Improving Wastewater Quality Using Ultrafiltration Technology for Sustainable Irrigation Reuse
Abstract
1. Introduction
2. Materials and Methods
2.1. Storage Tank
2.2. Filtration System Design
2.2.1. Design Considerations for the Filtration and Treatment System
- Contaminant removal efficiency: A multi-stage process includes a lamella clarifier, advanced membranes, and activated carbon and crushed glass media for enhanced filtration.
- Flow management and stability: Tanks and pumps ensure stable flow, with a 30 m3·h−1 feed pump, a 60 m3·h−1 backwash pump, and an air blower for media regeneration.
- Automation and monitoring: A PLC control system automates operations, minimizes errors, and provides real-time maintenance alerts.
- Durability and system longevity: Corrosion-resistant materials ensure long-term reliability in wastewater environments.
- Sustainability and environmental impact: Recycled crushed glass media promote sustainability, and the system supports water reuse.
- Scalability and adaptability: A modular design allows system expansion to meet future demands.
- Ease of performing maintenance operations.
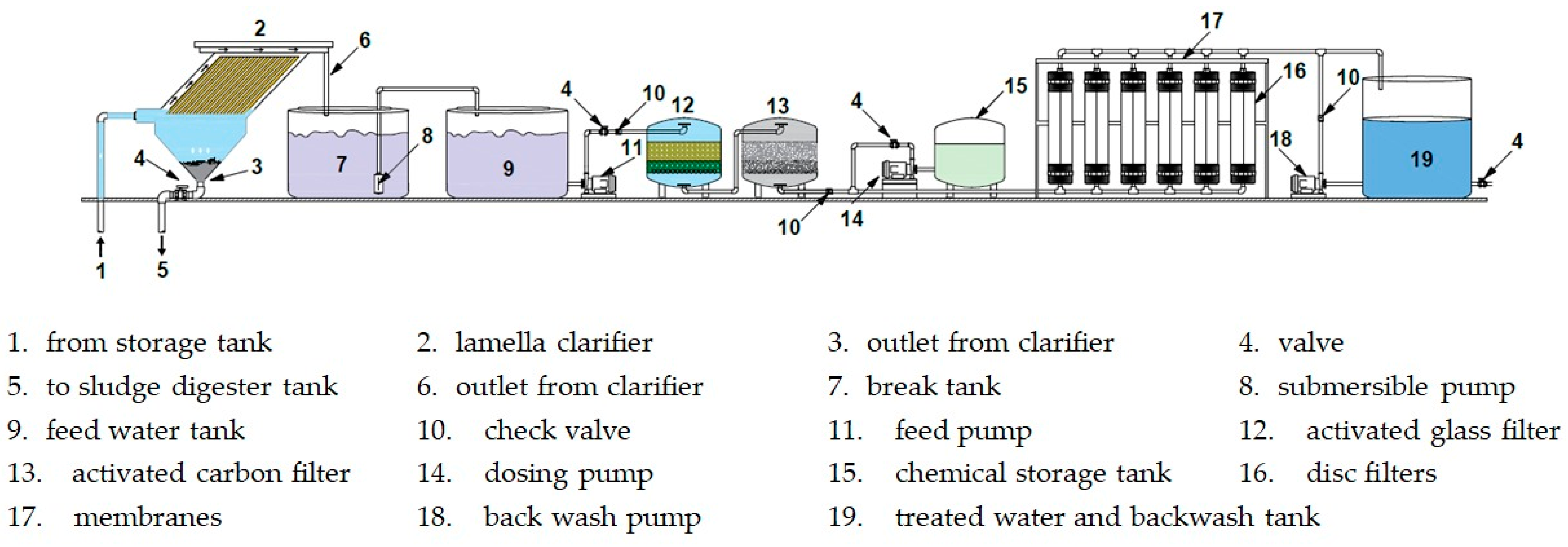
2.2.2. Filtration and Treatment System Components
- Clarifier unit
- 2.
- Break tank and feed water tanks
- 3.
- Filter vessels
- 4.
- Ultrafiltration Membranes
- 5.
- Pumps and blowers
- 6.
- Auxiliary components
- 7.
- Chemical treatment components
2.3. Field Experiment
2.4. Laboratory Measurements
2.4.1. Physical Parameters
2.4.2. Chemical Parameters
2.4.3. Biological Parameters
2.5. Evaluating Criteria
2.5.1. Removal Efficiency (η)
2.5.2. Water Quality Index (WQI)
2.5.3. Interpret the WQI
- WQI < 50: Excellent quality (suitable for irrigation);
- WQI 50–100: Good quality (minor treatment needed);
- WQI 100–200: Poor quality (requires significant treatment);
- WQI > 200: Unsuitable for irrigation.
2.6. Statistical Analysis
3. Results
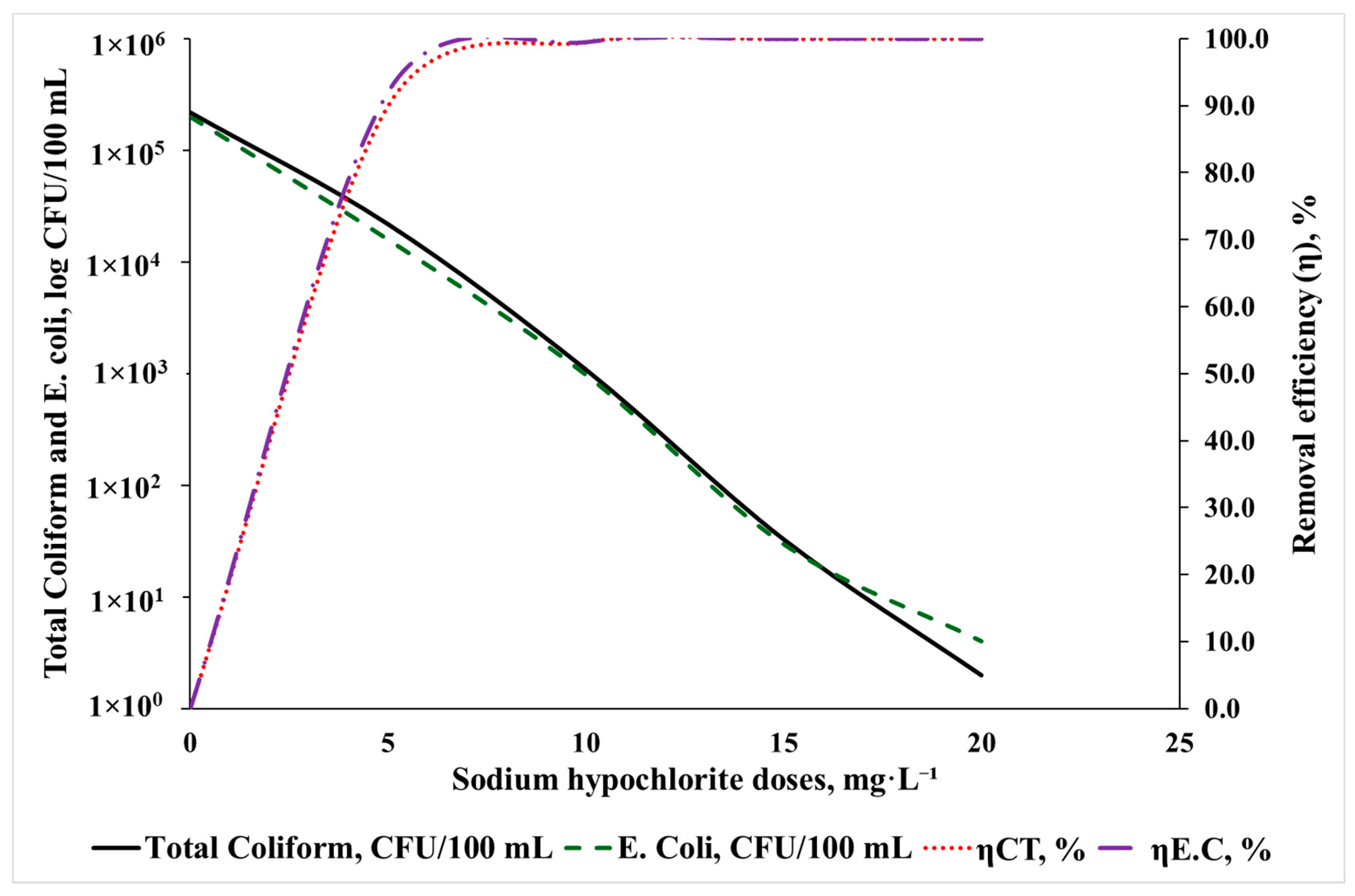
3.1. Preliminary Experiment
3.2. Properties of Wastewater
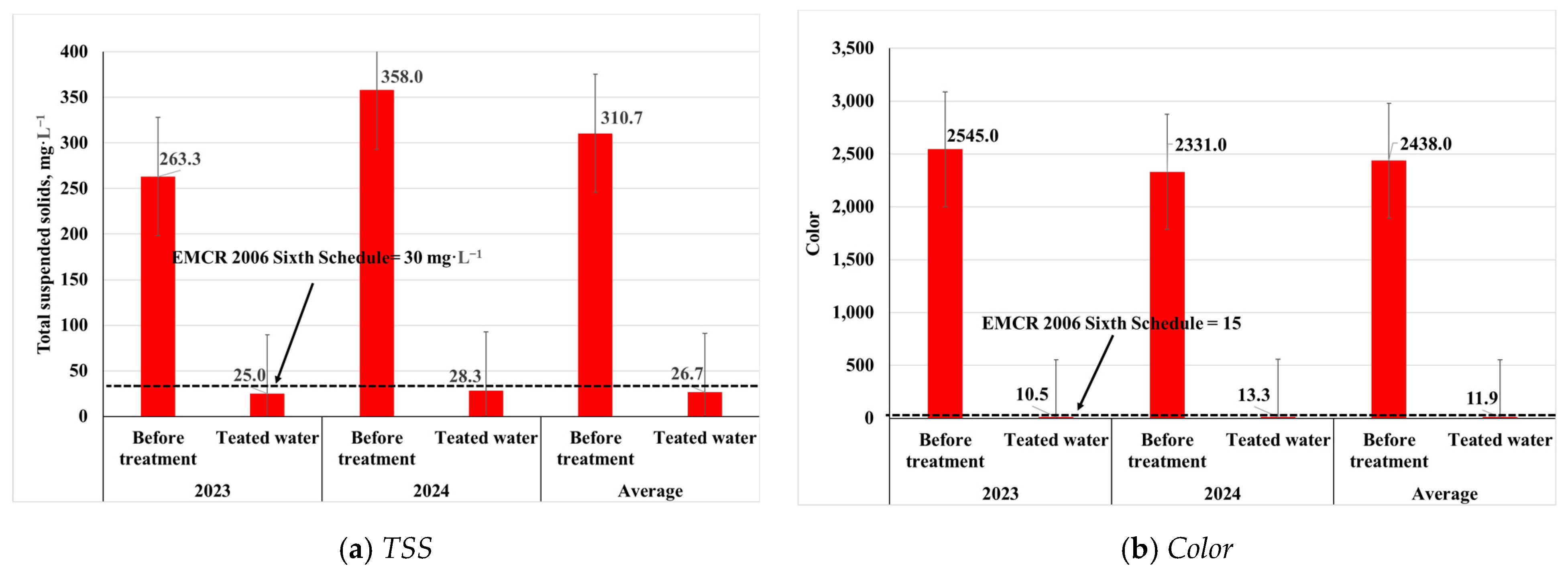
3.2.1. Physical Properties of Wastewater
3.2.2. Chemical Properties of Wastewater
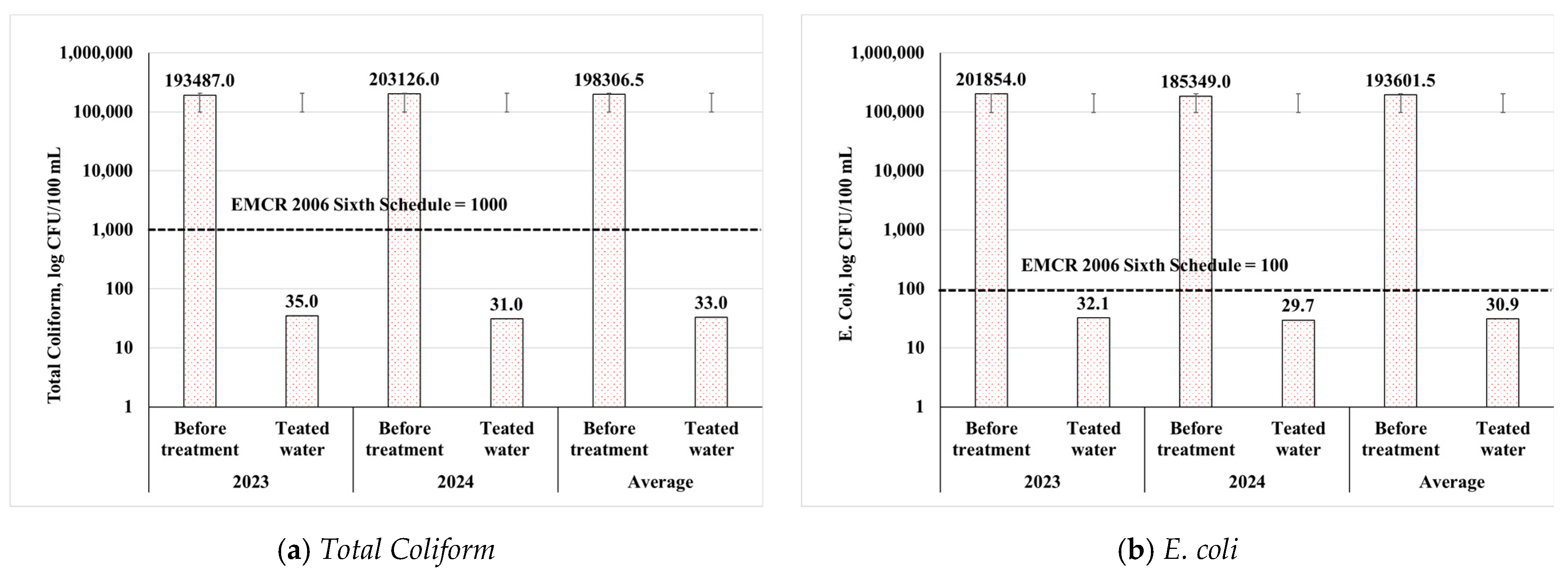
3.2.3. Biological Parameters
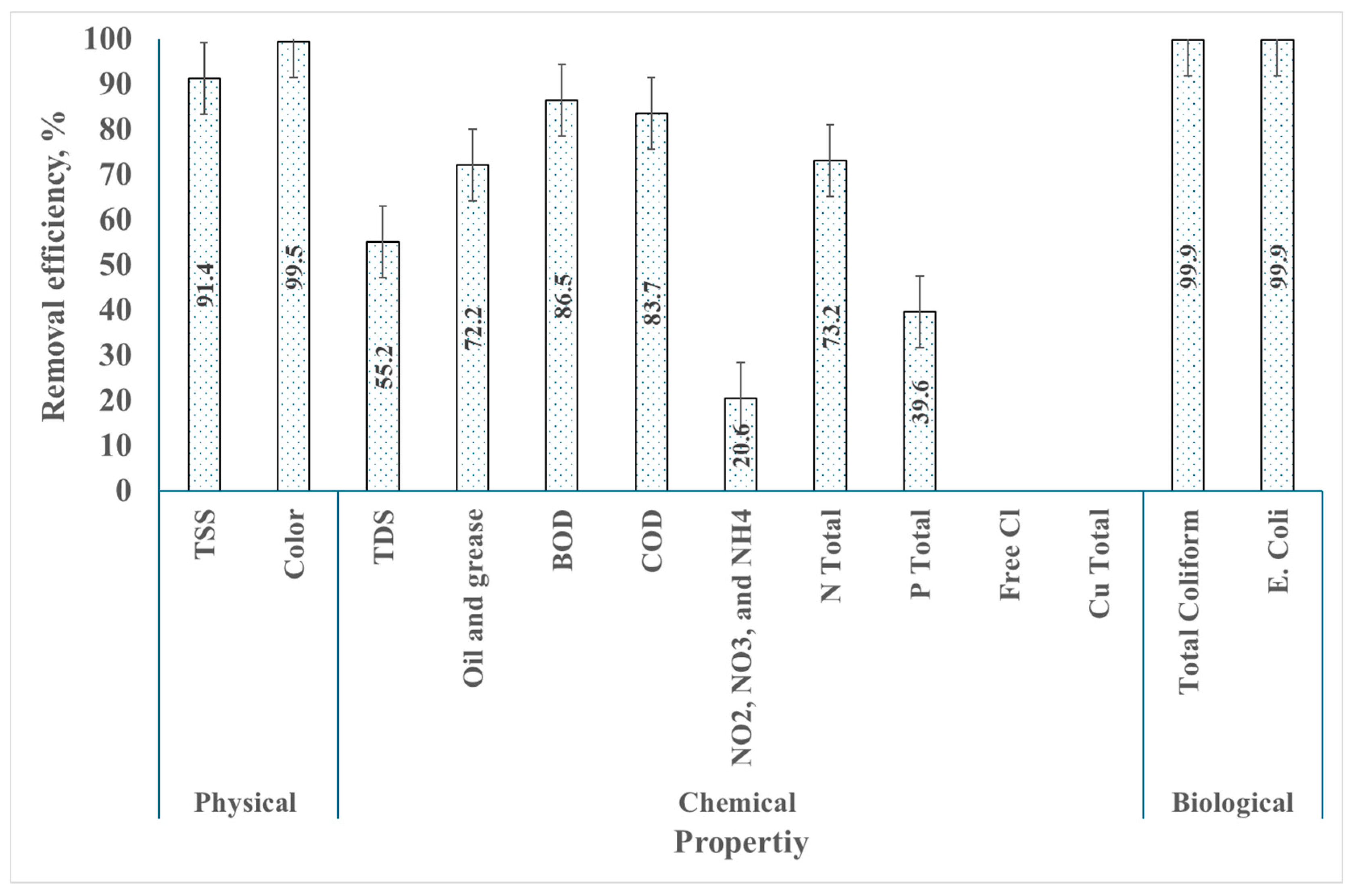
3.3. Removal Efficiency
3.3.1. Removal Efficiency of Physical Properties
3.3.2. Chemical Properties of Wastewater
3.3.3. Removal Efficiency of Biological Parameters
3.4. Water Quality Index (WQi)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Hazmi, H.E.; Mohammadi, A.; Hejna, A.; Majtacz, J.; Esmaeili, A.; Habibzadeh, S.; Mąkinia, J. Wastewater for reuse in agriculture: Prospects and challenges. Environ. Res. 2023, 236, 116711. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.M.; Al-Hazmi, H.E.; Śniatała, B.; Esmaeili, A.; Habibzadeh, S. Nanoparticles and nanofiltration for wastewater treatment: From polluted to fresh water. Environ. Res. 2023, 238, 117114. [Google Scholar] [CrossRef]
- Mateo-Sagasta, J.; Al-Hamdi, M.; AbuZeid, K. Water Reuse in the Middle East and North Africa: A Sourcebook; International Water Management Institute (IWMI): Battaramulla, Sri Lanka, 2023. (In Arabic) [Google Scholar]
- Lin, H.; Zhang, M. Advanced membrane technologies for wastewater treatment and recycling. Membranes 2023, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Alresheedi, M.T.; Albuaymi, A.M.; AlSaleem, S.S.; Haider, H.; Shafiquzzaman, M.; AlHarbi, A.; Ahsan, A. Low-cost ceramic filter bioreactor for treatment and reuse of residential septic tank effluent: A decentralized approach for small communities. Environ. Technol. Innov. 2023, 31, 103213. [Google Scholar] [CrossRef]
- Foglia, A.; González-Camejo, J.; Radini, S.; Sgroi, M.; Li, K.; Eusebi, A.L.; Fatone, F. Transforming wastewater treatment plants into reclaimed water facilities in water-unbalanced regions. An overview of possibilities and recommendations focusing on the Italian case. J. Clean. Prod. 2023, 410, 137264. [Google Scholar] [CrossRef]
- Meireles, A.C.M.; Andrade, E.M.D.; Chaves, L.C.G.; Frischkorn, H.; Crisostomo, L.A. A new proposal of the classification of irrigation water. Rev. Ciência Agronômica 2010, 41, 349–357. [Google Scholar] [CrossRef]
- Alresheedi, M.T.; Haider, H.; Albuaymi, A.M.; AlSaleem, S.S.; Shafiquzzaman, M.; Alharbi, A.; Ahsan, A. Sustainability of a low-cost decentralized treatment system for wastewater reuse: Resident perception-based evaluation for arid regions. Water 2023, 15, 3458. [Google Scholar] [CrossRef]
- Khanzada, N.K.; Farid, M.U.; Kharraz, J.A.; Choi, J.; Tang, C.Y.; Nghiem, L.D.; An, A.K. Removal of organic micropollutants using advanced membrane-based water and wastewater treatment: A review. J. Membr. Sci. 2020, 598, 117672. [Google Scholar] [CrossRef]
- Bhowmick, G.D.; Ghangrekar, M.M.; Zekker, I.; Kibena-Põldsepp, E.; Tammeveski, K.; Wilhelm, M.; Banerjee, R. Ultrafiltration membrane bio-fuel cell as an energy-efficient advanced wastewater treatment system. Int. J. Energy Res. 2022, 46, 20216–20227. [Google Scholar] [CrossRef]
- Yang, J.; Monnot, M.; Eljaddi, T.; Ercolei, L.; Simonian, L.; Moulin, P. Ultrafiltration as tertiary treatment for municipal wastewater reuse. Sep. Purif. Technol. 2021, 272, 118921. [Google Scholar] [CrossRef]
- Chew, C.M.; Aroua, M.K.; Hussain, M.A.; Ismail, W.M.Z.W. Evaluation of ultrafiltration and conventional water treatment systems for sustainable development: An industrial scale case study. J. Clean. Prod. 2016, 112, 3152–3163. [Google Scholar] [CrossRef]
- ECP 501; Egyptian Code of Practice for the Reuse of Treated Wastewater for Agricultural Purposes. The Ministry of Housing Utilities and Urban Communities: Cairo, Egypt, 2015. (In Arabic)
- Kesar, S.; Bhatti, M.S. Chlorination of secondary treated wastewater with sodium hypochlorite (NaOCl): An effective single alternate to other disinfectants. Heliyon 2022, 8, e11162. [Google Scholar] [CrossRef]
- Boni, M.R.; Copelli, S.; Raboni, M. Study of the performance of disinfection with sodium hypochlorite on a full-scale sewage treatment plant. Rev. Ambiente Água 2020, 15, e2652. [Google Scholar] [CrossRef]
- Al-Salmi, M.; Laqbaqbi, M.; Al-Obaidani, S.; Al-Maamari, R.S.; Khayet, M.; Al-Abri, M. Application of membrane distillation for the treatment of oil field produced water. Desalination 2020, 494, 114678. [Google Scholar] [CrossRef]
- Cooper, J. High-Value Organic Acid Recovery for the Valorisation of Bioethanol Dunder Using Two-Stage Membrane Processing. Ph.D. Dissertation, UNSW Sydney, Sydney, Australia, 2020. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Y.; Chen, G.; Yang, H.; Du, T. Water use efficiency was improved at leaf and yield levels of tomato plants by continuous irrigation using semipermeable membrane. Agric. Water Manag. 2018, 203, 430–437. [Google Scholar] [CrossRef]
- Sandoval-Olvera, I.G.; Gonzalez-Munoz, P.; Palacio, L.; Hernandez, A.; Avila-Rodriguez, M.; Pradanos, P. Ultrafiltration membranes modified by PSS deposition and plasma treatment for Cr (VI) removal. Sep. Purif. Technol. 2019, 210, 371–381. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Kumar, V.; Said, Z.; Paliwal, H.K. A review on the application of hybrid nanofluids for parabolic trough collector: Recent progress and outlook. J. Clean. Prod. 2021, 292, 126031. [Google Scholar] [CrossRef]
- Nagial, K.S.; Kumar, N.; Poddar, A. A comprehensive analysis of institutional wastewater for irrigating ornamental plants. Water Supply 2024, 24, 2830–2843. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Cuprys, A.K.; Maletskyi, Z.; Ratnaweera, H. Treatment trends and combined methods in removing pharmaceuticals and personal care products from wastewater—A review. Membranes 2023, 13, 158. [Google Scholar] [CrossRef]
- Ahmad, H.W.; Bibi, H.A.; Chandrasekaran, M.; Ahmad, S.; Kyriakopoulos, G.L. Sustainable Wastewater Treatment Strategies in Effective Abatement of Emerging Pollutants. Water 2024, 16, 2893. [Google Scholar] [CrossRef]
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. Oily wastewater treatment: Overview of conventional and modern methods, challenges, and future opportunities. Water 2021, 13, 980. [Google Scholar] [CrossRef]
- Sharma, R.; Verma, N.; Lugani, Y.; Kumar, S.; Asadnia, M. Conventional and advanced techniques of wastewater monitoring and treatment. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–48. [Google Scholar] [CrossRef]
- Patel, S. Industrial organic waste to energy. In Biodegradable Waste Processing for Sustainable Developments; Taylor & Francis Group: Boca Raton, FL, USA, 2024; p. 235. [Google Scholar] [CrossRef]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef]
- Raza, M.; Inayat, A.; Abu-Jdayil, B. Crude glycerol as a potential feedstock for future energy via thermochemical conversion processes: A review. Sustainability 2021, 13, 12813. [Google Scholar] [CrossRef]
- LeChevallier, M.W.; Au, K.K. Water Treatment and Pathogen Control; IWA Publishing: London, UK, 2004. [Google Scholar]
- Payment, P.; Waite, M.; Dufour, A. Introducing parameters for the assessment of drinking water quality. Assess. Microb. Saf. Drink. Water 2003, 4, 47–77. [Google Scholar]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality: Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Ashbolt, N.J. Microbial contamination of drinking water and human health from community water systems. Curr. Environ. Health Rep. 2015, 2, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Tsaridou, C.; Karabelas, A.J. Drinking water standards and their implementation—A critical assessment. Water 2021, 13, 2918. [Google Scholar] [CrossRef]
- Haile, Y.T. Performance Evaluation and Optimization of an Industrial Wastewater Treatment Plant (Case Study: Bahir Dar Textile Factory, Ethiopia). Master’s Thesis, Pan African University, Tlemcen, Algeria, 2018. [Google Scholar]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Reverse osmosis pretreatment technologies and future trends: A comprehensive review. Desalination 2019, 452, 159–195. [Google Scholar] [CrossRef]
- Pintor, A.M.; Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies. A critical review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]

| Parameter | Weight (Wi) |
|---|---|
| TDS | 0.10 |
| pH | 0.10 |
| Oil and Grease | 0.05 |
| BOD | 0.15 |
| COD | 0.15 |
| NO2 | 0.07 |
| N Total | 0.07 |
| P Total | 0.05 |
| Total Coliform | 0.13 |
| E. coli | 0.13 |
| Property | EMCR 2006 SIXTH SCHEDULE | 2023 | 2024 | Average | |||
|---|---|---|---|---|---|---|---|
| Before Treatment | Treated Water | Before Treatment | Treated Water | Before Treatment | Treated Water | ||
| TDS, mg·L−1 | 1200 | 863.3 | 368.7 | 813.0 | 382.3 | 838.2 | 375.5 |
| pH | 7.5 | 7.4 | 7.3 | 7.2 | 7.0 | 7.3 | 7.2 |
| Oil and grease | 1.2 | 0.3 | 0.6 | 0.2 | 0.9 | 0.3 | |
| BOD, mg·L−1 | 30 | 116.7 | 18.9 | 215.6 | 26.1 | 166.2 | 22.5 |
| COD, mg·L−1 | 50 | 249 | 45 | 235.0 | 34.1 | 242.0 | 39.6 |
| NO2, NO3, and NH4, mg·L−1 | 100 | 92.3 | 83.6 | 75.7 | 49.9 | 84.0 | 66.7 |
| N Total, mg·L−1 | 30 | 67.1 | 20.6 | 54.2 | 11.9 | 60.7 | 16.3 |
| P Total, mg·L−1 | 10 | 9.9 | 5.3 | 8.4 | 5.7 | 9.1 | 5.5 |
| Free Cl, mg·L−1 | 0.1 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 |
| Cu Total, mg·L−1 | 1 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 |
| Parameter | Cm | Cs | Qi | Wi | Qi × Wi |
|---|---|---|---|---|---|
| TDS, mg·L−1 | 375.5 | 1200 | 31.3 | 0.1 | 3.13 |
| pH | 7.2 | 7.5 | 4.6 | 0.1 | 0.46 |
| Oil and Grease | 0.3 | 1 | 25.0 | 0.05 | 1.25 |
| BOD, mg·L−1 | 22.5 | 30 | 75.0 | 0.15 | 11.25 |
| COD, mg·L−1 | 39.6 | 50 | 79.1 | 0.15 | 11.87 |
| NO2, mg·L−1 | 66.7 | 100 | 66.7 | 0.07 | 4.67 |
| N Total, mg·L−1 | 16.3 | 30 | 54.2 | 0.07 | 3.79 |
| P Total, mg·L−1 | 5.5 | 10 | 55.0 | 0.05 | 2.75 |
| Total Coliform, CFU/100 mL | 33.0 | 1000 | 3.3 | 0.13 | 0.43 |
| E. coli, CFU/100 mL | 30.9 | 100 | 30.9 | 0.13 | 4.02 |
| WQI | 43.61 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghonimy, M.; Alharbi, A.; Saad, S.A.H.; Hussein, N.S. Improving Wastewater Quality Using Ultrafiltration Technology for Sustainable Irrigation Reuse. Water 2025, 17, 870. https://doi.org/10.3390/w17060870
Ghonimy M, Alharbi A, Saad SAH, Hussein NS. Improving Wastewater Quality Using Ultrafiltration Technology for Sustainable Irrigation Reuse. Water. 2025; 17(6):870. https://doi.org/10.3390/w17060870
Chicago/Turabian StyleGhonimy, Mohamed, Abdulaziz Alharbi, Shereen A. H. Saad, and Nermin S. Hussein. 2025. "Improving Wastewater Quality Using Ultrafiltration Technology for Sustainable Irrigation Reuse" Water 17, no. 6: 870. https://doi.org/10.3390/w17060870
APA StyleGhonimy, M., Alharbi, A., Saad, S. A. H., & Hussein, N. S. (2025). Improving Wastewater Quality Using Ultrafiltration Technology for Sustainable Irrigation Reuse. Water, 17(6), 870. https://doi.org/10.3390/w17060870






