Pesticide Mobility in Surface and Subsurface Irrigation Return Flow in a Container Plant Production System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Nursery
2.2. Irrigation Monitoring and Control System
2.3. Irrigation Treatments
2.4. Plant Material and Substrate
2.5. Pesticide Application and Sampling
2.6. Pesticide Analysis
2.7. Experimental Design and Statistical Analysis
3. Results
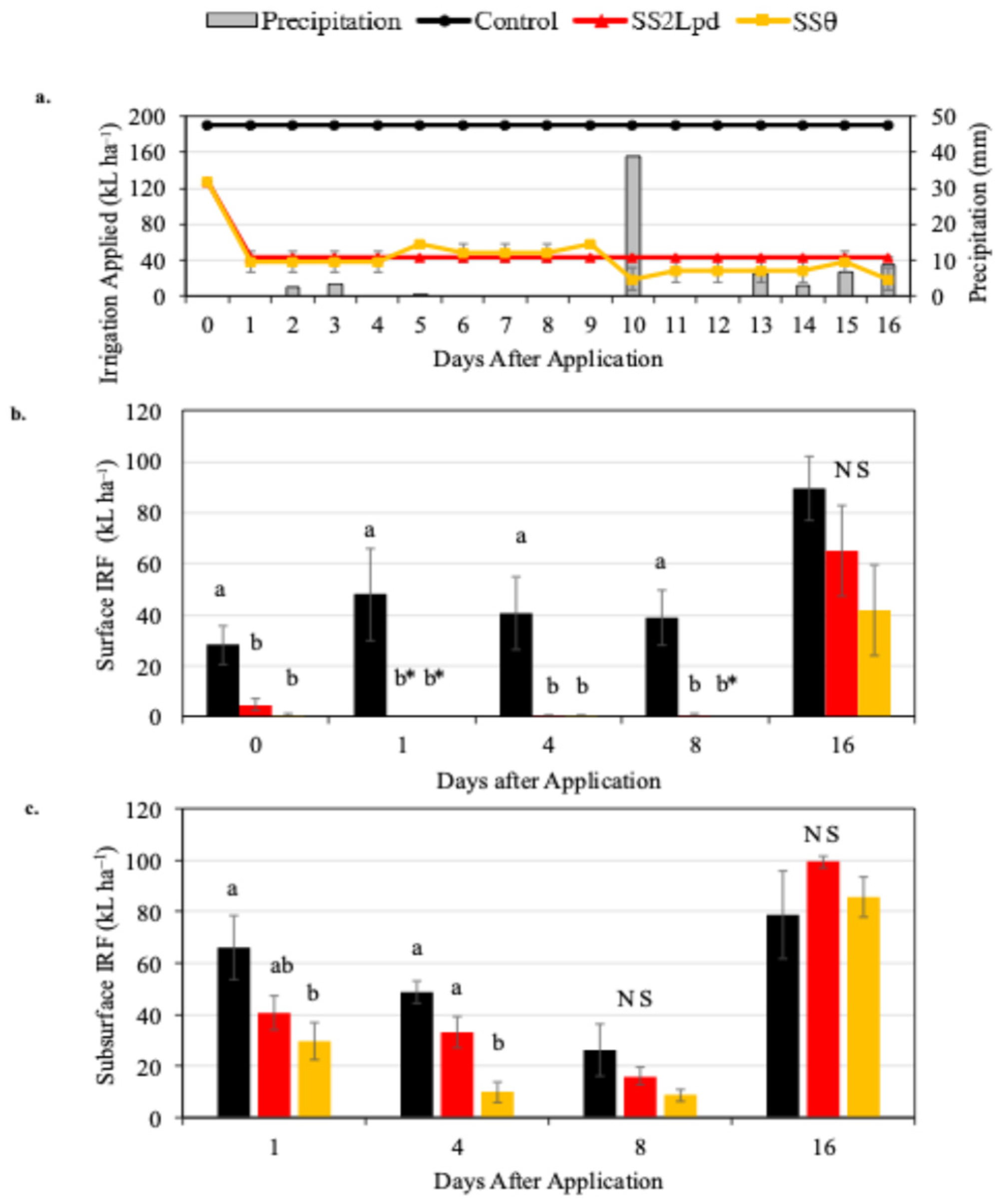
3.1. Irrigation and Irrigation Return Flow
3.1.1. Monitoring Period 1
3.1.2. Monitoring Period 2
3.1.3. Monitoring Period 3
3.2. Pesticide Transport
3.2.1. Monitoring Period 1
Acephate
Bifenthrin
Isoxaben
Mefenoxam
3.2.2. Monitoring Period 2
Chlorpyrifos
Glyphosate
Oxyfluorfen
Triflumizole
3.2.3. Monitoring Period 3
Glyphosate
Prodiamine
Thiophanate-Methyl (TPM)
4. Discussion
4.1. Irrigation and Irrigation Return Flow
4.2. Pesticide Mobility
4.2.1. Acephate
4.2.2. Bifenthrin
4.2.3. Isoxaben
4.2.4. Mefenoxam
4.2.5. Oxyfluorfen
4.2.6. Chloryprifos
4.2.7. Triflumizole
4.2.8. Glyphosate
4.2.9. Thiophanate-Methyl (TPM)
4.2.10. Prodiamine
4.3. Environmental, Ecological, and Plant Production Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOS | Day of sampling |
| IRF | Irrigation return flow |
| SS2Lpd | Spray stake irrigation at 2 L per day |
| SSθ | Spray stake irrigation based on sensor-derived substrate volumetric moisture content |
| θ | Substrate volumetric water content |
| TPM | Thiophanate-methyl |
Appendix A
Appendix A.1. Pesticide Analysis Methods
Appendix A.1.1. Extraction Procedure
Appendix A.1.2. LCMS Analysis
| Compound | Retention Time (min) | Precursor Ion | Product Ions | Dwell (ms) | Cone (V) | CE (V) |
|---|---|---|---|---|---|---|
| Acephate | 1.27 | 184 | 125, 143 | 0.5 | 25 | 17 |
| Isoxaben | 8.86 | 333 | 165 | 0.5 | 22 | 17 |
| Metalaxyl-m | 8.44 | 280 | 192, 220 | 0.5 | 17 | 17 |
| Thiophanate-methyl | 0.85 | 343 | 151, 226 | 0.5 | 20 | 17 |
| Triflumizole | 9.8 | 346 | 73, 278 | 0.5 | 22 | 17 |
| AMPA | 6.59 | 110 | 63, 79 | 0.5 | 20 | 21 |
| 13C-AMPA | 6.59 | 114 | 63.2, 79 | 0.5 | 23 | 19 |
| Glyphosate | 4.35 | 168 | 63, 79 | 0.5 | 20 | 20 |
| 13C-Glyphosate | 4.35 | 169.7 | 63.2, 79.1 | 0.5 | 23 | 17 |
Appendix A.1.3. GC-ECD Analysis
| Compound | Column Retention Time (min) | |
|---|---|---|
| DB-5MS + DG | DB-35MS | |
| Bifenthrin | 30.37 | 31.49 |
| Chlorpyrifos | 22.65 | 25.73 |
| Oxyfluorfen | 25.79 | 28.28 |
| Prodiamine | 21.96 | 24.30 |
Appendix A.1.4. Quality Control/Assurance
References
- Abdi, D.E.; Fernandez, R.T. Reducing water and pesticide movement in nursery production. HortTechnology 2019, 29, 730–735. [Google Scholar] [CrossRef]
- Abdi, D.E.; Owen, J.S., Jr.; Wilson, P.C.; Hinz, H.O.; Cregg, B.M.; Fernandez, R.T. Reducing Pesticide Transport in Surface and Subsurface Irrigation Return Flow in Specialty Crop Production. Agric. Water Manag. 2021, 256, 107124. [Google Scholar] [CrossRef]
- Vryzas, Z. Pesticide fate in soil-sediment-water environment in relation to contamination preventing actions. Curr. Opin. Environ. Sci. Health 2018, 4, 5–9. [Google Scholar] [CrossRef]
- Von Merey, G.; Manson, P.S.; Mehrsheikh, A.; Sutton, P.; Levine, S.L. Glyphosate and Aminomethylphosphonic acid chronic risk assessment for soil biota. Environ. Toxicol. Chem. 2016, 35, 2742–2752. [Google Scholar] [CrossRef]
- Agro, E.; Zheng, Y. Controlled-release fertilizer application rates for container nursery crop production in southwestern Ontario, Canada. HortScience 2014, 49, 1414–1423. [Google Scholar] [CrossRef]
- Majsztrik, J.C.; Fernandez, R.T.; Fisher, P.R.; Hitchcock, D.R.; Lea-Cox, J.; Owen, J.S., Jr.; Oki, L.R.; White, S.A. Water Use and Treatment in Container-grown specialty crop production: A review. Water Air Soil Pollut. 2017, 228, 151. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Harrison-Murray, R.; Atkinson, C.J.; Grant, O.M. Application of deficit irrigation to container-grown hardy ornamental nursery stock via overhead irrigation, compared to drip irrigation. Agric. Water Manag. 2016, 163, 244–254. [Google Scholar] [CrossRef]
- Million, J.B.; Yeager, T.H. Capture of sprinkler irrigation water by container-grown ornamental plants. HortScience 2015, 50, 442–446. [Google Scholar] [CrossRef]
- Pershey, N.A.; Fernandez, R.T.; Cregg, B.M.; Andresen, J.A. Irrigating based on daily water use reduces nursery effluent volume and nutrient load without reducing growth of four conifers. HortScience 2015, 50, 1553–1561. [Google Scholar] [CrossRef]
- Briggs, J.A.; Riley, M.B.; Whitwell, T. Quantification and remediation of pesticides in runoff water from containerized plant production. J. Environ. Qual. 1998, 27, 814–820. [Google Scholar] [CrossRef]
- Warsaw, A.L.; Fernandez, R.T.; Cregg, B.M.; Andresen, J.A. Container-grown ornamental plant growth and water runoff nutrient content and volume under four irrigation treatments. HortScience 2009, 44, 1573–1580. [Google Scholar] [CrossRef]
- Warsaw, A.L.; Fernandez, R.T.; Kort, D.R.; Cregg, B.M.; Rowe, B.; Vandervoort, C. Remediation of metalaxyl, trifluralin, and nitrate from nursery runoff using container-grown woody ornamentals and phytoremediation areas. Ecol. Eng. 2012, 47, 254–263. [Google Scholar] [CrossRef]
- Incrocci, L.; Marzialetti, P.; Incrocci, G.; Di Vita, A.; Balendonck, J.; Bibbiani, C.; Spagnol, S.; Pardossi, A. Sensor-based management of container nursery crops irrigated with fresh or saline water. Agric. Water Manag. 2019, 213, 49–61. [Google Scholar] [CrossRef]
- Lea-Cox, J.D.; Bauerle, W.L.; van Iersel, M.W.; Kantor, G.F.; Bauerle, T.L.; Lichtenberg, E.; King, D.M.; Crawford, L. Advancing wireless sensor networks for irrigation management of ornamental crops: An overview. HortTechnology 2013, 23, 717–724. [Google Scholar] [CrossRef]
- Gardner, D.S.; Branham, B.E. Effect of turfgrass cover and irrigation on soil mobility and dissipation of mefenoxam and propiconazole. J. Environ. Qual. 2001, 30, 1612–1618. [Google Scholar] [CrossRef]
- Lupi, L.; Bedmar, F.; Puricelli, M.; Marino, D.; Aparicio, V.C.; Wunderlin, D.; Miglioranza, K.S.B. Glyphosate runoff and its occurrence in rainwater and subsurface soil in the nearby area of agricultural fields in Argentina. Chemosphere 2019, 225, 906–914. [Google Scholar] [CrossRef]
- Riley, M.B.; Keese, R.J.; Camper, N.D.; Whitwell, T.; Wilson, P.C. Pendimethalin and oxyfluorfen residues in pond water and sediment from container plant nurseries. Weed Technol. 1994, 8, 299–303. [Google Scholar] [CrossRef]
- Hinz, F.O.; Fisher, P.R.; Wilson, P.C. Losses of selected pesticides in drainage water from containerized ornamental plants. J. Environ. Qual. 2020, 49, 1334–1346. [Google Scholar] [CrossRef]
- Abdi, D.E.; Owen, J.S., Jr.; Brindley, J.C.; Birnbaum, A.; Cregg, B.M.; Fernandez, R.T. Irrigation return flow and nutrient movement mitigation by irrigation method for container plant production. Irrig. Sci. 2021, 39, 567–585. [Google Scholar] [CrossRef]
- Text Recycling Research Project. Best Practices for Researchers. Available online: https://textrecycling.org/resources/best-practices-for-researchers/ (accessed on 14 November 2024).
- Abdi, D.E. Reducing Water and Agrochemical Movement from Container Nursery Production Using Bioreactors and Irrigation Management. Ph.D. Dissertation, Michigan State University, East Lansing, MI, USA, 2020. [Google Scholar]
- MSU Enviroweather. Available online: https://enviroweather.msu.edu/weather.php?stn=msu (accessed on 1 March 2023).
- Fare, D.C.; Gilliam, C.H.; Keever, G.J.; Olive, J.W. Cyclic irrigation reduces container nitrate-nitrogen concentration. HortScience 1994, 29, 1514–1517. [Google Scholar]
- Garber, M.P.; Ruter, J.M.; Midcap, J.T.; Bondari, K. Survey of container nursery irrigation practices in Georgia. HortTechnology 2002, 12, 727–731. [Google Scholar] [CrossRef]
- US Environmental Protection Agency Test Methods. Method 3510C: Separatory Funnel Liquid-Liquid Extraction; Revision 3; EPA: Washington, DC, USA, 1996; [CD-ROM].
- Lamack, W.F.; Niemera, A.X. Application method affects water application efficiency of spray stake-irrigated containers. HortScience 1993, 28, 625–627. [Google Scholar] [CrossRef]
- Burnett, S.E.; van Iersel, M.W. Morphology and irrigation efficiency of Gaura lindheimeri grown with capacitance sensor-controlled irrigation. HortScience 2008, 43, 1555–1560. [Google Scholar] [CrossRef]
- Van Iersel, M.W.; Dove, S.; Kang, J.G.; Burnett, S.E. Growth and water use of petunia as affected by substrate water content and daily light integral. HortScience 2010, 45, 277–282. [Google Scholar] [CrossRef]
- Bilderback, T.E. Water management is key in reducing nutrient runoff from container nurseries. HortTechnology 2002, 12, 541–544. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 1 March 2023).
- Yen, J.; Lin, K.; Wang, U. Potential of the insecticides acephate and methamidophos to contaminate groundwater. Ecotox Environ. Saf. 2000, 45, 79–86. [Google Scholar] [CrossRef]
- Sánchez-Camazano, M.; Gonzalez-Pozuelo, J.M.; Sanchez-Martin, M.J.; Crisanto, T. Adsorption and mobility of acephate in soils. Ecotox Environ. Saf. 1993, 29, 61–69. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, X.; Wang, L.; Huang, C.; Zhang, Y.; Zhao, M. Developmental toxicity of bifenthrin in embryo-larval stages of zebrafish. Aquat. Toxicol. 2009, 95, 347–354. [Google Scholar] [CrossRef]
- Lee, S.; Gan, J.; Kim, J.S.; Kabashima, J.N.; Crowley, D.E. Microbial transformation of pyrethroid insecticides in aqueous and sediment phases. Environ. Toxicol. Chem. 2004, 23, 1–6. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Ma, C.; Wu, N.; Li, C.; Yang, X. Simultaneous degradation of bifenthrin and chlorpyrifos by Pseudomonas sp. CB2. J. Environ. Sci. Health B 2018, 53, 304–312. [Google Scholar] [CrossRef]
- Sardiña, P.; Leahy, P.; Metzeling, L.; Stevenson, G.; Hinwood, A. Emerging and legacy contaminants across land-use gradients and the risk to aquatic ecosystems. Sci. Total Environ. 2019, 695, 133842. [Google Scholar] [CrossRef] [PubMed]
- Bertotto, L.B.; Dasgupta, S.; Vliet, S.; Dudley, S.; Gan, J.; Volz, D.C.; Schlenk, D. Evaluation of the estrogen receptor alpha as a possible target of bifenthrin effects in the estrogenic and dopaminergic signaling pathways in zebrafish embryos. Sci. Total Environ. 2019, 651, 2424–2431. [Google Scholar] [CrossRef] [PubMed]
- Brander, S.M.; Gabler, M.K.; Fowler, N.L.; Connon, R.E.; Schlenk, D. Pyrethroid pesticides as endocrine disruptors: Molecular mechanisms in vertebrates with a focus on fishes. Environ. Sci. Technol. 2016, 50, 8977–8992. [Google Scholar] [CrossRef]
- Weston, D.P.; Holmes, R.W.; You, J.; Lydy, M.J. Aquatic toxicity due to residential use of pyrethroid insecticides. Environ. Sci. Technol. 2005, 39, 9778–9784. [Google Scholar] [CrossRef]
- Camper, N.D.; Kim, J.H.; Riley, M.B. Degradation of isoxaben in soils and an aqueous system. J. Environ. Sci. Health B 2001, 36, 729–739. [Google Scholar] [CrossRef]
- Walker, A. Evaluation of a simulation model for prediction of herbicide movement and persistence in soil. Weed Res. 1987, 27, 143–152. [Google Scholar] [CrossRef]
- Triantafyllidis, V.; Hela, D.; Papadaki, M.; Bilalis, D.; Konstantinou, I. Evaluation of mobility and dissipation of mefenoxam and pendimethalin by application of CSTR model and field experiments using bare and tobacco tilled soil columns. Water Air Soil Pollut. 2012, 223, 1625–1637. [Google Scholar] [CrossRef]
- Alister, C.A.; Gomez, P.A.; Rojas, S.; Kogan, M. Pendimethalin and oxyfluorfen degradation under two irrigation conditions over four years application. J. Environ. Sci. Health B 2009, 44, 337–343. [Google Scholar] [CrossRef]
- Milhome, M.A.L.; Sousa, P.L.R.; Lima, F.A.F.; Nascimento, R.F. Influence the use of pesticides in the quality of surface and groundwater located in irrigated areas of Jaguaribe, Ceara, Brazil. Int. J. Environ. Res. 2015, 9, 255–262. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Seidler, F.J.; Wu, C.; MacKillop, E.A.; Linden, K.G. Ultraviolet photolysis of chlorpyrifos: Developmental neurotoxicity modeled in PC12 cells. Environ. Health Perspect. 2008, 117, 338–343. [Google Scholar] [CrossRef]
- Muñoz, A.; Ródenas, M.; Borrás, E.; Vázquez, M.; Vera, T. The gas-phase degradation of chlorpyrifos and chlorpyrifos-oxon towards OH radical under atmospheric conditions. Chemosphere 2014, 111, 522–528. [Google Scholar] [CrossRef]
- Briceño, G.; Fuentes, M.S.; Palma, G.; Jorquera, M.A.; Amoroso, M.J.; Diez, M.C. Chlorpyrifos biodegradation and 3,5,6-trichloro-2-pyridinol production by actinobacteria isolated from soil. Int. Biodeterior. Biodegrad. 2012, 73, 1–7. [Google Scholar] [CrossRef]
- Xi, J.; Shao, J.; Wang, Y.; Wang, X.; Yang, H.; Zhang, X.; Xiong, D. Acute toxicity of triflumizole to freshwater green algae Chlorella vulgaris. Pestic. Biochem. Physiol. 2019, 158, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Dion, H.M.; Harsh, J.B.; Hill, H.H., Jr. Competitive sorption between glyphosate and inorganic phosphate on clay minerals and low organic matter soils. J. Radioanal. Nucl. Chem. 2001, 249, 385–390. [Google Scholar] [CrossRef]
- Sasal, M.C.; Demonte, L.; Cislaghi, A.; Gabioud, E.A.; Oszust, J.D.; Wilson, M.G.; Michlig, N.; Beldoménico, H.R.; Repetti, M.R. Glyphosate loss by runoff and its relationship with phosphorus fertilization. J. Agric. Food Chem. 2015, 63, 4444–4448. [Google Scholar] [CrossRef]
- Saunders, L.E.; Pezeshki, R. Glyphosate in runoff waters and in the root-zone: A review. Toxics 2015, 3, 462–480. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Kolpin, D.W.; Scribner, E.A.; Kuivila, K.M.; Sandstrom, M.W. Glyphosate, other herbicides, and transformation products in Midwestern streams. J. Am. Water Resour. Assoc. 2005, 41, 323–332. [Google Scholar] [CrossRef]
- Briggs, J.; Whitwell, T.; Fernandez, R.T.; Riley, M.B. Effect of integrated pest management strategies on chlorothalonil, metalaxyl, and thiophanate-methyl runoff at a container nursery. J. Am. Soc. Hortic. Sci. 2002, 127, 1018–1024. [Google Scholar] [CrossRef]
- Cycoń, M.; Wójcik, M.; Piotrowska-Seget, Z. Biodegradation kinetics of the benzimidazole fungicide thiophanate-methyl by bacteria isolated from loamy sand soil. Biodegradation 2011, 22, 573–583. [Google Scholar] [CrossRef]
- National Pesticide Information Center. Available online: https://www.npic.orst.edu/ (accessed on 11 March 2025).
- Stearman, G.K.; George, D.B.; Hutchings, L.D. Removal of nitrogen, phosphorus, and prodiamine from a container nursery by a subsurface flow constructed wetland. J. Bioremediat. Biodegrad. 2012, S7, 1–5. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Available online: https://www.epa.gov/pesticides/epa-proposes-cancel-all-one-use-pesticide-acephate-protect-human-health#:~:text=Home-,EPA%20Proposes%20to%20Cancel%20All%20but%20One%20Use,Acephate%20to%20Protect%20Human%20Health (accessed on 11 March 2025).
- Environmental Protection Agency. Available online: https://www.epa.gov/pesticide-worker-safety/epa-update-use-pesticide-chlorpyrifos-food (accessed on 11 March 2025).
- European Commission. Available online: https://food.ec.europa.eu/plants/pesticides/approval-active-substances-safeners-and-synergists/renewal-approval/chlorpyrifos-chlorpyrifos-methyl_en (accessed on 11 March 2025).


| Active Ingredient: | Water Solubility (mg/L) z | Koc z | Vapor Pressure mm Hg z | Half-Life Soil (days) z | Half-Life Water (days) z | LC50 (µg L−1) Daphnia magna | LC50 (µg L−1) Onchorynchus mykiss | Mccalls (Koc Class) y | FAO Classification of Mobility (Koc) x | FAO Classification of Mobility (Solubility) x |
|---|---|---|---|---|---|---|---|---|---|---|
| Acephate | 818,000 | 4.7 | 1.7 × 10−6 | <3–32 | 6–20 | 1100–71,800 z,w,v | 1000–800,000 | Very High | Highly Mobile | Highly Soluble |
| Mefenoxam | 26,000 | 660 | 5.62 × 10−6 | 70 | 7–27 | 900–4100 u | 470 | Low | Moderately Mobile | Highly Soluble |
| Glyphosate | 10,500 | 2600–4900 | 1.84 × 10−7 | 47 | 3–91 | 234,000 z | 77,600–134,000 | Slightly | Slightly Mobile | Highly Soluble |
| Thiophanate-Methyl | 26.6 | 330 | 7.13 × 10−8 | 7 | 5 | 16,000 z | 7800–25,200 | Medium | Moderately Mobile | Readily Soluble |
| Triflumizole | 10.2 | 1400 | 1.4 × 10−6 | 18 | 4 | 1420 z,t | 580 | Low | Slightly Mobile | Readily Soluble |
| Isoxaben | 1.42 | 3300 | 4.13 × 10−9 | 100 | 14 | 1300 s | 1100 | Slightly | Slightly Mobile | Moderately Soluble |
| Chlorpyrifos | 1.4 | 995–31,000 | 2.02 × 10−5 | 7–120 | 35–78 | 1–3.7 z,r | 8–550 | Low to Immobile | Moderately to Hardly Mobile | Moderately Soluble |
| Oxyfluorfen | 0.116 | 8900 | 2 × 10−7 | 30–70 | 17–28 | 80 z,q | 410 | Immobile | Slightly Mobile | Slightly Soluble |
| Prodiamine | 0.013 | 5440–16,200 | 5.6 × 10−6 | 69–120 | 1.4–5.1 | 660 p | 830 | Immobile | Slightly to Hardly Mobile | Not Soluble |
| Bifenthrin | <0.001 | 8387–14,332 | 1.81 × 10−7 | 122–345 | 276–416 | 0.86–12.4 z | 0.15 | Immobile | Slightly to Hardly Mobile | Not Soluble |
| Monitoring Period 1 | Monitoring Period 2 | Monitoring Period 3 | |
|---|---|---|---|
| Total Precipitation (mm) | 70.09 | 22.83 | 16.75 |
| Total kL ha−1 Applied | |||
| Control | 3238 a z | 3238 a | 3238 a |
| SS2Lpd | 827 b | 763 b | 827 b |
| SSθ | 733 b | 641 b | 935 b |
| Total Days of Irrigation Applied | |||
| Control | 17 a | 17 a | 17 a |
| SS2Lpd | 17 a | 17 a | 17 a |
| SSθ | 12 b | 13 b | 15 a |
| Sample Day | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 8 | 16 | % Recovered | |
| Acephate Load (g) Insecticide applied as Acephate 97UP at 553 g ha−1 active ingredient | ||||||
| Treatment | Surface Return Flow | |||||
| Control | - | 56.74 | 12.11 a | 5.89 a | 2.93 a | 14.05 |
| SSLpd | - | 0 | 0.03 b | 0.97 b | 5.56 a | 1.19 |
| SSθ | - | 0 | 0.02 b | 0 b | 3.39 a | 0.62 |
| Subsurface Return Flow | ||||||
| Control | - | 26.07 a | 40.19 a | 18.97 a | 9.63 a | 17.15 |
| SSLpd | - | 1.17 a | 42.69 a | 15.88 a | 29.68 a | 16.17 |
| SSθ | - | 0.93 a | 3.59 a | 8.65 a | 21.66 a | 6.30 |
| Bifenthrin Load (mg) Insecticide applied as Talstar P at 130,000 mg ha−1 active ingredient | ||||||
| Surface Return Flow | ||||||
| Control | - | 405.27 | 4.42 a | 9.82 a | 34.79 a | 0.35 |
| SSLpd | - | 0 | 0.01 b | 0.08 a | 35.33 a | 0.03 |
| SSθ | - | 0 | 0.07 b | 0 a | 17.87 a | 0.01 |
| Subsurface Return Flow | ||||||
| Control | - | 10.15 a | 6.10 a | 3.26 a | 9.87 a | 0.02 |
| SSLpd | - | 4.07 ab | 5.14 a | 2.10 a | 12.43 a | 0.02 |
| SSθ | - | 3.44 b | 0.64 b | 1.11 a | 10.73 a | 0.01 |
| Isoxaben Load (g) Herbicide applied as Gallery 75DF at 867 g ha−1 active ingredient | ||||||
| Surface Return Flow | ||||||
| Control | 14.86 a | 36.08 | 9.73 a | 8.71 a | 0.15 a | 8.02 |
| SSLpd | 5.11 ab | 0 | 0.02 b | 0.13 b | 0.69 a | 0.69 |
| SSθ | 0.16 b | 0 | <0.01 b | 0 b | 0.36 a | 0.06 |
| Subsurface Return Flow | ||||||
| Control | - | 19.02 a | 13.65 a | 4.44 a | 0.29 a | 4.31 |
| SSLpd | - | 2.61 b | 2.19 b | 1.16 b | 0.90 a | 0.79 |
| SSθ | - | 0.58 b | 0.03 b | 0.06 b | 0.87 a | 0.18 |
| Mefenoxam Load (mg) Fungicide applied as Mefenoxam 2AQ at 18,200 mg ha−1 active ingredient | ||||||
| Surface Return Flow | ||||||
| Control | - | 2825.10 | 53.37 a | 31.12 a | 32.15 a | 16.16 |
| SSLpd | - | 0 | 0.04 b | 5.48 b | 63.16 a | 0.38 |
| SSθ | - | 0 | 0.07 b | 0 b | 46.03 a | 0.25 |
| Subsurface Return Flow | ||||||
| Control | - | 623.72 a | 241.70 a | 54.50 a | 59.45 a | 5.38 |
| SSLpd | - | 6.12 a | 131.30 ab | 55.21 a | 236.38 a | 2.36 |
| SSθ | - | 6.63 a | 4.87 b | 10.14 a | 145.08 a | 0.92 |
| Sample Day | ||||||
| 1 | 2 | 4 | 8 | 16 | % Recovered | |
| Chlorpyrifos Load (mg) Insecticide applied as Lorsban 4E at 1,145,800 mg ha−1 active ingredient | ||||||
| Treatment | Surface Return Flow | |||||
| Control | 11248.01 a | 1885.64 a | 1123.88 a | 118.24 a | 85.75 a | 1.26 |
| SSLpd | 103.94 b | 0 b | 126.30 b | 6.86 b | 0 b | 0.02 |
| SSθ | 1.00 b | 0 b | 0 b | 0 b | 0 b | <0.01 |
| Subsurface Return Flow | ||||||
| Control | 111.74 a | 90.70 a | 152.17 a | 45.22 a | 13.44 a | 0.04 |
| SSLpd | 22.20 b | 8.44 a | 2.00 b | 6.62 a | 1.87 a | <0.01 |
| SSθ | 7.22 b | 1.35 a | 3.91 b | 23.07 a | 9.19 a | <0.01 |
| Glyphosate Load (g) Herbicide applied as Roundup PowerMax at 2078 g ha−1 active ingredient | ||||||
| Surface Return Flow | ||||||
| Control | 1.07 a | 68.66 a | 9.84 a | 0.51 a | 0.08 a | 3.86 |
| SSLpd | 0.21 a | 0 a | 1.20 a | 0.01 a | 0 b | 0.07 |
| SSθ | 0.19 a | 0 a | 1.20 a | 0.01 a | 0 b | 0.07 |
| Subsurface Return Flow | ||||||
| Control | 0.43 a | 0.55 a | 0.61 a | 0.10 a | 0.08 a | 0.08 |
| SSLpd | 0.22 a | 0.58 a | 0.81 a | 2.37 a | 0.41 a | 0.21 |
| SSθ | 0.08 a | 0.03 a | 1.49 a | 2.00 a | 0.17 a | 0.18 |
| Oxyfluorfen Load (mg) Herbicide applied as Goaltender at 1,142,200 mg ha−1 active ingredient | ||||||
| Surface Return Flow | ||||||
| Control | 2902.85 a | 495.20 a | 948.82 a | 175.64 a | 183.95 a | 0.41 |
| SSLpd | 213.97 b | 0 b | 52.63 b | 6.22 b | 0 b | 0.02 |
| SSθ | 78.20 b | 0 b | 0 b | 0 b | 0 b | <0.01 |
| Subsurface Return Flow | ||||||
| Control | 16.32 a | 23.30 a | 29.72 a | 6.15 a | 2.58 a | <0.01 |
| SSLpd | 13.21 a | 12.97 a | 1.58 a | 7.30 a | 2.09 a | <0.01 |
| SSθ | 1.17 a | 3.82 a | 2.59 a | 6.23 a | 3.39 a | <0.01 |
| Triflumizole Load (mg) Fungicide applied as Terraguard SC at 288,500 mg ha−1 active ingredient | ||||||
| Surface Return Flow | ||||||
| Control | 2527.23 a | 730.37 a | 442.20 a | 20.01 a | 26.745 a | 1.30 |
| SSLpd | 90.04 b | 0 b | 41.28 ab | 3.78 ab | 0 b | 0.05 |
| SSθ | 0.41 b | 0 b | 0 b | 0 b | 0 b | <0.01 |
| Subsurface Return Flow | ||||||
| Control | 168.16 a | 397.91 a | 268.13 a | 22.48 a | 5.11 a | 0.30 |
| SSLpd | 13.41 b | 3.79 b | 1.81 b | 6.76 a | 2.45 a | 0.01 |
| SSθ | 2.12 b | 0.58 b | 2.95 b | 36.87 a | 8.48 a | 0.02 |
| Sample Day | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 11 | 16 | % Recovered | |
| Glyphosate Load (g) Herbicide applied as Roundup PowerMax at 2078 g ha−1 active ingredient | ||||||
| Treatment | Surface Return Flow | |||||
| Control | 84.35 a | 17.16 a | 2.17 a | 0.34 a | 0.21 a | 5.02 |
| SSLpd | 0.24 b | 0 b | 0 b | 10.73 a | 0 b | 0.53 |
| SSθ | 0 b | 0 b | 0 b | 0.58 a | 0 b | <0.01 |
| Subsurface Return Flow | ||||||
| Control | .z | 4.15 a | 0.95 a | . | 0.15 a | 0.25 |
| SSLpd | 0.23 a | 0.08 b | 0.08 b | . | 0.09 a | 0.02 |
| SSθ | 0.02 b | 0.01 b | <0.01 c | . | 0.07 a | 0.01 |
| Prodiamine Load (mg) Herbicide applied as Barricade 65WG at 1,697,900 mg ha−1 active ingredient | ||||||
| Surface Return Flow | ||||||
| Control | 406.01 a | 756.93 a | 249.02 a | 2302.24 a | 252.26 a | 0.23 |
| SSLpd | 8.71 b | 0 b | 0 b | 1245.00 a | 0 b | 0.07 |
| SSθ | 0 b | 0 b | 0 b | 61.45 a | 0 b | <0.01 |
| Subsurface Return Flow | ||||||
| Control | . | 25.39 a | 24.13 a | . | 94.21 a | 0.01 |
| SSLpd | 4.77 a | 4.25 b | 1.84 b | . | 4.19 b | <0.01 |
| SSθ | 0.51 a | 0.17 b | 0.09 b | . | 3.76 b | <0.01 |
| Thiophanate-Methyl Load (mg) Fungicide applied as Thiophanate Methyl 85WDG at 482,500 mg ha−1 active ingredient | ||||||
| Surface Return Flow | ||||||
| Control | 28,694.04 a | 3644.97 a | 4.68 a | 8.49 a | 5.16 a | 6.71 |
| SSLpd | 7.279 b | 0 b | 0 b | 38.13 a | 0 b | 0.01 |
| SSθ | 0 b | 0 b | 0 b | 18.16 a | 0 b | <0.01 |
| Subsurface Return Flow | ||||||
| Control | . | 106.49 a | 3.04 a | . | 3.63 a | 0.23 |
| SSLpd | 3.91 a | 2.367 b | 1.61 a | . | 2.40 a | <0.01 |
| SSθ | 0.51 b | 0.22 b | 0.09 b | . | 1.85 a | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdi, D.E.; Owen, Jr., J.S.; Wilson, P.C.; Hinz, F.O.; Cregg, B.M.; Fernandez, R.T. Pesticide Mobility in Surface and Subsurface Irrigation Return Flow in a Container Plant Production System. Water 2025, 17, 953. https://doi.org/10.3390/w17070953
Abdi DE, Owen, Jr. JS, Wilson PC, Hinz FO, Cregg BM, Fernandez RT. Pesticide Mobility in Surface and Subsurface Irrigation Return Flow in a Container Plant Production System. Water. 2025; 17(7):953. https://doi.org/10.3390/w17070953
Chicago/Turabian StyleAbdi, Damon E., James S. Owen, Jr., P. Christopher Wilson, Francisca O. Hinz, Bert M. Cregg, and R. Thomas Fernandez. 2025. "Pesticide Mobility in Surface and Subsurface Irrigation Return Flow in a Container Plant Production System" Water 17, no. 7: 953. https://doi.org/10.3390/w17070953
APA StyleAbdi, D. E., Owen, Jr., J. S., Wilson, P. C., Hinz, F. O., Cregg, B. M., & Fernandez, R. T. (2025). Pesticide Mobility in Surface and Subsurface Irrigation Return Flow in a Container Plant Production System. Water, 17(7), 953. https://doi.org/10.3390/w17070953







