Effect of Benthic Flux on the Nutrient Dynamics of Bottom Water during Stratification in an Artificial Brackish Lake
Abstract
:1. Introduction
2. Materials and Methods
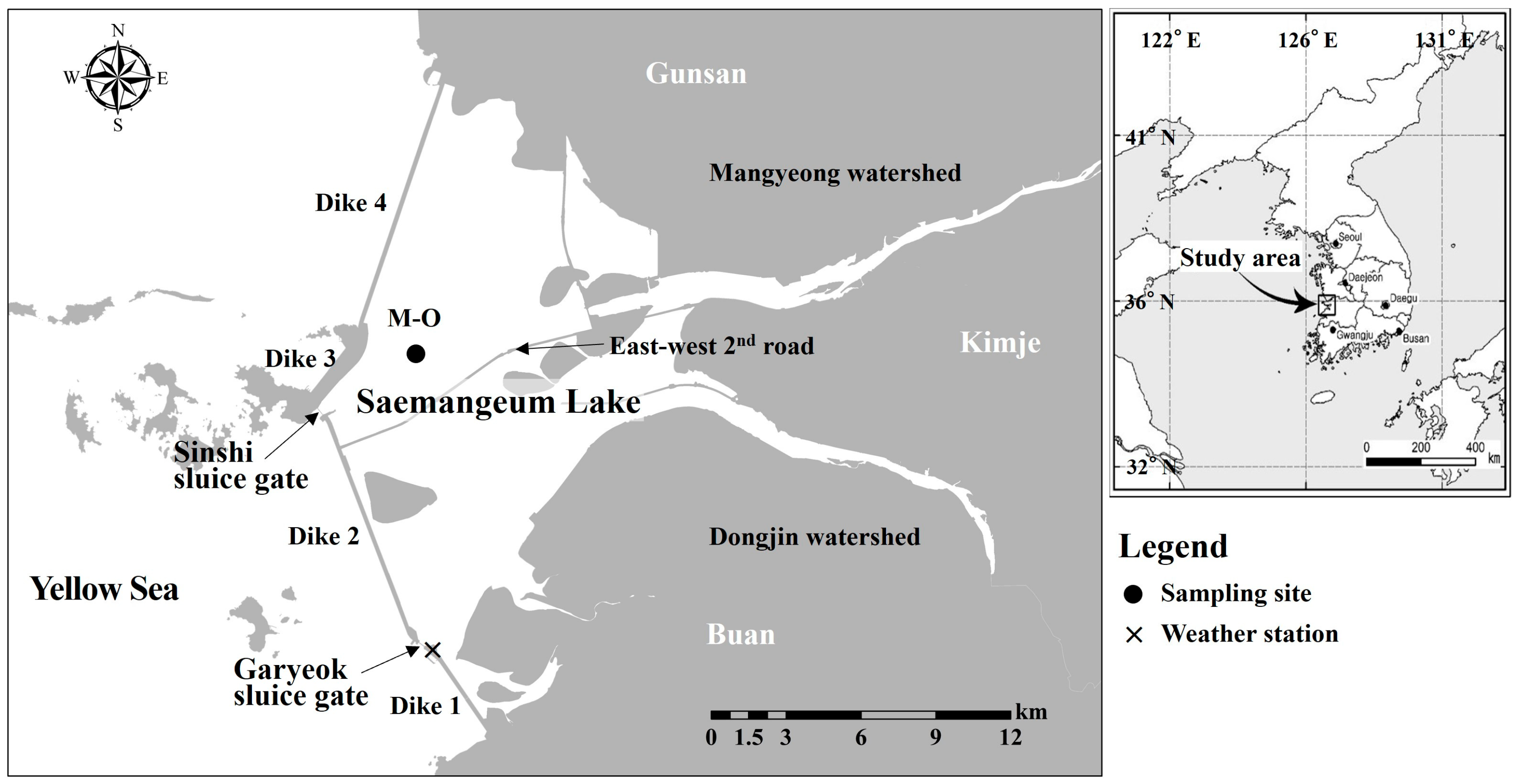
2.1. Study Area
2.2. Sampling
2.2.1. In Situ Measurement of Benthic Oxygen and Nutrient Fluxes
2.2.2. Laboratory Analysis
2.2.3. Potential Energy Anomaly and Benthic Flux Calculation
2.2.4. Other Data Sources
2.2.5. Statistical Analysis
3. Results
3.1. Hydrologic Conditions
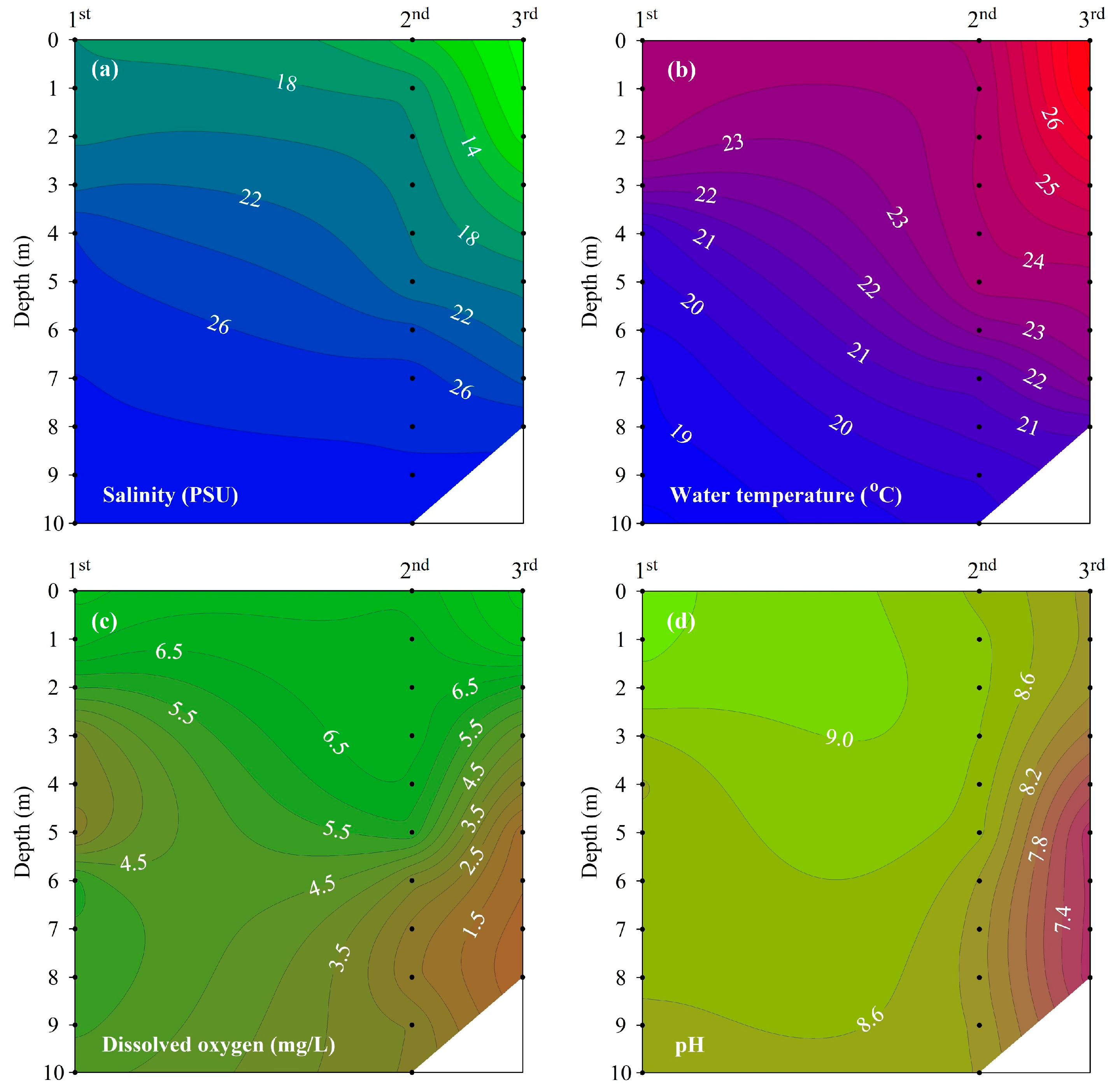
3.2. Vertical Profiles of Salinity, Water Temperature, DO, and pH
3.3. Vertical Profiles of TOC, Chl-a, and Nutrients
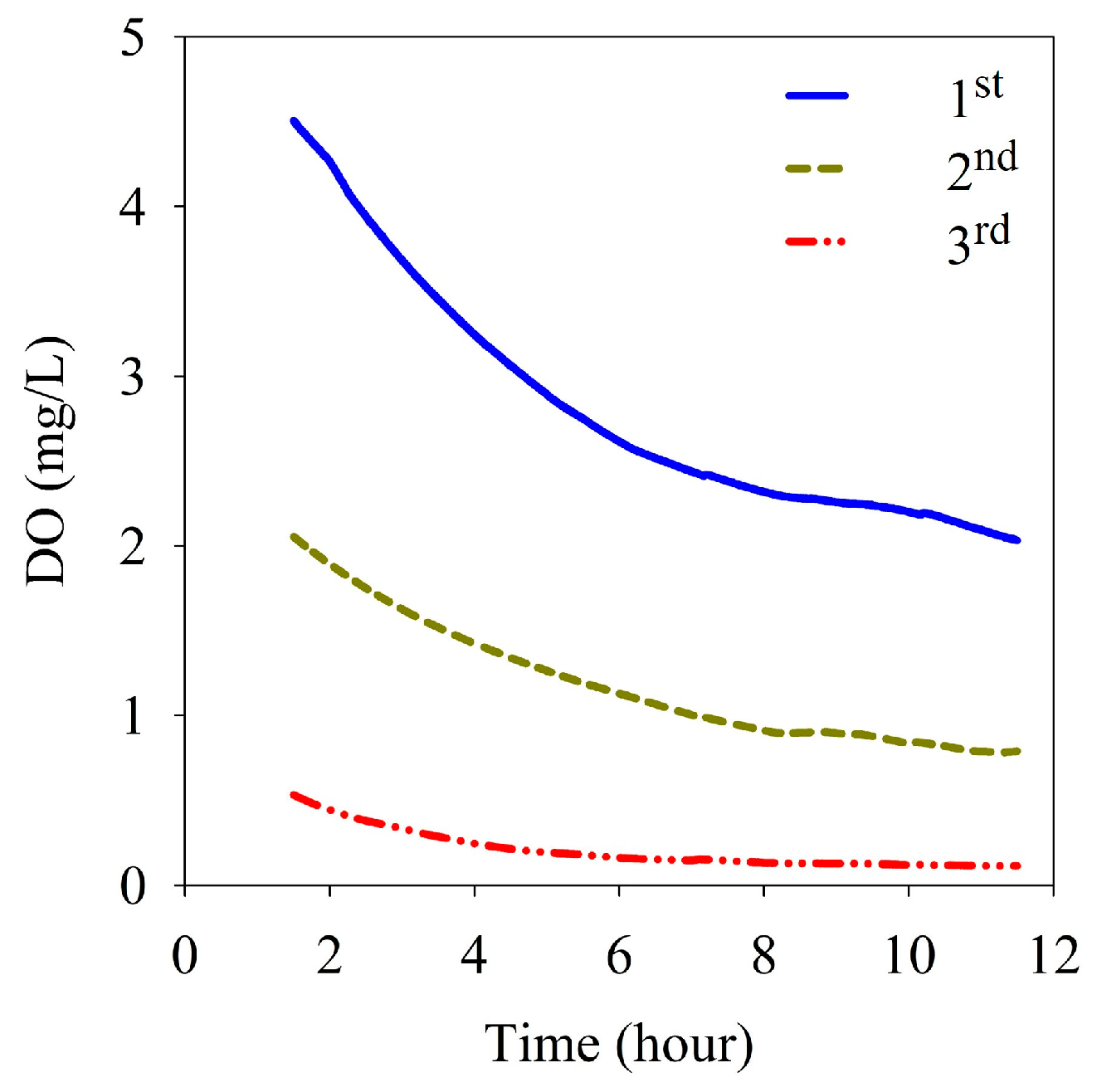
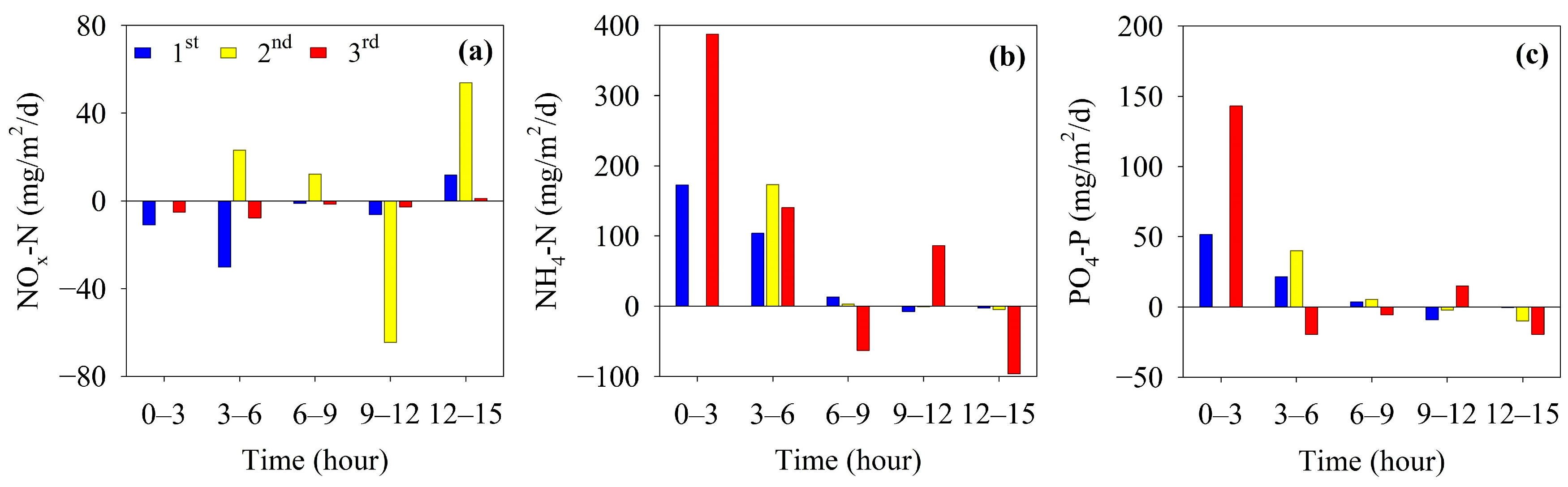
3.4. Sediment Oxygen Demand and Benthic Nutrient Fluxes
4. Discussion
4.1. Changes in Vertical Distribution of Water Quality and Development of Stratification
4.2. Benthic Nutrient Fluxes under Stratified Conditions
4.3. Contribution of Benthic Fluxes to Bottom Water during Stratification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Long, A.; Sun, L.; Shi, R.; Zhou, W.; Dang, A. Saltwater intrusion induced by a complete neap tide and its effect on nutrients variation in the estuary of Pearl River, China. J. Coast. Res. 2013, 29, 1158–1168. [Google Scholar] [CrossRef]
- Liu, B.; de Swart, H.E. Quantifying the Effect of Salinity Stratification on Phytoplankton Density Patterns in Estuaries. Estuaries Coast. 2017, 41, 453–470. [Google Scholar] [CrossRef]
- Shilei, Z.; Yue, S.; Tinglin, H.; Ya, C.; Xiao, Y.; Zizhen, Z.; Yang, L.; Zaixing, L.; Jiansheng, C.; Xiao, L. Reservoir water stratification and mixing affects microbial community structure and functional community composition in a stratified drinking reservoir. J. Environ. Manag. 2020, 267, 110456. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Li, S.; Liu, Q.; Jiang, F.; Hu, J. Distribution and partitioning of heavy metals in water and sediments of a typical estuary (Modaomen, South China): The effect of water density stratification associated with salinity. Environ. Pollut. 2021, 287, 117277. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Gong, G.C.; Shiah, F.K. Hypoxia in the East China Sea: One of the largest coastal low-oxygen areas in the world. Mar. Environ. Res. 2007, 64, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, K.T.; Lim, J.H.; Yoon, J.E.; Kim, I.N. Hypoxia in Korean coastal waters: A case study of the natural Jinhae Bay and artificial Shihwa Bay. Front. Mar. Sci. 2018, 5, 70. [Google Scholar] [CrossRef]
- Diaz, R.J. Overview of hypoxia around the world. J. Environ. Qual. 2001, 30, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; Eggelston, D.B. Effects of hypoxia on an estuarine predator-prey interaction: Foraging behavior and mutual interference in the blue crab Callinectes sapidus and the infaunal clam prey Mya arenaria. Mar. Ecol. Prog. Ser. 2000, 196, 221–237. [Google Scholar] [CrossRef]
- Purcell, J.E.; Decker, M.B.; Breitburg, D.L.; Broughton, K.J. Fine-scale vertical distributions of Mnemiopsis leidyi ctenophores: Predation on copepods relative to stratification and hypoxia. Mar. Ecol. Prog. Ser. 2014, 500, 103–120. [Google Scholar] [CrossRef]
- Li, M.; Zhong, L. Flood–ebb and spring–neap variations of mixing, stratification and circulation in Chesapeake Bay. Cont. Shelf Res. 2009, 29, 4–14. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Sun, Y.; Wang, J.; Wei, H. Contribution of sediment oxygen demand to hypoxia development off the Changjiang Estuary. Estuar. Coast. Shelf Sci. 2017, 192, 149–157. [Google Scholar] [CrossRef]
- Qian, W.; Gan, J.; Liu, J.; He, B.; Lu, Z.; Guo, X.; Wang, D.; Guo, L.; Huang, T.; Dai, M. Current status of emerging hypoxia in a eutrophic estuary: The lower reach of the Pearl River estuary, China. Estuar. Coast. Shelf Sci. 2018, 205, 58–67. [Google Scholar] [CrossRef]
- Schourup-Kristensen, V.; Larsen, J.; Maar, M. Drivers of hypoxia variability in a shallow and eutrophicated semi-enclosed fjord. Mar. Pollut. Bull. 2023, 188, 114621. [Google Scholar] [CrossRef] [PubMed]
- Middelburg, J.J. Reviews and syntheses: To the bottom of carbon processing at the seafloor. Biogeosciences 2018, 15, 413–427. [Google Scholar] [CrossRef]
- Berezina, N.A.; Maximov, A.A.; Vladimirova, O.M. Influence of benthic invertebrates on phosphorus flux at the sediment-water interface in the easternmost Baltic. Sea. Mar. Ecol. Prog. Ser. 2019, 608, 33–43. [Google Scholar] [CrossRef]
- Kendzierska, H.; Łukawska-Matuszewska, K.; Burska, D.; Janas, U. Benthic fluxes of oxygen and nutrients under the influence of macrobenthic fauna on the periphery of the intermittently hypoxic zone in the Baltic Sea. J. Exp. Mar. Biol. Ecol. 2020, 530, 151439. [Google Scholar] [CrossRef]
- Kopp, D.; Lefebvre, S.; Cachera, M.; Villanueva, M.C.; Ernande, B. Reorganization of a marine trophic network along an inshore–offshore gradient due to stronger pelagic–benthic coupling in coastal areas. Prog. Oceanogr. 2015, 130, 157–171. [Google Scholar] [CrossRef]
- Hopkinson, C.S.; Giblin, A.E.; Tucker, J. Benthic metabolism and nutrient regeneration on the continental shelf of eastern Massachusetts, USA. Mar. Ecol. Prog. Ser. 2001, 224, 1–19. [Google Scholar] [CrossRef]
- Lawrence, W.; Dagg, M.J.; Liu, H.; Cummings, S.R.; Ortner, P.B.; Kelble, C. Wind events and benthic-pelagic coupling in a shallow subtropical bay in Florida. Mar. Ecol. Prog. Ser. 2004, 266, 1–13. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, K.H.; Shim, J.; Han, J.H.; Choi, Y.H.; Khang, B.J. Massive sedimentation of fine sediment with organic matter and enhanced benthic-pelagic coupling by an artificial dyke in semi-enclosed Chonsu Bay, Korea. Mar. Pollut. Bull. 2012, 64, 153–163. [Google Scholar] [CrossRef]
- Hyun, J.H.; Kim, S.H.; Mok, J.S.; Lee, J.S.; An, S.U.; Lee, W.C. Impacts of long-line aquaculture of Pacific oyster (Crassostrea gigas) on sulfate reduction and diffusive nutrient flux in the coastal sediments of Jinhae-Tongyeong, Korea. Mar. Pollut. Bull. 2013, 74, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Spooner, D.R.; Maher, W. Benthic sediment composition and nutrient cycling in an Intermittently Closed and Open Lake Lagoon. J. Mar. Syst. 2009, 75, 33–45. [Google Scholar] [CrossRef]
- De Vittor, C.; Faganeli, J.; Emili, A.; Covelli, S.; Predonzani, S.; Acquavita, A. Benthic fluxes of oxygen, carbon and nutrients in the Marano and Grado Lagoon (northern Adriatic Sea, Italy). Estuar. Coast. Shelf Sci. 2012, 113, 57–70. [Google Scholar] [CrossRef]
- Petranich, E.; Covelli, S.; Acquavita, A.; De Vittor, C.; Faganeli, J.; Contin, M. Benthic nutrient cycling at the sediment-water interface in a lagoon fish farming system (northern Adriatic Sea, Italy). Sci. Total Environ. 2018, 644, 137–149. [Google Scholar] [CrossRef]
- Lehtoranta, J.; Ekholm, P.; Pitkänen, H. Coastal eutrophication thresholds: A matter of sediment microbial processes. AMBIO 2009, 38, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Jäntti, H.; Hietanen, S. The effects of hypoxia on sediment nitrogen cycling in the Baltic Sea. AMBIO 2012, 41, 161–169. [Google Scholar] [CrossRef]
- Matos, C.; Berrêdo, J.; Machado, W.; Metzger, E.; Sanders, C.; Faial, K.; Cohen, M. Seasonal changes in metal and nutrient fluxes across the sediment-water interface in tropical mangrove creeks in the Amazon region. Appl. Geochem. 2022, 138, 105217. [Google Scholar] [CrossRef]
- Qu, W.; Morrison, R.J.; West, R.J.; Su, C. Diagenetic stoichiometry and benthic nutrient fluxes at the sediment-water interface of Lake Illawarra, Australia. Hydrobiologia 2005, 537, 249–264. [Google Scholar] [CrossRef]
- Engelsen, A.; Hulth, S.; Pihl, L.; Sundbäck, K. Benthic trophic status and nutrient fluxes in shallow-water sediments. Estuar. Coast. Shelf Sci. 2008, 78, 783–795. [Google Scholar] [CrossRef]
- Grenz, C.; Rodier, M.; Seceh, C.; Varillon, D.; Haumani, G.; Pinazo, C. Benthic nutrients and oxygen fluxes at the water sediment interface in a pearl farming atoll (Ahe, Tuamotu, French Polynesia). Mar. Pollut. Bull. 2021, 173, 112963. [Google Scholar] [CrossRef] [PubMed]
- Belias, C.; Dassenakis, M.; Scoullos, M. Study of the N, P and Si fluxes between fish farm sediment and seawater. Results of simulation experiments employing a benthic chamber under various redox conditions. Mar. Chem. 2007, 103, 266–275. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kwak, D.H. Influence of external loading and halocline on phosphorus release from sediment in an artificial tidal lake. Int. J. Sediment Res. 2020, 35, 146–156. [Google Scholar] [CrossRef]
- Glud, R.N.; Gundersen, J.K.; Jørgensen, B.B.; Revsbech, N.P.; Schulz, H.D. Diffusive and total oxygen uptake of deep-sea sediments in the eastern South Atlantic Ocean:in situ and laboratory measurements. Deep-Sea Res. I Oceanogr. Res. Pap. 1994, 41, 1767–1788. [Google Scholar] [CrossRef]
- Hall, P.O.J.; Brunnegård, J.; Hulthe, G.; Martin, W.R.; Stahl, H.; Tengberg, A. Dissolved organic matter in abyssal sediments: Core recovery artifacts. Limnol. Oceanogr. 2007, 52, 19–31. [Google Scholar] [CrossRef]
- Lee, J.S.; Bahk, K.S.; Khang, B.J.; Kim, Y.T.; Bae, J.H.; Kim, S.S.; Park, J.J.; Choi, O.I. The development of a benthic chamber (BelcI) for benthic boundary layer studies. Sea J. Korean Soc. Oceanogr. 2010, 15, 41–50. [Google Scholar]
- Berelson, W.; McManus, J.; Severmann, S.; Rollins, N. Benthic fluxes from hypoxia-influenced Gulf of Mexico sediments: Impact on bottom water acidification. Mar. Chem. 2019, 209, 94–106. [Google Scholar] [CrossRef]
- Shin, Y.R.; Jung, J.Y.; Choi, J.H.; Jung, K.W. Hydrodynamic modeling of Saemangeum reservoir and watershed using HSPF and EFDC. J. Korean Soc. Water Environ. 2012, 28, 384–393. [Google Scholar]
- Kim, S.H.; Kim, K.; Lee, M.; Jeong, H.J.; Kim, W.J.; Park, J.G.; Yang, J.S. Enhanced benthic nutrient flux during monsoon periods in a coastal lake formed by tideland reclamation. Estuaries Coast. 2009, 32, 1165–1175. [Google Scholar] [CrossRef]
- Kwak, D.H.; Song, Y.S.; Choi, Y.H.; Kim, K.M.; Jeong, Y.H. Influence of sluice gate operation on salinity stratification and hypoxia development in a brackish estuary dam. Reg. Stud. Mar. Sci. 2023, 57, 102731. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Yang, J.S. The long-term variations of water qualities in the Saemangeum salt-water lake after the sea-dike construction. J. Korean Soc. Mar. Environ. Energy 2015, 18, 51–63. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kim, C.S.; Yang, J.S. Estimation of addition and removal processes nutrients from bottom water in the Saemangeum Salt-Water Lake by using missing model. J. Korean Soc. Mar. Environ. Energy 2014, 17, 306–317. [Google Scholar] [CrossRef]
- Kwak, D.H.; Jeon, Y.T.; Hur, Y.D. Phosphorus fractionation and release characteristics of sediment in the Saemangeum Reservoir for seasonal change. Int. J. Sediment Res. 2018, 33, 250–261. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kwak, D.H. Factors affecting behavior and distribution of dissolved organic matter in an artificial coastal reservoir. Reg. Stud. Mar. Sci. 2021, 44, 101786. [Google Scholar] [CrossRef]
- Sayles, F.L.; Dickinson, W.H. The ROLAI2D lander: A benthic lander for the study of exchange across the sediment–water interface. Deep Sea Res. A 1991, 38, 505–529. [Google Scholar] [CrossRef]
- Hammond, D.E.; Cummins, K.M.; McManus, J.; Berelson, W.M.; Smith, G.; Spagnoli, F. Methods for measuring benthic nutrient flux on the California Margin: Comparing shipboard core incubations to in situ lander results. Limnol. Oceanogr. Methods 2004, 2, 146–159. [Google Scholar] [CrossRef]
- Tengberg, A.; Stahl, H.; Gust, G.; Muller, V.; Arning, U.; Andersson, H.; Hall, P.O.J. Intercalibration of benthic flux chambers: I. Accuracy of flux measurements and influence of chamber hydrodynamics. Prog. Oceanogr. 2004, 59, 1–28. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, J.S.; Kim, K.T.; Kim, S.L.; Yu, O.H.; Lim, D.; Kim, S.H. Low benthic mineralization and nutrient fluxes in the continental shelf sediment of the northern East China Sea. J. Sea Res. 2020, 164, 101934. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984. [Google Scholar]
- Song, Y.; Jeong, Y.H.; Shin, C.M.; Kwak, D.H. Spatial distribution and comparative evaluation of phosphorus release rate in benthic sediments of an estuary dam. Int. J. Sediment Res. 2002, 37, 355–369. [Google Scholar] [CrossRef]
- Sierra, J.P.; Sánchez-Arcilla, A.; González Del Río, J.; Flos, J.; Movellán, E.; Mösso, C.; Martínez, R.; Rodilla, M.; Falco, S.; Romero, I. Spatial distribution of nutrients in the Ebro estuary and plume. Cont. Shelf Res. 2002, 22, 361–378. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Linker, L. Relative importance of nutrient load and wind on regulating interannual summer hypoxia in the Chesapeake Bay. Estuaries Coasts 2015, 38, 1048–1061. [Google Scholar] [CrossRef]
- Oviatt, C.; Smith, L.; Krumholz, J.; Coupland, C.; Stoffel, H.; Keller, A.; McManus, M.C.; Reed, L. Managed nutrient reduction impacts on nutrient concentrations, water clarity, primary production, and hypoxia in a north temperate estuary. Estuar. Coast. Shelf Sci. 2017, 199, 25–34. [Google Scholar] [CrossRef]
- McGlathery, K.J.; SundbÃfÂck, K.; Anderson, I.C. Eutrophication in shallow coastal bays and lagoons: The role of plants in the coastal filter. Mar. Ecol. Prog. Ser. 2007, 348, 1–18. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, H.; Song, J.; Li, X.; Duan, L. Hypoxia, acidification and nutrient accumulation in the Yellow Sea Cold Water of the South Yellow Sea. Sci. Total Environ. 2020, 745, 141050. [Google Scholar] [CrossRef] [PubMed]
- Murrell, M.C.; Hagy III, J.D.; Lores, E.M.; Greene, R.M. Phytoplankton production and nutrient distributions in a sub-tropical estuary: Importance of freshwater flow. Estuaries Coast. 2007, 30, 390–402. [Google Scholar] [CrossRef]
- Dortch, Q. The interaction between ammonium and nitrate uptake in phytoplankton. Mar. Ecol. Prog. Ser. 1990, 61, 183–201. [Google Scholar] [CrossRef]
- Boström, B.; Andersen, J.M.; Fleischer, S.; Jansson, M. Exchange of phosphorus across the sediment-water interface. Hydrobiologia 1988, 170, 229–244. [Google Scholar] [CrossRef]
- Noori, R.; Berndtsson, R.; Adamowski, J.F.; Abyaneh, M.R. Temporal and depth variation of water quality due to thermal stratification in Karkheh Reservoir, Iran. J. Hydrol. Reg. Stud. 2018, 19, 279–286. [Google Scholar] [CrossRef]
- Lacoste, É.; Bec, B.; Gall, P.L.; Boufahja, F.; Raimbault, P.; Messiaen, G.; Ouisse, V.; d’Orbcastel, E.R.; Munaron, D.; Fiandrino, A.; et al. Benthic-pelagic coupling under juvenile oyster influence in a French Mediterranean coastal lagoon (Thau Lagoon). Estuar. Coast. Shelf Sci. 2022, 267, 107779. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, Y.T.; Shin, K.H.; Hyun, J.H.; Kim, S.Y. Benthic nutrient fluxes at longline sea squirt and oyster aquaculture farms and their role in coastal ecosystems. Aquac. Int. 2011, 19, 931–944. [Google Scholar] [CrossRef]
- Herbert, R.A. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol. Rev. 1999, 23, 563–590. [Google Scholar] [CrossRef] [PubMed]
- Kleeberg, A.; Kozerski, H.P. Phosphorus release in Lake Großer Müggelsee and its implications for lake restoration. Hydrobiologia 1997, 342, 9–26. [Google Scholar] [CrossRef]
- Özkundakei, D.; Hamilton, D.P.; Gibbs, M.M. Hypolimnetic phosphorus and nitrogen dynamics in a small, eutrophic lake with a seasonally anoxic hypolimnion. Hydrobiologia 2011, 661, 5–20. [Google Scholar] [CrossRef]
- Chi, L.; Song, X.; Ding, Y.; Yuan, Y.; Wang, W.; Cao, X.; Wu, Z.; Yu, Z. Heterogeneity of the sediment oxygen demand and its contribution to the hypoxia off the Changjiang estuary and its adjacent waters. Mar. Pollut. Bull. 2021, 172, 112920. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.J.; Carini, S.A.; Liu, Z.; Ostrom, N.E.; Gardner, W.S. Oxygen consumption in the water column and sediments of the northern Gulf of Mexico hypoxic zone. Estuar. Coast. Shelf Sci. 2013, 123, 46–53. [Google Scholar] [CrossRef]
- Lam, P.; Lavik, G.; Jensen, M.M.; van de Vossenberg, J.; Schmid, M.; Woebken, D.; Gutiérrez, D.; Amann, R.; Jetten, M.S.M.; Kuypers, M.M.M. Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc. Natl. Acad. Sci. USA 2009, 106, 4752–4757. [Google Scholar] [CrossRef]
- Yoo, S.C.; Suh, S.W.; Lee, H.Y. Impacts on residence time and water quality of the Saemangeum reservoir caused by inner development. J. Korean Soc. Mar. Environ. Energy 2012, 15, 186–197. [Google Scholar] [CrossRef]





| SOD (g/m2/d) | Benthic Nutrient Fluxes (mg/m2/d) | |||
|---|---|---|---|---|
| NOx-N | NH4-N | PO4-P | ||
| 1st | 1.00 | −7.31 | 55.83 | 13.38 |
| 2nd | 0.52 | 9.59 | 68.88 | 14.64 |
| 3rd | 0.15 | −3.14 | 91.08 | 22.71 |
| DO | pH | TOC | Chl-a | T-N | NOx-N | NH4-N | Org-N | T-P | PO4-P | Org-P | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | |||||||||||
| Density | −0.483 | −0.807 ** | −0.800 ** | −0.833 ** | −0.828 ** | −0.917 ** | −0.594 | −0.817 ** | −0.753 * | −0.798 ** | −0.661 |
| DO | 0.723 * | 0.433 | 0.650 | 0.184 | 0.367 | −0.268 | 0.167 | 0.326 | 0.160 | 0.285 | |
| pH | 0.647 | 0.849 ** | 0.595 | 0.756 * | 0.224 | 0.571 | 0.646 | 0.508 | 0.646 | ||
| TOC | 0.700 * | 0.820 ** | 0.850 ** | 0.611 | 0.833 ** | 0.879 ** | 0.824 ** | 0.812 ** | |||
| Chl-a | 0.762 * | 0.883 ** | 0.435 | 0.717 * | 0.787 * | 0.773 * | 0.678 * | ||||
| T-N | 0.904 ** | 0.790 * | 0.996 ** | 0.824 ** | 0.916 ** | 0.714 * | |||||
| NOx-N | 0.711 * | 0.883 ** | 0.904 ** | 0.941 ** | 0.778 * | ||||||
| NH4-N | 0.778 * | 0.765 * | 0.806 ** | 0.731 * | |||||||
| Org-N | 0.803 ** | 0.899 ** | 0.695 * | ||||||||
| T-P | 0.873 ** | 0.945 ** | |||||||||
| PO4-P | 0.709 * | ||||||||||
| 2nd | |||||||||||
| Density | −0.952 ** | −0.888 ** | −0.927 ** | −0.733 * | −0.515 | −0.681 * | 0.401 | −0.806 ** | −0.394 | −0.202 | −0.689 * |
| DO | 0.906 ** | 0.903 ** | 0.564 | 0.564 | 0.626 | −0.353 | 0.830 ** | 0.479 | 0.337 | 0.640 * | |
| pH | 0.809 ** | 0.608 | 0.316 | 0.530 | −0.668 * | 0.644 * | 0.261 | 0.111 | 0.446 | ||
| TOC | 0.770 ** | 0.564 | 0.559 | −0.304 | 0.879 ** | 0.527 | 0.252 | 0.762 * | |||
| Chl-a | 0.236 | 0.450 | −0.304 | 0.503 | 0.248 | −0.043 | 0.476 | ||||
| T-N | 0.760 * | 0.158 | 0.806 ** | 0.879 ** | 0.742 * | 0.848 ** | |||||
| NOx-N | −0.049 | 0.602 | 0.693 * | 0.652 * | 0.719 * | ||||||
| NH4-N | −0.158 | 0.292 | 0.468 | −0.067 | |||||||
| Org-N | 0.636 * | 0.399 | 0.848 ** | ||||||||
| T-P | 0.902 ** | 0.750 * | |||||||||
| PO4-P | 0.500 | ||||||||||
| 3rd | |||||||||||
| Density | −1.000 ** | −0.883 ** | −0.967 ** | −0.967 ** | −0.833 ** | −0.967 ** | 0.950 ** | −0.883 ** | −0.217 | 0.377 | −0.736 * |
| DO | 0.883 ** | 0.967 ** | 0.967 ** | 0.833 ** | 0.967 ** | −0.950 ** | 0.883 ** | 0.217 | −0.377 | 0.736 * | |
| pH | 0.883 ** | 0.883 ** | 0.950 ** | 0.883 ** | −0.817 ** | 0.950 ** | 0.450 | −0.084 | 0.720 * | ||
| TOC | 0.950 ** | 0.833 ** | 0.950 ** | −0.933 ** | 0.933 ** | 0.183 | −0.393 | 0.644 | |||
| Chl-a | 0.883 ** | 1.000 ** | −0.867 ** | 0.867 ** | 0.267 | −0.318 | 0.728 * | ||||
| T-N | 0.883 ** | −0.733 * | 0.867 ** | 0.467 | −0.084 | 0.762 * | |||||
| NOx-N | −0.867 ** | 0.867 ** | 0.267 | −0.318 | 0.728 * | ||||||
| NH4-N | −0.867 ** | −0.050 | 0.536 | −0.669 * | |||||||
| Org-N | 0.217 | −0.285 | 0.636 | ||||||||
| T-P | 0.644 | 0.360 | |||||||||
| PO4-P | −0.452 |
| Net Loss (g/m2/day) | SOD (g/m2/day) | Contribution of SOD (%) | Net Flux (mg/m2/day) | Benthic Flux (mg/m2/day) | Contribution of Benthic Flux (%) | ||
|---|---|---|---|---|---|---|---|
| DO | 1.02 | 0.33 | 33 | T-N | 252.86 | 83.21 a | 33 |
| NH4-N | 144.43 | 79.98 | 55 | ||||
| T-P | 25.57 | 18.67 b | 73 | ||||
| PO4-P | 21.43 | 18.67 | 87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.-H.; Choi, Y.-H.; Kwak, D.-H. Effect of Benthic Flux on the Nutrient Dynamics of Bottom Water during Stratification in an Artificial Brackish Lake. Water 2024, 16, 958. https://doi.org/10.3390/w16070958
Jeong Y-H, Choi Y-H, Kwak D-H. Effect of Benthic Flux on the Nutrient Dynamics of Bottom Water during Stratification in an Artificial Brackish Lake. Water. 2024; 16(7):958. https://doi.org/10.3390/w16070958
Chicago/Turabian StyleJeong, Yong-Hoon, Yong-Ho Choi, and Dong-Heui Kwak. 2024. "Effect of Benthic Flux on the Nutrient Dynamics of Bottom Water during Stratification in an Artificial Brackish Lake" Water 16, no. 7: 958. https://doi.org/10.3390/w16070958
APA StyleJeong, Y.-H., Choi, Y.-H., & Kwak, D.-H. (2024). Effect of Benthic Flux on the Nutrient Dynamics of Bottom Water during Stratification in an Artificial Brackish Lake. Water, 16(7), 958. https://doi.org/10.3390/w16070958







