Rainwater Treatment Using Ecological Buffer Zones: Influence of Plant and Filler Collocation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory-Scale Experimental Setup
2.2. Experimental Materials
2.3. Experimental Design
2.4. Methods and Measurement
3. Results and Discussion
3.1. Removal Efficiency of Different Fillers for Initial Rainwater Pollutants Subsection
3.1.1. Effect of Different Fillers on the Removal of Total Nitrogen (TN) and Ammonium Nitrogen (NH4+-N)
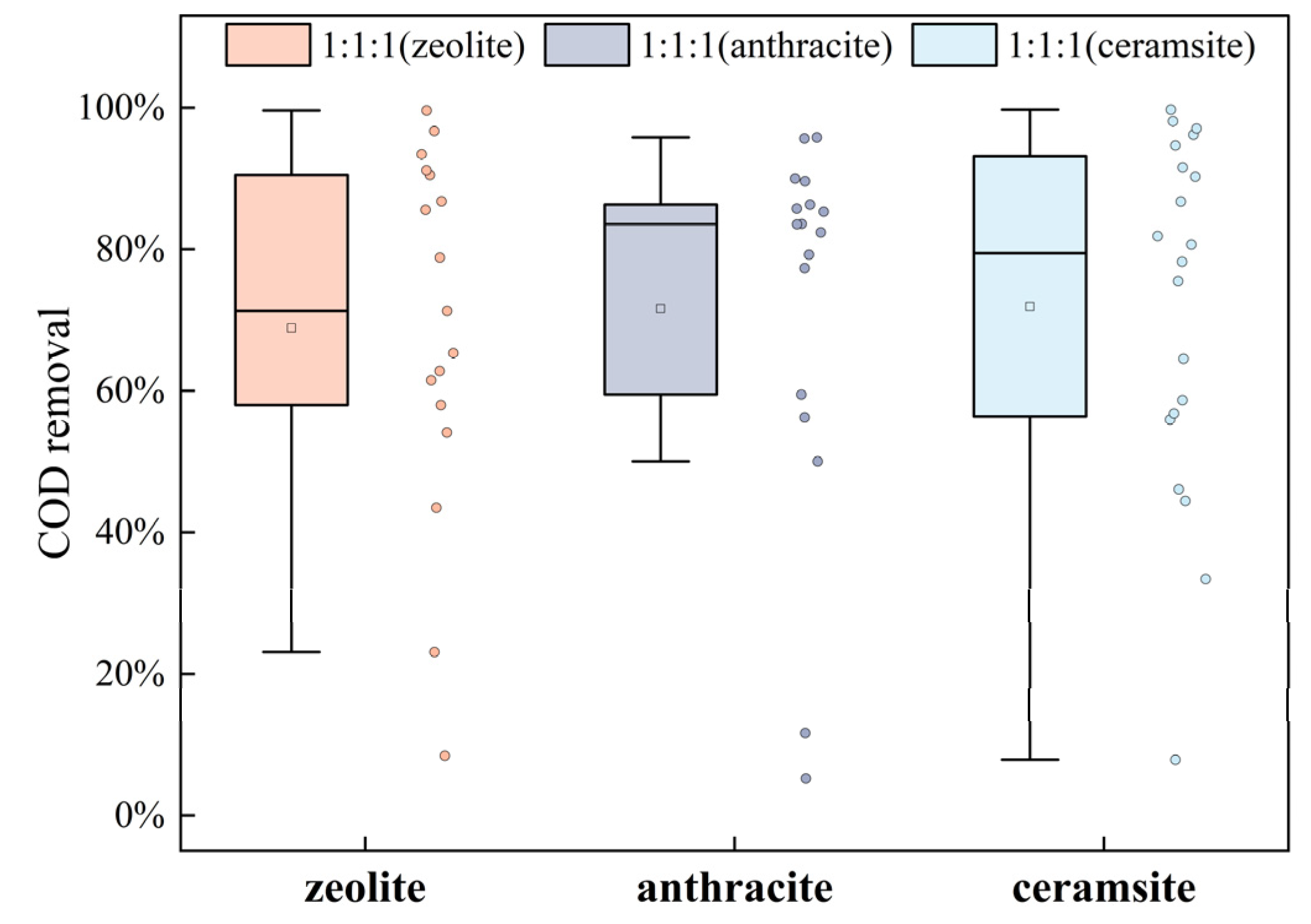
3.1.2. Effect of Different Fillers on Total Phosphorus (TP) and Chemical Oxygen Demand (COD) Removal
3.2. Microscopic Properties of the Fillers
3.2.1. Scanning Electron Microscope (SEM)
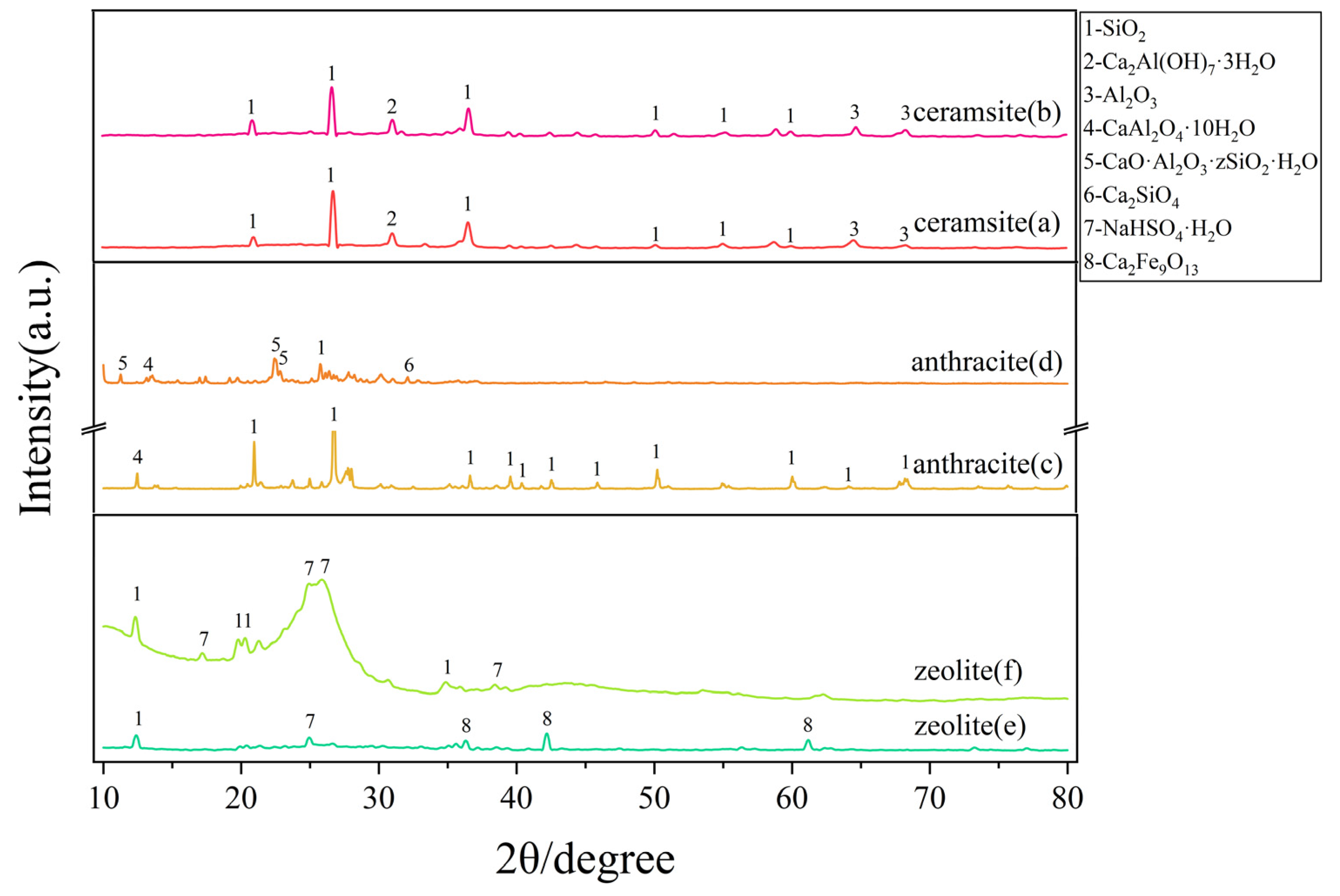
3.2.2. X-Ray Diffraction (XRD)
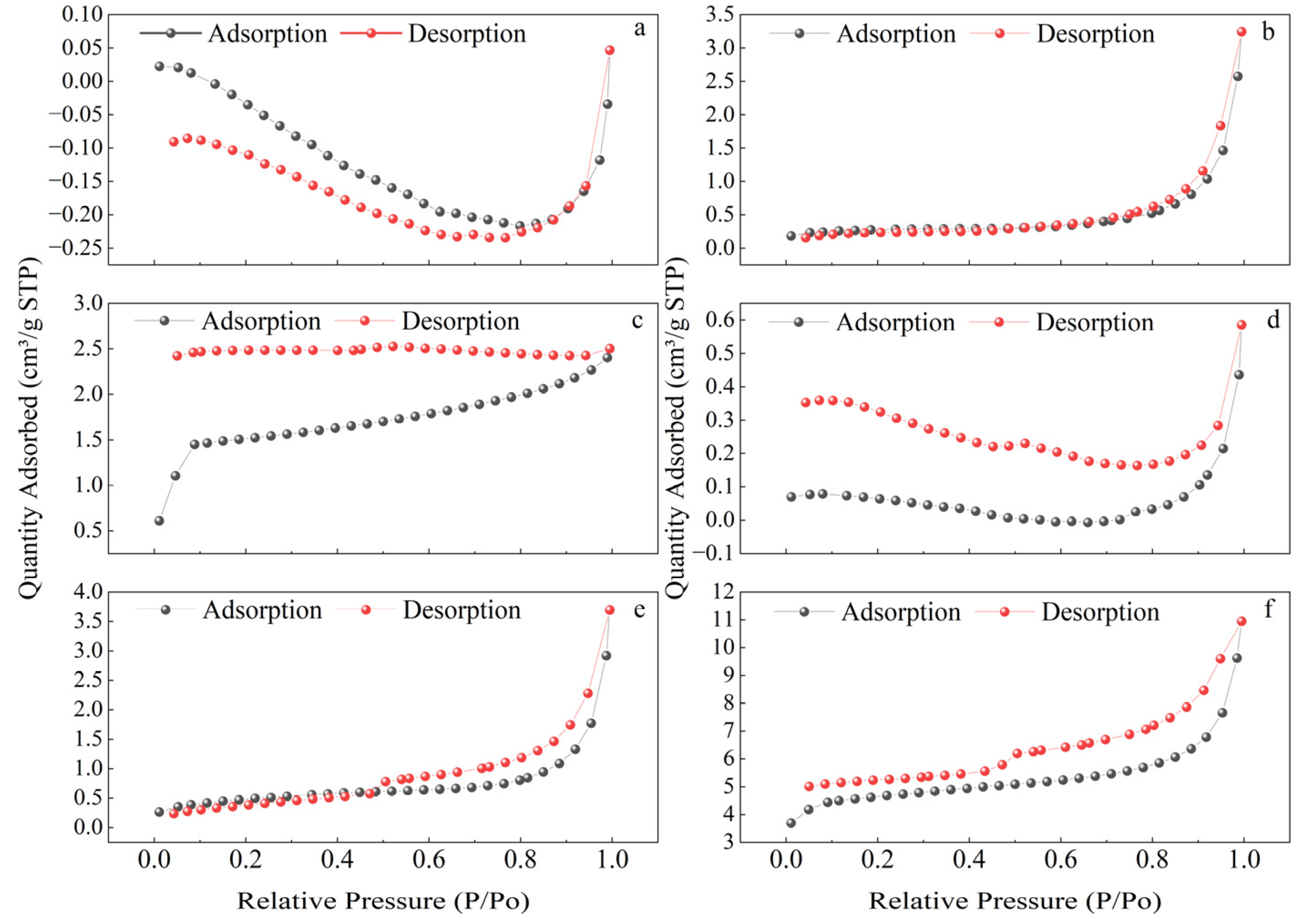
3.2.3. Specific Surface Area Measurement (BET)
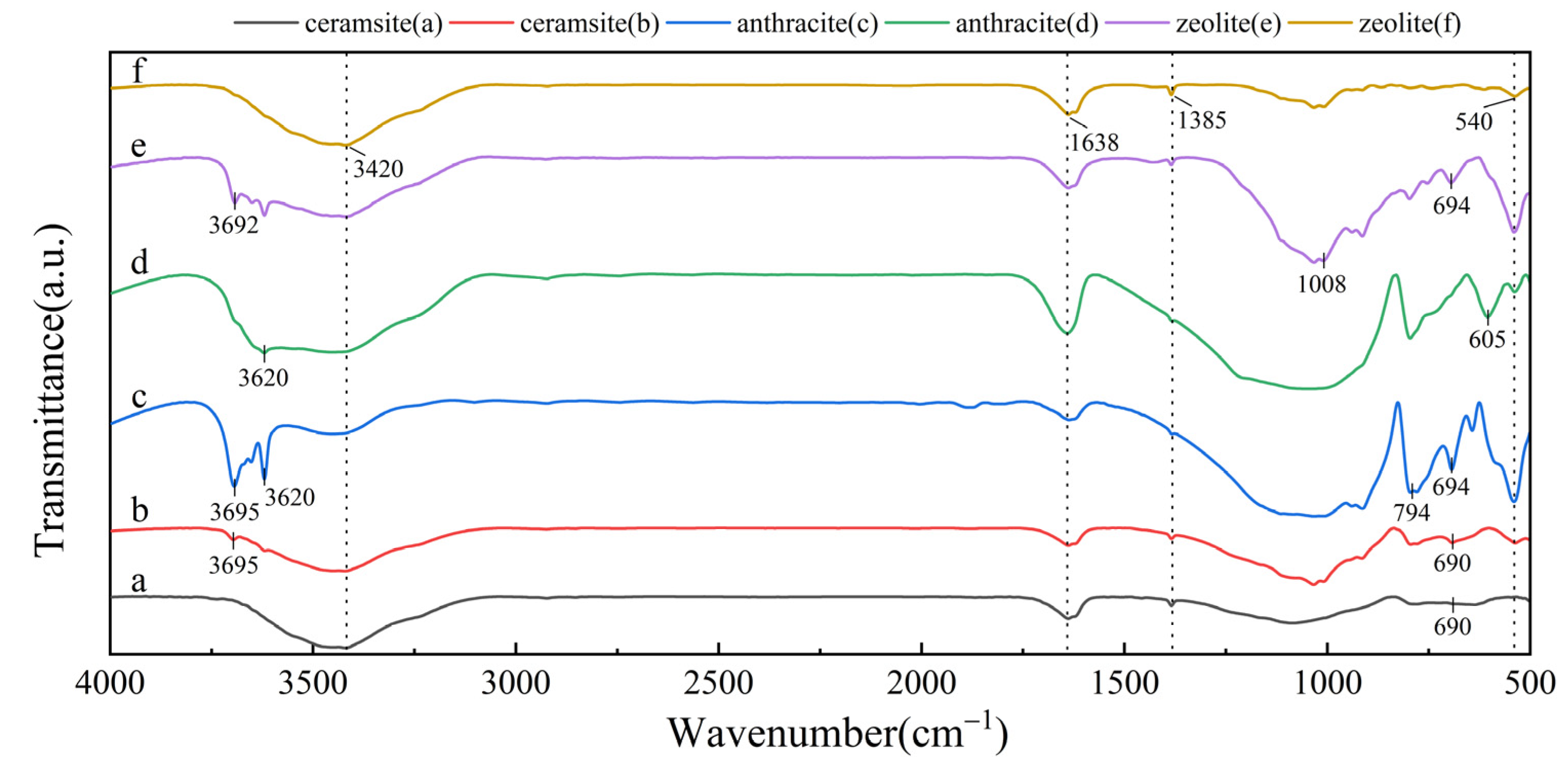
3.2.4. Fourier Transform Infrared Spectrometer (FTIR)
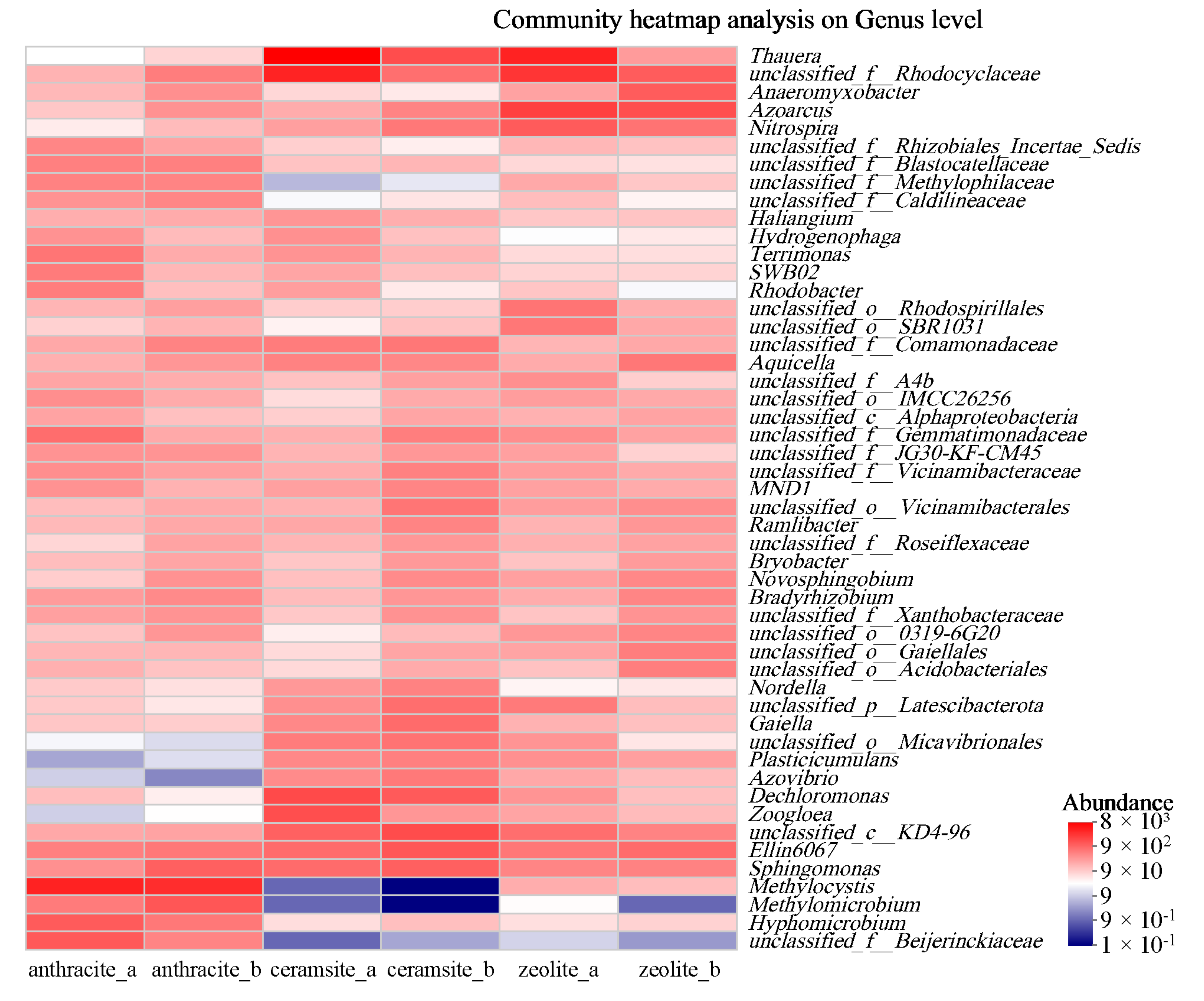
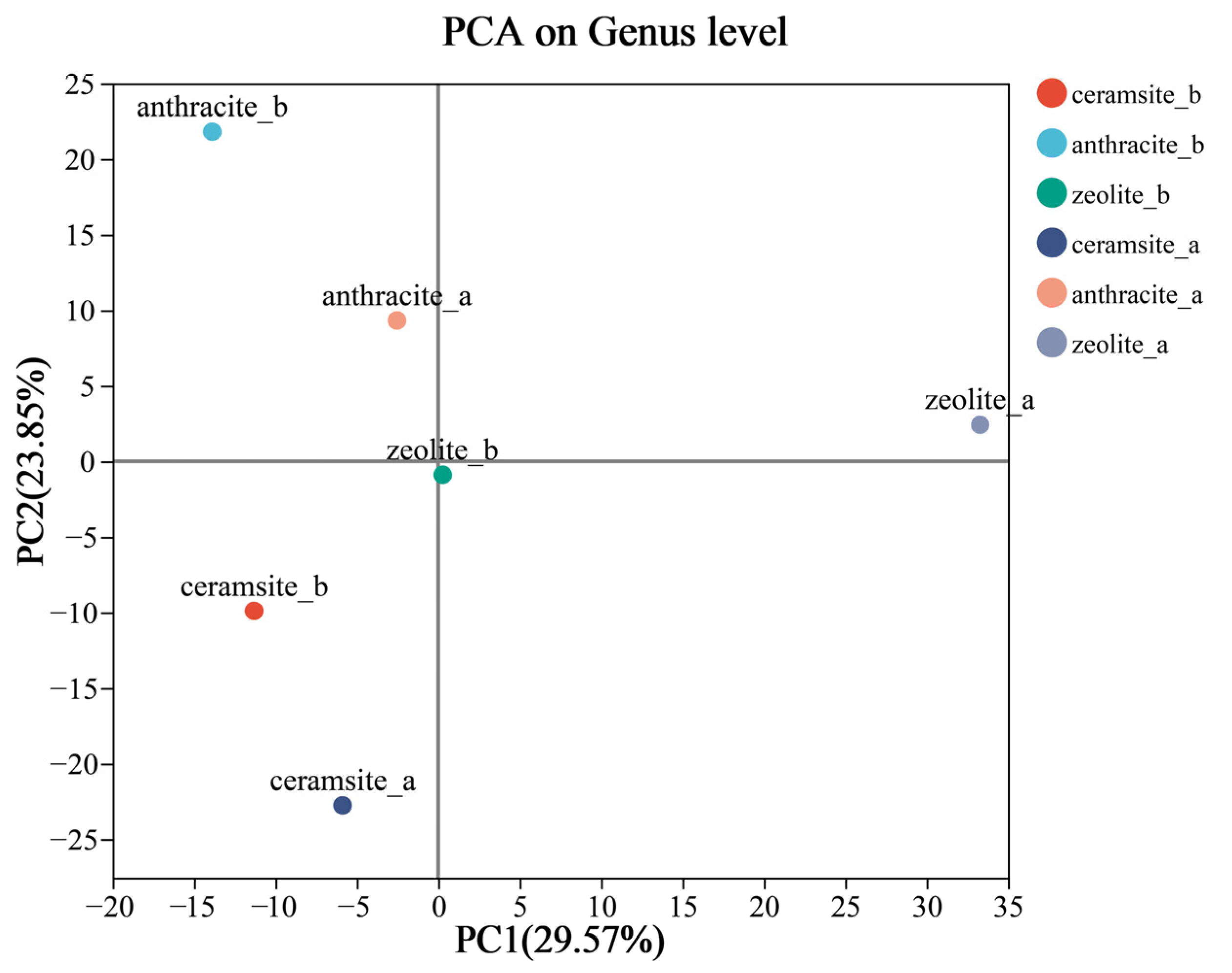
3.3. Microbial Community Diversity
4. Conclusions
- (1)
- In the plant combination of Pennisetum hybridum, Canna, and Lythrum virgatum (1:1:1), zeolite and ceramsite performed well in nitrogen removal, with zeolite achieving a total nitrogen removal rate of 96.79% and ammonium nitrogen removal rate of 92.77%, and ceramsite achieving total nitrogen removal rate of 93.76% and ammonium nitrogen removal rate of 91.49%. Ceramsite was more effective in removing total phosphorus 83.64% and COD 71.67%. Based on these results, a mixture of zeolite and ceramsite is recommended as the filler combination for the ecological buffer zone;
- (2)
- In the treatment of nitrogen-containing wastewater, zeolite not only adsorbs ammonium nitrogen but also achieves total nitrogen removal through an adsorption-desorption mechanism combined with biological processes. The presence of quartz and calcium aluminate hydrates in ceramsite, along with its larger specific surface area, contributes to the adsorption and precipitation of phosphorus. Additionally, the strong surface chemical adsorption capacity of quartz in ceramsite is one of the key factors behind its superior performance in COD removal;
- (3)
- The dominant microbial phyla in all three fillers were Bacteroidetes, Proteobacteria, and Actinobacteria. The dominant genera in ceramsite were Thauera and Dechloromonas, while in zeolite, the dominant genera were unclassified Rhodocyclaceae and Azoarcus. These genera played a significant role in the removal of initial stormwater pollutants;
- (4)
- Although synthetic rainwater was used in this study, the presence of suspended solids and colloids in real-world conditions could significantly affect the adsorption process and may lead to the clogging of porous media. This aspect should be addressed in future studies for more realistic assessments.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorgoglione, A.; Bombardelli, F.A.; Pitton, B.J.L.; Oki, L.R.; Haver, D.L.; Young, T.M. Uncertainty in the parameterization of sediment build-up and wash-off processes in the simulation of sediment transport in urban areas. Environ. Model. Softw. 2019, 111, 170–181. [Google Scholar] [CrossRef]
- Rio, M.; Salles, C.; Cernesson, F.; Marchand, P.; Tournoud, M.-G. An original urban land cover representation and its effects on rain event-based runoff and TSS modelling. J. Hydrol. 2020, 586, 124865. [Google Scholar] [CrossRef]
- Naves, J.; Rieckermann, J.; Cea, L.; Puertas, J.; Anta, J. Global and local sensitivity analysis to improve the understanding of physically-based urban wash-off models from high-resolution laboratory experiments. Sci. Total Environ. 2020, 709, 136152. [Google Scholar] [CrossRef]
- Deletic, A. The first flush load of urban surface runoff. Water Res. 1998, 32, 2462–2470. [Google Scholar] [CrossRef]
- Sánchez, A.; Cohim, E.; Kalid, R. A review on physicochemical and microbiological contamination of roof-harvested rainwater in urban areas. Sustain. Water Qual. Ecol. 2015, 6, 119–137. [Google Scholar] [CrossRef]
- Li, A.; Ji, G.; Xu, C.; Lichtfouse, E.; Huang, J.; Liu, H. Elevated purification of urban rainwater runoff using a calamus constructed wetland with multi-layer carrier fillers. J. Water Process Eng. 2023, 56, 104273. [Google Scholar] [CrossRef]
- Zheng, Z.; Duan, X.; Lu, S. The application research of rainwater wetland based on the Sponge City. Sci. Total Environ. 2021, 771, 144475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.; Hou, P.; Wan, W.; Ren, Y.; Ouyang, Z.; Yang, L. The temporal changes in road stormwater runoff quality and the implications to first flush control in Chongqing, China. Environ. Monit. Assess. 2013, 185, 9763–9775. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y. Design Strategy of Emergency Pools at Source Water Protection Areas. J. Archit. Res. Dev. 2022, 6, 35–40. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Zhou, K.; Feng, P.; Dong, L. The effects of surface pollution on urban river water quality under rainfall events in Wuqing district, Tianjin, China. J. Clean. Prod. 2021, 293, 126136. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, J.; Du, Z.; Zhang, Y.; Lai, X. Simulation of interception capacity of Nanhe initial rain storage tanks. IOP Conf. Ser. Earth Environ. Sci. 2021, 787, 012138. [Google Scholar] [CrossRef]
- Carluer, N.; Lauvernet, C.; Noll, D.; Munoz-Carpena, R. Defining context-specific scenarios to design vegetated buffer zones that limit pesticide transfer via surface runoff. Sci. Total Environ. 2017, 575, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Liu, X.; Tan, J.; Shao, X.; Cheng, J. Effect of Plant Buffer Zone–Antifouling Curtain Wall on Reducing Non-Point Source Pollution in Paddy Fields, China. Sustainability 2022, 14, 6044. [Google Scholar] [CrossRef]
- Syversen, N. Effect and design of buffer zones in the Nordic climate: The influence of width, amount of surface runoff, seasonal variation and vegetation type on retention efficiency for nutrient and particle runoff. Ecol. Eng. 2005, 24, 483–490. [Google Scholar] [CrossRef]
- Ramião, J.P.; Carvalho-Santos, C.; Pinto, R.; Pascoal, C. Modeling the Effectiveness of Sustainable Agricultural Practices in Reducing Sediments and Nutrient Export from a River Basin. Water 2022, 14, 3962. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, L.; Ma, C.; Wang, H.; Zhou, C. The Comprehensive Reduction Capacity of Five Riparian Vegetation Buffer Strips for Primary Pollutants in Surface Runoff. Appl. Sci. 2023, 13, 3898. [Google Scholar] [CrossRef]
- Hill, A.R. Groundwater nitrate removal in riparian buffer zones: A review of research progress in the past 20 years. Biogeochemistry 2019, 143, 347–369. [Google Scholar] [CrossRef]
- Piniewski, M.; Marcinkowski, P.; Kardel, I.; Giełczewski, M.; Izydorczyk, K.; Frątczak, W. Spatial Quantification of Non-Point Source Pollution in a Meso-Scale Catchment for an Assessment of Buffer Zones Efficiency. Water 2015, 7, 1889–1920. [Google Scholar] [CrossRef]
- Bu, X.; Xue, J.; Zhao, C.; Wu, Y.; Han, F.; Zhu, L. Sediment and nutrient removal by integrated tree-grass riparian buffers in Taihu Lake watershed, eastern China. J. Soil Water Conserv. 2016, 71, 129–136. [Google Scholar] [CrossRef]
- Lee, K.H.; Isenhart, T.M.; Schultz, R.C. Sediment and nutrient removal in an established multi-species riparian buffer. J. Soil Water Conserv. 2003, 58, 1–8. [Google Scholar]
- Dunn, R.M.; Hawkins, J.M.B.; Blackwell, M.S.A.; Zhang, Y.; Collins, A.L. Impacts of different vegetation in riparian buffer strips on runoff and sediment loss. Hydrol. Process. 2022, 36, e14733. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, C.S.C.; Rangel, A.O.S.S.; Castro, P.M.L. Constructed wetland systems vegetated with different plants applied to the treatment of tannery wastewater. Water Res. 2007, 41, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, K.; Bak, J.; Choi, S. Iris pseudacorus and Lythrum anceps as Plants Supporting the Process of Removing Microplastics from Aquatic Environments—Preliminary Research. Horticulturae 2024, 10, 631. [Google Scholar] [CrossRef]
- Thalla, A.K.; Devatha, C.P.; Anagh, K.; Sony, E. Performance evaluation of horizontal and vertical flow constructed wetlands as tertiary treatment option for secondary effluents. Appl. Water Sci. 2019, 9, 147. [Google Scholar] [CrossRef]
- Mander, Ü.; Tournebize, J.; Tonderski, K.; Verhoeven, J.T.A.; Mitsch, W.J. Planning and establishment principles for constructed wetlands and riparian buffer zones in agricultural catchments. Ecol. Eng. 2017, 103, 296–300. [Google Scholar] [CrossRef]
- Liu, M.; Wu, S.; Chen, L.; Dong, R. How substrate influences nitrogen transformations in tidal flow constructed wetlands treating high ammonium wastewater? Ecol. Eng. 2014, 73, 478–486. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Liu, R.; Morgan, D. Global development of various emerged substrates utilized in constructed wetlands. Bioresour. Technol. 2018, 261, 441–452. [Google Scholar] [CrossRef]
- Lima, M.; Carvalho, K.; Passig, F.; Borges, A.; Filippe, T.; Azevedo, J.; Nagalli, A. Performance of different substrates in constructed wetlands planted with E. crassipes treating low-strength sewage under subtropical conditions. Sci. Total Environ. 2018, 630, 1365–1373. [Google Scholar] [CrossRef]
- Drizo, A.; Frost, C.; Smith, K.; Grace, J. Phosphate and ammonium removal by constructed wetlands with horizontal subsurface flow, using shale as a substrate. Water Sci. Technol. 1997, 35, 95–102. [Google Scholar] [CrossRef]
- Vohla, C.; Kõiv, M.; Bavor, H.J.; Chazarenc, F.; Mander, Ü. Filter materials for phosphorus removal from wastewater in treatment wetlands—A review. Ecol. Eng. 2011, 37, 70–89. [Google Scholar] [CrossRef]
- Fu, G.; Wu, J.; Han, J.; Zhao, L.; Chan, G.; Leong, K. Effects of substrate type on denitrification efficiency and microbial community structure in constructed wetlands. Bioresour. Technol. 2020, 307, 123222. [Google Scholar] [CrossRef] [PubMed]
- Vispo, C.; Geronimo, F.K.; Jeon, M.; Kim, L.-H. Performance Evaluation of Various Filter Media for Multi-Functional Purposes to Urban Constructed Wetlands. Sustainability 2024, 16, 287. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, Z.; Pan, X.; Li, B.; Xie, S.; Guo, Q. Substrate influences on archaeal and bacterial assemblages in constructed wetland microcosms. Ecol. Eng. 2016, 94, 437–442. [Google Scholar] [CrossRef]
- Bowden, L.I.; Jarvis, A.P.; Younger, P.L.; Johnson, K.L. Phosphorus removal from waste waters using basic oxygen steel slag. Environ. Sci. Technol. 2009, 43, 2476–2481. [Google Scholar] [CrossRef] [PubMed]
- Abdelhakeem, S.G.; Aboulroos, S.A.; Kamel, M.M. Performance of a vertical subsurface flow constructed wetland under different operational conditions. J. Adv. Res. 2016, 7, 803–814. [Google Scholar] [CrossRef]
- Barca, C.; Meyer, D.; Liira, M.; Drissen, P.; Comeau, Y.; Andrès, Y.; Chazarenc, F. Steel slag filters to upgrade phosphorus removal in small wastewater treatment plants: Removal mechanisms and performance. Ecol. Eng. 2014, 68, 214–222. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Lin, X.; Wang, H.; Li, H.; Li, J. Purification effects of recycled aggregates from construction waste as constructed wetland filler. J. Water Process Eng. 2022, 50, 103335. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.-J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Feng, C.; Huang, L.; Yu, H.; Yi, X.; Wei, C. Simultaneous phenol removal, nitrification and denitrification using microbial fuel cell technology. Water Res. 2015, 76, 160–170. [Google Scholar] [CrossRef]
- Mayo, A.W.; Hanai, E.; Kibazohi, O. Nitrification–denitrification in a coupled high rate–water hyacinth ponds. Phys. Chem. Earth Parts A/B/C 2014, 72, 88–95. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, X.; Liu, H.; Wu, H. Elucidating the impact of influent pollutant loadings on pollutants removal in agricultural waste-based constructed wetlands treating low C/N wastewater. Bioresour. Technol. 2019, 273, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Li, L.; Zhao, Y.; Zhu, W.; Shen, J. An integrated model of substrate clogging in vertical flow constructed wetlands. J. Environ. Manag. 2013, 119, 67–75. [Google Scholar] [CrossRef]
- Xu, D.; Wang, L.; Li, H.; Li, Y.; Howard, A.; Guan, Y.; Li, J.; Xu, H. The forms and bioavailability of phosphorus in integrated vertical flow constructed wetland with earthworms and different substrates. Chemosphere 2015, 134, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, A.I.; Tsihrintzis, V.A. Use of zeolite and bauxite as filter media treating the effluent of Vertical Flow Constructed Wetlands. Microporous Mesoporous Mater. 2012, 155, 106–116. [Google Scholar] [CrossRef]
- Pansini, M. Natural zeolites as cation exchangers for environmental protection. Miner. Depos. 1996, 31, 563–575. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef]
- Arunbabu, V.; Sruthy, S.; Antony, I.; Ramasamy, E. Sustainable greywater management with Axonopus compressus (broadleaf carpet grass) planted in sub surface flow constructed wetlands. J. Water Process Eng. 2015, 7, 153–160. [Google Scholar] [CrossRef]
- Sun, J.; Yang, M.; Zeng, L.; Wang, M.; He, S.; Cai, S.; Li, L.; Liu, X.; Zhang, H. Adsorption Performance on Sediment Nutrients by Different Proportions of Zeolite and Shale Ceramsite (ZSC). Pol. J. Environ. Stud. 2020, 29, 2365–2372. [Google Scholar] [CrossRef]
- Hedström, A. Ion Exchange of Ammonium in Zeolites: A Literature Review. J. Environ. Eng. 2001, 127, 673–681. [Google Scholar] [CrossRef]
- Ji, Z.-Y.; Yuan, J.-S.; Li, X.-G. Removal of ammonium from wastewater using calcium form clinoptilolite. J. Hazard. Mater. 2007, 141, 483–488. [Google Scholar] [CrossRef]
- Pang, S.; Masjuki, H.; Kalam, M.; Hazrat, M. Liquid absorption and solid adsorption system for household, industrial and automobile applications: A review. Renew. Sustain. Energy Rev. 2013, 28, 836–847. [Google Scholar] [CrossRef]
- Li, N.; Lai, B.; Ding, L.; Li, J.; Liu, C.; Wu, L. Synchronous algae and phosphorus removal by Ceramsite@Fe2O3 (FC) via taking the algae as crystal nuclei of hydroxylapatite. Chem. Eng. J. 2021, 426, 130748. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Guo, Z.; Qin, J.; Liang, K. Effect of Fe2+/Fe3+ on high-strength ceramsite prepared by sintering geopolymers using iron ore tailings. Ceram. Int. 2022, 48, 5681–5688. [Google Scholar] [CrossRef]
- Monea, M.C.; Löhr, D.K.; Meyer, C.; Preyl, V.; Xiao, J.; Steinmetz, H.; Schönberger, H.; Drenkova-Tuhtan, A. Comparing the leaching behavior of phosphorus, aluminum and iron from post-precipitated tertiary sludge and anaerobically digested sewage sludge aiming at phosphorus recovery. J. Clean. Prod. 2020, 247, 119129. [Google Scholar] [CrossRef]
- Jiang, Z.; Wu, J.; Liu, X.; Yu, H.; Jiao, C.; Shen, J.-Y.; Pei, Y. Facile synthesis of MgAl layered double hydroxides by a co-precipitation method for efficient nitrate removal from water: Kinetics and mechanisms. New J. Chem. 2021, 45, 14580–14588. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, X.; Chen, C.; Zhang, H.; Wang, L. Ecological floating bed for decontamination of eutrophic water bodies: Using alum sludge ceramsite. J. Environ. Manag. 2022, 311, 114845. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Yu, J.; Si, W.; Fu, Y. Water supply sludge-based ceramsite denitrification filter: Pollutant removal and microbial community characteristics. J. Water Process Eng. 2023, 55, 104229. [Google Scholar] [CrossRef]
- Jiang, C.; Jia, L.; Zhang, B.; He, Y.; Kirumba, G. Comparison of quartz sand, anthracite, shale and biological ceramsite for adsorptive removal of phosphorus from aqueous solution. J. Environ. Sci. 2014, 26, 466–477. [Google Scholar] [CrossRef]
- Ahmed, S.J.; Taylor, H.F.W. Crystal Structures of the Lamellar Calcium Aluminate Hydrates. Nature 1967, 215, 622–623. [Google Scholar] [CrossRef]
- Christensen, A.N.; Jensen, T.R.; Lebech, B.; Hanson, J.C.; Jakobsen, H.J.; Skibsted, J. Thermal decomposition of monocalcium aluminate decahydrate (CaAl2O4·10H2O) investigated by in-situsynchrotron X-ray powder diffraction, thermal analysis and 27Al, 2H MAS NMR spectroscopy. Dalton Trans. 2008, 4, 455–462. [Google Scholar] [CrossRef]
- Chatterjee, N.D.; Johannes, W.; Leistner, H. The system CaO-Al2O3-SiO2-H2O: New phase equilibria data, some calculated phase relations, and their petrological applications. Contrib. Mineral. Petrol. 1984, 88, 1–13. [Google Scholar] [CrossRef]
- Meller, N.; Hall, C.; Kyritsis, K.; Giriat, G. Synthesis of cement based CaO–Al2O3–SiO2–H2O (CASH) hydroceramics at 200 and 250 °C: Ex-situ and in-situ diffraction. Cem. Concr. Res. 2007, 37, 823–833. [Google Scholar] [CrossRef]
- Saleh, H.I. Synthesis and formation mechanisms of calcium ferrite compounds. J. Mater. Sci. Technol. 2004, 20, 530–534. [Google Scholar]
- Amir, S.; Jouraiphy, A.; Meddich, A.; El Gharous, M.; Winterton, P.; Hafidi, M. Structural study of humic acids during composting of activated sludge-green waste: Elemental analysis, FTIR and 13C NMR. J. Hazard. Mater. 2010, 177, 524–529. [Google Scholar] [CrossRef]
- Xu, J.; Xu, X.; Liu, Y.; Li, H.; Liu, H. Effect of microbiological inoculants DN-1 on lignocellulose degradation during co-composting of cattle manure with rice straw monitored by FTIR and SEM. Environ. Prog. Sustain. Energy 2016, 35, 345–351. [Google Scholar] [CrossRef]
- Fan, Y.V.; Lee, C.-T.; Klemeš, J.J.; Chua, L.S.; Sarmidi, M.R.; Leow, C.W. Evaluation of Effective Microorganisms on home scale organic waste composting. J. Environ. Manag. 2017, 216, 41–48. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, Y.; Hu, Y.; Shi, H. The variation in microbial community structure under different heavy metal contamination levels in paddy soils. Ecotoxicol. Environ. Saf. 2019, 180, 557–564. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; Chen, Q.; Xia, H.; Zhang, Y. Novel Chitosan-FeS@ biochar-added constructed wetland microcosms for NH4+/NO3− and Pb removal: Performance and mechanism. J. Environ. Chem. Eng. 2023, 11, 110400. [Google Scholar] [CrossRef]
- Sha, H.; Song, X.; Abdullah Al-Dhabi, N.; Zeng, T.; Mao, Y.; Fu, Y.; Liu, Z.; Wang, G.; Tang, W. Effects of biochar layer position on treatment performance and microbial community in subsurface flow constructed wetlands for removal of cadmium and lead. Bioresour. Technol. 2024, 394, 130194. [Google Scholar] [CrossRef]
- Ren, T.; Chi, Y.; Wang, Y.; Shi, X.; Jin, X.; Jin, P. Diversified metabolism makes novel Thauera strain highly competitive in low carbon wastewater treatment. Water Res. 2021, 206, 117742. [Google Scholar] [CrossRef]
- Tian, M.; Zhao, F.; Shen, X.; Chu, K.; Wang, J.; Chen, S.; Guo, Y.; Liu, H. The first metagenome of activated sludge from full-scale anaerobic/anoxic/oxic (A2O) nitrogen and phosphorus removal reactor using Illumina sequencing. J. Environ. Sci. 2015, 35, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, Q.; Tan, B.; Li, M.; Peng, H.; He, J.; Zhang, Y.; Su, J. Microbial community and metabolic characteristics evaluation in start-up stage of electro-enhanced SBR for aniline wastewater treatment. J. Water Process Eng. 2022, 45, 102489. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Q.; Zhao, L.; Wu, W. Enhanced simultaneous removal of nitrate and phosphate using novel solid carbon source/zero-valent iron composite. J. Clean. Prod. 2021, 289, 125757. [Google Scholar] [CrossRef]
- Wang, L.; Pang, Q.; Zhou, Y.; Peng, F.; He, F.; Li, W.; Xu, B.; Cui, Y.; Zhu, X. Robust nitrate removal and bioenergy generation with elucidating functional microorganisms under carbon constraint in a novel multianode tidal constructed wetland coupled with microbial fuel cell. Bioresour. Technol. 2020, 314, 123744. [Google Scholar] [CrossRef]






| Analysis Indicator | Measurement Method |
|---|---|
| TN | Alkaline potassium persulfate digestion UV spectrophotometric method (HJ 636—2012) |
| TP | Ammonium molybdate spectrophotometric method (GB 11893-89) |
| NH4+-N | Nessler’s reagent spectrophotometric method (HJ 535-2009) |
| COD | Rapid digestion spectrophotometric method |
| Instruments and Equipment | Model | Test Content |
|---|---|---|
| Scanning Electron Microscope (SEM) | FEI Quanta 650 (Hillsboro, OR, USA) | Microscopic Structure |
| X-ray Diffraction (XRD) | Bruker D2 Phaser (Karlsruhe, Germany) | Mineral Composition |
| Automatic Surface Area and Porosity Analyzer (BET) | Micromeritics ASAP 2460 (Norcross, GA, USA) | Specific Surface Area |
| Fourier Transform Infrared Spectrometer (FTIR) | Thermo Scientific (Waltham, MA, USA) | Functional Groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhu, F.; Wang, W.; Zhou, X.; Li, J.; Fan, C. Rainwater Treatment Using Ecological Buffer Zones: Influence of Plant and Filler Collocation. Water 2025, 17, 741. https://doi.org/10.3390/w17050741
Xu J, Zhu F, Wang W, Zhou X, Li J, Fan C. Rainwater Treatment Using Ecological Buffer Zones: Influence of Plant and Filler Collocation. Water. 2025; 17(5):741. https://doi.org/10.3390/w17050741
Chicago/Turabian StyleXu, Jinchi, Feng Zhu, Wen Wang, Xiaolin Zhou, Juexiu Li, and Chunzhen Fan. 2025. "Rainwater Treatment Using Ecological Buffer Zones: Influence of Plant and Filler Collocation" Water 17, no. 5: 741. https://doi.org/10.3390/w17050741
APA StyleXu, J., Zhu, F., Wang, W., Zhou, X., Li, J., & Fan, C. (2025). Rainwater Treatment Using Ecological Buffer Zones: Influence of Plant and Filler Collocation. Water, 17(5), 741. https://doi.org/10.3390/w17050741







