Treatment Effect of Long-Term Subsurface-Flow Constructed Wetland on Mariculture Water and Analysis of Wetland Bacterial Community
Abstract
:1. Introduction
2. Materials and Methods
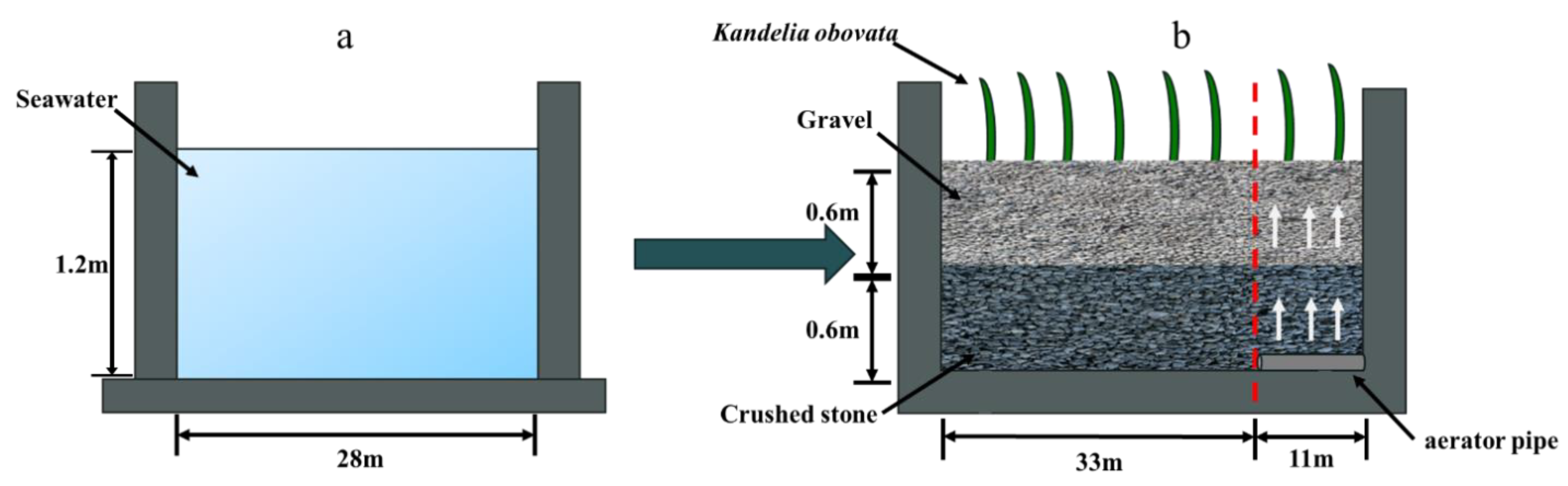
2.1. Subsurface-Flow Wetland System
2.2. Water Sampling and Analytical Methods
2.3. DNA Extraction and PCR Amplification
2.4. Data Analysis
Average Removal Amount
3. Results and Discussion
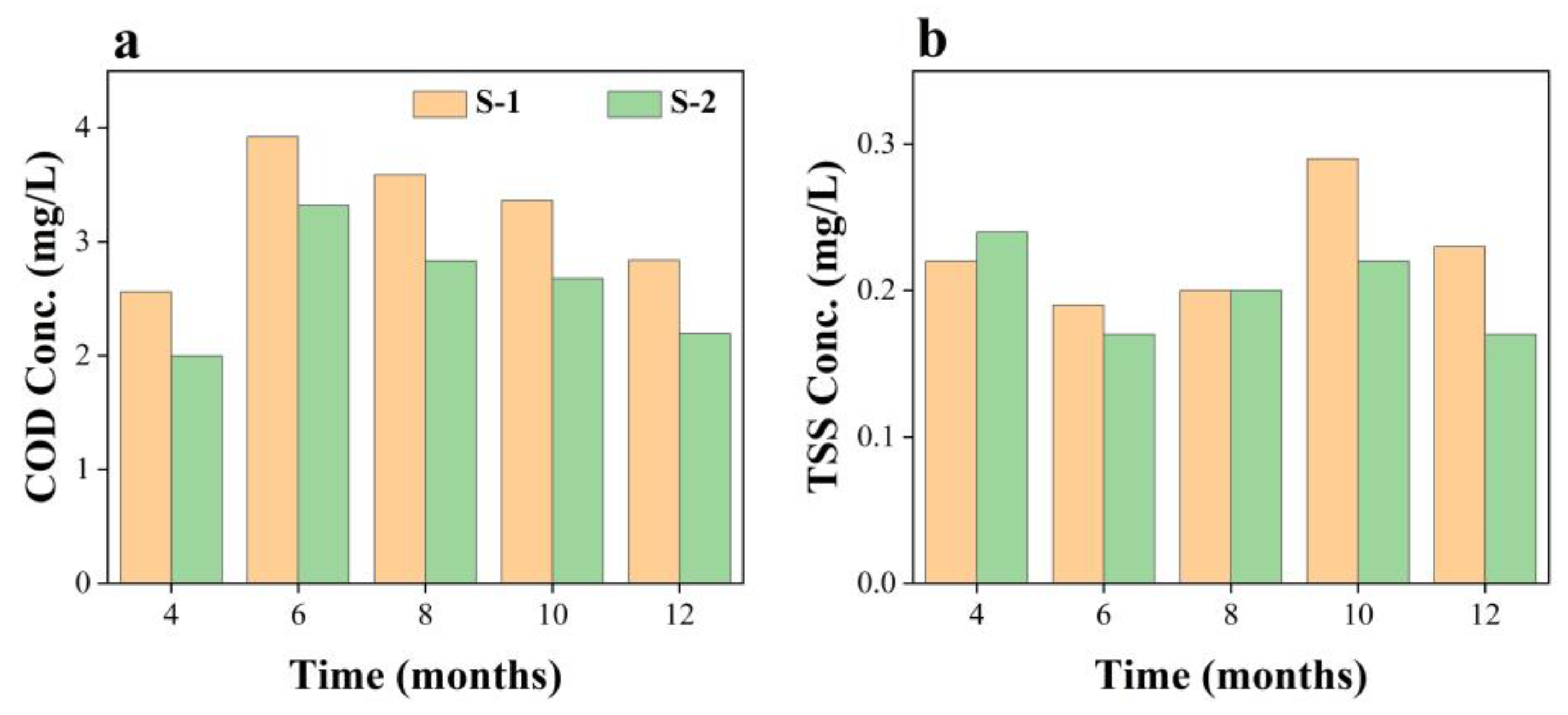
3.1. Variation in Water Quality
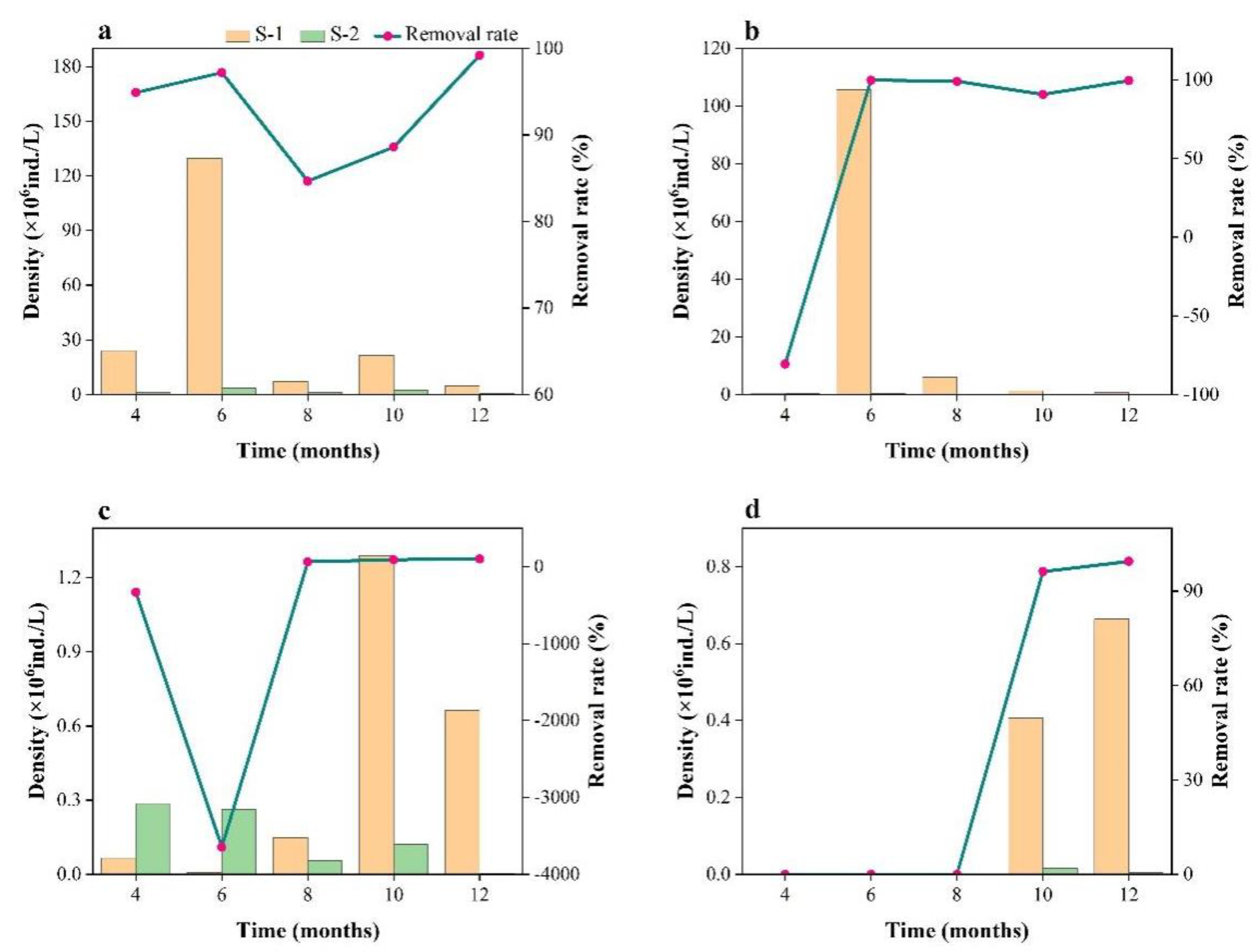
3.2. Variation in Phytoplankton Community Structure
3.3. Characteristics of Microbial Communities
3.3.1. Richness and Diversity of Microbial Communities
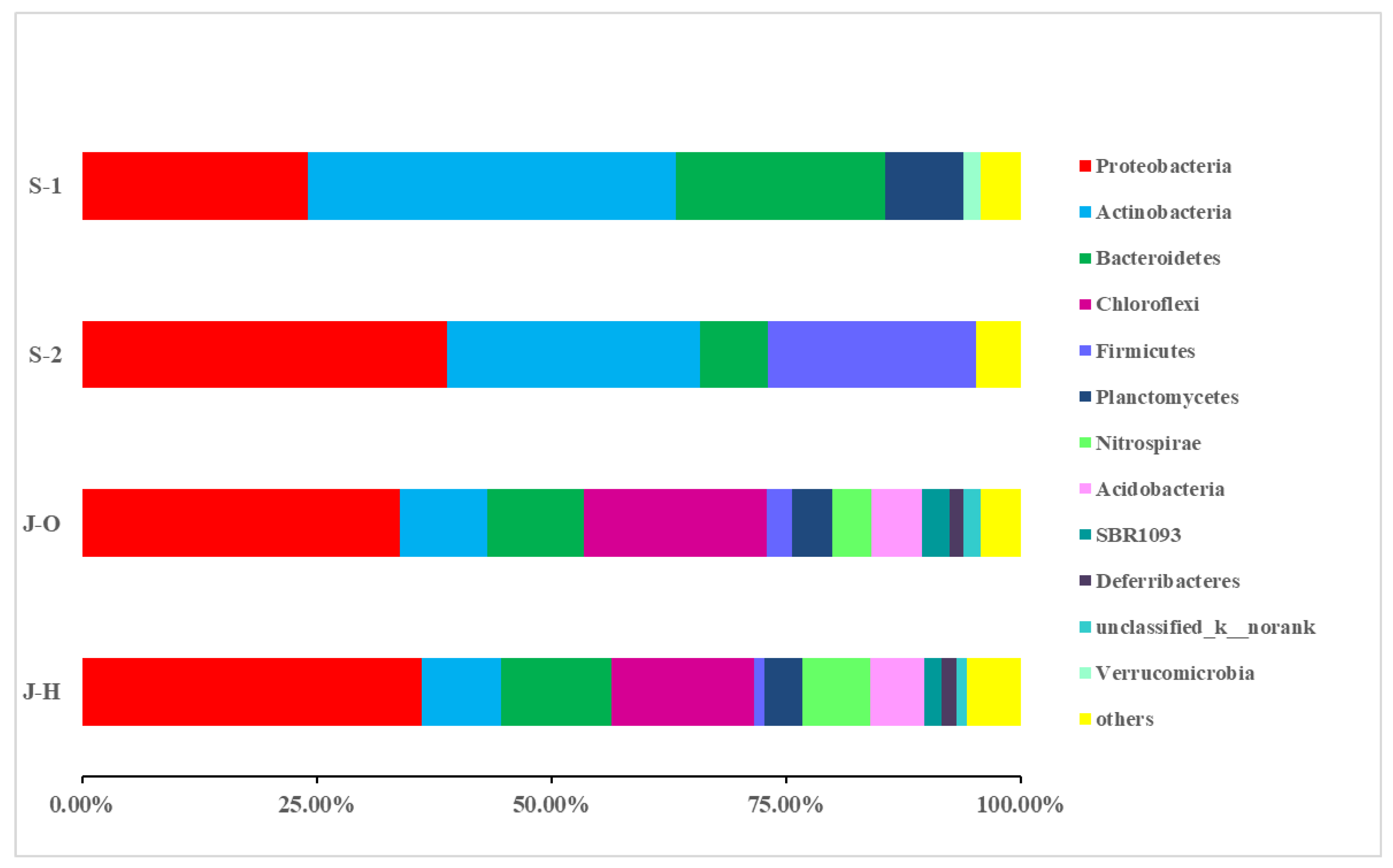
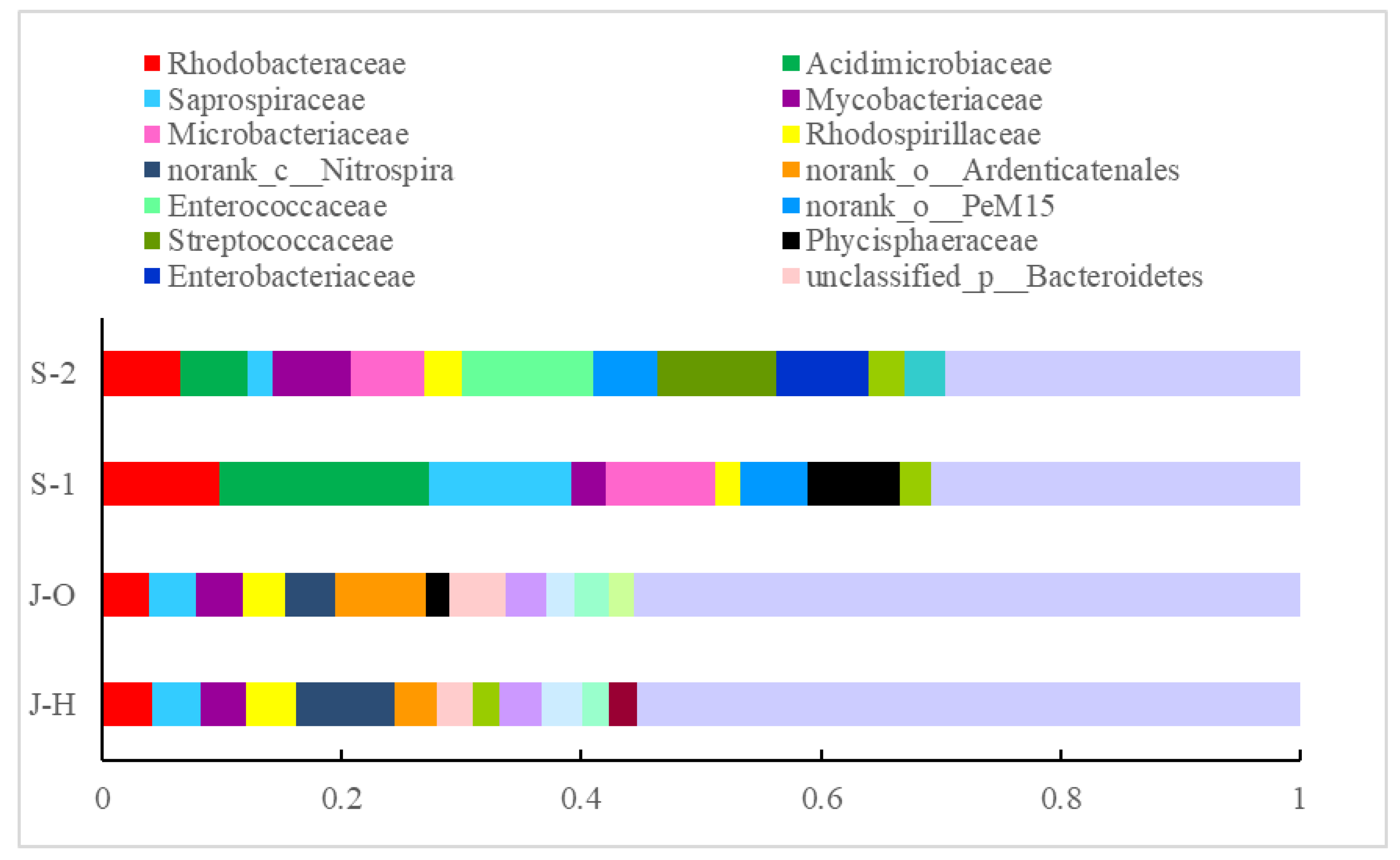
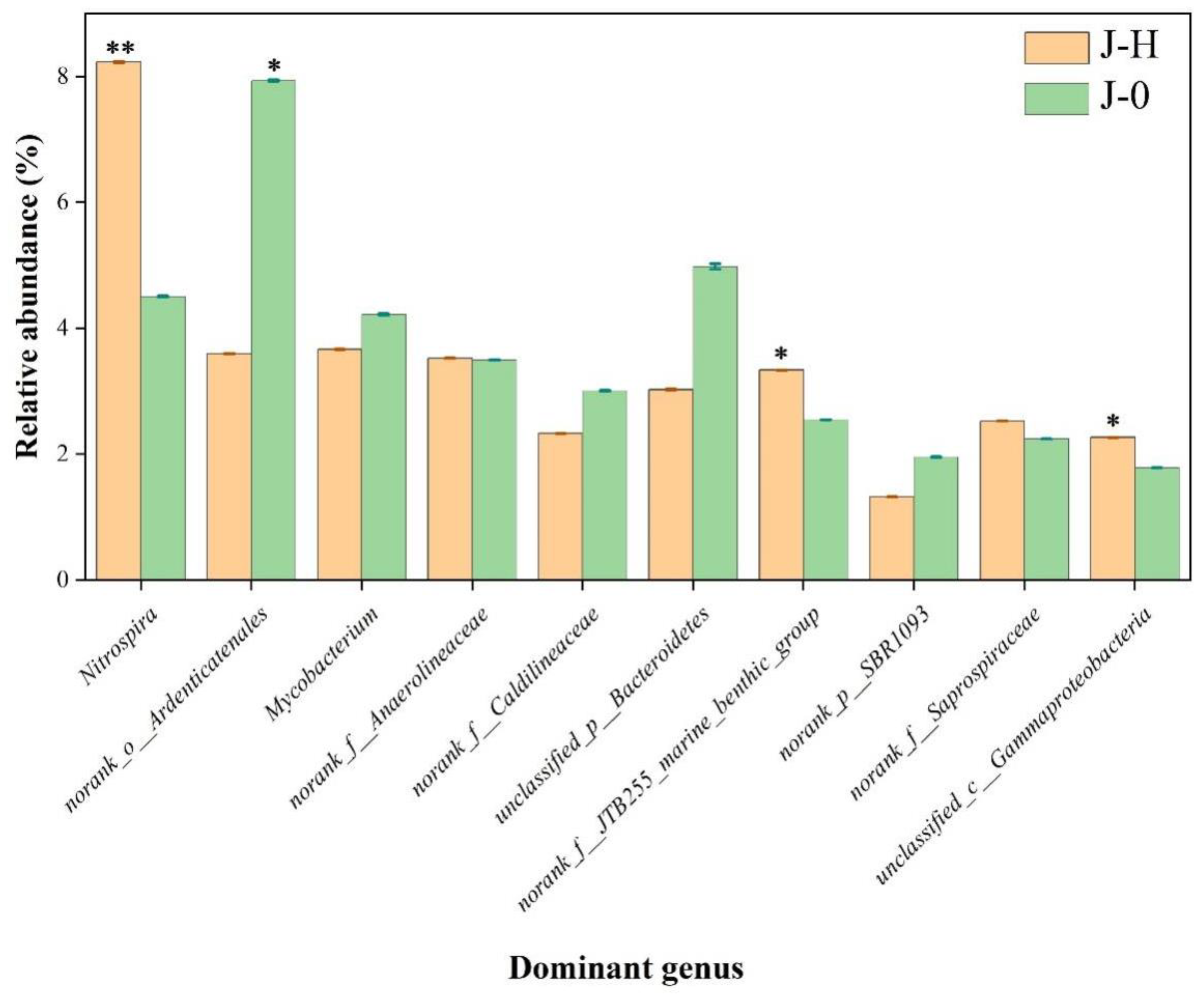
3.3.2. Composition and Distribution of Microbial Communities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, A.; Abdullah, S.R.S.; Abu Hasan, H.; Othman, A.R.; Ismail, N.I. Aquaculture industry: Supply and demand, best practices, effluent and its current issues and treatment technology. J. Environ. Manag. 2021, 287, 112271. [Google Scholar] [CrossRef] [PubMed]
- Hai, A.T.N.; Speelman, S. Economic-environmental trade-offs in marine aquaculture: The case of lobster farming in Vietnam. Aquaculture 2020, 516, 734593. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.-G. Ecological engineering in aquaculture—Potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, J.-W.; Wu, J.-W.; Yuan, Z.; Zhang, J.-W.; Gao, C.; Lin, Z.-Y. Comprehensive Assessment of Eutrophication in Xiamen Bay and Its Implications for Management Strategy in Southeast China. Int. J. Environ. Res. Public Health 2022, 19, 3055. [Google Scholar] [CrossRef]
- Kataki, S.; Chatterjee, S.; Vairale, M.G.; Dwivedi, S.K.; Gupta, D.K. Constructed wetland, an eco-technology for wastewater treatment: A review on types of wastewater treated and components of the technology (macrophyte, biolfilm and substrate). J. Environ. Manag. 2021, 283, 111986. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Kuschk, P.; Brix, H.; Vymazal, J.; Dong, R. Development of constructed wetlands in performance intensifications for wastewater treatment: A nitrogen and organic matter targeted review. Water Res. 2014, 57, 40–55. [Google Scholar] [CrossRef]
- Parde, D.; Patwa, A.; Shukla, A.; Vijay, R.; Killedar, D.J.; Kumar, R. A review of constructed wetland on type, treatment and technology of wastewater. Environ. Technol. Innov. 2021, 21, 101261. [Google Scholar] [CrossRef]
- Vymazal, J.; Zhao, Y.; Mander, U. Recent research challenges in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2021, 169, 106318. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Guo, W.; Liang, S.; Fan, J. Secondary effluent purification by a large-scale multi-stage surface-flow constructed wetland: A case study in northern China. Bioresour. Technol. 2018, 249, 1092–1096. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Shan, A.; Wang, W.; Kang, K.J.; Hou, D.; Luo, J.; Wang, G.; Pan, M.; Feng, Y.; He, Z.; Yang, X. The Removal of Antibiotics in Relation to a Microbial Community in an Integrated Constructed Wetland for Tail Water Decontamination. Wetlands 2020, 40, 993–1004. [Google Scholar] [CrossRef]
- Jia, L.; Gou, E.; Liu, H.; Lu, S.; Wu, S.; Wu, H. Exploring Utilization of Recycled Agricultural Biomass in Constructed Wetlands: Characterization of the Driving Force for High-Rate Nitrogen Removal. Environ. Sci. Technol. 2019, 53, 1258–1268. [Google Scholar] [CrossRef]
- Hu, H. The Freshwater Algae of China: Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006. [Google Scholar]
- GB 3097-1997; General Administration of Quality Supervision, Inspection and Quarantine of People’s Republic of China. Sea Water Quality Standard: Beijing, China, 1997.
- Wang, X.; Tian, Y.; Zhao, X.; Peng, S.; Wu, Q.; Yan, L. Effects of aeration position on organics, nitrogen and phosphorus removal in combined oxidation pond-constructed wetland systems. Bioresour. Technol. 2015, 198, 7–15. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, G.; Liu, J.; Zhu, Y.; Xu, J. Performance of a constructed wetland in treating brackish wastewater from commercial recirculating and super-intensive shrimp growout systems. Bioresour. Technol. 2011, 102, 9416–9424. [Google Scholar] [CrossRef]
- Muller, V. Betriebssicherheit von Pflanzenklaranlagen. Wasserwirtsch. Wassertech. 2000, 32–34. [Google Scholar]
- Banerjee, S.; Maity, S.; Guchhait, R.; Chatterjee, A.; Biswas, C.; Adhikari, M.; Pramanick, K. Toxic effects of cyanotoxins in teleost fish: A comprehensive review. Aquat. Toxicol. 2021, 240, 105971. [Google Scholar] [CrossRef]
- Huang, J.; Cai, W.; Zhong, Q.; Wang, S. Influence of temperature on micro-environment, plant eco-physiology and nitrogen removal effect in subsurface flow constructed wetland. Ecol. Eng. 2013, 60, 242–248. [Google Scholar] [CrossRef]
- Wang, T.; Xiao, L.; Lu, H.; Lu, S.; Li, J.; Guo, X.; Zhao, X. Nitrogen removal from summer to winter in a field pilot-scale multistage constructed wetland-pond system. J. Environ. Sci. 2022, 111, 249–262. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Zhang, W.; Wang, S.; Peng, H. Pollutants removal efficiency assessment of constructed subsurface flow wetlands in lakes with numerical models. J. Hydrol. 2021, 598, 126289. [Google Scholar] [CrossRef]
- Jozwiakowski, K.; Bugajski, P.; Kurek, K.; Caceres, R.; Siwiec, T.; Jucherski, A.; Czekala, W.; Kozlowski, K. Technological reliability of pollutant removal in different seasons in one-stage constructed wetland system with horizontal flow operating in the moderate climate. Sep. Purif. Technol. 2020, 238, 116439. [Google Scholar] [CrossRef]
- Kroupova, H.K.; Valentova, O.; Svobodova, Z.; Sauer, P.; Machova, J. Toxic effects of nitrite on freshwater organisms: A review. Rev. Aquac. 2018, 10, 525–542. [Google Scholar] [CrossRef]
- Hernandez-Crespo, C.; Gargallo, S.; Benedito-Dura, V.; Nacher-Rodriguez, B.; Rodrigo-Alacreu, M.A.; Martin, M. Performance of surface and subsurface flow constructed wetlands treating eutrophic waters. Sci. Total Environ. 2017, 595, 584–593. [Google Scholar] [CrossRef]
- Buley, R.P.; Gladfelter, M.F.; Fernandez-Figueroa, E.G.; Wilson, A.E. Complex effects of dissolved organic matter, temperature, and initial bloom density on the efficacy of hydrogen peroxide to control cyanobacteria. Environ. Sci. Pollut. Res. 2023, 30, 43991–44005. [Google Scholar] [CrossRef]
- Kinley-Baird, C.; Calomeni, A.; Berthold, D.E.; Lefler, F.W.; Barbosa, M.; Rodgers, J.H.; Laughinghouse, H.D. Laboratory-scale evaluation of algaecide effectiveness for control of microcystin-producing cyanobacteria from Lake Okeechobee, Florida (USA). Ecotoxicol. Environ. Saf. 2021, 207, 111233. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, M.-F.; Ye, L. 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J. 2012, 6, 1137–1147. [Google Scholar] [CrossRef]
- Guan, W.; Yin, M.; He, T.; Xie, S. Influence of substrate type on microbial community structure in vertical-flow constructed wetland treating polluted river water. Environ. Sci. Pollut. Res. 2015, 22, 16202–16209. [Google Scholar] [CrossRef]
- Liao, X.; Chen, C.; Zhang, J.; Dai, Y.; Zhang, X.; Xie, S. Operational performance, biomass and microbial community structure: Impacts of backwashing on drinking water biofilter. Environ. Sci. Pollut. Res. 2015, 22, 546–554. [Google Scholar] [CrossRef]
- Santos, F.; Ribeiro de Almeida, C.M.; Ribeiro, I.; Ferreira, A.C.; Mucha, A.P. Removal of veterinary antibiotics in constructed wetland microcosms Response of bacterial communities. Ecotoxicol. Environ. Saf. 2019, 169, 894–901. [Google Scholar] [CrossRef]
- Ansola, G.; Arroyo, P.; Saenz de Miera, L.E. Characterisation of the soil bacterial community structure and composition of natural and constructed wetlands. Sci. Total Environ. 2014, 473, 63–71. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Zhao, L.; Li, Y.; Dai, Y.; Xie, S. Distribution of sediment ammonia-oxidizing microorganisms in plateau freshwater lakes. Appl. Microbiol. Biotechnol. 2015, 99, 4435–4444. [Google Scholar] [CrossRef]
- Dong, W.; Lu, G.; Yan, L.; Zhang, Z.; Zhang, Y. Characteristics of pellets with immobilized activated sludge and its performance in increasing nitrification in sequencing batch reactors at low temperatures. J. Environ. Sci. 2016, 42, 202–209. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.Q.; Chen, F.R.; Zhang, L.L.; Ruan, J.J.; Wu, S.J.; Zhu, R.L. Effects of hydraulic loading rate and substrate on ammonium removal in tidal flow constructed wetlands treating black and odorous water bodies. Bioresour. Technol. 2021, 321, 124468. [Google Scholar] [CrossRef]
- Xia, Y.; Kong, Y.; Thomsen, T.R.; Nielsen, P.H. Identification and ecophysiological characterization of epiphytic protein-hydrolyzing Saprospiraceae (“Candidatus epiflobacter” spp.) in activated sludge. Appl. Environ. Microbiol. 2008, 74, 2229–2238. [Google Scholar] [CrossRef]
- Jeon, Y.; Li, L.; Calvillo, J.; Ryu, H.; Domingo, J.W.S.; Choi, O.; Brown, J.; Seo, Y. Impact of algal organic matter on the performance, cyanotoxin removal, and bio fi lms of biologically-active fi ltration systems. Water Res. 2020, 184, 116120. [Google Scholar] [CrossRef]
- Kim, N.-K.; Oh, S.; Liu, W.-T. Enrichment and characterization of microbial consortia degrading soluble microbial products discharged from anaerobic methanogenic bioreactors. Water Res. 2016, 90, 395–404. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Yuan, X.; Wang, R.; Liu, L.; Li, H.; Zhao, C.; Kong, Q. Characteristics of Bacterial and Archaeal Communities in Microbial-Enhanced Constructed Wetlands under NaCl Stress. Clean-Soil Air Water 2022, 50, 2100152. [Google Scholar] [CrossRef]
- Wei, G.; Shan, D.; Li, G.; Li, X.; Tian, R.; He, J.; Shao, Z. Prokaryotic communities vary with floc size in a biofloc-technology based aquaculture system. Aquaculture 2020, 529, 735632. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strategy Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Meky, N.; Elreedy, A.; Ibrahim, M.G.; Fujii, M.; Tawfik, A. Intermittent versus sequential dark-photo fermentative hydrogen production as an alternative for bioenergy recovery from protein-rich effluents. Energy 2021, 217, 119326. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]


| Grade | COD≤ (mg/L) | DIN≤ (mg/L) | Applicable Site |
|---|---|---|---|
| I | 2 | 0.20 | Marine fishery waters |
| II | 3 | 0.30 | Aquaculture areas |
| III | 4 | 0.40 | Industrial water function zone |
| IV | 5 | 0.50 | Ocean port waters |
| Index | Average Inlet (mg/L) | Average Outlet (mg/L) | Average Removal Amount (g/m2·d−1) | Average Removal (100%) |
|---|---|---|---|---|
| TN | 1.44 ± 0.41 | 0.90 ± 0.17 | 0.78 ± 0.43 | 36.94 |
| NO3−-N | 0.09 ± 0.03 | 0.13 ± 0.02 | −0.10 ± 0.08 | −52.02 |
| NO2−-N | 0.03 ± 0.02 | 0.01 ± 0.00 | 0.03 ± 0.02 | 78.54 |
| NH4+-N | 0.11 ± 0.02 | 0.06 ± 0.02 | 0.07 ± 0.02 | 45.07 |
| DIN | 0.23 | 0.20 | 0.04 | 10.88 |
| DON | 1.21 | 0.70 | 0.74 | 41.82 |
| Phyla | Genera | Frequency | Inlet Abundance (×103 ind./L) | Outlet Abundance (×103 ind./L) | Average Removal (100%) |
|---|---|---|---|---|---|
| Cyanophyta | Microcystis | 6 | 30,577.29 | 2957.55 | 90.33 |
| Synechocystis | 5 | 2673.70 | 65.94 | 97.53 | |
| Stanieria | 4 | 77.33 | 1.60 | 97.93 | |
| Oscillatoria | 4 | 2.40 | 13.22 | −450.87 | |
| Bacillariophyta | Phaeodactylum | 5 | 24,030.00 | 74.64 | 99.69 |
| Melosira | 5 | 135.30 | 25.55 | 81.12 | |
| Cylindrotheca | 5 | 40.12 | 3.33 | 91.70 | |
| Navicula | 6 | 39.73 | 12.39 | 68.81 | |
| Stephanopyxis | 6 | 38.98 | 13.66 | 64.96 | |
| Chlorophyta | Dunaliella | 4 | 148.69 | 691.80 | −365.25 |
| Chlamydomonas | 3 | 6.87 | 4.80 | 30.10 | |
| Ulothrix | 4 | 2.96 | 8.98 | −203.16 | |
| Platymonas | 1 | 19.60 | 3.60 | 81.63 | |
| Alexandrium | 2 | 346.94 | 15.28 | 95.60 |
| Sample | Shannon | Ace | Chao1 | Coverage |
|---|---|---|---|---|
| S-1 | 3.489 | 1315.87 | 1147.28 | 0.99 |
| S-2 | 4.43 | 1903.07 | 1860.98 | 0.99 |
| J-O | 6.25 | 3200.59 | 3200.45 | 0.98 |
| J-H | 6.56 | 3782.29 | 3778.42 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Yang, G.; Chen, X.; Li, P.; Chen, J.; Yan, M.; Guo, C. Treatment Effect of Long-Term Subsurface-Flow Constructed Wetland on Mariculture Water and Analysis of Wetland Bacterial Community. Water 2024, 16, 1054. https://doi.org/10.3390/w16071054
Chen C, Yang G, Chen X, Li P, Chen J, Yan M, Guo C. Treatment Effect of Long-Term Subsurface-Flow Constructed Wetland on Mariculture Water and Analysis of Wetland Bacterial Community. Water. 2024; 16(7):1054. https://doi.org/10.3390/w16071054
Chicago/Turabian StyleChen, Chen, Guijun Yang, Xuechu Chen, Pengquan Li, Jingfei Chen, Maocang Yan, and Chong Guo. 2024. "Treatment Effect of Long-Term Subsurface-Flow Constructed Wetland on Mariculture Water and Analysis of Wetland Bacterial Community" Water 16, no. 7: 1054. https://doi.org/10.3390/w16071054






