Dynamics of Water-Soluble Metals in Soil Moistened with Citrus Wastewaters Depends on Soil Reaction and Organic Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Citrus Wastewaters
2.2. Experimental Set-Up
2.3. Organic Acids Determinations by High Performance Liquid Chromatography (HPLC)
2.4. Soil Analyses
3. Results
3.1. Chemical Compositions of CWWs
3.2. Metal Content of CWWs
3.3. Amount of Metals Added to Soil by CWWs
3.4. Organic Acids in CWWs
3.5. Effect of CWWs Application on Soil pH
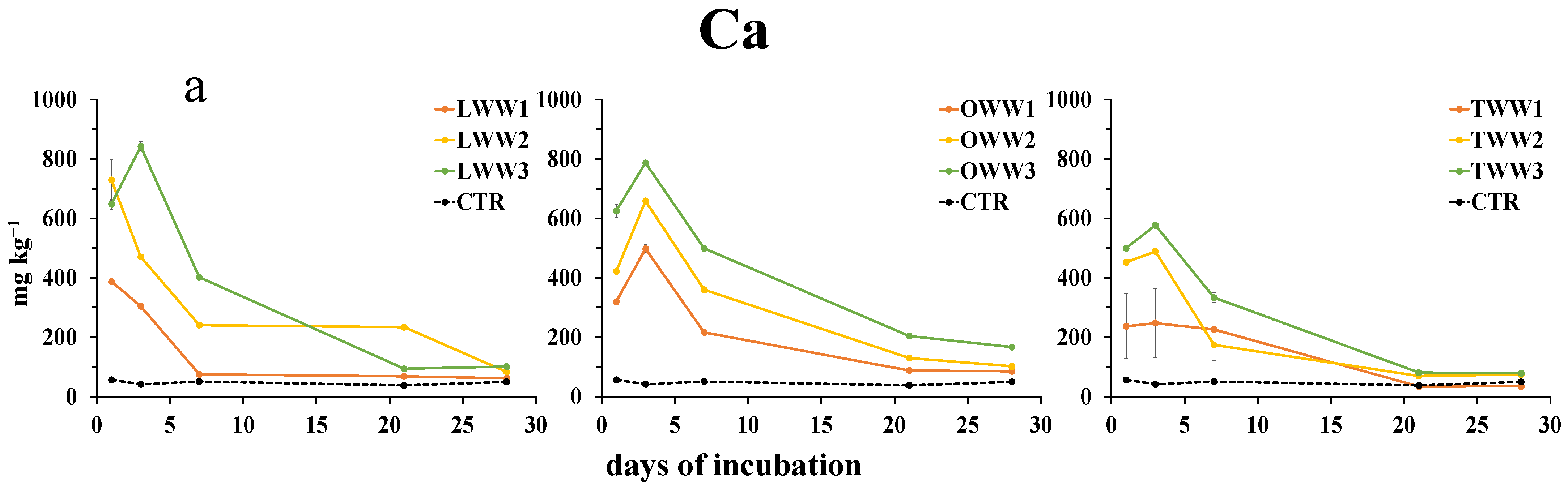
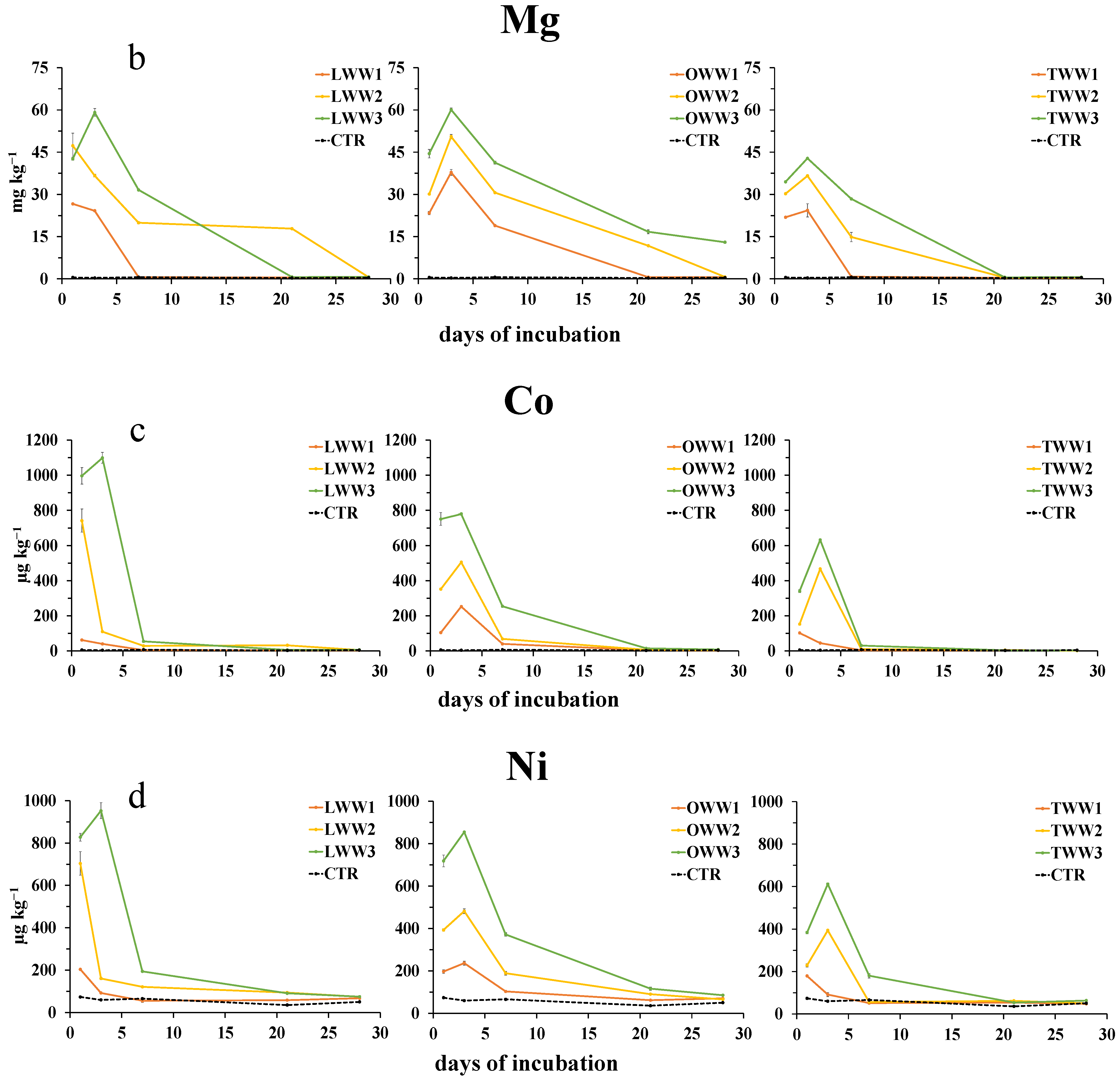
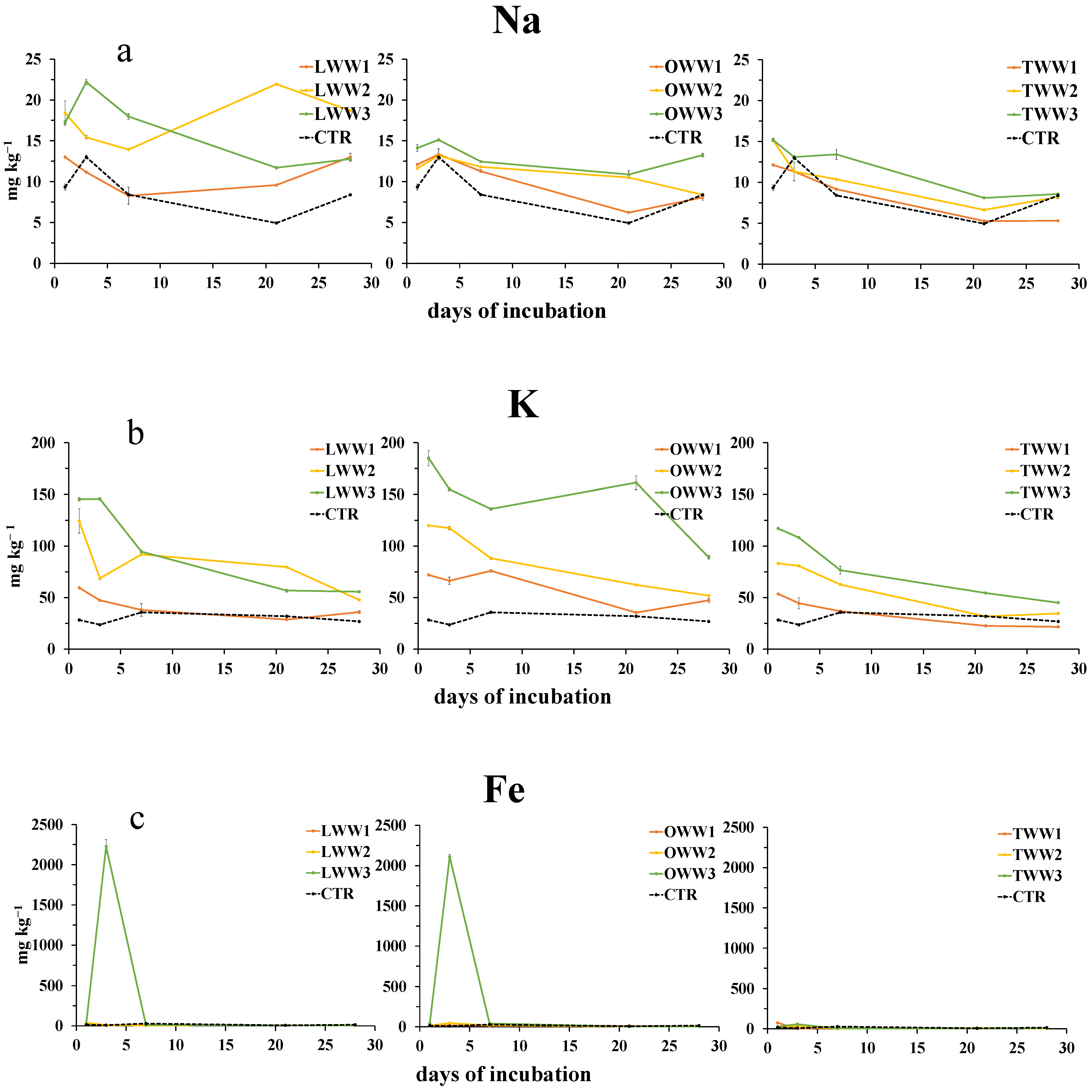
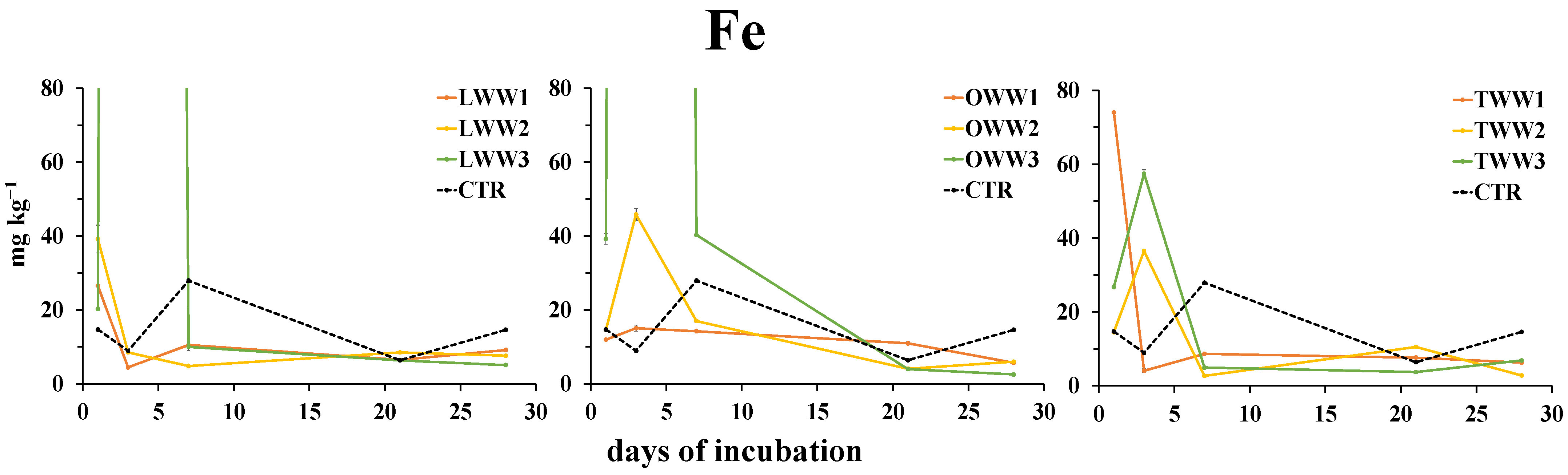
3.6. Effect of CWWs Application on Soil Water-Soluble Metals
4. Discussion
4.1. Water Dilution Effect on CWWs Characteristics
4.2. CWWs Effects on Soil pH
4.3. CWWs Effects on Soil Metals Availability
| Organic Acid | ||||||
|---|---|---|---|---|---|---|
| Metal | Citric | Ascorbic | Oxalic | Tartaric | Acetic | Malic |
| Ca | 3.18–3.50 a | 1.66–3.00 a | 0.45–1.18 a | 1.67–2.72 a | ||
| Mg | 3.27–3.37 a | 2.76–3.43 a | 0.50–1.27 a | 1.42–1.71 a | ||
| K | 0.59 a–0.84 b | 0.48 b | 0.11–0.4 a | |||
| Na | 0.89b | 1.06 b | 0.17–0.29 a | |||
| Al | 7.87 a | 3.86 c | 11.09 a | 1.51 a | ||
| Fe | 11.2 a | 7.59 a | 1.40 a | |||
| Cu | 5.90 d | 7.56 c | 4.84 d | 1.89 d | 3.33–3.60 a | |
| Zn | 4.98 d –5.90 a | 3.88 d | 3.31 a | 1.10 d–1.57 a | ||
| Mn | 4.15 a | 3.35 e | 1.94 e | 1.40 e | ||
| Ni | 5.35 a | 4.69 e | 2.41 e | 1.44 e | ||
| Cd | 4.54 a | 3.74 c | 3.35 e | 2.15 e | 1.92 e | |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mortimore, M.; Anderson, S.; Cotula, L.; Davies, J.; Faccer, K.; Hesse, C.; John Morton, J.; Nyangena, W.; Skinner, J.; Wolfangel, C. Dryland Opportunities: A New Paradigm for People, Ecosystems and Development; IUCN: Gland, Switzerland, 2009; p. 86. [Google Scholar]
- Modarres, R.; da Silva, V.R. Rainfall Trends in Arid and Semi-Arid Regions of Iran. J. Arid Environ. 2007, 70, 344–355. [Google Scholar] [CrossRef]
- Kulkarni, S.J. Recycle and Reuse of Water-A Review. Int. J. Res. 2014, 1, 802–805. [Google Scholar]
- FAO. Citrus Fruit Fresh and Processed Statistical Bulletin 2020; FAO: Roma, Italy, 2021. [Google Scholar]
- Lucia, C.; Laudicina, V.A.; Badalucco, L.; Galati, A.; Palazzolo, E.; Torregrossa, M.; Viviani, G.; Corsino, S.F. Challenges and opportunities for citrus wastewater management and valorisation: A review. J. Environ. Manag. 2022, 321, 115924. [Google Scholar] [CrossRef] [PubMed]
- Corsino, S.F.; Di Trapani, D.; Capodici, M.; Torregrossa, M.; Viviani, G. Optimization of acetate production from citrus wastewater fermentation. Water Resour. Ind. 2021, 25, 100140. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Dincă, M.; Zăbavă, B.Ș. Reuse of wastewater for irrigation, a sustainable practice in arid and semi-arid regions. In Proceedings of the 7th International Conference on Thermal Equipment, Renewable Energy and Rural Development (TE-RE-RD), Drobeta-Turnu Severin, Romania, 31 May–2 June 2018; pp. 379–384. [Google Scholar]
- Zema, D.A.; Bombino, G.; Andiloro, S.; Zimbone, S.M. Irrigation of energy crops with urban wastewater: Effects on biomass yields, soils and heating values. Agric. Water Manag. 2012, 115, 55–65. [Google Scholar] [CrossRef]
- Ioppolo, A.; Laudicina, V.A.; Badalucco, L.; Saiano, F.; Palazzolo, E. Wastewaters from citrus processing industry as natural biostimulants for soil microbial community. J. Environ. Manag. 2020, 273, 111137. [Google Scholar] [CrossRef]
- Bastida, F.; Torres, I.F.; Abadía, J.; Romero-Trigueros, C.; Ruiz-Navarro, A.; Alarcón, J.J.; García, C.; Nicolás, E. Comparing the impacts of drip irrigation by freshwater and reclaimed wastewater on the soil microbial community of two citrus species. Agric. Water Manag. 2018, 203, 53–62. [Google Scholar] [CrossRef]
- Corsino, S.F.; Di Trapani, D.; Torregrossa, M.; Viviani, G. Aerobic granular sludge treating high strength citrus wastewater: Analysis of pH and organic loading rate effect on kinetics, performance and stability. J. Environ. Manag. 2018, 214, 23–35. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Khan, S.A. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Eng. 2018, 120, 274–298. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Kumar, S.; Chadd, R.P.; Kumar, A. Trace elements in soil-vegetables interface: Translocation, bioaccumulation, toxicity and amelioration—A review. Sci. Total Environ. 2019, 6512, 2927–2942. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Factors affecting phytoextraction: A review. Pedosphere 2016, 26, 148–166. [Google Scholar] [CrossRef]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2022, 73, 13203. [Google Scholar] [CrossRef]
- Kim, J.O.; Lee, Y.W.; Chung, J. The role of organic acids in the mobilization of heavy metals from soil. KSCE J. Civ. Eng. 2013, 17, 1596–1602. [Google Scholar] [CrossRef]
- Huang, W.H.; Keller, W.D. Organic acids as agents of chemical weathering of silicate minerals. Nat. Phys. Sci. 1972, 239, 149–151. [Google Scholar] [CrossRef]
- Boudot, J.P.; Bel, H.; Brahim, A.; Steiman, R.; Seigle-Murandi, F. Biodegradation of Synthetic Organo-Metallic Complexes of Iron and Aluminum with Selected Metal to Carbon Ratios. Soil Biol. Biochem. 1989, 21, 961–966. [Google Scholar] [CrossRef]
- Terzano, R.; Cuccovillo, G.; Pascazio, S.; Crecchio, C.; Lettino, A.; Fiore, S.; Tomasi, N.; Pinton, R.; Mimmo, T.; Cesco, S. Degradation of citrate promotes copper co-precipitation within aluminium-hydroxides in calcareous soils. Biol. Fertil. Soils 2017, 53, 115–128. [Google Scholar] [CrossRef]
- Walkley, A.; Black, C.A. An examination of degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–83. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen—Total. In Methods of Soil Analysis. Part 3, Chemical Methods; Sparks, D.L., Ed.; SSSA Book Series No. 5; Soil Science Society of America & American Society of Agronomy: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Hedge, J.E.; Hofreiter, B.T. Carbohydrate Chemistry 17; Whistler, R.L., Be Miller, J.N., Eds.; Academic Press: New York, NY, USA, 1962. [Google Scholar]
- Yebra, M.C.; Gallego, M.; Valcárcel, M. Automatic determination of reducing sugars by atomic absorption spectrometry. Anal. Chim. Acta 1993, 276, 385–391. [Google Scholar] [CrossRef]
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; Devries, J.W.; Furda, I. Determination of insoluble, soluble, and total dietary fiber in foods and Food products: Interlaboratory study. J. Assoc. Off. Anal. Chem. 1988, 71, 5. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Zaman, M.; Shahid, S.A.; Heng, L. Irrigation water quality. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; p. 5. [Google Scholar]
- Jones, D.L.; Dennis, P.G.; Owen, A.G.; van Hees, P.A.W. Organic acid behaviour in soils-misconceptions and knowledge gaps. Plant Soil 2003, 248, 31–41. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soil, 14th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Regulation EU 2020/741, 2020. Regulation EU 2020/741, Minimum Requirements for Water Reuse. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0741 (accessed on 28 February 2024).
- Mekki, A.; Dhouib, A.; Sayadi, S. Review: Effects of olive mill wastewater application on soil properties and plants growth. Int. J. Recycl. Org. Waste Agric. 2013, 2, 15. [Google Scholar] [CrossRef]
- Najafi, S.; Jalali, M. Effect of heavy metals on pH buffering capacity and solubility of Ca, Mg, K, and P in non-spiked and heavy metal-spiked soils. Environ. Monit. Assess 2016, 1886, 342. [Google Scholar] [CrossRef] [PubMed]
- Almas, A.R.; Lofts, S.; Mulder, J.; Tipping, E. Solubility of major cations and Cu, Zn and Cd in soil extracts of some contaminated agricultural soils near a zinc smelter in Norway: Modelling with a multi surface extension of WHAM. Eur. J. Soil Sci. 2007, 58, 1074–1086. [Google Scholar] [CrossRef]
- Uroz, S.; Oger, P.; Lepleux, C.; Collignon, C.; Frey-Klett, P.; Turpault, M.P. Bacterial weathering and its contribution to nutrient cycling in temperate forest ecosystems. Res. Microbiol. 2011, 1629, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Kirchman, D.L. Microbial growth, biomass production, and controls. In Processes in Microbial Ecology, 2nd ed.; Oxford University Press: New York, NY, USA, 2018; pp. 133–153. [Google Scholar]
- Mortensen, J.L. Complexing of Metals by Soil Organic Matter. Soil Sci. Soc. Am. J. 1963, 272, 179–186. [Google Scholar] [CrossRef]
- Rieuwerts, J.S.; Thornton, I.; Farago, M.E.; Ashmore, M.R. Factors influencing metal bioavailability in soils: Preliminary investigations for the development of a critical loads approach for metals. Chem. Speciat. Bioavailab. 1998, 102, 61–75. [Google Scholar] [CrossRef]
- Evans, L.J. Chemistry of metal retention by soils. Environ. Sci. Technol. 1989, 23, 1046–1056. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Holm, P.E.; Strobel, B.W. Potential of dissolved organic matter DOM to extract As, Cd, Co, Cr, Cu, Ni, Pb and Zn from polluted soils: A review. Geoderma 2019, 343, 235–246. [Google Scholar] [CrossRef]
- Liu, X.; Tournassat, C.; Grangeon, S.; Kalinichev, A.; Takahashi, Y.; Fernandes, M. Molecular-level understanding of metal ion retention in clay-rich materials. Nat. Rev. Earth. Environ. 2022, 3, 461–476. [Google Scholar] [CrossRef]
- Javanbakht, V.; Alavi, S.A.; Zilouei, H. Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci.Technol. 2014, 69, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Renella, G.; Landi, L.; Nannipieri, P. Degradation of low molecular weight organic acids complexed with heavy metals in soil. Geoderma 2004, 122, 311–315. [Google Scholar] [CrossRef]
- Schwab, A.P.; Zhu, D.S.; Banks, M.K. Influence of organic acids on the transport of heavy metals in soil. Chemosphere 2008, 72, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, R.; Nwangburuka, C.; Oboirien, B. Origins, roles and fate of organic acids in soils: A review. S. Afr. J. Bot. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Goss, S.L.; Lemons, K.A.; Kerstetter, J.E.; Bogner, R.H. Determination of calcium salt solubility with changes in pH and PCO2, simulating varying gastrointestinal environments. J. Pharm. Pharmacol. 2007, 59, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M.; Martell, A.E. Critical Stability Constants; Plenum Press: New York, NY, USA, 1974; Volume 1, p. 135. [Google Scholar]
- Huang, W.H.; Keller, W.D. Geochemical mechanisms for the dissolution, transport, and deposition of aluminum in the zone of weathering. Clay Clay Min. 1972, 20, 69–74. [Google Scholar] [CrossRef]
- McBride, M.B. Cu2+ adsorption characteristics of aluminum hydroxide and oxyhydroxides. Clay Clay Min. 1982, 30, 21–28. [Google Scholar] [CrossRef]
- Martinez, C.E.; McBride, M.B. Aging of coprecipitated Cu in alumina: Changes in structural location, chemical form, and solubility. Geochim. Cosmochim. Acta 2000, 64, 1729–1736. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E. Critical Stability Constants; Plenum Press: New York, NY, USA, 1977; Volume 3, p. 512. [Google Scholar]
- Smith, R.M.; Martell, A.E. Critical Stability Constants; Plenum Press: New York, NY, USA, 1989; Volume 6, p. 643. [Google Scholar]
- Zelenina, T.E.; Zelenin, O.Y. Complexation of citric and tartaric acids with Na and K ions in aqueous solution. Russ. J. Coord. Chem. 2005, 31, 235–242. [Google Scholar] [CrossRef]
- Chandrathilaka, A.M.D.S.; Ileperuma, O.A.; Hettiarachchi, C.V. Spectrophotometric and pH-metric studies on Pb(II), Cd(II), Al(III) and Cu(II) complexes of paracetamol and ascorbic acid. J. Natl. Sci. Found. 2013, 41, 337–344. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, R.; Lu, T.; Qi, W.; Zhu, Y.; Lu, M.; Qi, Z.; Chen, W. Enhanced transport of heavy metal ions by low-molecular-weight organic acids in saturated porous media: Link complex stability constants to heavy metal mobility. Chemosphere 2022, 290, 133339. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Topsoil | |
|---|---|---|
| Clay | % | 16 |
| Sand | % | 65 |
| Slit | % | 19 |
| pH | 7.2 | |
| Electrical conductivity | dS m−1 | 0.1 |
| Total carbon | g kg−1 | 15.5 |
| Total nitrogen | g kg−1 | 1.2 |
| Total carbonates | n.d. | |
| Ca | g kg−1 | 2.3 |
| Mg | g kg−1 | 2.6 |
| K | g kg−1 | 1.3 |
| Na | g kg−1 | 0.2 |
| Al | g kg−1 | 6.3 |
| Fe | g kg−1 | 17.6 |
| Cu | mg kg−1 | 25 |
| Zn | mg kg−1 | 40 |
| Mn | mg kg−1 | 320 |
| Ni | mg kg−1 | 30 |
| Cd | mg kg−1 | 5.0 |
| Parameters | LWW | OWW | TWW | |
|---|---|---|---|---|
| Density | g cm−3 | 1.01 a | 1.04 a | 1.01 a |
| Total carbon | % | 2.5 b | 6.9 a | 2.7 b |
| Total nitrogen | % | 0.28 b | 0.54 a | 0.16 b |
| Total carbon/nitrogen | 8.9 c | 12.8 b | 19.9 a | |
| Ashes | % | 2.6 a | 1.4 b | 2.2 a |
| Total soluble monosaccharides | % | 1.1 c | 3.1 a | 2.0 b |
| Total carbohydrates | % | 1.4 c | 3.6 a | 2.9 b |
| Total fibers | % | 0.9 b | 1.1 a | 0.3 c |
| Total phosphorus | mg L−1 | 1.59 b | 4.64 a | 1.36 b |
| Ca | mg L−1 | 5.0 b | 10.3 a | 3.7 b |
| Mg | mg L−1 | 1.5 b | 7.7 a | 2.5 b |
| K | mg L−1 | 60 b | 206 a | 69 b |
| Na | mg L−1 | 0.5 b | 1.3 a | 0.4 b |
| Al | μg L−1 | 244 a | 188 b | 132 c |
| Cd | μg L−1 | 0.01 a | 0.01 a | 0.01 a |
| Co | μg L−1 | 0.19 b | 0.14 a | 0.16 a |
| Cu | μg L−1 | 43 a | 43 a | 42 a |
| Fe | μg L−1 | 94 a | 68 b | 62 b |
| Ni | μg L−1 | 2.1 b | 1.6 c | 2.3 a |
| Zn | μg L−1 | 37 ab | 36 b | 41 a |
| Treatment | Dose | pH |
|---|---|---|
| LWW | 1/3 | 2.88 Ba |
| 2/3 | 2.82 Ba | |
| 3/3 | 2.78 Ba | |
| OWW | 1/3 | 3.39 Aa |
| 2/3 | 3.48 Aa | |
| 3/3 | 3.45 Aa | |
| TWW | 1/3 | 3.23 Aa |
| 2/3 | 3.29 Aa | |
| 3/3 | 3.28 Aa |
| Metal | LWW | OWW | TWW | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1/3 | 2/3 | 3/3 | 1/3 | 2/3 | 3/3 | 1/3 | 2/3 | 3/3 | |
| mg kg−1 | |||||||||
| Ca | 0.39 | 0.78 | 1.17 | 0.80 | 1.60 | 2.40 | 0.29 | 0.58 | 0.86 |
| Mg | 0.12 | 0.23 | 0.35 | 0.60 | 1.20 | 1.80 | 0.19 | 0.39 | 0.58 |
| K | 4.69 | 9.38 | 14.07 | 16.03 | 32.06 | 48.09 | 5.37 | 10.73 | 16.10 |
| Na | 0.04 | 0.08 | 0.12 | 0.10 | 0.20 | 0.30 | 0.03 | 0.06 | 0.09 |
| µg kg−1 | |||||||||
| Al | 18.97 | 37.94 | 56.91 | 14.60 | 29.19 | 43.79 | 10.28 | 20.57 | 30.85 |
| Cd | 0.0008 | 0.0016 | 0.0023 | 0.0008 | 0.0016 | 0.0023 | 0.0008 | 0.0016 | 0.0023 |
| Co | 0.04 | 0.03 | 0.01 | 0.03 | 0.02 | 0.01 | 0.04 | 0.02 | 0.01 |
| Cu | 3.36 | 6.72 | 10.08 | 3.33 | 6.65 | 9.98 | 3.29 | 6.57 | 9.86 |
| Fe | 7.31 | 14.63 | 21.94 | 5.32 | 10.64 | 15.96 | 4.80 | 9.60 | 14.40 |
| Ni | 0.16 | 0.32 | 0.48 | 0.13 | 0.26 | 0.38 | 0.18 | 0.36 | 0.55 |
| Zn | 2.88 | 5.76 | 8.64 | 2.79 | 5.59 | 8.38 | 3.19 | 6.38 | 9.58 |
| CWWs | Organic Acid (g L−1) | |||||
|---|---|---|---|---|---|---|
| Citric | Ascorbic | Oxalic | Tartaric | Acetic | Malic | |
| LWW | 10.4 a | 0.43 a | 0.12 a | 0.09 b | 0.20 a | 0.16 a |
| OWW | 4.8 b | 0.22 b | 0.12 a | 0.34 a | 0.17 a | 0.09 b |
| TWW | 2.8 c | 0.12 c | 0.05 b | 0.08 b | 0.07 b | 0.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pampinella, D.; Laudicina, V.A.; Saiano, F.; Palazzolo, E.; Badalucco, L.; Ioppolo, A. Dynamics of Water-Soluble Metals in Soil Moistened with Citrus Wastewaters Depends on Soil Reaction and Organic Acids. Water 2024, 16, 1112. https://doi.org/10.3390/w16081112
Pampinella D, Laudicina VA, Saiano F, Palazzolo E, Badalucco L, Ioppolo A. Dynamics of Water-Soluble Metals in Soil Moistened with Citrus Wastewaters Depends on Soil Reaction and Organic Acids. Water. 2024; 16(8):1112. https://doi.org/10.3390/w16081112
Chicago/Turabian StylePampinella, Daniela, Vito Armando Laudicina, Filippo Saiano, Eristanna Palazzolo, Luigi Badalucco, and Antonino Ioppolo. 2024. "Dynamics of Water-Soluble Metals in Soil Moistened with Citrus Wastewaters Depends on Soil Reaction and Organic Acids" Water 16, no. 8: 1112. https://doi.org/10.3390/w16081112
APA StylePampinella, D., Laudicina, V. A., Saiano, F., Palazzolo, E., Badalucco, L., & Ioppolo, A. (2024). Dynamics of Water-Soluble Metals in Soil Moistened with Citrus Wastewaters Depends on Soil Reaction and Organic Acids. Water, 16(8), 1112. https://doi.org/10.3390/w16081112







