low- to high-flow conditions, whereas groundwater temperature remained relatively stable (Figure S1), which may be related to the varying degrees of influence of air temperature. Furthermore, the water temperature showed a significant interaction effect between different water types and hydrological conditions (p < 0.05; Table 2), implying the complexity of the karst water system. Both DO and ORP exhibited statistically significant differences in water types and hydrological conditions (p < 0.05 or p < 0.001, Table 2). A marked rise in DO from low- to high-flow conditions was observed in all analyzed water except Quaternary groundwater (Figure S1). Furthermore, surface water had the highest DO level, possibly due to its exposure to the atmosphere. Spring water and karst GW had relatively lower DO contents, which indicate a depleted state of oxygen in the aquifer. It is worth noting that all water samples are oxic (DO > 0), which could limit the denitrification process but favor the bacterial metabolism of certain organic compounds. With regard to ORP, all four types of water showed an increase from low- to high-flow conditions (Figure S1). Surface water and karst GW had relatively lower ORP, which may be ascribed to sewage inputs or industrial waste. EC only demonstrated significant differences in hydrological conditions (p < 0.05, Table 2), i.e., the EC of high-flow conditions was higher than that of low-flow conditions. This result may be associated with an enhanced degree of water–rock interaction or the input of contaminants after rainy episodes. In addition, EC was ranked as Quaternary GW > spring water > surface water > karst GW during low-flow conditions, and Quaternary GW > spring water > karst GW > surface water during high-flow conditions. Quaternary GW showed the highest EC level among the four types of water, regardless of hydrological conditions, possibly due to its susceptibility to evaporation and sewage infiltration. Moreover, extensively varying ranges in EC value were observed for all types of water, which may indicate the spatiotemporal variability of hydrochemistry in the JSC.
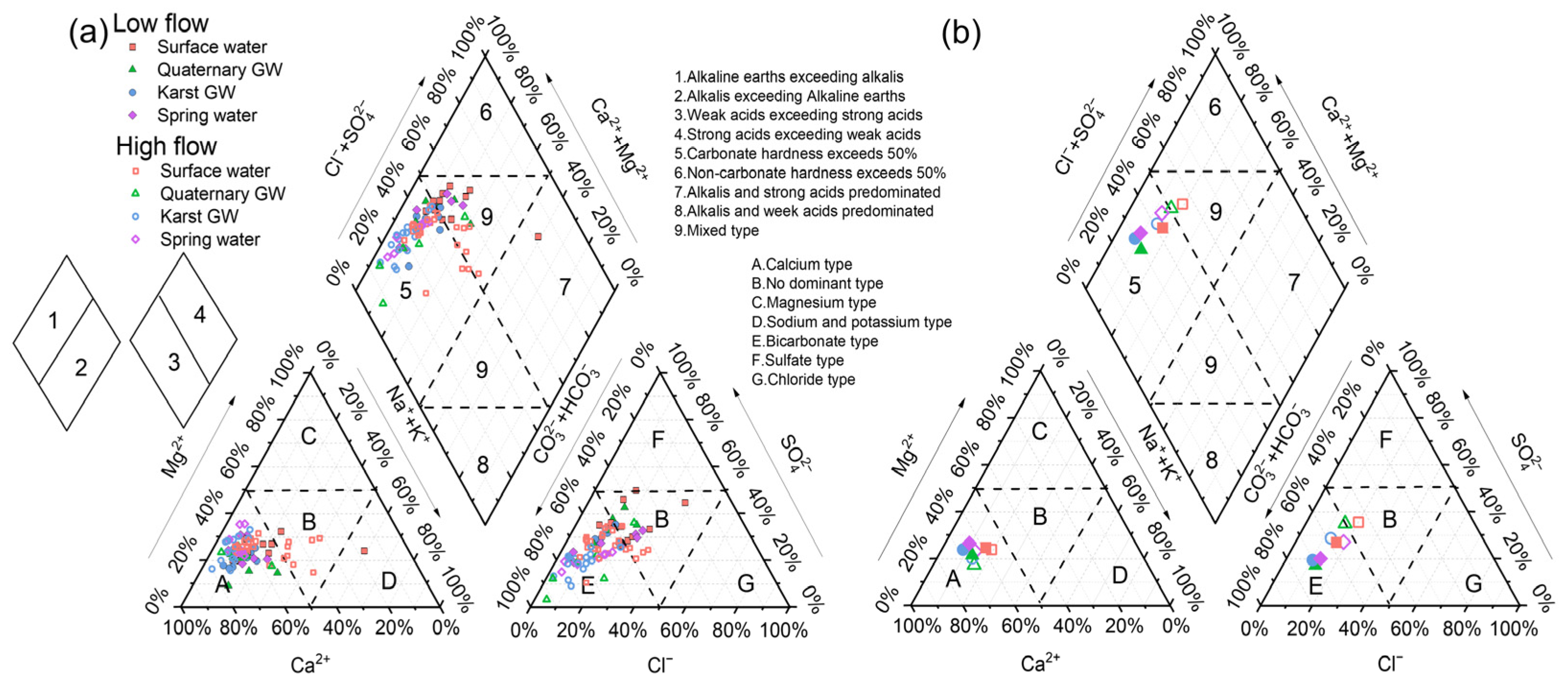
The Piper diagram is presented in Figure 6 to characterize the principal hydrochemical facies and evolutionary trend of water in the study area. For surface water and groundwater during low-flow conditions, most data were scattered in zone A of the cation triangle, indicating the dominance of the calcium type, or located in zones E and B of the anion triangle, suggesting the dominance of the bicarbonate type and no dominant type, respectively (Figure 6). After projection to the upper rhombus, the data were dispersed in zones 5 and 9, implying calcium and mixed types, respectively (Figure 6). The major hydrochemical facies of the surface water and groundwater were Ca-HCO3·SO4 (56%), Ca-HCO3·SO4·Cl (14%), and Ca·Mg-HCO3·SO4 (13%). From the perspective of ion abundance, the major cations were dominated by Ca2+, followed by (in decreasing order) Mg2+ > Na+ > K+. Notably, Ca2+ alone accounts for 62.3% of the total cation budget, whereas Ca2+ and Mg2+ account for 84.1%, more than five times that of Na+ and K+ (15.9%), indicating the predominance of carbonate weathering. On the other hand, the anion proportions were ranked as HCO3− > SO42− > Cl− > NO3−. The dominant anion is HCO3−, which accounts for 45.6%, followed by SO42− and Cl− with 30.2% and 16.4%, respectively. NO3− was the least abundant anion in the area, with an average proportion of 7.8%. The contribution of HCO3− and SO42− is more than twice that of Cl−, suggesting that the weathering of carbonate and evaporite could be crucial processes that affect water chemistry in the study area. As regards the surface and groundwater samples collected during high-flow conditions, data were concentrated in zone 5, with cations and anions falling in zones A and E, respectively (Figure 6). Ca-HCO3 (37%), Ca·Mg-HCO3 (25%), and Ca·Mg-HCO3·SO4 (23%) were the common hydrochemical facies. The abundance patterns of cations and anions were the same as those of low-flow conditions, although the contributions of each ion changed somewhat. The overall evolution of water chemistry from low- to high-flow conditions shows an increase in Ca2+, Mg2+, and HCO3− proportions and a decrease in Na+, Cl−, SO42−, and NO3− proportions (Figure 6b).
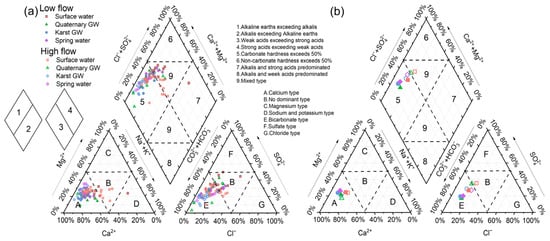
Figure 6.
Piper diagram showing the ionic proportions of all water samples during low- and high-flow conditions (a) and mean chemical compositions (b).
The major chemical components of surface water and groundwater are shown in Figure 5. Surface water was less mineralized compared with groundwater, with mean TDS of 391.8 mg/L and 495.3 mg/L during low- and high-flow conditions, respectively. The principal hydrochemical facies in surface water were Ca-HCO3·SO4 and Ca·Mg-HCO3·SO4 during low- and high-flow conditions, respectively, showing the dominance of Ca2+, Mg2+, and HCO3− (Figure 6). However, the Ca2+ and HCO3− contents in surface water were significantly less than in Quaternary GW, karst GW, and spring water (p < 0.05, Figure 5). Moreover, surface water featured lower levels for major ions, except for Na+, K+, and Cl−, than groundwater, possibly due to its susceptibility to dilution by various recharge sources, e.g., aquifers and local tributaries. The average Na+ contents in surface water during low- and high-flow conditions were 34.2 mg/L and 35.1 mg/L, respectively, significantly higher than karst GW (p < 0.05, Figure 5). The enriched levels of Na+ were accompanied by increased Cl− levels, both of which varied extensively during both low- and high-flow conditions. Additionally, surface waters exhibited K+ concentrations significantly higher than Quaternary GW, karst GW, and spring water (p < 0.05, Figure 5). The NO3− concentrations in surface water ranged from 0.7 to 57.4 mg/L (mean = 22.0 mg/L) during low-flow conditions and from 0.8 to 57.2 mg/L (mean = 23.6 mg/L) during high-flow conditions, showing that surface water is not contaminated by nitrate, except at a few sites (i.e., J18 and JN77). It is worth noting that surface water showed higher concentrations of all analyzed chemical components during high-flow conditions than in low-flow conditions (Figure 5). This suggests the enrichment of major ions in surface water with rising flow conditions, which could result from potential mechanisms like chemical weathering, road salts, and sewage inputs [52].
Quaternary GW featured the highest TDS values of the four types of water throughout the study periods, ranging from 397.1 to 705.0 mg/L (554.2 mg/L) during low-flow conditions and from 249.5 to 949.3 mg/L (643.3 mg/L) during high-flow conditions. The major hydrochemical facies in Quaternary GW during low- and high-flow conditions were Ca-HCO3·SO4 and Ca-HCO3, respectively, showing a considerable reduction in SO42− (Figure 5). This suggests the occurrence of processes of dilution or removing SO42− from the Quaternary aquifer. For Quaternary GW, relatively higher concentrations of major ions (except Na+, Cl−, and SO42−) were observed during high-flow conditions (Figure 6), suggesting an enriched solute load after rainy episodes. The SO42− concentration in Quaternary GW was the highest of all four types of water during low-flow conditions and substantially reduced during high-flow conditions (Figure 5). In addition, Quaternary GW showed Na+ and Cl− contents close to surface water, higher than those of karst GW and spring water regardless of the season (Figure 5). In contrast, the Ca2+ and HCO3− levels of Quaternary GW were comparable to karst GW and spring water and significantly higher than surface water throughout the study period (p < 0.05, Figure 5). Such differences between Na+ and Cl− and Ca2+ and HCO3− may suggest possible hydrogeochemical interactions between Quaternary GW, surface water, and karst GW. Furthermore, Quaternary GW NO3− concentrations ranged from 16.1 to 53.2 mg/L (mean = 33.8 mg/L) and 9.1 to 130.6 mg/L (mean = 48.1 mg/L) during low- and high-flow conditions, respectively. Thereinto, three of the eleven samples exceeded the WHO limit value (50 mg/L) for drinking water [53].
For karst GW, the TDS ranged from 184.4 to 702.0 mg/L (mean = 428.3 mg/L) and 359.0 to 795.9 mg/L (mean = 569.9 mg/L) during low- and high-flow conditions, respectively, showing an obvious increasing trend (Figure 5). The principal hydrochemical facies of karst GW transformed from Ca-HCO3·SO4 during low-flow conditions to Ca-HCO3 and Ca·Mg-HCO3 during high-flow conditions, showing increased Ca2+, Mg2+, and HCO3− abundance and decreased [Na++K+], Cl−, and SO42− abundance (Figure 5). From the perspective of ion contents, Ca2+, Mg2+, HCO3−, Cl−, and NO3− increased from low- to high-flow conditions, while Na+, K+, and SO42− decreased more or less (Figure 5). In karst GW, Ca2+ and HCO3− are the dominant ions, exhibiting significantly higher contents than surface water regardless of the season, indicating the predominance of carbonate weathering. The concentration of Mg2+ in karst GW was lower than other types of water during low-flow conditions, whereas it increased substantially during high-flow conditions (Figure 5), reflecting varying degrees of dolomite dissolution. Furthermore, karst GW had the lowest Na+, K+, and Cl− contents (Table 1), were significantly lower than surface water (p < 0.05, Figure 5), especially during high-flow conditions. Karst groundwater also showed the lowest SO42− levels during both flow conditions, varying extensively within space (Table 2). This result is probably associated with the evaporite weathering that occurs locally in the formation. The NO3− concentrations in karst GW ranged from 4.9 to 88.7 mg/L (mean = 44.6 mg/L) and 15.3 to 107.1 mg/L (mean = 50.5 mg/L) during low- and high-flow conditions, respectively, showing an increasing trend. Notably, ten of twenty-two samples exceeded the WHO standard for drinking water (WHO, 2011). The wide range of NO3− levels could be related to the high instability of nitrate in the karst aquifer and the spatial variability of its input or transformation in the study area.
Spring water was characterized by TDS ranging from 327.2 to 547.5 mg/L (mean = 450.4 mg/L) and 417.7 to 989.5 mg/L (mean = 635.1 mg/L) during low- and high-flow conditions, respectively, showing an obvious increasing trend. The main hydrochemical facies of spring water changed from Ca-HCO3·SO4 and Ca-HCO3·SO4·Cl during low-flow conditions to Ca-HCO3 and Ca·Mg-HCO3 during high-flow conditions, with reduced [Na+ + K+], Cl−, and SO42− abundance. In terms of ion contents, all major ions except Na+ increased during high-flow conditions (Figure 5). The hydrochemistry of spring water appeared fairly close to that of karst GW (Figure 5), and the latter is often the primary source of spring water. However, spring water was slightly more mineralized than karst GW, as were the concentrations of major ions (Table 1). This could be related to mineralization along flow paths, mixing with polluted water, or sewage infiltration near spring areas. Additionally, spring water had greater Cl− levels than karst GW, comparable to surface and Quaternary GW (Table 1). It is worth noting that spring water had the highest NO3− contents among the four types of water, regardless of the season, ranging from 23.7 to 94.4 mg/L (mean = 45 mg/L) and 22.3 to 170.7 mg/L (mean = 58.0 mg/L) during low-flow and high-flow conditions. The spring water NO3− level was significantly higher than surface water and increased from low- to high-flow conditions (p < 0.05, Figure 4). In particular, the Beiqiu Spring (i.e., J25, JN80) showed NO3− contents of 94.4 mg/L and 171 mg/L in May and October, which far exceeded the WHO drinking water quality standard.
Overall, the aqueous chemistry of these four waters (surface water, Quaternary GW, karst GW, and spring water) was somewhat similar in terms of different parameters, implying that they may have undergone comparable geochemical processes or hydrogeochemical interactions in the subsurface continuum.
4.2. Stable Isotopes (δ18O and δ2H) in Surface Water and Groundwater
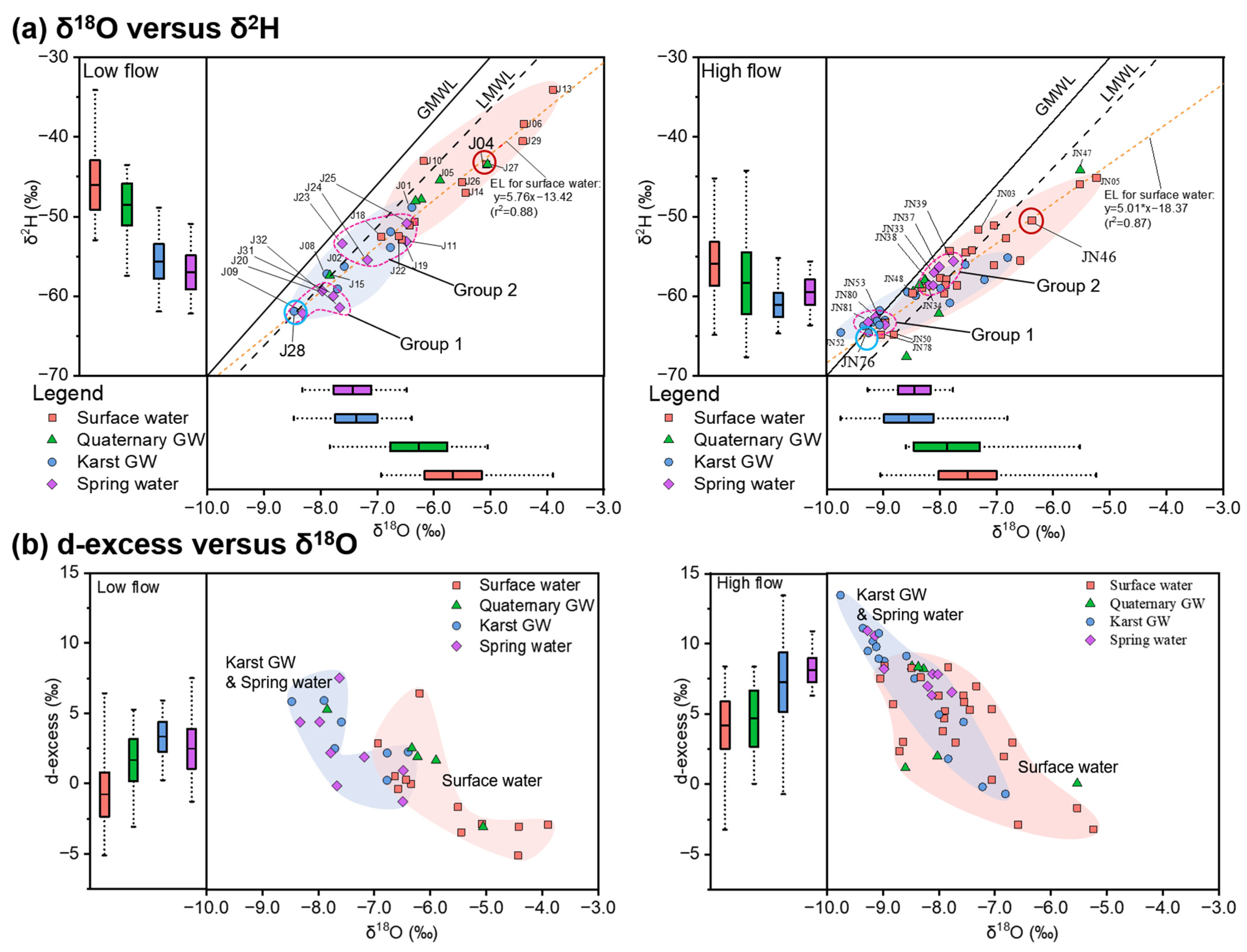
The isotopic signatures of the water samples collected during low- and high-flow conditions were generally distributed close to the LMWL and GMWL for the duration of the study (Figure 7a), indicating the overall meteoric origin of surface water and groundwater in the study area. There were distinct differences (persisting beyond seasonal variation) between the isotopic compositions of surface water, Quaternary GW, karst GW, and spring water (p ≤ 0.001, Table 2). In addition, the δ18O and δ2H in all four types of water showed significant temporal trends (p < 0.05, Figure 7), i.e., shifting towards isotopic enrichment during low-flow conditions and vice versa, which reflects the effect of atmospheric meteorological conditions in the study area.
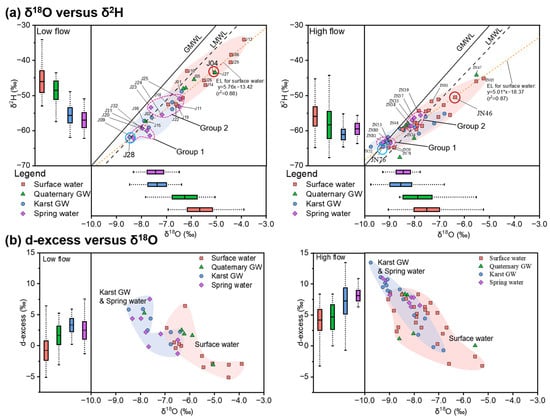
Figure 7.
Dual isotope plot (center) and box plots (left, bottom) showing δ18O versus δ2H, along with the evaporation line (EL) (a) and d-excess versus δ18O (b). The global meteoric water line (GMWL, δ2H = 8 δ18O + 10 [54]) and local meteoric water line (LMWL, δ2H = 7.46 δ18O + 0.9 [55]) are given for reference.
Plotting data from two sampling campaigns in the isotope plot (Figure 7a) revealed noticeable differences between the more enriched rivers and streams and the more depleted springs and karst GW. Surface water was characterized by the most enriched isotopic compositions throughout the study period, with mean values of up to −5.7‰ for δ18O and −46‰ for δ2H (Table 1). Moreover, the majority of surface water samples were distributed to the lower right of the LMWL (Figure 7a), indicating the impact of evaporation, which was also evident from the slopes of the evaporation line (EL) for surface water samples during low- (5.76) and high-flow conditions (5.06). By contrast, spring water and karst GW featured the most depleted isotopic compositions during low- and high-flow conditions, respectively, both of which were significantly lighter than that of surface water (p < 0.05, Table 1). Moreover, much narrower ranges of isotopes were observed in karst GW and spring water relative to surface water (Figure 7). Karst GW showed δ18O and δ2H values of −8.5‰ to −6.4‰ (mean = −7.4‰) and −62‰ to −49‰ (mean = −56‰) during low-flow conditions, and −9.8‰ to −6.8‰ (mean = −8.6‰) and −65‰ to −55‰ (mean = −61‰) during high-flow conditions. The isotopic signatures of spring water resembled karst GW during low-flow conditions, with δ18O of −9.5‰ to −6.5‰ and δ2H of −62‰ to −51‰, whereas they were slightly more enriched during high-flow conditions, with δ18O of −9.3‰ to −7.8‰ and δ2H of −64‰ to −56‰. Quaternary GW featured an intermediate isotopic composition between surface water and karst GW, or spring water. Mean isotopic values changed from −6.5‰ for δ18O and −49‰ for δ2H during low-flow conditions to −7.9‰ for δ18O and −58‰ for δ2H during high-flow conditions. The isotopic signature of Quaternary GW was higher than karst GW and spring water (p < 0.05, Figure 7a), slightly lower than surface water, but without significant difference. Therefore, from the perspective of stable isotopes, Quaternary GW could have close connectivity with surface water and karst GW. Furthermore, Quaternary GW samples from high-flow conditions exhibited broader variation ranges of δ18O and δ2H values compared with the low-flow conditions. This may be associated with evaporation processes or recharge from evaporated river water.
5. Discussion
5.1. Mixing Processes and Karst Water Flow System
5.1.1. Stable Isotopes as Indicators of Water Origins
Water stable isotopes (δ18O and δ2H) are natural and conservative tracers of the water cycle, the variation of which is generally driven by hydrological processes in the basin [56]. Thus, stable isotopes are widely used for evaluating hydrological processes across space and time at multiple scales [13]. In this study, the isotopic compositions of water were studied in an effort to investigate water sources, subsequent evaporation, and mixing processes between different waters. In the JSC, surface water and groundwater are mainly derived from atmospheric precipitation, as confirmed by the approximately linear relationship of δ18O and δ2H along the LMWL (Figure 7). The overall trend of δ18O and δ2H in Figure 7 also showed clear seasonal changes, with more enriched isotopes during low-flow conditions and more depleted isotopes during high-flow conditions. This result reflects the impact of meteorological conditions and fits with the precipitation isotope characteristics of the monsoon climate region in China [53]. It should be noted that there was significant overlap in the isotopic ranges of the four water types during both low- and high-flow conditions, possibly indicating the general existence of water mixing in the JSC. Additionally, some data were distributed above the LMWL with a negative shift in δ18O, especially pronounced during high-flow conditions. This could be due to the exchange with CO2, which is common in systems with high ratios of CO2 to water, e.g., CO2 geological sequestration [57].
The heavier isotopic compositions are indicative of evaporative enrichment in corresponding water samples, which tend to deviate towards the right of the LMWL. Based on the higher isotopic values and larger deviation from the LWML (Figure 7), it can be inferred that the effect of evaporation was stronger during low-flow conditions relative to high-flow conditions. This finding is further confirmed by the d-excess (d-excess = δ2H – 8*δ18O [58]), which displayed a clear increment from low-flow to high-flow conditions. Moreover, two clusters of water samples could be identified for each sampling period according to the plot of d-excess versus δ18O (Figure 7b). Karst GW and spring water samples typically fell in the upper-left of the diagram, with lower δ18O and greater d-excess values, forming the karst GW-related cluster. The other cluster is mainly composed of surface water samples with higher δ18O and lower d-excess values, plotted in the bottom-right of the diagram, making up the surface water cluster of the JSC. By comparison, we can distinguish the degree of evaporation in these two clusters of water samples. Surface water featured significantly lower d-excess values than karst GW-related clusters (p < 0.05, Figure 7b), indicating a higher extent of direct evaporation in the rivers and streams. Furthermore, changes in the overlap between the karst GW and surface water clusters may indicate variations in the interaction between surface water and groundwater (Figure 7b).
The variation in water isotope values reflects the influence of prior precipitation and can reveal the input of water from different sources [59]. Karst GW was depleted in heavy isotopes and mainly distributed along the LMWL, with some data even falling exactly on the LMWL (Figure 7). This indicates that karst GW in the JSC primarily originates from precipitation, corroborating the findings of Wang [40]. Karst water flow systems typically have widespread heterogeneous porosity, such as sinkholes, caves, conduits, faults, fractures, and fissures, which can act as preferential channels for subsurface flow in the karstic terrain and permit the rapid infiltration of rainfall [15]. Thus, the karst GW collected in the southern mountainous area retains a pristine isotopic composition similar to prior precipitation and can represent one of the water sources involved in mixing processes in the JSC. Surface water had a clear enrichment in stable isotopes and deviated from the LMWL during both flow conditions (Figure 7). This result demonstrates the prevalence of evaporative fractionation in surface water, which is also confirmed by the d-excess values (Figure 7b). Additionally, δ18O and δ2H in surface water were progressively enriched from upstream to downstream, indicating that the degree of evaporation increased as the rivers and streams flowed towards the urban area. Consequently, the lower reaches of the Yufu River (J04 and JN05) showed the most enriched isotopic compositions during both sampling campaigns (Figure 7). And the recharge from the Yufu River to other water may cause an increment in δ18O and δ2H. Previous surveys found that downstream sinking zones allow rapid infiltration of water from the Yufu River into the underlying aquifers [60]. Therefore, the Yufu River seems to be another water source involved in the mixing processes in the JSC. As shown in Figure 7, there is a higher overlap between surface water and karst GW isotopic compositions during high-flow conditions than during low-flow conditions. This may indicate an enhanced interaction between surface water and groundwater as flow increases. Notably, several surface water samples (e.g., J17, J21, and J22 during the low-flow period and JN35, JN36, and JN62 during the high-flow period) showed depleted isotopic signatures comparable to karst GW (Figure 7). These samples were taken from spring-fed lakes or tributaries, or from water bodies that receive recharge from the karst aquifer, which are largely distributed in the discharge area of the catchment. The isotopic signatures of spring water are highly overlapping with karst GW (Figure 7), demonstrating that the latter is the primary source of spring water, which is consistent with the prior findings [61]. However, it is worth noting that spring water samples are approximately clustered into two groups during each sampling period (Figure 7), which may indicate the effect of different water sources. Springs in Group 1 (e.g., J09, J31, and J32 from LF and JN53, JN80, and JN81 from HF) showed lighter δ18O and δ2H, comparable to karst GW (Figure 7). This observation indicates that they were primarily replenished by the karst aquifer, which is derived from atmospheric precipitation that rapidly infiltrates the epikarst through fractures and fissures with little evaporation. These springs were found in the southern mountainous area, thus being termed “mountainous springs”, such as Beiqiu Spring, Cheziyu Spring, and Niyu Spring. Springs in Group 2 (e.g., J11, J23, J24, and J25 from LF and JN33, JN37, JN38, and JN39 from HF) exhibited surface water-like isotopic compositions (Figure 7). These springs were located in the northern urban area, termed “urban springs”, such as Baotu Spring, Heihu Spring, Wulongtan Spring, and Zhenzhu Spring. And the similarities in the isotopic compositions of urban springs and surface water suggest recent communication between springs and the surface environment. This may be due to the interaction of karst GW with sinking surface water during its transfer from the mountainous area towards the alluvial plain. The widespread fractures and conduits in karst terrains typically serve as large channels for surface water leakage and groundwater flow [62]. Furthermore, multiple tectonic movements have generated a large number of faults and fractures in the JSC, which act as water-conducting pathways, enhance water–rock interaction, and promote the development of conduits and fissures [58]. Other researchers have also reported the role of faults and fractures in connecting surface water, shallow groundwater, and underlying aquifers [10,63]. Therefore, the enrichment in δ18O and δ2H in urban springs is most likely caused by recharge from the Yufu River, which is related to the sinking zone and the high-angle fractures near the river. In addition, the altitude effect of water isotopes could also have a certain contribution to the discrepancy in δ2H and δ18O of mountainous springs and urban springs [12]. The fact that the average elevation of the southern mountainous area (405 m) is noticeably higher than that of the urban area (31 m) lends evidence to this explanation. Quaternary GW had enriched isotopic signatures comparable to surface water during low-flow conditions, whereas Quaternary GW showed depleted isotopic compositions similar to karst GW during high-flow conditions. This result could signify better connection of Quaternary aquifers with surface water during low-flow conditions and the predominance of connection with the karst aquifer during high-flow conditions. The apparent change reflects variations in surface–groundwater interactions, possibly due to fluctuations in groundwater levels caused by varying flow conditions.
5.1.2. Mixing Processes in the JSC
Based on what was discussed in the last section, it can be observed that there were significant differences and overlaps between the isotopic ranges of surface water and karst GW. On the other hand, the isotopic signatures of spring water and Quaternary GW were approximately linearly distributed between surface water and karst GW. Hence, a likely binary mixing of surface water and karst GW can be inferred in the JSC. This means that, in a simplified context (ignoring other possible end-members due to lack of data), there could be two distinct water sources involved in the mixing system of water in the study area: (1) karst GW (EM1, (δ18O)KG: −8.5‰ in May and −9.2‰ in October) collected at relatively high elevations that experiences little evaporation; (2) surface water from the Yufu River in the downstream leakage section (EM2, (δ18O)SW: −5.0‰ in May and −6.3‰ in October) that shows clear evidence of evaporative fractionation. Thus, these two end-members were chosen to represent a variety of water compositions in this study, from Quaternary GW that was impacted by evaporative enrichment (with higher δ18O) to spring water less affected by such enrichment (fresh and with lower δ18O).
An end-member mixing analysis (EMMA) was carried out to quantify the connectivity between surface water and karst GW. Stable isotopes and chloride are routinely conservative indicators that can be used in the EMMA [13,36]. However, within the JSC, where halite is commonly found in the limestone strata, stable isotopes are likely to be more accurate than chloride. Therefore, only δ18O data were assessed in mixing calculations this time. The results of the EMMA are summarized in Figures S2 and S3. For karst GW, the estimated surface water mixing fractions (fSW) ranged from 0 to 53% (mean = 23%) and from 0 to 83% (mean = 25%) during low- and high-flow conditions, respectively. Among them, the fSW in J28, JN85, JN51, and JN52 was 0, indicating that karst GW at these sites received negligible recharge from surface water at the time of sampling. In contrast, the fSW in karst GW samples near the rivers, e.g., J01, JN45, JN74, and JN76, showed relatively higher fSW (up to 83%). This suggests the mixing process between the river and karst GW and also reflects the strong infiltration capacity of the streambed. From the perspective of spatial variability, the karst GW samples in IRA showed lower fSW values than those in DRA and DA, possibly due to topographic and hydrological conditions (Figure S3). Since the IRA is situated in mountainous surroundings with steep hills and rugged terrain, it is not favorable for surface runoff to infiltrate. Moreover, the relatively higher groundwater level in this area could lessen or dilute the river water leakage via the streambed. The DRA, on the other hand, is located in the alluvial plain with gentle terrain and considerable sinking zones, which facilitates significant surface water leakage. For surface water, the mixing fractions (fSW) ranged from 35% to 100% (mean = 72%) during low-flow conditions and from 6% to 100% (mean = 56%) during high-flow conditions. It is worth noting that some of the surface water samples have significantly lower fSW values (down to 9%), implying that these sampling sites receive substantial replenishment from the karst aquifer. In particular, Daming Lake (J22/JN36) and Baihua Lake (J17/JN35) featured fSW values of 45–55% throughout the study period, which confirms the notion that they are spring-fed lakes. As for spring water, an obvious discrepancy emerged between the fSW of mountainous and urban springs (Figure S2). Urban springs showed higher fSW values with an increasing trend from low- to high-flow conditions, whereas mountainous springs had lower fSW values with little seasonal variation. This indicates that urban springs are hydraulically connected to rivers or lakes and receive a considerable contribution from them during high-flow conditions. In contrast, mountainous springs have a weak hydraulic connection to surface water and remain in a relatively natural state. Quaternary GW featured larger ranges of fSW, varying from 5% to 98% (mean = 50%) and 22% to 100% (mean = 42%), respectively, for low- and high-flow conditions (Figure S2). Notably, J27 and JN47 exhibited fSW values of 98% and 100%, respectively, revealing these locations as being replenished by primarily sinking surface water. The results of end-member mixing analysis revealed that surface water–groundwater interactions are prevalent in JSC. However, such connections between surface water and groundwater may vary throughout the catchment and an entire hydrological year due to the heterogeneity of geological characteristics and changing flow conditions in the JSC.
5.2. Factors Controlling the Hydrochemistry of the Jinan Spring Catchment
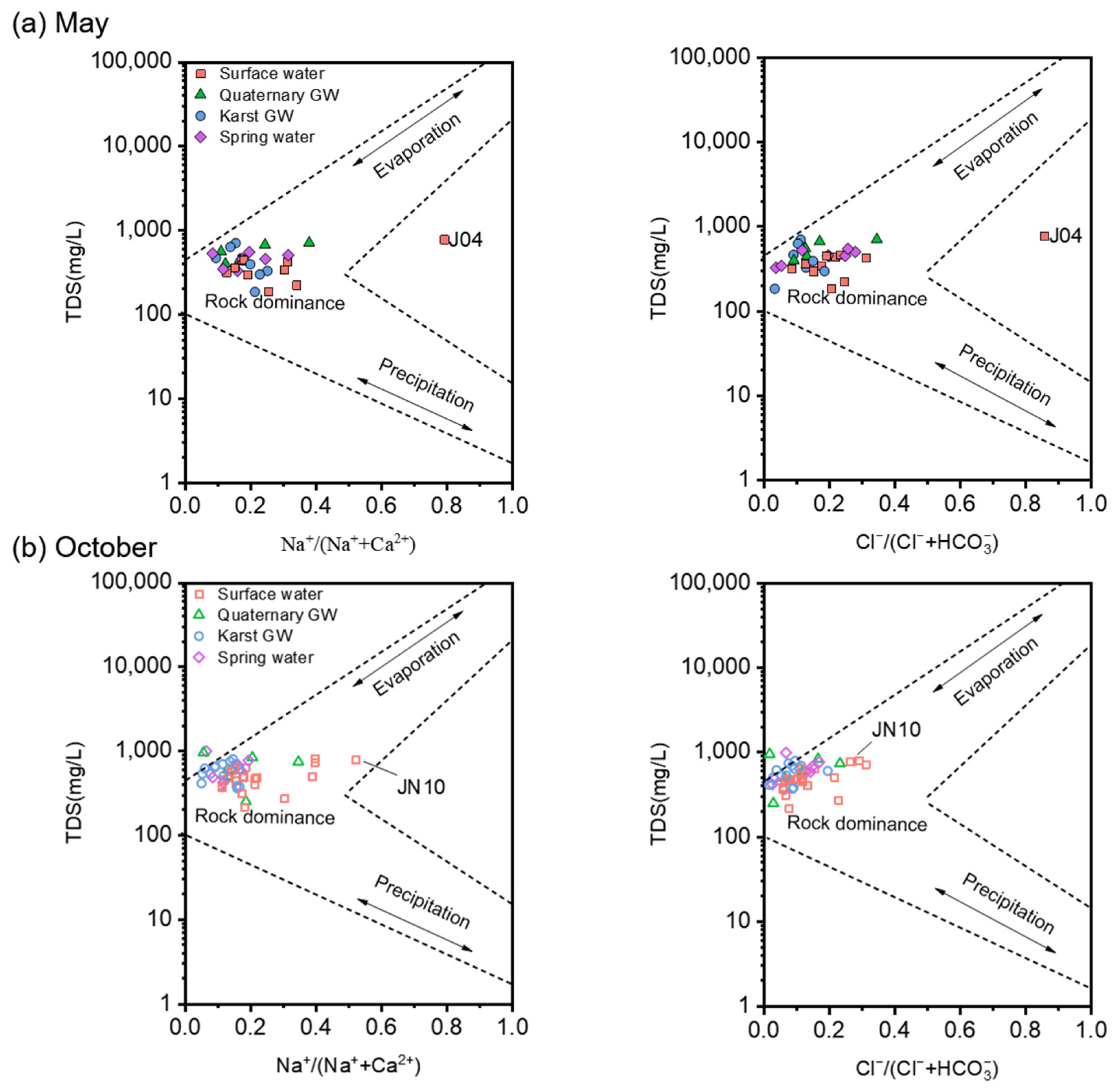
The hydrochemistry of natural water is the result of the long-term interaction of water with the surrounding environment during the water cycle and can reflect information about flow paths [64]. In general, the chemical compositions of water come from atmospheric precipitation, rock weathering, and regional geologic and anthropogenic inputs [65]. The semi-logarithmic diagrams proposed by Gibbs [66] are widely used to identify the major factors controlling water chemistry [67]. As shown in Figure 8, the water samples from May and October mainly fell in the rock weathering dominance zone, illustrating the dominance of geogenic factors on water chemistry during both low- and high-flow conditions. In other words, rock-dominated geochemical processes (i.e., water–rock interactions) are the prevailing mechanisms responsible for the chemical compositions of the sampled waters, consistent with the karstic nature of the JSC. It is noteworthy that groundwater (i.e., Quaternary GW, karst GW, and spring water) had relatively higher TDS values and lower Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−) ratios compared to surface water, indicating that rock weathering plays a greater role in underground aquifers than in rivers, streams, or reservoirs. Moreover, groundwater samples showed a marked increase in TDS values and a decrease in Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−) ratios from low- to high-flow conditions (Figure 8). This result suggests that the enhancement of carbonate dissolution (releasing Ca2+ and HCO3−) following the rainy episode outweighed the dilution effect caused by rising flow. Furthermore, such seasonal variation in dissolved ions may reflect the climatic control over certain geochemical processes in the study area [68]. On the other hand, surface water showed wider ranges of Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−) than groundwater, suggesting the impact of multiple factors apart from rock weathering. And the TDS values of surface water exhibited no discernible variation as the Na+/(Na+ + Ca2+) ratio increased from low to medium. This may be associated with the exchange between Ca2+ in the water and Na+ in the rock, seeing that the molar mass of Ca2+ (40) is nearly twice that of Na+ (23). For instance, J04 and JN10 were plotted in the middle or right of the Gibbs diagram with Na+/(Na+ + Ca2+) ratios greater than 0.5, suggesting the occurrence of cation exchange.
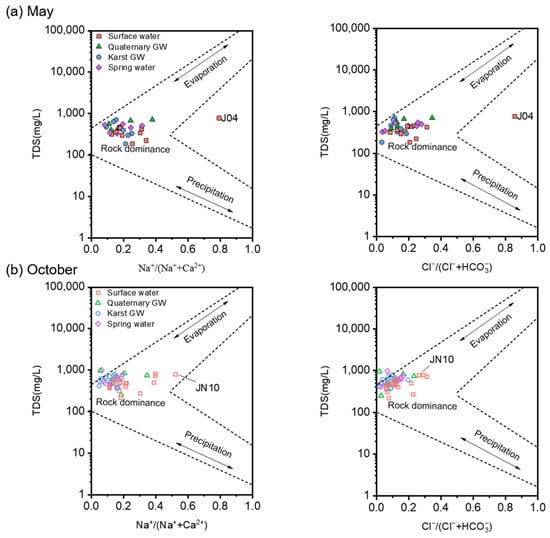
Figure 8.
Gibbs diagrams illustrating the most likely controlling mechanism of the water chemistry in the study area. Subplot (a) includes the water samples collected in May 2020. Subplot (b) includes the water samples collected in October 2020.
The results of the Gibbs diagram for this study are in good agreement with those obtained for basins draining from similar lithological and climatic environments [69,70]. However, it should be noted that the Gibbs diagrams cannot determine the influence of human inputs on water chemistry. Nitrogen concentrations in October were substantially higher than those in May, which may reflect a higher risk of anthropogenic NO3− pollution during high-flow conditions.
5.3. Sources of Major Ions
5.3.1. Hydrogeochemical Processes
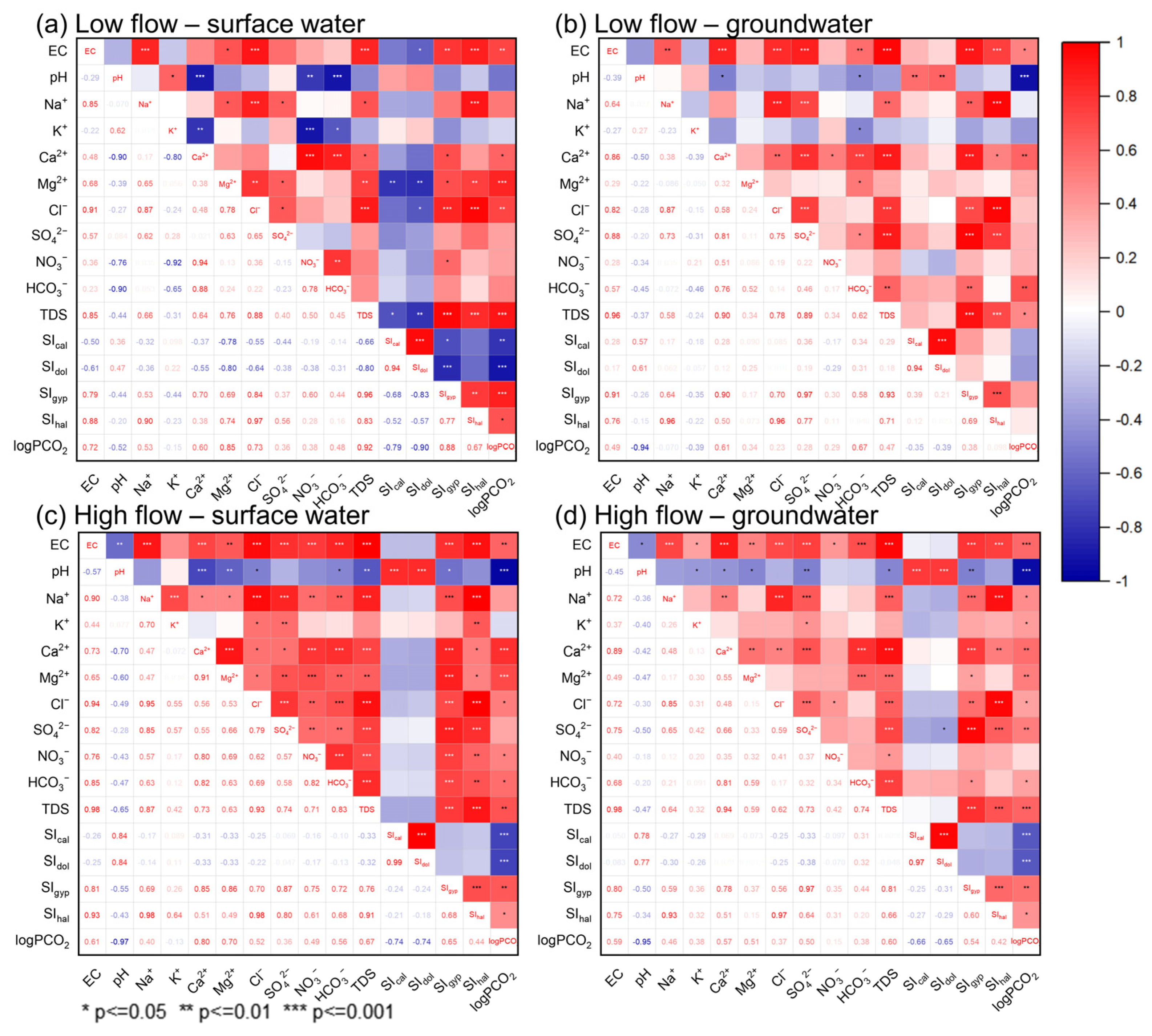
Correlation analysis and R-type clustering were used in this study to identify the linkage between hydrochemical variables of the surface and groundwater, as compiled in Figure 9 and Figure S4. EC had the best positive correlations with TDS, as expected, since adding dissolved ions or constituents to the water makes the electrical conductivity go up [71]. Furthermore, EC showed correlations with most of the ions in surface and groundwater during both low- and high-flow conditions, possibly due to the extensive water–rock interaction in the area (Figure 9). Thereinto, EC had relatively better correlations with carbonate system-related parameters such as HCO3−, Ca2+, Mg2+, and logPCO2, indicating the prevalence of carbonate weathering in the study area.
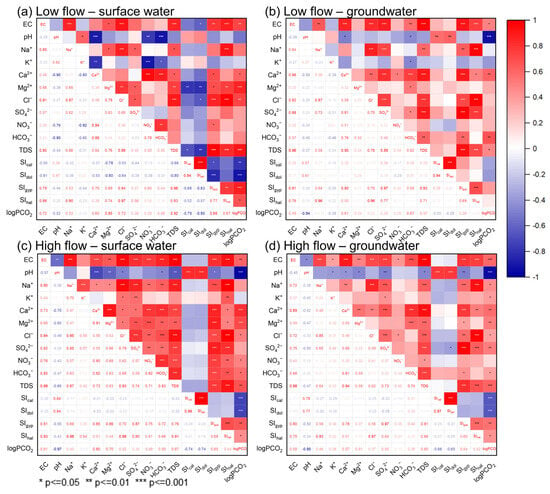
Figure 9.
Correlation coefficient matrix of major hydrochemical variables in surface water and groundwater samples during low-flow and high-flow conditions.
Since the main formation lithology in the JSC is carbonate rock, carbonate minerals (calcite and dolomite) tend to dissolve into the surface runoff and underground flow, as shown in Equations (2) and (3). And this statement is supported by the significant correlations of Ca2+-HCO3−, Mg2+-HCO3−, and Ca2+-Mg2+ pairs. It is worth mentioning that the correlation coefficient of Ca2+-HCO3− for groundwater slightly increased from low- to high-flow conditions (Figure 9), illustrating enhanced dissolution of carbonate along with rising flow. Moreover, the increasing partial pressure of CO2 during high-flow conditions (Table 1) and the positive correlation of logPCO2-HCO3− in surface and groundwater reflect the local reactivity between recently generated runoff and soluble rocks. As the groundwater flow rate rises after rain episodes, the erosion capacity of groundwater also markedly improves, thus leading to enhanced weathering of aquifer matrix rocks [72]. Furthermore, the logPCO2 showed negative correlations with pH and positive correlations with EC, Ca2+, and Mg2+ (Figure 9). This result indicates that CO2 levels regulate the acidity of natural water in the study area and, as a consequence, affect a range of chemical and physical processes, especially the dissolution and precipitation of carbonates [16,73]. Therefore, CO2 dissolution could be one of the principal factors influencing the chemical characteristics of water in the JSC. SO42− showed strong correlations with Ca2+ in both surface and groundwater, suggesting a close relationship between water, evaporites, and soil sulfate. This is consistent with previous findings that the dissolution of gypsum and anhydrite often occurs in karst areas [10]. Additionally, Ca2+ and SO42− exhibited higher correlation coefficients in groundwater than in surface water, implying that gypsum was more adequately dissolved in the groundwater environment. Mg2+ and SO42− showed significant correlations in surface water but not in groundwater, possibly illustrating different origins of SO42− in sampled waters. This result corroborates the finding of Zhang [74], who reported that the dissolved sulfate in the JSC derives from a mix of multiple sources. Throughout the study period, Na+ and Cl− showed significant positive correlations in both surface and groundwater. This observation demonstrates the lithogenic origins of the Na+ and Cl−. The highly positive correlations between Na+ and SIhalite also supported the ongoing interaction of halite with water. Furthermore, Na+ and Ca2+ showed significant correlations in both surface and groundwater during high-flow conditions but no correlations in either surface or groundwater during low-flow conditions. This difference may be related to the effect of cation exchange in rainy seasons, which affects the concentrations of Na+ and Ca2+ [75]. Another explanation for this relationship could be the joint dissolution of halite and gypsum, as can be inferred from the good correlations of SO42−-Cl− and SIgypsum-SIhalite pairs.
CaCO3 + CO2 + H2O ↔ Ca2+ + 2HCO3−
CaMg(CO3)2 + 2CO2 + 2H2O ↔ Ca2+ + Mg2+ + 4HCO3−
CaCO3 + CaMg(CO3)2 + 3CO2 + 3H2O ↔ 2Ca2+ + Mg2+ + 6HCO3−
Finally, it is noteworthy that NO3− and EC showed significant correlations in both surface and groundwater during high-flow conditions while exhibiting no correlations during low-flow conditions. This discrepancy could suggest that nitrate contamination became more severe with rising flow, as also seen from the elevated NO3− level from May to October (Figure 5 and Table 1). Additionally, significant correlations between NO3−, Cl−, and SO42− were observed in surface water during high-flow conditions. This may be indicative of anthropogenic inputs (e.g., agricultural fertilizers, sewage), as is discussed in more detail below.
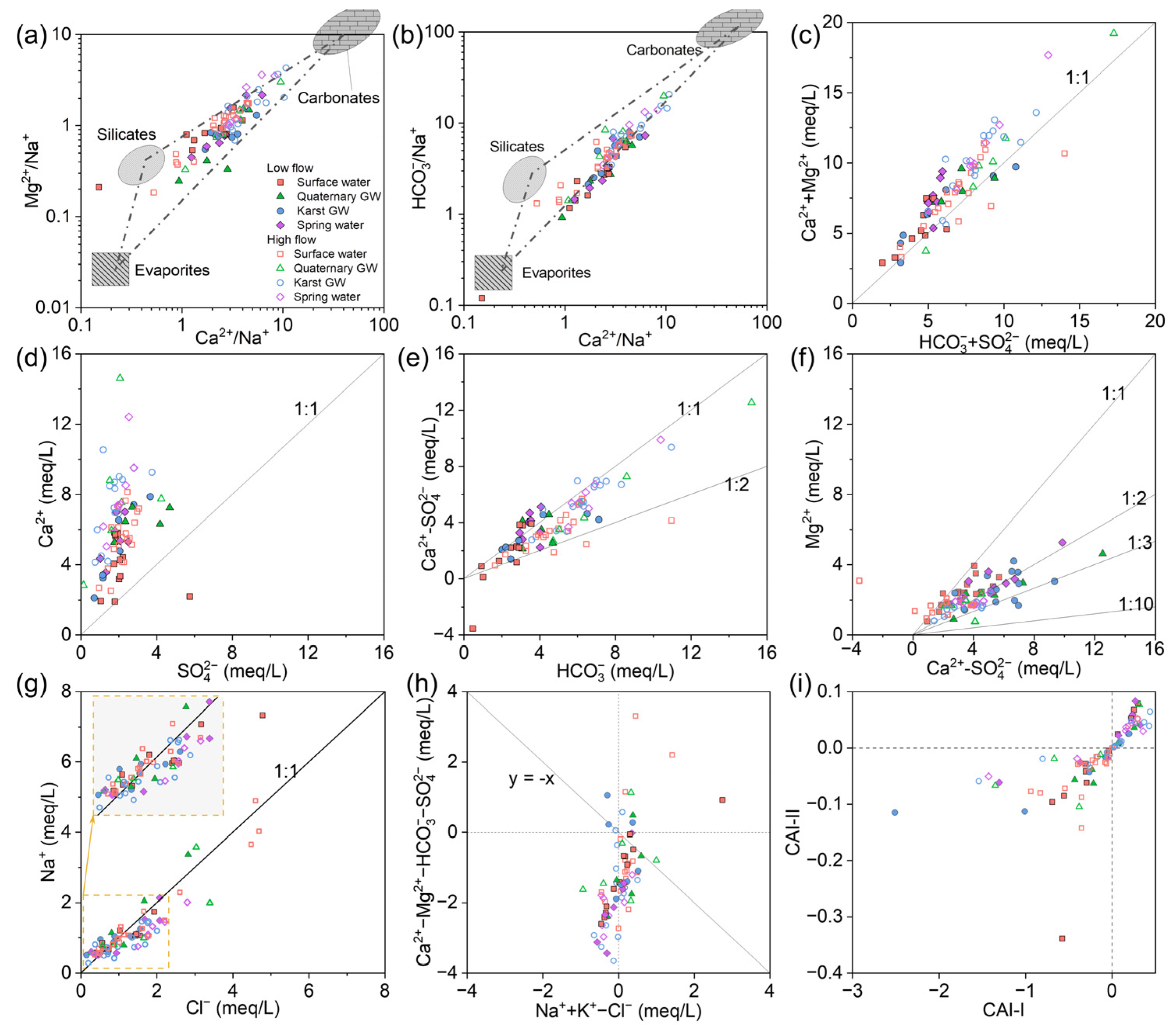
Based on the discussion above, the weathering of local rocks is the dominant mechanism influencing the water chemistry in the JSC. The stoichiometric relationship among the major ions can further determine the possible reactive minerals. Gaillardet et al. [76] developed an end-member diagram depicting the ion ratios between Ca2+, Mg2+, Na+, and HCO3− (molar concentration) to clarify the impact of the three principal weathered rocks (carbonate, silicate, and evaporite) on the hydrochemistry. As shown in Figure 10a,b, water samples were primarily plotted in the triangle-shaped zone formed by the three end-members, illustrating that carbonate, silicate, and evaporate contribute together to the water mineralization in the study area. Notably, surface and groundwater shifted towards silicate and carbonate, respectively, during high-flow conditions. This trend may indicate that silicate weathering in surface water and carbonate weathering in groundwater intensify after rainy episodes.
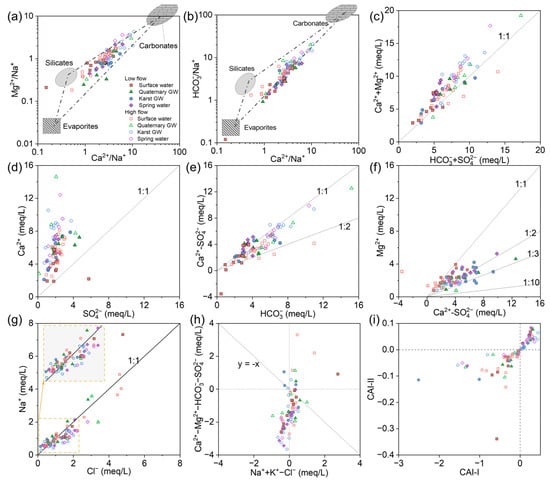
Figure 10.
(a–i) Stoichiometric relation among major ions of surface water and groundwater in the JSC.
Theoretically, the stoichiometric relation between [Ca2+ + Mg2+] and [HCO3− + SO42−] would follow 1:1 if carbonate and gypsum are the primary sources of corresponding ions. As shown in Figure 10c, water samples were predominantly spread along the 1:1 line, supporting the dominance of carbonate and gypsum dissolution. Particularly, considerable samples fell close to the upper left side of the 1:1 line, showing the excess of [Ca2+ + Mg2+], which could be related to silicate weathering. Reverse cation exchange could also lead to excessive [Ca2+ + Mg2+] over [HCO3− + SO42−]. This process usually occurs in the low-permeability zones (like clay) or the immobile zones within the local fracture system, which can be facilitated by the storage of sodium-rich water [77]. And in the plot of Ca2+ versus SO42−, most samples were concentrated along the upper left portion of the 1:1 line (Figure 10d). It seems that gypsum dissolution was not the primary factor affecting water chemistry in the study area, but it did have some effect, possibly acting as a weathering agent for the carbonate rocks.
In Figure 10e, most of the water samples were distributed between the 1:1 and 1:2 lines, suggesting the combined impact of calcite and dolomite dissolution. The congruent dissolution of calcite and dolomite was an important process affecting water chemistry [78]. Since calcite dissolution yields only Ca2+, while dolomite dissolution yields both Ca2+ and Mg2+, the ratio of Mg2+ to Ca2+ can reflect the relative contributions of calcite and dolomite to water mineralization. According to Equation (4), the ratio of Mg2+ versus non-gypsum sourced Ca2+ (i.e., Ca2+–SO42−) should be 1:1 when only dolomite dissolves, 1:2 when calcite and dolomite dissolve in equal amounts, and less than 1:10 when only calcite dissolves. In Figure 10f, surface water samples mainly fell between the lines of 1:1 and 1:2, indicating the dominance of dolomite dissolution in the surface water environment. By contrast, groundwater samples were mostly plotted between the 1:2 and 1:10 lines, illustrating the predominance of calcite dissolution in underground aquifers. Furthermore, several samples were spread out over the 1:1 line, showing an excess of Mg2+ over Ca2+. This could be related to dedolomitization, an irreversible process that is often observed in karst water systems containing evaporite sulfate [79,80]. Gypsum or anhydrite dissolution adds Ca2+ to the water, which may cause oversaturation and precipitation of calcite, thereby reducing Ca2+ and HCO3− and further promoting dolomite dissolution. The net result is the transformation of dolomite to calcite in the aquifer matrix and increasing Mg2+ and SO42− levels in the water. The significant correlations between SIgypsum and Mg2+ in surface and groundwater support this explanation (Figure S5). Moreover, SIgypsum and Mg2+ showed higher correlation coefficients in October than May, suggesting that there is more intensive dedolomitization in the surface–groundwater system of the JSC after rain episodes.
As halite dissolution releases equal amounts of Na+ and Cl−, Na/Cl will be close to 1 if Na+ derives from halite. In Figure 10g, the majority of data were distributed in the lower left corner and along the 1:1 line, illustrating that halite was the primary source of low Na+ and Cl− levels. Furthermore, several samples were markedly biased above the 1:1 line, indicating additional contributions of Na+ from other sources. Silicate weathering, hydrolysis of Na-bearing minerals (e.g., mirabilite, feldspar, muscovite), and cation exchange can cause excess Na+ over Cl−. Anthropogenic input can also lead to excessive Na+ in the water [81].
Ion exchange also affects the water chemistry of a region. The relationship of [Ca2+ + Mg2+-HCO3−-SO42−] versus [Na+ + K+-Cl−] is often used to identify the cation exchange [82]. The chloro-alkaline indices (CAIs) were applied to further assess the ion exchange reactions between water and its host rock [83]. The indices can be calculated using the following formula:
where all ions are given in meq/L. A CAI < 0 indicates cation exchange, with Ca2+ (or Mg2+) being absorbed by the rocks and Na+ (or K+) being released into the water, whereas a CAI > 0 indicates that the Na+ (or K+) in the water is replaced by the Ca2+ (or Mg2+) from the rocks. Figure 10i shows that surface water samples primarily fell into the CAI < 0 area, while GW samples were mainly located in the CAI > 0 area. This discrepancy is particularly noticeable during high-flow conditions. After rainy episodes, the groundwater level may rise, resulting in the replenishment of surface water and an increase in the Ca2+ content in rivers or lakes. As a consequence, this promotes cation exchange in favorable locations. On the other hand, the large amount of Na+ in surface runoff could be transferred to the aquifer through the epikarst and sinking zones, facilitating the reverse cation exchange between groundwater and the aquifer matrix.
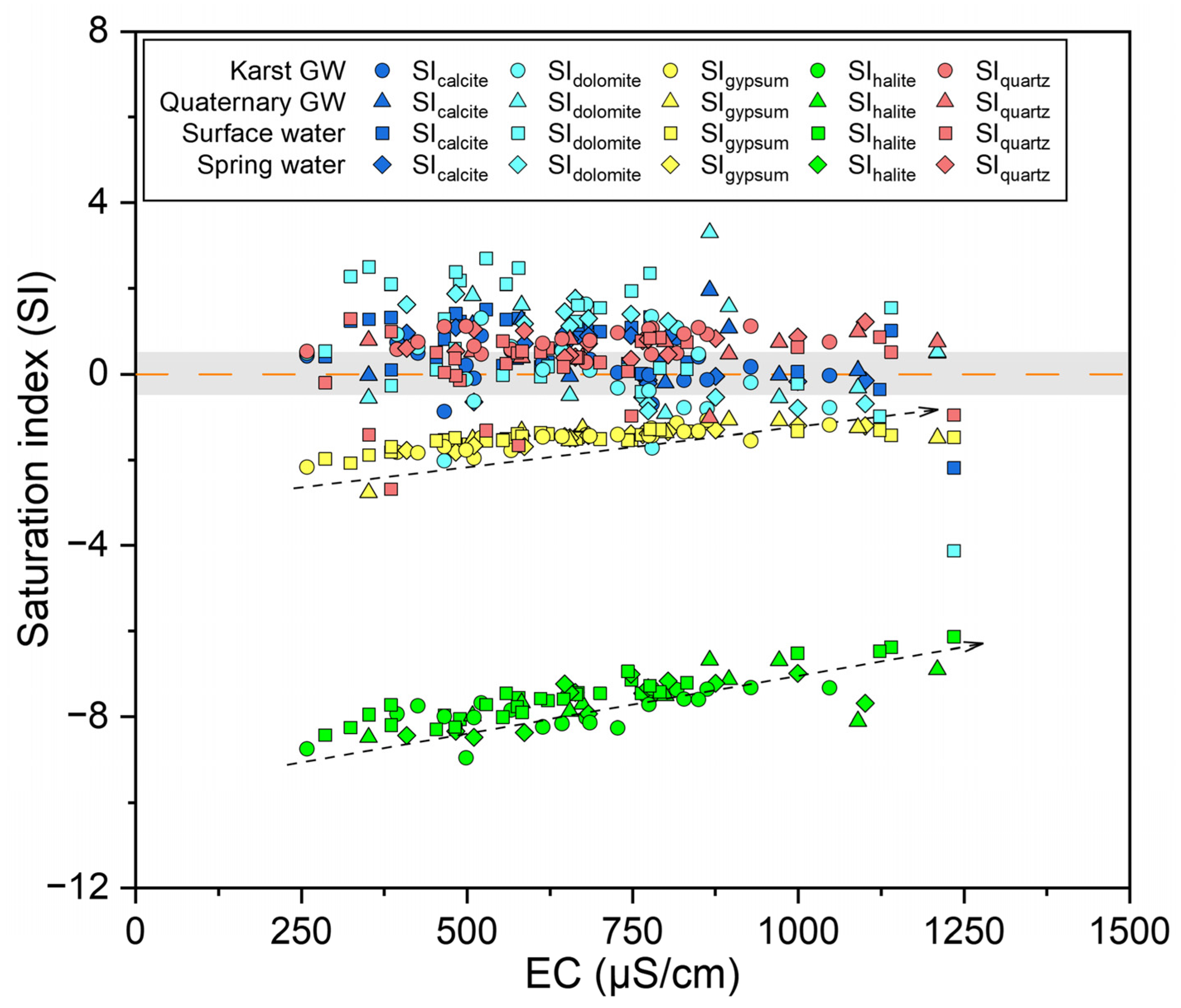
Comprehending hydrogeochemical processes relies on the grasp of mineral dissolution and precipitation. The saturation index (SI) values are commonly employed to assess the saturation states of various mineral phases in water [84]. A SI > 0 or SI < 0 implies that the corresponding mineral is either oversaturated or undersaturated. As depicted in Figure 11, the SIhalite and SIgypsum values for all water samples were below zero, indicating that halite and gypsum were unsaturated and tended to dissolve in water. Furthermore, SIhalite and SIgypsum increased slightly with EC, suggesting that halite and gypsum played a joint role in the mineralization of water within the study area (Figure 11). In contrast, SIcalcite and SIdolomite had values over zero for considerable samples, suggesting a prevalence of calcite and dolomite oversaturation. However, it should be noted that there were still certain samples at undersaturation or near equilibrium. Moreover, SIcalcite and SIdolomite decreased somewhat with EC and displayed a tendency to precipitate (Figure 11). This finding implies that the dissolution of calcite and dolomite has already affected the hydrochemistry of the JSC, which could also be seen from the dominance of Ca-HCO3 or Ca·Mg-HCO3 types in hydrochemical facies. Furthermore, the majority of SIquartz was distributed above the line of SI = 0 except for a few surface water samples, indicating that quartz was well-precipitated in the water (Figure 11). In order to appreciate the seasonal variation in mineral dissolution and precipitation, the statistical results of SI values are presented in Figure S6. Both SIcalcite and SIdolomite showed an obvious decline from low-flow to high-flow conditions, possibly due to the dilution effect caused by increasing discharge. And an enhancement in carbonate dissolution can be inferred from the rising logPCO2 from low- to high-flow conditions (Figure S6). Additionally, SIgypsum and SIquartz exhibited a modest rise from low- to high-flow conditions, indicating that the dissolution of gypsum and quartz became stronger in response to rising flow conditions.
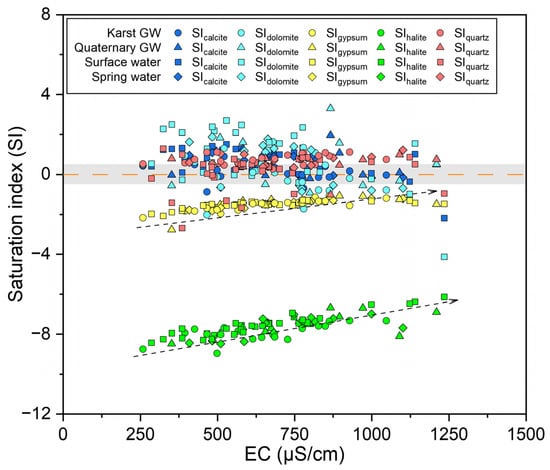
Figure 11.
Saturation index of different minerals for surface and groundwater samples.
The above findings reveal that mineral dissolution, CO2 dissolution/exsolution, and cation exchange are the main hydrogeochemical processes controlling the water chemistry in the JSC. Calcite and dolomite are the major mineral phases, followed by gypsum, halite, and quartz. However, such patterns may vary in space and time due to the variable nature of lithological and hydrogeological conditions in karst regions, as well as anthropogenic interferences.
5.3.2. Anthropogenic Influences
A number of global locations are undergoing massive industrialization and urbanization, leading to human activities having a non-negligible impact on the chemical composition of water [85,86]. The JSC has seen rapid population and economic growth in recent decades, along with a profound rise in sewage, landfills, and human-induced pollutants. Hence, it is critical to identify anthropogenic influences when evaluating the controlling mechanisms of hydrochemistry in the JSC.
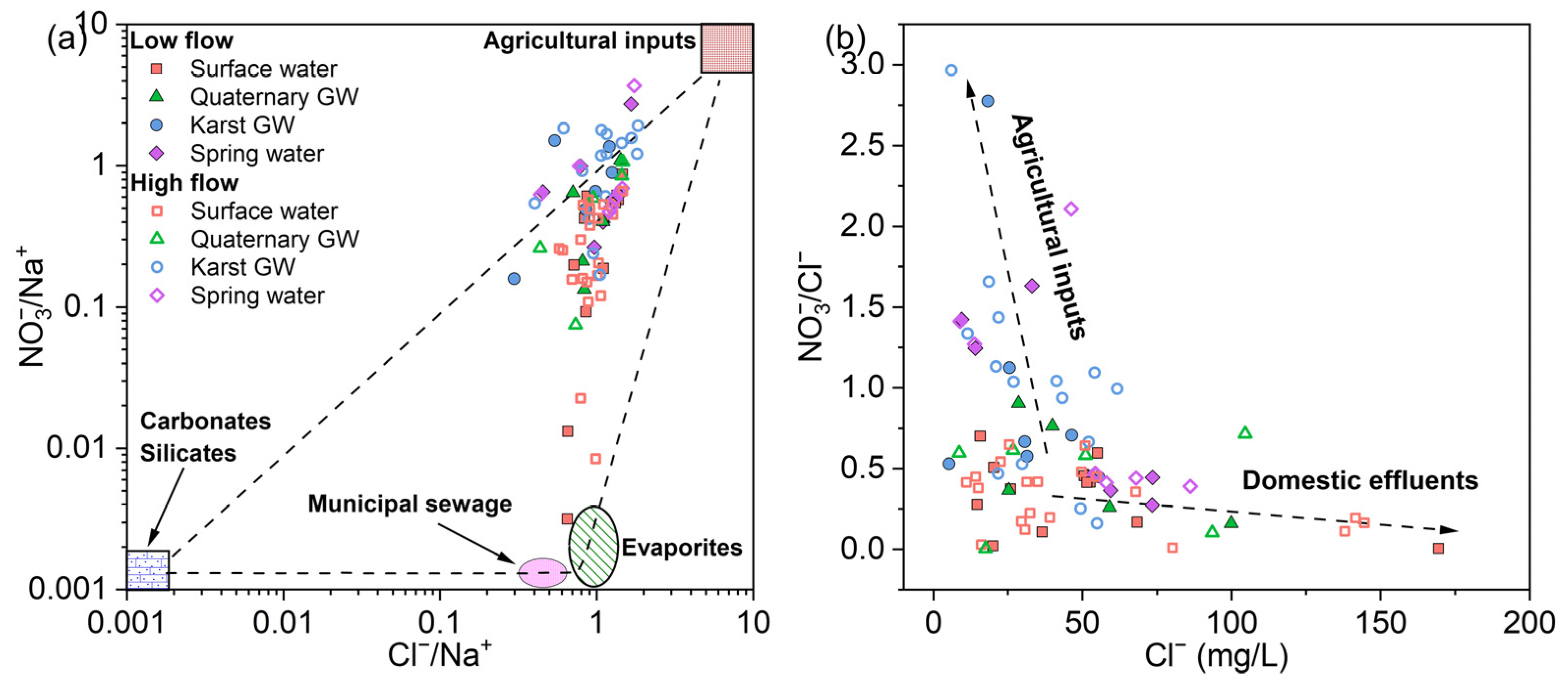
According to Figure 5 and Table 1, karst GW and spring water had relatively higher NO3− levels. This could be indicative of anthropogenic NO3− sources associated with land use, such as agricultural fertilizer and domestic sewage. In the JSC, the dominant land use pattern is woodland (36.6%), arable land, and build-up area [87], which were consistent with the observations during the field survey and sampling. Furthermore, the intricate surface–groundwater interaction, coupled with rapid groundwater velocity and poor filtration in the epikarst, makes the karst aquifer vulnerable to human contamination [88]. Therefore, the high NO3− levels found in the karst aquifer were most likely caused by anthropogenic nitrogen inputs coming from the large areas of arable land and dense human settlements in the study area. Elevated molar ratios of Cl−/Na+ or NO3−/Na+ are typical characteristics of human-contaminated water [89]. As shown in Figure 12a, a large number of samples were located near the upper right corner. This suggests that agricultural activities are the main source of nitrate in the surface–groundwater system of JSC, whereas the input from municipal sewage is minor. Agricultural inputs (e.g., fertilizers) generally have high NO3− contents and low Cl− contents, while domestic effluents such as human excreta, animal excrement, and septic sewage show high Cl− values and low NO3−/Cl− ratios [90]. In Figure 12b, karst GW exhibited relatively higher NO3−/Cl− ratios with lower Cl− concentrations, suggesting the dominance of agricultural inputs on nitrate pollution in the karst aquifer. In contrast, surface water and Quaternary GW samples displayed lower NO3−/Cl− ratios, reflecting the impact of domestic effluents. The anthropogenic impacts on nitrate and chloride might be particularly noticeable during high-flow conditions, as shown by the significantly positive correlation between NO3− and Cl− (p < 0.05, Figure 9). Notably, there was a marked discrepancy in spring water samples; urban springs had lower NO3−/Cl− ratios and higher Cl− contents, whereas mountain springs showed the opposite trend. This distinction may suggest that the nitrate in these springs comes from different sources. And this result also reflects the spatial variability of major nitrate sources across the study area. Furthermore, denitrification is supposed to be minor given that all samples had over 3 mg/L of dissolved oxygen—an aerobic environment.
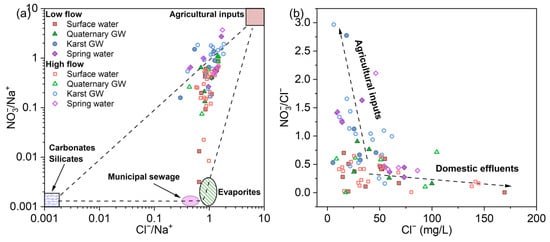
Figure 12.
Plots of (a) Cl−/Na+ versus NO3−/Na+ and (b) Cl− versus NO3−/Na+ molar ratios illustrating the possible human influences on the sampled waters.
The use of synthetic nitrogen fertilizer and animal manure for farming has long been blamed for increasing the risk of NO3− contamination [91,92]. When more fertilizers are applied than the crop can absorb, excess ammonium (the main ingredient in nitrogen fertilizer) is stored in the soil. In aerobic environments, soil microbes convert ammonium into nitrate by nitrification [93], as described below:
Step I: 2NH4+ + 3O2 → 2NO2− + 2H2O + 4H+
Step II: 2NO2− + O2 → 2NO3−
Note that H+ is produced during nitrification, which may reduce the pH level and promote carbonate dissolution. This could partly explain the negative correlations between NO3− and pH and the positive correlations between NO3− and HCO3− in surface water samples (Figure 9), which in turn confirm the occurrence of the nitration process in rivers or streams. Therefore, the significant correlations found between NO3− and Ca2+, and SO42− in surface water may be associated with the use of Ca(NO3)2 and (NH4)2SO4 fertilizers. And the observed strong correlations between NO3− and Na+ and K+ may support the notion that anthropogenic activities also had an impact on Na+ and K+ levels in the study area. This agrees with prior reports that the main human-influenced ions in the JSC are NO3−, Cl−, and SO42−, followed by Na+ and K+ [41,71]. In karst areas, surface pollutants like nutrients and pesticides can be injected straight into deep aquifers due to the high degree of surface–groundwater interaction and the rapid infiltration nature of karst landscapes [94]. Hence, the elevated NO3− levels in groundwater in October may be because rainfall mobilized the nitrate that had accumulated in the epikarst and flushed it to the aquifer through the fissures and conduits. And this process counteracted the dilution effect due to rising flows. The observed significant correlation between NO3− and EC in groundwater during high-flow conditions supports this explanation (Figure 9), which means that groundwater mineralization increases with nitrate concentrations. In addition, surface water showed no significant increase in NO3− (Figure 5), indicating that the nitrate inputs from leaking sewers may be rather stable or masked by the dilution effect. Overall, it is likely that the combination of precipitation and leakage aggravates nitrate pollution in karst GW and spring water, whereas, on the other hand, it decreases the nitrate burden in surface water and Quaternary GW. This hypothesis fits with recent studies that have reported the impact of surface water leakage and antecedent rainfall on NO3− concentrations in karst regions [95].
It is worth noting that the NO3− concentrations observed in this study are higher than those reported by Wang (2016) [39], who found that deep aquifers had NO3− contents below 15 mg/L and received little recharge from upper unconfined aquifers. According to the declining trend in nitrogen fertilizer consumption in Jinan over the past two decades (SPBS, 2000–2021 [96]; Figure S7), the alarming NO3− levels in karst GW may not be a recent occurrence but rather a residual consequence of prior nitrate pollution. Given the long history of farming activities in the JSC, agricultural practices such as the application of synthetic fertilizers and animal manure for tillage and the post-dumping of crop residues could have already raised the soil nitrate contents in soil or water. The accumulated nitrate loads in the epikarst, together with the practice of traditional flood irrigation, would contribute to elevated groundwater NO3− concentrations. Furthermore, extensive groundwater pumping may alter the previous hydrodynamic conditions, enhancing the downward leakage and irrigation return flow. Consequently, this could result in an increased NO3− input to the groundwater or perhaps cause contamination. On the other hand, the NO3− enrichment in karst aquifers is hard to eliminate given the relative lack of microbes in confined aquifers [97]. Therefore, the increase in nitrate levels in the JSC is probably due to the combined effect of local hydrogeological settings and cumulative anthropogenic activities.
5.4. Hydrogeochemical Evolution during Flow Paths
The inverse geochemical simulation was used to ascertain the water–rock interactions and the hydrogeochemical evolution as groundwater flows from the southern mountainous area to the northern urban area. Two flow paths were selected to simulate the groundwater geochemical processes based on the groundwater flow direction in the study area. The water samples collected along the flow paths include J09, J01, J10, and J24 in May and JN53, JN83, JN48, and JN39 in October, which correspond to the section locations in Figure 4. Based on the formation lithology of the study area, the preceding discussion of groundwater chemistry, and mineral SI values, the mineral phases for inverse modeling were determined. Calcite, dolomite, and gypsum were considered to quantify carbonate dissolution and precipitation, sulfate dissolution, and dedolomitization. Halite and quartz dissolution were also anticipated, as mentioned above, and therefore included in the model. Furthermore, CO2 gas was expected in water flow and added to the flow path since the studied surface and groundwater in the JSC generally evolve under open carbonated system conditions. The inverse modeling results using PHREEQC software are presented in Table 3.

Table 3.
Results for the inverse geochemical modeling of selected water samples, showing molar transfer in the mineral properties along the flow paths (unit: mmol/L).
As a typical karst fissure water system in North China, the JSC is characterized by large-scale and deep burial with low flow velocities and long flow paths, causing more adequate water–rock interactions [98]. Water chemistry in karst regions is the result of the water–carbonate rock–CO2 gas–aquatic organism interaction, significantly impacted by soil CO2 and rainfall [98]. Therefore, the main drivers of hydrogeochemical evolution in the study area may be climate (rainfall and temperature) and topography. During low-flow conditions, the groundwater flow along Path A exhibited the dissolution of calcite, dolomite, gypsum, and halite and the precipitation of quartz. The hydrogeochemical processes were dominated by the dissolution of carbonate and evaporite minerals. From IRA to DRA, the relatively long flow path and groundwater residence time allow for extensive water–rock interaction, which results in substantial mineral dissolution and CO2 uptake during water flow. In the case of Path B, the groundwater flow dissolved halite and gypsum and precipitated calcite, dolomite, and quartz, accompanied by the degassing of CO2 (Table 3). Groundwater with high PCO2 issuing as springs and resurgent streams will lose CO2 as it approaches equilibrium with the atmosphere [99,100]. In DA, groundwater overflows from the underground to the surface in the form of springs, causing a sudden drop in air pressure, which could lead to massive outgassing of CO2 and substantial precipitation of carbonates from the groundwater. Therefore, the hydrogeochemical evolution from DRA to DA showed a predominant occurrence of evaporite dissolution and carbonate precipitation. Additionally, dedolomitization caused gypsum dissolution and calcite precipitation. Furthermore, quartz remains in a precipitated state in both flow paths, implying its minor influence on water chemistry. During high-flow conditions, the hydrogeochemical processes along Path A included the dissolution of calcite, dolomite, halite, and gypsum, the precipitation of quartz, and the dissolution of CO2. This observation was basically the same as that during low-flow conditions, most likely the result of long-term interaction between groundwater and the surrounding environment, indicating the essential role of lithology and topography in hydrogeochemical evolution from IRA to DRA. As for groundwater flow along Path B, the hydrogeochemical processes mainly involved the precipitation of calcite, the dissolution of dolomite, halite, quartz, and gypsum, and the absorption of CO2. It seems that the hydrogeochemical evolution of the DRA to the DA in October involved more dissolved minerals compared to that in May, suggesting more complex and intense water–rock interactions due to enhanced flow conditions.
The inverse modeling demonstrates that the hydrogeochemical evolution in the JSC is closely linked to the dissolution and precipitation of carbonate, as well as the dissolution of halite and gypsum and the effects of dedolomitization. However, the main dissolved mineral phases may vary in different flow paths and flow conditions.
5.5. Conceptual Model and Environmental Implications
Karst groundwater is particularly susceptible to degradation because of frequent surface–groundwater interaction, rapid groundwater flow velocities, and inadequate filtration through the high-permeability networks of fissures and conduits [62]. Human activity exacerbates this vulnerability; many karst regions are experiencing large population growth, urbanization, and industrialization, together with increases in wastewater, landfills, and other sources of anthropogenic pollution [1]. A conceptual model for water sources and hydrogeochemical evolution in the JSC was proposed to comprehend the karst water flow system in the context of human activity, based on isotopic and hydrochemical analyses (Figure 13).
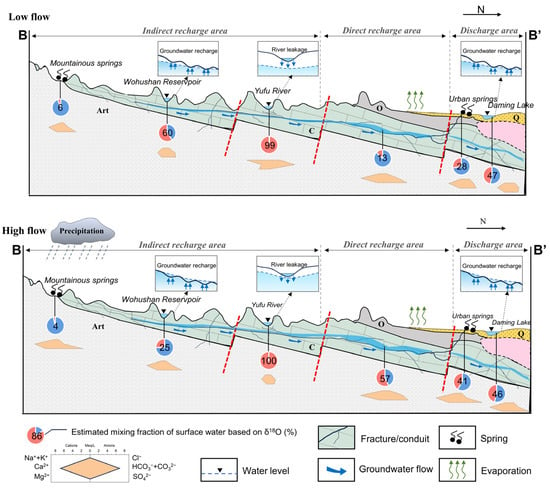
Figure 13.
Idealized conceptual model of the Jinan Spring Catchment showing the surface–groundwater interaction and hydrogeochemical evolution under different hydrological conditions.
The JSC covers the indirect recharge area (IRA), direct recharge area (DRA), and discharge area (DA), stretching from steep mountainous areas to the alluvial plain of the Yellow River. The isotopic signatures of surface water, karst GW, and spring water display obvious seasonal and spatial variance, reflecting the recharge–discharge relationship and the regional hydrologic cycle in this area (Figure 7). Atmospheric precipitation from Mount Tai is the principal source for groundwater and surface water in the IRA, which shows comparatively lower isotopic values given the temperature and altitude effects of precipitation isotopes. Groundwater flows from south to north along the topographic gradient and overflows as springs in the DA due to blocks of magmatic rocks. Apart from springs, karst GW also discharges via artificial exploitation, vertical flow into the Quaternary aquifer, and recharge to rivers and lakes. Remarkably, springs in different areas of the catchment exhibit varying isotopic compositions, indicating the influence of different water sources. The mountainous springs showed lighter δ18O and δ2H values, reflecting recharge from fresh precipitation and less evaporation due to rapid infiltration through karst features. The urban springs exhibited heavier δ18O and δ2H values, similar to the Yufu River, suggesting replenishment from river leakage. Moreover, the highly permeable formations cause frequent interactions between surface and groundwater in karst regions, which may vary throughout different sites or seasons due to the varying geological backgrounds and hydrological conditions. The results of end-member mixing analysis revealed temporal and spatial variability of surface–groundwater interactions in the JSC (Figures S3 and S4). The IRA is located in the southern mountainous area with a shallow groundwater table depth. In this area, groundwater samples showed lower mixing fractions (fSW) than those in the direct recharge and discharge areas, due to hydrological and topographic conditions. A relatively shallower groundwater table depth may retard and dilute the arrival of leakage from rivers, and this is more pronounced when the groundwater level rises during high-flow conditions [13]. Additionally, the steep slopes of the mountainous surroundings in the IRA make it unfavorable for surface runoff to infiltrate. Mountainous springs distributed in this area show poor hydraulic connections with surface water and remain relatively pristine. The DRA, on the other hand, lies in a piedmont-inclined plain with gentle terrain and numerous sinking zones, allowing massive surface water leakage. Recharge from sinkholes and sinking rivers is concentrated through dissolution-enlarged fissures and conduits and rapidly reaches the water table. Relatively higher fSW values were observed in groundwater near rivers, indicating the good hydraulic connection between the river and aquifer, accompanied by the strong infiltration capacity of the riverbed. As for the DA, karst GW typically discharges in the form of springs or into gaining streams. Urban springs in this area show steady hydraulic linkages to the Yufu River and obtain significant replenishment through riverbed leakage, particularly during high-flow conditions. Furthermore, the Yufu River is regulated by Wohushan Reservoir and intercepted for irrigation and fisheries, which could affect the surface–groundwater interaction in the JSC. During low-flow conditions, the Wohushan Reservoir stores water, resulting in lower river levels downstream, which is not conducive to river seepage. As a result, the springs and karst GW had lower fSW values. During high-flow conditions, the reservoir discharges the prior-stored water, leading to increased water levels in downstream rivers and enhanced river leakage in the sinking zone of the Yufu River. Urban springs and some karst GW near rivers showed a noticeable increase in fSW values at that time. Additionally, irrigation return flow becomes a non-negligible recharge source for karst aquifers due to the long-term overpumping of groundwater and the practice of traditional flood irrigation in the region. Moreover, springs may serve as recharge sites during floods and provide an additional source of surface water for karst aquifers, thereby impacting biogeochemical reactions [101].
In the JSC, karst GW flows from the hillslope to the urban area and generates deeper and slower groundwater runoff, resulting in extensive interactions between water and aquifer matrix rocks. Similar to most regions underlain by carbonate rock, bedrock architecture and meteorological conditions are the essential factors shaping the aqueous geochemistry in the JSC [69,70,102]. Local mineral dissolution, CO2 dissolution/exsolution, and cation exchange are the main hydrogeochemical processes that control the hydrogeochemical evolution in this region. The major mineral phases involved are calcite, dolomite, gypsum, halite, and quartz, in descending order. Along the flow path from south to north, ion chemistry accumulates, and the dominant anion phase changes from HCO3− to SO42− to Cl−. The increasing partial pressure of CO2 in surface and groundwater after rain episodes and the positive correlation of logPCO2-HCO3 reflect the local reactivity between recently generated runoff, aquifer matrix rocks, and the riverbed carbonate sediments (Figure 9). During high-flow conditions, rainfall causes the enrichment of CO2 in surface and groundwater, resulting in an increase in the aggressiveness of water and facilitating the dissolution of carbonate and evaporite rocks. During low-flow conditions, the aggressiveness of water decreases as CO2 degassing progresses, and as a result, mineral dissolution tends to decline (water becomes equilibrated with the atmosphere). The JSC involves urban and suburban areas; therefore, human activities are also influencing the water chemistry. Anthropogenic sources like domestic sewage, industrial wastewater, agricultural fertilizer, irrigation return flow, and surface runoff from farming can rapidly enter the aquifer through high-permeability networks of fissures and conduits, degrading groundwater quality in the study area [103]. The high degree of surface water–groundwater interaction in karst terrain can cause the injection of pollutants in surface water, such as pesticides and metals from runoff, directly into groundwater [104]. Rapid flow velocities and the geometry of voids can lead to turbulent flow, enabling the transport of contaminants [105]. The antecedent rainfall and land use are also important factors when exploring nutrient cycling and biogeochemical reactions in a karst water system [95].
Overall, our findings highlighted the variability of surface–groundwater interactions in the JSC across different locations and flowing conditions. This may have a significant impact on water chemistry, especially the transportation and transformation of nitrate, given the multiple anthropogenic influences. We recommend comprehensive groundwater management strategies that consider the hydrogeochemical evolution and flow dynamics of the JSC when coping with the complex water environment in this region.
6. Conclusions
In this study, stable isotopes, hydrochemical parameters, and data analysis methods were integrated to gain new insights into the origin and hydrogeochemical evolution of groundwater in the JSC in northern China. The isotopes of hydrogen and oxygen in surface water, karst GW, and spring water display obvious seasonal and spatial variance, illustrating the recharge–discharge relationship and the regional hydrologic cycle in this area. Groundwater in the region is principally derived from local precipitation and interacts with surface water during the flow from the indirect recharge area to the discharge area. Remarkably, spring water that outflows in different areas shows an obvious discrepancy in isotopic compositions, reflecting multiple water sources and intricate mixing processes. The mountainous springs had lighter δ18O and δ2H values, suggesting recharge from fresh precipitation (high altitude) and less evaporation due to rapid infiltration through karst features. The urban springs exhibited heavier δ18O and δ2H values because of additional replenishment from river leakage, signifying a close connectivity between surface water and aquifer. To assess the possible mixing processes in the JSC, an end-member mixing analysis (EMMA) with δ18O as the main conservative tracer was conducted. The mixing results showed that the mean contribution of the surface water in the mountainous springs was 6% and 4% during low-flow and high-flow conditions, respectively, indicating a poor hydraulic connection between mountainous springs and surface water. In other words, over 90% of the discharge from the main perennial springs in the indirect recharge area comes from the hosting karst aquifer, evidencing the pristine state of mountainous springs. In contrast, the mean contribution of surface water in urban springs was 28% and 41% during low-flow and high-flow conditions. Relatively gentle terrain and extensive development of sinking zones in the indirect recharge area make it conducive to infiltration of surface water. Therefore, urban springs could receive considerable replenishment from the surface water, especially during rising flow conditions after rain episodes; in an extreme case, up to 50% of the spring discharge was found to be from the sinking Yufu River. This observation reveals the hydraulic dependence of spring overflows on river leakage and its impact on the hydrochemistry of the spring water.
The karstic nature of lithology is the essential factor shaping the aqueous geochemistry of the JSC. Natural hydrogeochemical processes (including local mineral dissolution/precipitation, CO2 dissolution/exsolution, and cation exchange) determine the major ion chemistry of water and control its hydrogeochemical evolution. During high-flow conditions, rainfall causes the enrichment of CO2 and results in an increase in the aggressiveness of water, facilitating the dissolution of carbonate and evaporite minerals. During low-flow conditions, the aggressiveness of water decreases as CO2 degasses, leading to a decline in mineral dissolution. Anthropogenic influences, involving the discharge of domestic effluents and industrial wastewater, agricultural activities, and irrigation return flow, contribute to the degradation of water quality. Surface water showed low NO3− concentrations, whereas karst GW and spring water featured noteworthy NO3− levels, with low-flow averages of 45.6 mg/L and 45.0 mg/L and high-flow averages of 50.5 and 58.0 mg/L. Agricultural inputs are responsible for the nitrate contamination in the karst aquifer, while domestic sewage is the dominant source of nitrate in surface water. Additionally, the remarkable NO3− contents in the karst aquifer are likely a legacy problem of previous overuse of fertilizer and groundwater exploitation. Antecedent rainfall and land use patterns are also important factors when exploring the formation of localized nitrate pollution.
The results of this study reveal the hydraulic connectivity between urban springs and surface water and confirm that the local government’s efforts at artificial recharge have been effective in achieving the goal of maintaining perennial spring outflow. However, spring water quality degrades over time because of multiple water sources and anthropogenic impacts. More field investigations and high-precision groundwater flow and solute transport models are necessary to quantify the hydrological and human influences on karst GW. Urban rivers in karst regions require competent administration because of frequent surface–groundwater interactions and the substantial amount of anthropogenic input through conduits and fissures, particularly during high-flow conditions. Water management in the JSC should pay particular attention to the control and mitigation of groundwater nitrate pollution.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16060829/s1, Figure S1: Box plots for pH, EC, T, ORP, and DO in water samples during low flow and high flow conditions; Figure S2: Fraction of surface water (fSW) in the sampled waters during low and high flow conditions; Figure S3: Maps showing the fSW of the sampled waters during low and high flow conditions; Figure S4: Dendrogram showing the result of the R-type cluster; Figure S5. The relationship between the SIgyp and Mg2+ concentrations; Figure S6: Box plots for saturation index values of calcite (a), dolomite (b), gypsum (c), halite (d), quartz (e) and partial pressure of CO2 (f) in water samples; Figure S7: The annual consumption of nitrogen fertilizer, phosphate fertilizer, and potash fertilizer in Jinan City from 2000 to 2021, collected from the Shandong Statistical Yearbook (2001–2022).
Author Contributions
Conceptualization, Y.K. and X.S.; methodology, Y.K. and X.S.; formal analysis, Y.K.; investigation, L.Y.; resources, X.S.; data curation, Y.K.; writing—original draft preparation, Y.K.; writing—review and editing, Y.K. and X.S.; visualization, Y.K. and X.S.; supervision, X.S. and S.Y.; project administration, X.S.; funding acquisition, X.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 41730749.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank Yoshitaka Komatsu, Zekang He, Dongxu Yao, Yue Li, and Yiran Li for their assistance in the field work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Goldscheider, N.; Chen, Z.; Auler, A.S.; Bakalowicz, M.; Broda, S.; Drew, D.; Hartmann, J.; Jiang, G.; Moosdorf, N.; Stevanovic, Z.; et al. Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 2020, 28, 1661–1677. [Google Scholar] [CrossRef]
- Olarinoye, T.; Gleeson, T.; Marx, V.; Seeger, S.; Adinehvand, R.; Allocca, V.; Andreo, B.; Apaéstegui, J.; Apolit, C.; Arfib, B. Global karst springs hydrograph dataset for research and management of the world’s fastest-flowing groundwater. Sci. Data 2020, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.B.; Kurz, M.J.; Khadka, M.B. Climate control of decadal-scale increases in apparent ages of eogenetic karst spring water. J. Hydrol. 2016, 540, 988–1001. [Google Scholar] [CrossRef]
- Gutiérrez, F.; Parise, M.; De Waele, J.; Jourde, H. A review on natural and human-induced geohazards and impacts in karst. Earth-Sci. Rev. 2014, 138, 61–88. [Google Scholar] [CrossRef]
- An, L.; Hao, Y.; Yeh, T.-C.J.; Liu, Y.; Liu, W.; Zhang, B. Simulation of karst spring discharge using a combination of time–frequency analysis methods and long short-term memory neural networks. J. Hydrol. 2020, 589, 125320. [Google Scholar] [CrossRef]
- Leopold, M.; Gupanis-Broadway, C.; Baker, A.; Hankin, S.; Treble, P. Time lapse electric resistivity tomography to portray infiltration and hydrologic flow paths from surface to cave. J. Hydrol. 2021, 593, 125810. [Google Scholar] [CrossRef]
- Cholet, C.; Steinmann, M.; Charlier, J.-B.; Denimal, S. Characterizing fluxes of trace metals related to dissolved and suspended matter during a storm event: Application to a karst aquifer using trace metals and rare earth elements as provenance indicators. Hydrogeol. J. 2019, 27, 305–319. [Google Scholar] [CrossRef]
- Ni, M.; Li, S. Spectroscopic indices trace spatiotemporal variability of dissolved organic matter in a river system with Karst characteristic. J. Hydrol. 2020, 590, 125570. [Google Scholar] [CrossRef]
- Jiang, Y.; Cao, M.; Yuan, D.; Zhang, Y.; He, Q. Hydrogeological characterization and environmental effects of the deteriorating urban karst groundwater in a karst trough valley: Nanshan, SW China. Hydrogeol. J. 2018, 26, 1487–1497. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G.; Zhan, H.; Chen, X.; Liu, M.; Wang, M. Identification of hydrogeochemical processes and transport paths of a multi-aquifer system in closed mining regions. J. Hydrol. 2020, 589, 125344. [Google Scholar] [CrossRef]
- White, W.B. A brief history of karst hydrogeology: Contributions of the NSS. J. Cave Karst Stud. 2007, 69, 13–26. [Google Scholar]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Koit, O.; Barberá, J.A.; Marandi, A.; Terasmaa, J.; Kiivit, I.-K.; Martma, T. Spatiotemporal assessment of humic substance-rich stream and shallow karst aquifer interactions in a boreal catchment of northern Estonia. J. Hydrol. 2020, 580, 124238. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.-Y. Seasonal and interannual variations of hydrochemical characteristics and stable isotopic compositions of drip waters in Furong Cave, Southwest China based on 12 years’ monitoring. J. Hydrol. 2019, 572, 40–50. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Lian, J.; Fu, Z.; Nie, Y. Seasonal recharge of spring and stream waters in a karst catchment revealed by isotopic and hydrochemical analyses. J. Hydrol. 2020, 591, 125595. [Google Scholar] [CrossRef]
- Barberá, J.; Andreo, B. River-spring connectivity and hydrogeochemical interactions in a shallow fractured rock formation. The case study of Fuensanta river valley (Southern Spain). J. Hydrol. 2017, 547, 253–268. [Google Scholar] [CrossRef]
- Carrière, S.D.; Ruffault, J.; Cakpo, C.B.; Olioso, A.; Doussan, C.; Simioni, G.; Chalikakis, K.; Patris, N.; Davi, H.; MartinSt-Paul, N.K. Intra-specific variability in deep water extraction between trees growing on a Mediterranean karst. J. Hydrol. 2020, 590, 125428. [Google Scholar] [CrossRef]
- Filippini, M.; Squarzoni, G.; De Waele, J.; Fiorucci, A.; Vigna, B.; Grillo, B.; Riva, A.; Rossetti, S.; Zini, L.; Casagrande, G. Differentiated spring behavior under changing hydrological conditions in an alpine karst aquifer. J. Hydrol. 2018, 556, 572–584. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bicalho, C.C.; Batiot-Guilhe, C.; Seidel, J.; Van Exter, S.; Jourde, H. Geochemical evidence of water source characterization and hydrodynamic responses in a karst aquifer. J. Hydrol. 2012, 450, 206–218. [Google Scholar] [CrossRef]
- Musgrove, M.; Opsahl, S.P.; Mahler, B.J.; Herrington, C.; Sample, T.L.; Banta, J.R. Source, variability, and transformation of nitrate in a regional karst aquifer: Edwards aquifer, central Texas. Sci. Total Environ. 2016, 568, 457–469. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Yue, F.-J.; Lu, J.; Wang, Y.-C.; Qin, C.-Q.; Ding, H.; Xue, L.-L.; Li, S.-L. New insight into the response and transport of nitrate in karst groundwater to rainfall events. Sci. Total Environ. 2022, 818, 151727. [Google Scholar] [CrossRef]
- Mudarra, M.; Hartmann, A.; Andreo, B. Combining Experimental Methods and Modeling to Quantify the Complex Recharge Behavior of Karst Aquifers. Water Resour. Res. 2019, 55, 1384–1404. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.; Wang, W.; Zhu, C.; Chen, Y.; Liu, X.; Zhang, T. Water quality and interaction between groundwater and surface water impacted by agricultural activities in an oasis-desert region. J. Hydrol. 2023, 617, 128937. [Google Scholar] [CrossRef]
- Chu, H.B.; Wei, J.H.; Wang, R.; Xin, B.D. Characterizing the interaction of groundwater and surface water in the karst aquifer of Fangshan, Beijing (China). Hydrogeol. J. 2017, 25, 575–588. [Google Scholar] [CrossRef]
- Rugel, K.; Golladay, S.W.; Jackson, C.R.; Rasmussen, T.C. Delineating groundwater/surface water interaction in a karst watershed: Lower Flint River Basin, southwestern Georgia, USA. J. Hydrol. Reg. Stud. 2016, 5, 1–19. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, X.; Zhao, C.; Tang, C.; Shen, H.; Wang, Z.; Wang, Y. Review: Characterization, evolution, and environmental issues of karst water systems in Northern China. Hydrogeol. J. 2018, 26, 1371–1385. [Google Scholar] [CrossRef]
- Li, C. Analysis on karst resources and preservation of famous springs in Jinan. Carsologica Sin. 1985, 4, 37–45, (In Chinese with English Abstract). [Google Scholar]
- Kang, F.X.; Jin, M.G.; Qin, P.R. Sustainable yield of a karst aquifer system: A case study of Jinan springs in northern China. Hydrogeol. J. 2011, 19, 851–863. [Google Scholar] [CrossRef]
- Guan, Q.; Li, F.; Wang, A.; Feng, P.; Tian, C.; Chen, X.; Liu, D. Hydrochemistry characteristics and evolution of karst spring groundwater system in Jina. Carsologica Sin. 2019, 38, 653–662, (In Chinese with English Abstract). [Google Scholar]
- Qian, J.; Zhan, H.; Wu, Y.; Li, F.; Wang, J. Fractured-karst spring-flow protections: A case study in Jinan, China. Hydrogeol. J. 2006, 14, 1192–1205. [Google Scholar] [CrossRef]
- Luo, Q.; Yang, Y.; Qian, J.; Wang, X.; Chang, X.; Ma, L.; Li, F.; Wu, J. Spring protection and sustainable management of groundwater resources in a spring field. J. Hydrol. 2020, 582, 124498. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Xiang, H.; Gai, Y.; Li, F. Relationship between Groundwater in Western Jinan and Jinan Spring Area Based on Correlation Degree of Water Table Fluctuation. J. China Hydrol. 2018, 38, 31–36, 96. [Google Scholar]
- Wang, G.-F.; Wu, Y.-X.; Lu, L.; Li, G.; Shen, J.S. Investigation of the geological and hydrogeological environment with relation to metro system construction in Jinan, China. Bull. Eng. Geol. Environ. 2019, 78, 1005–1024. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, J.; Xu, X.; Wang, Q.; Wang, M.; Feng, J.; Fu, T. Temporal variations of spring water in karst areas: A case study of Jinan Spring Area, Northern China. Water 2020, 12, 1009. [Google Scholar] [CrossRef]
- Guo, Y.; Qin, D.; Sun, J.; Li, L.; Li, F.; Huang, J. Recharge of River Water to Karst Aquifer Determined by Hydrogeochemistry and Stable Isotopes. Water 2019, 11, 479. [Google Scholar] [CrossRef]
- Qi, X.; Wang, Y.; Yang, L.; Liu, Z.; Wang, W.; Li, W. Time lags variance of groundwater level response to precipitation of Jinan karst spring watershed in recent 50 years. Carsologica Sin. 2016, 35, 384–393, (In Chinese with English Abstract). [Google Scholar]
- Chi, G.; Xing, L.; Zhu, H.; Hou, X.; Xiang, H.; Xing, X. The study of quantitative relationship between the spring water and the dynamic change of the atmospheric precipitation in Jinan. Ground Water 2017, 39, 8–11, (In Chinese with English Abstract). [Google Scholar]
- Wang, J.L.; Jin, M.G.; Jia, B.J.; Kang, F.X. Hydrochemical characteristics and geothermometry applications of thermal groundwater in northern Jinan, Shandong, China. Geothermics 2015, 57, 185–195. [Google Scholar] [CrossRef]
- Wang, J.L.; Jin, M.G.; Lu, G.P.; Zhang, D.; Kang, F.X.; Jia, B.J. Investigation of discharge-area groundwaters for recharge source characterization on different scales: The case of Jinan in northern China. Hydrogeol. J. 2016, 24, 1723–1737. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, O.; Qin, D.; Teng, Z.; Dong, Y. Factors Controlling Hydrochemical Characteritics of Karstic Water in Jinan. China Rural Water Hydropower 2012, 7, 31–37, (In Chinese with English Abstract). [Google Scholar]
- Xing, L.; Zhou, J.; Song, G.; Xing, X. Mixing ratios of recharging water sources for the four largest spring groups in Jinan. Earth Sci. Front. 2018, 25, 260–272, (In Chinese with English Abstract). [Google Scholar]
- Wang, Q.; Duan, X.; Gao, Z.; Xu, H.; Yin, X.; Li, W.; Zhou, Y. Reginal groundwater level monitoring in Jinan Karstic Spring Basin. Hydrogeol. Eng. Geol. 2007, 12, 1–7, (In Chinese with English Abstract). [Google Scholar]
- Sun, B.; Peng, Y.; Li, C.; Lin, G. Division of Karst Water System and Hydraulic Connection of Typical Spring Fields in Jinan City. Shandong Land Resour. 2016, 32, 31–34, (In Chinese with English Abstract). [Google Scholar]
- Hong-hai, L.; Cheng, Z. The Variations of Groundwater Quality and Its Relationship with Human Activity. Res. Soil Water Conserv. 2007, 24, 238–240, (In Chinese with English Abstract). [Google Scholar]
- JWRB (Jinan Water Resources Bureau); JURWAB (Jinan Urban and Rural Water Affairs Bureau). Water Resources Bulletin of Jinan; JWRB/JURWAB: Jinan, China, 2017. (In Chinese) [Google Scholar]
- Parkhurst, D.L.; Appelo, C. Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geol. Surv. Tech. Methods 2013, 6, 497. [Google Scholar]
- Mendiguchía, C.; Moreno, C.; García-Vargas, M. Evaluation of natural and anthropogenic influences on the Guadalquivir River (Spain) by dissolved heavy metals and nutrients. Chemosphere 2007, 69, 1509–1517. [Google Scholar] [CrossRef]
- Clark, I. Groundwater Geochemistry and Isotopes; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Gabrovšek, F.; Dreybrodt, W. Role of mixing corrosion in calcite-aggressive H2O-CO2-CaCO3 solutions in the early evolution of karst aquifers in limestone. Water Resour. Res. 2000, 36, 1179–1188. [Google Scholar] [CrossRef]
- Romanov, D.; Gabrovsek, F.; Dreybrodt, W. The impact of hydrochemical boundary conditions on the evolution of limestone karst aquifers. J. Hydrol. 2003, 276, 240–253. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Duan, S.; Doody, T.R.; Haq, S.; Smith, R.M.; Johnson, T.A.N.; Newcomb, K.D.; Gorman, J.; Bowman, N.; Mayer, P.M. Human-accelerated weathering increases salinization, major ions, and alkalinization in fresh water across land use. Appl. Geochem. 2017, 83, 121–135. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality; WHO chronicle 2011; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, X.; Yuan, G.; Sun, X.; Liu, X.; Wang, S. Characteristics of δ18O in precipitation over Eastern Monsoon China and the water vapor sources. Chin. Sci. Bull. 2010, 55, 200–211. [Google Scholar] [CrossRef]
- Cartwright, I.; Weaver, T.; Cendón, D.I.; Swane, I. Environmental isotopes as indicators of inter-aquifer mixing, Wimmera region, Murray Basin, Southeast Australia. Chem. Geol. 2010, 277, 214–226. [Google Scholar] [CrossRef]
- Pang, Z.; Kong, Y.; Li, J.; Tian, J. An isotopic geoindicator in the hydrological cycle. Procedia Earth Planet. Sci. 2017, 17, 534–537. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Segura, C.; Noone, D.; Warren, D.; Jones, J.A.; Tenny, J.; Ganio, L.M. Climate, landforms, and geology affect baseflow sources in a mountain catchment. Water Resour. Res. 2019, 55, 5238–5254. [Google Scholar] [CrossRef]
- Xi, D.Y.; Li, X.Z.; Shao, Z. Hydrogeological Survey Report of Keeping Spring Spurting and Water Supply in Jinan City, Shandong Province. 1988. Available online: https://www.ngac.cn/dzzlfw_sjgl/d2d/dse/category/detail.do?method=cdetail&_id=102_81672&tableCode=ty_qgg_edmk_t_ajxx&categoryCode=dzzlk (accessed on 10 January 2024).
- Guo, Y.; Qin, D.; Li, L.; Sun, J.; Li, F.; Huang, J. A Complicated Karst Spring System: Identified by Karst Springs Using Water Level, Hydrogeochemical, and Isotopic Data in Jinan, China. Water 2019, 11, 947. [Google Scholar] [CrossRef]
- Bakalowicz, M. Karst groundwater: A challenge for new resources. Hydrogeol. J. 2005, 13, 148–160. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Wang, W.; Zhang, X.; Zhang, X.; Jiang, C.; Wang, Y. Hydro-biogeochemical processes of surface water leakage into groundwater in large scale karst water system: A case study at Jinci, northern China. J. Hydrol. 2021, 596, 125691. [Google Scholar] [CrossRef]
- Drever, J.I. The Geochemistry of Natural Waters: Surface and Groundwater Environments; Wiley Press: Prentice Hall, NJ, USA, 1997; p. 436. [Google Scholar]
- Yuan, R.; Wang, M.; Wang, S.; Song, X. Water transfer imposes hydrochemical impacts on groundwater by altering the interaction of groundwater and surface water. J. Hydrol. 2020, 583, 124617. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Yuan, R.; Zhang, Y.; Long, X. Deep groundwater circulation in a syncline in Rucheng County, China. J. Hydrol. 2022, 610, 127824. [Google Scholar] [CrossRef]
- Singh, A.K.; Hasnain, S.I. Major ion chemistry and weathering control in a high altitude basin: Alaknanda River, Garhwal Himalaya, India. Hydrol. Sci. J. 1998, 43, 825–843. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Wang, L.; Shi, L.; Song, X.; Yeh, T.-C.J.; Zhen, P. Coupling hydrochemistry and stable isotopes to identify the major factors affecting groundwater geochemical evolution in the Heilongdong Spring Basin, North China. J. Geochem. Explor. 2019, 205, 106352. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Li, C.; Gao, Z.; Chen, S. Characterization of the hydrochemistry of water resources of the Weibei Plain, Northern China, as well as an assessment of the risk of high groundwater nitrate levels to human health. Environ. Pollut. 2021, 268, 115947. [Google Scholar] [CrossRef]
- Rajmohan, N.; Elango, L. Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ. Geol. 2004, 46, 47–61. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, J.; Yang, L.; Wu, S.; Dai, E. The interactive effects of elevation, precipitation and lithology on karst rainfall and runoff erosivity. Catena 2021, 207, 105588. [Google Scholar] [CrossRef]
- Class, H.; Bürkle, P.; Sauerborn, T.; Trötschler, O.; Strauch, B.; Zimmer, M. On the Role of Density-Driven Dissolution of CO2 in Phreatic Karst Systems. Water Resour. Res. 2021, 57, e2021WR030912. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, M.; Cao, M.; Huang, X.; Zhang, Z.; Zhang, L. Sources and behaviors of dissolved sulfate in the Jinan karst spring catchment in northern China identified by using environmental stable isotopes and a Bayesian isotope-mixing model. Appl. Geochem. 2021, 134, 105109. [Google Scholar] [CrossRef]
- Panagopoulos, G.; Lambrakis, N.; Tsolis-Katagas, P.; Papoulis, D. Cation exchange processes and human activities in unconfined aquifers. Environ. Geol. 2004, 46, 542–552. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allegre, C. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Zaidi, F.K.; Nazzal, Y.; Jafri, M.K.; Naeem, M.; Ahmed, I. Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: A case study from northwestern Saudi Arabia. Environ. Monit. Assess. 2015, 187, 607. [Google Scholar] [CrossRef]
- Jia, H.; Qian, H.; Zheng, L.; Feng, W.; Wang, H.; Gao, Y. Alterations to groundwater chemistry due to modern water transfer for irrigation over decades. Sci. Total Environ. 2020, 717, 137170. [Google Scholar] [CrossRef] [PubMed]
- Hanshaw, B.B.; Back, W. Major geochemical processes in the evolution of carbonate—Aquifer systems. J. Hydrol. 1979, 43, 287–312. [Google Scholar] [CrossRef]
- Plummer, L. Defining reactions and mass transfer in part of the Floridan aquifer. Water Resour. Res. 1977, 13, 801–812. [Google Scholar] [CrossRef]
- Liu, F.; Song, X.; Yang, L.; Han, D.; Zhang, Y.; Ma, Y.; Bu, H. The role of anthropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subei Lake basin, Ordos energy base, Northwestern China. Sci. Total Environ. 2015, 538, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Carol, E.; Mas-Pla, J.; Kruse, E. Interaction between continental and estuarine waters in the wetlands of the northern coastal plain of Samborombón Bay, Argentina. Appl. Geochem. 2013, 34, 152–163. [Google Scholar] [CrossRef]
- Schoeller, H. Qualitative evaluation of groundwater resources. In Methods and Techniques of Groundwater Investigations and Development; UNESCO: Paris, France, 1965; Volume 5483. [Google Scholar]
- Deutsch, W.J.; Siegel, R. Groundwater Geochemistry: Fundamentals and Applications to Contamination; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Khatri, N.; Tyagi, S. Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front. Life Sci. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Li, P.; Tian, R.; Xue, C.; Wu, J. Progress, opportunities, and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ. Sci. Pollut. Res. 2017, 24, 13224–13234. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Zhang, S.; Li, R.; Yan, C.; Wu, S. China Multi-Period Land Use Remote Sensing Monitoring Dataset; Resource and Environmental Science Data Registration and Publishing System: Beijing, China, 2018. [Google Scholar] [CrossRef]
- White, W.B. Karst hydrology: Recent developments and open questions. Eng. Geol. 2002, 65, 85–105. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Pinzon, J.; Oppenheimer, J.; Jacangelo, J.G. Sources of nutrients impacting surface waters in Florida: A review. J. Environ. Manag. 2012, 109, 80–92. [Google Scholar] [CrossRef]
- Liu, C.-Q.; Li, S.-L.; Lang, Y.-C.; Xiao, H.-Y. Using δ15N-and δ18O-values to identify nitrate sources in karst ground water, Guiyang, Southwest China. Environ. Sci. Technol. 2006, 40, 6928–6933. [Google Scholar] [CrossRef]
- Amiel, R.B.; Grodek, T.; Frumkin, A. Characterization of the hydrogeology of the sacred Gihon Spring, Jerusalem: A deteriorating urban karst spring. Hydrogeol. J. 2010, 18, 1465–1479. [Google Scholar] [CrossRef]
- Anornu, G.; Gibrilla, A.; Adomako, D. Tracking nitrate sources in groundwater and associated health risk for rural communities in the White Volta River basin of Ghana using isotopic approach (δ15N, δ18ONO3 and 3H). Sci. Total Environ. 2017, 603, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, A.; Loehr, R.; Prakasam, T.; Srinath, E. Inhibition of nitrification by ammonia and nitrous acid. J. Water Pollut. Control Fed. 1976, 48, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Vesper, D.J.; Loop, C.M.; White, W.B. Contaminant transport in karst aquifers. Theor. Appl. Karstology 2001, 13, 101–111. [Google Scholar]
- Zhang, P.; Yue, F.-J.; Wang, X.-D.; Chen, S.-N.; Li, X.-Z.; Liu, T.-Z.; Yang, C. Antecedent rainfall and land use controlling the fate of nitrogen in karst urban rivers, elucidated by an isotopic approach. J. Hydrol. 2021, 592, 125803. [Google Scholar] [CrossRef]
- SPBS. Shandong Statistical Yearbook. 2000–2021. Available online: http://tjj.shandong.gov.cn/ (accessed on 10 January 2024).
- Postma, D.; Boesen, C.; Kristiansen, H.; Larsen, F. Nitrate reduction in an unconfined sandy aquifer: Water chemistry, reduction processes, and geochemical modeling. Water Resour. Res. 1991, 27, 2027–2045. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Sun, H.; Wang, J. Seasonal, diurnal and storm-scale hydrochemical variations of typical epikarst springs in subtropical karst areas of SW China: Soil CO2 and dilution effects. J. Hydrol. 2007, 337, 207–223. [Google Scholar] [CrossRef]
- Herman, J.S.; Lorah, M.M. CO2 outgassing and calcite precipitation in Falling Spring Creek, Virginia, USA. Chem. Geol. 1987, 62, 251–262. [Google Scholar] [CrossRef]
- Crossey, L.J.; Karlstrom, K.E.; Springer, A.E.; Newell, D.; Hilton, D.R.; Fischer, T. Degassing of mantle-derived CO2 and He from springs in the southern Colorado Plateau region—Neotectonic connections and implications for groundwater systems. Geol. Soc. Am. Bull. 2009, 121, 1034–1053. [Google Scholar] [CrossRef]
- Gulley, J.; Martin, J.B.; Screaton, E.J.; Moore, P.J. River reversals into karst springs: A model for cave enlargement in eogenetic karst aquifers. Bulletin 2011, 123, 457–467. [Google Scholar] [CrossRef]
- Zilberbrand, M.; Gimburg, A.; Doroshev, A.; Mirlas, V.; Anker, Y. Chemical evolution of runoff in Eastern Mediterranean mountainous karstic terrains. J. Hydrol. 2022, 605, 127388. [Google Scholar] [CrossRef]
- Lukač Reberski, J.; Terzić, J.; Maurice, L.D.; Lapworth, D.J. Emerging organic contaminants in karst groundwater: A global level assessment. J. Hydrol. 2022, 604, 127242. [Google Scholar] [CrossRef]
- Mahler, B.; Massei, N. Anthropogenic contaminants as tracers in an urbanizing karst aquifer. J. Contam. Hydrol. 2007, 91, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Gale, S.J. The hydraulics of conduit flow in carbonate aquifers. J. Hydrol. 1984, 70, 309–327. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).