Elucidating the Electrochemical Corrosion of a Water Pump Impeller in an Industrial Cooling System with Zero Liquid Discharge
Abstract
1. Introduction
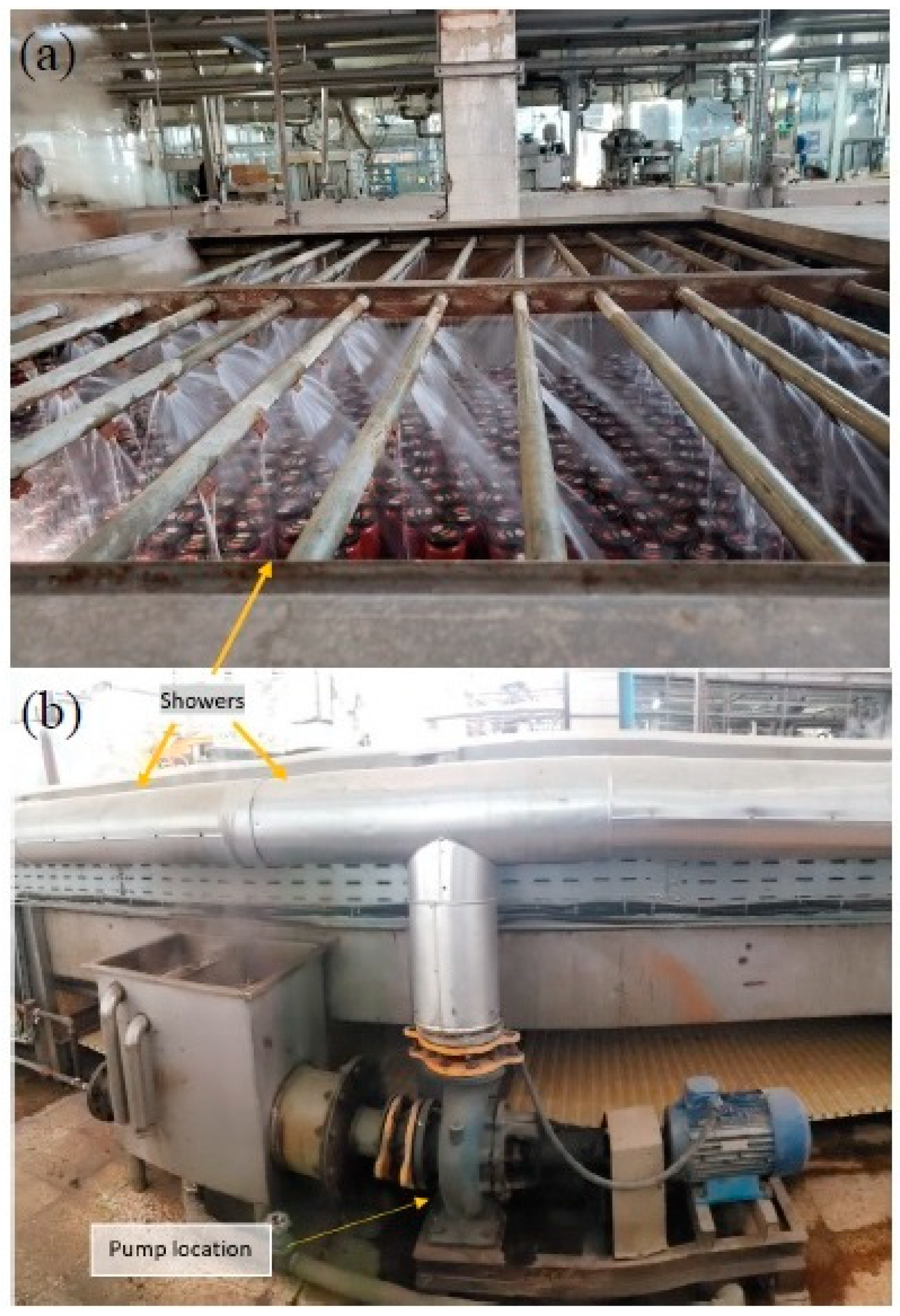
2. Materials and Methods
3. Results and Discussion
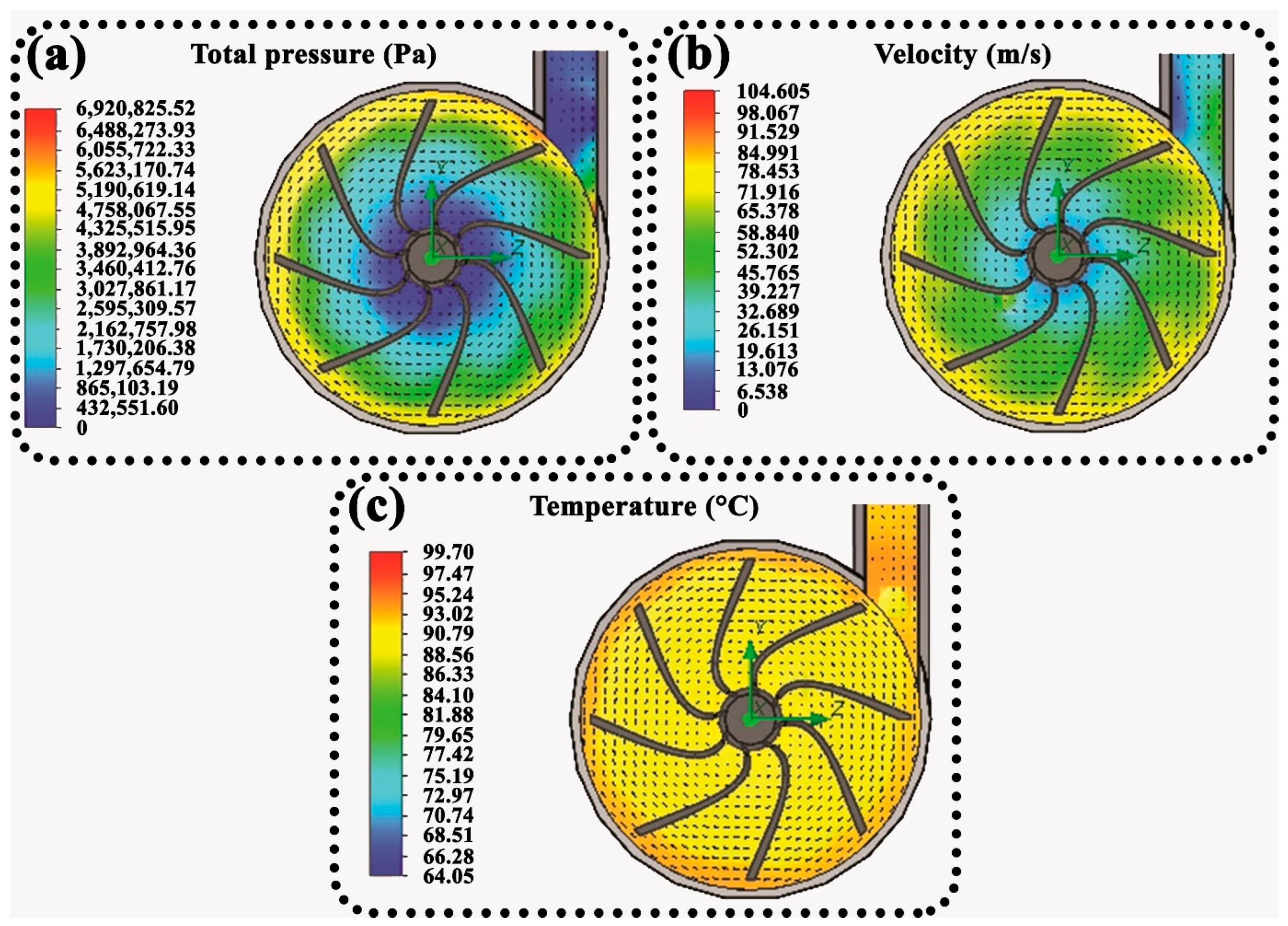
3.1. CFD Simulation
3.2. Visual Inspection
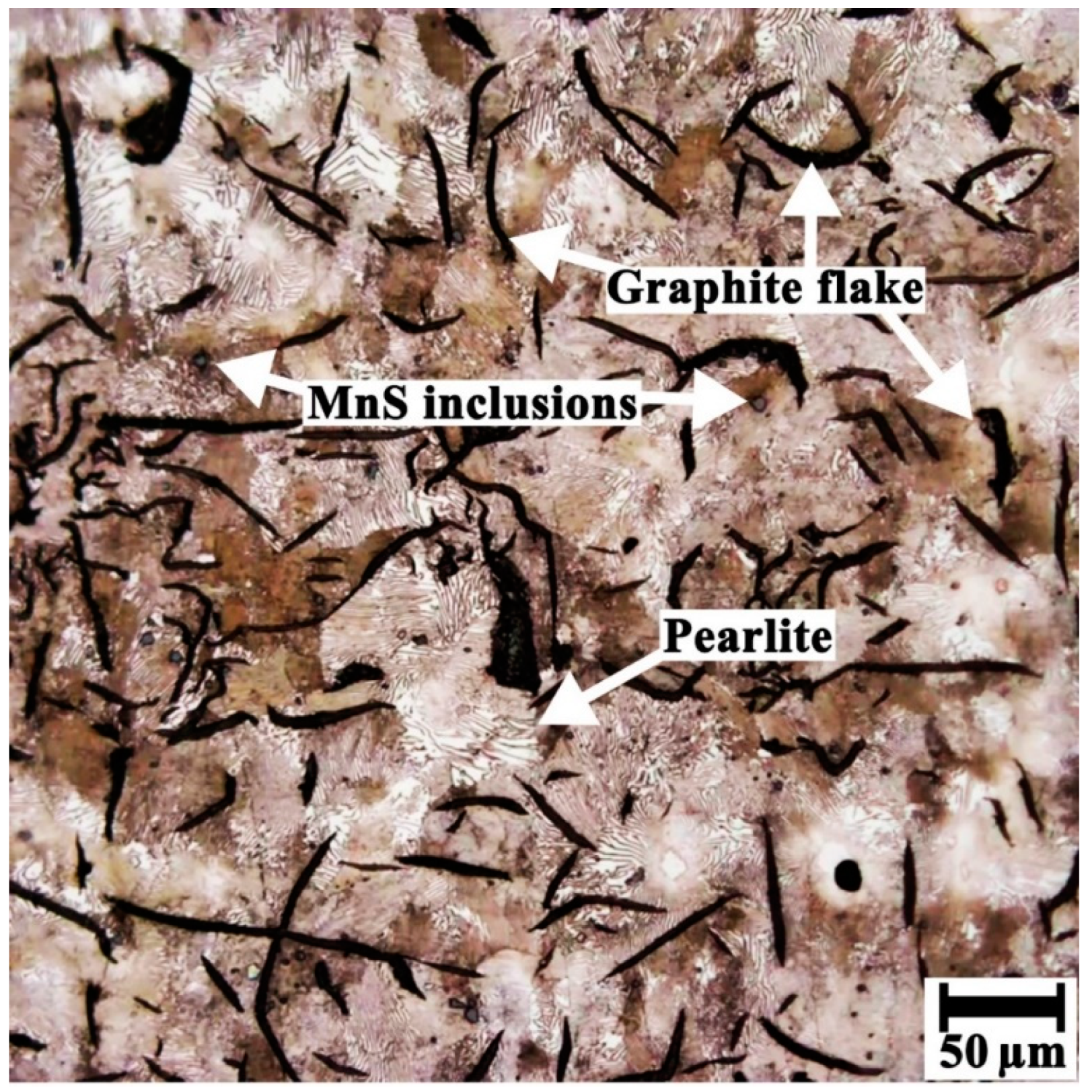
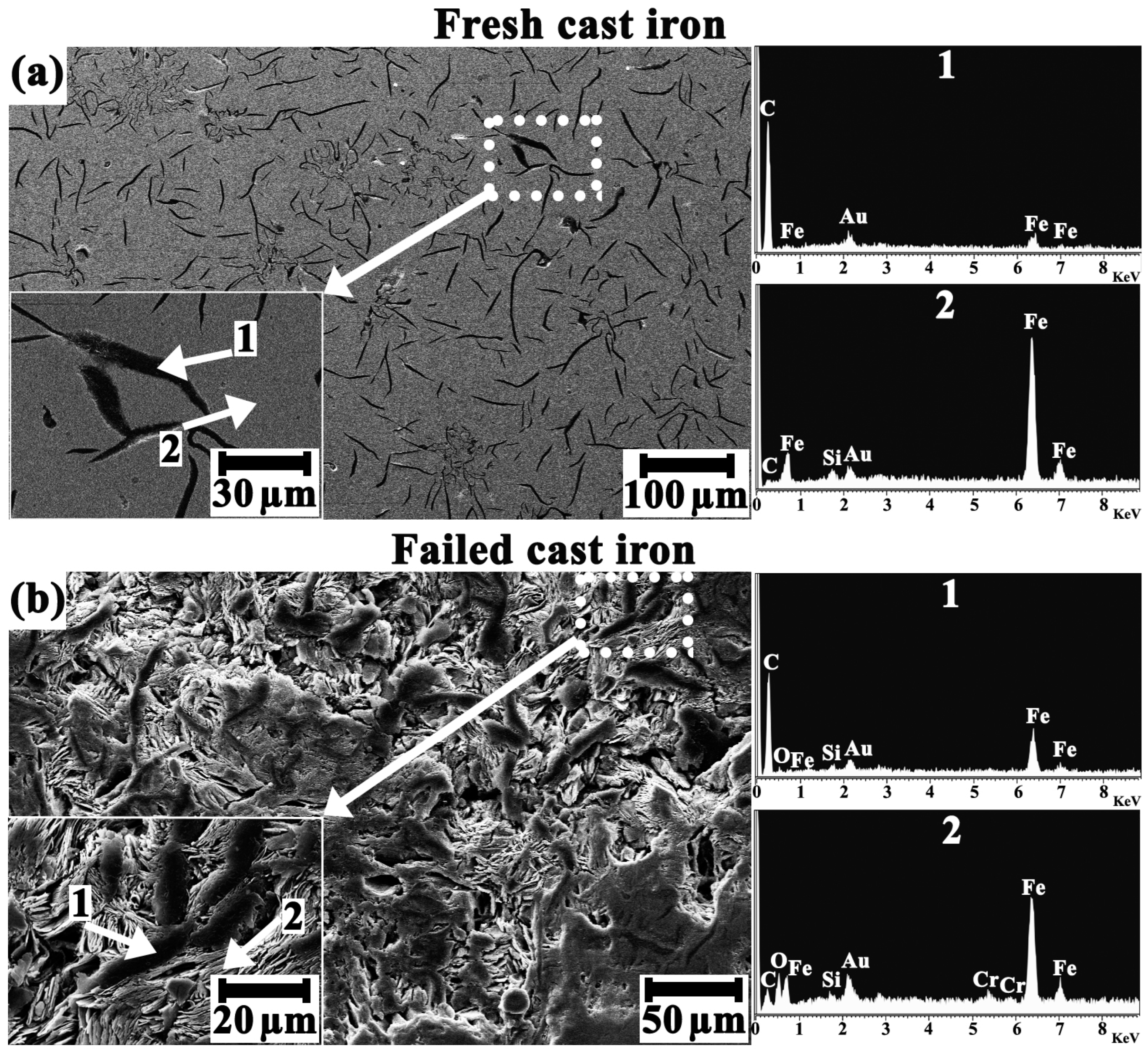
3.3. Microscopic Examination
3.4. Water Chemical Analysis
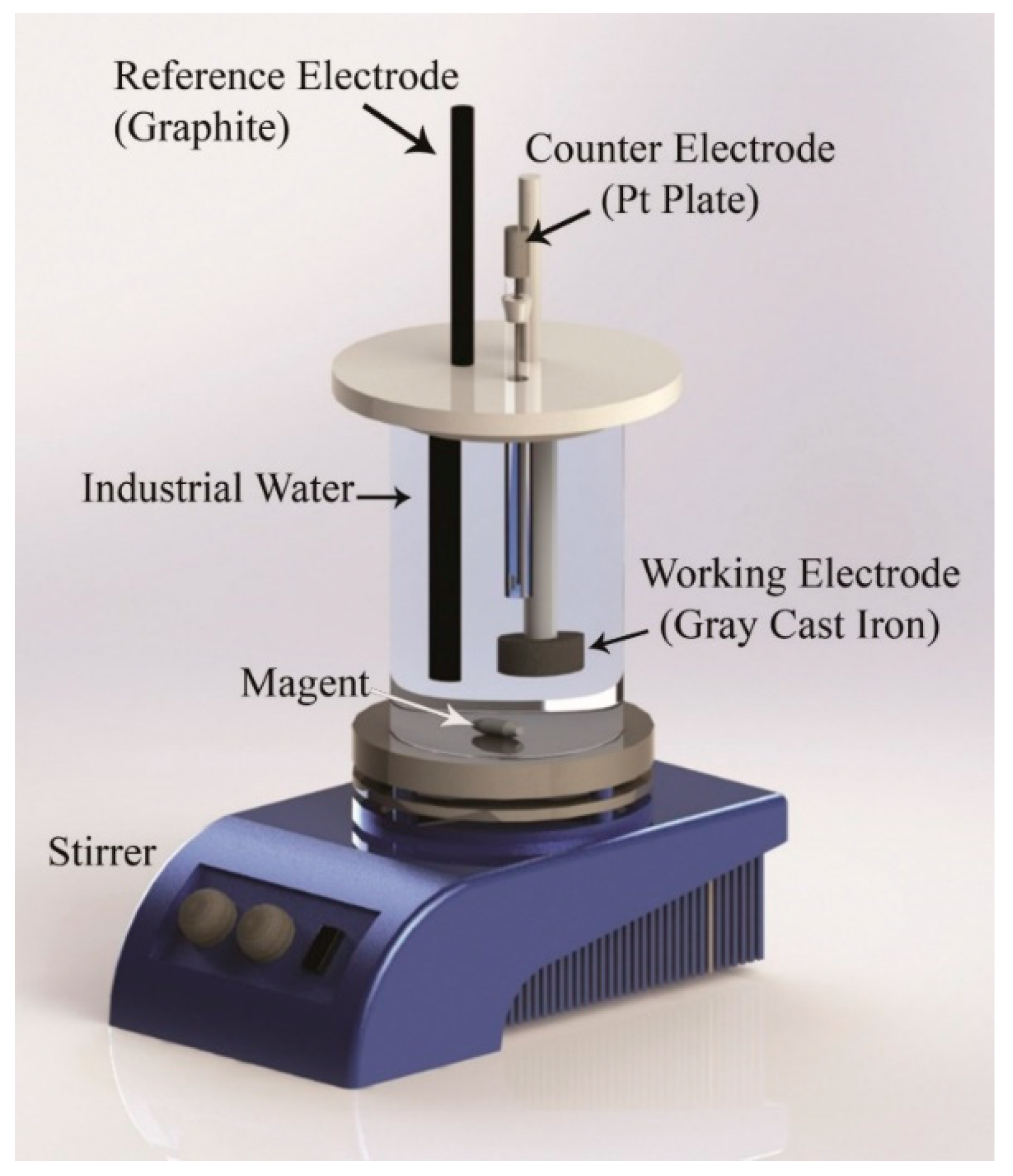
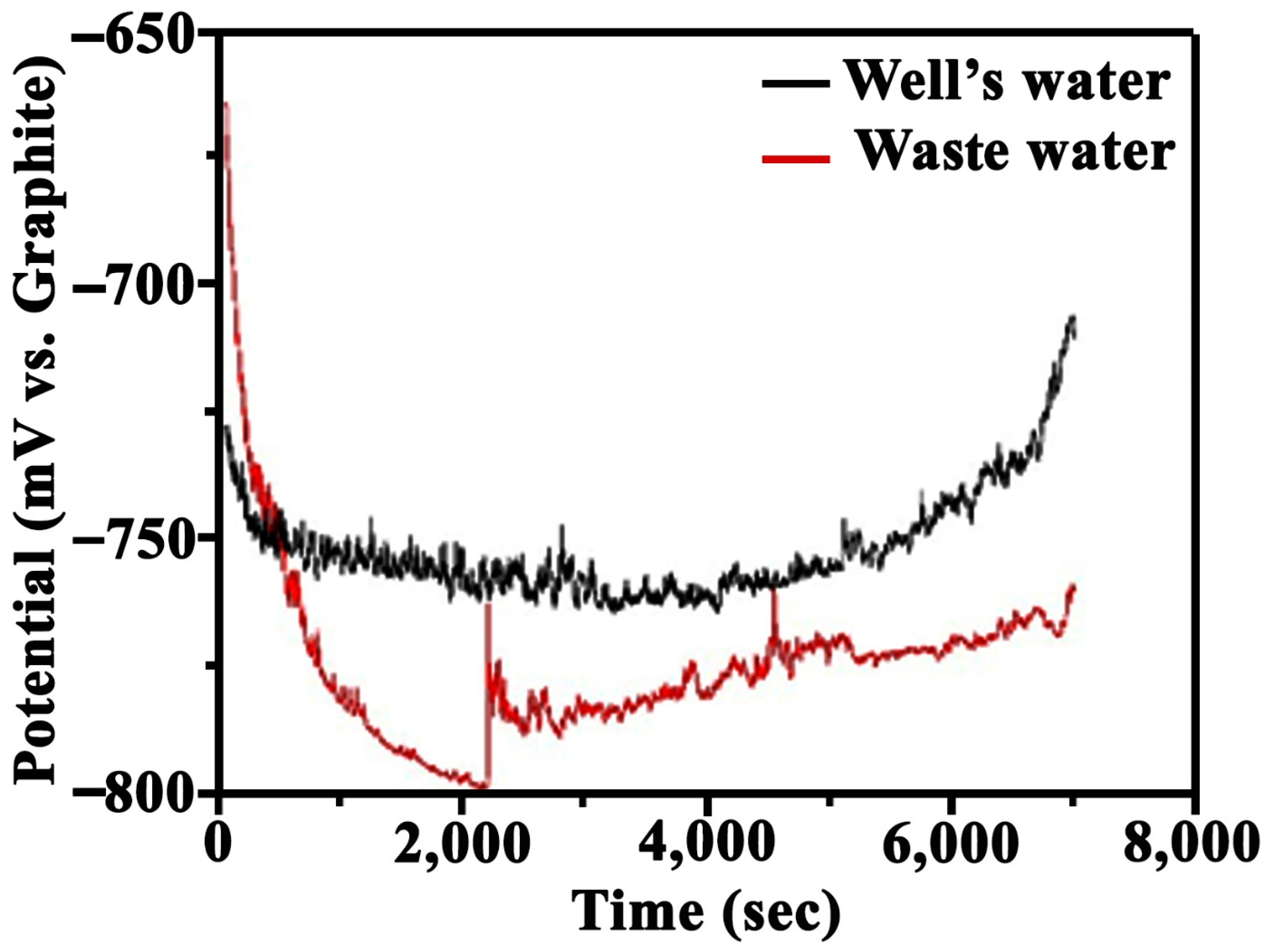
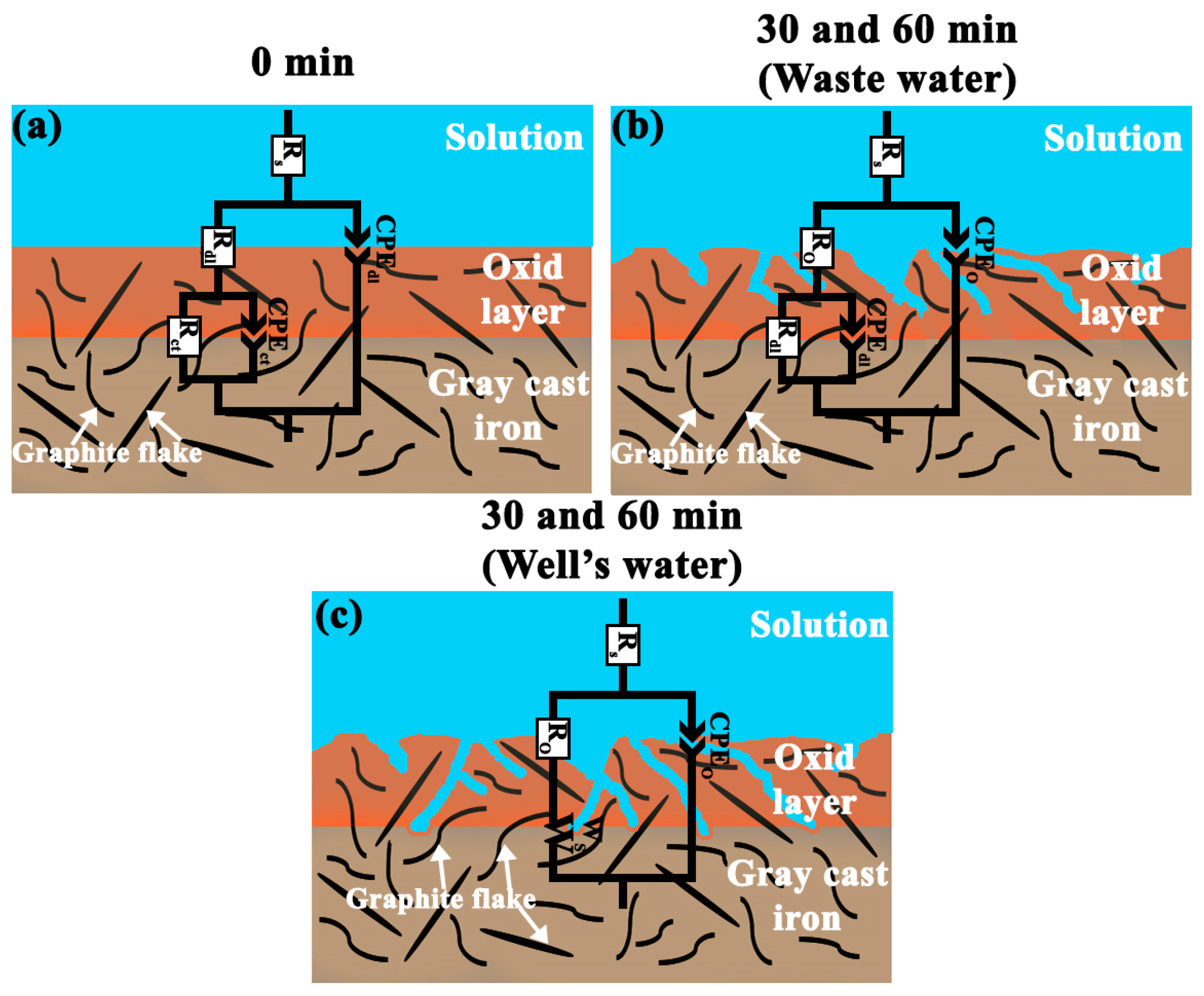
3.5. Electrochemical Corrosion Study
4. Conclusions
- The premature electrochemical corrosion failure of a single-suction centrifugal water pump impeller made of gray cast iron operating at 85 °C was investigated experimentally and numerically in two electrolytes, including groundwater (well water) and treated wastewater. The groundwater was more contaminated with Ca, Mg, Na, Si, and S elements and possessed a higher conductivity, hardness, and pH (basic) and more suspended solids than the wastewater.
- According to the CFDs simulation results, increased total fluid pressure, velocity, and temperature at the trailing edges, i.e., turbulent flow regions, exacerbated the corrosion of the impeller blades, causing ruptures and cracks in the areas adjacent to the trailing edges, visually verified.
- In essence, emerging micro-galvanic cells between graphite flakes (noble cathodic sites) and the pearlitic matrix (active anodic sites) promoted the graphitic corrosion of the impeller blades.
- A porous-scale equivalent electrical circuit with two parallel time constants deconvoluted the EIS profiles of the gray cast iron impeller. The EIS profiles showed the non-ideal capacitive behavior of the iron oxide layer caused by the distribution of surface properties such as conductivity, roughness, and porosity due to the inhomogeneous microstructure of the gray cast iron.
- A kinetic control electrochemical mechanism was predominant at the electrochemical interface under free corrosion conditions at (a) the 0 min test point for both the well water and wastewater and (b) the 30 and 60 min test points for the wastewater.
- A mixed diffusion–kinetic control electrochemical mechanism emerged under free corrosion conditions (OCP) in the well water at the 30 and 60 min test points because a short Warburg diffusional impedance emerged in the corresponding equivalent electrical circuit supported by a diffusive tail in the bode plots of phase angle at low frequencies.
5. Recommendations
- This study showed the significance of establishing an integrated water treatment strategy in industrial units that employ rotating equipment such as pumps to improve equipment integrity and service life. According to Zahrani [23], sustainable wastewater treatment can reduce water corrosivity by controlling pH, hardness, and total suspended solids and eliminating dissolved Ca, Mg, Na, Si, and S elements.
- Despite its low cost and ease of fabrication, gray cast iron suffers from poor mechanical properties and corrosion resistance due to inhomogeneous microstructure and flake graphite [33,34,55]. Considering more durable materials with improved mechanical and corrosion properties could be an option in critical applications despite higher manufacturing and materials costs, which might be a significant shortcoming in the Global South. Some alternative alloys for manufacturing centrifugal water pump impellers discussed in the literature were as follows: Cu-Al alloys [56], Zn [57], bronze [57], 17-4PH stainless steel [58,59], and ductile Ni-resistant cast iron [60]. Except for ductile Ni-resistant cast iron, which could be recommended for general applications due to its reasonable cost, the other alternative alloys could be considered for more advanced critical applications wherein process safety and integrity requirements support costly material options. Pitting corrosion resistance and quality of the alloys are important considerations when applying active-passive alloys, e.g., stainless steel, in water pump impellers [61].
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, A.O.; Ighodaro, O.O.; Fasogbon, S.K.; Orumwense, E.F.; Waheed, M.A. Failure investigation of the tube of a dual fired steam boiler in a western nigerian food and beverage manufacturing plant. Eng. Fail. Anal. 2023, 143, 106906. [Google Scholar] [CrossRef]
- Ro, Y.; Choi, W.S.; Park, H.; Shin, K.C. Failure analysis of casting Ni-Cr-Mo impeller after long-term weak acid exposure. J. Fail. Anal. Preven. 2024, 24, 510–519. [Google Scholar] [CrossRef]
- da Silveira, N.N.A.; Meghoe, A.A.; Tinga, T. Integration of multiple failure mechanisms in a life assessment method for centrifugal pump impellers. Adv. Mech. Eng. 2023, 15, 18. [Google Scholar] [CrossRef]
- Yu, T.; Shuai, Z.; Jian, J.; Wang, X.; Ren, K.; Dong, L.; Li, W.; Jiang, C. Numerical study on hydrodynamic characteristics of a centrifugal pump influenced by impeller-eccentric Effect. Eng. Fail. Anal. 2022, 138, 106395. [Google Scholar] [CrossRef]
- Nezhad, A.H.N.; Zahrani, E.M.; Alfantazi, A.M. Erosion-corrosion of electrodeposited superhydrophobic Ni-Al2O3 nanocomposite coatings under jet saline-sand slurry impingement. Corros. Sci. 2022, 197, 110095. [Google Scholar] [CrossRef]
- Zhao, S.; Jing, Y.; Liu, T.; Zhao, W.; Li, F. Corrosion behavior and mechanism of carbon steel in industrial circulating cooling water system operated by electrochemical descaling technology. J. Clean. Prod. 2024, 434, 139817. [Google Scholar] [CrossRef]
- Stringer, L.C.; Mirzabaev, A.; Benjaminsen, T.A.; Harris, R.M.B.; Jafari, M.; Lissner, T.K.; Stevens, N.; der Pahlen, C.T.-V. Climate change impacts on water security in global drylands. One Earth 2021, 4, 851–864. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Zhang, M.; Li, M.; Zhao, Z.; Liu, L. Failure analysis of the outlet pipeline and ball valve of the circulating pump in the desulfurization liquid concentration tower. Eng. Fail. Anal. 2024, 161, 108338. [Google Scholar] [CrossRef]
- Kannaujia, V.; Bhore, S.P.; Goyal, H.S. A case study on the failure analysis of 1 hp house hold electrical submersible pump in Prayagraj India. Eng. Fail. Anal. 2024, 156, 107742. [Google Scholar] [CrossRef]
- Pan, H.; Li, D.; Hu, H. Failure analysis of mechanical seal of nitrobenzene sulfuric acid pump. Eng. Fail. Anal. 2022, 141, 106660. [Google Scholar] [CrossRef]
- Karassik, I.J.; Messina, J.P.; Cooper, P.; Heald, C.C. Pump Handbook, 4th ed.; McGRAW-HILL: New York, NY, USA, 2008; Available online: https://www.accessengineeringlibrary.com/content/book/9780071460446 (accessed on 20 February 2021).
- Park, I.-C.; Kim, S.-J. Effect of pH of the sulfuric acid bath on cavitation erosion behavior in natural seawater of electroless nickel plating coating. Appl. Surf. Sci. 2019, 483, 194–204. [Google Scholar] [CrossRef]
- Yu, R.; Liu, J. Failure analysis of centrifugal pump impeller. Eng. Fail. Anal. 2018, 92, 343–349. [Google Scholar] [CrossRef]
- Myszka, D. Cast iron–based alloys. In High-Performance Ferrous Alloys; Radhakanta, R., Ed.; Springer: Cham, Switzerland, 2021; pp. 153–210. [Google Scholar] [CrossRef]
- Stefanescu, D.M. (Ed.) Corrosion of Cast Irons, Cast Iron Science and Technology. In ASM Handbook; ASM International: Novelty, OH, USA, 2017; Volume 1A, pp. 502–510. [Google Scholar] [CrossRef]
- Spence, R.; Amaral-Teixeira, J. A CFD parametric study of geometrical variations on the pressure pulsations and performance characteristics of a centrifugal pump. Comput. Fluids 2009, 38, 1243–1257. [Google Scholar] [CrossRef]
- Yousefi, H.; Noorollahi, Y.; Tahani, M.; Fahimi, R.; Saremian, S. Numerical simulation for obtaining optimal impeller’s blade parameters of a centrifugal pump for high-viscosity fluid pumping. Sustain. Energy Technol. 2019, 34, 16–26. [Google Scholar] [CrossRef]
- Li, W. Effect of exit blade angle, viscosity and roughness in centrifugal pumps investigated by CFD computation. Task Q. 2011, 15, 21–41. Available online: https://task.gda.pl/files/quart/TQ2011/01/tq115b-e.pdf (accessed on 14 January 2018).
- Liang, Y.; Lin, X.; Kong, X.; Duan, Q.; Wang, P.; Mei, X.; Ma, J. Making waves: Zero liquid discharge for sustainable industrial effluent management. Water 2021, 13, 2852. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, S.; Li, B.; Li, H.; Ling, Z.; Zhang, G.; Liu, M. Current status of zero liquid discharge technology for desulfurization wastewater. Water 2024, 16, 900. [Google Scholar] [CrossRef]
- Romo, S.A.; Elhashimi, M.; Abbasi, B.; Srebric, J. Mapping of a novel zero-liquid discharge desalination system based on humidification–dehumidification onto the field of existing desalination technologies. Water 2022, 14, 2688. [Google Scholar] [CrossRef]
- Van Bennekom, A.; Berndt, F.; Rassool, M. Pump impeller failures—A compendium of case studies. Eng. Fail. Anal. 2001, 8, 145–156. [Google Scholar] [CrossRef]
- Zahrani, E.M. Premature failure of grade-316Ti stainless steel tubing in a boiler feed-water heat exchanger in a steel complex. J. Fail. Anal. Prev. 2021, 21, 61–73. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Brennen, C.E. Hydrodynamics of Pumps, 1st ed.; Cambridge University Press: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Barrio, R.; Blanco, E.; Parrondo, J.; González, J.; Fernández, J. The Effect of impeller cutback on the fluid-dynamic pulsations and load at the blade-passing frequency in a centrifugal pump. J. Fluids Eng. 2008, 130, 111102. [Google Scholar] [CrossRef]
- Cai, J.; Pan, J.; Guzzomi, A. The flow field in a centrifugal pump with a large tongue gap and back blades. J. Mech. Sci. Technol. 2014, 28, 4455–4464. [Google Scholar] [CrossRef]
- Al-Yasiri, M.; Al-Khateeb, M.; Wen, D. Examination of drill pipe corrosion in water-based drilling fluids under wellbore conditions, Corros. Eng. Sci. Technol. 2017, 53, 183–187. [Google Scholar] [CrossRef]
- Prasetia, A.E.; Salazar, A.T.N.; Toralde, J.S.S. Corrosion control in geothermal aerated fluids drilling projects in Asia Pacific. In Proceedings of the World Geothermal Congress, Bali, Indonesia, 25–29 April 2010. [Google Scholar]
- Badiea, A.; Mohana, K. Effect of temperature and fluid velocity on corrosion mechanism of low carbon steel in presence of 2-hydrazino-4,7-dimethylbenzothiazole in industrial water medium. Corros. Sci. 2009, 51, 2231–2241. [Google Scholar] [CrossRef]
- Tchada, A.M.; Tchoumboué, N.K.; Mesquita, A.L.A.; Hendrick, P. Enhancing Pump as Turbine (PAT) performances: A numerical investigation into the impact of impeller leading edge rounding. Heliyon 2024, 10, e34663. [Google Scholar] [CrossRef] [PubMed]
- Collini, L.; Nicoletto, G.; Konečná, R. Microstructure and mechanical properties of pearlitic gray cast iron. Mater. Sci. Eng. A 2008, 488, 529–539. [Google Scholar] [CrossRef]
- Sancy, M.; Gourbeyre, Y.; Sutter, E.; Tribollet, B. Mechanism of corrosion of cast iron covered by aged corrosion products: Application of electrochemical impedance spectrometry. Corros. Sci. 2010, 52, 1222–1227. [Google Scholar] [CrossRef]
- Xu, W.; Ferry, M.; Wang, Y. Influence of alloying elements on as-cast microstructure and strength of gray iron. Mater. Sci. Eng. A 2005, 390, 326–333. [Google Scholar] [CrossRef]
- Muhmond, H.M.; Fredriksson, H. Relationship between inoculants and the morphologies of MnS and graphite in gray cast iron. Metall. Mater. Trans. B 2013, 44, 283–298. [Google Scholar] [CrossRef][Green Version]
- Al-Hashem, A.; Abdullah, A.; Riad, W. Cavitation corrosion of nodular cast iron (NCI) in seawater: Microstructural effects. Mater. Charact. 2001, 47, 383–388. [Google Scholar] [CrossRef]
- Jur, T.A.; Middleton, J.I.; Yurko, A.A.; Windham, R.L.; Grey, J.R., Jr. Case studies in graphitic corrosion of cast iron pipe. J. Fail. Anal. Prev. 2021, 21, 376–386. [Google Scholar] [CrossRef]
- Rahmati, M.; Zahrani, E.M.; Atapour, M.; Nezhad, A.H.N.; Hakimizad, A.; Alfantazi, A.M. In situ synthesis and electrochemical corrosion behavior of plasma electrolytic oxidation coating containing an osteoporosis drug on AZ31 magnesium alloy. Mater. Chem. Phys. 2024, 315, 128983. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Sharma, P. Premature failure of ductile iron pump impeller in cooling tower system. J. Fail. Anal. Prev. 2014, 14, 303–306. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corr. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- ASTM G102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2010. [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy, the Electrochemical Society Series, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Moradighadi, N.; Nesic, S.; Tribollet, B. Identifying the dominant electrochemical reaction in electrochemical impedance spectroscopy. Electrochim. Acta 2021, 400, 139460. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Jorcin, J.B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Kerroum, Y.; Skal, S.; Guenbour, A.; Bellaouchou, A.; Boulif, R.; Anton, J.G.; Zarrouk, A. A novel investigation on the cast iron corrosion in polluted phosphoric acid. Surf. Interfaces 2020, 19, 100481. [Google Scholar] [CrossRef]
- Brug, J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedance complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Zhang, R.; Sur, D.; Li, K.; Witt, J.; Black, R.; Whittingham, A.; Scully, J.R.; Hattrick-Simpers, J. Bayesian assessment of commonly used equivalent circuit models for corrosion analysis in electrochemical impedance spectroscopy. npj Mater. Degrad. 2024, 8, 120. [Google Scholar] [CrossRef]
- Orazem, M.E. Measurement model for analysis of electrochemical impedance data. J. Solid State Electrochem. 2024, 28, 1273–1289. [Google Scholar] [CrossRef]
- Vivier, V.; Orazem, M.E. Impedance analysis of electrochemical systems. Chem. Rev. 2022, 122, 11131–11168. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Z.; Song, C.; Wang, H.; Zhang, J. Electrochemical Impedance Spectroscopy in PEM Fuel Cells: Fundamentals and Applications; Springer: London, UK, 2009. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Khalid, Y.A.; Sapuan, S.M. Wear analysis of centrifugal slurry pump impellers. Ind. Lubr. Tribol. 2007, 59, 18–28. [Google Scholar] [CrossRef]
- Im, M.H. Cavitation characteristics on impeller materials of centrifugal pump for ship in sea water and fresh water. Corros. Sci. Technol. 2011, 10, 218–224. [Google Scholar]
- Babalola, P.O.; Omada, J.E.; Kilanko, O.; Banjo, S.; Ozour, O. Performance evaluation of a centrifugal pump with different impeller materials. J. Phys. Conf. Ser. 2019, 1378, 022091. [Google Scholar] [CrossRef]
- Huang, P.H.; Guo, M.J. A study on the investment casting of 17-4PH stainless steel helical impeller on centrifugal pump. Mater. Res. Innov. 2015, 19, S977–S981. [Google Scholar] [CrossRef]
- Wang, C.; Shi, W.; Si, Q.; Zhou, L. Numerical calculation and finite element calculation on impeller of stainless steel multistage centrifugal pump. J. Vibroeng. 2014, 16, 1723–1734. Available online: https://www.jvejournals.com/article/15031 (accessed on 21 March 2020).
- Alzafin, Y.A.; Mourad, A.H.; AbouZour, M.; Abuzeid, O.A. A study on the failure of pump casting made of ductile Ni-resist cast irons used in desalination plants. Eng. Fail. Anal. 2007, 14, 1294–1300. [Google Scholar] [CrossRef]
- Zahrani, E.M.; Saatchi, A.; Alfantazi, A. Pitting of 316L stainless steel in flare piping of a petrochemical plant. Eng. Fail. Anal. 2010, 17, 810–817. [Google Scholar] [CrossRef]







| Element | C | Si | Mn | S | Mo | Cr | P | Ti | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Gray Cast Iron | 4.516 | 2.084 | 0.263 | 0.160 | 0.006 | 0.184 | 0.053 | 0.018 | 0.235 | Bal. |
| Element/Parameter | Concentration (mg/L) | |
|---|---|---|
| Well Water | Wastewater | |
| B | 0.440 | 0.215 |
| Ca | 102.842 | 58.367 |
| Fe | - | 0.329 |
| K | 10.466 | 22.166 |
| Li | 0.871 | 0.379 |
| Mg | 30.319 | 18.645 |
| Na | 278.947 | 163.206 |
| P | 0.014 | 0.344 |
| S | 54.433 | 22.100 |
| Se | 0.024 | 0.038 |
| Si | 41.807 | 34.581 |
| Sr | 1.858 | 1.056 |
| Zn | 0.176 | 0.069 |
| Cl (ppm) | <1 | <1 |
| pH | 7.60 | 6.84 |
| Conductivity (µs) | 1433 | 963.0 |
| TSS (mg/lit) | 230 | 30.0 |
| Electrolyte | Exposure Time (min) | βa (V/dec.) | icorr (µA/cm2) | Ecorr (V) | RP (Ω cm2) |
|---|---|---|---|---|---|
| Wastewater | 0 | 0.22 | 259 | −0.53 | 369 |
| 30 | 0.32 | 153 | −0.62 | 908 | |
| 60 | 0.32 | 148 | −0.65 | 939 | |
| Well water | 0 | 0.25 | 310 | −0.61 | 350 |
| 30 | 0.30 | 309 | −0.67 | 421 | |
| 60 | 0.32 | 261 | −0.66 | 534 |
| Water | Time (min) | (Ω cm2) | (µS sn/cm2) | (Ω cm2) | (µS sn/cm2) | (Ω cm2) | Short Warburg Impedance | (µF/cm2) | (µF/cm2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(Ω cm2) | (s) | ||||||||||||
| Waste | 0 | 155.4 | 1570 | 0.74 | 60.14 | 1870 | 0.71 | 912.7 | - | - | - | 690 | 2310 |
| 30 | 134.9 | 1490 | 0.62 | 578.1 | 29,900 | 0.98 | 534.9 | - | - | - | 1350 | 31,570 | |
| 60 | 140.2 | 1780 | 0.63 | 326.5 | 10,900 | 0.53 | 929.4 | - | - | - | 1300 | 82,600 | |
| Well | 0 | 139.1 | 1390 | 0.62 | 311.7 | 2650 | 0.58 | 335 | - | - | - | 820 | 2430 |
| 30 | 119.8 | 5870 | 0.59 | 310 | - | - | - | 1356 | 12.75 | 0.47 | 8820 | - | |
| 60 | 53.15 | 5080 | 0.58 | 310.3 | - | - | - | 426.4 | 55.45 | 0.62 | 7030 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi Jarrahi, M.; Khajavian, E.; Noorbakhsh Nezhad, A.H.; Mohammadi Zahrani, E.; Alfantazi, A. Elucidating the Electrochemical Corrosion of a Water Pump Impeller in an Industrial Cooling System with Zero Liquid Discharge. Water 2025, 17, 173. https://doi.org/10.3390/w17020173
Mousavi Jarrahi M, Khajavian E, Noorbakhsh Nezhad AH, Mohammadi Zahrani E, Alfantazi A. Elucidating the Electrochemical Corrosion of a Water Pump Impeller in an Industrial Cooling System with Zero Liquid Discharge. Water. 2025; 17(2):173. https://doi.org/10.3390/w17020173
Chicago/Turabian StyleMousavi Jarrahi, Mina, Ehsan Khajavian, Amir Hossein Noorbakhsh Nezhad, Ehsan Mohammadi Zahrani, and Akram Alfantazi. 2025. "Elucidating the Electrochemical Corrosion of a Water Pump Impeller in an Industrial Cooling System with Zero Liquid Discharge" Water 17, no. 2: 173. https://doi.org/10.3390/w17020173
APA StyleMousavi Jarrahi, M., Khajavian, E., Noorbakhsh Nezhad, A. H., Mohammadi Zahrani, E., & Alfantazi, A. (2025). Elucidating the Electrochemical Corrosion of a Water Pump Impeller in an Industrial Cooling System with Zero Liquid Discharge. Water, 17(2), 173. https://doi.org/10.3390/w17020173









