Alternative and Classical Processes for Disinfection of Water Polluted by Fungi: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Eligibility Criteria
2.3. Search for Articles
2.4. Data Extraction
2.5. Bibliometric Analysis
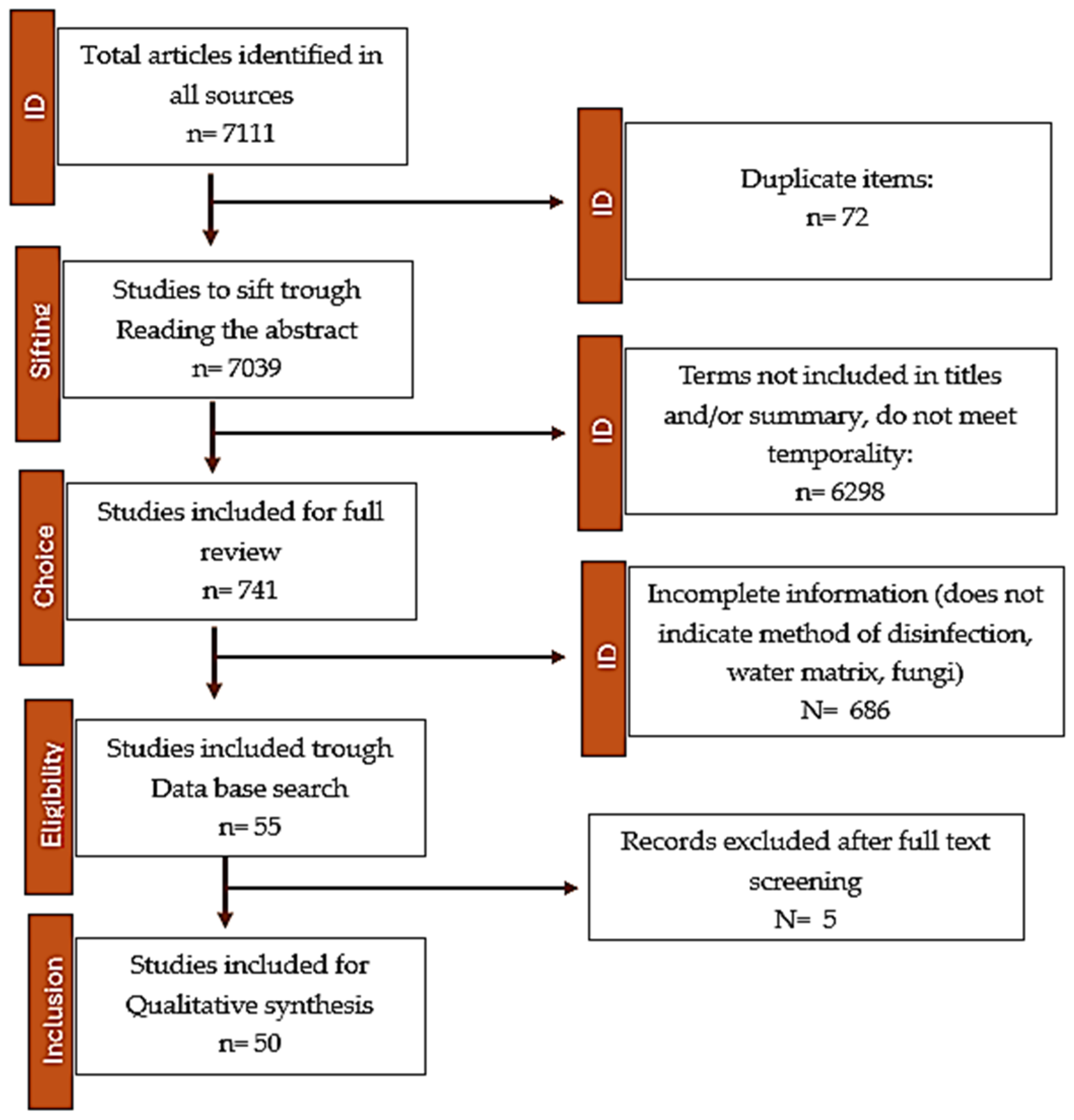
2.5.1. Search for Results
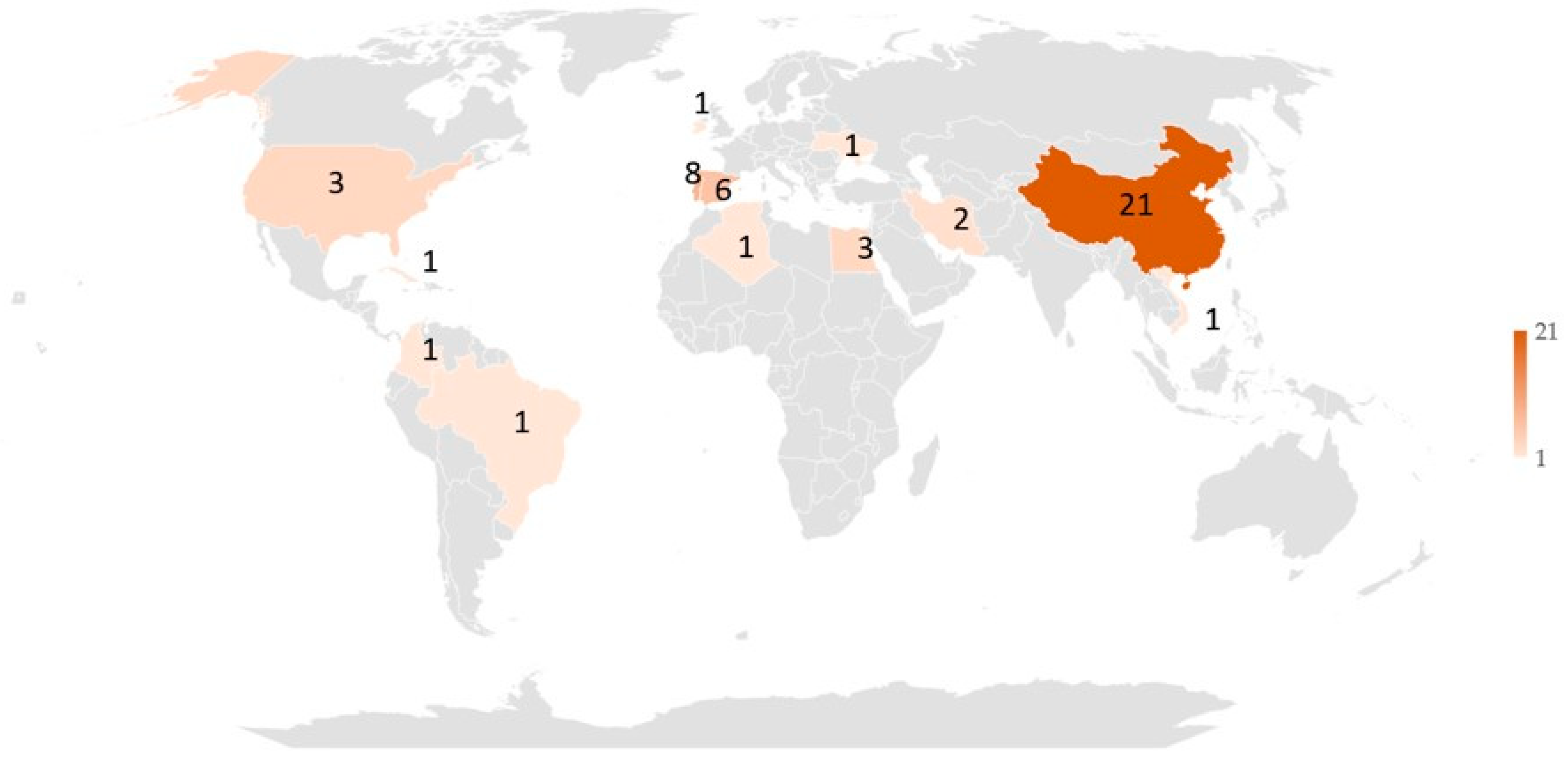
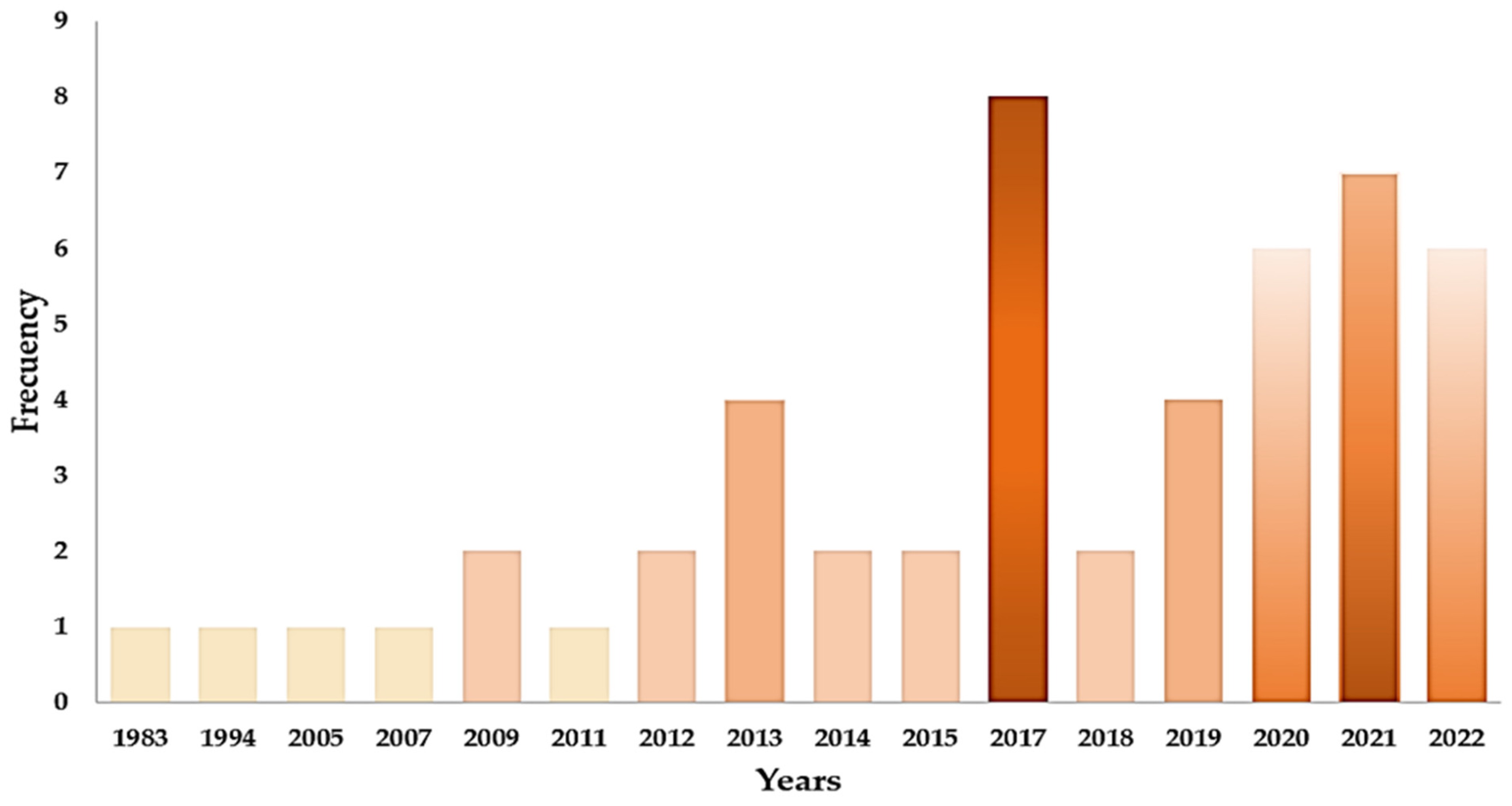
2.5.2. Characteristics of the Included Studies
3. Medical Impact of Fungi and Pollution of Aquatic Media by Fungi
3.1. Health Problems Related to Fungi
3.2. Fungi in Water Samples
4. Antifungals in the Environment and Antifungal Susceptibility/Resistance
4.1. Antifungals in Aquatic Systems
4.2. Susceptibility, Resistance to Antifungals, and Resistant Fungi in the Aquatic Systems
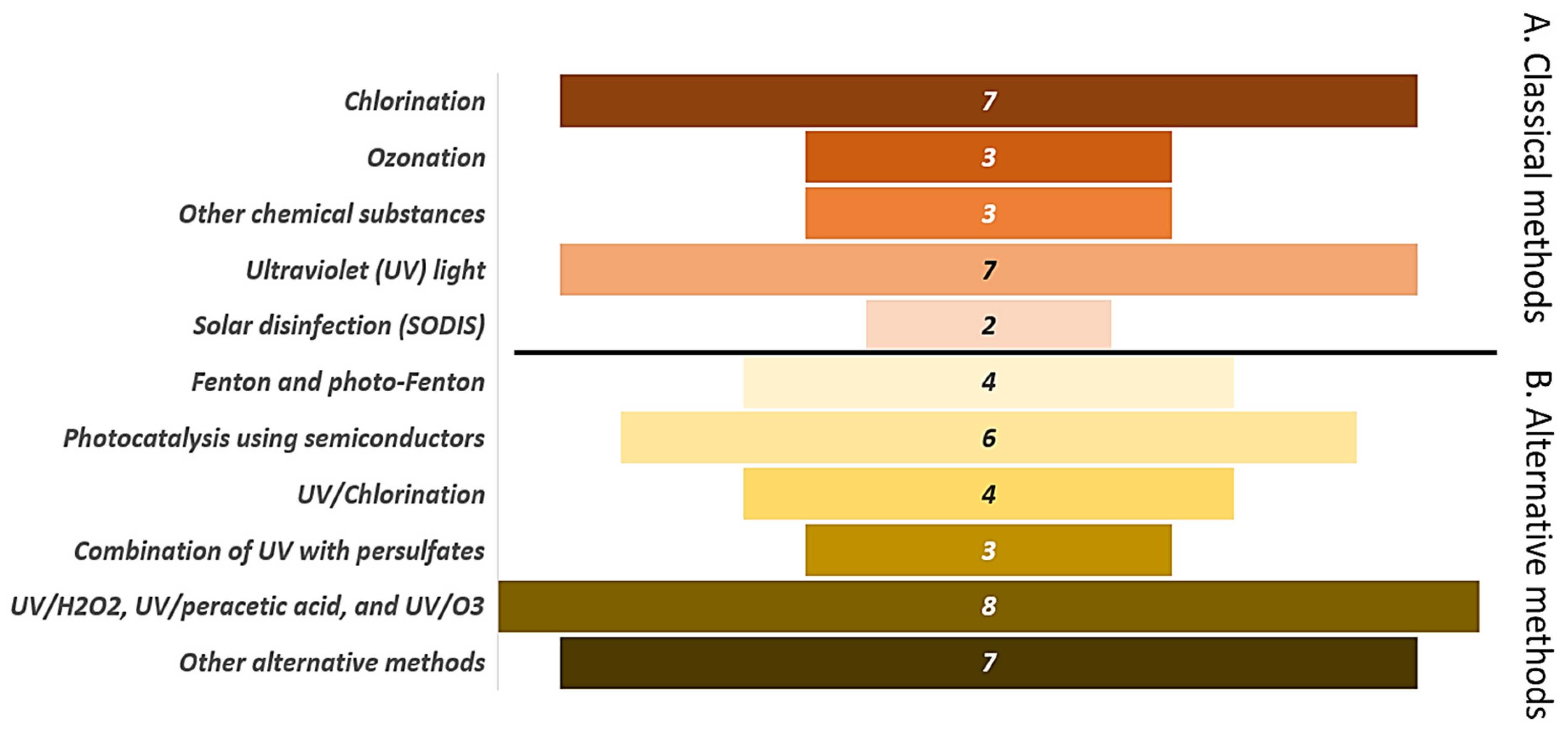
5. The Methods for Fungi Elimination in Aquatic Systems
5.1. Classical Processes for Fungi Inactivation
5.1.1. Chlorination
5.1.2. Ozonation
5.1.3. Other Disinfection Methods Using Chemical Substances
5.1.4. Ultraviolet (UV) Light for Fungi Inactivation
5.1.5. Solar Disinfection (SODIS) for Fungi Inactivation
5.2. Advanced Oxidation Process as Alternative Methods for Fungi Inactivation
5.2.1. Fenton and Photo-Fenton Processes
5.2.2. Photocatalysis Using Semiconductors
5.2.3. UV/Chlorination
5.2.4. Combination of UV with Persulfates
5.2.5. UV/H2O2, UV/Peracetic Acid, and UV/O3 for Fungi Inactivation in Water
5.2.6. Other Alternative Methods of Fungi Disinfection
6. Conclusions and Outlooks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez-Jimenez, I.; Lucio, J.; Menéndez-Fraga, M.D.; Mellado, E.; Peláez, T. Hospital environment as a source of azole-resistant aspergillus fumigatus strains with TR34/L98H and g448s cyp51a mutations. J. Fungi 2021, 7, 22. [Google Scholar] [CrossRef]
- Lemaire, B.; Normand, A.-C.; Forel, J.-M.; Cassir, N.; Piarroux, R.; Ranque, S. Hospitalized Patient as Source of Aspergillus fumigatus, 2015. Emerg. Infect. Dis. 2018, 24, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; Ying, G.G. Occurrence, fate and ecological risk of five typical azole fungicides as therapeutic and personal care products in the environment: A review. Environ. Int. 2015, 84, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.E.; Riedel, J.; Sae-Ong, T.; Kang, K.; Brabetz, W.; Panagiotou, G.; Deising, H.B.; Kurzai, O. Effects of agricultural fungicide use on Aspergillus fumigatus Abundance, Antifungal Susceptibility, and Population Structure. Appl. Environ. Sci. 2020, 11, e02213-20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jimenez, L.L.; Snelders, E.; Debets, A.J.M.; Rietveld, A.G.; Verweij, P.E.; Schoustra, S.E.; Zwaan, B.J. Dynamics of Aspergillus fumigatus in Azole Fungicide- Containing Plant Waste in the Netherlands (2016–2017) Jianhua. Appl. Environ. Microbiol. 2021, 87, e02295-20. [Google Scholar] [CrossRef] [PubMed]
- Maza-Márquez, P.; Vílchez-Vargas, R.; González-Martínez, A.; González-López, J.; Rodelas, B. Assessing the abundance of fungal populations in a full-scale membrane bioreactor (MBR) treating urban wastewater by using quantitative PCR (qPCR). J. Environ. Manag. 2018, 223, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Assress, H.A.; Selvarajan, R.; Nyoni, H.; Ogola, H.J.O.; Mamba, B.B.; Msagati, T.A.M. Azole antifungal resistance in fungal isolates from wastewater treatment plant effluents. Environ. Sci. Pollut. Res. 2021, 28, 3217–3229. [Google Scholar] [CrossRef] [PubMed]
- Milanezi, A.C.M.; Witusk, J.P.D.; van der Sand, S.T. Antifungal susceptibility of yeasts isolated from anthropogenic watershed. An. Acad. Bras. Ciências 2019, 91, e20170369. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Cao, R.; Wen, G.; Xu, X.; Xia, Y.; Wu, G. Sequential use of UV-LEDs irradiation and chlorine to disinfect waterborne fungal spores: Efficiency, mechanism and photoreactivation. J. Hazard. Mater. 2022, 423, 127102. [Google Scholar] [CrossRef]
- Chen, J.; Loeb, S.; Kim, J.-H. LED revolution: Fundamentals and prospects for UV disinfection applications. Environ. Sci. Water Res. Technol. 2017, 3, 188–202. [Google Scholar] [CrossRef]
- Kim, J.Y. Human fungal pathogens: Why should we learn? J. Microbiol. 2016, 54, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.N.M.; Cseresny, Z.; Hartung, S.; Blickensdorf, M.; Saffer, C.; Rennert, K. Invasive aspergillosis-on-chip: A quantitative treatment study of human Aspergillus fumigatus infection. Biomaterials 2022, 283, 121420. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.J.; Marques, R.; Marques, M.; Benoliel, M.J.; Barreto Crespo, M.T. Free chlorine inactivation of fungi in drinking water sources. Water Res. 2013, 47, 517–523. [Google Scholar] [CrossRef]
- Wen, G.; Xu, X.; Huang, T.; Zhu, H.; Ma, J. Inactivation of three genera of dominant fungal spores in groundwater using chlorine dioxide: Effectiveness, influencing factors, and mechanisms. Water Res. 2017, 125, 132–140. [Google Scholar] [CrossRef]
- Wen, G.; Liang, Z.; Xu, X.; Cao, R.; Wan, Q.; Ji, G.; Lin, W.; Wang, J.; Yang, J.; Huang, T. Inactivation of fungal spores in water using ozone: Kinetics, influencing factors and mechanisms. Water Res. 2020, 185, 116218. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Cao, R.; Wen, G.; Xu, X.; Xia, Y.; Wu, G.; Li, Y.; Wang, J.; Xu, H.; Lin, Y.; et al. Efficacy of UV-LED based advanced disinfection processes in the inactivation of waterborne fungal spores: Kinetics, photoreactivation, mechanism and energy requirements. Sci. Total Environ. 2022, 803, 150107. [Google Scholar] [CrossRef]
- Wen, G.; Xu, X.; Zhu, H.; Huang, T.; Ma, J. Inactivation of four genera of dominant fungal spores in groundwater using UV and UV/PMS: Efficiency and mechanisms. Chem. Eng. J. 2017, 328, 619–628. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X. Removal of antibiotic resistance genes (ARGs) in various wastewater treatment processes: An overview. Crit. Rev. Environ. Sci. Technol. 2022, 52, 571–630. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, B.; Cui, X.; Ren, Q.; Ren, T.; Zhou, Y. Distribution and dynamics of antibiotic resistance genes in a three-dimensional multifunctional biofilm during greywater treatment. Environ. Pollut. 2023, 327, 121533. [Google Scholar] [CrossRef]
- Wan, Q.; Wen, G.; Cui, Y.; Cao, R.; Xu, X.; Wu, G.; Wang, J.; Huang, T. Occurrence and control of fungi in water: New challenges in biological risk and safety assurance. Sci. Total Environ. 2023, 860, 160536. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zhang, T.Y.; Wang, H.; Hu, C.Y.; Tang, Y.L.; Xu, B. Occurrence of fungal spores in drinking water: A review of pathogenicity, odor, chlorine resistance and control strategies. Sci. Total Environ. 2022, 853, 158626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ren, X.; Chen, Y.; Feng, H.; Yu, J.; Peng, K.; Zhang, Y.; Chen, W.; Tang, J.; Wang, J.; et al. Bacteria inactivation by sulfate radical: Progress and non-negligible disinfection by-products. Front. Environ. Sci. Eng. 2023, 17, 29. [Google Scholar] [CrossRef]
- Garvey, M.; Rowan, N.J. Pathogenic Drug Resistant Fungi: A Review of Mitigation Strategies. Int. J. Mol. Sci. 2023, 24, 1584. [Google Scholar] [CrossRef] [PubMed]
- Visconti, V.; Coton, E.; Rigalma, K.; Dantigny, P. Effects of disinfectants on inactivation of mold spores relevant to the food industry: A review. Fungal Biol. Rev. 2021, 38, 44–66. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. Ann. Intern. Med. 2009, 151, W-65–W-94. [Google Scholar] [CrossRef]
- Siscar-Lewin, S.; Hube, B.; Brunke, S. Emergence and evolution of virulence in human pathogenic fungi. Trends Microbiol. 2022, 30, 693–704. [Google Scholar] [CrossRef]
- Paul, S.; Joshi, S.R. Industrial Perspectives of Fungi. In Industrial Microbiology and Biotechnology; Springer: Singapore, 2022; pp. 81–105. [Google Scholar]
- Babič, M.N.; Gunde-Cimerman, N.; Vargha, M.; Tischner, Z.; Magyar, D.; Veríssimo, C.; Sabino, R.; Viegas, C.; Meyer, W.; Brandão, J. Fungal contaminants in drinking water regulation? A tale of ecology, exposure, purification and clinical relevance. Int. J. Environ. Res. Public Health 2017, 14, 636. [Google Scholar] [CrossRef]
- Bohner, F.; Gacser, A. Epidemiological Attributes of Candida Species in Tropical Regions. Curr. Trop. Med. Rep. 2021, 8, 59–68. [Google Scholar] [CrossRef]
- Maza-Márquez, P.; Vilchez-Vargas, R.; Kerckhof, F.; Aranda, E.; González-López, J.; Rodelas, B. Community structure, population dynamics and diversity of fungi in a full-scale membrane bioreactor (MBR) for urban wastewater treatment. Water Res. 2016, 105, 507–519. [Google Scholar] [CrossRef]
- Shearer, C.A.; Descals, E.; Kohlmeyer, B.; Kohlmeyer, J.; Marvanová, L.; Padgett, D.; Porter, D.; Raja, H.A.; Schmit, J.P.; Thorton, H.A.; et al. Fungal biodiversity in aquatic habitats. Biodivers. Conserv. 2007, 16, 49–67. [Google Scholar] [CrossRef]
- Krauss, G.J.; Solé, M.; Krauss, G.; Schlosser, D.; Wesenberg, D.; Bärlocher, F. Fungi in freshwaters: Ecology, physiology and biochemical potential. FEMS Microbiol. Rev. 2011, 35, 620–651. [Google Scholar] [CrossRef]
- Arvanitidou, M.; Kanellou, K.; Constantinides, T.C.; Katsouyannopoulos, V. The occurrence of fungi in hospital and community potable waters. Lett. Appl. Microbiol. 1999, 29, 81–84. [Google Scholar] [CrossRef]
- Wurzbacher, C.M.; Bärlocher, F.; Grossart, H.P. Fungi in lake ecosystems. Aquat. Microb. Ecol. 2010, 59, 125–149. [Google Scholar] [CrossRef]
- Oliveira, H.M.B.; Santos, C.; Paterson, R.R.M.; Gusmão, N.B.; Lima, N. Fungi from a groundwater-fed drinkingwater supply system in Brazil. Int. J. Environ. Res. Public Health 2016, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wang, Z.; Li, T.; Lu, F.; Sheng, D.; Huang, W. Immunosuppressed Patients with Clinically Diagnosed Invasive Fungal Infections: The Fungal Species Distribution, Antifungal Sensitivity and Associated Risk Factors in a Tertiary Hospital of Anhui Province. Infect. Drug Resist. 2022, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.O.; Kohler, L.M.; Hamdan, J.S.; Missagia, B.S.; Barbosa, F.A.R.; Rosa, C.A. Diversity and antifungal susceptibility of yeasts from tropical freshwater environments in Southeastern Brazil. Water Res. 2008, 42, 3921–3929. [Google Scholar] [CrossRef] [PubMed]
- Monapathi, M.E.; Bezuidenhout, C.C.; Rhode, O.H.J. Water quality and antifungal susceptibility of opportunistic yeast pathogens from rivers. Water Sci. Technol. 2017, 75, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.; Laurent, J.; Edel-Hermann, V.; Barbezant, M.; Sixt, N.; Dalle, F.; Aho, S.; Bonnin, A.; Hartemann, P.; Sautour, M. Adaptation of Fusarium oxysporum and Fusarium dimerum to the specific aquatic environment provided by the water systems of hospitals. Water Res. 2015, 76, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Anversa, L.; Lara, B.R.; Romani, C.D.; Saeki, E.K.; Nascentes, G.A.N.; Bonfietti, L.X.; de Souza Carvalho Melhem, M.; da Silva Ruiz, L.; Camargo, C.H.; Pereira, V.B.R. Fungi in dialysis water and dialysate: Occurrence, susceptibility to antifungal agents and biofilm production capacity. J. Water Health 2021, 19, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Mataraci-Kara, E.; Ataman, M.; Yilmaz, G.; Ozbek-Celik, B. Evaluation of antifungal and disinfectant-resistant Candida species isolated from hospital wastewater. Arch. Microbiol. 2020, 202, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Montanari, L.B.; Sartori, F.G.; Ribeiro, D.B.M.; Leandro, L.F.; Pires, R.H.; Melhem, M.d.S.C.; de Mello, C.A.; Martins, C.H.G. Yeast isolation and identification in water used in a Brazilian hemodialysis unit by classic microbiological techniques and Raman spectroscopy. J. Water Health 2018, 16, 311–320. [Google Scholar] [CrossRef]
- Ivanov, M.; Ćirić, A.; Stojković, D. Emerging Antifungal Targets and Strategies. Int. J. Mol. Sci. 2022, 23, 2756. [Google Scholar] [CrossRef]
- Doughty, K.J.; Sierotzki, H.; Semar, M.; Goertz, A. Selection and amplification of fungicide resistance in aspergillus fumigatus in relation to DMI fungicide use in agronomic settings: Hotspots versus coldspots. Microorganisms 2021, 9, 2439. [Google Scholar] [CrossRef]
- Snelders, E.; Van Der Lee, H.A.L.; Kuijpers, J.; Rijs, A.J.M.M.; Varga, J.; Samson, R.A.; Mellado, E.; Donders, A.R.T.; Melchers, W.J.G.; Verweij, P.E. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008, 5, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Assress, H.A.; Selvarajan, R.; Nyoni, H.; Mamba, B.B.; Msagati, T.A.M. Antifungal Azoles and Azole Resistance in the Environment: Current Status and Future Perspectives—A Review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 1011–1041. [Google Scholar] [CrossRef]
- Stevenson, E.M.; Gaze, W.H.; Gow, N.A.R.; Hart, A.; Schmidt, W.; Usher, J.; Warris, A.; Wilkinson, H.; Murray, A.K. Antifungal Exposure and Resistance Development: Defining Minimal Selective Antifungal Concentrations and Testing Methodologies. Front. Fungal Biol. 2022, 3, 918717. [Google Scholar] [CrossRef] [PubMed]
- Assress, H.A.; Nyoni, H.; Mamba, B.B.; Msagati, T.A.M. Occurrence and risk assessment of azole antifungal drugs in water and wastewater. Ecotoxicol. Environ. Saf. 2020, 187, 109868. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Vermeulen, E.; Lagrou, K.; Verweij, P.E. Azole resistance in Aspergillus fumigatus: A growing public health concern. Curr. Opin. Infect. Dis. 2013, 26, 493–500. [Google Scholar] [CrossRef]
- Kang, S.E.; Sumabat, L.G.; Melie, T.; Mangum, B.; Momany, M.; Brewer, M.T. Evidence for the agricultural origin of resistance to multiple antimicrobials in Aspergillus fumigatus, a fungal pathogen of humans. G3 Genes Genomes Genet. 2022, 12, jkab427. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Health and Food Security-TCLocal. Science 2018, 742, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-Y.; Lee, D.-C. Comparative Efficacy of Antifungal Agents for Aquaculture Fish and their Eggs. Korean J. Fish. Aquat. Sci. 2009, 42, 34–40. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, J.L.; Liu, Y.S.; Zhang, Q.Q.; Jiang, Y.X.; Liu, S.; Liu, W.R.; Yang, Y.Y.; Ying, G.G. Personal care products in wild fish in two main Chinese rivers: Bioaccumulation potential and human health risks. Sci. Total Environ. 2018, 621, 1093–1102. [Google Scholar] [CrossRef]
- Kacprzak, M.; Warchol, M.; Widawska, U. Microfungal species composition in raw and treated wastewater from selected wastewater treatment plants. In Environmental Engineering Studies; Pawlowski, L., Dudzinska, M.R., Pawlowski, A., Eds.; Springer: New York, NY, USA, 2003; pp. 167–173. [Google Scholar] [CrossRef]
- Monapathi, M.E.; Oguegbulu, J.C.; Adogo, L.; Klink, M.; Okoli, B.; Mtunzi, F.; Modise, J.S. Pharmaceutical pollution: Azole antifungal drugs and resistance of opportunistic pathogenic yeasts in wastewater and environmental water. Appl. Environ. Soil Sci. 2021, 2021, 9985398. [Google Scholar] [CrossRef]
- Reddy, G.K.K.; Padmavathi, A.R.; Nancharaiah, Y.V. Fungal infections: Pathogenesis, antifungals and alternate treatment approaches. Curr. Res. Microb. Sci. 2022, 3, 100137. [Google Scholar] [CrossRef]
- YujiKim, J.Y.U.; Bessegato, G.G.; de Souza, B.C.; da Silva, J.J.; Zanoni, M.V.B. Efficient treatment of swimming pool water by photoelectrocatalytic ozonation: Inactivation of Candida parapsilosis and mineralization of Benzophenone-3 and urea. Chem. Eng. J. 2019, 378, 122094. [Google Scholar] [CrossRef]
- Szymański, M.; Chmielewska, S.; Czyżewska, U.; Malinowska, M.; Tylicki, A. Echinocandins–structure, mechanism of action and use in antifungal therapy. J. Enzyme Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef]
- Barantsevich, N.; Barantsevich, E. Diagnosis and Treatment of Invasive Candidiasis. Antibiotics 2022, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin b and other polyenes—Discovery, clinical use, mode of action and drug resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Gabaldón, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Flörl, C.; Perlin, D.S. Drug-resistant fungi: An emerging challenge threatening our limited antifungal armamentarium. Antibiotics 2020, 9, 877. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, D.F.; Balena, F.; Ronga, L.; Signorile, F.; Romanelli, F.; Stolfa, S.; Sparapano, E.; De Carlo, C.; Mosca, A.; Monno, L.; et al. Emerging issue of fluconazole-resistant candidemia in a tertiary care hospital of southern italy: Time for antifungal stewardship program. J. Med. Mycol. 2022, 32, 101206. [Google Scholar] [CrossRef] [PubMed]
- Buil, J.B.; Snelders, E.; Denardi, L.B.; Melchers, W.J.G.; Verweij, P.E. Trends in Azole Resistance in the Netherlands 1994–2016. Emerg. Infect. Dis. 2019, 25, 176–178. [Google Scholar] [CrossRef]
- Friedman, D.Z.P.; Schwartz, I.S. Emerging fungal infections: New patients, new patterns, and new pathogens. J. Fungi 2019, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.R.; Verweij, P.E.; Castanheira, M.; Dannaoui, E.; White, P.L.; Arendrup, M.C. Molecular mechanisms of acquired antifungal drug resistance in principal fungal pathogens and EUCAST guidance for their laboratory detection and clinical implications. J. Antimicrob. Chemother. 2022, 77, 2053–2073. [Google Scholar] [CrossRef] [PubMed]
- Ener, B.; Ergin, Ç.; Gülmez, D.; Aǧca, H.; Tikveşli, M.; Aksoy, S.A.; Otkun, M.; Siǧ, A.K.; Öǧünç, D.; Özhak, B.; et al. Frequency of azole resistance in clinical and environmental strains of Aspergillus fumigatus in Turkey: A multicentre study. J. Antimicrob. Chemother. 2022, 77, 1894–1898. [Google Scholar] [CrossRef]
- Enoch, D.A.; Ludlam, H.A.; Brown, N.M. Invasive fungal infections: A review of epidemiology and management options. J. Med. Microbiol. 2006, 55, 809–818. [Google Scholar] [CrossRef]
- Kluge, S.; Strauß, R.; Kochanek, M.; Weigand, M.A.; Rohde, H.; Lahmer, T. Aspergillosis: Emerging risk groups in critically ill patients. Med. Mycol. 2021, 60, myab064. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect. Dis. 2019, 6, 16–18. [Google Scholar] [CrossRef]
- Rivelli Zea, S.M.; Toyotome, T. Azole-resistant Aspergillus fumigatus as an emerging worldwide pathogen. Microbiol. Immunol. 2022, 66, 135–144. [Google Scholar] [CrossRef]
- Castorena-Montoya, M. A Translational Approach to Improve Clinical Outcomes of Invasive Fungal Infections through Disease Surveillance and Antifungal Drug Discovery; University of Rochester: New York, NY, USA, 2020; 233p. [Google Scholar]
- Lockhart, S.R.; Frade, J.P.; Etienne, K.A.; Pfaller, M.A.; Diekema, D.J.; Balajee, S.A. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob. Agents Chemother. 2011, 55, 4465–4468. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gamboa, R.A. New Tools in laboratory diagnosis of invasive fungal infections. In Tha Impact of Climate Change of Fungal Diseases. Fungal Biology; Springer: Cham, Switzerland, 2022; pp. 257–276. [Google Scholar] [CrossRef]
- Kone, A.K.; Thera, M.A. Resistance of Candida isolates to antifungals using VITEK system at the Rodolphe Mérieux Centre, Bamako, Mali. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Ira, V.B.; Roger, F.; Noell, J. Identification, Prevalence and Susceptibility Profile of Candida Isolates at the Pasteur Institute in Côte D ’ ivoire From 2017 to 2019. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Jacobs, J.L.; Dennis, E.K.; Taimur, S.; Rana, M.; Patel, D.; Gitman, M.; Patel, G.; Schaefer, S.; Iyer, K.; et al. Candida auris Pan-Drug-Resistant to Four Classes of Antifungal Agents. Antimicrob. Agents Chemother. 2022, 66, e0005322. [Google Scholar] [CrossRef]
- Brandão, L.R.; Medeiros, A.O.; Duarte, M.C.; Barbosa, A.C.; Rosa, C.A. Diversity and antifungal susceptibility of yeasts isolated by multiple-tube fermentation from three freshwater lakes in Brazil. J. Water Health 2010, 8, 279–289. [Google Scholar] [CrossRef]
- Steffen, H.; Bosch, C.; Wolfaardt, G.; Botha, A. Rising environmental temperatures and polluted surface waters: The prelude to the rise of mycoses in South Africa. Water SA 2022, 48, 199–216. [Google Scholar] [CrossRef]
- Cupozak-Pinheiro, W.J.; Araújo de Almeida-Apolonio, A.; Sasaki, M.H.; Maran, N.H.; Pires de Araújo, R.; Beraldo dos Santos Silva, D.; de Andrade dos Santos, J.V.; Barufatti, A.; Rodrigues Chang, M.; Pires de Oliveira, K.M. Candida species contamination in drinking groundwater from residence wells in three municipalities of midwestern Brazil and the potential human health risks. Microb. Pathog. 2022, 169, 105660. [Google Scholar] [CrossRef]
- Pérez-García, A.; Abad, R.F.; Pestaña, M. Infecciones en el paciente inmunocomprometido (II). Pacientes con trasplante de órgano sólido. Medicine 2022, 13, 3288–3297. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.O. Micosis en pacientes inmunocomprometidos. Med.-Programa Form. Médica Contin. Acreditado 2022, 13, 3415–3425. [Google Scholar] [CrossRef]
- González, L.; Sarabia, D. Prevalencia de Infecciones Fúngicas Oportunistas en Pacientes Hospitalizados en la Unidad de Cuidados Intensivos del Hospital Teodoro Maldonado Carbo en el Periodo Comprendido Entre Enero 2016 a Enero 2021; Universidad Católica de Santiago de Guayaquil: Guayaquil, Ecuador, 2022. [Google Scholar]
- Araya-Rojas, F.; Lasso-Barreto, M. Aspergilosis pulmonar asociada a COVID-19 en pacientes críticos: Experiencia de un hospital público chileno. Rev. Chil. Infectología 2021, 38, 754–760. [Google Scholar] [CrossRef]
- Sánchez Martín, C.; Madrid Martínez, E.; González Pellicer, R.; Armero Ibáñez, R.; Martínez González, E.; Llau Pitarch, J.V. Invasive pulmonary aspergillosis in patients with acute respiratory syndrome by COVID-19. Rev. Española Anestesiol. Reanim. (Engl. Ed.) 2022, 69, 48–53. [Google Scholar] [CrossRef]
- Vinayagamoorthy, K.; Pentapati, K.C.; Prakash, H. Prevalence, risk factors, treatment and outcome of multidrug resistance Candida auris infections in Coronavirus disease (COVID-19) patients: A systematic review. Mycoses 2022, 65, 613–624. [Google Scholar] [CrossRef]
- Rybak, J.M.; Fortwendel, J.R.; Rogers, P.D. Emerging threat of triazole-resistant Aspergillus fumigatus. J. Antimicrob. Chemother. 2019, 74, 835–842. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Hubka, V.; Watanabe, A.; Nagi, M.; Miyazaki, Y.; Yaguchi, T.; Kamei, K. Prevalence of Antifungal Resistance, Genetic Basis of Acquired Azole and Echinocandin Resistance, and Genotyping of Candida krusei Recovered from an International Collection. Antimicrob. Agents Chemother. 2022, 66, e01856-21. [Google Scholar] [CrossRef] [PubMed]
- Domán, M.; Makrai, L.; Bányai, K. Molecular Phylogenetic Analysis of Candida krusei. Mycopathologia 2022, 187, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Vahedi-Shahandashti, R.; Dietl, A.M.; Binder, U.; Nagl, M.; Wurzner, R.; Lass-Florl, C. Aspergillus terreus and the Interplay with Amphotericin B: From Resistance to Tolerance? Antimicrob. Agents Chemother. 2022, 66, e0227421. [Google Scholar] [CrossRef] [PubMed]
- Angiolella, L. Virulence Regulation and Drug-Resistance Mechanism of Fungal Infection. Microorganisms 2022, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Wan, Q.; Deng, X.; Cao, R.; Xu, X.; Chen, Z.; Wang, J.; Huang, T. Reactivation of fungal spores in water following UV disinfection: Effect of temperature, dark delay, and real water matrices. Chemosphere 2019, 237, 124490. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Cai, H.Q.; Qu, S.Y.; Lin, W.H.; Liang, C.C.; Liu, H.; Xie, Z.X.; Yuan, Y.J. Genomic Variation-Mediating Fluconazole Resistance in Yeast. Biomolecules 2022, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Niimi, M.; Niimi, K.; Tanabe, K.; Cannon, R.D.; Lamping, E. Inhibitor-Resistant Mutants Give Important Insights into Candida albicans ABC Transporter Cdr1 Substrate Specificity and Help Elucidate Efflux Pump Inhibition. Antimicrob. Agents Chemother. 2022, 66, e01748-21. [Google Scholar] [CrossRef]
- Maheronnaghsh, M.; Teimoori, A.; Dehghan, P.; Fatahinia, M. The evaluation of the overexpression of the ERG-11, MDR-1, CDR-1, and CDR-2 genes in fluconazole-resistant Candida albicans isolated from Ahvazian cancer patients with oral candidiasis. J. Clin. Lab. Anal. 2022, 36, e24208. [Google Scholar] [CrossRef]
- Gonçalves, P.; Melo, A.; Dias, M.; Almeida, B.; Caetano, L.A.; Veríssimo, C.; Viegas, C.; Sabino, R. Azole-resistant aspergillus fumigatus harboring the tr34/l98h mutation: First report in portugal in environmental samples. Microorganisms 2021, 9, 57. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.; Lavergne, R.A.; Hagen, F.; Morio, F.; Meis, J.F.; Le Pape, P. Fungicide-driven alterations in azole-resistant Aspergillus fumigatus are related to vegetable crops in Colombia, South America. Mycologia 2019, 111, 217–224. [Google Scholar] [CrossRef]
- Brizzotti-Mazuchi, N.S.; Cunha, K.C.; Siqueira, J.P.Z.; Almeida, B.G.; Lemes, T.H.; Maschio-Lima, T.; Caetano, M.H.; Ribeiro, M.D.; Rodrigues, C.R.; Castilho, E.M.; et al. Diversity, seasonality, and antifungal susceptibility of yeasts in the public drinking water supply in a municipality of southeastern Brazil. Ecohydrol. Hydrobiol. 2020, 20, 450–455. [Google Scholar] [CrossRef]
- Hollomon, D. Does agricultural use of azole fungicides contribute to resistance in the human pathogen Aspergillus fumigatus? Pest Manag. Sci. 2017, 73, 1987–1993. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Flores, D.; Lana, D.; Kelly, G.; Carneiro, M. Resistance of filamental fungi iResistance of filamental fungi in opportunistic mycoses: Literature review. Res. Soc. Dev. 2022, 11, e2011426198. [Google Scholar] [CrossRef]
- Pham, C.D.; Reiss, E.; Hagen, F.; Meis, J.F.; Lockhart, S.R. Passive surveillance for azole-resistant Aspergillus fumigatus, United States, 2011–2013. Emerg. Infect. Dis. 2014, 20, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, J.W.M.; Arendrup, M.C.; Warris, A.; Lagrou, K.; Pelloux, H.; Hauser, P.M.; Chryssanthou, E.; Mellado, E.; Kidd, S.E.; Tortorano, A.M.; et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg. Infect. Dis. 2015, 21, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, J.W.M.; Arendrup, M.C.; Melchers, W.J.G.; Verweij, P.E. Multiple fungicide-driven alterations in azole-resistant Aspergillus fumigatus, Colombia, 2015. Emerg. Infect. Dis. 2016, 22, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.S.; Whitley, R.D.; Plummer, C.E.; Richardson, R.L.; Hamor, R.E.; Wellehan, J.F.X. In vitro antifungal susceptibility of Fusarium species and Aspergillus fumigatus cultured from eleven horses with fungal keratitis. Vet. Ophthalmol. 2022, 25, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Morin, N.; Eric, C.; Guorui, L.; Balaram, V.; Rita, A.; Ribeiro, L. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Oke, L.; Maseko, O.B. Occurrence of antibiotics in wastewater from hospital and convectional wastewater treatment plants and their impact on the effluent receiving rivers: Current knowledge between 2010 and 2019. Environ. Monit. Assess. 2022, 194, 2–25. [Google Scholar] [CrossRef]
- Castelo-Branco, D.; Lockhart, S.R.; Chen, Y.-C.; Santos, D.A.; Hagen, F.; Hawkins, N.J.; Lavergne, R.-A.; Meis, J.F.; Le Pape, P.; Rocha, M.F.G.; et al. Collateral consequences of agricultural fungicides on pathogenic yeasts: A One Health perspective to tackle azole resistance. Mycoses 2022, 65, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-izquierdo, A.; Berman, J.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; Kuijper, E.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Lota, M.M.M.; Chua, A.Q.; Azupardo, K.; Lumangaya, C.; Reyes, K.A.V.; Yvette, S.; Villanueva, A.M.; Legido-Quigley, H.; Roxas, E.A. A Qualitative Study on the Design and Implementation of the National Action Plan on Antimicrobial Resistance in the Philippines. Antibiotics 2022, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, Z.; Vidović, J.; Erdeljan, M.; Cincović, M.; Ružić, Z.; Galić, I.; Kukurić, T.; Stojanac, N.; Horvat, O. Veterinary Practitioners ’ Standpoints and Comprehension towards Antimicrobial Use-Are There Opportunities for Antimicrobial Stewardship Improvement? Antibiotics 2022, 11, 867. [Google Scholar] [CrossRef]
- Al-gabr, H.M.; Zheng, T.; Yu, X. Efficacy of two chemical coagulants and three different filtration media on removal of Aspergillus flavus from surface water. J. Environ. Sci. 2014, 26, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Albolafio, S.; Marín, A.; Allende, A.; García, F.; Sim, P.J.; Abell, M.; Gil, M.I. Strategies for mitigating chlorinated disinfection byproducts in wastewater treatment plants. Chemosphere 2022, 288, 132583. [Google Scholar] [CrossRef]
- Foschi, J.; Bianchi, G.F.; Turolla, A.; Antonelli, M. Disinfection efficiency prediction under dynamic conditions: Application to peracetic acid disinfection of wastewater. Water Res. 2022, 222, 118879. [Google Scholar] [CrossRef]
- Ocampo-Rodríguez, D.B.; Vázquez-Rodríguez, G.A.; Martínez-Hernández, S.; Iturbe-Acosta, U.; Coronel-Olivares, C. Water disinfection: A review of conventional and advanced treatments with chlorine and peracetic acid. Ing. Agua 2022, 26, 185–204. [Google Scholar] [CrossRef]
- Venkobachar, C.; Iyengar, L.; Prabhakara Rao, A.V.S. Mechanism of disinfection: Effect of chlorine on cell membrane functions. Water Res. 1977, 11, 727–729. [Google Scholar] [CrossRef]
- Wan, Q.; Xia, Y.; Li, Y.; Wu, G.; Wang, J.; Huang, T.; Wen, G. Enhanced solar inactivation of fungal spores by addition of low-dose chlorine: Efficiency and mechanism. Water Res. 2022, 222, 118964. [Google Scholar] [CrossRef] [PubMed]
- Adefisoye, M.A.; Olaniran, A.O. Does Chlorination Promote Antimicrobial Resistance in Waterborne Pathogens? Mechanistic Insight into Co-Resistance and Its Implication for Public Health. Antibiotics 2022, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wan, Q.; Tan, L.; Xu, X.; Wu, G.; Wang, J.; Xu, H.; Huang, T.; Wen, G. Evaluation of the vital viability and their application in fungal spores’ disinfection with flow cytometry. Chemosphere 2021, 269, 128700. [Google Scholar] [CrossRef] [PubMed]
- Deborde, M.; von Gunten, U. Reactions of chlorine with inorganic and organic compounds during water treatment—Kinetics and mechanisms: A critical review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, W.D.; Minnigh, H.A.; Pipes, W.O. Chlorine demand and inactivation of fungal propagules. Appl. Environ. Microbiol. 1983, 45, 182–186. [Google Scholar] [CrossRef]
- Ma, X.; Baron, J.L.; Vikram, A.; Stout, J.E.; Bibby, K. Fungal diversity and presence of potentially pathogenic fungi in a hospital hot water system treated with on-site monochloramine. Water Res. 2015, 71, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Bibby, K. Free chlorine and monochloramine inactivation kinetics of Aspergillus and Penicillium in drinking water. Water Res. 2017, 120, 265–271. [Google Scholar] [CrossRef]
- Luo, X.; Xu, X.; Cao, R.; Wan, Q.; Wang, J.; Xu, H.; Lin, Y.; Wen, G.; Huang, T. The formation kinetics and control of biofilms by three dominant fungi species isolated from groundwater. J. Environ. Sci. 2021, 109, 148–160. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, X.; Tan, L.; Liang, Z.; Cao, R.; Wan, Q. The aggregation of Aspergillus spores and the impact on their inactivation by chlorine-based disinfectants. Water Res. 2021, 204, 117629. [Google Scholar] [CrossRef]
- Lim, S.; Lily, J.; von Gunten, U.; Mccurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef]
- Wolf, C.; von Gunten, U.; Kohn, T. Kinetics of Inactivation of Waterborne Enteric Viruses by Ozone. Environ. Sci. Technol. 2018, 52, 2170–2177. [Google Scholar] [CrossRef]
- Costa, L.R.d.C.; Féris, L.A. Use of ozonation technology to combat viruses and bacteria in aquatic environments: Problems and application perspectives for SARS-CoV-2. Environ. Technol. 2022, 44, 2490–2502. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rey, R.; Cháez, H.; Baluja, C. Ozone Inactivation of Biologically-Risky Wastewaters. Ozone Sci. Eng. 1995, 17, 499–509. [Google Scholar] [CrossRef]
- Liang, Z.; Xu, X.; Cao, R.; Wan, Q.; Xu, H.; Wang, J.; Lin, Y.; Huang, T.; Wen, G. Synergistic effect of ozone and chlorine on inactivating fungal spores: Influencing factors and mechanisms. J. Hazard. Mater. 2021, 420, 126610. [Google Scholar] [CrossRef]
- Huang, N.; Wang, W.-L.; Xu, Z.-B.; Lee, M.-Y.; Wu, Q.-Y.; Hu, H.-Y. A study of synergistic oxidation between ozone and chlorine on benzalkonium chloride degradation: Reactive species and degradation pathway. Chem. Eng. J. 2020, 382, 122856. [Google Scholar] [CrossRef]
- Diaz Fernandez, J.O.S.E. Ecuaciones y Cálculos para el Tratamiento de Aguas; Ediciones Paraninfo S.A.: Madrid, Spain, 2019; ISBN 8428341524. [Google Scholar]
- Zhang, C.; Brown, P.J.B.; Hu, Z. Thermodynamic properties of an emerging chemical disinfectant, peracetic acid. Sci. Total Environ. 2018, 621, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Xu, X.; Wan, Q.; Cao, R.; Liang, Z.; Xu, H.; Li, K.; Huang, T.; Wen, G.; Ma, J. Inactivation of fungal spores in water with peracetic acid: Efficiency and mechanism. Chem. Eng. J. 2022, 427, 131753. [Google Scholar] [CrossRef]
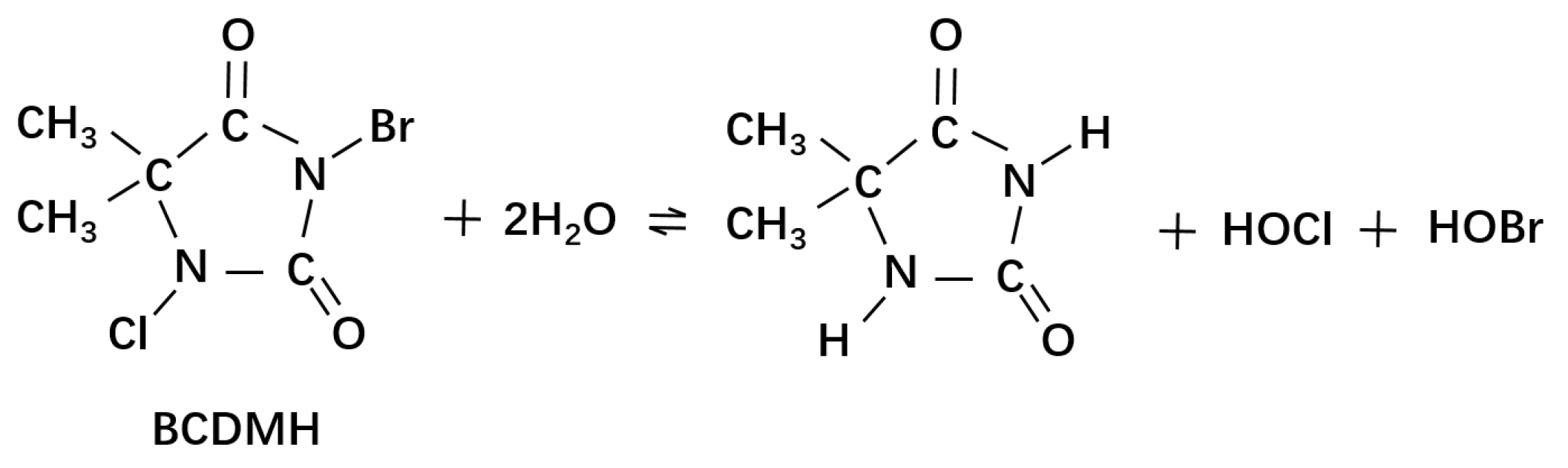
- Wen, G.; Tan, L.; Cao, R.; Wan, Q.; Xu, X.; Wu, G. Inactivation of waterborne fungal spores by 1-bromo-3-chloro-5, 5- dimethylhydantoin: Kinetics, in fl uencing factors and mechanisms. Chemosphere 2021, 274, 129764. [Google Scholar] [CrossRef]
- Xu, X.; Ran, Z.; Wen, G.; Liang, Z.; Wan, Q.; Chen, Z.; Lin, Y.; Li, K.; Wang, J.; Huang, T. Efficient inactivation of bacteria in ballast water by adding potassium peroxymonosulfate alone: Role of halide ions. Chemosphere 2020, 253, 126656. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Li, W.; Qiang, Z. A review of the fluence determination methods for UV reactors: Ensuring the reliability of UV disinfection. Chemosphere 2022, 286, 131488. [Google Scholar] [CrossRef]
- Khan, M.; Mcdonald, M.; Mundada, K. Efficacy of Ultraviolet Radiations against Coronavirus, Bacteria, Fungi, Fungal Spores and Biofilm. Hygiene 2022, 2, 120–131. [Google Scholar] [CrossRef]
- Mukunda, D.C.; Joshi, V.K.; Mahato, K.K. Light emitting diodes (LEDs) in fluorescence-based analytical applications: A review. Appl. Spectrosc. Rev. 2022, 57, 1–38. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J. Oxidatively Generated Damage to Cellular DNA by UVB and UVA Radiation. Photochem. Photobiol. 2015, 91, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.Y.; Murray, V. The influence of DNA methylation on the sequence specificity of UVB- and UVC-induced DNA damage. J. Photochem. Photobiol. B Biol. 2021, 221, 112225. [Google Scholar] [CrossRef] [PubMed]
- Braga, G.U.L.; Rangel, D.E.N.; Fernandes, É.K.K.; Flint, S.D.; Roberts, D.W. Molecular and physiological effects of environmental UV radiation on fungal conidia. Curr. Genet. 2015, 61, 405–425. [Google Scholar] [CrossRef] [PubMed]
- Goldman, G.H.; Kafer, E. Aspergillus nidulans as a model system to characterize the DNA damage response in eukaryotes. Fungal Genet. Biol. 2004, 41, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, S.; Rosado, D.; Moreno-andrés, J.; Cartuche, L.; Cruz, D.; Acevedo-merino, A.; Nebot, E. Inactivation of a wild isolated Klebsiella pneumoniae by photo-chemical processes: UV-C, UV-C/H2O2 and UV-C/H2O2/Fe3+. Catal. Today 2018, 313, 94–99. [Google Scholar] [CrossRef]
- Xu, X.; Zuo, J.; Wan, Q.; Cao, R.; Xu, H.; Li, K. Effective inactivation of fungal spores by the combined UV/PAA: Synergistic effect and mechanisms. J. Hazard. Mater. 2022, 430, 128515. [Google Scholar] [CrossRef]
- França, M.; Deborah, D.; Freitas, L.; Cristina, E.; Leticia, M.; Dutra, C.; Gabriel, L.; Juliana, F.; Araújo, C. De Effects of activated sludge and UV disinfection processes on the bacterial community and antibiotic resistance profile in a municipal wastewater treatment plant. Environ. Sci. Pollut. Res. 2022, 29, 36088–36099. [Google Scholar] [CrossRef]
- Rodríguez, R.A.; Navar, C.; Sangsanont, J.; Linden, K.G. UV inactivation of sewage isolated human adenovirus. Water Res. 2022, 218, 118496. [Google Scholar] [CrossRef] [PubMed]
- Nourmoradi, H.; Nikaeen, M.; Stensvold, C.R.; Mirhendi, H. Ultraviolet irradiation: An effective inactivation method of Aspergillus spp. in water for the control of waterborne nosocomial aspergillosis. Water Res. 2012, 46, 5935–5940. [Google Scholar] [CrossRef]
- Pereira, V.J.; Ricardo, J.; Galinha, R.; Benoliel, M.J.; Crespo, M.T.B. Occurrence and low pressure ultraviolet inactivation of yeasts in real water sources. Photochem. Photobiol. Sci. 2013, 12, 626–630. [Google Scholar] [CrossRef]
- Wan, Q.; Wen, G.; Cao, R.; Xu, X.; Zhao, H.; Li, K.; Wang, J.; Huang, T. Comparison of UV-LEDs and LPUV on inactivation and subsequent reactivation of waterborne fungal spores. Water Res. 2020, 173, 115553. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.R.; Crespo, M.T.B.; Pereira, V.J. Small but powerful: Light-emitting diodes for inactivation of Aspergillus species in real water matrices. Water Res. 2020, 168, 115108. [Google Scholar] [CrossRef]
- Oliveira, B.R.; Marques, A.P.; Asif, M.; Crespo, M.T.B.; Pereira, V.J. Light-emitting diodes effect on Aspergillus species in filtered surface water: DNA damage, proteome response and potential reactivation. Environ. Pollut. 2021, 287, 117553. [Google Scholar] [CrossRef]
- Moreira, C.J.S.; Pereira, C.S.; Oliveira, B.R.; Marques, A.P.; Ressurreiç, M.; Crespo, T.B.; Pereira, V.J. Inactivation of Aspergillus species in real water matrices using medium pressure mercury lamps. J. Photochem. Photobiol. B Biol. 2021, 221, 112242. [Google Scholar] [CrossRef]
- Severin, B.F. Kinetic modeling of UV disinfetion of water. Water Res. 1983, 17, 1669–1678. [Google Scholar] [CrossRef]
- Geeraerd, A.H.; Herremans, C.H.; Van Impe, J.F. Structural model requirements to describe microbial inactivation during a mild heat treatment. Int. J. Food Microbiol. 2000, 59, 185–209. [Google Scholar] [CrossRef]
- Rattanakul, S.; Oguma, K. Inactivation kinetics and ef fi ciencies of UV-LEDs against Pseudomonas aeruginosa, Legionella pneumophila, and surrogate microorganisms. Water Res. 2018, 130, 31–37. [Google Scholar] [CrossRef]
- Mcguigan, K.G.; Conroy, R.M.; Mosler, H.; Ubomba-jaswa, E.; Fernandez-iba, P. Solar water disinfection (SODIS): A review from bench-top to roof-top. J. Hazard. Mater. 2012, 235–236, 29–46. [Google Scholar] [CrossRef]
- Berney, M.; Weilenmann, H.; Egli, T. Flow-Cytometric study of vital cellular functions in Escherichia coli during solar disinfection (SODIS) Flow-cytometric study of vital cellular functions in Escherichia coli during solar disinfection (SODIS). Microbiology 2006, 152, 1719–1729. [Google Scholar] [CrossRef]
- Kadir, K.; Nelson, K.L. Sunlight mediated inactivation mechanisms of Enterococcus faecalis and Escherichia coli in clear water versus waste stabilization pond water. Water Res. 2013, 50, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Lonnen, J.; Kilvington, S.; Kehoe, S.C.; Al-Touati, F.; McGuigan, K.G. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Res. 2005, 39, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wan, Q.; Xu, X.; Cao, R.; Li, Y.; Wang, J. Solar disinfection of fungal spores in water: Kinetics, influencing factors, mechanisms and regrowth. Chem. Eng. J. 2022, 428, 132065. [Google Scholar] [CrossRef]
- Isaac, S.; Salmer, I. Solar-driven free chlorine advanced oxidation process for simultaneous removal of microcontaminants and microorganisms in natural water at pilot-scale. Chemosphere 2022, 288, 132493. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; Wan, Q.; Xu, X.; Cao, R.; Wang, J. Inactivation of fungal spores in water by CuO-activated peracetic acid: Kinetics, mechanism and regrowth. J. Hazard. Mater. 2022, 439, 129611. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.R.; Maura, R.; Franco, B.; Guadagnini, R.A.; Urbano, L. Giardia duodenalis: Number and Fluorescence Reduction Caused by the Advanced Oxidation Process (H2O2/UV). Int. Sch. Res. Not. 2014, 2014, 525719. [Google Scholar] [CrossRef]
- Augugliaro, V.; Litter, M.; Palmisano, L.; Soria, J. The combination of heterogeneous photocatalysis with chemical and physical operations: A tool for improving the photoprocess performance. J. Photochem. Photobiol. C Photochem. Rev. 2006, 7, 127–144. [Google Scholar] [CrossRef]
- Alarco, D.C.; Maldonado, M.I.; Malato, S.; Gernjak, W. Photocatalytic decontamination and disinfection of water with solar collectors. Catal. Today 2007, 122, 137–149. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Surbhi, B.; Ameta, S.C. Photocatalysis. In Advanced Oxidation Processes for Wastewater Treatment; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, UK, 2018; pp. 135–175. [Google Scholar]
- Guerra-Rodriguez, S.; Rodríguez-Chueca, J.; Peres, J.A.; Lucas, M.S. Inactivation of Pathogenic Microorganisms with Sulfate Radical-based Advanced Oxidation Processes. In Persulfate-Based Oxidation Processes in Environmental Remediation; Zhu, M., Bian, Z., Zhao, C., Eds.; The Royal Society of Chemistry: London, UK, 2022; pp. 229–251. ISBN 978-1-83916-308-1. [Google Scholar]
- Khan, J.A.; Sayed, M.; Khan, S.; Shah, N.S.; Dionysiou, D.D.; Boczkaj, G. Advanced oxidation processes for the treatment of contaminants of emerging concern. In Contaminants of Emerging Concern in Water and Wastewater; Hernández-Maldonado, A.J., Blaney, L., Eds.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 299–365. [Google Scholar]
- Ioannou-ttofa, L.; Raj, S.; Prakash, H.; Fatta-kassinos, D. Solar photo-Fenton oxidation for the removal of ampicillin, total cultivable and resistant E. coli and ecotoxicity from secondary-treated wastewater effl uents. Chem. Eng. J. 2019, 355, 91–102. [Google Scholar] [CrossRef]
- Carbajo, J.; Silveira, J.E.; Pliego, G.; Zazo, J.A.; Casas, J.A. Increasing Photo-Fenton process Efficiency: The effect of high temperatures. Sep. Purif. Technol. 2021, 271, 118876. [Google Scholar] [CrossRef]
- Polo-López, M.I.; García-Fernández, I.; Velegraki, T.; Katsoni, A.; Oller, I.; Mantzavinos, D.; Fernández-Ibáñez, P. Mild solar photo-Fenton: An effective tool for the removal of Fusarium from simulated municipal effluents. Appl. Catal. B Environ. 2012, 111–112, 545–554. [Google Scholar] [CrossRef]
- Polo-López, M.I.; Castro-Alférez, M.; Oller, I.; Fernández-Ibáñez, P. Assessment of solar photo-Fenton, photocatalysis, and H2O2 for removal of phytopathogen fungi spores in synthetic and real effluents of urban wastewater. Chem. Eng. J. 2014, 257, 122–130. [Google Scholar] [CrossRef]
- Oller, I.; Fernández-ibá, P. Benefits of photo-Fenton at low concentrations for solar disinfection of distilled water. A case study: Phytophthora capsici. Catal. Today 2013, 209, 181–187. [Google Scholar] [CrossRef]
- Aguas, Y.; Hincapie, M.; Fernández-Ibáñez, P.; Polo-López, M.I. Solar photocatalytic disinfection of agricultural pathogenic fungi (Curvularia sp.) in real urban wastewater. Sci. Total Environ. 2017, 607–608, 1213–1224. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Naeem, K.; Ouyang, F. Preparation of Fe3+-doped TiO2 nanoparticles and its photocatalytic activity under UV light. Phys. B Phys. Condens. Matter 2010, 405, 221–226. [Google Scholar] [CrossRef]
- Polo-López, M.I.; Fernández-Ibáñez, P.; García-Fernández, I.; Oller, I.; Salgado-Tránsito, I.; Sichel, C. Resistance of Fusarium sp. spores to solar TiO2 photocatalysis: Influence of spore type and water (scaling-up results). J. Chem. Technol. Biotechnol. 2010, 85, 1038–1048. [Google Scholar] [CrossRef]
- Makoday, N.M.; Saprykina, M.N.; Soboleva, N.M.; Savluk, O.S.; Goncharuk, V.V. Inactivation of Candida Albicans in the UV/TiO2/Fe3+ system. J. Water Chem. Technol. 2015, 37, 140–144. [Google Scholar] [CrossRef]
- Rodrigues-Silva, C.; Miranda, S.M.; Lopes, F.V.S.; Silva, M.; Dezotti, M.; Silva, A.M.T.; Faria, J.L.; Boaventura, R.A.R. Bacteria and fungi inactivation by photocatalysis under UVA irradiation: Liquid and gas phase. Environ. Sci. Pollut. Res. 2017, 24, 6372–6381. [Google Scholar] [CrossRef] [PubMed]
- Rengifo-Herrera, J.A.; Pulgarin, C. Why five decades of massive research on heterogeneous photocatalysis, especially on TiO2, has not yet driven to water disinfection and detoxification applications? Critical review of drawbacks and challenges. Chem. Eng. J. 2023, 477, 146875. [Google Scholar] [CrossRef]
- Yeom, Y.; Han, J.; Zhang, X.; Shang, C.; Zhang, T.; Li, X.; Duan, X.; Dionysiou, D.D. A review on the degradation efficiency, DBP formation, and toxicity variation in the UV/chlorine treatment of micropollutants. Chem. Eng. J. 2021, 424, 130053. [Google Scholar] [CrossRef]
- Wan, Q.; Wen, G.; Cao, R.; Zhao, H.; Xu, X. Simultaneously enhance the inactivation and inhibit the photoreactivation of fungal spores by the combination of UV-LEDs and chlorine: Kinetics and mechanisms. Water Res. 2020, 184, 116143. [Google Scholar] [CrossRef] [PubMed]
- Al-gabr, H.M.; Zheng, T.; Yu, X. Inactivation of Aspergillus flavus in drinking water after treatment with UV irradiation followed by chlorination. Sci. Total Environ. 2013, 463–464, 525–529. [Google Scholar] [CrossRef]
- Ali, E.; Abdelrahman, T.; Sayed, M.; Abd Al Khalek, S. Occurrence of Fungi in Drinking Water Sources and Their Treatment by Chlorination and UV-Irradiation. Egypt. J. Bot. 2017, 57, 621–632. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, H.; Wan, Q.; Xu, X.; Cao, R.; Li, K.; Wang, J.; Huang, T.; Lu, J.; Wen, G. Inactivation and subsequent reactivation of Aspergillus species by the combination of UV and monochloramine: Comparisons with UV/chlorine. J. Environ. Sci. 2022, 117, 105–118. [Google Scholar] [CrossRef]
- Rehman, F.; Sayed, M.; Khan, J.A.; Shah, N.S.; Khan, H.M.; Dionysiou, D.D. Oxidative removal of brilliant green by UV/S2O8 2null, UV/HSO5 null and UV/H2O2 processes in aqueous media: A comparative study. J. Hazard. Mater. 2018, 357, 506–514. [Google Scholar] [CrossRef]
- Wen, G.; Deng, X.; Wan, Q.; Xu, X.; Huang, T. Photoreactivation of fungal spores in water following UV disinfection and their control using UV-based advanced oxidation processes. Water Res. 2019, 148, 1–9. [Google Scholar] [CrossRef]
- Ashrafivala, M.; Borhan, S.; Zeinali, S.; Heidari, M.; Mohammadpourfard, M.; Aslani, H. Investigation of H2O2/UV advanced oxidation process on the removal rate of coliforms from the industrial effluent: A pilot-scale study. Int. J. Hydrogen Energy 2022, 47, 33530–33540. [Google Scholar] [CrossRef]
- Sichel, C.; Fernández-Ibáñez, P.; de Cara, M.; Tello, J. Lethal synergy of solar UV-radiation and H2O2 on wild Fusarium solani spores in distilled and natural well water. Water Res. 2009, 43, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Polo-López, M.I.; Garcia-Fernández, I.; Oller, I.; Fernández-Ibáñez, P. Solar disinfection of fungal spores in water aided by low concentrations of hydrogen peroxide. Photochem. Photobiol. Sci. 2011, 2, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.M.; Macedo, G.; Pedrosa, M.; Becerra-Castro, C.; Castro-Silva, S.; Pereira, M.F.R.; Silva, A.M.T.; Nunes, O.C.; Manaia, C.M. Ozonation and UV254 nm radiation for the removal of microorganisms and antibiotic resistance genes from urban wastewater. J. Hazard. Mater. 2017, 323, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Liu, B. Mechanism study on the effect of peracetic acid (PAA), UV/PAA and ultrasonic/PAA oxidation on ultrafiltration performance during algae-laden water treatment. Water Res. 2022, 220, 118705. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Komasa, S.; Morimoto, Y.; Sekino, T.; Kawazoe, T.; Okazaki, J. UV/ozone irradiation manipulates immune response for antibacterial activity and bone regeneration on titanium. Mater. Sci. Eng. C 2021, 129, 112377. [Google Scholar] [CrossRef] [PubMed]
- Oliva, G.; Comia, J.R.; Senatore, V.; Zarra, T.; Ballestreros, F.; Belgiorno, V.; Naddeo, V. Degradation of gaseous volatile organic compounds (VOCs) by a novel UV-ozone technology. Sci. Rep. 2022, 12, 11112. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Chen, Z.; Wan, Q.; Zhao, D.; Xu, X.; Wang, J.; Li, K.; Huang, T. Activation of PMS by pipe corrosion products for fungi disinfection in water: Performance and mechanisms. Chem. Eng. J. 2020, 382, 123003. [Google Scholar] [CrossRef]
- Rodriguez-Chueca, J.; Moreira, I.; Lucas, M.S.; Tavares, P.B.; Sampaio, A. Disinfection of simulated and real winery wastewater using sulphate radicals: Peroxymonosulphate/transition metal/UV-A LED oxidation. J. Clean. Prod. 2017, 149, 805–817. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-liethy, M.A.; Ahmed, M.S.; Ezzat, S.M.; Kamel, M.M. Facile synthesis of magnetic disinfectant immobilized with silver ions for water pathogenic microorganism’s deactivation. Environ. Sci. Pollut. Res. 2018, 25, 22797–22809. [Google Scholar] [CrossRef]
- Le, T.T.H.; Ngo, T.T.; Nguyen, T.H.H.; Pham, T.D.; Vu, T.X.H.; Tran, Q.V. Green Nanoarchitectonics Using Cleistocalyx Operculatus Leaf Extract in the Preparation of Multifunctional Graphene Oxide/Fe3O4/Ag Nanomaterials for Water Decontamination and Disinfection. J. Inorg. Organomet. Polym. Mater. 2022, 32, 547–559. [Google Scholar] [CrossRef]
- Hadi, D.M.; Hossein, M.A.; Reza, J.G.; Razieh, S. Investigation and evaluation of ultrasound reactor for reduction of fungi from sewage. J. Zhejiang Univ. Sci. B 2007, 8, 493–497. [Google Scholar] [CrossRef]
- El-sayyad, G.S.; Elkodous, M.A.; El-khawaga, A.M.; Elsayed, M.A.; El-batal, A.I.; Gobara, M. Merits of photocatalytic and antimicrobial applications of gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite for pyridine removal and pathogenic bacteria/fungi disinfection: Implication for wastewater treatment. R. Soc. Chem. 2020, 10, 5241–5259. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.N.; Paiva, M.A.N.; Sampaio, C.M.S.; Castelo-Branco, D.S.C.M.; Teixeira, C.E.C.; de Alencar, L.P.; Bandeira, T.J.P.G.; Monteiro, A.J.; Cordeiro, R.A.; Pereira-Neto, W.A.; et al. Azole Resistance in Candida Spp. Isolated from Catú Lake, Ceará, Brazil: An Efflux-Pump-Mediated Mechanism. Braz. J. Microbiol. 2016, 47, 33–38. [Google Scholar] [CrossRef]
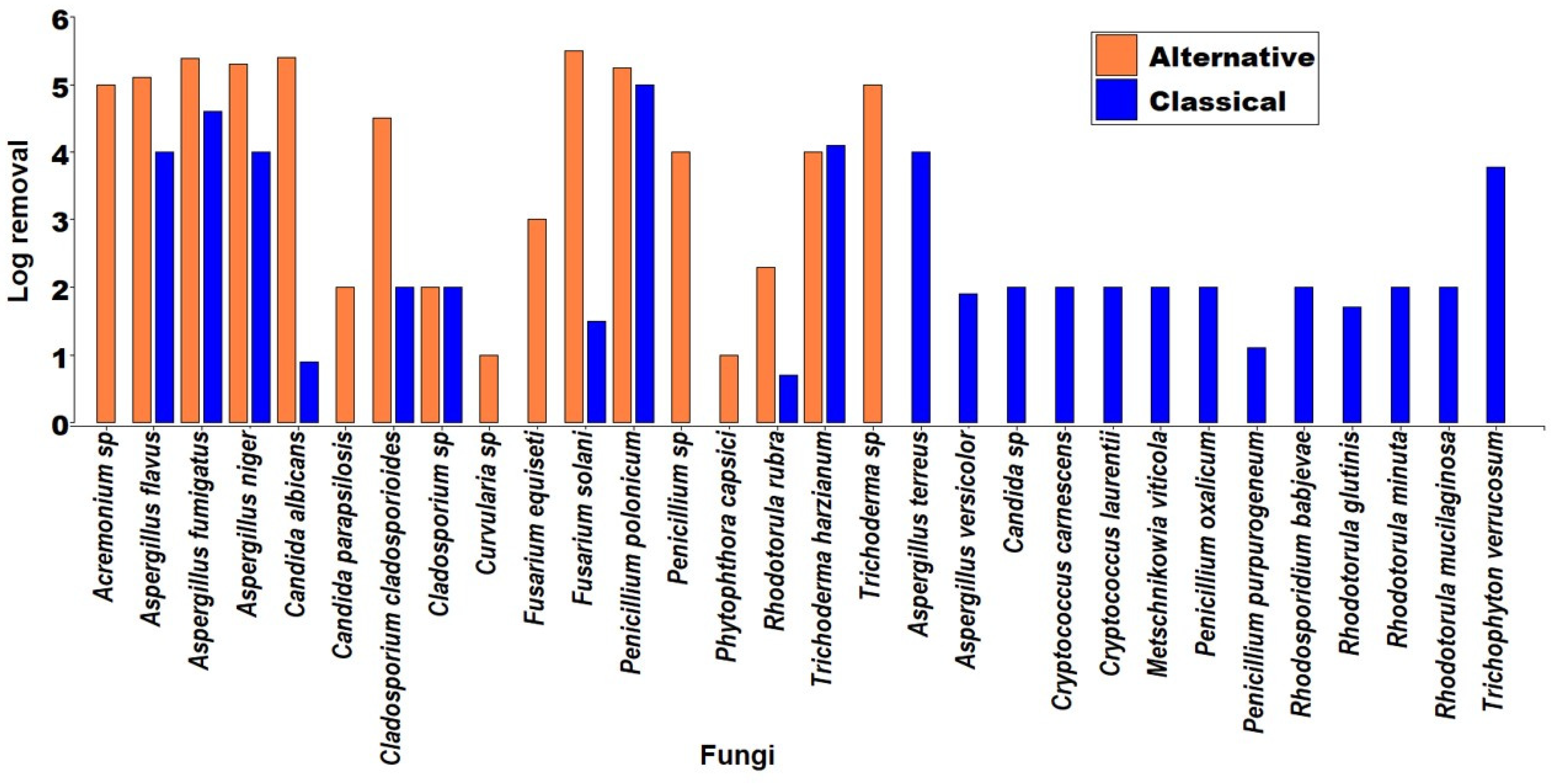
| Main Topics about Fungi Inactivation in Water | Reference |
|---|---|
| Fungi treatment by classical systems and advanced disinfection processes are discussed. However, kinetics models are not presented. | [20] |
| Fungi inactivation in drinking water. The main focus is on classical and UV-based processes. | [21] |
| Disinfection strategies for addressing fungal pathogens in medical devices and surgical instruments (medicine field but no water systems). | [23] |
| Focus only on sulfate radical-based processes and bacteria more than fungi. | [22] |
| Treatment of mold spores in the food industry. | [24] |
| Systematic review of the different classical and alternative processes, and kinetic aspects of water disinfection and antifungal resistance; in addition to a bibliometric analysis on fungi inactivation. | This work |
| Date | Matrix * | Fungi | Method | Experimental Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| 1983 | DW | A. fumigatus A. niger Cladosporium sp. C. laurentii P. oxalicum R.glutinis R. rubra | Cl2 | [Cl2]Free: 7 mg L−1. [conidia] 1.0 × 105 to 5.0 × 106 [Yeast]: 105 to 106 pH: 5, 7, or 8. t: 10, 30, 60 min, Inactivate by Na2S2O3 0.25% | pH 5 > 7 > 8. Inactivation level: Conidia’s: 1.0 log yeast: 1.0 log. Cl2 demand (mg per cell or conidium) after 60 min: A. fumigatus 1.2 × 10−8 A. niger 3.2 × 10−8 Cladosporium sp. 3.6 × 10−9 C. laurentii 8.0 × 10−9 P. oxalicum 5.9 × 10−9 R. glutinis, 2.6 × 10−9 R. rubra 2.4 × 10−9 | [122] |
| 2013 | LGW SW | C. tenuissimum, C. cladosporioides P. glomerata A. terreus A. fumigatus P. griseofulvum P. citrinum | Cl2 | [Cl2]Free: 1 mg L−1 in LGW and SW: 3 mg L−1 pH 7; 21° C. t: 1, 4, 10, 15, 20 min: 104 spores mL−1. Stop reaction by Ascorbic acid 25 mg L−1 | SW, 80% inactivation using a Ct: 60 mg min L−1. The Ct value is required to reach 0.7 log removal: A. fumigatus (946) A. terreus (1404) C. cladosporoides (139) C. tenuissimum (71) P. citrinum (959) P. griseofulvum (107) P. glomerata (152) | [13] |
| 2015 | HWS | Penicillium Aspergillus Peniophora Cladosporium Rhodospiridium Aureobasidium Fusarium | Cl2 NH2Cl. | NH2Cl. (mg L−1 as Cl2). Fungal ecology was then analyzed by high throughput sequencing of the fungal ITS1 region. | Penicillium was the dominant fungal genus. Average relative abundance: 88.89 ±6,37%. The central fungal biome consisted of the genera: Penicillium (100%) Aspergillus (90%), Peniophora (56.67%), Cladosporium (50%) Rhodosporidium (50%). Aureobasidium (43.33%) Fusarium (40%), Pichia (19.78%). No significant change in the fungal community structure | [123] |
| 2017 | DW | P. purpurogenum A. fumigatus, A. versicolor | Cl2 NH2Cl. | [Cl2] 1 mg L−1 of 5% NaClO [NH2Cl] 4–10 mg L−1 pH 7.0 for Cl2 pH 8.0 for NH2Cl [Spore]0: 4.3 log spore’s mL−1. t: 0, 5, 15, 30, 60 min. Stirred: 300 rpm, T: 22.5 °C. | Ct for Cl2 free 60 mg min/L. A. fumigatus: 2.9 log. A. fumigatus 4.6 log A. versicolor: 1.9 log P. purpurogenum: 0.9 log The disinfection kinetics: Chick–Watson model incorporating an initial lag phase and Markov Chain Monte Carlo model. | [124] |
| 2017 | GW | C. cladosporiodes T. harzianum P. polonicum | ClO2 | 5 ± 0.5 × 105 CFU mL−1, [ClO2] 0.5–3 mg L−1. 10 or 27 °C, 130 rpm. 10, 20, 30, 50, and 60 s Na2S2O3: 0.1 mol L−1 pH 6.0 and 7.0. [Humic acid]: 0, 0.5, 2.0, or 4.0 mg/L, as total organic carbon (TOC). The inactivation kinetics Chick, Chick–Watson, and Hom models. | Penicillium sp., Trichoderma sp., and Cladosporium sp. 100%, 99.6%, and 70% for t: 60 s. kinactivation to ClO2: Penicillium sp., Trichoderma sp., Cladosporium sp., and E. coli were 1.182, 0.615, and 0.398, kinactivation to Cl2: Penicillium sp., Trichoderma sp., and Cladosporium sp. were 0.045, 0.079, and 0.037. | [14] |
| 2021 | GW | A. niger P. polonicum T. harzianum | Cl2 ClO2 NH2Cl | [Spores]0 (4.5–5.5) × 105 CFU mL−1, pH: 7.2–7.4, T: 28 ± 1 °C. [Cl2], [ClO2], [NH2Cl]: 0.1, 0.3, 0.5, 0.7 and 1.0 mg L−1. | [Cl2]0 1.0 mg L−1: kmax A. niger, P. polonicum, and T. harzianum 25.0%,43.6%, and 34.6%, respectively. [ClO2]0 1.0 mg L−1: kmax A. niger, P. polonicum, and T. harzianum decreased by 26.7, 44.4%, and 37.8%, respectively. [NH2Cl]0 1.0 mg L−1, kmax A. niger, P. polonicum, and T. harzianum decreased by 23.7%, 32.4%, and 31.8%, respectively. | [125] |
| 2021 | PFW | A. flavus A. fumigatus | Cl2 ClO2 NH2Cl | [Spores]0: 5–8 × 105 CFU mL−1 [Cl2]: 2.0, 3.0 mg L−1 pH: 7.0 ± 0.2; T: 25 ± 2 °C | The inactivation rate constants of Aspergillus fumigatus at 30% and 63% aggregation degree were 1.5- and 4-fold lower than that of monodisperse spores, respectively. | [126] |
| Date | Matrix * | Fungi | Method | Experimental Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| 1994 | WW | T. verrucosum | O3 | [O3] up to 25 mg L−1 Flow rate: 4 L h−1 6 × 103 CFU mL−1 | Total inactivation (100%). Ozone consumption 200–210 mg O3 min L−1 | [130] |
| 2020 | GW | T. harzianum P. polinicum A. niger | O3 | 1–2 × 105 CFU mL−1 [O3]0: 2.0 mg L−1 T: 20 °C, pH = 7.0 40 mmol L−1 with 20 mmol L−1 t-BuOH | O3 to inactivation 2 log (99%) (mg min L−1): A. niger 5.65 T. harzianum 2.36 P. polonicum 0.82 | [15] |
| 2021 | GW | A. niger T. harzianum | O3 Cl2 | [O3]0 = 1.0, 2.0 mg L−1 [Cl2]0 = 1.0, 2.0, and 3.0 mg L−1 in 40 mM PBS pH = 7.0, T = 20 °C; [Spores]0: 1–2 × 105 CFU mL−1. | The log reduction in survival of A. niger and T. harzianum spores at 10 min: 0.28- and 0.13 log. Ct O3 and Cl2 inactivation 2log A. niger: 2.56 mg O3 min L−1 and 9.68 mg chlorine min L−1 T. harzianum 2.09 mg O3 min L−1 and 6.59 mg chlorine min L−1 | [131] |
| Date | Matrix * | Fungi | Method | Experimental Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| 2012 | DW | A. fumigatus A. flavus A. niger | UV | UV fluence: 4.15–25 mJ cm−2 102–103 CFU mL−1 t: 5–30 s Turbidity 1–5 NTU [Fe2+]: 0.1–0.5 mg L−1 | 4 log inactivation achieved at UV fluence (mJ cm−2), respectively: A. fumigatus 12.45 A. flavus 16.6 A. niger 20.75 | [149] |
| 2013 | UDW GW SW | Candida sp. C. carnescens M. viticola C. kofuensis R. babjevae R. minuta R. mucilaginosa | LPUP 254 nm. | UV fluences 0, 5, 10, 20, 30, 40, 70, and 100 mJ cm−2 T: 21 ± 2 °C. 2 and 6 × 106 cells mL−1. t: up to 13 min. | 2 log inactivation for all yeasts using UV fluences lower than 111 mJ cm−2. UV fluences lower than 32 mJ cm−2 to achieve 99% inactivation levels for the tested Cryptococcus, Candida, and Metschnikowia species | [150] |
| 2019 | GW | P. polonicum, A. niger T. harzianum | UV 254 nm | UV fluence: 0.112 mW cm−2. [Spore]0: 106 CFU mL−1. Room temperature. After 2 log10 UV inactivation: photoreactivation and dark repair | Fungal spores were more resistant compared with E. coli. The photoreactivation (k) rate constant of T. harzianum, A. niger, and P. polonicum: 0.0066 min−1, 0.0054 min−1, 0.0107 min−1. respectively. | [93] |
| 2020 | GW | A. niger P. polonicum, T. harzianum | UV/LEDs LPUV | Irradiance 0.215 mW/cm2 for the 265 nm LEDs, 0.214 mW cm−2 for the 280 nm LEDs, 0.185 mW cm−2 for the 265/280 nm combination UV-LEDs and 0.120 mW/cm2 for the 254 nm (LP) Initial spore 106 CFU mL−1. | UV inactivation efficiency (UV-LEDs and LP UV) was not influenced by the incubation time of spores. UV-LEDs emitting at 265, 280, and 265/280 nm were more effective compared with the 254 nm (LP). | [151] |
| 2020 | GW | A. niger A. terreus A. fumigatus | LED (255 nm, 265 nm) | 108 spores mL−1, pH 7, Temperature 20 °C. t: 0, 0.5, 1, 5, 10, 15, 30, 45, and 60 min. UV fluence of 2.33 mJ cm−2 Monitoring disinfection by plate counting, flow cytometry with viability staining, and electron microscopy. | A. fumigatus: 2 log reduction, with 10 min. A. terreus: 3 log reduction, with 1.64 mJ cm−2 (5 min) for the 265 nm. A. niger: reduction < 1 log was obtained even for the highest UV fluence (60 min) using both LEDs. | [152] |
| 2021 | GW | A. niger A. terreus A. fumigatus | LEDs (255 nm, 265 nm) | Irradiance 54 μW cm−2 (255 nm) and 250 μW cm−2 (265 nm). 108 spores/mL Exposure: 0, 30 and 60 min Natural light and dark as controls. | LEDs (255 nm) are less efficient in the inactivation of A. fumigatus and A. terreus, having no inactivation effect on A. niger. LEDs that emit at 265 nm showed 3 log, 2 log, and 4 log reduction for A. fumigatus, A. niger, and A. terreus, respectively | [153] |
| 2021 | GW | A. niger A. terreus A. fumigatus | UV Mercury lamp and UVH-Lamp Type Z. | 108 spores/mL, pH 7, T: 20 °C. t: 0, 0.5, 1, 5, 10, 15, 30, 45, and 60 min Efficacy monitoring by plate counting, scanning electron microscopy, flow cytometry analysis, DNA damage, proteome analysis, photoreactivation, and dark repair experiments. | A. fumigatus, A. niger, and A. terreus were 3.05 log, 0.23 log, and 3.50 log after 1 min of inactivation and 5.58 log, 1.90 log, and 5.63 log after 45 min of inactivation. Resistance: A. niger > A. fumigatus > A. terreus to UV MP radiation. | [154] |
| Date | Matrix | Fungi | Method | Experimental Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| 2005 | Drinking water | C. albicans F. solani | SODIS | [C. albicans]0 = 2.5 × 105 CFUmL−1 [F. solani]0 = 3.4 × 105 CFU mL−1 Global irradiances of 870 W m−2 300 nm–10 μm range and 200 W m−2 in the 300–400 nm UV range | C. albicans 4.1 log inactivation after 2 h; total inactivation 5.4 log at 6 h. Conidia of F. solani, 1.75 log inactivation occurred at 2 h and a total (5.5 log) inactivation at 8 h | [161] |
| 2022 | Groundwater | A. niger P. polonicum | SODIS | [Spores]0: 1–2 × 105 CFU mL−1 λ ≥ 300 nm. pH = 7.4 ± 0.2. T = 20 ± 2 °C. Irradiance simulated sunlight: 900 W m−2 300–800 nm. Agitation at 200 rpm. The pH 5–9; T: 30–40 °C for A. niger, and 20–30 °C for P. polonicum. | SL: A. niger (64.30 min) > P. polonicum (32.27 min) (p < 0.05), kmax: A. niger (0.033 min−1)< P. polonicum (0.062 min−1) (p < 0.05). 110 min P. polonicum to achieve 2 log inactivation, while for A. niger, 220 min. pH: 5.0–9.0 and [humic acid]: 1.0, 3.0 mg L−1 did not affect the solar inactivation of spores. | [162] |
| Date | Matrix * | Fungi | Method | Experimental Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| 2012 | SMWWE | F. solani | LDFO Solar radiation H2O2 oxidation in the dark | [Spore]0: 103 CFU mL−1 LDFO: [Fe2+]: 5 mg L−1, [H2O2]: 10 mg L−1 pH 3, Solar radiation 21.1 kJ L−1, pH 3–8 H2O2 oxidation alone up to 20 mg L−1 in the dark | Solar irradiation + 10 mg L−1 peroxide = ≤2 CFU mL−1, at 11.9 kJ L−1, pH 3 and 16.9 kJ L−1 at pH 4–8, but no mineralization occurred. Complete inactivation required 17.1 kJ L−1 accompanied by 36% mineralization. | [173] |
| 2013 | DW | P. capsici | Photo-Fenton Fe2+/H2O2 | H2O2/solar radiation: 2.5, 5, and 10 mg L−1 of H2O2 Photo-Fenton 1/2.5 mg L−1, 2.5/5 mg L−1, 5/10 mg L−1 of Fe2+/H2O2 and Solar photo-inactivation. [Spores]0 315 (±85) CFU mL−1. | Best inactivation results were achieved with 10 mg L−1 of H2O2 which required only 1 h of solar exposure (4 kJ L−1 of QUV) to attain the detection limit (2 CFU mL−1). 1 log spore reduction was attained with 5 mg L−1 of Fe3+ | [175] |
| 2014 | DW, SMWWE MWWE | F. solani | Photo-Fenton (Fe2+, Fe3+) | Sunlight and 10 mg L−1 H2O2 in dW. Photo-Fenton with FeSO4 in SMWWE at several concentrations. | The best F. solani inactivation rate was with photo-Fenton treatment (10/20 mg L−1 of Fe2+/H2O2) at pH 3, followed by H2O2/Solar (10 mg L−1) and finally TiO2/Solar was the slowest. Complete inactivation with 27 kJ L−1. | [174] |
| 2017 | WW, DW | Curvularia sp. | Solar/H2O2 Solar photo-Fenton | Several oxidant concentrations, 10, 20, 30, 40, and 60 mg L−1 in DW under natural solar irradiation. Acid and near-neutral pH | Complete inactivation with 30, 40, and 60 mg L−1 of H2O2 with solar UV dose between 14.7 and 15.2 kJ L−1 | [176] |
| Date | Matrix * | Fungi | Method | Experimental Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| 2005 | dW | C. albicans F. solani | SPC-DIS | [C. albicans]0: 2.5 × 105 CFU mL−1 [F. solani]0: 3.4 × 105 CFU mL−1 | C. albicans 4.1 log inactivation after 2 h with a total inactivation of 5.4 log at 4 h. Conidia of F. solani, 1.75 log inactivation occurred at 2 h and a total (5.5 log) inactivation at 4 h | [161] |
| 2009 | DW, WW | F. equiseti F. solani | PC | 5 or 6 h exposure Natural sunlight. [TiO2]: 0, 50, and 100 mg L−1, 30 L min−1 of flow rate | The highest Fusarium spore inactivation with 100 mg L−1 TiO2. Resistant: Chlamydospores > macroconidia > microconidia | [179] |
| 2014 | DW, SMWWE, MWWE | F. solani | PC Solar photoassis-ted H2O2 | Solar photocatalysis [TiO2]:100 mg L−1 SMWWE: Fe2+/H2O2 DW: Fe3+/H2O2 Solar photo-Fenton (FeSO4, pH: 3), TiO2, and H2O2 in MWWE. PC, Fe(NO3)3 in SMWWE at pH 3 and several concentrations. | Complete spore inactivation, 31.8 kJ/L of QUV were required, and DOC was reduced 56% at the end of the experimental time with 55.42 kJ/L of QUV. The highest temperature was 44.1 °C and the pH was 7.8. | [174] |
| 2015 | TP | C. albicans | UV/TiO2/Fe3+ | [Spores]0:1 × 106 CFU cm−3. λ = 289–365 nm pH 3, 7 and 9. [FeCl3]:1 × 10−2, 1 × 10−3, 1 × 10−4 mol L−1. | Log removal: pH 9: 5 log, pH 3: 3.5 log pH 7: 4 log [TiO2] 0.5; 0.25 and 0.1 g L−3 constitutes, respectively, 0.24, 0.37, and 0.5 log | [180] |
| 2017 | WW | A. fumigatus Penicillium spp. | PC (UVA/TiO2) | [TiO2]P25: 0.125 mg L−1 | Inactivation after 60 min of irradiation. Inhibition of 98.5% and 99.7% were by A. fumigatus and Penicillium sp. respectively, after 180 min under UVA irradiation | [181] |
| 2017 | WW, DW | Curvularia sp. | SPC-DIS with H2O2 | TiO2/solar and TiO2/H2O2/solar in vessel reactors. [TiO2]: 35, 50, and 100 mg L−1 Natural solar radiation in dW, [H2O2]: 10, 20, 30, 40 and 60 mg L−1 | (~1 log). light inactivation under natural sunlight. The water temperature varied from 26.1 to 38.2 ± 0.1 °C. 3 h. | [176] |
| Date | Matrix * | Fungi | Method | Experimental Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| 2013 | dW | A. flavus | UV Cl2 UV-Cl2 | UV fluence: 73.33 to 2239.5 mJ cm2. Contact time 1–60 s. [NaClO]: 0.5 to 3 mg L−1. Contact time: 1, 5, 10, 15, 30, 60, 120, 240, 480, and 1440 min. 10 °C. Ascorbic acid (25 mg L−1) was used to quench Cl2, pH: 7. | UV of 1119 mJ cm2: 2 log after the 30 s Chlorination: 0.5 mg L−1: 3 log at 120 min 1 mg L−1: 3 log at 60 min 2 mg L−1: 3 log: 10 min, 4 log: 2 h. 100% elimination: 24 h. 3 mg L−1: 2 log after 1 min, 4 log after 2 h, 100% inactivation after 24 h. UV for 5 s followed by Cl2 (0.5–3 mg L−1) showed better results than treatments used alone. | [185] |
| 2017 | dW RSW | A. fumigatus A. flavus A. niger A. terreus A. alutaceus A. sulfurous P. chrysogenum P. gloprum T. viride | UV Cl2 UV-Cl2 | UV: t:15, 30, 60, 90, 120, 150 and 180 s [NaClO]: 0.5–4 mg L−1. t: 5, 15, 30, 60 and 120 min. [Ascorbic acid]: 25 mg L−1 to quench Cl2. UV- Cl2: UV exposure 15–120 s and [NaClO]: 1–0.125 mg L−1 | [NaClO] 0.125 mg L−1 and UV exposure 15 s were required to eliminate the fungal contamination from a water sample. | [186] |
| 2020 | GW | P. polonicum A. niger T. harzianum | UV-LEDs/Cl2 LPUV/Cl2 UV | UV, Cl2, UV-LEDs/Cl2 and LPUV/Cl2. [Spores]0: 2–4 × 106 CFU mL−1, [Cl2]: 2 mg L−1. UVA intensity: 0.25 mW cm−2. | UV-LEDs/Cl2 exhibited better inactivation compared to UV alone and Cl2 alone. The inactivation rate constants (k) by Cl2 alone for P. polonicum, A. niger, and T. harzianum were only 0.022, 0.011, and 0.008 min−1, respectively. | [184] |
| 2022 | GW | Penicillium polonicum Aspergillus niger Trichoderma harzianum | UV-Cl2, Cl2-UV, UV/Cl2-UV, UV-UV/Cl2, | UV fluence: 40 mJ cm−2 [Cl2]: 2 mg L−1 and 30 min at each stage. | UV-Cl2 (UV265-Cl2, UV280-Cl2), treatments by UV265 and UV280 with the fluence of 40 mJ cm−2 caused the LCR of 1.75 log and 2.23 log, 2.20 log and 2.10 log, 0.76 log and 0.87 log for P. polonicum, A. niger, and T. harzianum respectively. | [9] |
| Date | Matrix * | Fungi | Method | Experimental Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| 2022 | GW | T. harzianum P. polinicum A. niger | UV-LEDs/PS UV-LEDs/PMS | [Spores]0: 2–4 × 10 6 CFU mL−1 T: 25 ± 2 °C. UV irradiance 254, 265, 280 and 265/280 nm was 0.215, 0.214, 0.185 and 0.120 mW cm−1 respectively [PS or PMS]: 0.1 mmol L−1 | 2 log P. polonicum y T. harzianum: fluence 20–40 mJ cm−2 2 log A. niger: fluence 60 mJ cm−2 | [16] |
| 2019 | GW | A. niger T. harzianum P. polinicum | UV/PMS | The final concentration of fungal spores: 106 CFU mL−1. UV fluence: 0.109 mW cm−2. UVA365nm irradiance: 0.10–0.25 mW cm−2. 1 mL Na2S2O3 (1 mol L−1) to terminate the process. 1 mL reagent (PMS) was added to 98 mL of PBS solution without a chloride | UV inactivation rate constants (k) of T. harzianum, P. polonicum, and A. niger: 0.0638, 0.0859, and 0.0368 mJ cm−2 respectively. | [189] |
| 2017 | PBS GW | Trichoderma sp. Acremonium sp. Penicillium sp. Cladosporium sp. | UV/PMS | UV254 in PBS solution pH = 7.0 T = 20 ± 2 °C Fluence rate: 0.109 mW cm−2. [Spores]0: 2–7 × 105 CFU mL−1 UV-AOPs (UV/PMS, UV/PS): [PMS] = [PS] = [H2O2] = 0.1 mmol L−1 UV dose 40 mJ cm−2 | UV dose (mJ cm−2) for 99% inactivation in PBS: Trichoderma sp. 45; Acremonium sp. 50; Penicillium sp. 65; Cladosporium sp. 130. UV + PMS dose (mJ cm−2) for 99% inactivation in PBS: Trichoderma sp. 35; Acremonium sp. 35; Penicillium sp. 45; Cladosporium sp. 85. | [17] |
| Date | Matrix | Fungi | Method | Experimental Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| 2011 | DW SWWE | F. equiseti | H2O2/UV-Vis | 10 mg L−1 of H2O2 in 60 L CPC photoreactor 325 (±70) CFU mL−1 Exposure: 2–5 h | Removal: 2 log dW: 27.1 kJ L−1 SWWE: 31.8 kJ L−1 DW: 44.5 kJ L−1 | [192] |
| 2009 | DW | F. solani | H2O2/ UV solar | [H2O2]: 5–500 mg L−1 Triplicates Temperature: >25 °C Sunlight + H2O2 | Log removal: 0.6 log; 1.5 log 30.4 kJ L−1; 38.5 kJ L−1 | [191] |
| 2017 | SWWE | A. niger R. rubra | UV/O3 | UV 254 nm Contact time: 30 min Density: 103 CFU/100 mL | Log inactivation 2.3 ± 0.7 for Rhodotorula rubra and Aspergillus niger, corresponding to 99.98 ± 0.03% and 98.25 ± 2.20%, respectively. 30 min was sufficient time to achieve log reductions of 3.3 ± 0.2 for fungi | [193] |
| 2019 | GW | A. niger T. harzianum P. polinicum | UV/H2O2 | The final concentration of fungal spores: 106 CFU/mL. UV fluence rate: 0.109 mW cm−1. UVA365nm irradiance intensity: 0.10–0.25 mW cm−1. An aliquot of 1 mL reagent (H2O2) was added to 98 mL PBS without a chloride ion. | The UV inactivation rate constants (k) of T. harzianum, P. polonicum, and A. niger are 0.0638, 0.0859, and 0.0368 cm−2 mJ, respectively. | [189] |
| 2022 | GW | T. harzianum P. polinicum A. niger | UV-LEDs/H2O2 | Initial concentration: 2–4 × 106 CFU/mL Temperatura (25 ± 2 °C). The irradiance for the 265, 280, and 265/280 nm combination was 0.215, 0.214, 0.185, and 0.120 mW cm−2, respectively [H2O2]: 0.1 mM | UV fluence to achieve 2 log reduction in P. polonicum y T. harzianum: 20–40 mJ cm−2 for each wavelength. UV fluence to achieve 2 log reduction in A. niger: 60 mJ/cm2 | [16] |
| 2022 | PBS SW | A. niger A. flavus | UV/PAA | [PAA]0 = 7.0 mg L−1, UV irradiance = 0.120 mW cm−2; pH = 7.2 ± 0.2; T = 25 ± 2 °C; initial concentration of fungal spores: 2.5 × 105 CFU mL−1. Concentrations of PAA (5.0, 7.0, and 10.0 mg L−1). pH value (5.0, 7.0 and 9.0). | The k of A. niger and A. flavus was similar at pH 5.0 and 7.0, while it decreased 60.00% and 39.13% at pH 9.0 compared with that at pH 7.0. The inactivation of A. niger in the UV/PAA system: 48.23%. The inactivation of A. flavus: 64.91%. k of A. niger by UV/PAA: 0.48 min−1, and k of A. flavus by the UV/PAA: 0.91 min−1. | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caicedo-Bejarano, L.D.; Morante-Caicedo, A.; Castro-Narváez, S.P.; Serna-Galvis, E.A. Alternative and Classical Processes for Disinfection of Water Polluted by Fungi: A Systematic Review. Water 2024, 16, 936. https://doi.org/10.3390/w16070936
Caicedo-Bejarano LD, Morante-Caicedo A, Castro-Narváez SP, Serna-Galvis EA. Alternative and Classical Processes for Disinfection of Water Polluted by Fungi: A Systematic Review. Water. 2024; 16(7):936. https://doi.org/10.3390/w16070936
Chicago/Turabian StyleCaicedo-Bejarano, Luz Dary, Alejandra Morante-Caicedo, Sandra Patricia Castro-Narváez, and Efraím A. Serna-Galvis. 2024. "Alternative and Classical Processes for Disinfection of Water Polluted by Fungi: A Systematic Review" Water 16, no. 7: 936. https://doi.org/10.3390/w16070936
APA StyleCaicedo-Bejarano, L. D., Morante-Caicedo, A., Castro-Narváez, S. P., & Serna-Galvis, E. A. (2024). Alternative and Classical Processes for Disinfection of Water Polluted by Fungi: A Systematic Review. Water, 16(7), 936. https://doi.org/10.3390/w16070936







