Abstract
Currently, the world faces serious challenges in meeting the growing demand for clean water. The present paper demonstrates the possibility of using the ultrafiltration (UF) process to reuse water from wastewater generated in car washes. Car washes commonly use foaming agents with dyes, which, although they are not necessary for washing cars, may hinder water reuse. For this reason, the aim of this work was to investigate the effect of the dyes present in car wash wastewater on the membrane fouling intensity. For this purpose, experimental tests were conducted with the application of a pilot plant with an industrial PCI B1 membrane module. The module was equipped with tubular FP100 (100 kDa) polyvinylidene fluoride (PVDF) membranes. For the feed, two types of cleaning agents and synthetic wastewater were used. The results obtained in the current study demonstrated that the UF membranes allowed the obtainment of the permeate characterized by high quality. In addition, it has been shown that the presence of Indigo carmine dye in the wastewater led to an increase in the fouling intensity. To sum up, it should be pointed out that the findings presented in the current study may be of key importance in the design of pilot installations used for the treatment of car wash wastewater.
Keywords:
car wash; dye; foam solution; fouling; industrial membrane; pilot installation; PVDF; ultrafiltration; water reuse; wastewater 1. Introduction
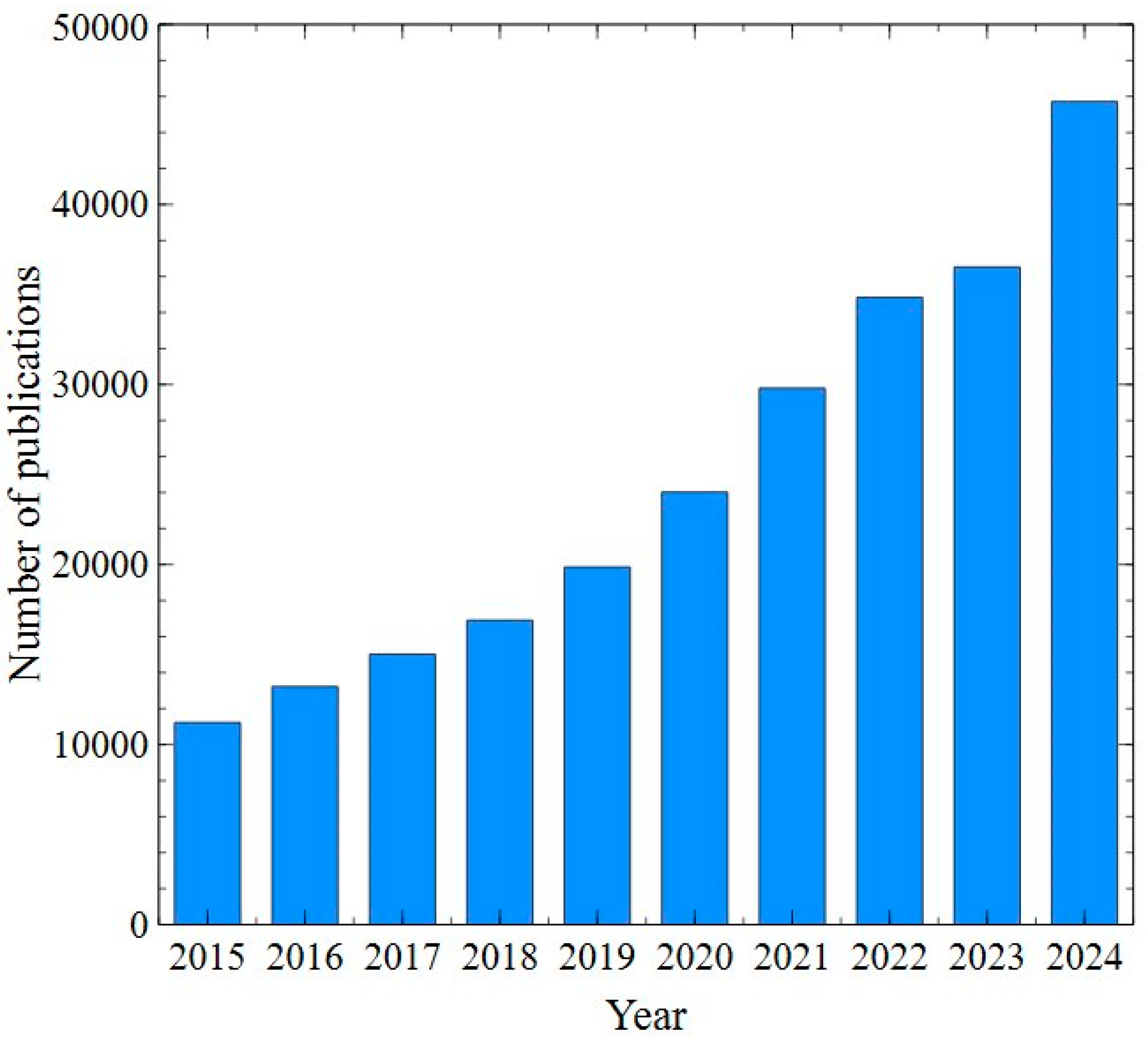
The world is currently facing significant challenges in meeting the growing demand for clean water. Only 3% of the Earth’s water is available as freshwater resources suitable for drinking [1]. In line with global trends in environmental protection and the circular economy, many countries have made efforts in wastewater recovery, establishing stringent regulations to encourage water reuse. As it has been pointed out in [2], in most developing countries, the provision of clean water is prioritized over other services. Reused water is defined as the wastewater that has been treated with the use of various treatment processes for meeting target water quality [3]. Worldwide, the total volume of reused water is approximately 14.2 billion m3 per year [4]. The literature analysis has shown that the number of published articles focused on this issue has significantly increased in recent years (Figure 1).
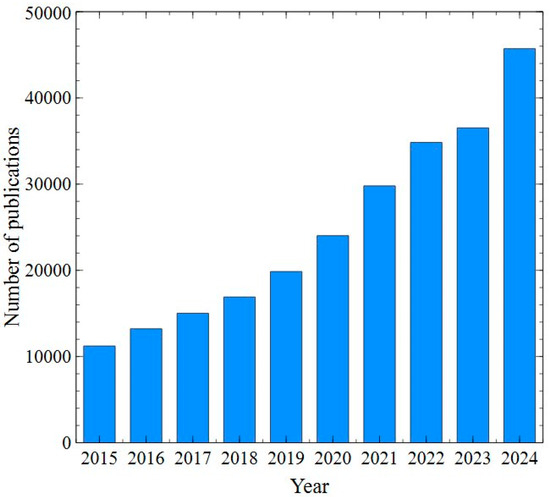
Figure 1.
The number of publications according to Science Direct. Keyword: water reuse. Data retrieved: 8 December 2024.
At the same time, the car wash industry is known as an industry that produces a high amount of wastewater. Moreover, it is expected to grow [5]. Hence, due to legislation and water scarcity, there is a requirement for the application of solutions ensuring the reuse of water in this industry. Accordingly, modern technologies for car washing including water recycling and reuse are expected to be implemented. Nevertheless, it is a great technological challenge since wastewater released from car wash centers can contain many pathogenic microorganisms [6] and a wide range of contaminants, such as the following: (i) suspended particles, (ii) oil, (iii) oil and grease, (iv) anionic surfactants, (v) fuel residues, and (vi) metals [7,8,9,10,11]. It is worth noting that the composition of wastewater may vary depending on the type and size of car being washed and the chemical products, soaps, and detergents used. The characteristics of car wash wastewater have been presented in a recently published review paper [12]. Wastewater also contains dyes that are added to the foaming solution to make the foam more attractive, yet removing dyes from wastewater is a great challenge [13,14] and a serious threat to the environment [15].
Obviously, untreated or insufficiently cleaned car wash wastewater discharged into natural water bodies may contaminate the land and water [16]. Therefore, the main aim of an appropriate treatment of this wastewater type is to reduce the negative impact of car washing on the environment and to reuse water resources [7,8]. To date, several studies have proven that the treatment of car wash wastewater may be ensured by numerous conventional technologies. The advantages and disadvantages of the above-mentioned techniques have been described in several studies [11,17,18,19].
Importantly, in recent years, the use of membrane processes, especially ultrafiltration (UF) for this purpose, has received significant research attention, e.g., [20,21,22,23,24,25]. This is due to the fact that UF membranes allow the removal of pathogens, contaminants, and suspended particles presented in a feed [26]. Moreover, compared to most conventional technologies, membrane technology provides high-quality permeation, offers a more holistic solution, is cheaper, and is easier to use and manage [27,28,29,30]. Polyvinylidene fluoride (PVDF) is widely used for the UF process due to its good thermal stability, high mechanical strength, and excellent chemical and aging resistance [13,31]. However, it is well known that one of the challenges for full-scale applications of UF technology is fouling. This is defined as the phenomenon which causes the accumulation of suspended or dissolved solids on the membrane surface and/or its pores [32,33]. It is a complex issue which may include adsorption, pore blocking, and cake formation [34,35,36]. Obviously, it leads to a decline in the process performance and may affect the economic profitability of the membranes technology. For these reasons, the application of the UF process in a car wash requires the use of an effective cleaning agent which allows the restoration of the maximum permeate flux. It should be pointed out that in addition to detergents, cleaning agents used in car washes often contain dyes. This includes, for instance, Indigo carmine dye, that may lead to the human health deterioration and several environmental issues [37,38]. For this dye, the most intensive fouling of polypropylene (PP) and polytetrafluoroethylene (PTFE) membranes was observed during the filtration of car wash wastewaters containing yellow, green, red or blue dye [39]. The intensive adsorption of dyes on the surface of PVDF membranes was also demonstrated in the study [13]. It has been widely documented that UF may be an effective process for the separation of dyes present mainly in textile wastewater [26,40,41,42,43,44]. However, performing the literature review allowed us to find that up to now, the impact of dyes present in car wash wastewaters on UF process performance has not been determined. Therefore, in the current study, the impact of Indigo carmine dye, which is often added to cleaning agents used for washing cars, was investigated.
In a few studies [13,14], the influence of UF membrane performance, wastewater composition, and dye types on dye rejection degree was investigated. However, reclaimed water in a car wash station does not have to be completely free of all the components (e.g., dye or surfactants) which are present in wastewater [45]. Indeed, from a practical point of view, limiting the fouling intensity to maintain high permeate flux is of significant importance.
It is recognized that the studies, with the use of a pilot-scale installation, are crucial for considering the possibility of the industry implementing the UF process for the treatment of car wash wastewater. Nevertheless, in most of the studies, the experimental investigations with the use of UF membranes for this purpose were performed on a laboratory scale. On such a scale, the representation of industrial conditions is a great challenge, which makes it difficult to investigate the effect of the dyes’ presence in car wash wastewater on the fouling intensity. It was assumed that the dyes present in wastewater adsorb on membranes and change their properties, which affects the intensity of fouling during the separation process. To imitate the UF process and evaluate the effects of the applied membrane washing procedure in the current study, an industrial membrane module was used.
2. Materials and Methods
2.1. Experimental Set-Up
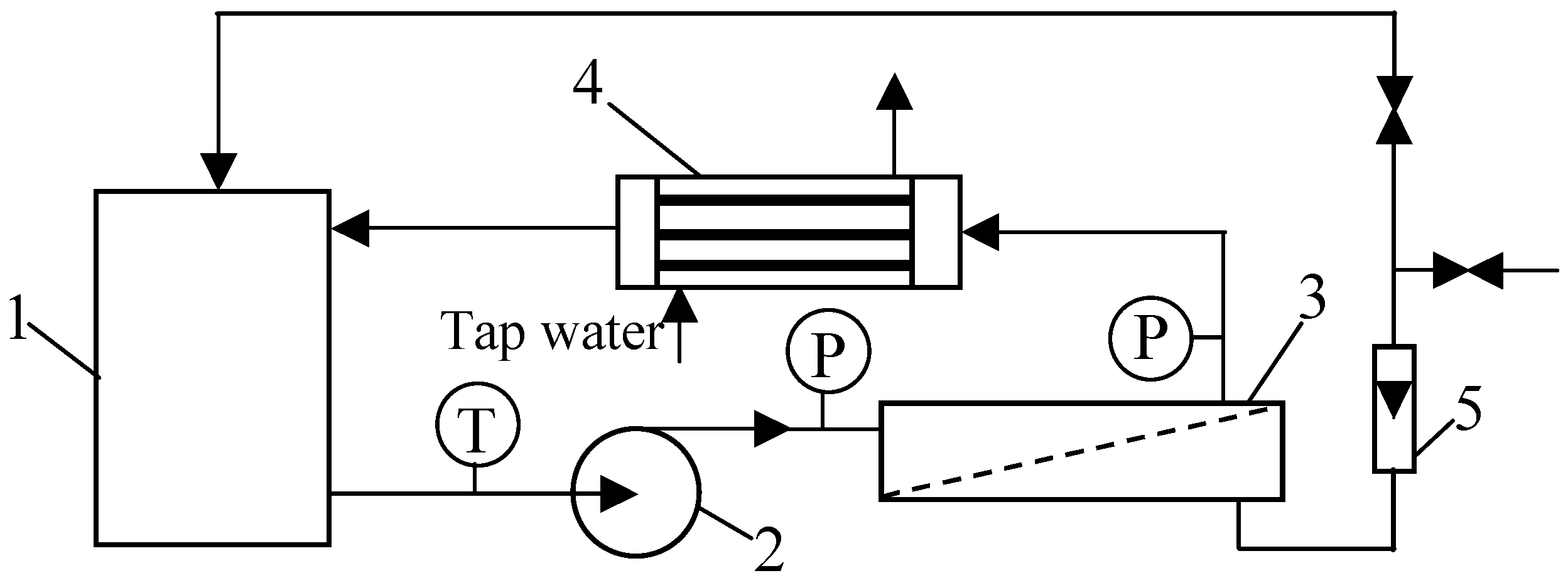
In the current work, a pilot-scale installation was used (Figure 2). It was equipped with 18 tube FP100 membranes made of polyvinylidene fluoride (PVDF) (PCI Membranes, Kostrzyn Wielkopolski, Poland). The membranes’ diameter and length were equal to 1.25/1.27 cm and 120 cm, respectively. The total membrane area was 0.9 m2. The molecular weight cut-off (MWCO) declared by the manufacturer was 100 kDa. The membranes were mounted in a stainless steel PCI B1 module with a diameter of 10 cm (29% packing density).
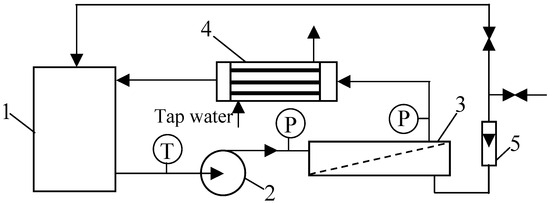
Figure 2.
Experimental set-up of the pilot-scale UF unit with the tubular membrane module: 1—feed tank, 2—pump, 3—UF tubular module, 4—heat exchanger, 5—rotameter, T—thermometer, P—manometer.
The UF process was performed at transmembrane pressure (TMP) in the range from 0.05 to 0.2 MPa. The feed was pressurized by a centrifugal pump (type VNR-8, Grundfos, Bjerringbro, Denmark) and flowed inside the tubular membranes with a velocity equal to 1.2 m/s. A parallel flow head was mounted in the B1 module, which allowed for the obtainment of similar process conditions in each FP100 tube. As a result, the obtained test results are the average of 18 membrane samples.
The Pilot UF was equipped with a feed tank with a capacity of 200 L, and the feed line volume was 20 L. The valve system in the permeate line allowed for the continuous collection of permeate or its return to the feed tank (constant concentration option). For all types of the solutions tested, the feed temperature was 300 ± 1 K. Temperature control was ensured by a shell-and-tube cooler (made of AISI 321 stainless steel) through which the feed flowed.
The pilot installation was operated in a university laboratory; therefore, due to safety regulations, UF tests were conducted with a night break. After completing a given series of tests, the installation was rinsed twice with tap water softened in the nanofiltration process (50–60 µS/cm). Additionally, chemical cleaning was also used periodically, for 10 or 30 min. For washing the tested membranes, the PCI manufacturer recommends P3 Ultrasil 11 cleaning agent, which contains NaOH and detergents (SUTURAMED, Szczecin, Poland). For membrane washing, 0.1 wt.% solutions (pH = 11.7) were used.
2.2. Feed Solutions and Process Conditions
In the first stage of the experimental research, tests were carried out with the use of two types of cleaning agents (EuroEcol, Łódź, Poland):
- (i)
- Euro Turbo Foam (‘White’), without added dye;
- (ii)
- Euro Turbo Foam Color Blue (‘Blue’), which has the same composition as White; however, this is enriched with Indigo carmine dye (5,5′-indigodisulfonic acid sodium salt). It is among the most commonly employed dyes [46]. Indeed, it is popularly used in food and other consumables (E132), cosmetics, and as a medicine contrast agent. It is also used as a fabric dye, which indicates its strong adsorption properties [47].
The composition of the above-mentioned cleaning agents is presented in [48]. The manufacturer supplies them in the form of liquid concentrates containing diethylene gly-col butyl ether, benzenesulfonic acid, and polymers (each component in the amount of 1–5 wt.%).
The UF tests were conducted for TMP = 0.1 MPa. The feed tank (200 L) was filled with water (NF permeate, 55 μS/cm) and the process was conducted for at least 1 h until a stable permeate flux was obtained. Then, ‘White’ or ‘Blue’ concentrate was added to the feed to obtain a 0.5 vol.% solution. Importantly, this concentration is similar to that used in car washes. After the permeate flux had stabilized, the feed solution was concentrated. The process was completed when its volume decreased from 200 to 50 L.
Additionally, during the UF process, the effect of pressure on the permeate flow was determined for water and solutions (VCR = 1, 2, and 4). In this case, the pressure was reduced to TMP = 0.05 MPa and increased every 5 min to 0.1, 0.15, and 0.2 MPa. After taking readings, the pressure was reduced to TMP = 0.1 MPa and the UF process was continued.
In a final experiment, the UF process was carried out with the use of synthetic wastewater consisting of a mixture of 0.5 vol.% foaming agent solution (‘White’ or ‘Blue’) and 0.2 vol.% Hydrowax (EuroEcol, Łódź, Poland). This agent is used to polish the car and contains diethylene glycol monobutyl ether polymer (10–20 wt.%) and polymeric waxes. In this case, the studies were performed at a constant feed concentration, which was achieved by returning the permeate to the feed tank. In order to increase the fouling intensity, the TMP value was increased to 0.2 MPa.
2.3. Analytical Methods
The turbidity of the test solutions was determined using a portable turbidimeter, model 2100 AN IS (Hach Company, Loveland, CO, USA). The values of electrical conductivity were measured using a 6P Ultrameter (Myron L Company, Carlsbad, CA, USA).
The Hach cuvette tests were used to determine the concentration of surfactants (LCK 334—nonionic, LCK 344—anionic) and chemical oxygen demand—COD (LCK 1014).
The rejection efficiency [%] was determined as follows:
where Cp [mg/L] and Cf [mg/L] are the measured oil concentrations of the permeate and feed, respectively.
The water recovery coefficient [%] was calculated with the following equation:
where VF and VP are the volumes of the feed and permeate, respectively.
The membrane surface was examined by atomic force microscopy (AFM). A multi-Mode 8 AFM apparatus equipped with a Nanoscope V converter from Bruker (Billerica, MA, USA) characterized the membrane roughness in the scanasyst mode. The Ra and Rq parameters were evaluated on a basis of at least five AFM images (10 μm × 10 μm).
3. Results and Discussion
3.1. UF of White Foam Solution
Before carrying out tests using cleaning agents as a feed, the UF installation was cleaned with P3 Ultrasil 11 solution and then rinsed with water twice (100 L each). Subsequently, the installation was filled with a new portion of water (200 L) and the UF process was carried out for 2 h at TMP of 0.1 MPa, obtaining a stable water flux of 240 LMH.
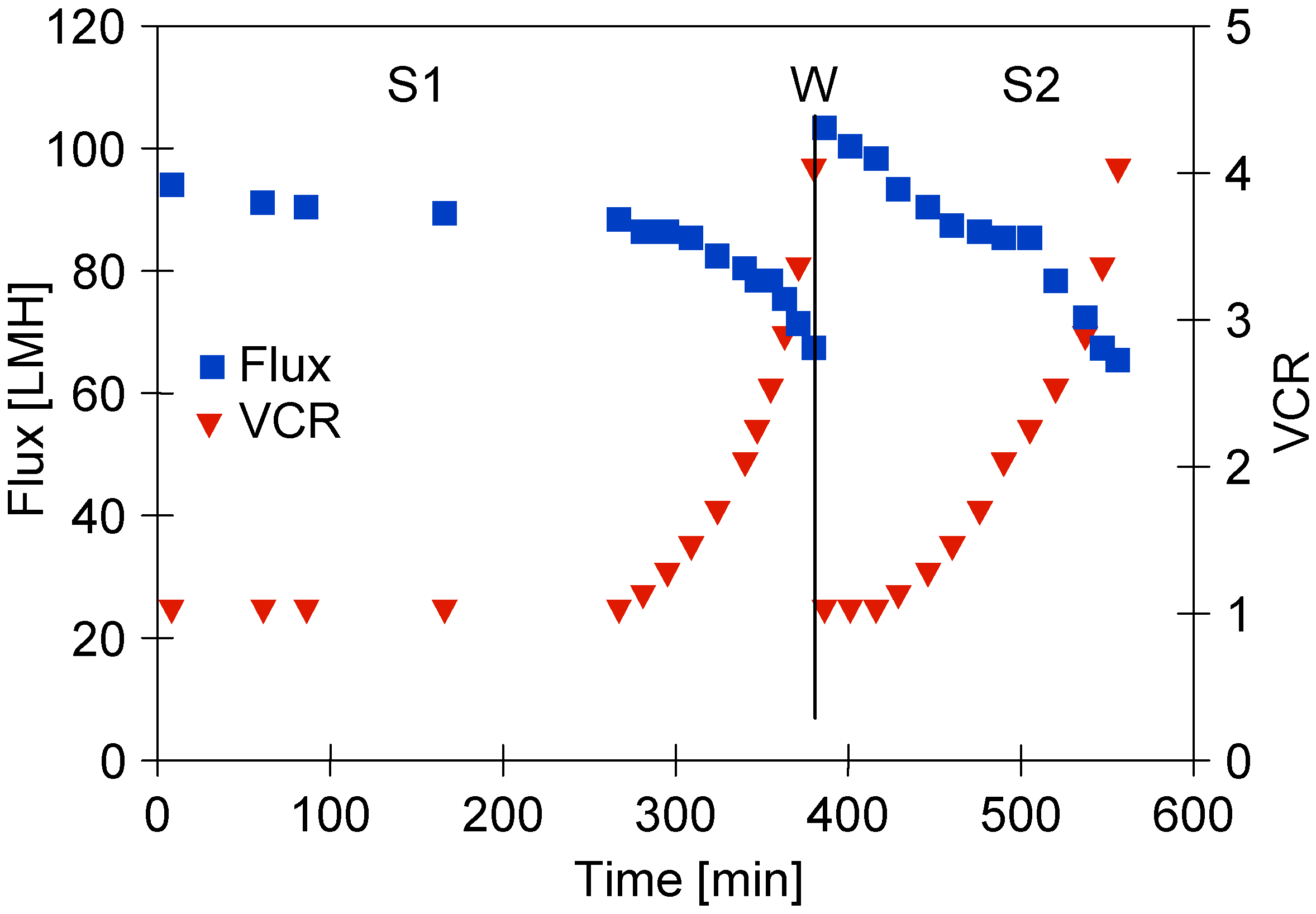
It has been noted that adding the ‘White’ agent concentrate to water (0.5 vol.% solution) caused the flux decline to 95 LMH (Figure 3, ‘S1 Series’). The initial flux was equal to 39% of the water flux, which indicates pore blocking [49]. For the first 260 min of the process run, the obtained permeate was recycled to the feed tank (VCR = 1) and a stable permeate flux of 88 LHM was obtained. Then, the permeate was successively collected, which led to the subsequent flux decline to 67 LHM (VCR = 4). This could be due to the increasing feed concentration and a significant increase in its turbidity which led to an increase in the intensity of concentration polarization and fouling. This finding is consistent with the results presented in several previous studies wherein it has been documented that during the pressure-driven membrane processes, the permeate flux is influenced by the feed turbidity. This result was recorded for the following feed types: reactive dye [40], seasonal high-turbidity surface water [50], water [51], and fermentation broths [52].
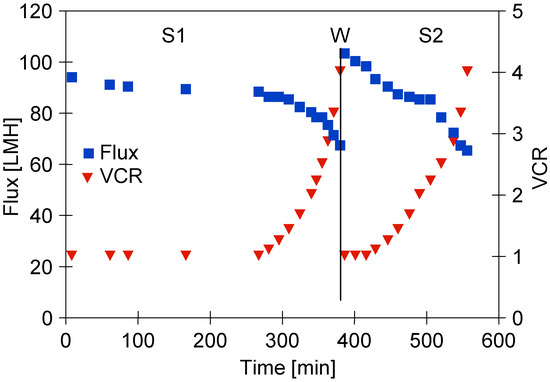
Figure 3.
The permeate flux during the UF process of ‘White’ solution under TMP of 0.1 MPa, S1—series 1, S2—series 2. W—installation washed with P3 Ultrasil 11 solution and rinsed twice with water.
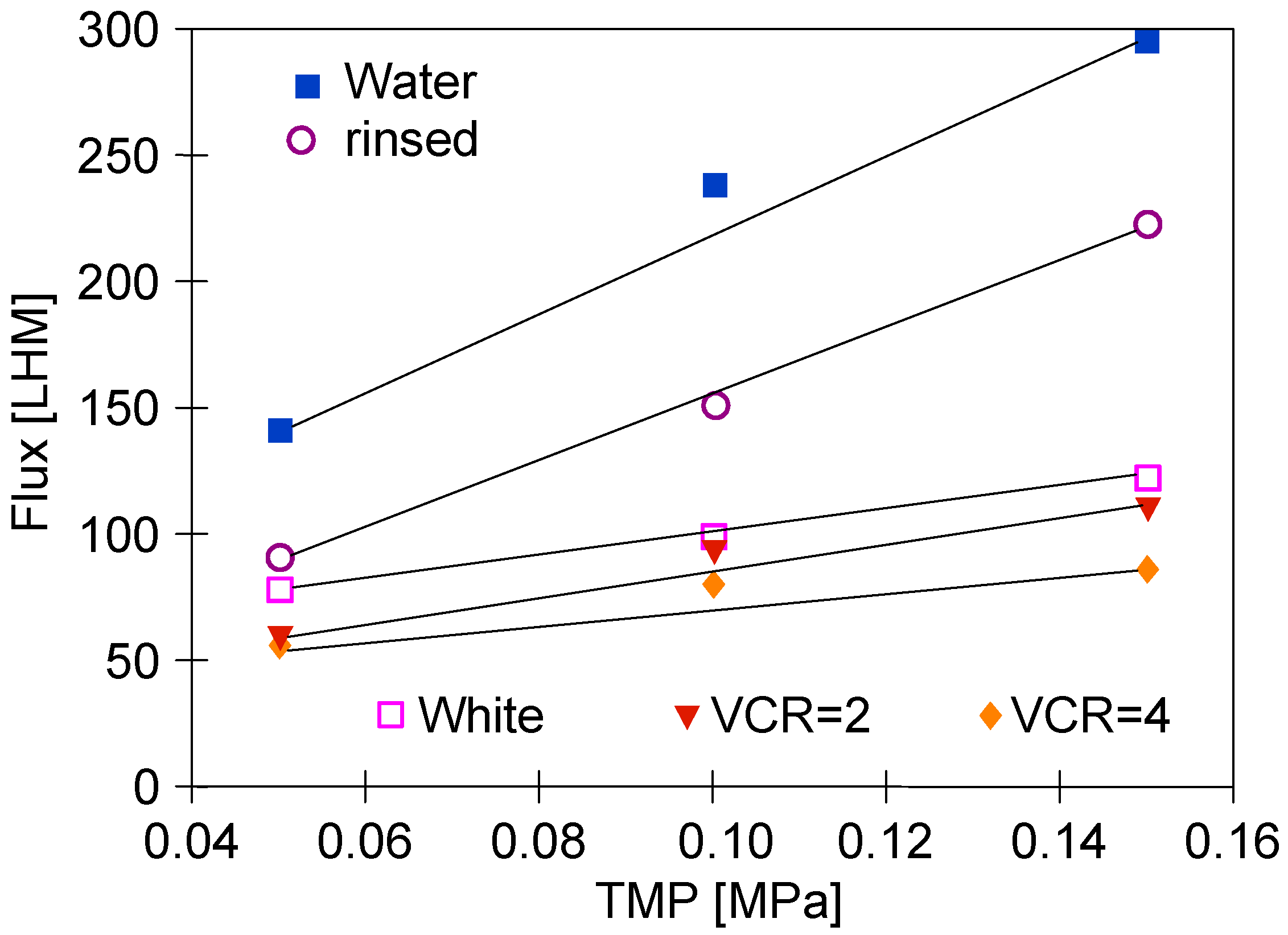
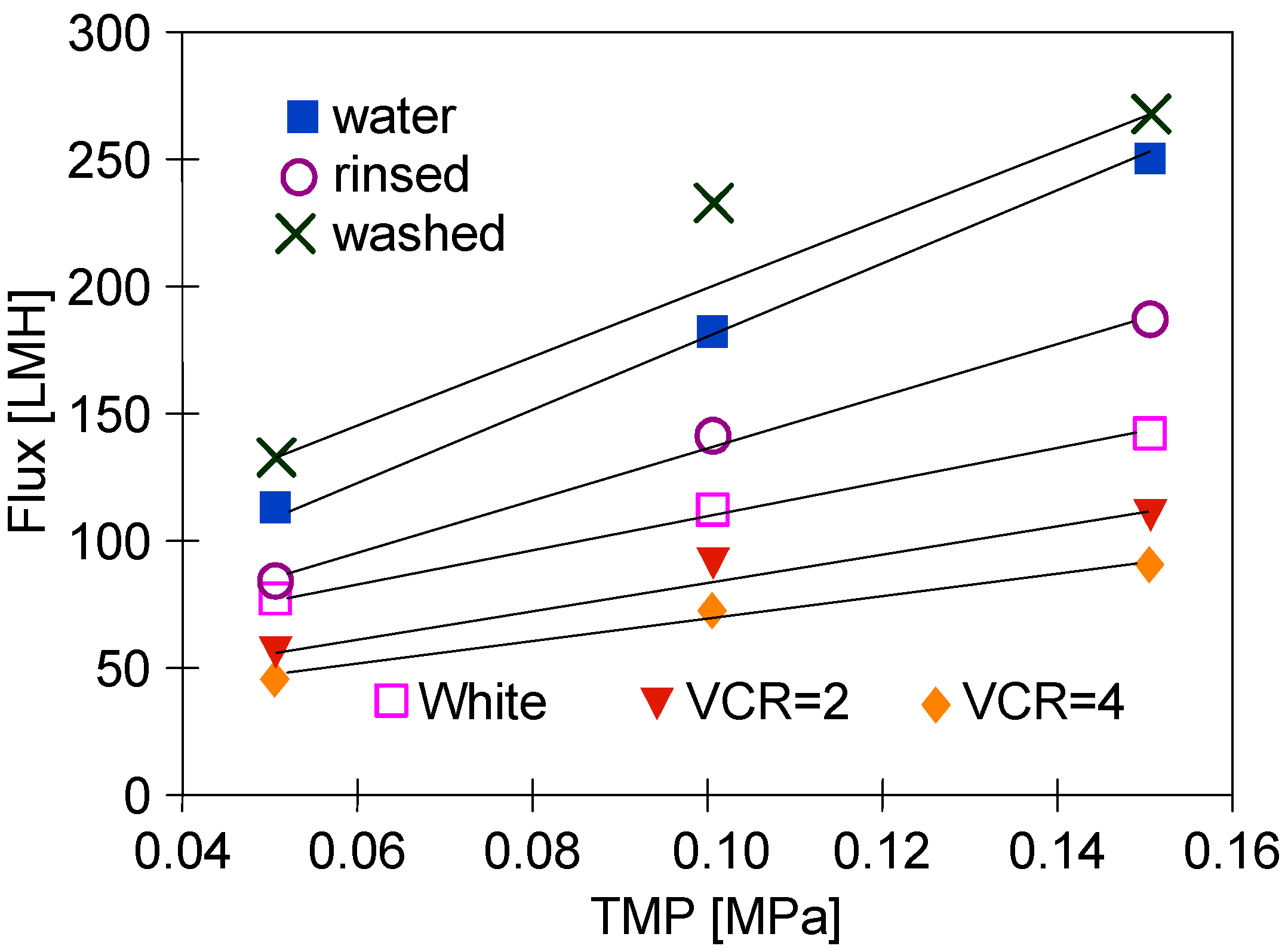
The changes in the membranes’ performance that occurred during series S1 were analyzed by measuring the dependence of permeate flux on TMP (Figure 4). Overall, since TMP is a driving force of the UF process, if the membrane permeability does not change, the permeate flux increases linearly with an increase in TMP. In the figure, some of the points for TMP = 0.1 MPa lie above the straight line marked by the extreme points. This indicated the formation of a fouling layer, which, due to compression, reduced the flux for TMP = 0.15 MPa. It is worth noting that this effect increased with increasing feed concentration (Figure 4, ‘VCR = 2 and VCR = 4’). This change for the initial water flux (Figure 4, ‘Water’) was the result of another factor. There were also large pores (0.1 μm) on the surface of the FP100 membranes, which were cleaned by washing with P3 Ultrasil 11, which increases the initial flux [15]. During UF, these pores become blocked by deposits, which is facilitated by increasing TMP [49]. Module rinsing with water does not remove deposits from the pores’ interior, hence, in this case, a good convergence of the measurement points with the straight line was obtained (Figure 4, ‘rinsed’). The results of the study confirmed that under conditions favoring the increase in fouling and gel layer thickness (Figure 4, ‘White, VCR = 2 and VCR = 4’), the increase in TMP caused a smaller increase in permeate flux.
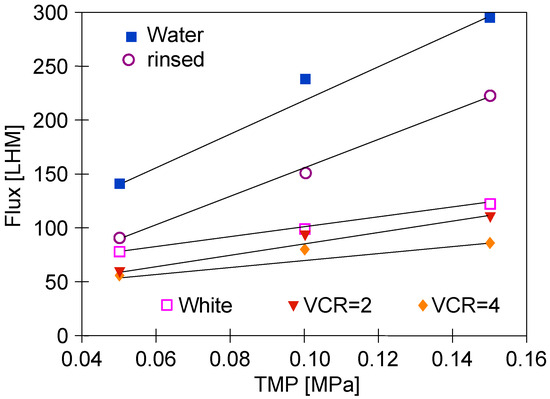
Figure 4.
Changes in the permeate flux during UF tests. Feed: ‘White’ solution (Figure 3, Series S1) and NF permeate (water and rinsed).
After each completed measurement series, the system was thoroughly cleaned three times using water. As a matter of fact, it did not allow for the restoration of the initial membranes performance (Figure 4, ‘rinsed’). For instance, after the membranes were rinsed, the flux for water at TMP of 0.1 MPa was equal to 150 LMH. From this analysis, it can be clearly seen that the main reason of the flux decline during the UF process was the irreversible fouling phenomenon. For this reason, before the second series of ‘White’ solution separations, the membranes were washed with P3 Ultrasil 11 solution (Figure 3, ‘W’), which allowed for an increase in the water flux to 175 LMH; however, after adding the ‘White’ agent, it decreased to 103 LMH (series S2). Moreover, it is worth noting that during the second series of the UF process (Figure 3, ‘S2 series’), the initial flux of 103 LMH decreased to 98 LHM when the feed concentration was started. In this case, a higher flux was obtained initially than during the S1 series (Figure 3, ‘S1 Series’). This can be explained by the fact that in this case, the feed was characterized by the lower turbidity (Figure 5, ‘White 2’).
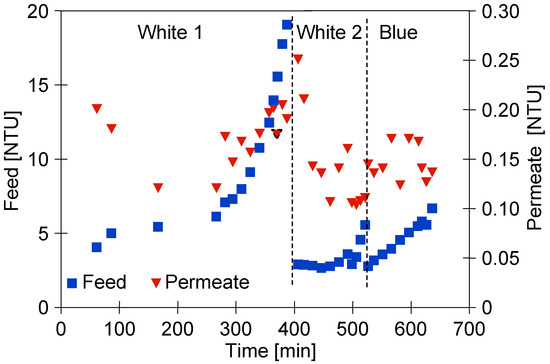
Figure 5.
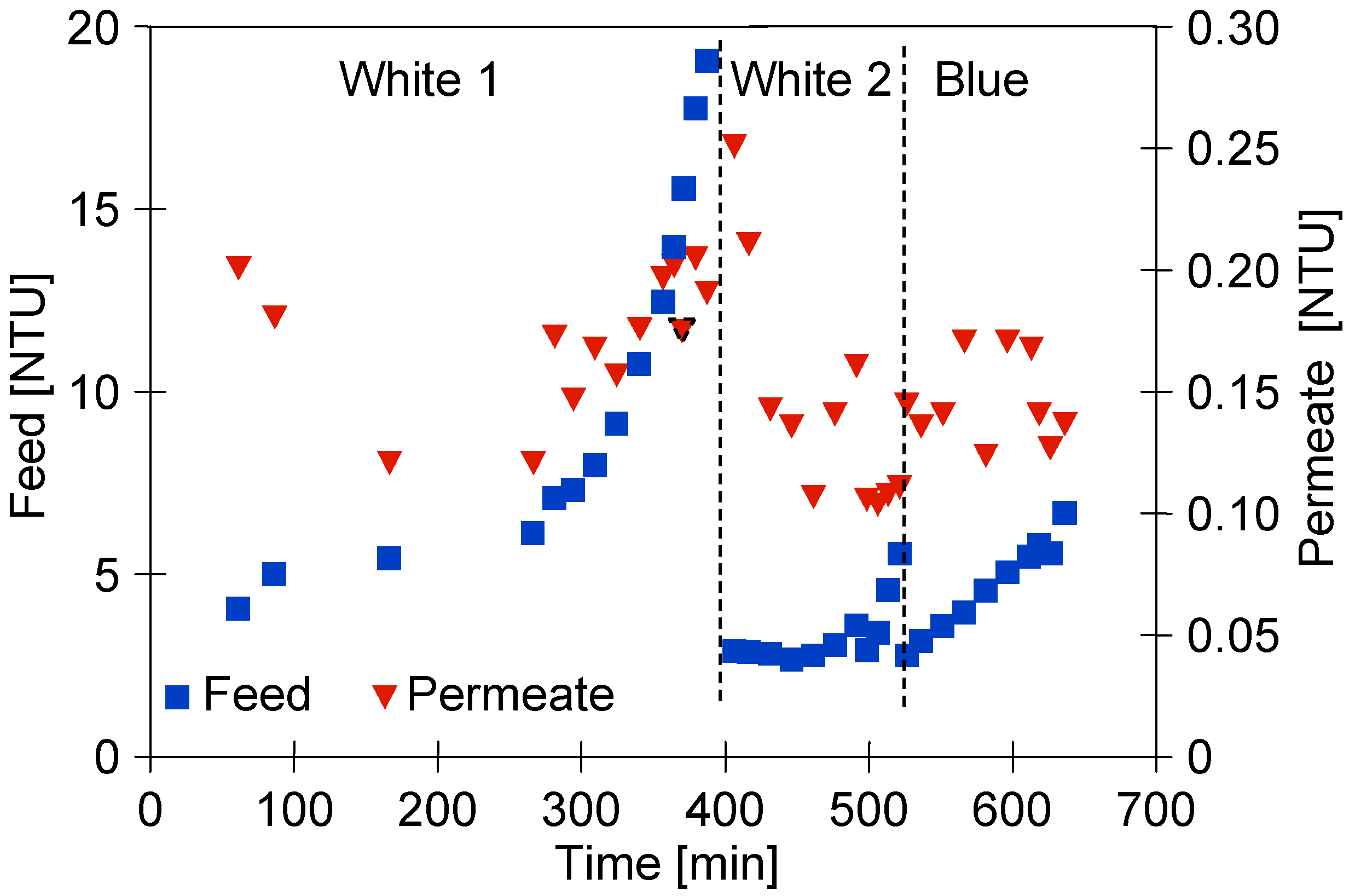
Changes in feed and permeate turbidity during separation of ‘White’ and ‘Blue’ foaming solutions.
Before performing the research presented in the current paper, the installation (Figure 2) was used to investigate other issues related to the separation of car wash wastewater. Despite the repeated cleaning of the set-up, after resuming its use, the remaining contaminants were probably detached from the pipe surfaces by the cleaning agent in the S1 series. In the next series focused on the separation of ‘Blue’ solution, the feed turbidity was already at a similarly lower level (Figure 5, ‘Blue’).
As it has been indicated by Yang et al. [4], the permeate quality is critical for the realization of water reuse. In the present study, despite the differences in the feed turbidity, the permeate turbidity was equal to 0.1–0.2 NTU (Figure 5). Slightly higher permeate turbidity values were recorded for the washed membranes. As shown in a previous work [21], the chemical cleaning of membranes successfully removes foulants from their surface and opens larger membrane pores. With respect to these findings, it should be clearly pointed out that the UF membranes used in this study allowed the obtainment of the permeate characterized by high quality. Indeed, for all measurement series, regardless of the feed type, the permeate turbidity did not exceed 0.3 NTU. Accordingly, a turbidity reduction higher than 98% was noted.
During S2 series (Figure 3), after receiving 150 L of permeate (VCR = 4), the process efficiency decreased to 65 LMH, which is similar to that obtained during the S1 series. However, after rinsing the installation with water, the obtained flux (TMP = 0.15 MPa) was 185 LMH (Figure 6, ‘rinsed’), which was lower than that obtained during the S1 series tests (220 LMH, Figure 4). This value increased to 265 LMH after washing the installation with P3 Ultrasil 11 solution. Increasing the membrane cleaning time to 30 min allowed the removal of the deposits from the large pores, which resulted in a significant increase in the permeate flux. During the TMP effect measurements, these pores were blocked again, which limited the flux value for TMP = 0.15 MPa. As a result, for similar reasons as for the initial water flux (Figure 4, ‘Water’), the point for TMP = 0.1 MPa also lies above the line (Figure 6, ‘washed’).
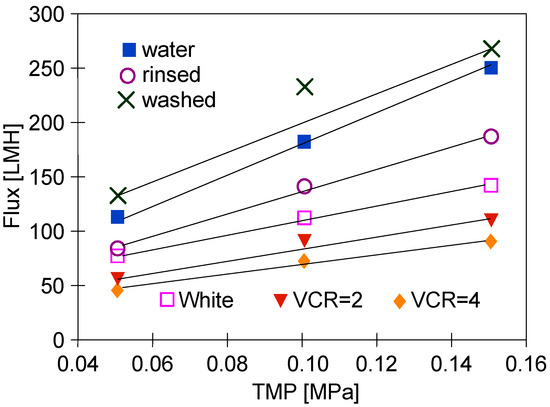
Figure 6.
Changes in the permeate flux changes during UF tests. Feed: ‘White’ solution (series S2) and water. Water—membranes washed P3 Ultrasil 11 (10 min) after the end of the S1 series, washed—membranes washed with P3 Ultrasil 11 solution (30 min) and rinsed twice with water after the end of the S2 series (Figure 3).
3.2. UF of Blue Foam Solution
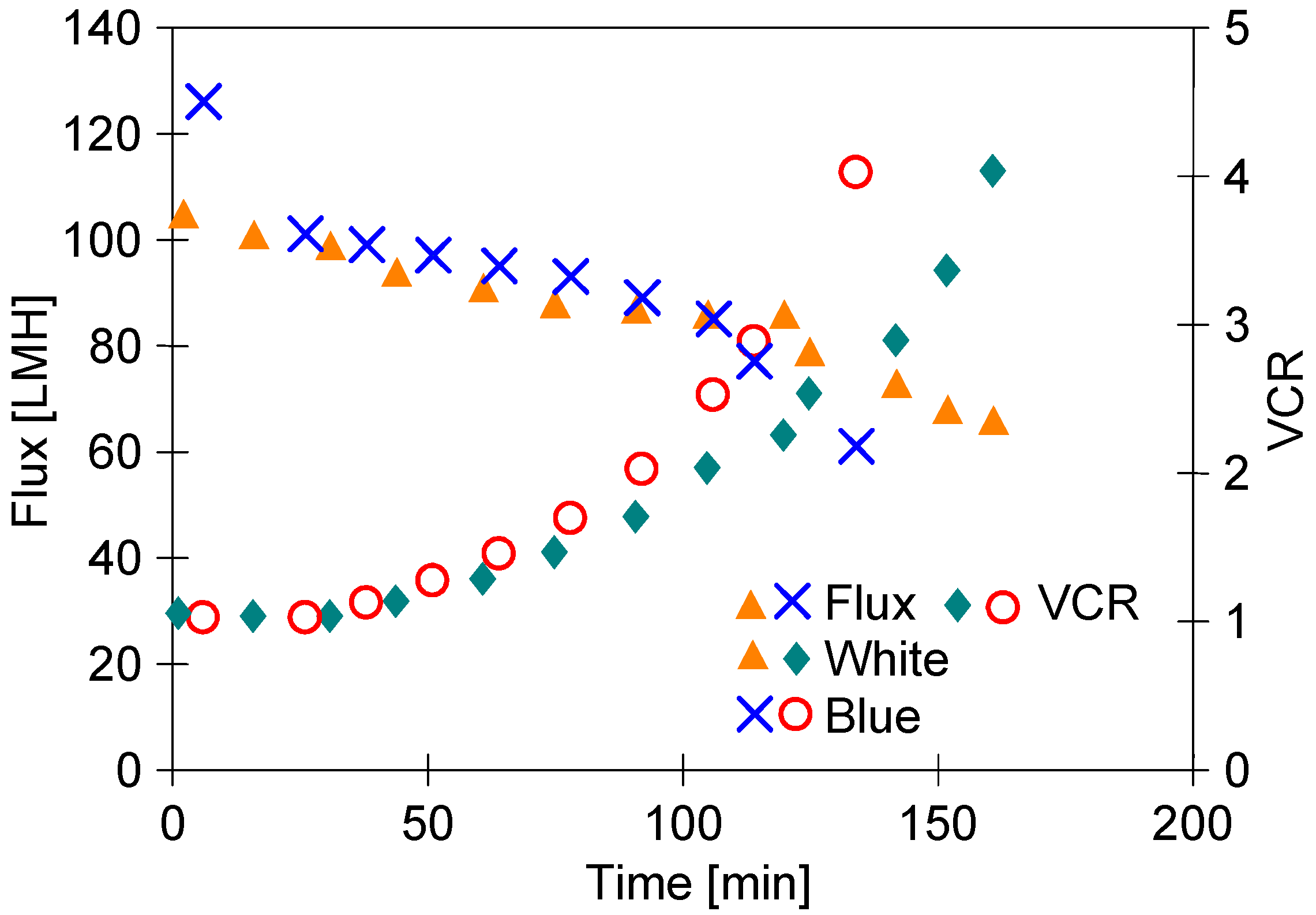
In the next step of the presented research, the UF process of the ‘Blue’ solution was conducted (Figure 7). It has been found that the separation of the ‘Blue’ solution allowed us to receive a slightly higher permeate flux than that noted during the separation of the ‘White’ solution. Hence, the concentration of the feed to VCR = 4 was obtained after 108 min, while for ‘White’ this was after 130 min. This result was influenced by the fact that after chemical washing, the initial efficiency (TMP = 0.1 MPa) during the separation of the ‘White’ and ‘Blue’ solutions was 103 LMH and 125 LMH, respectively.
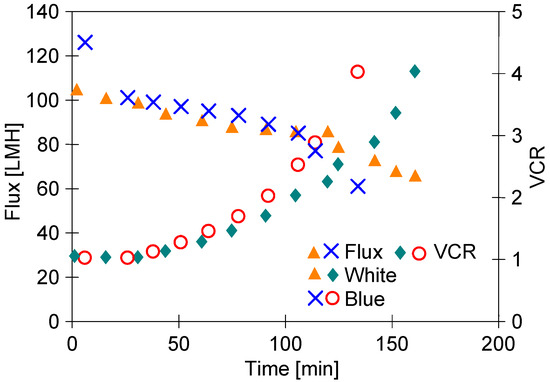
Figure 7.
The comparison of performance of UF process of ‘Blue’ and ‘White’ (series S2) solution under a TMP of 0.1 MPa.
The second reason could be differences in the fouling mechanism. The solutions tested contained anionic and nonionic surfactants. The surfactants present in the feed form a gel/fouling layer which reduces the permeability of UF membranes [49]. Nonionic surfactants adsorbed on hydrophobic membranes cause a more pronounced flux decline than ionic surfactants [53]. Anionic dyes with surfactants form agglomerates with a negative charge, which limits their deposition on the surface of PVDF membranes [13]. This phenomenon may explain the slight increase in permeate flux for the ‘Blue’ feed.
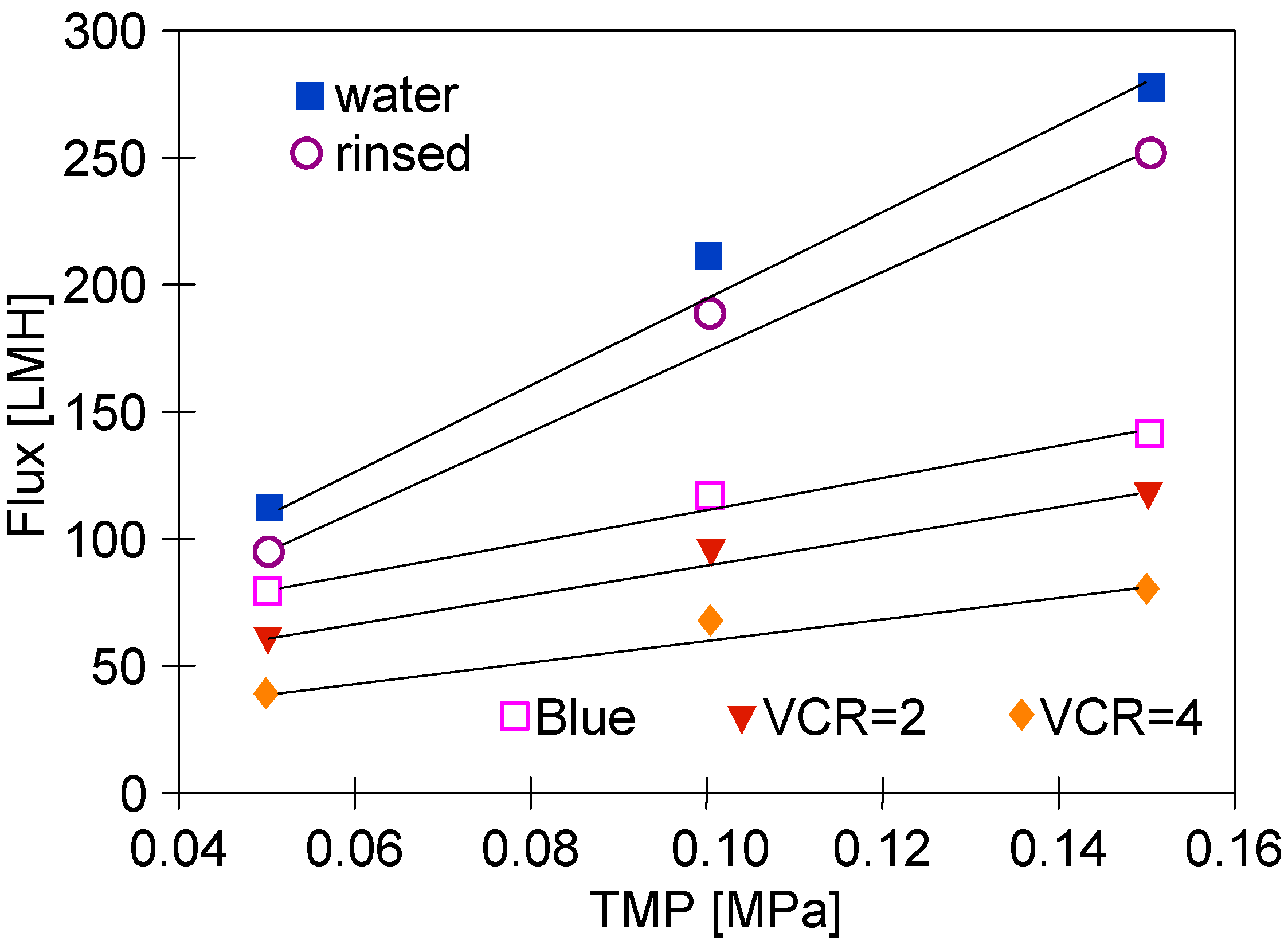
It is necessary to point out that after concentrating the ‘Blue’ solution, washing the system with water allowed the obtainment of a much larger flux (Figure 8) than in the case of testing the ‘White’ solution (Figure 6). This can be attributed to the fact that the outer layer PVDF membrane is similar to the anionic structure of the studied blue dye carrying the negative charges [13]. The separation mechanisms of the binary dye mixtures using a PVDF ultrafiltration membrane led to the Donnan effect and their intermolecular character [13]. In contrast to the ‘White’ solution tests, during the separation of ‘Blue’ solutions, all points for TMP = 0.1 MPa were above the line. This indicates that in this case, the gel/fouling layer formation was greater.
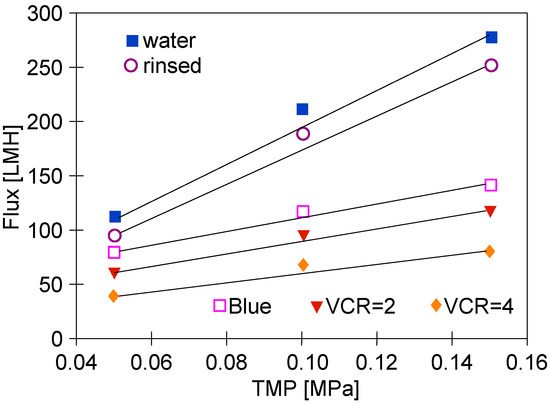
Figure 8.
Changes in the permeate flux changes during UF tests. Feed: ‘Blue’ solution and water.
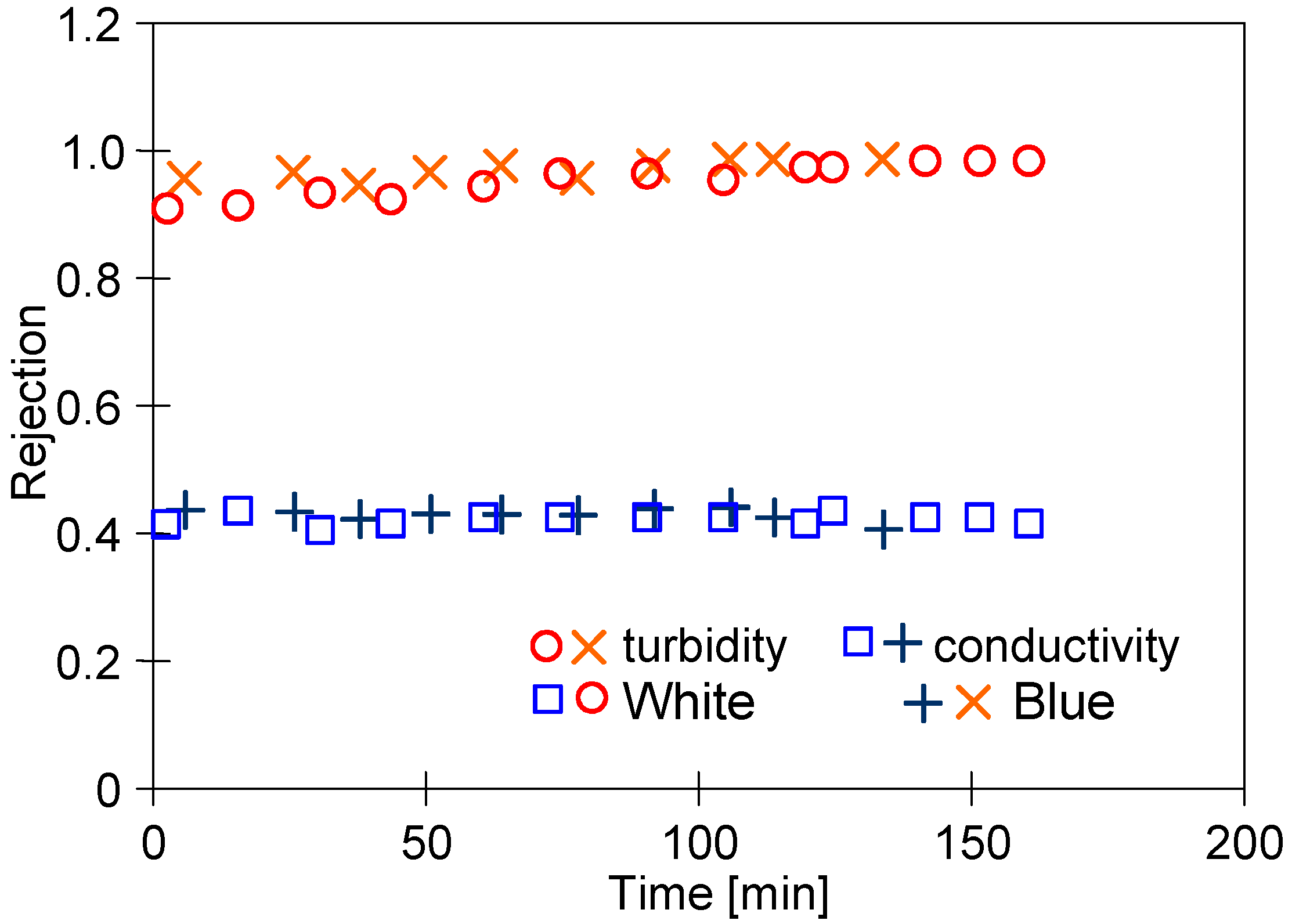
The membrane selectivity is of crucial importance based on the intention of the separation performed [30]. Hence, in the study, the retention degree for suspensions and dissociable compounds has been investigated with the use of both feed types (Figure 9). It has been found the suspension (turbidity) retention in both cases was equal to 98%. In addition, it has been noted that the permeate conductivity was less than two times lower compared to the conductivity feed. This can be explained by the fact that generally, UF membranes ensure ions are retained to a limited extent [54]. Anionic dyes are retained by PVDF membranes to a greater extent due to electrostatic interactions [13]. In the case studied, however, it was not found to cause a significant increase in the level of rejection calculated for conductivity. Higher values were obtained for the separation of surfactants and COD (Table 1).
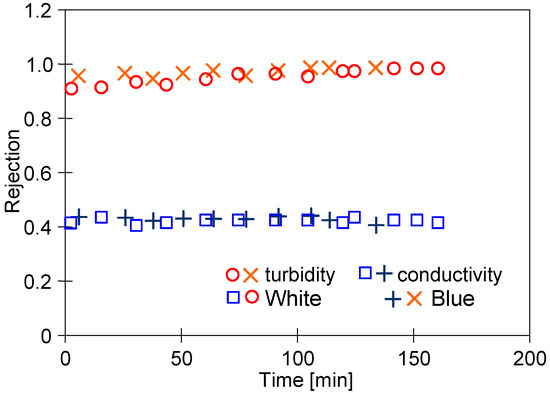
Figure 9.
Rejection degree for suspensions and dissociable compounds (conductivity).

Table 1.
UF separation results: COD and surfactants (anionic and nonionic). P—permeate, F—feed, R—rejection, S1—series 1, S2—series 2.
The concentration of the feed caused the content of organic solutes to systematically increase, in most cases more than twice (Table 1). Despite this, their content in the permeate increased only by 10–17%. This indicates that the growth of the gel/fouling layer during concentration increased the retention rate. This is also evidenced by the fact that for the membranes used with a MWCO of 100 kDa, a reduction in the COD value was obtained that was 10–20% higher than that obtained during the separation of dye solutions by a more dense polyethersulfone (PES) membrane (MWCO of 10 kDa) [49].
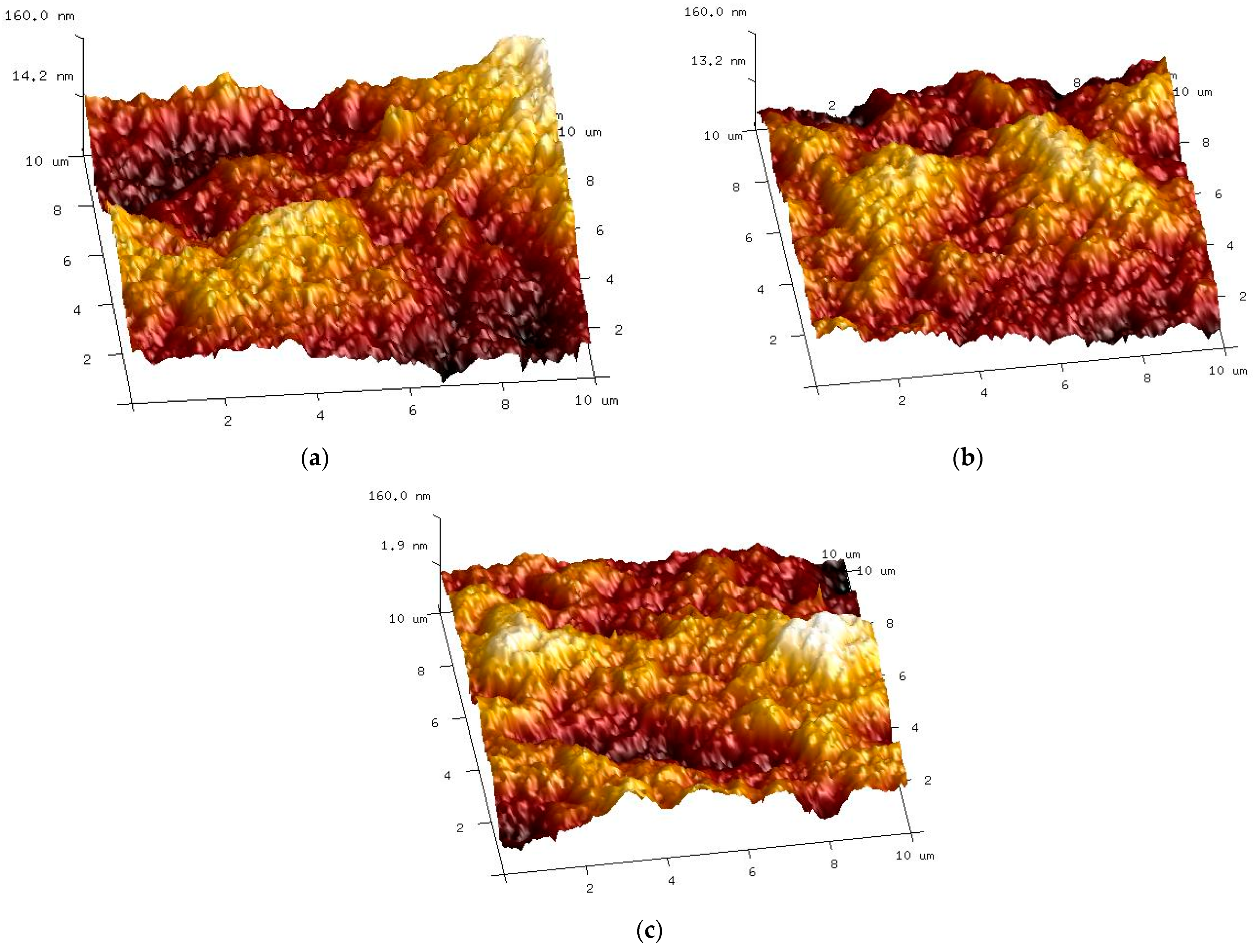
The AFM studies showed that there were no significant differences between the characteristics of the new membrane and membranes used for the separation of ‘White’ and ‘Blue’ solutions (Figure 10). This also confirms the above presented result demonstrating that there was no significant difference in the permeate flux obtained (Figure 7) and in the separation degree (Figure 9, Table 1). Surface roughness analysis showed only a slight increase, especially for the membrane used for the separation of the ‘Blue’ solution (Table 2).
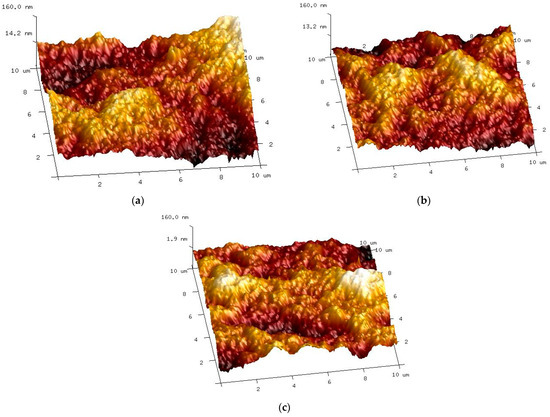
Figure 10.
AFM images of the membrane surface: (a) new membrane; (b) membrane used for the separation of the ‘White’ solution; (c) membrane used for the separation of the ‘Blue’ solution.

Table 2.
Membrane surface roughness.
This result indicated that the dye could permanently adsorb locally on the surface of the tested PVDF membranes. To confirm this, the FP100 membrane sample was immersed for 24 h in a 0.5 vol.% ‘Blue’ solution. After this period, the blue-dyed membrane was intensively rinsed in distilled water. Despite this, the membrane was still slightly blue, which indicated that small amounts of the dye remained on the membrane surface. This irreversible fouling caused by the dyes was also found in another work, which resulted in an approximately 8% decrease in the initial permeate flux [49].
3.3. UF of Synthetic Wastewater
In the present study, the synthetic car wash wastewater was prepared from a mixture of 0.5 vol.% foaming agent (‘White’ or ‘Blue’) solution with 0.2 vol.% Hydrowax. The Hydrowax solution used in the final stage of car washing contains substances that create a protective hydrophobic layer on the car surface. As shown in previous works, the filtration of such a mixture led to a significant fouling phenomenon [48,55].
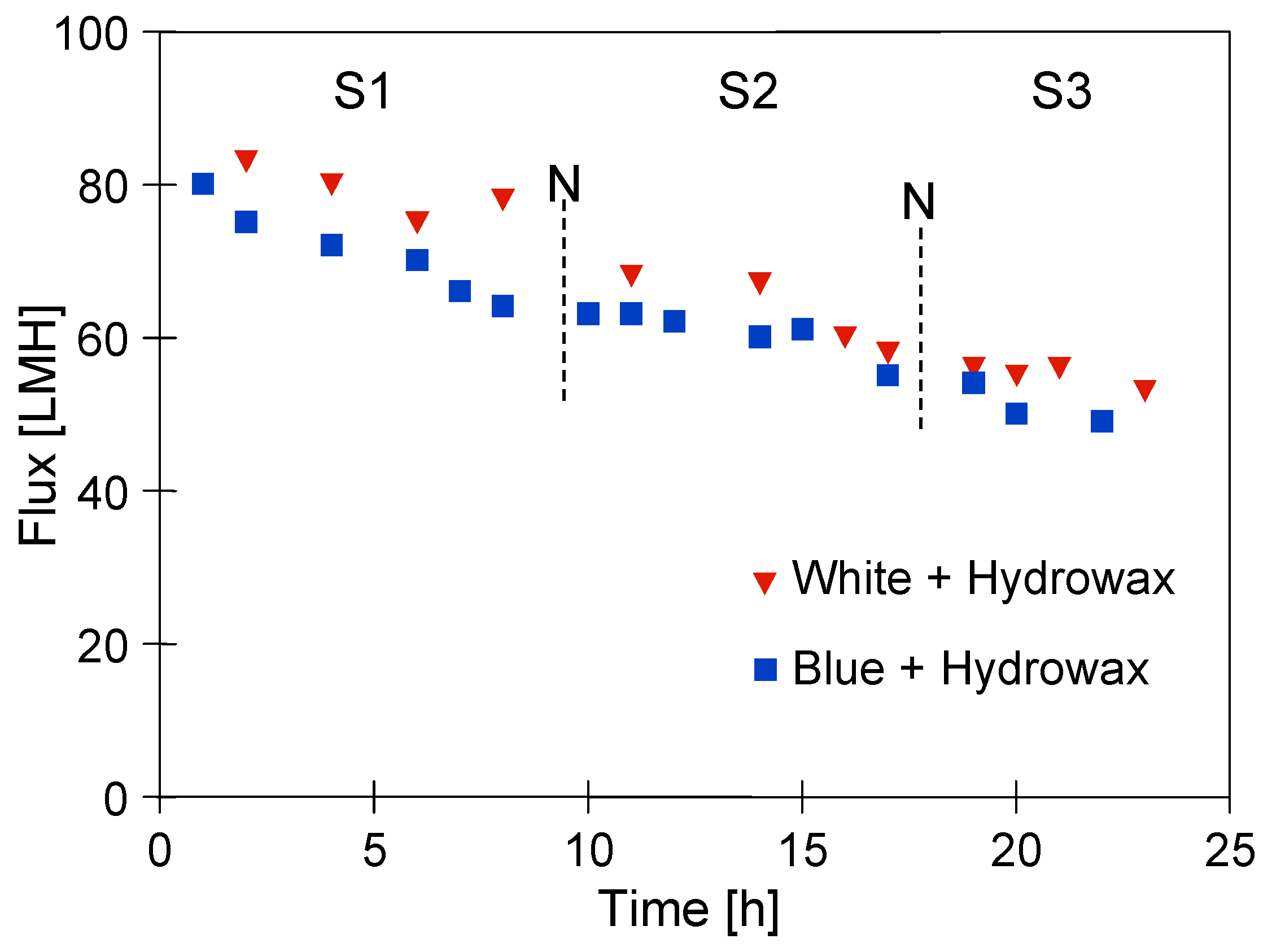
In the conducted research, three measurement series were performed (Figure 11). During the downtimes (12 h—night break), no action was taken and the tested solution remained in the installation. It is clear from these details that for both of the synthetic wastewater mixtures, a significant decrease in the flux was observed. However, a greater decrease in the process performance flux was noted for the mixture with the ‘Blue’ solution. Indeed, a slightly higher UF process performance after the end of Series S3 was noted for the mixture with a ‘White’ solution. Hence, it can be concluded that the presence of Indigo carmine dye in the wastewater led to an increase in the fouling intensity. This can be explained by the fact that it has adsorption properties which caused an increase in the feed–membrane surface interactions, contributing to the formation of a filter cake and the blocking of the membrane pores. During the UF process of the clean ‘Blue’ solution, the decrease in permeate flux was much less significant (Figure 7). This indicated that in complex mixtures, there may be interactions between the dye and its components, the products of which may intensify the fouling phenomenon.
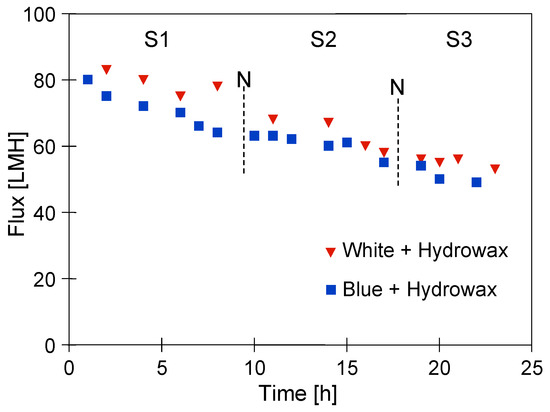
Figure 11.
The permeate flux during the UF process of synthetic wastewaters under TMP of 0.2 MPa. N—12 h break.
Finally, it is interesting to note that the plant downtimes of the installation did not have a significant impact on the UF process performance. This finding may be of key importance in the design of pilot installations intended for the treatment of car wash wastewater.
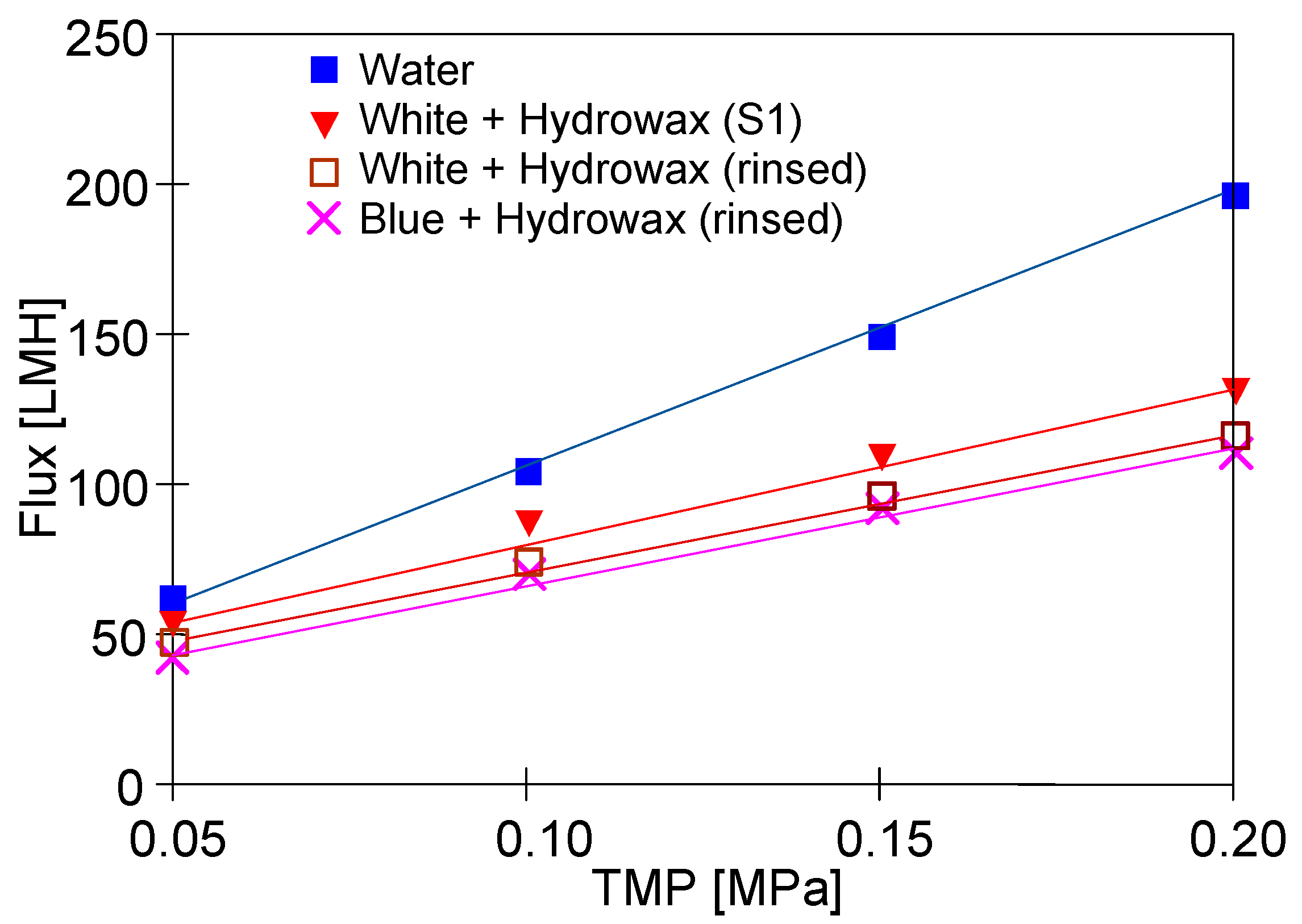
The results presented in Figure 12 confirm that the addition of ‘Hydrowax’ to a feed led to the more significant decline in the permeate flux. In the case of the ‘White’ solution for TMP = 0.15 MPa, the initial flux was 140 LHM (Figure 6, ‘White’), while in the mixture with Hydrowax, the flux at the beginning of the S1 series, it was only 110 LHM (Figure 12, ‘White + Hydrowax S1’). This finding clearly indicates that in this case, the fouling intensity became more severe. Moreover, the ‘Hydrowax’ presence decreased the efficiency of the membrane rinsing. After the UF process of the “White” and ‘Blue” solutions, the rinsing module with water always resulted in an increase in permeate flux (Figure 6 and Figure 8, ‘rinsed’). Meanwhile, for Hydrowax mixtures, it has been found that the lowest value of permeate flux (feed–water) has been obtained for membranes which have been used for the UF process of the wastewater that contained the ‘White solution’. Lower water flux values were recorded after the UF process of wastewater that contained the ‘Blue solution’ (Figure 12, ‘Blue + Hydrowax’ (rinsed)). Therefore, it can be concluded that the presence of Indigo carmine dye led to an increase in the irreversible fouling intensity.
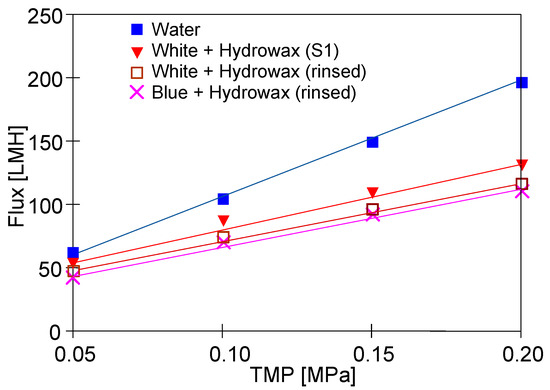
Figure 12.
Changes in the permeate flux during UF tests. Feed: synthetic wastewater and water.
After completing the measurement series, the UF installation was rinsed with water, washed with P3 Ultrasil 11 solution (30 min), and then washed twice with water. The performing of this procedure allowed the obtainment of a water flux close to the initial one.
4. Conclusions
The main aim of this study was to determine the effect of the dyes present in car wash wastewater on the fouling intensity and the efficiency of the UF process using an industrial membrane module. The results presented in the current study may contribute to more efficient and sustainable solutions for car wash wastewater on a pilot scale.
It has been demonstrated that the PVDF tubular membranes used in the current study allowed the obtainment of the UF permeate characterized by a turbidity that did not exceed 0.3 NTU. Importantly, water of this purity can be successfully used for washing cars.
The applied FP100 membranes rejected surfactants at the level of 65%, which due to the formation of a fouling layer, increased to over 80%, which resulted in a reduction in COD by 76%. Adding dye (Indigo carmine) to the foaming solution did not cause any significant changes in the obtained results.
Moreover, it has been found that the application of 75% water recovery (VCR = 4) caused the permeate flux to decrease to 60–70 LHM (TMP = 0.1 MPa), both for foaming solution with and without dye. Washing the membranes with water allowed the recovery of the initial permeate flux at about 75% (White) and 82% for the solution containing dye (Blue). It is important to note that the initial flux was recovered after performing membrane washing (30 min) with P3 Ultrasill 11 solution (pH = 11.7).
After the UF of synthetic wastewaters containing the tested foaming solution and the mixture of polymeric waxes (Hydrowax), rinsing the membranes with water resulted in a slight increase in water flux, which indicated a higher intensity of the irreversible fouling phenomenon. It has been shown that the presence of Indigo carmine dye in the wastewater led to the increase in the membrane fouling intensity. However, in both cases, the initial permeate flux was recovered after intensive membrane washing with P3 Ultrasill 11 solution.
Moreover, it has been noted that the plant downtimes of the pilot installation did not have a significant impact on the UF process performance.
Author Contributions
Conceptualization, W.T. and M.G.; methodology, P.W. and M.G.; validation, W.T. and M.G.; formal analysis, W.T. and M.G.; investigation, P.W.; resources, M.G.; data curation, W.T. and M.G.; writing—original draft preparation, W.T.; writing—review and editing, M.G. and W.T.; visualization, M.G.; supervision, M.G.; project administration, M.G.; funding acquisition, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was financed from the Polish budget within the framework of the program of the Minister of Education and Science in Poland entitled “Science for the Society”, project No. NdS/538617/2021/2022: total amount of funding—PLN 352 135.

Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Musie, W.; Gonfa, G. Fresh Water Resource, Scarcity, Water Salinity Challenges and Possible Remedies: A Review. Heliyon 2023, 9, e18685. [Google Scholar] [CrossRef] [PubMed]
- Tortajada, C. Contributions of Recycled Wastewater to Clean Water and Sanitation Sustainable Development Goals. npj Clean Water 2020, 3, 22. [Google Scholar] [CrossRef]
- Zaneti, R.; Etchepare, R.; Rubio, J. More Environmentally Friendly Vehicle Washes: Water Reclamation. J. Clean. Prod. 2012, 37, 115–124. [Google Scholar] [CrossRef]
- Yang, J.; Monnot, M.; Eljaddi, T.; Ercolei, L.; Simonian, L.; Moulin, P. Ultrafiltration as Tertiary Treatment for Municipal Wastewater Reuse. Sep. Purif. Technol. 2021, 272, 118921. [Google Scholar] [CrossRef]
- Talebzadeh, M.; Valeo, C.; Gupta, R.; Constabel, C. Exploring the Potential in LID Technologies for Remediating Heavy Metals in Carwash Wastewater. Sustainability 2021, 13, 8727. [Google Scholar] [CrossRef]
- Woźniak, P.; Dubicki, M.; Gryta, M. Microbiological Hazard Analysis of Car WashWastewater. Pol. J. Environ. Stud. 2023, 32, 3871–3882. [Google Scholar] [CrossRef]
- Fayed, M.; Shewitah, M.A.; Dupont, R.R.; Fayed, M.; Badr, M.M. Treatability Study of Car Wash Wastewater Using Upgraded Physical Technique with Sustainable Flocculant. Sustainability 2023, 15, 8581. [Google Scholar] [CrossRef]
- Kuan, W.-H.; Hu, C.-Y.; Ke, L.-W.; Wu, J.-M. A Review of On-Site Carwash Wastewater Treatment. Sustainability 2022, 14, 5764. [Google Scholar] [CrossRef]
- Torkashvand, J.; Farzadkia, M.; Younesi, S.; Gholami, M. A Systematic Review on Membrane Technology for Carwash Wastewater Treatment: Efficiency and Limitations. Desalination Water Treat. 2021, 210, 81–90. [Google Scholar] [CrossRef]
- Canales, F.A.; Plata-Solano, D.; Cantero-Rodelo, R.; Pereira, Y.Á.; Díaz-Martínez, K.; Carpintero, J.; Kaźmierczak, B.; Tavera-Quiroz, H. Assessment of Carwash Wastewater Reclamation Potential Based on Household Water Treatment Technologies. Water Resour. Ind. 2021, 26, 100164. [Google Scholar] [CrossRef]
- Kashi, G.; Younesi, S.; Heidary, A.; Akbarishahabi, Z.; Kavianpour, B.; Rezaei Kalantary, R. Carwash Wastewater Treatment Using the Chemical Processes. Water Sci. Technol. 2021, 84, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, M.; Zarei, A.A.; Ghahrchi, M.; Sepehrnia, B.; Meshkinian, A.; Moein, H.; Nakhaei, S.; Bazrafshan, E. Carwash Wastewater Characteristics—A Systematic Review Study. Desalination Water Treat. 2021, 225, 112–148. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Kumar, S.R.; Lue, S.J. Separation Mechanisms of Binary Dye Mixtures Using a PVDF Ultrafiltration Membrane: Donnan Effect and Intermolecular Interaction. J. Membr. Sci. 2019, 575, 38–49. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; Gómez, M.; Murcia, M.D.; Serrano, M.; Rodríguez-Schmidt, R.; Escudero, P.A. Behaviour of Polysulfone Ultrafiltration Membrane for Dyes Removal. Water Sci. Technol. 2018, 77, 2093–2100. [Google Scholar] [CrossRef]
- Shishegaran, A.; Boushehri, A.N.; Ismail, A.F. Gene Expression Programming for Process Parameter Optimization during Ultrafiltration of Surfactant Wastewater Using Hydrophilic Polyethersulfone Membrane. J. Environ. Manag. 2020, 264, 110444. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Alzghoul, T.M.; Amr, S.A.; Bangalore Ramu, M.; Nassani, D.E. Bibliometric Insights into Car Wash Wastewater Treatment Research: Trends and Perspectives. Water 2024, 16, 2034. [Google Scholar] [CrossRef]
- Sarmadi, M.; Foroughi, M.; Najafi Saleh, H.; Sanaei, D.; Zarei, A.A.; Ghahrchi, M.; Bazrafshan, E. Efficient Technologies for Carwash Wastewater Treatment: A Systematic Review. Environ. Sci. Pollut. Res. 2020, 27, 34823–34839. [Google Scholar] [CrossRef]
- Torkashvand, J.; Pasalari, H.; Gholami, M.; Younesi, S.; Oskoei, V.; Farzadkia, M. On-Site Carwash Wastewater Treatment and Reuse: A Systematic Review. Int. J. Environ. Anal. Chem. 2022, 102, 3613–3627. [Google Scholar] [CrossRef]
- Ullah, M.; Innocenzi, V.; Ayedi, K.; Vegliò, F.; Ippolito, N.M. Automotive Wastewater Treatment Processes and Technologies: A Review. ACS EST Water 2024, 4, 3663–3680. [Google Scholar] [CrossRef]
- Gryta, M.; Woźniak, P. Polyethersulfone Membrane Fouling Mitigation during Ultrafiltration of Wastewaters from Car Washes. Desalination 2024, 574, 117254. [Google Scholar] [CrossRef]
- Woźniak, P.; Gryta, M. Application of Polymeric Tubular Ultrafiltration Membranes for Separation of Car Wash Wastewater. Membranes 2024, 14, 210. [Google Scholar] [CrossRef]
- Woźniak, P.; Gryta, M. Carwash Oily Wastewater Separated by Ultrafiltration. Separations 2024, 11, 164. [Google Scholar] [CrossRef]
- Moazzem, S.; Wills, J.; Fan, L.; Roddick, F.; Jegatheesan, V. Performance of Ceramic Ultrafiltration and Reverse Osmosis Membranes in Treating Car Wash Wastewater for Reuse. Environ. Sci. Pollut. Res. 2018, 25, 8654–8668. [Google Scholar] [CrossRef] [PubMed]
- Wills, J.; Moazzem, S.; Jegatheesan, V. Treating Car Wash Wastewater by Ceramic Ultrafiltration Membranes for Reuse Purposes. In Water Scarcity and Ways to Reduce the Impact; Pannirselvam, M., Shu, L., Griffin, G., Philip, L., Natarajan, A., Hussain, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 63–73. ISBN 978-3-319-75198-6. [Google Scholar]
- Uçar, D. Membrane Processes for the Reuse of Car Washing Wastewater. J. Water Reuse Desalination 2018, 8, 169–175. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Najafi, M.; Golgoli, M.; Asif, A.H.; Khiadani, M.; Razmjou, A.; Zargar, M. Microplastics and Dye Removal from Textile Wastewater Using MIL-53 (Fe) Metal-Organic Framework-Based Ultrafiltration Membranes. Chemosphere 2024, 364, 143170. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Hruza, J.; Yalcinkaya, F. Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review. Polymers 2021, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, W.; Gryta, M. Application of Capillary Polypropylene Membranes for Microfiltration of Oily Wastewaters: Experiments and Modeling. Fibers 2021, 9, 35. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Long-Term Performance of Ultrafiltration Membranes: Corrosion Fouling Aspect. Materials 2023, 16, 1673. [Google Scholar] [CrossRef] [PubMed]
- Goh, P.S.; Wong, K.C.; Ismail, A.F. Membrane Technology: A Versatile Tool for Saline Wastewater Treatment and Resource Recovery. Desalination 2022, 521, 115377. [Google Scholar] [CrossRef]
- Poonguzhali, E.; Kapoor, A.; Prabhakar, S. Membrane Assisted Process Intensification and Optimization for Removal and Recovery of Phenol from Industrial Effluents. Sep. Purif. Technol. 2023, 319, 124026. [Google Scholar] [CrossRef]
- Zulkefli, N.F.; Alias, N.H.; Jamaluddin, N.S.; Abdullah, N.; Abdul Manaf, S.F.; Othman, N.H.; Marpani, F.; Mat-Shayuti, M.S.; Kusworo, T.D. Recent Mitigation Strategies on Membrane Fouling for Oily Wastewater Treatment. Membranes 2021, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, W.; Gryta, M. Energy-Efficient AnMBRs Technology for Treatment of Wastewaters: A Review. Energies 2022, 15, 4981. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and Cleaning of Ultrafiltration Membranes: A Review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, K.; Wang, X.; Liang, S.; Wei, C.; Wen, X.; Huang, X. Outlining the Roles of Membrane-Foulant and Foulant-Foulant Interactions in Organic Fouling During Microfiltration and Ultrafiltration: A Mini-Review. Front. Chem. 2020, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, A.; Tomaiuolo, G.; Guido, S. Membrane Fouling Phenomena in Microfluidic Systems: From Technical Challenges to Scientific Opportunities. Micromachines 2021, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current Treatment Technologies and Mechanisms for Removal of Indigo Carmine Dyes from Wastewater: A Review. J. Mol. Liq. 2020, 318, 114061. [Google Scholar] [CrossRef]
- Imad, H.U.; Mahar, R.B.; Pathan, A.A.; Khatri, A. Exploring Effective Methods for Indigo Dye Removal and Recovery from Textile Effluent: A Sustainable Approach towards Resource Recovery. Int. J. Environ. Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Gryta, M.; Woźniak, P. Application of Polypropylene Microfiltration Membranes for Separation of Wastewater from Car Wash. Sep. Purif. Technol. 2024, 331, 125707. [Google Scholar] [CrossRef]
- Alventosa-deLara, E.; Barredo-Damas, S.; Alcaina-Miranda, M.I.; Iborra-Clar, M.I. Ultrafiltration Technology with a Ceramic Membrane for Reactive Dye Removal: Optimization of Membrane Performance. J. Hazard. Mater. 2012, 209–210, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Ye, K.; Deng, J.; Lin, J.; Ye, W.; Zhao, S.; Van Der Bruggen, B. Conventional Ultrafiltration As Effective Strategy for Dye/Salt Fractionation in Textile Wastewater Treatment. Environ. Sci. Technol. 2018, 52, 10698–10708. [Google Scholar] [CrossRef]
- Srivastava, H.P.; Arthanareeswaran, G.; Anantharaman, N.; Starov, V.M. Performance of Modified Poly(Vinylidene Fluoride) Membrane for Textile Wastewater Ultrafiltration. Desalination 2011, 282, 87–94. [Google Scholar] [CrossRef]
- Aouni, A.; Fersi, C.; Cuartas-Uribe, B.; Bes-Pía, A.; Alcaina-Miranda, M.I.; Dhahbi, M. Reactive Dyes Rejection and Textile Effluent Treatment Study Using Ultrafiltration and Nanofiltration Processes. Desalination 2012, 297, 87–96. [Google Scholar] [CrossRef]
- Yang, C.; Xu, W.; Nan, Y.; Wang, Y.; Hu, Y.; Gao, C.; Chen, X. Fabrication and Characterization of a High Performance Polyimide Ultrafiltration Membrane for Dye Removal. J. Colloid Interface Sci. 2020, 562, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, P.; Gryta, M. Influence of Reclaimed Water on the Visual Quality of Automotive Coating. Materials 2024, 17, 5382. [Google Scholar] [CrossRef] [PubMed]
- Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C.; González-López, F.; Cuartas-Uribe, B.; Mendoza-Roca, J.A. Treatment of Water from the Textile Industry Contaminated with Indigo Dye: A Hybrid Approach Combining Bioremediation and Nanofiltration for Sustainable Reuse. Case Stud. Chem. Environ. Eng. 2023, 8, 100498. [Google Scholar] [CrossRef]
- Ristea, M.-E.; Zarnescu, O. Indigo Carmine: Between Necessity and Concern. JoX 2023, 13, 509–528. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. The Application of Polyethersulfone Ultrafiltration Membranes for Separation of Car Wash Wastewaters: Experiments and Modelling. Membranes 2023, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Simonič, M. Efficiency of Ultrafiltration for the Pre-Treatment of Dye-Bath Effluents. Desalination 2009, 245, 701–707. [Google Scholar] [CrossRef]
- Luo, J.; Hu, Y.; Guo, X.; Wang, A.; Lin, C.; Zhang, Y.; Wang, H.; Wang, Y.; Tang, X. Effect of In Situ Aeration on Ultrafiltration Membrane Fouling Control in Treating Seasonal High-Turbidity Surface Water. Water 2024, 16, 2195. [Google Scholar] [CrossRef]
- Park, W.; Jeong, S.; Im, S.-J.; Jang, A. High Turbidity Water Treatment by Ceramic Microfiltration Membrane: Fouling Identification and Process Optimization. Environ. Technol. Innov. 2020, 17, 100578. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Comparison of Polypropylene and Ceramic Microfiltration Membranes Applied for Separation of 1,3-PD Fermentation Broths and Saccharomyces Cerevisiae Yeast Suspensions. Membranes 2021, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Majewska-Nowak, K. Fouling of Hydrophilic Ultrafiltration Membranes Applied to Water Recovery from Dye and Surfactant Solutions. Environ. Prot. Eng. 2005, 31, 229–241. [Google Scholar]
- Muthumareeswaran, M.R.; Alhoshan, M.; Agarwal, G.P. Ultrafiltration Membrane for Effective Removal of Chromium Ions from Potable Water. Sci. Rep. 2017, 7, 41423. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, W.; Woźniak, P.; Gryta, M.; Grzechulska-Damszel, J.; Daniluk, M. Cleaning of Ultrafiltration Membranes: Long-Term Treatment of Car Wash Wastewater as a Case Study. Membranes 2024, 14, 159. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).