Hydrogeochemical Evolution, Isotopic Insights, and Genetic Models of Geothermal Water in Anhui Province, China
Abstract
:1. Introduction
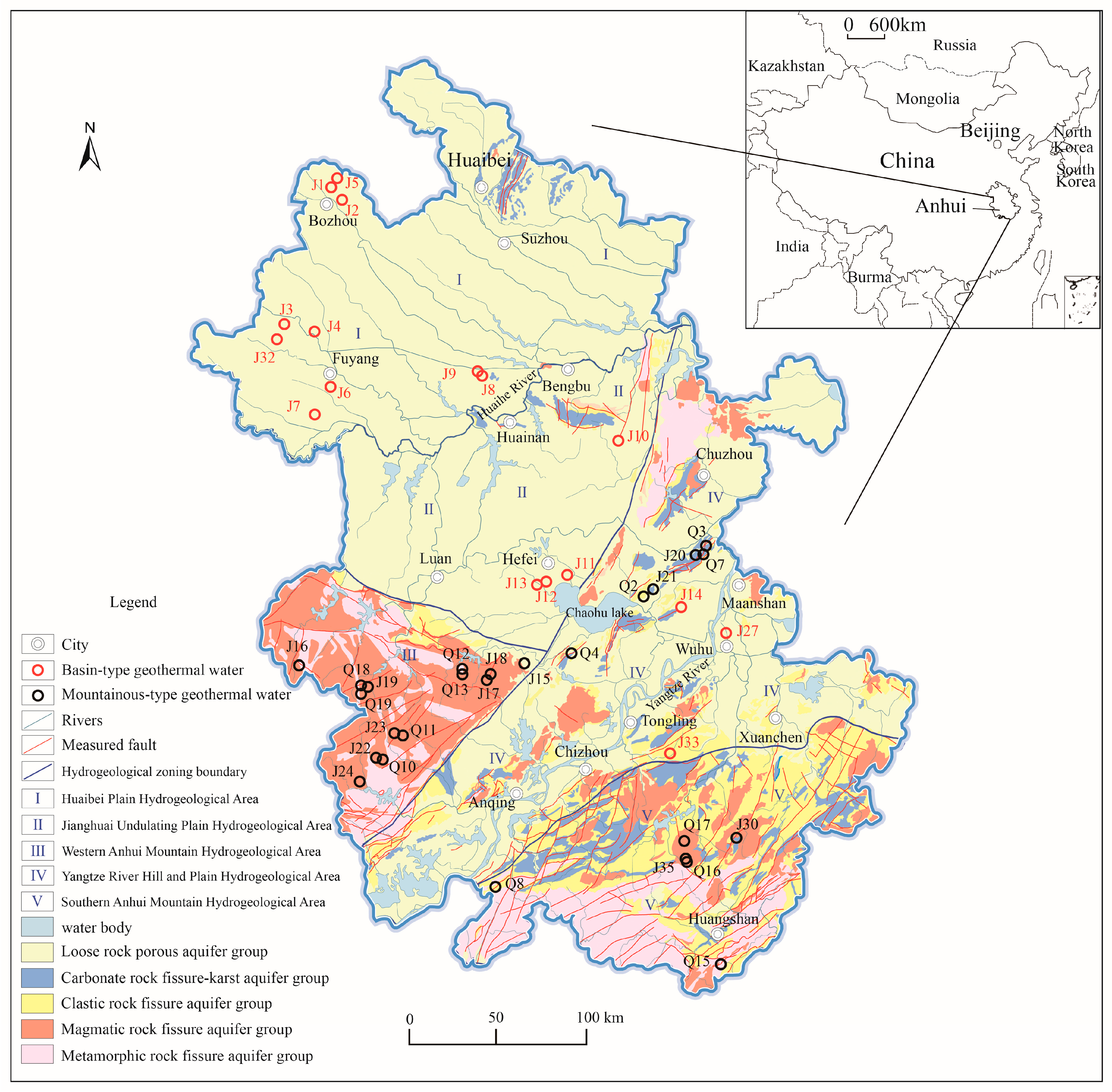
2. Study Area
2.1. Physical Geography
2.2. Geology and Structural Features
2.2.1. Geology
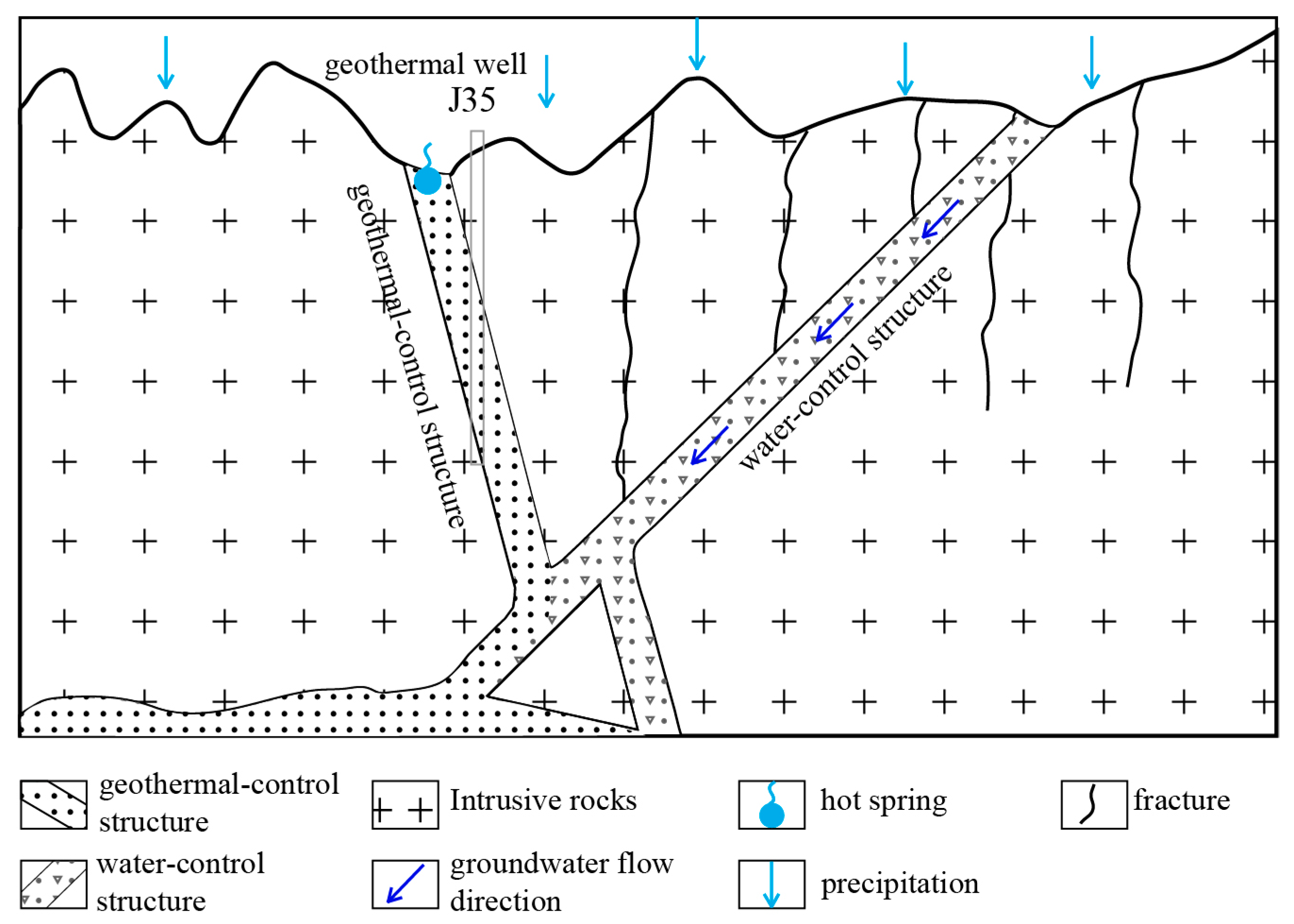
2.2.2. Structural Features
2.3. Geothermal Distribution and Geothermal Geological Conditions
2.4. Sample Collection and Testing
3. Results
3.1. Hydrochemical Characteristics
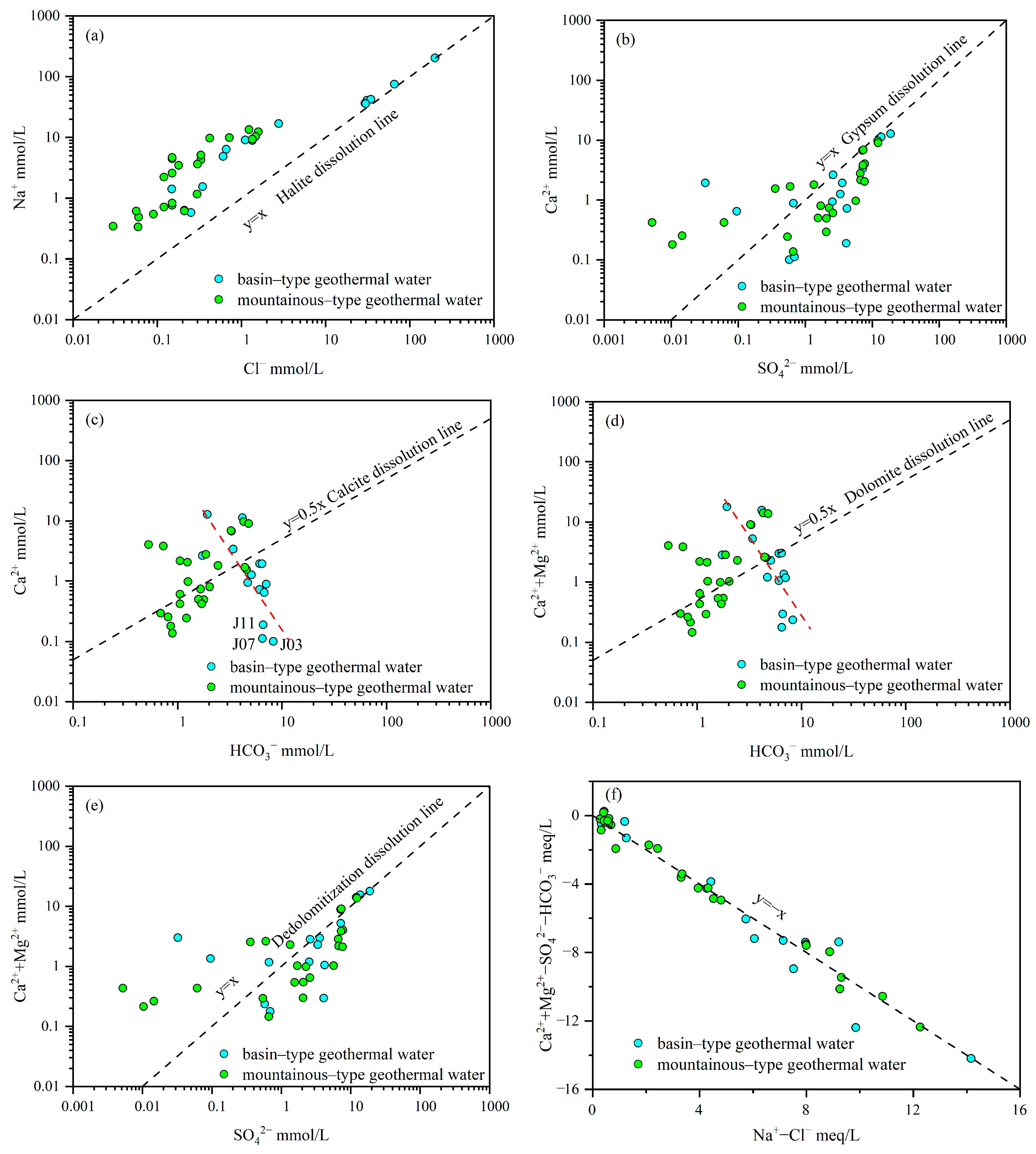
3.2. Hydrochemical Evolution
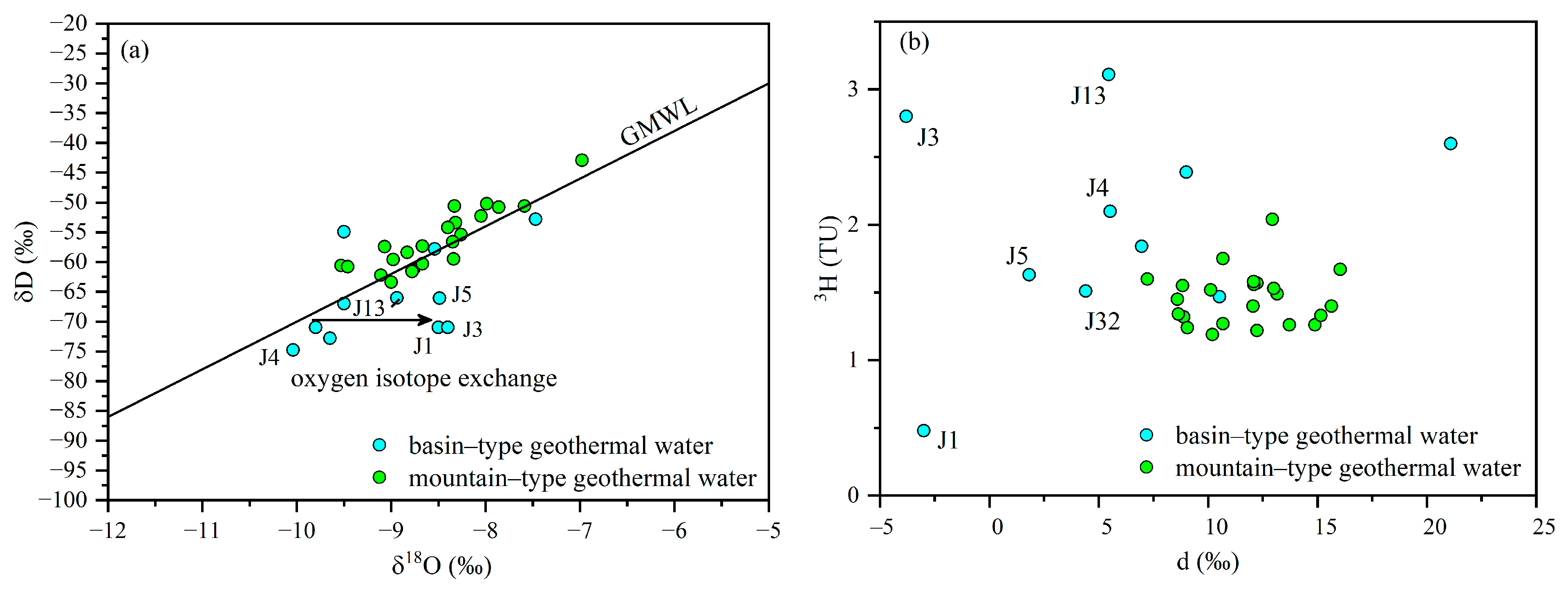
3.3. Hydrogen and Oxygen Isotope Analysis
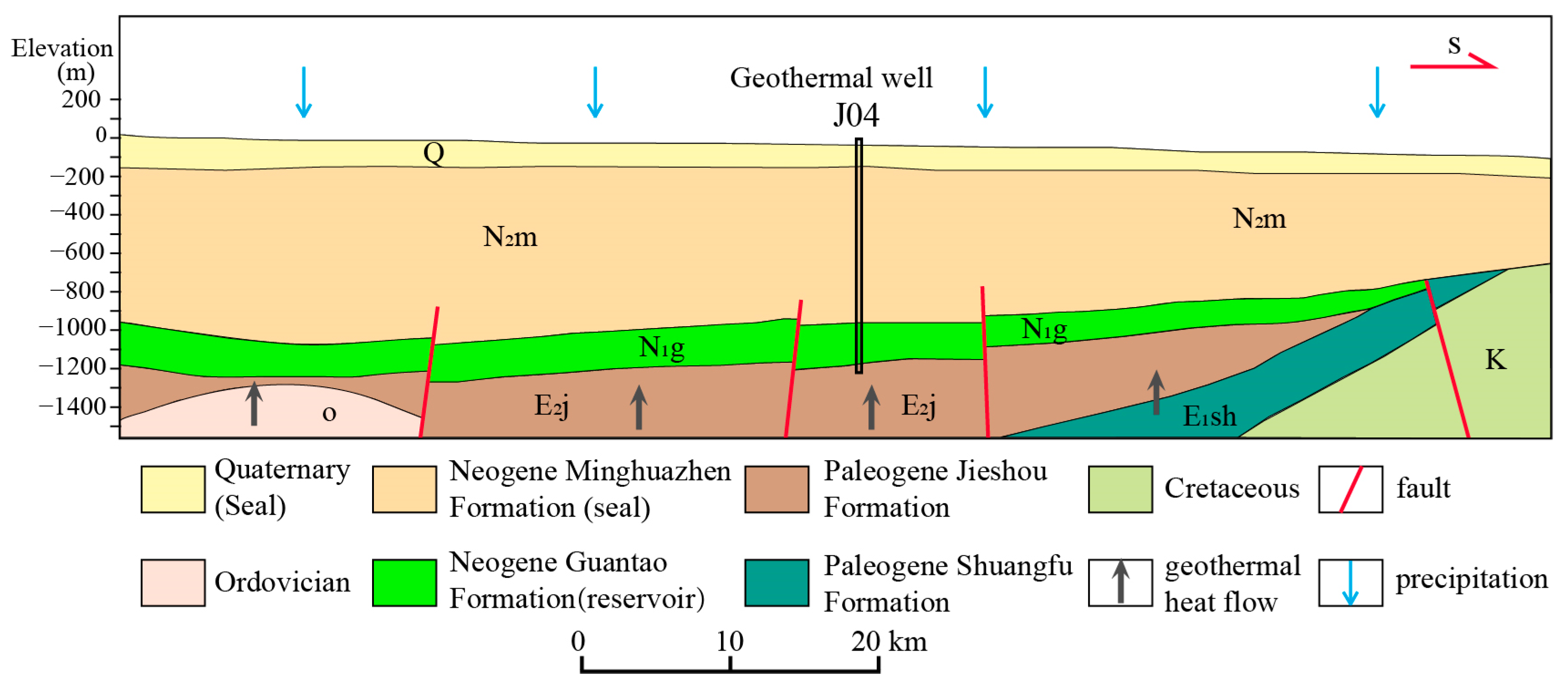
4. Genetic Models of Geothermal Systems
5. Conclusions
- (1)
- Basin–type geothermal water is primarily of the Cl–Na type, characterized by a closed system with slow groundwater flow. Dominant water–rock interactions include halite dissolution, gypsum dissolution, dedolomitization, and silicate hydrolysis.
- (2)
- Mountainous-type geothermal water predominantly exhibits HCO3–Na·Ca, SO4–Na·Ca, and SO4–Na hydrochemical types. It is characterized by an open system with fast groundwater flow. The major chemical components result from calcite dissolution, dolomite dissolution, gypsum dissolution, halite dissolution, dedolomitization, and silicate hydrolysis.
- (3)
- Isotope analysis indicates that both types of geothermal water receive mixed recharge from sub-modern and modern waters. However, the basin-type system is more closed off, with limited recharge and renewal capacity, whereas the mountainous-type system benefits from fault-induced fractures, allowing for sufficient recharge.
- (4)
- Geothermal resources in Anhui Province exhibit two primary genetic models: Basin-type geothermal resources are formed in closed reservoirs with restricted recharge. Mountainous-type geothermal resources are controlled by fault structures, with efficient precipitation recharge and faster groundwater flow.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, Y.; Lei, Y.; Liu, J. Economic impacts of the geothermal industry in Beijing, China: An input–output approach. Math. Geosci. 2019, 51, 353–372. [Google Scholar] [CrossRef]
- Subić, J.; Nastić, L.; Bekić, B. Economic effects of using geothermal waters in the production of flowers and vegetables in glasshouses. Proc. Econ. Financ. 2015, 22, 131–140. [Google Scholar] [CrossRef]
- Gomes, L.M.F.; Guedes, J.F.; Da Costa, T.C.G.; Ferreira, P.J.C.; Trota, A.P.N. Geothermal potential of Portuguese granitic rock masses: Lessons learned from deep boreholes. Environ. Earth Sci. 2014, 73, 2963–2979. [Google Scholar] [CrossRef]
- Begy, R.C.; Savin, C.F.; Suele, D.K.; Giagias, E. Radiological survey of geothermal water resources in Romania and dose estimation from their use in balneotherapy. Eur. Phys. J. Spec. Top. 2023, 232, 1595–1605. [Google Scholar] [CrossRef]
- Hussein, M.T.; Lashin, A.; Bassam, A.A.; Arifi, N.A.; Zahrani, I.A. Geothermal power potential at the western coastal part of Saudi Arabia. Renew. Sustain. Energy Rev. 2013, 26, 668–684. [Google Scholar] [CrossRef]
- Mishra, G.S.; Glassley, W.E.; Yeh, S. Realizing the geothermal electricity potential–water use and consequences. Environ. Res. Lett. 2011, 6, 034023. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.; Tomaszewska, B.; Operacz, A. Sustainable utilization of low enthalpy geothermal resources to electricity generation through a cascade system. Energies 2020, 13, 2495. [Google Scholar] [CrossRef]
- Banks, D. Thermogeological assessment of open–loop well–doublet schemes: A review and synthesis of analytical approaches. Hydrogeol. J. 2009, 17, 1149–1155. [Google Scholar] [CrossRef]
- Halilovic, S.; Bttcher, F.; Zosseder, K.; Hamacher, T. Spatial analysis of thermal groundwater use based on optimal sizing and placement of well doublets. Energy 2024, 304, 132058. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, C.; Ji, B.; Zhang, R.; Niu, J. A simplified assessment method for estimating recoverable geothermal resources. Geothermics 2019, 79, 145–151. [Google Scholar] [CrossRef]
- Ciurba, A.P.; Haidu, I.; Ianc, D. Administrative Aspects Regarding the Valorisation of Geothermal Waters for Balneological Purposes in Bihor County, Romania. Sustainability 2023, 15, 10320. [Google Scholar] [CrossRef]
- Yousefi, H.; Roumi, S.; Armannsson, H.; Noorollahi, Y. Cascading uses of geothermal energy for a sustainable energy supply for Meshkinshahr city, northwest, Iran. Geothermics 2019, 79, 152–163. [Google Scholar] [CrossRef]
- Zhou, X.; Zhuo, L.; Wu, Y.; Tao, G.; Ma, J.; Jiang, Z.; Sui, L.; Wang, Y.; Wang, C.; Cui, J. Origin of some hot springs as conceptual geothermal models. J. Hydrol. 2023, 624, 129927. [Google Scholar] [CrossRef]
- Li, J.; Yang, G.; Sagoe, G.; Li, Y. Major hydrogeochemical processes controlling the composition of geothermal waters in the Kangding geothermal field, western Sichuan Province. Geothermics 2018, 75, 154–163. [Google Scholar] [CrossRef]
- Duan, R.; Li, P.; Wang, L.; He, X.; Zhang, L. Hydrochemical characteristics, hydrochemical processes and recharge sources of the geothermal systems in Lanzhou city, northwestern China. Urban Clim. 2022, 43, 101152. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Chen, K. Geothermal geology characteristic and origin analysis of Shilin basin in Yunnan province. Adv. Mater. Res. 2013, 779, 1449–1452. [Google Scholar] [CrossRef]
- Yan, B.; Qiu, S.; Liu, Z.; Xiao, C. Characteristics of the geothermal water in changbai mountain volcanic region, northeast of China. Arab. J. Geosci. 2017, 10, 261. [Google Scholar] [CrossRef]
- Chen, L.; Ma, T.; Du, Y.; Xiao, C.; Chen, X.; Liu, C.; Wang, Y. Hydrochemical and isotopic (2H, 18O and 37Cl) constraints on evolution of geothermal water in coastal plain of southwestern Guangdong province, China. J. Volcanol. Geotherm. Res. 2016, 318, 45–54. [Google Scholar] [CrossRef]
- Ma, Z.; Li, X.; Zheng, H.; Li, J.; Pei, B.; Guo, S.; Zhang, X. Origin and classification of geothermal water from Guanzhong basin, NW China: Geochemical and isotopic approach. J. Earth Sci. 2017, 28, 719–728. [Google Scholar] [CrossRef]
- Hao, Y.; Pang, Z.; Kong, Y.; Tian, J.; Wang, Y.; Liao, D.; Fan, Y. Chemical and isotopic constraints on the origin of saline waters from a hot spring in the eastern coastal area of China. Hydrogeol. J. 2020, 28, 2457–2475. [Google Scholar] [CrossRef]
- Vengosh, A.; Helvacı, C.; Karamanderesi, I.H. Geochemical constraints for the origin of thermal waters from western turkey. Appl. Geochem. 2002, 17, 163–183. [Google Scholar] [CrossRef]
- Levet, S.; Toutain, J.P.; Munoz, M.; Berger, G.; Negrel, P.; Jendrzejewski, N.; Agrinier, P.; Sortino, F. Geochemistry of the Bagneres-de-Bigorre thermal waters from the North Pyrenean Zone (France). Geofluids 2002, 2, 25–40. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Li, Q.; You, S. Hydrochemical and isotope characteristics of spring water and travertine in the Baishuitai area (SW China) and their meaning for paleoenvironmental reconstruction. Environ. Geol. 2003, 44, 698–704. [Google Scholar] [CrossRef]
- Bianchini, G.; Pennisi, M.; Cioni, R.; Muti, A.; Cerbai, N.; Kloppmann, W. Hydrochemistry of the high–boron groundwaters of the Cornia aquifer (Tuscany, Italy). Geothermics 2005, 34, 297–319. [Google Scholar] [CrossRef]
- Petrini, R.; Italiano, F.; Ponton, M.; Slejko, F.; Aviani, U.; Zini, L. Geochemistry and isotope geochemistry of the Monfalcone thermal waters (northern Italy): Inference on the deep geothermal reservoir. Hydrogeol. J. 2013, 21, 1275–1287. [Google Scholar] [CrossRef]
- Lambrakis, N.; Zagana, E.; Katsanou, K. Geochemical patterns and origin of alkaline thermal waters in Central Greece (Platystomo and Smokovo areas). Environ. Earth Sci. 2013, 69, 2475–2486. [Google Scholar] [CrossRef]
- Haklidir, F.T. Hydrogeochemical evaluation of thermal, mineral and cold waters between Bursa city and Mount Uludağ in the South Marmara region of Turkey. Geothermics 2013, 48, 132–145. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.; Xing, L.; Li, T.; Zhao, J. Geochemical response of deep geothermal processes in the Litang region, western Sichuan. Energy Explor. Exploit. 2019, 37, 626–645. [Google Scholar] [CrossRef]
- Shoedarto, R.M.; Tada, Y.; Kashiwaya, K.; Koike, K.; Iskandar, I. Specifying recharge zones and mechanisms of the transitional geothermal field through hydrogen and oxygen isotope analyses with consideration of water–rock interaction. Geothermics 2020, 86, 101797. [Google Scholar] [CrossRef]
- Li, Y.; Dor, J.; Zhang, C.; Wang, G.; Zhang, B.; Zhang, F.; Xing, Y. Genesis of the Xifeng Low-Temperature Geothermal Field, Guizhou, SW China: Constrains From Geology, Element Geochemistry, and D-O Isotopes. Front. Earth Sci. 2021, 9, 782943. [Google Scholar] [CrossRef]
- Bao, Y.; Pang, Z.; Li, Y.; Tian, J.; Luo, J.; Fan, Y.; Yang, F.; Qian, T.; Chen, F.; Sun, C.; et al. Gas isotope geochemistry of Tangquan hot spring system in Nanjing (China). Appl. Geochem. 2024, 173, 106118. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Chen, A. The Geochemical Characteristics and Genesis Mechanisms of the Zaozigou Geothermal Field. Sustainability 2024, 16, 6790. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, X.; Chen, Y.; Liu, J.; Zhan, C.; Lv, G.; Hu, J.; Sun, M.; Zhang, Y. Geochemical Evidence Constraining Genesis and Mineral Scaling of the Yangbajing Geothermal Field, Southwestern China. Water 2024, 16, 24. [Google Scholar] [CrossRef]
- Yin, H.Y.; Zhou, W.; Dong, F.; Liu, Y.; Shi, C.; Meng, M.; Cheng, W. Hydrochemical characteristics and genetic mechanism of porous sandstone geothermal water in northern Jinan, Shandong, China. Environ. Sci. Pollut. Res. 2024, 31, 24180–24196. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, D.; Wei, W.; Fang, T.; Cheng, C.; Cheng, P.; Gao, X.; Song, Y.; Huang, J. Study on hydrochemical characteristics of underground hot water in typical granite thermal storage and hot water development model in Dabie mountain, Anhui province. Environ. Earth Sci. 2024, 83, 138. [Google Scholar] [CrossRef]
- Xu, J.; Gui, H.; Chen, J.; Chen, L.; Li, Y.; Zhao, C.; Guo, Y. Hydrogeochemical characteristics and formation mechanisms of the geothermal water in the Qingdong coal mine, northern Anhui province, China. Mine Water Environ. 2022, 41, 1015–1026. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Y.; Cheng, H.; Kang, J.; Liao, A. Characteristics and potential evaluation of geothermal resources in Anhui of Yangtze River Economic Zone. Geol. China 2022, 49, 1765–1777. (In Chinese) [Google Scholar]
- Fang, Z.; Huang, X.; Wang, X.; Yang, Y.; Ni, H.; Zhang, B. The post seismic effect of far-field strong earthquakes of water radon and its mechanism analysis for L01 well of Lujiang geothermal hot spring. Acta Seismol. Sin. 2020, 42, 732–744. (In Chinese) [Google Scholar]
- Su, L.; Zhou, X.; Li, Z.; Xu, Y.; Jiang, Z. Hydrochemical and isotopic characteristics and genesis of hot springs in the Chuhe fault zone, Anhui. Geol. Rev. 2022, 68, 2022030009. (In Chinese) [Google Scholar]
- Armannsson, H. The fluid geochemistry of Icelandic high temperature geothermal areas. Appl. Geochem. 2016, 66, 14–64. [Google Scholar] [CrossRef]
- Lu, L.; Pang, Z.; Kong, Y.; Guo, Q.; Wang, Y. Geochemical and Isotopic Evidence on the recharge and circulation of geothermal water in the Tangshan geothermal system near Nanjing, China: Implications for sustainable development. Hydrogeol. J. 2018, 26, 1705–1719. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water–analyses. Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar]
- Hanshaw, B.B.; Back, W.; Deike, R.G. A geochemical hypothesis for dolomitization by ground water. Econ. Geol. 1971, 66, 710–724. [Google Scholar] [CrossRef]
- Back, W.; Hanshaw, B.B. Comparison of chemical hydrogeology of the carbonate peninsulas of Florida and Yucatan. J. Hydrol. 1970, 10, 330–368. [Google Scholar] [CrossRef]
- Hanshaw, B.B.; Back, W. Major geochemical processes in the evolution of carbonate–aquifer systems. J. Hydrol. 1979, 43, 287–312. [Google Scholar] [CrossRef]
- Gastmans, D.; Chang, H.K.; Hutcheon, I. Groundwater geochemical evolution in the northern portion of the Guarani Aquifer System (Brazil) and its relationship to diagenetic features. Appl. Geochem. 2010, 25, 16–33. [Google Scholar] [CrossRef]
- Toth, J. A theoretical analysis of groundwater flow in small drainage basins. J. Geophys. Res. 1963, 68, 4795–4812. [Google Scholar] [CrossRef]
- Yin, G.; Ni, S. Deuterium excess parameter and geohydrology significance. Bull. Mineral. Petrol. Geochem. 2001, 20, 409–411. [Google Scholar]
- Dansgaard, W.; Johnson, S.J.; Clausen, H.B.; Dahl-Jensen, D.; Gundestrup, N.; Hammer, C.U.; Oeschger, H. North Atlantic climatic oscillations revealed by deep Greenland ice cores, Climate Processes and Climate Sensitivity, Geophys. Clim. Process. Clim. Sensit. 1984, 29, 288–298. [Google Scholar]
- Wang, X.; Qiao, W.; Chen, J.; Liu, X.; Yang, F. Understanding the Burial and Migration Characteristics of Deep Geothermal Water Using Hydrogen, Oxygen, and Inorganic Carbon Isotopes. Water 2018, 10, 7. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmengtal Isotopes in Hydrogeology; Lewis Publishers: Boca Raton, FL, USA, 1997; pp. 27–271. [Google Scholar]
- Cook, P.G. Introduction to Isotopes and Environmengtal Tracers as Indicators of Groundwater Flow; The Groundwater Project: Guelph, ON, Canada, 2020. [Google Scholar]



| Category | Parameter | No. of Samples | Min | Max | Mean | Median | Std. | Skewness |
|---|---|---|---|---|---|---|---|---|
| Basin-type geothermal water | T (°C) | 14 | 25.50 | 53.00 | 36.66 | 32.1 | 9.12 | 0.55 |
| pH | 14 | 7.10 | 8.68 | 7.93 | 7.98 | 0.52 | −0.22 | |
| K (mg/L) | 14 | 1.26 | 44.00 | 7.63 | 4.86 | 11.25 | 3.00 | |
| Na (mg/L) | 14 | 13.27 | 4686.00 | 795.34 | 299.23 | 1227.10 | 2.78 | |
| Ca (mg/L) | 14 | 4.01 | 512.24 | 111.23 | 44.28 | 162.35 | 2.06 | |
| Mg (mg/L) | 14 | 1.59 | 123.83 | 28.50 | 12.52 | 38.50 | 1.90 | |
| Cl (mg/L) | 14 | 5.27 | 7068.74 | 1005.87 | 73.23 | 1884.67 | 2.92 | |
| SO4 (mg/L) | 14 | 3.10 | 1810.00 | 426.50 | 286.16 | 526.83 | 1.88 | |
| HCO3 (mg/L) | 14 | 105.59 | 504.00 | 326.10 | 371.38 | 118.53 | −0.71 | |
| TDS (mg/L) | 14 | 353.70 | 14,817.21 | 2607.85 | 1602.46 | 3766.78 | 2.98 | |
| 18O‰ | 11 | −10.04 | −7.47 | −8.98 | −8.94 | 0.78 | 0.44 | |
| D‰ | 11 | −74.80 | −52.80 | −65.93 | −67 | 7.51 | 0.79 | |
| 3H (TU) | 10 | 0.48 | 3.11 | 1.99 | 1.97 | 0.77 | −0.47 | |
| d‰ | 11 | −3.80 | 21.10 | 5.94 | 5.52 | 6.77 | 0.75 | |
| Mountainous-type geothermal water | Parameter | No. of Samples | Min | Max | Mean | Median | Std. | Skewness |
| T (°C) | 25 | 28.50 | 66.00 | 46.76 | 47 | 10.45 | 0.19 | |
| pH | 25 | 6.90 | 8.86 | 7.95 | 8.22 | 0.57 | −0.31 | |
| K (mg/L) | 25 | 0.05 | 16.76 | 5.35 | 4.58 | 4.21 | 0.93 | |
| Na (mg/L) | 25 | 7.70 | 310.00 | 94.58 | 59.3 | 97.37 | 1.01 | |
| Ca (mg/L) | 25 | 5.50 | 387.70 | 93.37 | 39.01 | 113.02 | 1.59 | |
| Mg (mg/L) | 25 | 0.10 | 114.02 | 16.39 | 1.2 | 32.22 | 2.36 | |
| Cl (mg/L) | 25 | 0.1 | 56.39 | 13.99 | 6.38 | 16.97 | 1.69 | |
| SO4 (mg/L) | 25 | 0.50 | 1175.77 | 370.51 | 199.7 | 367.39 | 0.81 | |
| HCO3 (mg/L) | 25 | 32.17 | 292.26 | 119.72 | 96.5 | 81.84 | 1.09 | |
| TDS (mg/L) | 25 | 97.00 | 1929.00 | 724.96 | 457 | 569.51 | 0.68 | |
| 18O‰ | 23 | −9.53 | −6.98 | −8.53 | −8.67 | 0.59 | 0.64 | |
| D‰ | 23 | −63.40 | −42.90 | −56.55 | −57.4 | 5.16 | 0.88 | |
| 3H (TU) | 23 | 1.19 | 2.04 | 1.46 | 1.45 | 0.20 | 1.02 | |
| d‰ | 23 | 7.22 | 16.04 | 11.66 | 12.08 | 2.48 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Peng, Y.; Li, Y.; Sang, H. Hydrogeochemical Evolution, Isotopic Insights, and Genetic Models of Geothermal Water in Anhui Province, China. Water 2025, 17, 236. https://doi.org/10.3390/w17020236
Zhou X, Peng Y, Li Y, Sang H. Hydrogeochemical Evolution, Isotopic Insights, and Genetic Models of Geothermal Water in Anhui Province, China. Water. 2025; 17(2):236. https://doi.org/10.3390/w17020236
Chicago/Turabian StyleZhou, Xiaoping, Yinxue Peng, Yunfeng Li, and Honghui Sang. 2025. "Hydrogeochemical Evolution, Isotopic Insights, and Genetic Models of Geothermal Water in Anhui Province, China" Water 17, no. 2: 236. https://doi.org/10.3390/w17020236
APA StyleZhou, X., Peng, Y., Li, Y., & Sang, H. (2025). Hydrogeochemical Evolution, Isotopic Insights, and Genetic Models of Geothermal Water in Anhui Province, China. Water, 17(2), 236. https://doi.org/10.3390/w17020236





