Abstract
The Dongting Lake Plain is a major ecological reserve for river and lake wetlands in the Yangtze River Basin, with complex river and lake relationships and frequent water flow exchange. Studies on the hydrochemical characteristics and the mechanism of interaction between groundwater and surface water will actively promote the scientific management, utilization of water resources, and protection of the ecological environment in the Dongting Lake Plain. Based on hydrogeochemical statistics, Gibbs diagrams, ion ratios, rock weathering end-element diagrams, hydrogen–oxygen isotope relationship diagrams, and other technical methods, the chemical characteristics, ion sources, and the distribution of hydrogen–oxygen isotopes of groundwater and surface water in “the Three Inlets” and “the Four Rivers” water system areas as well as the Dongting Lake water were analyzed. Additionally, the interactions between groundwater and surface water and the proportions of these contributions were discussed. The results show that both groundwater and surface water in the Dongting Lake Plain are weakly acidic or alkaline, and the anions are mainly HCO3−, the cations are mainly Ca2+and Mg2+, with the hydrochemical types being mainly HCO3−Ca−Mg and HCO3−Ca. The chemical characteristics of groundwater and surface water are mainly affected by the interaction between water and rock; the ions in surface water mainly come from the weathered dissolution of carbonate and silicate rocks, while the ions in groundwater mainly come from the weathered dissolution of carbonate and silicate rocks, with the dissolution of evaporite rocks locally. Groundwater and surface water are mainly distributed near the local meteoric water line (LMWL), and the slope of the local evaporation line is less than that of the LMWL, which indicates that atmospheric rainfall is an important recharge source for groundwater and surface water and that at the same time, it is affected by evaporation to a certain extent. Part of the groundwater in the Dongting Lake Plain is discharged into the surface rivers in “the Three Inlets” and “the Four Rivers” water system areas, and the other part is directly discharged into Dongting Lake. According to the mass balance relationship of isotopes, the proportions of surface water in “the Three Inlets” and “the Four Rivers” water system areas contributing to Dongting Lake’s water are 18.48% and 60.38%, respectively, and the proportion of groundwater in the lake plain contributing to Dongting Lake water is 21.14%.
1. Introduction
Water resources are important for human survival and the stable development of ecosystems [1,2]. These resources are indispensable, highly valuable, and important sources of safe drinking water, agricultural irrigation, and industrial water and are basic guarantees for promoting socioeconomic development [3]. Groundwater and surface water, as important components of water resources, are both interrelated and relatively independent [4]. With global climate change and intensified human activities, the interactions between groundwater and surface water and water cycle processes have changed, causing uneven spatial and temporal distributions of water resources and the degradation of water ecosystems, which have affected the production and life activities of humans as well as socioeconomic development. Therefore, scientific evaluation of the interactions between groundwater and surface water, accurate understanding of the water cycle evolution process, and exploration of the quality and quantity characteristics and spatiotemporal distribution patterns of water resources in a region will have important practical significance for the effective evaluation of scientific management, rational utilization of water resources, and healthy development of the ecological environment.
The transformation between groundwater and surface water is a key step in the water cycle process in the basin [5,6]. Groundwater and surface water exhibit unique hydrochemical parameter characteristics in different material sources and transport and transformation processes, and hydrochemical analysis has become one of the most important technical methods for identifying the interactions between groundwater and surface water [7,8,9,10,11]. Methods such as hydrogeochemical mathematical statistics [2,3], principal component analysis [12,13], correlation diagrams [14], and ion ratios [15,16] are widely used to identify the sources of hydrochemical components and their transport and transformation as the basis for determining the relationship between groundwater and surface water; however, the accuracy of the hydrochemical parameters is mostly affected by complex geological conditions, water and rock actions [17], and human activities [18], and there are uncertainties in the hydrochemical characteristics of the interactions between groundwater and surface water [19,20]. As isotope tracer technology is used in water cycle research [21], hydrogen and oxygen isotope technology, which can quantify the interactions between groundwater and surface water, is maturing and widely used [22]. Hydrogen and oxygen isotopes, which are components of water molecules, have different characteristics and relatively stable contents in different water sources [20,23]. These isotopic compositions can be very good indicators of the source of groundwater recharge and are conducive to determining the interactions between groundwater and surface water [24]. Therefore, the combination of hydrochemical methods and hydrogen- and oxygen-stable isotope tracers has become an effective technical method for determining the interactions between groundwater and surface water [25,26]. This approach is widely used in identifying the recharge sources of groundwater and surface water and interpreting the effects of natural and anthropogenic factors on hydrogeochemical processes [20,27]. Song, X. et al. [28] combined water chemistry and hydrogen- and oxygen-stable isotopes with topographic, geological, and hydrological characterizations to obtain the contribution ratio of the surface and subsurface runoff to river runoff. Lei, Y. et al. [29] determined the transformation relationship between groundwater and surface water and the ratio of complementarity by analyzing the isotopic and hydrochemical characteristics in river water and groundwater. Hao, S et al. [30] revealed the replenishment mechanism between precipitation, surface water, and groundwater in the Abi Lake Basin of Xinjiang based on hydrogen- and oxygen-stable isotope characteristics.
Dongting Lake, the second-largest freshwater lake in the Yangtze River Basin, is also a core water resource gathering area in the Dongting Lake Ecological and Economic Zone. Water resources have become the key resources for the green transformation and high-quality development of the Dongting Lake Ecological and Economic Zone. Under the influence of human activities and global climate change, flood disasters in the wet season and seasonal water shortages in the dry season are common, and ecology and environment are obviously degrading in Dongting Lake Plain [31]. These conditions greatly restrict the socioeconomic development and ecological protection of the Dongting Lake Plain. Thus, there is an urgent need to ascertain the sources of water resources and the interaction relationship between groundwater and surface water to identify effective solutions. In the past, the Dongting Lake Plain mainly focused on the dynamic analysis of water storage [32,33,34], the exchange of Dongting Lake water with the Yangtze River [35,36], and the discharge and material migration of groundwater in the lake plain [37,38]. However, there has been relatively little research on the relationship and feedback between groundwater and surface water. To this end, this article analyzes the hydrogeological conditions and groundwater movement in the Dongting Lake plain and explores the interactions between groundwater and surface water by means of water chemistry and isotope methods to provide support for the development and utilization of water resources and protection and restoration of the ecological environment in the lake plain.
2. Overview of the Study Area
Dongting Lake is located on the south bank of the middle Yangtze River (Figure 1). It is the second-largest freshwater lake in China and one of the most important international wetlands. The geographic location is as follows: longitude range of 110°50′ E~113°45′ E, and latitude range of 27°39′ N~30°20′ N. The area of the lake plain is 28,737 km2, of which the area of natural lakes amounts to 2691 km2. Dongting Lake Plain is an alluvial–lacustrine deposit plain with densely covered lakes, intertwined river networks, and a flat topography that is mostly below 50 m in elevation. Located in a subtropical, humid monsoon climate, the lake plain is warm and humid, with four distinct seasons. The average temperatures over many years are 16.4~17.0 °C; the temperature gradually decreases from south to north, and rainfall is abundant. Additionally, the average rainfall over many years is 1200~2000 mm. The rainfall is mainly concentrated from April to August, and the interannual variation is large. The average evaporation for many years reaches 1270.5 mm, and evaporation is mainly concentrated from April to October. The rainfall is basically accompanied by high temperatures.
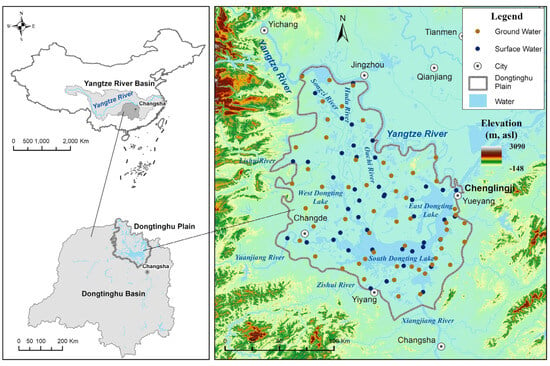
Figure 1.
Geographical location and sampling distribution map of Dongting Lake Plain.
Dongting Lake mainly consists of three lakes, namely, East Dongting Lake, South Dongting Lake, and West Dongting Lake (Figure 1). The northern part of the lake plain is drained into Dongting Lake by “the Three Inlets”, namely, Songzi Kou (Songzi River), Taiping Kou (Hudu River), and Ouchi Kou (Ouchi River), and the western and southern parts are drained into Dongting Lake by “the Four Rivers”, namely, the Xiangjiang River, Zishui River, Yuanjiang River, and Lishui River. Dongting Lake, after receiving water flow from “the Three Inlets” and “the Four Rivers”, contains water that is centrally injected into the Yangtze River from Chenglingji in Yueyang. The Dongting Lake Plain is mainly distributed in the Quaternary strata, with a small amount of exposed pre-Quaternary strata. Quaternary Holocene and Pleistocene strata are relatively complete, with a depth of up to 240~290 m. Additionally, clay, sandy soil, silt, medium–coarse sand, and sand and gravel layers dominate and constitute the three-layered water-bearing rock groups from top to bottom: submerged shallow-pore water-bearing rock groups (Qh), upper pore-pressurized water-bearing rock groups (Qp2+3), and lower pore-fractured water-bearing rock groups (Qp1) [39]. The surface river systems in the lake plain are developed and controlled by the topography and geological structural conditions, and the groundwater and surface water frequently connect, presenting characteristics of seasonal mutual transformation.
3. Materials and Methods
3.1. Sample Collection and Testing
In June–July 2022, water samples were collected from Dongting Lake Plain; 100 sets of samples for a comprehensive analysis of the water quality and hydrogen- and oxygen-stable isotopes analysis were collected, 44 sets of which were surface water samples and 56 sets of which were groundwater samples (Figure 1). Samples from surface water were collected mainly from the main streams of “the Three Inlets” and the depositional areas of “the Four Rivers” and Dongting Lake, while samples from the groundwater were collected mainly from civil wells and a small number of machine wells, with depths ranging from 5 to 50 m. The distribution of sampling points was relatively uniform, with different hydrogeological units and different types of water bodies. Samples were collected in 20 mL, 100 mL, and 1 L polypropylene bottles, of which 20 mL were used for hydrogen- and oxygen-stable isotope analyses and should be refrigerated for storage without bubbles; 100 mL was used for the cations analysis, filtered through a 0.45 µm membrane, acidified with nitric acid to a pH < 2, and sealed for storage; and 1 L bottles were used for the anions analysis and sealed for storage. The collection and storage of the samples complied with the provisions of “ Methods for analysis of groundwater quality-Part 2: Collection and preservation of water samples” (DZ/T 0064.2-2021), and if there was no provision in the standard analysis method, an appropriate amount of preservative should be added. The “Water quality-Technical regulation of the preservation and handling of samples” (HJ 493-2009) was followed, and the tests were completed at the Central South Mineral Resources Testing Center of the Ministry of Natural Resources.
The pH, oxidation-reduction potential (ORP), dissolved oxygen (DO), conductivity (EC), and water temperature (t) were measured by using the portable multi-parameter water quality meter (model: Manta 2.0, Eureka Co., Ltd., Houston, TX, USA). HCO3− was titrated on-site using HCl (0.025 mol/L), and the error was controlled to less than ±5%. δ18O and δD were measured by a liquid water isotope analyzer (model: Los Gatos Research IWA-35EP, USA), and the detection accuracy was D ± 1‰, 18O ± 0.1‰. The cations (K+, Na+, Ca2+, and Mg2+) were determined by plasma emission spectrometry (ICP–OES), with a detection limit of 0.005 mg/L; the anions (Cl−, SO42−, and NO3−) were determined by ion chromatography (model: ICS-5000+DC, Diane Co., Ltd., Fullerton, CA, USA), with detection limits of 0.005 mg/L, 0.004 mg/L, and 0.002 mg/L, respectively; PO43− was determined by the continuous flow injection method, and the detection limit was 0.004 mg/L. The relative errors of the anions’ and cations’ charge equilibrium were within ±5%, and the results were reliable.
3.2. Data Processing and Methods
ArcGIS10.5 was used to compile the location map of the study area and deployment diagram of the sampling points; SPSS22.0 was used to carry out the descriptive statistical analysis of the water quality data; and the minimum, maximum, mean, standard deviation, and coefficient of variation for the main ionic components of the groundwater and surface water samples were also carried out. The characteristics and differences of the statistical values can reveal the hydrochemical distribution characteristics and spatial differences from different water sources by simultaneously combining the significant correlations between ions from different water sources to identify mutual correlations [40,41]. In order to explore the interactions between groundwater and surface water, Aqua Chem 9.0 software was used to draw a Piper trilinear diagram, Origin 2018 software was used to draw Gibbs diagrams, ion–ratio relationship diagrams, rock-weathering end-element diagrams, and hydrogen–oxygen isotope diagrams, as well as to analyze the sources of recharge and the controlling factors for chemical elements in groundwater and surface water. The research methods and technical ideas are shown in Figure 2.
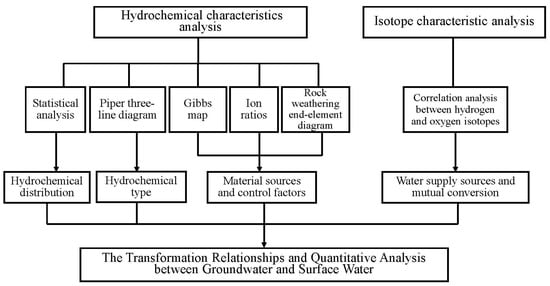
Figure 2.
Technical roadmap for research.
4. Results and Analysis
4.1. Characterization of the Hydrochemical Composition in Groundwater and Surface Water
Due to the different sources of recharge and the structure differences of the water flow systems in Dongting Lake Plain, groundwater and surface water in “the Three Inlets” and “the Four Rivers” water system areas and Dongting Lake water were separated for statistics. The statistics of the water quality indexes in each water system area of Dongting Lake Plain are shown in Table 1.

Table 1.
Statistical characteristic values of major ions from different water sources in Dongting Lake Plain.
Groundwater and surface water in “the Three Inlets” water system area have a similar pH, which is moderately to weakly alkaline, with the pH of surface water ranging from 6.81 to 7.53 and an average value of 7.29; that of the groundwater ranges from 6.81 to 7.72 and has an average value of 7.27; and the TDS and the concentrations of major anions and cations are generally ρ (groundwater) > ρ (surface water). The TDS of surface water ranges from 145 to 245 mg/L, with an average value of 224 mg/L; the TDS of groundwater ranges from 145 to 938 mg/L, with an average value of 407.75 mg/L. These values are characteristic of the freshwater category, and the spatial variation in the groundwater concentration is greater than that in the surface water concentration, which is related to the differences in groundwater runoff pathways and interactions between water and rock. The concentrations of anions and cations from groundwater and surface water are in the following order: HCO3− > SO42− > Cl− > NO3− and Ca2+ > Na+ > Mg2+ > K+; the distribution patterns along the course are the same.
There is a slight difference in the pH of groundwater and surface water in “the Four Rivers” water system area, with surface water being moderately to weakly alkaline and groundwater being moderately to weakly acidic; the pH of the surface water ranges from 7.00 to 8.26, with an average value of 7.6; and that of groundwater ranges from 6.45 to 7.56, with an average value of 6.91. The TDS and the concentrations of major anions and cations are close to each other. The TDS of surface water ranges from 82.6 to 327 mg/L, with an average value of 199.61 mg/L, and the TDS of groundwater ranges from 69.7 to 507 mg/L, with an average value of 186.27 mg/L, and all are part of the freshwater category. The concentrations of anions and cations from the surface water are in the following order: HCO3− > SO42− > Cl− > NO3−; Ca2+ > Mg2+ > Na+ > K+; those of groundwater are in the following order: HCO3− > SO42− > NO3− > Cl−; Ca2+ > Na+ > Mg2+ > K+.
The pH of Dongting Lake water ranges from 7.26 to 7.87, with an average value of 7.55, indicating weak alkalinity. The TDS of the lake water ranges from 59.4 to 914 mg/L, with an average value of 335.79 mg/L; these values are attributed to the freshwater category. The TDS of the lake water is generally greater than that of the surface water from “the Three Inlets” and “the Four Rivers” water system areas and less than that in the groundwater from “the Three Inlets “and” the Four Rivers” water system areas, indicating that the lake water receives a certain amount of groundwater directly mixed with recharge, and there is a transformation process between the groundwater and surface water. The concentrations of anions and cations from the lake water are in the following order: HCO3− > SO42− > Cl− > NO3−; Ca2+ > Mg2+ > Na+ > K+.
4.2. Types of Water Chemistry
The Piper trilinear diagram is a common method for analyzing changes in water chemistry and water chemistry types and is not controlled by human factors [42]. As shown in Figure 3a, the distribution of groundwater and surface water samples in “the Three Inlets” water system area is mostly close to the Ca2+-Mg2+ end of the distribution, and part of the groundwater is close to the Na+ +K+ end of the distribution. The cations are mainly Ca2+ and Mg2+, while some groundwater is mainly Na+ and Ca2+, and the anions are mainly close to the HCO3− end of the distribution, while the anions are basically HCO3−. According to Sukharev’s classification, the hydrochemical types of surface water are mainly HCO3-Ca-Mg, and the hydrochemical types of groundwater are mainly HCO3 -Ca-Mg, followed by HCO3-Na-Ca-Mg and HCO3-Na-Ca. The groundwater and surface water exhibit the same hydrochemical types and distribution patterns, implying the same source. Figure 3b shows that the distribution of surface water samples from “the Four Rivers“ water system area is close to the Ca2+-Mg2+ end of the distribution, with Ca2+ and Mg2+ as the main cations; additionally, the anions are close to the HCO3− end of the distribution, and the anions are mainly HCO3−. In addition, the distribution of the groundwater samples is at the Ca2+, Mg2+, and Na+ +K+ ends of the distribution, and the majority of the anions are close to the HCO3− end of the distribution, while the anions are mainly HCO3−. The hydrochemical types of surface water are HCO3-Ca-Mg and HCO3-Ca, and the hydrochemical types of groundwater are mainly HCO3-Ca, HCO3-Ca-Mg, and HCO3-Na-Ca-Mg. Groundwater originates from “the Four Rivers” basins, and the stratigraphy is characterized by diverse lithologies and different degrees of human activity. The groundwater types are diverse, with HCO3-Cl, HCO3-SO4, and other water types sporadically distributed in local areas. As shown in Figure 3c, the distribution of the Dongting Lake water samples is close to the Ca2+-Mg2+ end of the distribution, with Ca2+ and Mg2+ as the major cations; additionally, the anions are close to the HCO3− end of the distribution. Moreover, the anions are all HCO3−, and the water chemistry types are HCO3-Ca-Mg and HCO3-Ca. Generally, the anions of groundwater and surface water in the Dongting Lake Plain are mainly HCO3−, and the cations are mainly Ca2+ and Mg2+. The relatively high content of Na+ at some of the sample points is related to the lithological differences in water-bearing rock groups and the interactions between water and rock.
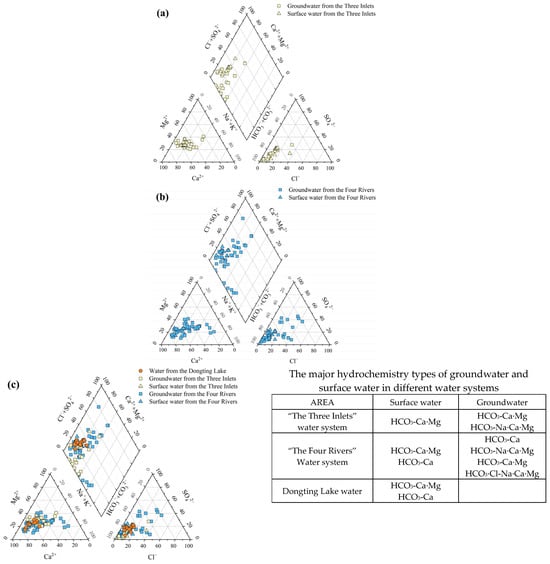
Figure 3.
Piper three-line diagram of groundwater and surface water in Dongting Lake Plain: (a) Piper three-line diagram in “the Three Inlets“ water system; (b) Piper three-line diagram in “the Four Rivers“ water system; and (c) Piper three-line diagram in lake area water system.
4.3. Interrelationships of Chemical Indicators
A correlation analysis of indicator ions was performed to identify the connections between different water sources by inferring the ionic sources [3,43]. Table 2 shows the correlation matrix between the chemical components in the different water system areas in Dongting Lake Plain. The TDS of the surface water from “the Three Inlets“ water system area is positively correlated with Ca2+, HCO3−, and soluble SiO2 and has a certain positive correlation with Mg2+. The TDS of the groundwater is strongly correlated with Ca2+, Mg2+, and HCO3− (R > 0.9) and is obviously correlated with Cl− and SO42−. The TDSs of the groundwater and surface water have the most significant correlation with Ca2+ and HCO3−, and the spatial trends are the same. Ca2+ and HCO3− are the most important component sources of the TDS, and the HCO3− of groundwater and surface water has a strong positive correlation with Ca2+ and Mg2+, which indicates that the sources of HCO3− are the same as those of Ca2+ and Mg2+ and may originate from the weathering and dissolution of carbonate rocks. The TDS of the surface water from “the Four Rivers “water system area has the highest correlation with soluble SiO2, NO3−, and SO42−, while Ca2+ and Mg2+ are highly correlated with Cl− and SO42−, indicating that these four ions may be derived from the dissolution of silicate and carbonate rocks, and the high correlation with NO3− indicates that the agricultural activities in the lake plain are related to the TDS. There is a significant correlation between the TDS and Ca2+, Mg2+, HCO3−, and SO42− in groundwater, and the groundwater may have originated from carbonate or gypsum dissolution; moreover, the high correlation between Ca2+, Mg2+, and Na+ indicates that the groundwater originated from the weathering and dissolution of the same material. The TDS of the Dongting Lake water is strongly correlated with NO3− and soluble SiO2, which is influenced by human activities, and the correlation of each ion is relatively high, indicating that the sources of Dongting Lake water recharge are diverse and complex. In addition to surface water flows from “the Three Inlets” and “the Four Rivers”, groundwater in the lake plain may experience direct drainage of Dongting Lake water, which is consistent with the results of the TDS analysis.

Table 2.
Correlation between conventional indicators of groundwater and surface water in Dongting Lake Plain (Pearson correlation).
4.4. Hydrogen and Oxygen Isotope Characterization
According to the statistical results of the hydrogen and oxygen isotope values in the Dongting Lake Plain (Table 3), δD and δ18O of the surface water in “the Three Inlets” water system area range from −15.7 to −67.2 and −2.07 to −9.87, respectively, while δD and δ18O of the groundwater range from −28.6 to −51.4 and −4.28 to −7.44, respectively; and the linear relationships are y = 6.31x − 3.5 (R2 = 0.995) and y = 6.22x − 0.44 (R = 0.79), respectively. δD and δ18 O of the groundwater and surface water show similar proportions; the slope is similar, and δD and δ18O of the groundwater are more concentrated in the study region. δD and δ18O of the surface water are dispersed in the enriched and infertile ends of the range and tend to represent medium and average changes (Figure 4a). It can be speculated that there is a spatial complementary relationship between groundwater and surface water. δD and δ18O of the surface water in “the Four Rivers” water system area range from −32.6 to −72.9 and −5.62 to −10.6, respectively; δD and δ18O of the groundwater range from −21.1 to −76.2 and −3.4 to −10.68, respectively; and the linear relationships are y = 8.14x + 13.92 (R2 = 0.988), and y = 7.88x + 11.44 (R2 = 0.953), respectively. The isotopes of the groundwater and surface water exhibit consistent changes in the spatial regions and are interrelated, and δD and δ18O of the groundwater are more enriched than surface water (Figure 4b). These indicate that the groundwater recharges to surface water in “the Four Rivers” water system area. δD and δ18O of the Dongting Lake water range from −19.5 to −73.8 and from −2.93 to −11.05, respectively, and the linear relationship is y = 6.89x + 3.15 (R2 = 0.99), with a wide range and uniform dispersion of δD and δ18O (Figure 4c). These findings indicate that the Dongting Lake water obviously receives a mixed recharge from multiple sources, which is in agreement with the results of the correlation analysis of the water chemistry.

Table 3.
Statistical characteristic values of hydrogen and oxygen isotopes from different water sources in Dongting Lake Plain.
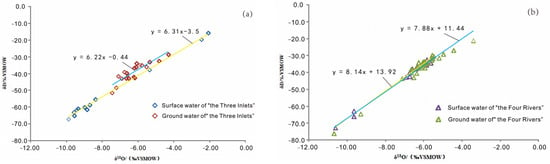
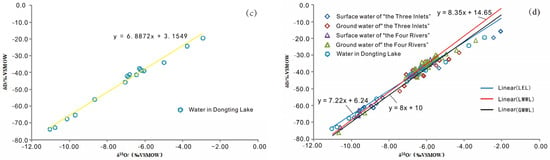
Figure 4.
δ 18O- δ D correlation diagram of groundwater and surface water in Dongting Lake Plain. (a) “The Three Inlets” water system; (b) “the Four Rivers” water system; (c) Dongting Lake water; and (d) lake area water system.
Craig proposed the global meteoric water line (GMWL) [44]. The use of the GMWL can provide the basis for the isotopic inference of recharge conditions, evaporation loss, and mixing [45]. Due to differences in geographical and environmental conditions, different regions have local meteoric water lines (LMWL). Dongting Lake Plain is closest to Changsha (Figure 1), so the Changsha meteoric water line is adopted as the meteoric water line for Dongting Lake Plain, and its δ18O-δD relationship equation is y = 8.35x + 14.65 [46]. Figure 4d shows that the meteoric water line in Changsha is closer to the GMWL, most of the samples of groundwater and surface water in the Dongting Lake Plain are distributed near the LMWL, and the slope of the local evaporation line composed of the samples is less than that of the meteoric water LMWL, which indicates that atmospheric rainfall is an important source of recharge for groundwater and surface water and is also affected by evaporation to a certain extent. Some of the surface water samples in “the Three Inlets” water system area deviate from the LMWL. In addition to recharging by atmospheric rainfall, the surface water in “the Three Inlets” water system area also originates from the Yangtze River, which receives groundwater recharge during its convergence from Dongting Lake. The samples plot closer to the LMWL. Groundwater and surface water samples in “the Four Rivers” water system area are mainly distributed around the LMWL. Atmospheric rainfall is the major source of recharge. Groundwater and surface water samples are relatively concentrated and dense, and the range of the numerical variation is similar, which indicates that there is a close hydraulic connection between groundwater and surface water. Dongting Lake water deviates from the LMWL and is mainly located in the lower-right area, which is caused by secondary evaporation of the lake water after receiving rainfall recharge and convergence, and the spatial change is also consistent with the recharge sources of the groundwater and surface water in “the Three Inlets” and “the Four Rivers” water system areas.
5. Discussion
5.1. Water Chemical Ion Sources and Control Factors
The Gibbs model [47], which considers the different types of ions and concentrations in rivers, lakes, and oceans, highlights the influence of the chemical characteristics of natural water bodies based on the mechanism, namely, evaporation–crystallization, rock weathering, and atmospheric precipitation, as a direct way to distinguish the controlling factors of hydrochemistry. The Gibbs diagram of the groundwater and surface water in “the Three Inlets” and “the Four Rivers” water system areas and Dongting Lake water (Figure 5, Figure 6 and Figure 7) reveal that the equivalent concentrations of groundwater and surface water in the Dongting Lake Plain are relatively concentrated and are mainly located in the rock weathering area, indicating that the ion sources are mainly affected by rock weathering and dissolution. Some water samples from “the Three Inlets” water system area and Dongting Lake are inclined towards the evaporation and concentration areas, indicating that the ion sources are evaporation and concentration, in addition to the dominant rock dissolution. On the one hand, water samples from “the Three Inlets” water system area were dominantly collected from shallow water, and strong evaporation occurred in the area. On the other hand, for the surficial river water in “the Three Inlets” water system area, the Dongting Lake water is exposed at the surface, and direct evaporation of the water body occurs, and for the surface water in “the Three Inlets” water system area, groundwater performance depends on a similar ion source mechanism. These findings indicate that the two have a certain transformation relationship, and at the same time, direct drainage of groundwater into Dongting Lake occurs. “The Four Rivers” water system area is partially biased toward atmospheric rainfall (Figure 6); the source area of “the Four Rivers” has a high depth of groundwater in the terrain, the effect of evaporation is weak, and the ion sources of groundwater are dominated by the natural weathering of rocks, and the surface water receiving atmospheric rainfall is controlled by certain atmospheric rainfall effects. Meanwhile, the ion sources of groundwater and surface water in “the Four Rivers” water system area are mainly dominated by the natural weathering of rocks, indicating that groundwater is likely to mix and replenish surface water. Some of the groundwater samples in the Dongting Lake Plain plot outside the dotted line delineating the control area (Figure 7), and it is hypothesized that these samples may be influenced by other factors, such as cation exchange and human activities [20].
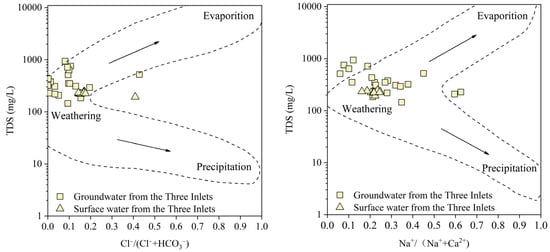
Figure 5.
Gibbs map of groundwater and surface water in “the Three Inlets” water system area.
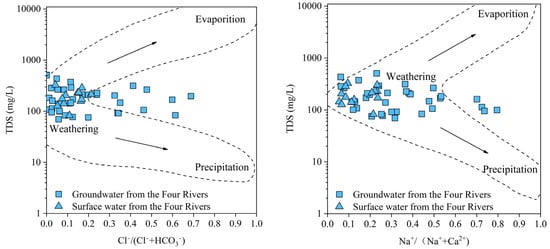
Figure 6.
Gibbs map of groundwater and surface water in “the Four Rivers” water system area.
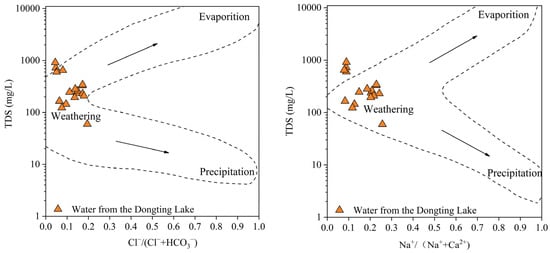
Figure 7.
Gibbs map of Dongting Lake water.
The ion sources of groundwater and surface water in the Dongting Lake Plain are dominated by the dissolution effect caused by rock weathering. Combined with the relationships between the ratios of Ca2+/Na+, Mg2+/Na+, and HCO3−/Na+, the dominant type of rock weathering can be further identified [48,49]. The ion end-element map (Figure 8) shows that surface water in the Dongting Lake Plain is mainly present within the carbonate and silicate rocks end of the distribution, indicating that surface water in the lake plain is affected by the weathering of carbonate and silicate rocks, mainly because “the Four Rivers” basin is located in the mountainous bedrock area where carbonate rocks and granite are distributed; these basins include Wuling–Xuefeng and Nanling–Luoxiao. In the mountainous bedrock area, the products of bedrock weathering and dissolution infiltrate groundwater within the water chemistry system. In “the Three Inlets” and “the Four Rivers” water system areas, groundwater is mainly present within the carbonate and silicate rocks end of the distribution; it is scattered around the surface water, with a numerical range greater than that of the surface water. Groundwater and surface water exhibit a close hydraulic relationship, and groundwater recharges surface water. Moreover, groundwater also undergoes dissolution of evaporite saline, and an ion exchange occurs in the process of migration along the way or exchange with surface water. Additionally, the mechanism of evaporation is rarely observed in surface water.
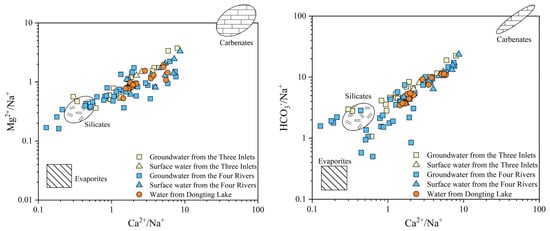
Figure 8.
Mg2+/Na+, HCO3−/Na+, and Ca2+/Na+ ratios of groundwater and surface water in Dongting Lake Plain.
5.2. Transformational Ratio between Groundwater and Surface Water
The above hydrochemical characteristics and isotope analysis reveal that there is a hydraulic connection and transformation between groundwater and surface water in the Dongting Lake Plain, and the groundwater in “the Three Inlets” and “the Four Rivers” water system areas drains and recharges Songzi River, Hudu River, and Ouchi River, as well as Xiangjiang River, Zishui River, Yuanjiang River, Lishui River, and other river systems overall. The river system reaches Dongting Lake after the direct or indirect collection or direct mixing of the discharge of Dongting Lake water, and the groundwater is indirectly or directly transformed into Dongting Lake water. To better analyze the transformation between groundwater and surface water and their contribution ratios, δ18O was based on the mass balance equation of the isotope, selected as a tracer element to obtain the contribution ratio of surface water in “the Three Inlets” and “the Four Rivers” water system areas and the groundwater in the Dongting Lake Plain that becomes Dongting Lake water. The formulas are as follows [50]:
where Qs is the flow rate of the water body after mixing; Cs is the tracer concentration of the water body after mixing; Qu and Cv are the flow rates of the water body involved in mixing; Cu and Cv are the tracer concentrations of the water body before mixing; and δ18 O was chosen as the tracer in this case.
Qs·Cs = Qu·Cu + (Qs − Qu)·Cv
Fu = Qu/Qs × 100% = (Cs − Cv)/(Cu − Cv) × 100%
Fv = Qv/Qs × 100% = (Cs − Cu)/(Cv − Cu) × 100%
Dongting Lake water (Cs = −7.12) receives surface water (Cu = −7.38) and groundwater (Cv = −6.15) recharge from “the Three Inlets” and “the Four Rivers” water system areas, and based on Equations (1)–(3), the contribution ratios of groundwater and surface water to Dongting Lake water are 21.14% and 78.86%, respectively, which are close to the contribution ratios of the groundwater and surface water recharges to the Dongting Lake water obtained by Du, Y. et al. [51]. According to the statistics of He, Q. et al. [52], after the operation of the Three Gorges Reservoir, the multiyear average runoff values of surface water in “the Three Inlets” and “the Four Rivers” were 487.71 × 108 m3 and 1593.69 × 108 m3, respectively. Therefore, through the conversion of the flow ratio, it can be determined that the contribution ratios of surface water from “the Three Inlets” and “the Four Rivers” to the Dongting Lake water are 18.48% and 60.38%, respectively, and that the contribution ratio of groundwater in the lake plain is 21.14%.
The quantification of the interaction between groundwater and surface water, as well as the contribution of the groundwater’s and surface water’s input and output is crucial for exploring the stable development of lake ecosystems. However, the identification and quantification of fluxes are difficult and have always attracted the attention of many scholars. Scholars have comprehensively applied various methods (ground penetrating radar, temperature measures in the hydraulic zone, groundwater dynamics, stable isotope measures) to explore the process and water flow path of the mutual transformation for groundwater and surface water [53,54,55,56]. The research on the quantitative transformation relationship between groundwater and surface water is still ongoing, and the coupling of hydrochemical characteristics and stable isotope methods has been widely applied and is relatively effective. However, due to heavy sampling work and high testing costs, it is difficult to dynamically monitor this during the wet and dry seasons, and accurate quantification is not easy in areas with significant seasonal changes regarding the interaction between groundwater and surface water. Further identification and dynamic evaluation using other methods, such as numerical models, are needed.
6. Conclusions
Groundwater and surface water in the Dongting Lake Plain are generally weakly acidic and weakly alkaline. The TDS variation range of the surface water is 59.4~914 mg/L, and groundwater is 69.4~938 mg/L, both of which are freshwater. The TDS of the lake water is greater than the surface water in “the Three Inlets” and “the Four Rivers” water system areas and is less than the groundwater in the lake plain. Anions in groundwater and surface water are dominantly HCO3−, and cations are dominantly Ca2+ and Mg2+. The water chemistry types of surface water in “the Three Inlets” water system area are mainly HCO3-Ca-Mg; the water chemistry types of groundwater are mainly HCO3-Ca-Mg and HCO3-Na-Ca-Mg, and the water chemistry types of surface water in “the Four Rivers” water system area are mainly HCO3-Ca-Mg and HCO3-Ca. The water chemistry types of groundwater are mainly HCO3-Ca, HCO3-Ca-Mg, and HCO3-Na-Ca-Mg, and the water chemistry types of Dongting Lake water are mainly HCO3-Ca-Mg and HCO3-Ca.
δD and δ18O of surface water in “the Three Inlets” water system area range from −15.7 to −67.2 and −2.07 to −9.87, respectively; δD and δ18O of groundwater range from −28.6 to −51.4 and −4.28 to −7.44, respectively; δD and δ18O of surface water in “the Four Rivers” water system area range from −34.4 to −72.9 and −5.62 to −10.6, respectively; δD and δ18O of groundwater range from −21.1 to −76.2 and −3.4 to −10.68, respectively; and δD and δ18O of Dongting Lake water range from −19.5 to −73.8 and −2.93 to −11.05, respectively. In the Dongting Lake Plain, the groundwater and surface water samples are mainly located near the LMWL, and the slope of the local evaporation line in the Dongting Lake Plain is less than that of the LMWL, which indicates that atmospheric rainfall is an important source of recharge for groundwater and surface water and is also affected by evaporation to a certain extent.
According to the Gibbs model and the ratios of Ca2+/Na+, Mg2+/Na+, and HCO3−/Na+ in the end-element diagram, the hydrochemical characteristics of groundwater and surface water in the Dongting Lake Plain are mainly affected by water–rock interactions; the main source of ions is the weathering and dissolution of rocks, parts of the water bodies are related to cation exchange interactions, human activities, and other factors that affect them. The hydrochemical characteristics of surface water are mainly affected by the weathering of carbonate rocks and silicate rocks; groundwater is mainly affected by the weathering of carbonate rocks, silicate rocks, and the dissolution of evaporated saline rocks in some areas; and groundwater and surface water are closely hydraulically connected with each other.
Part of the groundwater in the Dongting Lake Plain is discharged to the surface rivers in “the Three Inlets” and “the Four Rivers” water system areas, which then converge into Dongting Lake, while the other part is directly discharged to Dongting Lake, undergoing a transformation between groundwater and surface water. Based on the equation of isotope mass balance and the conversion of the flow ratio, the contribution ratios of surface water in “the Three Inlets” and “the Four Rivers” water system areas recharge into Dongting Lake water and are 18.48% and 60.38%, respectively, and the contribution ratio of groundwater recharge in the lake plain is 21.14%.
Author Contributions
Data curation, P.X.; and X.Z. Supervision, J.C.; Methodology, J.W.; Visualization, X.W.; Software, X.Z.; and Writing—review and editing, P.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Geological Survey Project of China Geological Survey (DD20221755).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, P.; Qian, H. Water resources research to support as sustainable China. Int. J. Water Resour. Dev. 2018, 34, 327–336. [Google Scholar]
- Gao, Z.; Wan, Z.; He, K.; Wei, K.; Liu, J. Hydro-chemical characteristics and controlling factors of karst groundwater in middle and upper reaches of Dawen River basin. Bull. Geol. Sci. Technol. 2022, 41, 264–272. [Google Scholar]
- Chen, J.; Jiang, S.; Yang, X.; Wang, H.; Liu, W. Study on hydro-chemical characteristics and controlling factors of the upper reach of Dagu River basin. Geol. Rev. 2022, 68, 1853–1862. [Google Scholar]
- Wang, G.; Zhang, Y.; Gou, Q.; Zhang, Z.; Sun, J. Hydro-chemical characteristics of surface water and groundwater in oasis edge in the middle reaches of the Heihe River basin. Sci. Geogr. Sin. 2022, 42, 1818–1828. [Google Scholar]
- Yi, B.; Liu, J.; Lu, X.; He, W.; Zhu, L. Hydro-chemical and Isotopic Evidence for Groundwater Conversion of Surface Water in Alpine Ard Areas: A Case Study of the Datong River Basin. Environ. Sci. 2023, 44, 752–760. [Google Scholar]
- Pei, S.; Duan, L.; Miao, P.; Pan, H.; Cui, C. Water Chemical isotope Characteristics and Water Transformation Relationship in Mongolian Section of the Yellow River Basin. Environ. Sci. 2023, 44, 4863–4873. [Google Scholar] [CrossRef]
- Lambs, L. Interactions between groundwater and surface water at river banks and the confluence of rivers. J. Hydrol. 2004, 288, 312–326. [Google Scholar] [CrossRef]
- Nie, Z.; Chen, Z.; Cheng, X.; Hao, M.; Zhang, G. The chemical information of the interaction of unconfined Groundwater and Surface water along the Heihe River, northwestern China. J. Jilin Univ. (Earth Sci. Ed.) 2005, 35, 48–53. [Google Scholar]
- Xiao, Y.; Shao, J.; Cui, Y. Groundwater circulation and hydrogeochemical evolution in Nomhon of Qaidam Basin, Northwest China. J. Earth Syst. Sci. 2017, 126, 26. [Google Scholar]
- Liang, B. The Application of Hydro-Chemical Characteristics on Transform Relationship between Surface Water and Groundwater in the Hotan River Basin; Xinjiang University: Wulumuqi, China, 2018. [Google Scholar]
- Yang, H.; Wei, J.; Ren, Q. Interaction between surface water and groundwater and hydro-chemical characteristics in the typical watersheds of the Qaidam Basin. Arid Zone Res. 2022, 39, 1543–1554. [Google Scholar]
- Busico, G.; Cuoco, E.; Kazakis, N. Multivariate statistical analysis to characterize discriminate between anthropogenic and geogenic trace elements occurrence in the Campania Plain, Southern Italy. Environ. Pollut. 2018, 234, 260–269. [Google Scholar] [CrossRef]
- Dong, J.; Duan, Q.; Zhao, D. A combined method for the source apportionment of sediment organic carbon in rivers. Sci. Total Environ. 2021, 752, 141840. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Lai, C.; Ding, Y.; Wang, Z.; Cheng, Z. Natural water chemistry change in the surface water of Chengdu and impact factors. Environ. Sci. 2021, 42, 5364–5374. [Google Scholar]
- Liu, W.; Gao, Z.; Xu, Y.; Han, C.; Luo, Z.; Zhao, Z. Hydro-chemical characteristics and water quality evaluation of karst groundwater in Jinan City. Carsologica Sin. 2022, 42, 220–232. [Google Scholar]
- Lu, J.; An, Y.; Wu, Q.; Luo, J.; Jiang, H. Hydro-chemical Characteristics and Sources of Qingshuijiang River Basin at Wet Season in Guizhou Province. Environ. Sci. 2015, 36, 1565–1572. [Google Scholar]
- Song, J.; Cheng, D.; Zhang, J. Estimating spatial pattern of hyporheic water exchange in slack water pool. J. Geogr. Sci. 2019, 29, 377–388. [Google Scholar]
- Schmalz, B.; Springer, P.; Fohrer, N. Variability of water quality in a riparian wetland with interacting shallow groundwater and surface water. J. Plant Nutr. Soil Sci. 2009, 172, 757–768. [Google Scholar] [CrossRef]
- Kong, X.; Wang, S.; Liu, B. Impact of water transfer on interaction between surface water and groundwater in the lowland area of North China Plain. Hydrol. Process. 2018, 32, 2044–2057. [Google Scholar] [CrossRef]
- Lei, M.; Zhou, J.; Zhang, J.; Chen, Y.; Teng, J. Hydro-chemical characteristics and transformation relationship of surface water and groundwater in the plain area of Bortala River basin, Xinjiang. Environ. Sci. 2022, 43, 1873–1884. [Google Scholar]
- Ping, J.; Cao, J.; Su, X.; Ye, X.; Jiang, J. Application of isotopic technique in the research of the affected range of lateral seepage of the down-Yellow River water. J. Jilin Univ. (Earth Sci. Ed.) 2004, 34, 400–404. [Google Scholar]
- Zhao, W.; Ma, J.; He, J. Groundwater recharge and geochemical evolution in the Dunhuang basin of Danghe River, northwest. China. Arid Land Geogr. 2015, 38, 1133–1141. [Google Scholar]
- Zhang, H.; Yu, K.; Li, Z.; Li, P.; Zhao, B. Characteristics of hydrogen and oxygen isotopes in different water bodies in hilly and gully regions of the loess plateau. Environ. Sci. 2019, 40, 3030–3038. [Google Scholar]
- Zhu, P.; Su, X.; Zhang, S.; Huang, Y.; Yang, F. Study on the Interaction Relationship Between Surface Water and Groundwater in Nalingguole River Alluvial-Proluvial Fan. Yellow River 2014, 36, 60–64. [Google Scholar]
- Zhang, B.; Song, X.; Zhang, Y. A study of the interrelation between surface water and groundwater using isotopes and chlorofluorocarbons in Sanjiang plain, Northeast China. Environ. Earth Sci. 2014, 72, 3901–3913. [Google Scholar]
- Freitas, J.G.; Furquim, A.C.; Aravena, R. Interaction between lakes’ surface water and groundwater in the Pantanal wetland, Brazil. Environ. Earth Sci. 2019, 78, 139. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Zhang, L. Watershed scale spatiotemporal nitrogen transport and source tracing using dual isotopes among surface water, sediments and groundwater in the Yiluo River Watershed, middle of China. Sci. Total Environ. 2022, 833, 155180. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, X.; Xia, J.; Yu, J.; Tang, C. A study of interaction between surface water and Groundwater using environmental isotope in Huaisha River basin. Sci. China Ser. D Earth Sci. 2006, 49, 1299–1310. [Google Scholar]
- Lei, Y.; Cao, S.; Cao, G.; Yang, Y.; Lan, K.; Ji, Y.; Li, H. Study on surface water and groundwater interaction of Shaliu River basin in Qinghai Lake in different periods. J. Nat. Resour. 2020, 35, 2528–2538. [Google Scholar]
- Hao, S.; Li, F.; Li, Y.; Gu, C.; Zhang, Q.; Qiao, Y.; Jiao, L.; Zhu, N. Stable isotope evidence for identifying the recharge mechanisms of precipitation, surface water, and groundwater in the Ebinur Lake basin. Sci. Total Environ. 2018, 657, 1041–1050. [Google Scholar]
- Li, J. Optimization of Water Resources Utilization in the Process of Urbanization in Dongting Lake District; Hunan Normal University: Changsha, China, 2013. [Google Scholar]
- Zhang, J.; Xu, K.; Yang, Y.; Qi, L.; Hayashi, S.; Watanabe, M. Measuring water storage fluctuations in lake Dongting, China, by topex/poseidon satellite altimetry. Environ. Monit. Assess. 2006, 115, 23–37. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, S.; Huang, G.; Zhang, R. Analysis of Long-Term Water Level Variation in Dongting Lake, China. Water 2016, 8, 306. [Google Scholar] [CrossRef]
- Wang, H.; Huang, L.; Guo, Q.; Zhu, Y.; Yang, H.; Jiao, X.; Zhou, H. Evaluation of ecohydrological regime and its driving forces in the Dongting Lake, China. J. Hydrol. Reg. Stud. 2022, 41, 101067. [Google Scholar] [CrossRef]
- Hu, C.; Fang, C.; Cao, W. Shrinking of Dongting Lake and its weakening connection with the Yangtze River: Analysis of the impact on flooding. Int. J. Sediment Res. 2015, 30, 256–262. [Google Scholar]
- Dai, M.; Wang, J.; Zhang, M.; Chen, X. Impact of the Three Gorges Project operation on the water exchange between Dongting Lake and the Yangtze River. Int. J. Sediment Res. 2017, 32, 506–514. [Google Scholar] [CrossRef]
- Sun, X.; Du, Y.; Deng, Y.; Fan, H.; Ma, T. Contrasting lacustrine groundwater discharge and associated2 nutrient loads in different geological conditions. Hydrol. Earth Syst. Sci. 2021. [Google Scholar] [CrossRef]
- Sun, X.; Du, Y.; Deng, Y.; Fan, H.; Ma, T.; Gan, Y. Contrasting nutrients input along with groundwater discharge to east Dongting Lake, central China: A geological perspective. Ecol. Indic. 2022, 145, 109658. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, W.; Xiao, P.; Guan, Y.; Yao, T. Discussion on Compilation Thought and Mothodology of 1:450000 Hydrogeological Map of the Jianghan-Dongting Plain. South China Geol. 2020, 36, 396–403. [Google Scholar]
- Lu, S.; Zhou, N.; Jiang, S.; Zheng, X. Combining hydrochemistry and environmental isotopes to study hydrogeochemical evolution of karst groundwater in the Jinci spring area, North China. Carbonates Evaporites 2023, 38, 36. [Google Scholar] [CrossRef]
- Zheng, T.; Qin, X.; Wu, J. Hydrochemical Characteristics and Its Origin of Surface Water and Groundwater in Dianbu River Basin. Environ. Sci. 2024, 45, 813–825. [Google Scholar]
- Zhang, T.; Cai, W.; Li, Y. Major Ionic Features and Their Possible Controls in the Water of the Niyang River Basin. Environ. Sci. 2017, 38, 4537–4545. [Google Scholar]
- Ren, C.; Zhang, Q. Groundwater chemical characteristics and controlling factors in a region of Northern China with intensive human activity. Int. J. Environ. Res. Public Health 2020, 17, 9126. [Google Scholar] [CrossRef] [PubMed]
- CRAIG, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [PubMed]
- Zhang, P. Hydrogen-Oxygen Isotope Geochemistry and Environmental Indication of River Water in the Dabieshan Area, the Upper Watershed of Huaihe River, China; China University of Geosciences: Beijing, China, 2019. [Google Scholar]
- Zhang, L.; Chen, Z.; Nie, Z.; Liu, F.; Jia, Y.; Zhang, X. Correlation between 18O in precipitation and surface air temperature on different time-scale in China. Nucl. Tech. 2008, 31, 715–720. [Google Scholar]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar]
- Gaillardet, J.; Dupre, B.; Louvat, P. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar]
- Li, S.; Han, X.; Wang, W.; Li, Z. Hydro-chemical characteristics and controlling factors of surface water and groundwater in Wuding River basin. Environ. Seience 2022, 43, 220–229. [Google Scholar]
- Wen, G.; Wang, W.; Duan, L.; Gu, X.; Li, Y.; Zhao, J. Quantitative evaluation of the transformation relationship between surface water and groundwater in Bayin River Basin Based on hydrochemistry and stable isotopes. Arid. Area Geogrsphy 2018, 41, 734–743. [Google Scholar]
- Sun, X.; Du, Y.; Deng, Y.; Tao, Y.; Ma, T. Contribution and Its Temporal variation of Groundwater Discharge to the Water Mass Balance of Dongting Lake from 1996 to 2017. Earth Sci. 2021, 46, 2555–2564. [Google Scholar]
- He, Q.; Yu, D.; Yu, S.; Li, C.; Luo, W.; Yang, L.; Zou, J. Changes of Water Resources amount in Dongting Lake before and after the Operation of the Three Gorges Reservoir. Earth Sci. 2021, 46, 293–307. [Google Scholar]
- Lewandowski, J.; Meinikmann, K.; Krause, S. Groundwater–Surface Water Interactions: Recent Advances and Interdisciplinary Challenges. Water 2020, 12, 296. [Google Scholar] [CrossRef]
- Turner, J.V. Estimation and Prediction of the Exchange of Groundwater and Surface Water: Field Methodologies; E Water Technical Report; E Water Cooperative Research Centre: Canberra, Australia, 2009. [Google Scholar]
- Vrzel, J.; Kip Solomon, D.; Blažeka, Ž.; Ogrinc, N. The study of the interactions between groundwater and Sava River water in the Ljubljansko polje aquifer system (Slovenia). J. Hydrol. 2018, 556, 384–396. [Google Scholar]
- Langhoff, J.H.; Rasmussen, K.R.; Christensen, S. Quantification and regionalization of groundwater–surface water interaction along an alluvial stream. J. Hydrol. 2006, 320, 342–358. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).