Adsorption Performance of Fe2O3-Modified Dolomite Composite (DFC) for Congo Red Removal
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of DFC
2.3. Characterization of DFC
2.4. Recycling and Regeneration of DFC
2.5. Adsorption Capability Evaluation
3. Results and Discussion
3.1. DFC Characterization
3.1.1. XRF Analysis
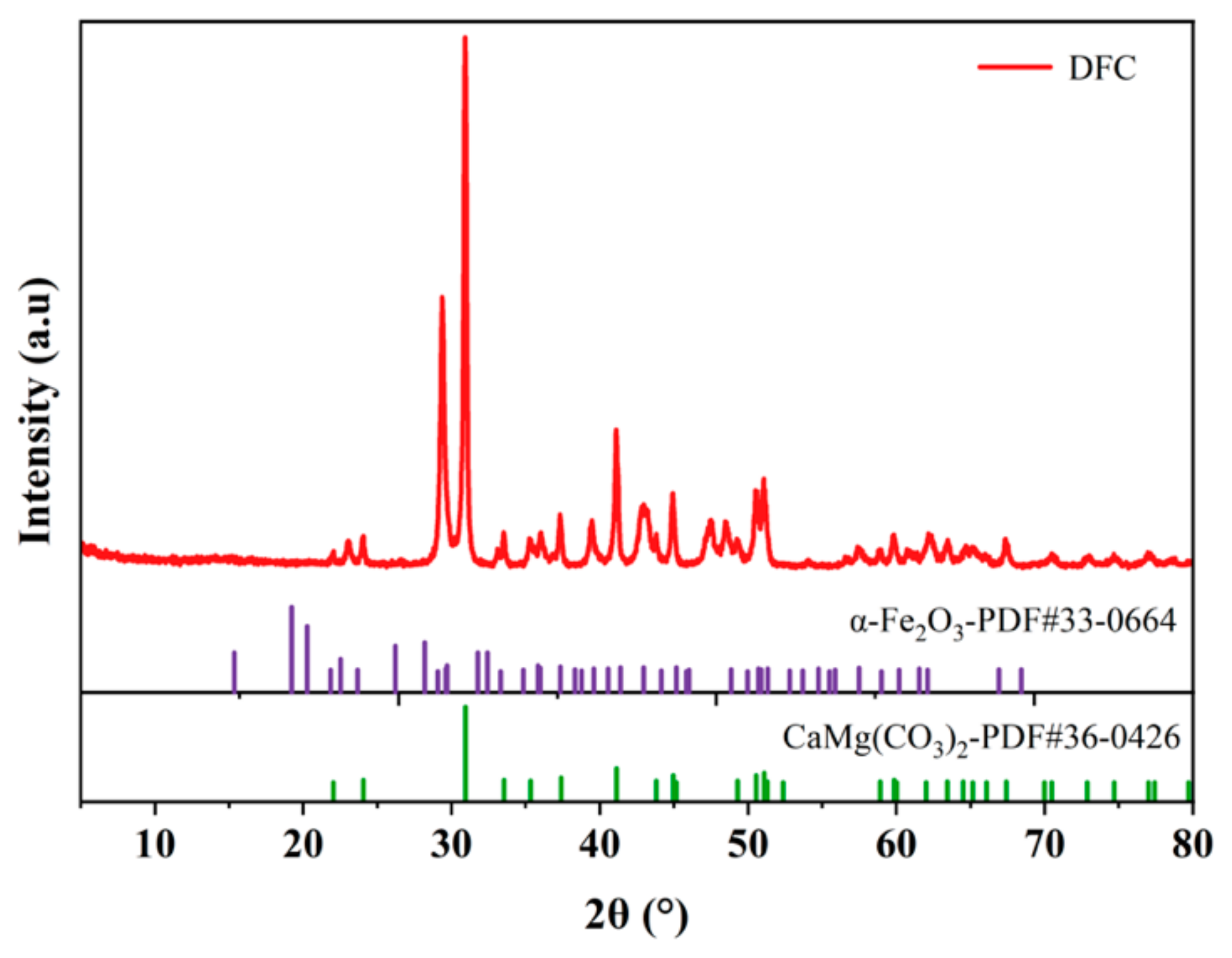
3.1.2. XRD Analysis
3.1.3. FTIR Analysis
3.1.4. XPS Analysis
3.1.5. SEM and TEM Analysis
3.2. Surface and Thermal Properties
3.3. Adsorption Properties
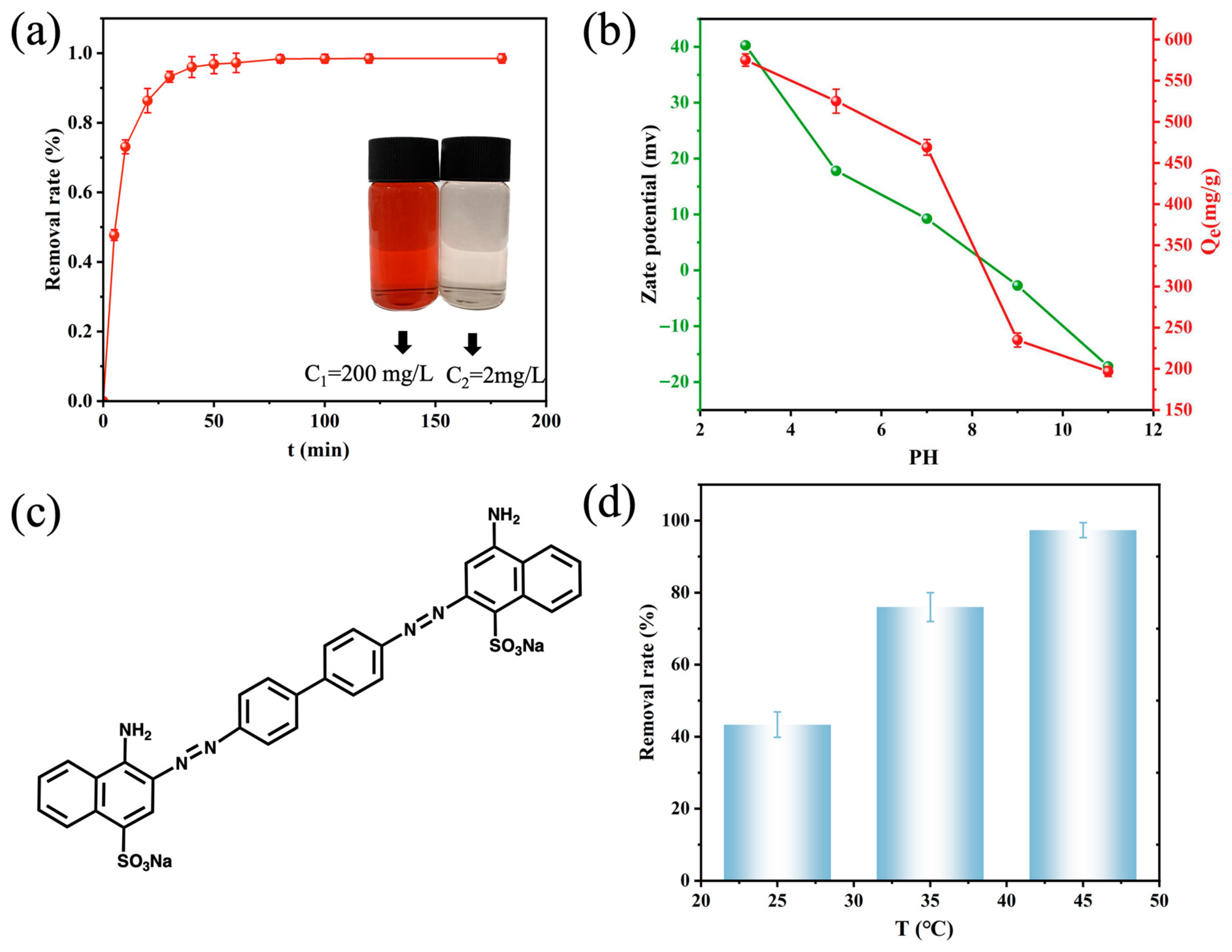
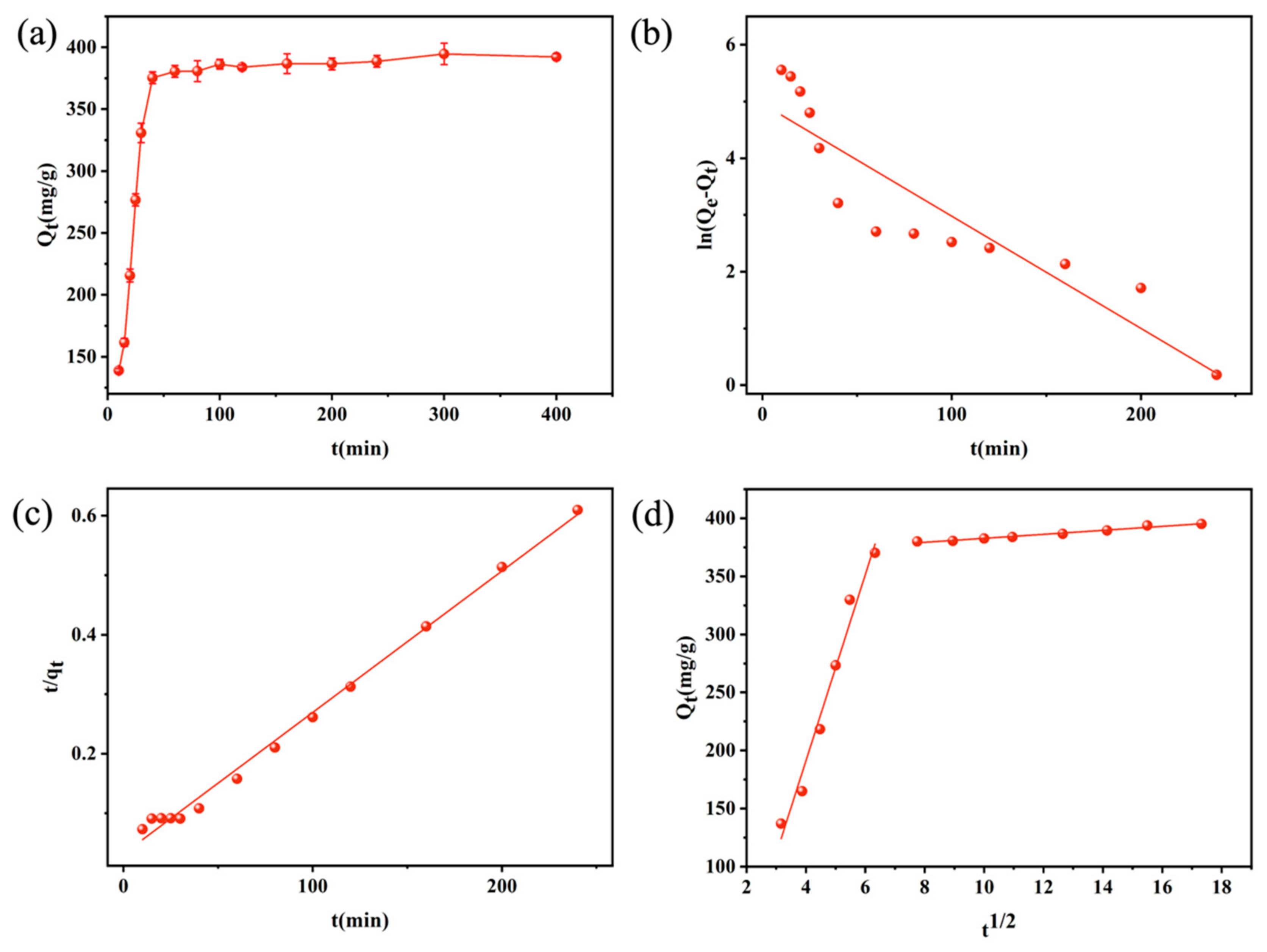
3.3.1. Temporal Variation in CR Removal by DFC
3.3.2. Structural Characteristics of CR
3.3.3. Effect of pH on CR Adsorption and Zeta Potential of DFC
3.3.4. Effect of Temperature on CR Adsorption
3.4. Analysis of Adsorption Isotherms
3.4.1. Langmuir Isotherm Model
3.4.2. Freundlich Isotherm Model
3.4.3. Dubinin–Radushkevich (D-R) Isotherm Model
3.5. Mechanism Study of Adsorption
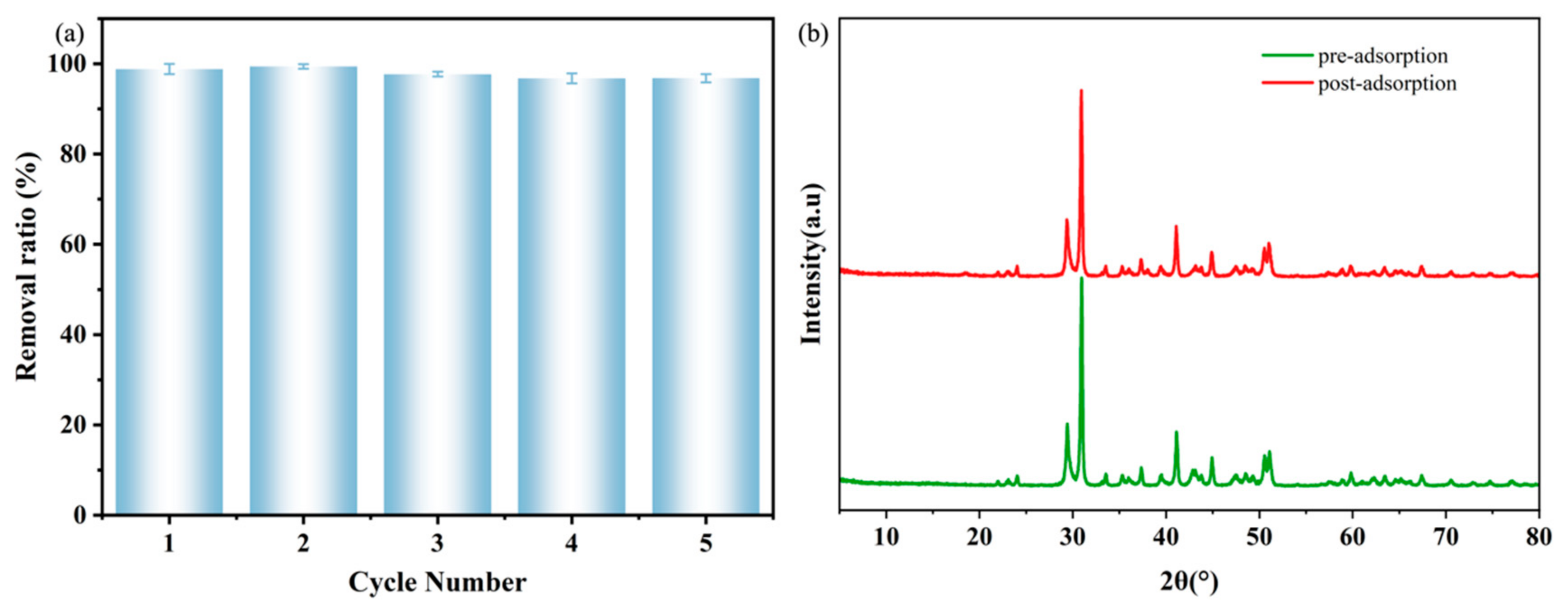
3.6. Cycling Stability and Performance of the DFC
4. Practical Implications and Challenges
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, M.V.P.; Shankar, K.R. Next-generation hybrid technologies for the treatment of pharmaceutical industry effluents. J. Environ. Manag. 2024, 353, 120197. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Sehar, S.; Guan, X.; Aftab, S.; Manaa, H.; Mahmood, T.; Iqbal, J.; Akram, F.; Ali, N.; Wu, T. Four-in-one strategy to boost the performance of 3-dimensional MoS2 nanostructures for industrial effluent treatment and hydrogen evolution reactions. J. Alloys Compd. 2024, 976, 173104. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ye, W.; Xie, M.; Seo, D.H.; Luo, J.; Wan, Y.; Van der Bruggen, B. Environmental impacts and remediation of dye-containing wastewater. Nat. Rev. Earth Environ. 2023, 4, 785–803. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater treatment by advanced oxidation process and their worldwide research trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef]
- Ponnusami, A.B.; Sinha, S.; Ashokan, H.; Paul, M.V.; Hariharan, S.P.; Arun, J.; Gopinath, K.; Le, Q.H.; Pugazhendhi, A. Advanced oxidation process (AOP) combined biological process for wastewater treatment: A review on advancements, feasibility and practicability of combined techniques. Environ. Res. 2023, 237, 116944. [Google Scholar] [CrossRef]
- Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Natural-based coagulants/flocculants as sustainable market-valued products for industrial wastewater treatment: A review of recent developments. RSC Adv. 2023, 13, 19335–19355. [Google Scholar] [CrossRef]
- Vasiljević, S.; Vujić, M.; Agbaba, J.; Federici, S.; Ducoli, S.; Tomić, R.; Tubić, A. Efficiency of coagulation/flocculation for the removal of complex mixture of textile fibers from water. Processes 2023, 11, 820. [Google Scholar] [CrossRef]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, Z.; Wu, Y.; Wang, X.; Xu, H.; Li, L. Removing multiple refractory organic pollutants in wastewater by cell bioaugmentation and genetic bioaugmentation with Rhodococcus sp. strain p52. J. Water Process Eng. 2024, 66, 106093. [Google Scholar] [CrossRef]
- Zeng, B.; Tao, B.; Pan, Z.; Shen, L.; Zhang, J.; Lin, H. A low-cost and sustainable solution for nitrate removal from secondary effluent: Macroporous ion exchange resin treatment. J. Environ. Manag. 2023, 347, 119142. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Sadia, M.; Azeem, M.; Ahmad, M.Z.; Umar, M.; Abbas, Z.U. Ion Exchange Resins and their Applications in Water Treatment and Pollutants Removal from Environment: A Review: Ion Exchange Resins and their Applications. Futur. Biotechnol. 2023, 3, 12–19. [Google Scholar] [CrossRef]
- Tomczyk, A.; Kubaczyński, A.; Szewczuk-Karpisz, K. Assessment of agricultural waste biochars for remediation of degraded water-soil environment: Dissolved organic carbon release and immobilization of impurities in one-or two-adsorbate systems. Waste Manag. 2023, 155, 87–98. [Google Scholar] [CrossRef]
- Vakili, A.; Zinatizadeh, A.; Rahimi, Z.; Zinadini, S.; Mohammadi, P.; Azizi, S.; Karami, A.; Abdulgader, M. The impact of activation temperature and time on the characteristics and performance of agricultural waste-based activated carbons for removing dye and residual COD from wastewater. J. Clean. Prod. 2023, 382, 134899. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Kumar, N.; Pandey, A.; Sharma, Y.C. A review on sustainable mesoporous activated carbon as adsorbent for efficient removal of hazardous dyes from industrial wastewater. J. Water Process Eng. 2023, 54, 104054. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Przybek, A. Assessment of Physico-Chemical Behavior and Sorptivity—Diatomaceous Earth as Support for Paraffinic Phase-Change Materials. Materials 2024, 17, 4691. [Google Scholar] [CrossRef]
- Taghavi, R.; Rostamnia, S.; Farajzadeh, M.; Karimi-Maleh, H.; Wang, J.; Kim, D.; Jang, H.W.; Luque, R.; Varma, R.S.; Shokouhimehr, M. Magnetite metal–organic frameworks: Applications in environmental remediation of heavy metals, organic contaminants, and other pollutants. Inorg. Chem. 2022, 61, 15747–15783. [Google Scholar] [CrossRef]
- De Smedt, J.; Heynderickx, P.M.; Arauzo, P.J.; Ronsse, F. Adsorption mechanism of different dyes on chemical activated carbon as quantitative assessment for wastewater treatment: Comparative study between ZnCl2 and its eutectic. Sep. Purif. Technol. 2024, 334, 126002. [Google Scholar] [CrossRef]
- Ritter, M.T.; Lobo-Recio, M.Á.; Padilla, I.; Nagel-Hassemer, M.E.; Romero, M.; López-Delgado, A. Adsorption of Safranine-T dye using a waste-based zeolite: Optimization, kinetic and isothermal study. J. Ind. Eng. Chem. 2024, 136, 177–187. [Google Scholar] [CrossRef]
- Chen, H.; Gao, R.; Ma, Q. Preparation and adsorption kinetics and thermodynamics of sodium humate-modified diatomite. Int. J. Appl. Ceram. Technol. 2024, 21, 1395–1407. [Google Scholar] [CrossRef]
- Liu, P.; Lyu, J.; Bai, P. Synthesis of mixed matrix membrane utilizing robust defective MOF for size-selective adsorption of dyes. Sep. Purif. Technol. 2025, 354, 128672. [Google Scholar] [CrossRef]
- Ren, Q.; Chen, P.; Yang, Y.; Wei, F.; Chen, H.; Wang, S.; Liang, Z. Synthesis of metal–organic frameworks for the adsorption of Congo Red from wastewater. Sci. Adv. Mater. 2023, 15, 1159–1165. [Google Scholar] [CrossRef]
- Liu, J.; Wang, N.; Zhang, H.; Baeyens, J. Adsorption of Congo red dye on FexCo3-xO4 nanoparticles. J. Environ. Manag. 2019, 238, 473–483. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Q.; Li, X.; Ca, I.O. Mg2+ with dolomite (1 0 4) surface and its effect on caproic acid adsorption: DFT calculation. Appl. Surf. Sci. 2023, 614, 156244. [Google Scholar] [CrossRef]
- Guo, M.; Wang, Z.; Xiao, J.; Chen, D.; Lv, Z. The role of hydrotalcite in the chloride binding of hydrated C3A pastes containing dolomite. Constr. Build. Mater. 2023, 402, 132975. [Google Scholar] [CrossRef]
- Van Pham, V.; La, H.P.P.; Le, T.Q.; Nguyen, P.H.; Van Le, T.; Cao, T.M. Fe2O3/diatomite materials as efficient photo-Fenton catalysts for ciprofloxacin removal. Environ. Sci. Pollut. Res. 2023, 30, 33686–33694. [Google Scholar] [CrossRef]
- Li, Y.; Gao, L.; Lu, Z.; Wang, Y.; Wang, Y.; Wan, S. Enhanced removal of heavy metals from water by hydrous ferric oxide-modified biochar. ACS Omega 2020, 5, 28702–28711. [Google Scholar] [CrossRef]
- Oyehan, T.A.; Laoui, T.; Tawabini, B.; Patel, F.; Olabemiwo, F.A.; Atieh, M.A. Enhancing the adsorptive capacity of carbon nanofibers by impregnation with ferric oxide for the removal of cadmium from aqueous solution. J. Water Process Eng. 2021, 42, 102130. [Google Scholar] [CrossRef]
- Duojie, Z.; Chen, K.; Chen, J.; Zeng, Q.; Bai, J.; Li, T.; Ma, C.; Zhang, M. Tailoring Morphology of MgO with Mg-MOF for the Enhanced Adsorption of Congo Red. ACS Omega 2024, 9, 41676–41686. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.F.A.; Saleh, S.S.M.; Mohammad, N.F.; Idris, M.S.; Saliu, H. Effect of thermal treatment on natural dolomite. J. Phys. Conf. Ser. 2021, 2080, 012009. [Google Scholar] [CrossRef]
- Ermrich, M.; Opper, D. X-Ray powder diffraction, XRD for the Analyst. In Getting Acquainted with the Principles; PANalytical GmbH: Kassel, Germany, 2011; pp. 63–85. [Google Scholar]
- Silva, M.A.; Hilliou, L.; de Amorim, M.P. Fabrication of pristine-multiwalled carbon nanotubes/cellulose acetate composites for removal of methylene blue. Polym. Bull. 2020, 77, 623–653. [Google Scholar] [CrossRef]
- Zhuravlev, Y.N.; Atuchin, V.V. First-principle studies of the vibrational properties of carbonates under pressure. Sensors 2021, 21, 3644. [Google Scholar] [CrossRef]
- Xu, B.; Poduska, K.M. Linking crystal structure with temperature-sensitive vibrational modes in calcium carbonate minerals. Phys. Chem. Chem. Phys. 2014, 16, 17634–17639. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Ye, Y.; Wang, C.; Liu, D.; Shi, X.; Wang, S.; Zhu, X. In-situ high-temperature XRD and FTIR for calcite, dolomite and magnesite: Anharmonic contribution to the thermodynamic properties. J. Earth Sci. 2019, 30, 964–976. [Google Scholar] [CrossRef]
- Arunkumar, B.; Jothibas, M.; Jeyakumar, S.J.; Jeyaseelan, S.J. Analysis of the magnetic possessions of α-Fe2O3 and the stimulation of α- Fe2O3 by Zn doping at different weight ratios. Appl. Phys. A 2023, 129, 679. [Google Scholar] [CrossRef]
- Bagus, P.S.; Nelin, C.J.; Brundle, C.; Crist, B.V.; Lahiri, N.; Rosso, K.M. Origin of the complex main and satellite features in Fe 2p XPS of Fe2O3. Phys. Chem. Chem. Phys. 2022, 24, 4562–4575. [Google Scholar] [CrossRef]
- Yao, C.; Wang, R.; Wang, Z.; Lei, H.; Dong, X.; He, C. Highly dispersive and stable Fe3+ active sites on 2D graphitic carbon nitride nanosheets for efficient visible-light photocatalytic nitrogen fixation. J. Mater. Chem. A 2019, 7, 27547–27559. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, T.; He, H.; Wang, N. Bifunctional interface modification for efficient and UV-robust α- Fe2O3-based planar organic–inorganic hybrid perovskite solar cells. Adv. Compos. Hybrid Mater. 2022, 5, 3212–3222. [Google Scholar] [CrossRef]
- Xu, M.; Guo, Z.; Li, L.; Zhu, Z.; Zhang, Q.; Hong, J. Degradation of azo dyes by sodium percarbonate activated with nanosheet Mn2O3@ α-Fe3O4. Chem. Ind. Eng. Prog. 2022, 41, 1043. [Google Scholar]
- Bo, J.; Shi, B. Performances of residues from hydrolyzed corn-cobs for the adsorption of Congo red. Ind. Crops Prod. 2024, 220, 119311. [Google Scholar] [CrossRef]
- Khan, O.; Anjikar, N.D.; Nalabothu, M.K.; Dunn, M.E.; Sweilem, W.B.; Yang, S. The Synthesis of Amino-Acid-Anchored Two-Dimensional Silicoaluminophosphates and Congo Red Adsorption Application. Langmuir 2024, 40, 10526–10533. [Google Scholar] [CrossRef]
- Hamd, A.; Salah, D.; Alyafei, H.F.; Soliman, N.K.; El-Reedy, A.A.; Elzanaty, A.M.; Al-Saeedi, S.I.; Al-Ghamdi, A.; Shaban, M.; El-Sayed, R. NaOH-activated natural glauconite for low-cost adsorption of Congo Red dye. Water 2023, 15, 3753. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, H.; Sang, M.; Liu, F.; Nie, G. Fabrication of a 3D Cellulose/MoS2 Aerogel for Efficient and Regenerative Adsorption of Congo Red. Water Air Soil Pollut. 2023, 234, 401. [Google Scholar] [CrossRef]
- Galvani, G.M.; Zito, C.A.; Perfecto, T.M.; Malafatti, J.O.D.; Paris, E.C.; Volanti, D.P. Two-dimensional NiO nanosheets for efficient Congo red adsorption removal. Mater. Chem. Phys. 2022, 290, 126591. [Google Scholar] [CrossRef]
- Zhai, S.; Chen, R.; Liu, J.; Xu, J.; Jiang, H. N-doped magnetic carbon aerogel for the efficient adsorption of Congo red. J. Taiwan Inst. Chem. Eng. 2021, 120, 161–168. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, T.; Li, J.; Hua, Y.; Dou, J.; Chen, X.; Li, S. Potassium citrate-derived porous carbon with high CO2 capture and Congo red adsorption performance. Environ. Sci. Eur. 2023, 35, 9. [Google Scholar] [CrossRef]
- Aghaei, F.; Tangestaninejad, S.; Bahadori, M.; Moghadam, M.; Mirkhani, V.; Mohammadpoor, I.; Khalaji, M.; Asadi, V. Green synthesize of nano-MOF-ethylcellulose composite fibers for efficient adsorption of Congo red from water. J. Colloid Interface Sci. 2023, 648, 78–89. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings; applications; solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Vareda, J.P. On validity; physical meaning, mechanism insights and regression of adsorption kinetic models. J. Mol. Liq. 2023, 376, 121416. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]




| No. | Element | Result | Unit |
|---|---|---|---|
| 1 | O | 55.871 | Mass% |
| 2 | Ca | 31.082 | Mass% |
| 3 | Mg | 12.784 | Mass% |
| 4 | other | 0.263 | Mass% |
| Sample | Specific Surface Area (m2⋅g−1) | Total Pore Volume (cm3⋅g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| Dolomite | 1.47 | 0.006 | 16.61 |
| DFC | 4.89 | 0.040 | 32.66 |
| Author | Material | Time (h) | Qe (mg/g) | Temperature (K) | Ref. |
|---|---|---|---|---|---|
| Q. Ren | Co-MOF1 | 0.6 | 1100 | 298 | [25] |
| J. Liu | FexCo3−xO4 | 4 | 128.6 | 298 | [26] |
| P.F. Silva | A-1/1 | 2.5 | 652.5 | 303 | [44] |
| O. Khan | 2D SAPO | 6 | 26.6 | 298 | [45] |
| A. Hamd | GLACT2M | 6 | 11.9 | 298 | [46] |
| S. Qiu | CAM | 2.5 | 334.92 | 298 | [47] |
| G.M. Galvani | NiO300 | 2 | 259.74 | 308 | [48] |
| S. Zhai | N-MCA | 0.8 | 431 | 318 | [49] |
| S. Wang | PCMCA-900 | 35 | 652.3 | 308 | [50] |
| F. Aghaei | EC/ZIF-67 | 0.8 | 357.42 | 298 | [51] |
| Pengfei yang | DFC | 3 | 3790.06 | 318 | This work |
| Model | Parameters | Values |
|---|---|---|
| Langmuir | qm | 3790.06 mg⋅g−1 |
| KL | 0.03274 L⋅mg−1 | |
| R2 | 0.996 | |
| Freundlich | KF | 378.6 mg⋅g−1 |
| n | 202.6 | |
| R2 | 0.901 | |
| D-R | Qm | 3265.7 mg⋅g−1 |
| E | 73.4 KJ⋅mol−1 | |
| R2 | 0.973 |
| Model | Parameters | Values |
|---|---|---|
| Pseudo-first-order | qe,cal | 142.17 mg⋅g−1 |
| K1 | 0.0197 min−1 | |
| R2 | 0.838 | |
| Pseudo-second-order | qe,cal | 420.17 mg⋅g−1 |
| K2 | 0.00018 g·mg−1·min−1 | |
| R2 | 0.994 | |
| Weber–Morris | C1 | −129.33 |
| K1 | 80.18 mg⋅g−1·min−0.5 | |
| R2 | 0.976 | |
| C2 | 365.519 | |
| K2 | 1.17 mg⋅g−1·min−0.5 | |
| R2 | 0.977 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Pan, L.; Lan, J.; Ye, Y.; Ao, R.; Xie, X.; Chen, Y.; Lan, X. Adsorption Performance of Fe2O3-Modified Dolomite Composite (DFC) for Congo Red Removal. Water 2025, 17, 1198. https://doi.org/10.3390/w17081198
Yang P, Pan L, Lan J, Ye Y, Ao R, Xie X, Chen Y, Lan X. Adsorption Performance of Fe2O3-Modified Dolomite Composite (DFC) for Congo Red Removal. Water. 2025; 17(8):1198. https://doi.org/10.3390/w17081198
Chicago/Turabian StyleYang, Pengfei, Lizhi Pan, Junfeng Lan, Youming Ye, Ran Ao, Xuezhen Xie, Yanmeng Chen, and Xingxian Lan. 2025. "Adsorption Performance of Fe2O3-Modified Dolomite Composite (DFC) for Congo Red Removal" Water 17, no. 8: 1198. https://doi.org/10.3390/w17081198
APA StyleYang, P., Pan, L., Lan, J., Ye, Y., Ao, R., Xie, X., Chen, Y., & Lan, X. (2025). Adsorption Performance of Fe2O3-Modified Dolomite Composite (DFC) for Congo Red Removal. Water, 17(8), 1198. https://doi.org/10.3390/w17081198





