Electrocoagulation Process as an Efficient Method for the Treatment of Produced Water Treatment for Possible Recycling and Reuse
Abstract
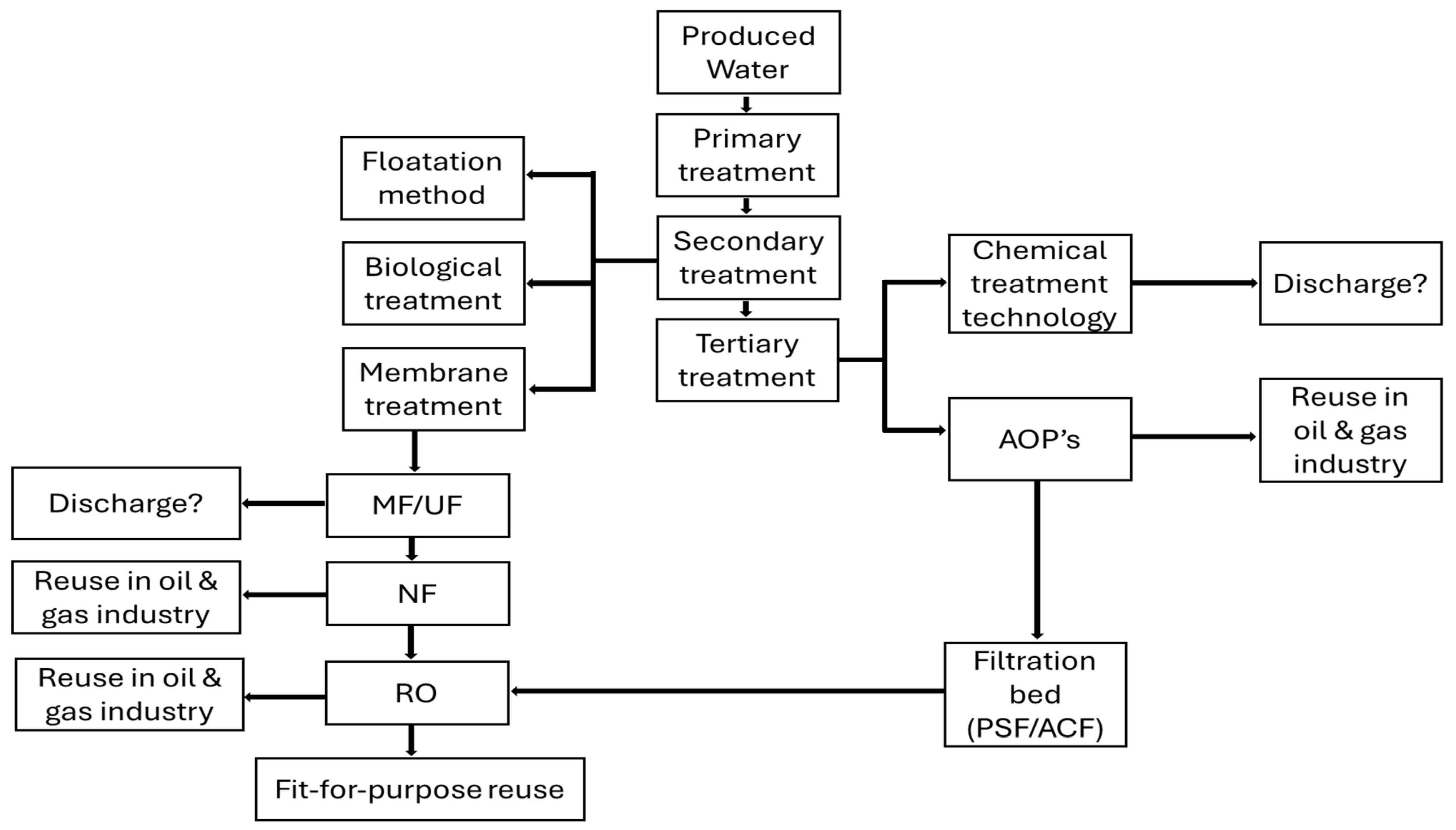
1. Introduction
2. Literature Review
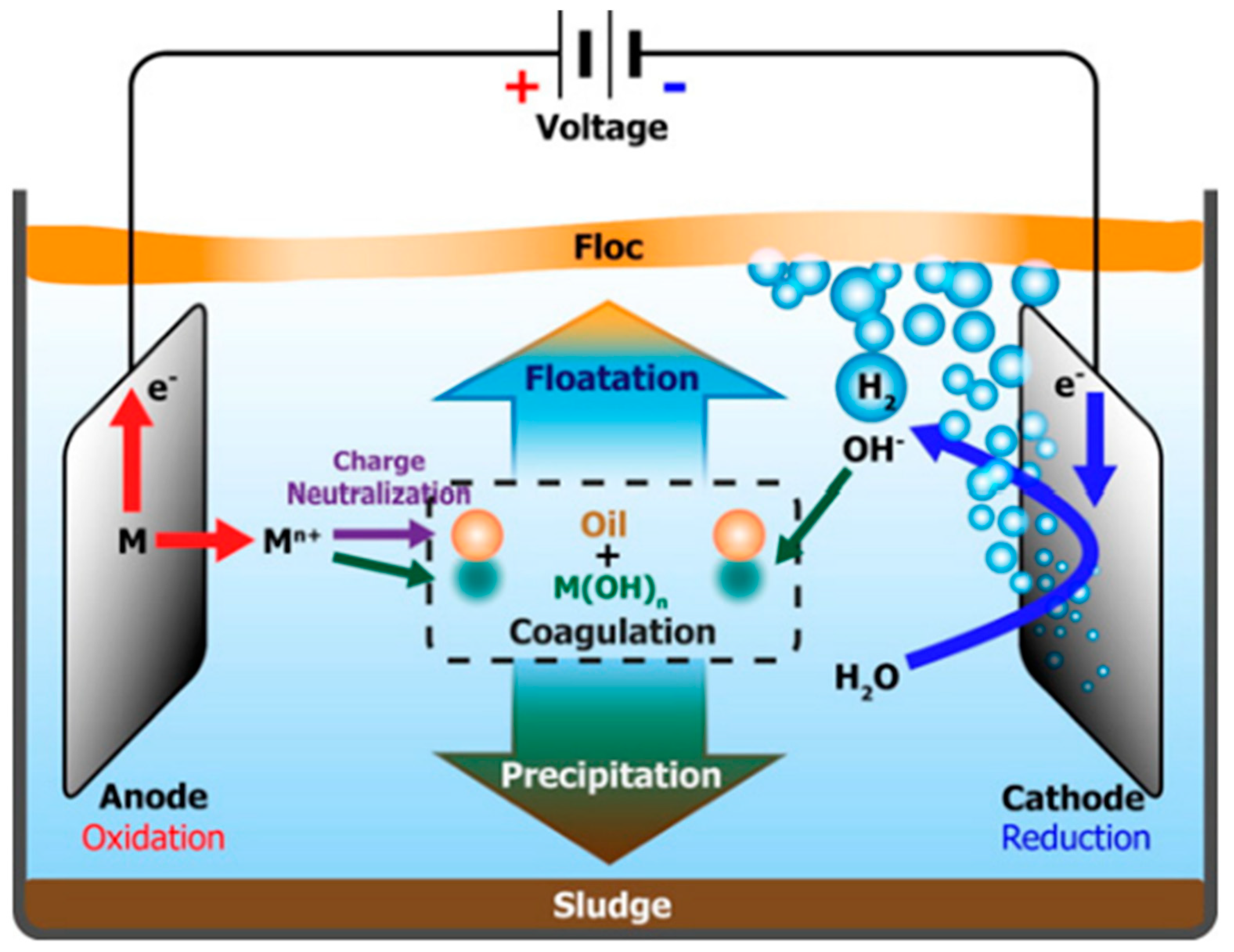
3. Mechanism of Electrocoagulation
- Anodic Reaction (Oxidation)
- Cathodic Reaction (Reduction)
- Hydroxide Formation
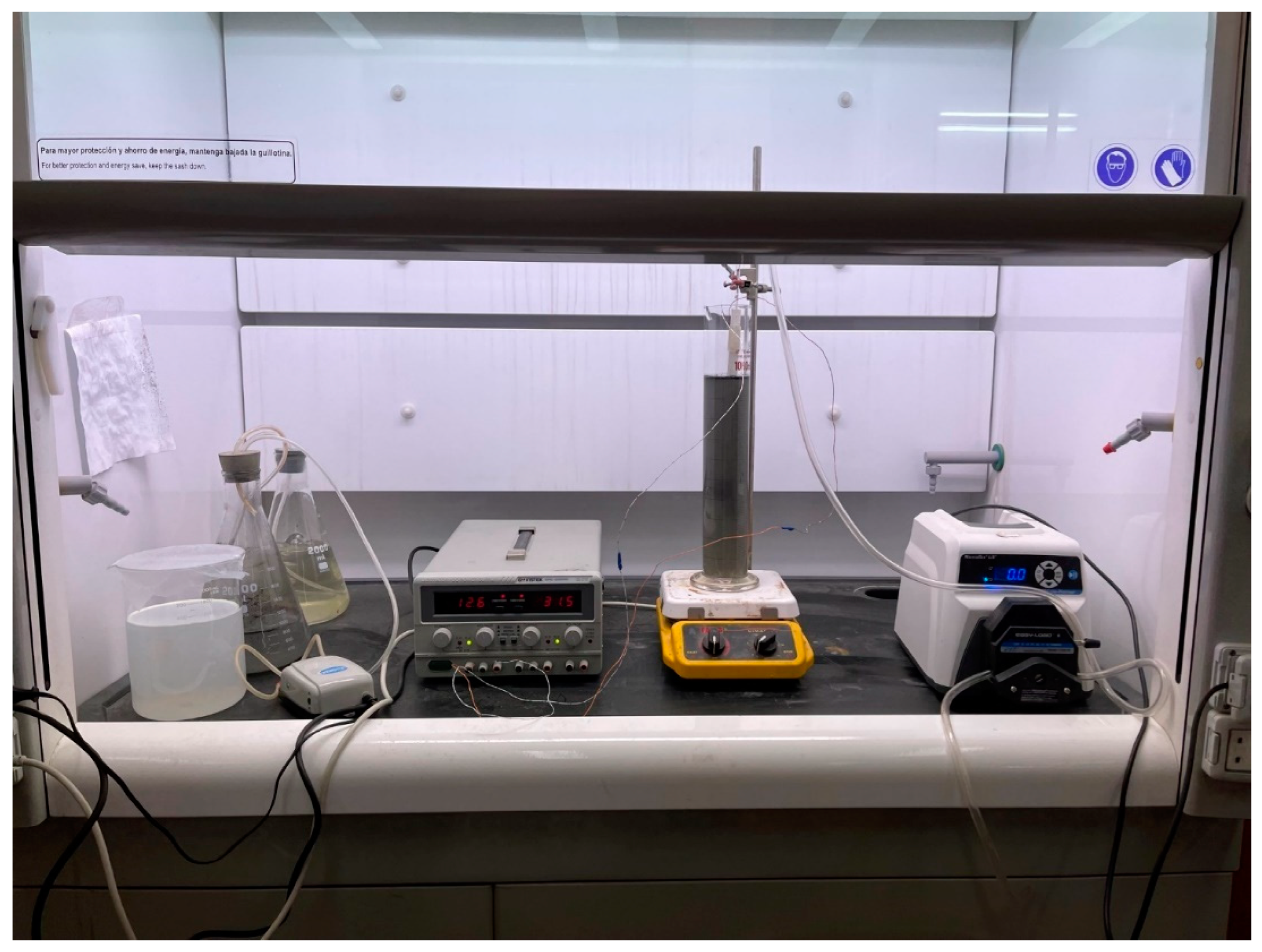
4. Materials and Methods
4.1. PW Pre-Treatment
4.2. Electrocoagulation Tests
4.3. Electrode Type Selection
5. Results and Discussions
5.1. PW Characterization
5.2. Electrocoagulation (EC) of PW
5.2.1. Turbidity Removal
5.2.2. Effect of EC on the pH of PW
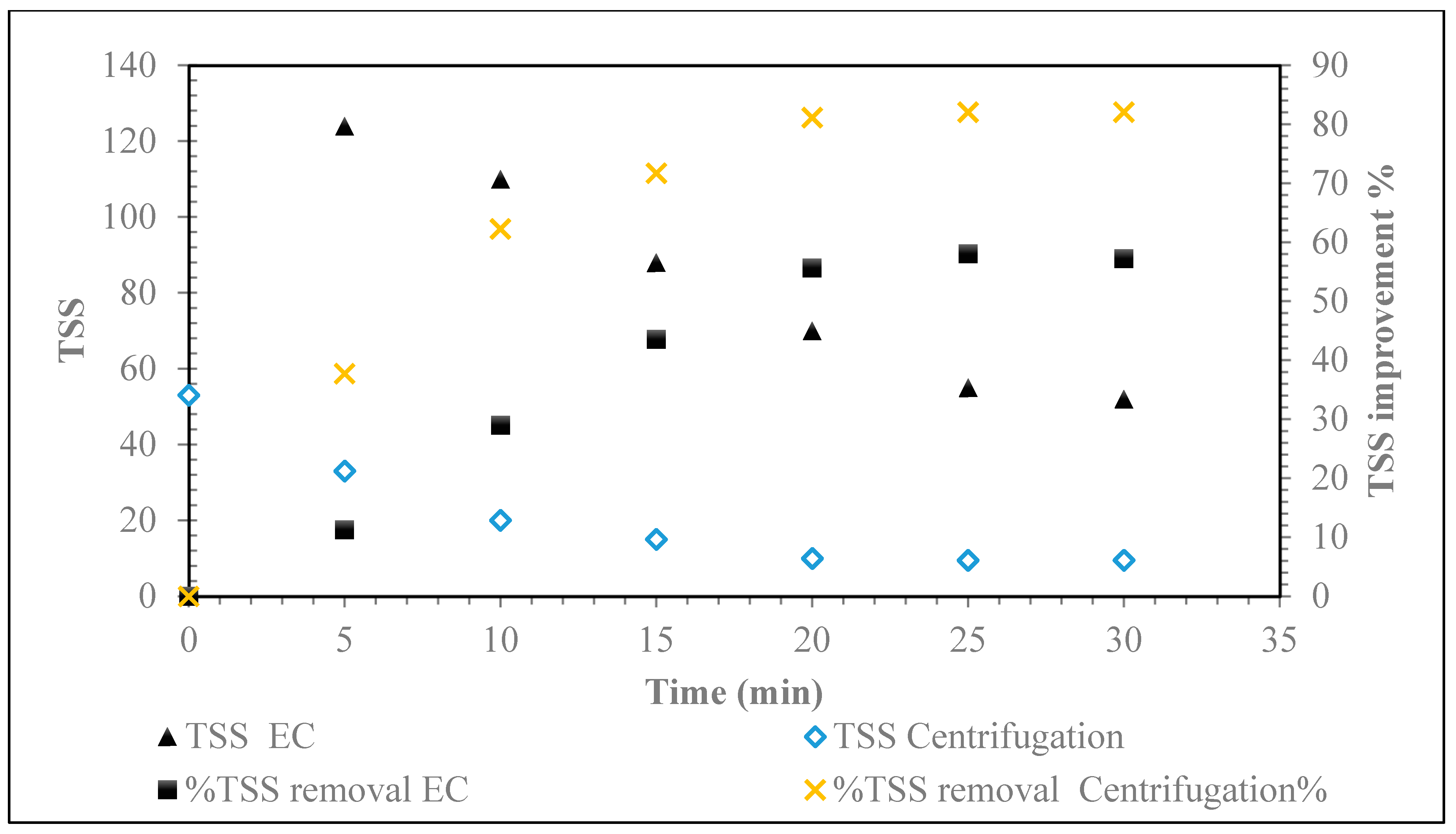
5.2.3. Total Suspended Solids (TSS) Removal
5.2.4. Effect of EC on Conductivity of PW
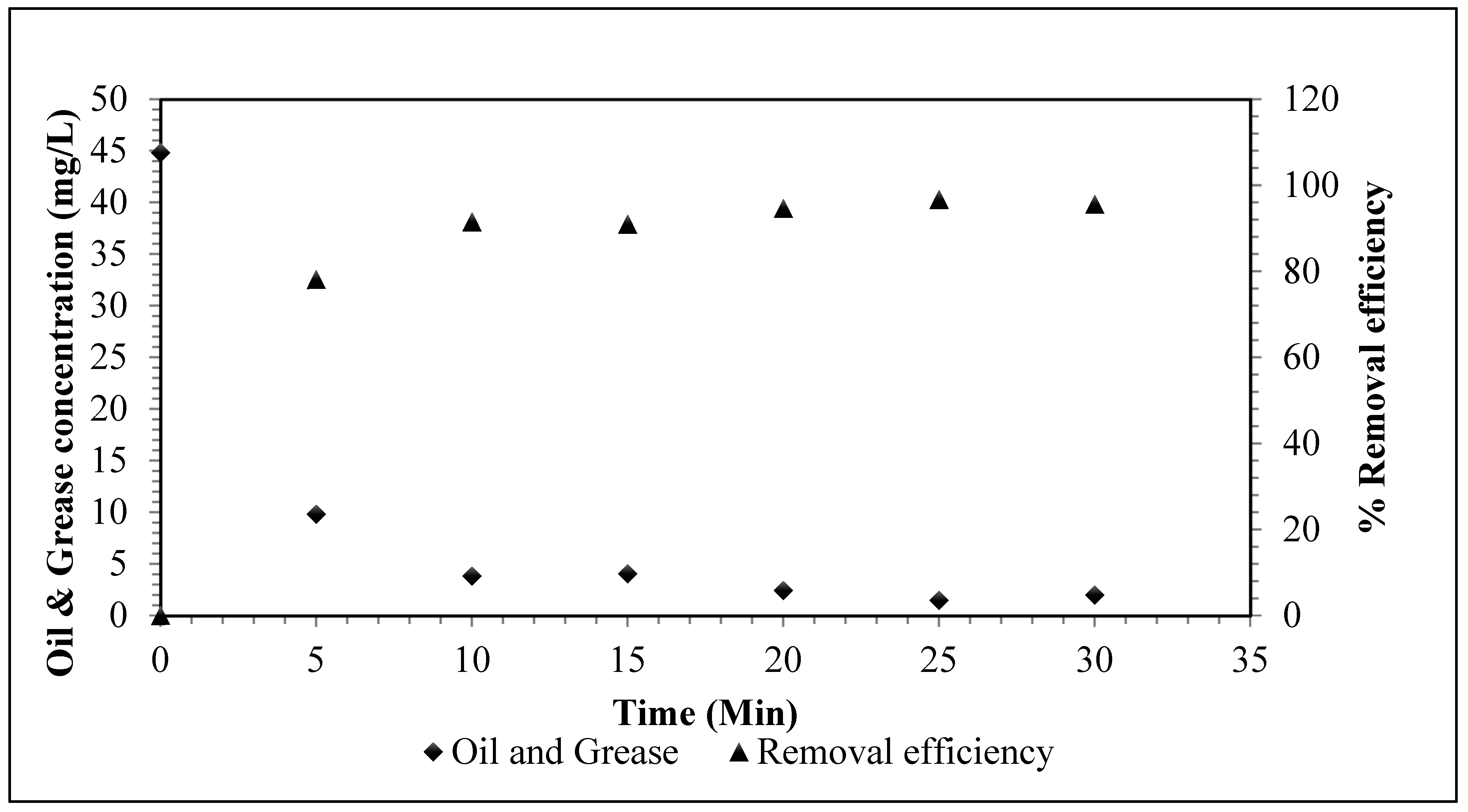
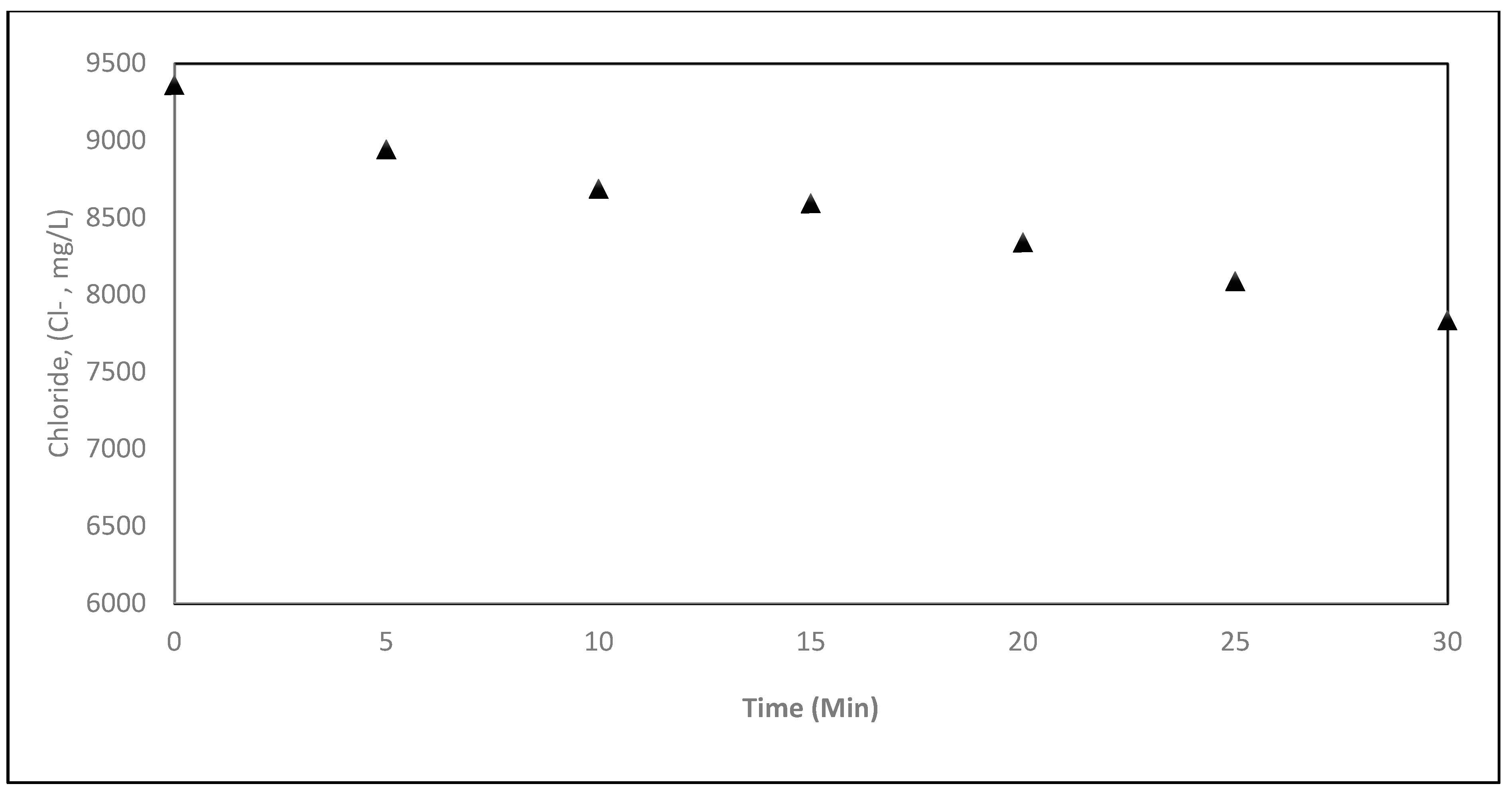
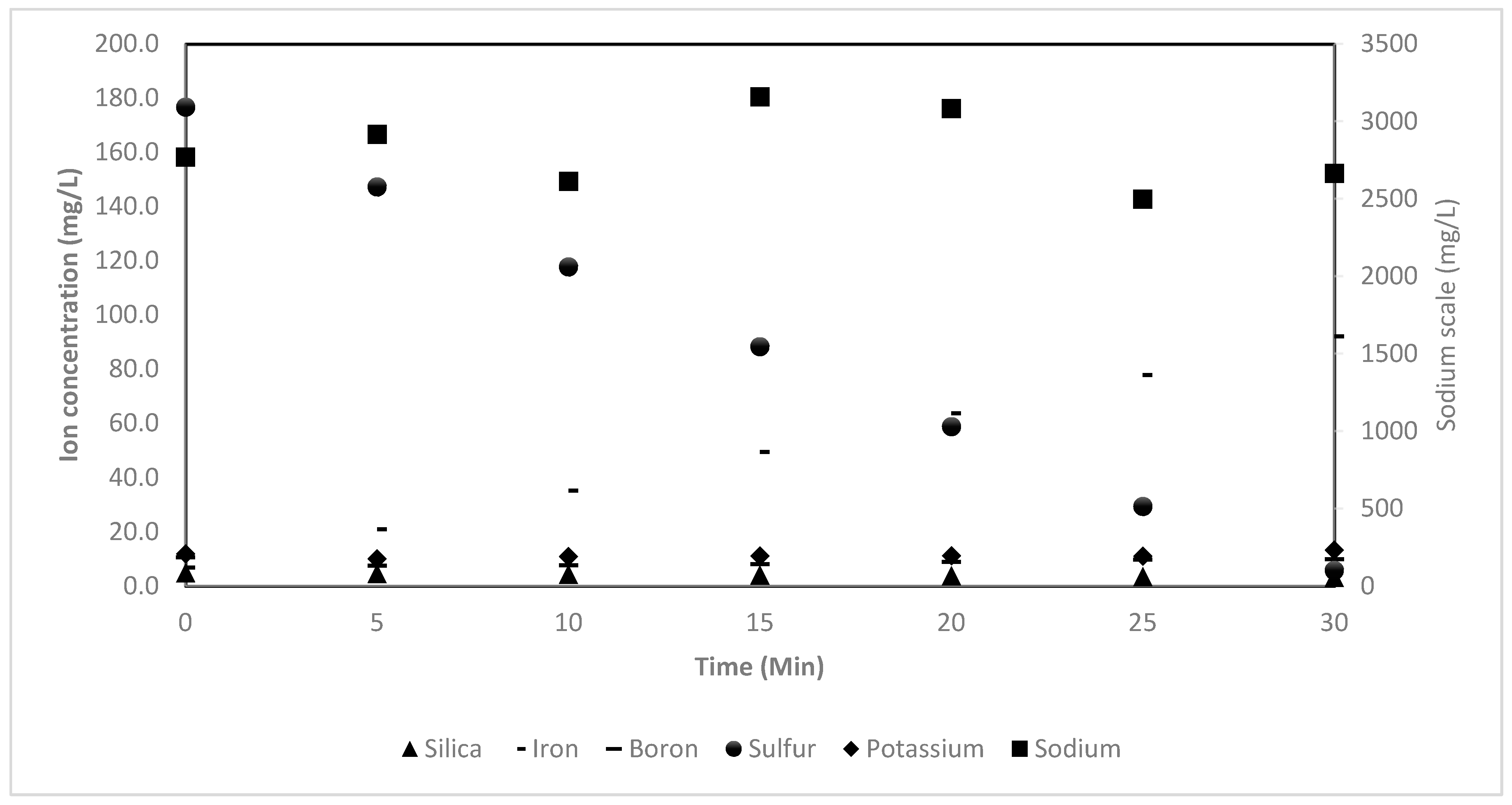
5.2.5. Removal of Other Contaminates via EC Process
5.2.6. Characteristics of Treated PW
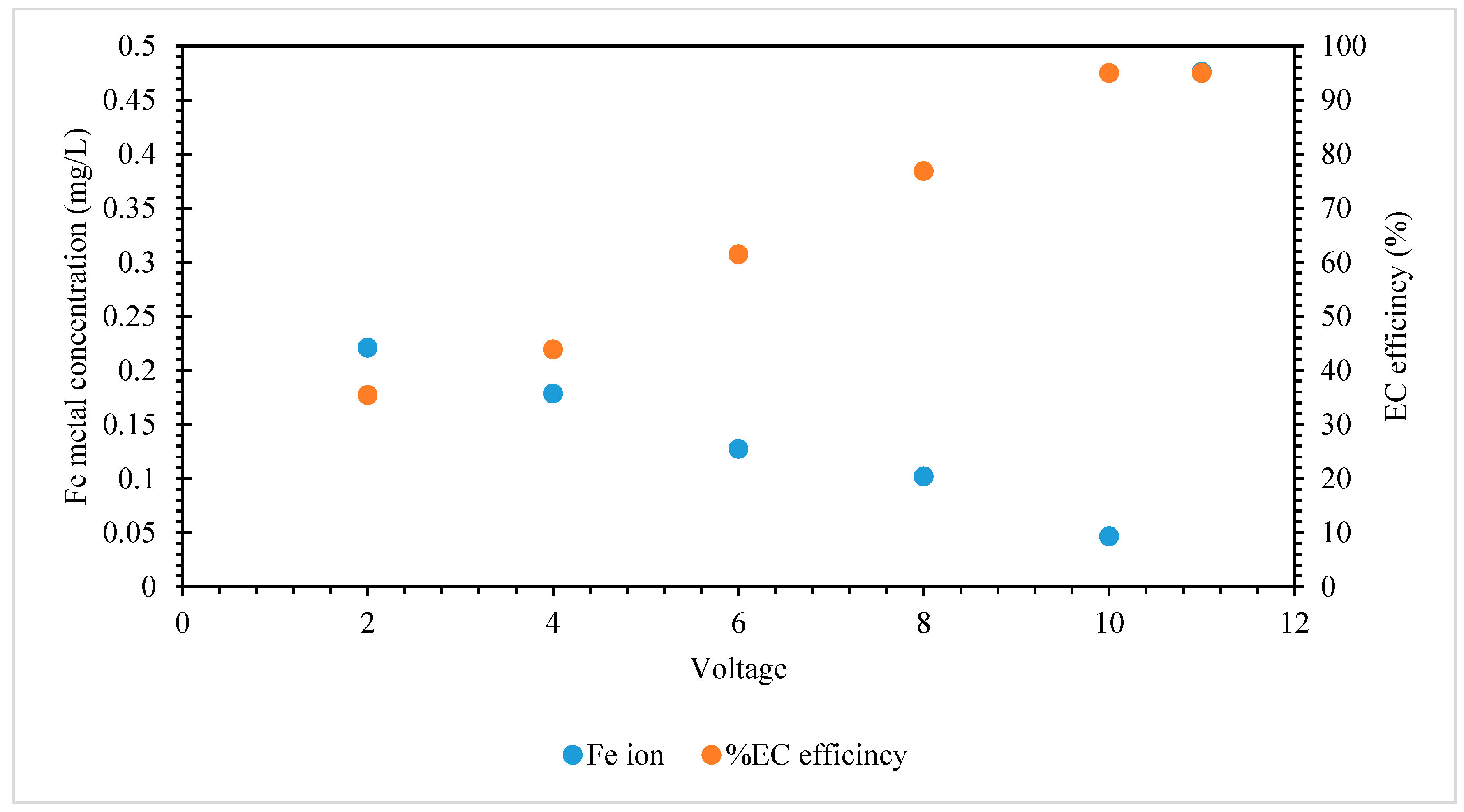
5.2.7. Effect of Voltage on EC Performance
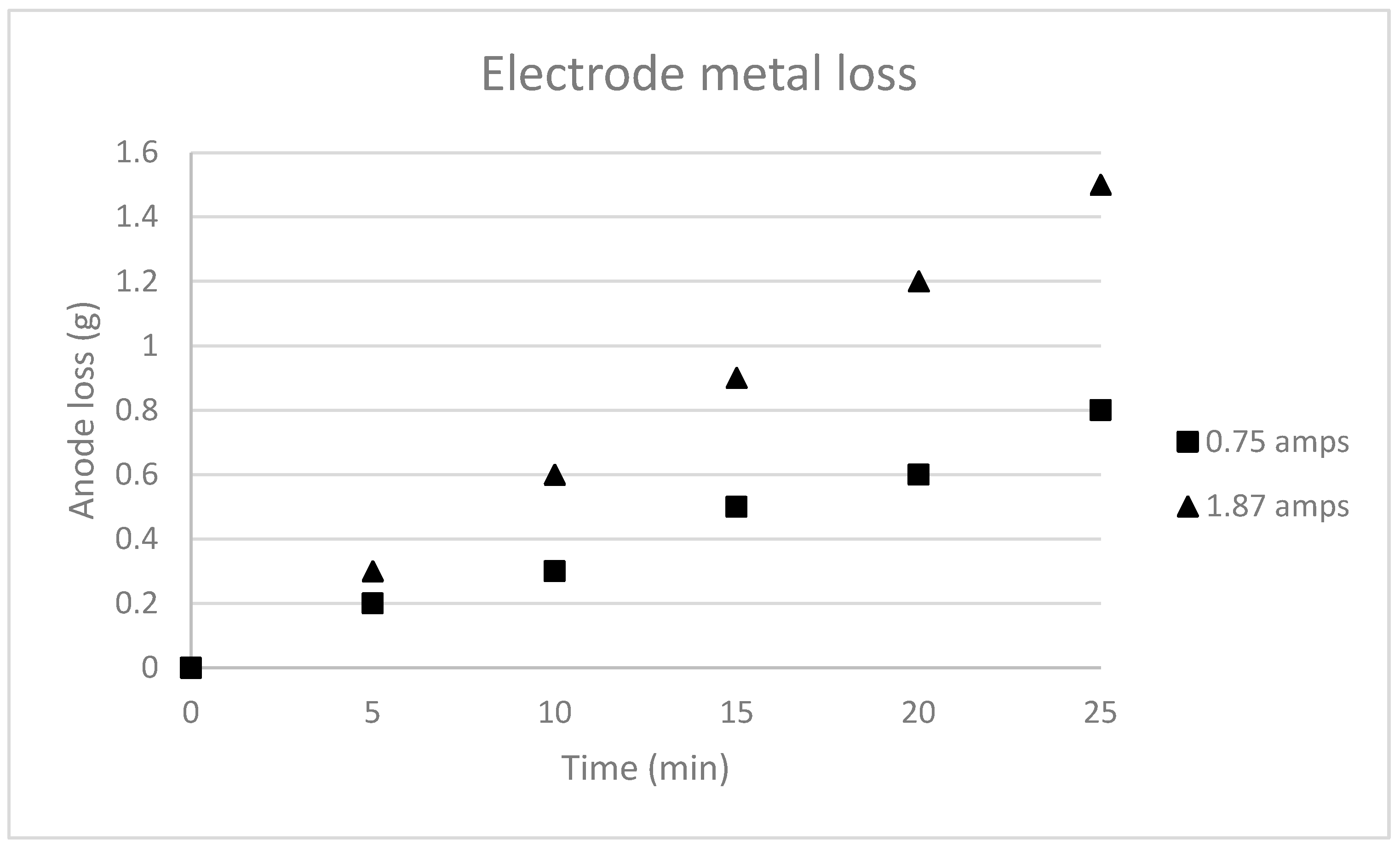
5.3. Key Challenges of the EC Process
- The phenomenon of electrode fouling: A notable obstacle encountered was the pronounced fouling on the electrodes, impeding the reaction process and diminishing the efficacy of pollutant elimination. Although the use of sandpaper to clean the electrode plates after each cycle provided a transient enhancement, the problem of fouling necessitates additional examination;
- The Impact of Voltage on Electrochemical Performance: The research conducted revealed that the manipulation of voltage had an impact on the concentration of iron ions present in the treated water. A drop in EC efficiency was seen as the iron ion content decreased at higher voltages. The data indicate that the maximum removal efficiency for iron was observed at a voltage of 18 volts. However, it also highlights the necessity of careful power supply control to maintain the effectiveness of EC during the full duration of the operation.
- Further investigation is necessary to gain a comprehensive understanding of the fouling phenomena and to devise more enduring strategies for its prevention or mitigation;
- Further investigation is required to assess the influence of metal deterioration on the performance of EC. One such approach is to examine various electrode materials or coatings that exhibit resistance to deterioration;
- A proposal has been made to carry out the experiment with a hybrid system that combines EC with a Forward Osmosis (FO) membrane in order to enhance water quality. This suggests that although EC is efficient, it may be necessary to incorporate it into a multi-stage treatment procedure.
6. Conclusions
Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Kaabi, M.A.; Zouari, N.; Da’na, D.A.; Al-Ghouti, M.A. Adsorptive batch and biological treatments of produced water: Recent progresses, challenges, and potentials. J. Environ. Manag. 2021, 290, 112527. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent advances on the treatment technology of oil and gas produced water for sustainable energy industry-mechanistic aspects and process chemistry perspectives. Chem. Eng. J. Adv. 2020, 4, 100049. [Google Scholar] [CrossRef]
- Zainab, R.; Hasnain, M.; Ali, F.; Dias, D.A.; El-Keblawy, A.; Abideen, Z. Exploring the bioremediation capability of petroleum-contaminated soils for enhanced environmental sustainability and minimization of ecotoxicological concerns. Environ. Sci. Pollut. Res. 2023, 30, 104933–104957. [Google Scholar] [CrossRef]
- Amakiri, K.T.; Ogolo, N.A.; Angelis-Dimakis, A.; Albert, O. Physicochemical assessment and treatment of produced water: A case study in Niger delta Nigeria. Pet. Res. 2023, 8, 87–95. [Google Scholar] [CrossRef]
- Eldos, H.I.; Khan, M.; Zouari, N.; Saeed, S.; Al-Ghouti, M.A. Characterization and assessment of process water from oil and gas production: A case study of process wastewater in Qatar. Case Stud. Chem. Environ. Eng. 2022, 6, 100210. [Google Scholar] [CrossRef]
- Ghafoori, S.; Omar, M.; Koutahzadeh, N.; Zendehboudi, S.; Malhas, R.N.; Mohamed, M.; Al-Zubaidi, S.; Redha, K.; Baraki, F.; Mehrvar, M. New advancements, challenges, and future needs on treatment of oilfield produced water: A state-of-the-art review. Sep. Purif. Technol. 2022, 289, 120652. [Google Scholar] [CrossRef]
- Gökkuş, Ö.; Yildiz, Y.Ş. Application of electrocoagulation for treatment of medical waste sterilization plant wastewater and optimization of the experimental conditions. Clean Technol. Environ. Policy 2015, 17, 1717–1725. [Google Scholar] [CrossRef]
- Othmani, A.; Kadier, A.; Singh, R.; Igwegbe, C.A.; Bouzid, M.; Aquatar, M.O.; Khanday, W.A.; Bote, M.E.; Damiri, F.; Gökkuş, Ö.; et al. A comprehensive review on green perspectives of electrocoagulation integrated with advanced processes for effective pollutants removal from water environment. Environ. Res. 2022, 215, 114294. [Google Scholar] [CrossRef]
- Conrad, C.L.; Ben Yin, Y.; Hanna, T.; Atkinson, A.J.; Alvarez, P.J.J.; Tekavec, T.N.; Reynolds, M.A.; Wong, M.S. Fit-for-purpose treatment goals for produced waters in shale oil and gas fields. Water Res. 2020, 173, 115467. [Google Scholar] [CrossRef]
- Miranda, M.A.; Ghosh, A.; Mahmodi, G.; Xie, S.; Shaw, M.; Kim, S.; Krzmarzick, M.J.; Lampert, D.J.; Aichele, C.P. Treatment and Recovery of High-Value Elements from Produced Water. Water 2022, 14, 880. [Google Scholar] [CrossRef]
- Kassab, M.A.; Abbas, A.E.; Elgamal, I.; Shawky, B.M.; Mubarak, M.F.; Hosny, R. Review on the Estimating the Effective Way for Managing the Produced Water: Case Study. Open J. Mod. Hydrol. 2021, 11, 19–37. [Google Scholar] [CrossRef]
- Salem, F.; Thiemann, T. Produced Water from Oil and Gas Exploration—Problems, Solutions and Opportunities. J. Water Resour. Prot. 2022, 14, 142–185. [Google Scholar] [CrossRef]
- Dawoud, H.D.; Saleem, H.; Alnuaimi, N.A.; Zaidi, S.J. Characterization and treatment technologies applied for produced water in Qatar. Water 2021, 13, 3573. [Google Scholar] [CrossRef]
- Lusinier, N.; Seyssiecq, I.; Sambusiti, C.; Jacob, M.; Lesage, N.; Roche, N. A comparative study of conventional activated sludge and fixed bed hybrid biological reactor for oilfield produced water treatment: Influence of hydraulic retention time. Chem. Eng. J. 2021, 420, 127611. [Google Scholar] [CrossRef]
- Santos, M.A.; Capponi, F.; Ataíde, C.H.; Barrozo, M.A.S. Wastewater treatment using DAF for process water reuse in apatite flotation. J. Clean. Prod. 2021, 308, 127285. [Google Scholar] [CrossRef]
- Daverey, A.; Pandey, D.; Verma, P.; Verma, S.; Shah, V.; Dutta, K.; Arunachalam, K. Recent advances in energy efficient biological treatment of municipal wastewater. Bioresour. Technol. Rep. 2019, 7, 100252. [Google Scholar] [CrossRef]
- Silva, P.C.; Ferraz, N.P.; Perpetuo, E.A.; Asencios, Y.J.O. Oil Produced Water Treatment Using Advanced Oxidative Processes: Heterogeneous-Photocatalysis and Photo-Fenton. J. Sediment. Environ. 2019, 4, 99–107. [Google Scholar] [CrossRef]
- Jiménez, S.; Andreozzi, M.; Micó, M.M.; Álvarez, M.G.; Contreras, S. Produced water treatment by advanced oxidation processes. Sci. Total Environ. 2019, 666, 12–21. [Google Scholar] [CrossRef]
- Alomar, T.S.; Hameed, B.H.; Usman, M.; Almomani, F.A.; Ba-Abbad, M.M.; Khraisheh, M. Recent advances on the treatment of oil fields produced water by adsorption and advanced oxidation processes. J. Water Process Eng. 2022, 49, 103034. [Google Scholar] [CrossRef]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Gholami, M.; Abbasi Souraki, B.; Pendashteh, A. Electro-activated persulfate oxidation (EC/PS) for the treatment of real oilfield produced water: Optimization, developed numerical kinetic model, and comparison with thermal/EC/PS and EC systems. Process Saf. Environ. Prot. 2021, 153, 384–402. [Google Scholar] [CrossRef]
- Madhavan, M.A.; Antony, S.P. Effect of polarity shift on the performance of electrocoagulation process for the treatment of produced water. Chemosphere 2021, 263, 128052. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Singh, T.S.A. Arsenic removal by electrocoagulation process: Recent trends and removal mechanism. Chemosphere 2017, 181, 418–432. [Google Scholar] [CrossRef]
- An, C.; Huang, G.; Yao, Y.; Zhao, S. Emerging usage of electrocoagulation technology for oil removal from wastewater: A review. Sci. Total Environ. 2017, 579, 537–556. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Jiang, W.-M.; Liu, Y.; Kang, Y. Quantitative contribution study and comparison between electrocoagulation, anode-electrocoagulation and chemical coagulation using polymer-flooding sewage. Chemosphere 2020, 250, 126128. [Google Scholar] [CrossRef]
- Tegladza, I.D.; Xu, Q.; Xu, K.; Lv, G.; Lu, J. Electrocoagulation processes: A general review about role of electro-generated flocs in pollutant removal. Process Saf. Environ. Prot. 2021, 146, 169–189. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Abbasi, S.; Zinatizadeh, A.A.; Mirghorayshi, M.; Zinadini, S.; McKay, T. Electrocoagulation technique for continuous industrial licorice processing wastewater treatment in a single reactor employing Fe-rod electrodes: Process modeling and optimization and operating cost analysis. J. Environ. Chem. Eng. 2022, 10, 106686. [Google Scholar] [CrossRef]
- Sasson, M.B.; Calmano, W.; Adin, A. Iron-oxidation processes in an electroflocculation (electrocoagulation) cell. J. Hazard. Mater. 2009, 171, 704–709. [Google Scholar] [CrossRef]
- Ghosh, D.; Solanki, H.; Purkait, M.K. Removal of Fe(II) from tap water by electrocoagulation technique. J. Hazard. Mater. 2008, 155, 135–143. [Google Scholar] [CrossRef]
- Ezechi, E.H.; Isa, M.H.; Muda, K.; Kutty, S.R.M. A comparative evaluation of two electrode systems on continuous electrocoagulation of boron from produced water and mass transfer resistance. J. Water Process Eng. 2020, 34, 101133. [Google Scholar] [CrossRef]
- Mehri, M.; Fallah, N.; Nasernejad, B. Mechanisms of heavy metal and oil removal from synthetic saline oilfield produced water by electrocoagulation. NPJ Clean Water 2021, 4, 45. [Google Scholar] [CrossRef]
- Sivaranjani; Gafoor, A.; Ali, N.; Kumar, S.; Ramalakshmi; Begum, S.; Rahman, Z. Applicability and new trends of different electrode materials and its combinations in electro coagulation process: A brief review. Mater. Today Proc. 2020, 37, 377–382. [Google Scholar] [CrossRef]
- Ingelsson, M.; Yasri, N.; Roberts, E.P.L. Electrode passivation, faradaic efficiency, and performance enhancement strategies in electrocoagulation—A review. Water Res. 2020, 187, 116433. [Google Scholar] [CrossRef] [PubMed]
- Khor, C.M.; Wang, J.; Li, M.; Oettel, B.A.; Kaner, R.B.; Jassby, D.; Hoek, E.M.V. Performance, energy and cost of produced water treatment by chemical and electrochemical coagulation. Water 2020, 12, 3426. [Google Scholar] [CrossRef]
- Alam, P.N.; Yulianis; Pasya, H.L.; Aditya, R.; Aslam, I.N.; Pontas, K. Acid mine wastewater treatment using electrocoagulation method. Mater. Today Proc. 2022, 63, S434–S437. [Google Scholar] [CrossRef]
- Jyoti Biswal, H.; Vundavilli, P.R.; Gupta, A. Investigations on the effect of electrode gap variation over pulse-electrodeposition profile. IOP Conf. Ser. Mater. Sci. Eng. 2019, 653, 012046. [Google Scholar] [CrossRef]
- Almukdad, A.; Hafiz, M.A.; Yasir, A.T.; Alfahel, R.; Hawari, A.H. Unlocking the application potential of electrocoagulation process through hybrid processes. J. Water Process Eng. 2021, 40, 101956. [Google Scholar] [CrossRef]
- Espinoza-Quiñones, F.R.; Romani, M.; Borba, C.E.; Módenes, A.N.; Utzig, C.F.; Dall’Oglio, I.C. A mathematical approach based on the Nernst-Planck equation for the total electric voltage demanded by the electrocoagulation process: Effects of a time-dependent electrical conductivity. Chem. Eng. Sci. 2020, 220, 115626. [Google Scholar] [CrossRef]
- Ojagh, S.M.A.; Fallah, N.; Nasernejad, B. Biological treatment of organic compounds in produced water with use of halotolerant bacteria. J. Environ. Chem. Eng. 2020, 8, 104412. [Google Scholar] [CrossRef]
- Sannino, D.; Vaiano, V.; Ciambelli, P.; Isupova, L.A. Structured catalysts for photo-Fenton oxidation of acetic acid. Catal. Today 2011, 161, 255–259. [Google Scholar] [CrossRef]
- Manilal, A.M.; Soloman, P.A.; Basha, C.A. Removal of Oil and Grease from Produced Water Using Electrocoagulation. J. Hazard. Toxic Radioact. Waste 2020, 24, 04019023. [Google Scholar] [CrossRef]
- Kong, F.-x.; Chen, Y.-X.; Wang, Y.-k.; Chen, J.-f. Simultaneous electrocoagulation and E-peroxone coupled with ultrafiltration membrane for shale gas produced water treatment. Chemosphere 2024, 355, 141834. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z.; Yang, K.; Wei, J.; Li, Z.; Ma, C.; Yang, X.; Wang, T.; Zeng, G.; Yu, G.; et al. Removal of chloride from water and wastewater: Removal mechanisms and recent trends. Sci. Total Environ. 2022, 821, 153174. [Google Scholar] [CrossRef] [PubMed]
- Alsarayreh, M.; Almomani, F.; Khraisheh, M.; Nasser, M.S.; Soliman, Y. Biological-Based Produced Water Treatment Using Microalgae: Challenges and Efficiency. Sustainability 2022, 14, 499. [Google Scholar] [CrossRef]
- Tawabini, B.S.; Plakas, K.V.; Karabelas, A.J. A pilot study of BTEX removal from highly saline water by an advanced electrochemical process. J. Water Process Eng. 2020, 37, 101427. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Du, J.; Zhang, B.; Li, J.; Lai, B. Decontamination of heavy metal complexes by advanced oxidation processes: A review. Chinese Chem. Lett. 2020, 31, 2575–2582. [Google Scholar] [CrossRef]
- Al-Kaabi, M. Enhancing produced water quality using modified activated carbon. QU 2016, 16, ii. [Google Scholar] [CrossRef]
- Weiss, S.F.; Christensen, M.L.; Jørgensen, M.K. Mechanisms behind pH changes during electrocoagulation. AIChE J. 2021, 67, e17384. [Google Scholar] [CrossRef]
- Heidmann, I.; Calmano, W. Removal of Cr(VI) from model wastewaters by electrocoagulation with Fe electrodes. Sep. Purif. Technol. 2008, 61, 15–21. [Google Scholar] [CrossRef]








| Tested Parameter | Test Method | Results Obtained | Units |
|---|---|---|---|
| Chemical Oxygen Demand | SMWW 5220 D | 3790 | mg/L |
| Total Organic Carbon | SMWW 5310 B | 1320.3 | mg/L |
| Bromide (Br-) | SMWW 4110-B Br | 59.70 | mg/L |
| Chloride (Cl-) | SMWW 4110-B Cl | 9360.00 | mg/L |
| Oil and Grease | SMWW 5520 B | 44.80 | mg/L |
| Fluoride (F-) | SMWW 4110-B F | 0.59 | mg/L |
| Nitrite (N) | SMWW 4110-B NO2 | <0.01 | mg/L |
| Phosphate (PO43-) | SMWW 4110-B PO4 | 2.60 | mg/L |
| Sulfate (SO42-) | SMWW 4110-B SO4 | 76.90 | ml/L |
| Bromate (BrO3-) | SMWW 4110 D | <5 | µg/L |
| Chlorite (ClO2-) | SMWW 4110 D | 0.57 | mg/L |
| Chlorate (ClO3-) | SMWW 4110 D | 69.86 | mg/L |
| Nitrate (NO3) | SMWW 4110-B NO3 | 0.38 | mg/L |
| Metals | |||
| Cadmium | US EPA 6010C/3005A | <0.005 | mg/L |
| Chromium | US EPA 6010C/3005A | 0.096 | mg/L |
| Potassium | US EPA 6010C/3005A | 208.322 | mg/L |
| Sodium | US EPA 6010C/3005A | 2768.980 | mg/L |
| Silica | US EPA 6010C/3005A | 4.922 | mg/L |
| Copper | US EPA 6010C/3005A | 0.067 | mg/L |
| Iron | US EPA 6010C/3005A | 6.791 | mg/L |
| Lead | US EPA 6010C/3005A | 0.089 | mg/L |
| Mercury | US EPA 6010C/3005A | <0.01 | µg/L |
| Boron | US EPA 6010C/3005A | 10.600 | mg/L |
| Manganese | US EPA 6010C/3005A | 0.279 | mg/L |
| Barium | US EPA 6010C/3005A | 0.223 | mg/L |
| Zinc | US EPA 6010C/3005A | 0.148 | mg/L |
| Arsenic | US EPA 6010C/3005A | <0.200 | µg/L |
| Selenium | US EPA 6010C/3005A | <0.200 | µg/L |
| Sulfur | ICP-OES | 176.700 | mg/L |
| Strontium | US EPA 6010C/3005A | 29.852 | mg/L |
| Aluminum | US EPA 6010C/3005A | 0.698 | mg/L |
| Lithium | US EPA 6010C/3005A | 4.074 | mg/L |
| Molybdenum | US EPA 6010C/3005A | 0.036 | mg/L |
| BTEX | |||
| Toluene | US EPA 5030 C/8260 C | 36,330.0 | µg/L |
| Ethyl Benzene | US EPA 5030 C/8260 C | 1322.0 | µg/L |
| Xylenes | US EPA 5030 C/8260 C | 13,430.0 | µg/L |
| Benzene | US EPA 5030 C/8260 C | 72,272.0 | µg/L |
| PW | EC | ||||||
|---|---|---|---|---|---|---|---|
| Parameter (Unit) | Initial | 5 min | 10 min | 15 min | 20 min | 25 min | 30 min |
| COD (mg/L) | 3790 | 2600 | 2620 | 2545 | 2450 | 2615 | 2525 |
| TOC (mg/L) | 1320.3 | 740.0 | 704.0 | 776.5 | 561.5 | 585.5 | 619.0 |
| BROMIDE (Br−) (mg/L) | 59.70 | 67.70 | 71.90 | 65.60 | 34.00 | 62.0 | 60.30 |
| CHLORIDE, (CL−) (mg/L) | 9360.00 | 8943.0 | 8688.0 | 8593.0 | 8340.54 | 8088.08 | 7835.62 |
| Oil and Grease (MG/L) | 44.80 | <10 | <10 | <10 | <10 | <10 | <10 |
| Sulfate () (mg/L) | 76.90 | 74.00 | 88.00 | 84.00 | 80.00 | 72.0 | 77.00 |
| Bromate () | <5 | <5 | <5 | <5 | <5 | <5 | <5 |
| Metals | |||||||
| Cadmium (mg/L) | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 |
| Chromium | 0.096 | 0.023 | 0.024 | 0.022 | 0.039 | 0.035 | 0.029 |
| Potassium | 208.322 | 175.142 | 191.650 | 194.567 | 195.907 | 193.89 | 231.155 |
| Sodium (mg/L) | 2768.9 | 2915.8 | 2613.9 | 3159.9 | 3082.2 | 2497.8 | 2664.1 |
| Silica (mg/L) | 4.922 | 4.641 | 4.359 | 4.078 | 3.797 | 3.515 | 3.234 |
| Iron (mg/L) | 6.791 | 21.022 | 35.253 | 49.484 | 63.715 | 77.946 | 92.177 |
| Boron (mg/L) | 10.600 | 7.490 | 7.633 | 8.104 | 8.913 | 9.735 | 9.961 |
| Barium (mg/L) | 0.223 | 0.114 | 0.113 | 0.122 | 0.141 | 0.121 | 0.124 |
| Zinc (mg/L) | 0.148 | 0.011 | 0.053 | 0.024 | 0.026 | 0.037 | 0.034 |
| Sulfur (mg/L) | 176.700 | 147.232 | 117.764 | 88.296 | 58.828 | 29.360 | 5.786 |
| Strontium (mg/L) | 29.852 | 23.571 | 25.273 | 25.800 | 24.374 | 25.60 | 28.780 |
| Lithium (mg/L) | 4.074 | 2.899 | 3.083 | 3.126 | 3.351 | 3.45 | 3.855 |
| BTEX | |||||||
| Toluene (µg/L) | 36,330.0 | 1674.0 | 1061.0 | 706.0 | 786.0 | 543.0 | 432.0 |
| Ethyl benzene (µg/L) | 1322.0 | 101.0 | 44.0 | 28.0 | 27.0 | 15.0 | 11.0 |
| Xylenes (µg/L) | 13,430.0 | 811.0 | 404.0 | 255.0 | 270.0 | 156.0 | 129.0 |
| Benzene (µg/L) | 72,272.0 | 2801.0 | 1761.0 | 1083.0 | 1584.0 | 1228.0 | 1024.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ajmi, F.; Al-Marri, M.; Almomani, F. Electrocoagulation Process as an Efficient Method for the Treatment of Produced Water Treatment for Possible Recycling and Reuse. Water 2025, 17, 23. https://doi.org/10.3390/w17010023
Al-Ajmi F, Al-Marri M, Almomani F. Electrocoagulation Process as an Efficient Method for the Treatment of Produced Water Treatment for Possible Recycling and Reuse. Water. 2025; 17(1):23. https://doi.org/10.3390/w17010023
Chicago/Turabian StyleAl-Ajmi, Fahad, Mohammed Al-Marri, and Fares Almomani. 2025. "Electrocoagulation Process as an Efficient Method for the Treatment of Produced Water Treatment for Possible Recycling and Reuse" Water 17, no. 1: 23. https://doi.org/10.3390/w17010023
APA StyleAl-Ajmi, F., Al-Marri, M., & Almomani, F. (2025). Electrocoagulation Process as an Efficient Method for the Treatment of Produced Water Treatment for Possible Recycling and Reuse. Water, 17(1), 23. https://doi.org/10.3390/w17010023







